Summary
Activated retina-specific T cells that have acquired the ability to break through the blood-retinal barrier are thought to be causally involved in autoimmune uveitis, a major cause of human blindness. It is unclear where these autoreactive T cells first become activated, given that their cognate antigens are sequestered within the immune privileged eye. We demonstrate in a novel mouse model of spontaneous uveitis that activation of retina-specific T cells is dependent on gut commensal microbiota. Retina-specific T cell activation involved signaling through the autoreactive T cell receptor (TCR) in response to non-cognate antigen in the intestine, and was independent of the endogenous retinal autoantigen. Our findings not only have implications for etiology of human uveitis, but also raise the possibility that activation of autoreactive TCRs by commensal microbes may be a more common trigger of autoimmune diseases than is currently appreciated.
INTRODUCTION
The skin and mucosal surfaces are densely populated by different kinds of commensal microorganisms that include bacteria, fungi and viruses that have co-evolved with their vertebrate hosts during millions of years. During recent years it has become clear that commensals affect multiple aspects of host physiology and homeostasis, including the normal development and functioning of the immune system (for reviews see Cerf-Bensussan and Gaboriau-Routhiau, 2010; Hooper et al., 2012). Consequently, dysregulation of the endogenous microbiota can have profound effects on host immune function (Lee et al., 2011; Proal et al., 2009; Round and Mazmanian, 2009; Turnbaugh and Gordon, 2009; Wen et al., 2008). The contribution of gut commensal microbiota to development of inflammatory and autoimmune diseases has been studied in various animal models (Berer et al., 2011; Garrett et al., 2010; Wu et al., 2010). A number of microorganisms, including Klebsiella pneumoniae, Proteus mirabilis and segmented filamentous bacteria (SFB) have been implicated as necessary, and in some cases sufficient (Garrett et al., 2010; Wu et al., 2010). However, while it is well established that IL-17-producing T cells are activated by microbial components in the gut environment (Ivanov et al., 2009; Wu et al., 2010), it is unclear how this relates to activation of tissue-specific autoimmune T cells. The recently published evidence that the Th17 cells elicited by SFB are actually specific to SFB antigens (Goto et al., 2014; Yang et al., 2014) makes the connection to tissue-specific pathology even less clear.
An extra layer of complexity arises for immune privileged sites, whose antigens are separated from the immune system by blood-tissue barriers. The eye is the prototypic immune-privileged tissue, but nevertheless it is subject to destructive autoimmunity (Caspi, 2010). Autoimmune uveitis is a major cause of blindness, having incidence similar to that of multiple sclerosis and accounting for up to 15% of severe visual handicap in the Western world (Durrani et al., 2004; Gritz and Wong, 2004). The disease affects working age population and has a significant public health impact. Patients often have detectable immune responses to unique retinal proteins involved in visual function such as retinal arrestin and interphotoreceptor retinoid binding protein (IRBP), which, upon immunization of laboratory rodents, elicit experimental autoimmune uveitis (EAU) (Caspi, 2010). However, unlike the experimental disease, most cases of human uveitis cannot be connected to an exposure of the immune system to ocular antigens. This presents a paradox, because in order to enter the healthy eye through the blood-retinal barrier the pathogenic T cells must first be activated, but the target antigens are sequestered within the eye and are not expressed in the periphery (Gonzalez-Fernandez et al., 1993). It therefore remains a major unresolved question where such retina-specific T cells become activated. To address this, we recently developed mice that express the R161 T cell receptor (TCR) specific to residues 161–180 of IRBP, a major uveitogenic epitope for uveitis susceptible B10.RIII mice (Horai et al., 2013). Mice of the R161H line develop uveitis spontaneously starting around weaning age and reach 100% incidence by 2 months, making them a robust and reproducible model of autoimmune uveitis (Horai et al., 2013).
In the present study we used the R161H mouse model of uveitis to study natural triggers of the disease. Our data indicate that a microbiota-dependent signal activates retina-specific T cells in the gut lamina propria that precedes clinical onset of the disease in the eyes. More importantly, activation of these T cells is independent of the endogenous antigen and involves signaling through the clonotypic autoreactive TCR by microbiota-dependent stimuli. Our study thus uncovers a novel mechanism whereby engagement of the specific TCR by non-cognate stimuli in the gut activates autoreactive T cells and contributes to autoimmune disease.
RESULTS
Spontaneous uveitis in R161H mice is associated with an enhanced Th17 phenotype in the gut
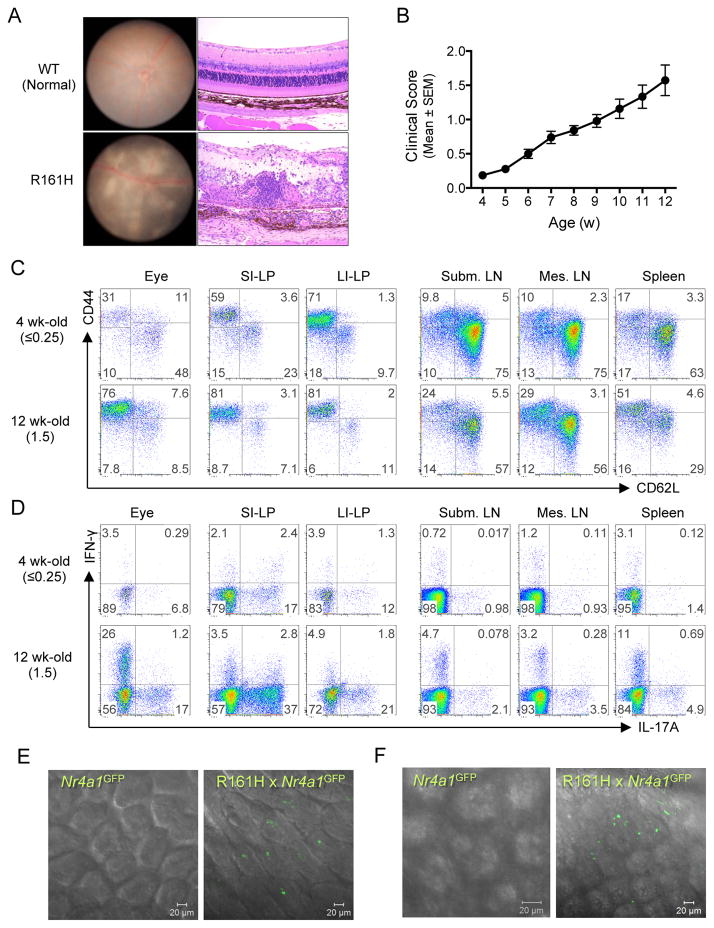
R161H mice develop uveitis spontaneously around weaning age (4 wk-old) reaching peak disease around 12 weeks (Figure 1A and 1B). In an attempt to define when and where the pathogenic cells become activated, thus acquiring the ability to actively cross the blood-retinal barrier, we searched for T cells with an activated phenotype in different tissues of young (4 wk-old) and adult (12 wk-old) R161H mice. Although 4 wk-old R161H mice had minimal or no detectable clinical disease and minimal ocular leukocytic infiltrate, they displayed high percentages of T cells with an activated/memory phenotype (CD44hiCD62Llo) in the small and large intestinal lamina propria (LP). This was in sharp contrast to peripheral lymphoid tissues, including the eye-draining submandibular lymph nodes (Figure 1C). Furthermore, these T cells could produce IL-17A (Figure 1D), a cytokine that our previous studies identified as pathogenic in autoimmune uveitis (Chi et al., 2008; Luger et al., 2008; Wang et al., 2012). To directly visualize the activation in vivo, we crossed the R161H mice to Nr4a1GFP reporter mice, in which intensity of GFP fluorescence reflects the level of Nur77 expression, and hence the strength of antigen receptor signaling (Moran et al., 2011). As early as 17 days of age, and well before clinical onset of disease, GFP signal in freshly explanted intestinal tissue of R161H-Nr4a1GFP mice was prominent, especially in the ileum, compared to “polyclonal” Nr4a1GFP controls (Figure 1E, and Movies S1 and S2), and was similar in intensity to the signal in adult R161H mice (Figure 1F and Movies S3 and S4). In contrast, the GFP signal in the submandibular lymph nodes as well as other peripheral lymph nodes was not enhanced (Figure S1). Collectively, these data support the notion that activation of T cells in the gut of R161H mice precedes the clinical onset of disease.
Figure 1. R161H mice develop spontaneous uveitis that is associated with presence of activated T cells in the gut.
(A) Spontaneous uveitis in R161H mice. Fundus photography (left) and histology (right) of healthy WT and uveitic R161H retina.
(B) Kinetics of disease development in R161H mice. Clinical scores were determined weekly by fundus examination.
(C and D) R161H CD4+ T cells in the intestine show an effector/memory phenotype. Representative CD4+ T cell plots for activation/memory markers (C) and Th17 phenotype (D) in various tissues from 4 wk-old (clinical score ≤0.25) and 12-wk old (clinical score 1.5). Intracellular IL-17A and IFN- γ were detected following 4 h ex vivo stimulation with PMA and ionomycin in the presence of Brefeldin A.
(E and F) Expression of Nr4a1 (encoding Nur77) was detected by GFP (green) in fresh ileum samples of Nr4a1GFP reporter mice at 17 days of age (E, before disease onset) and adult (F, after onset). Single plane of the Z stack is shown. Also see Figure S1, Movies 1–4.
Elimination of commensals attenuates uveitis and reduces activation of Th17 cells in the intestine
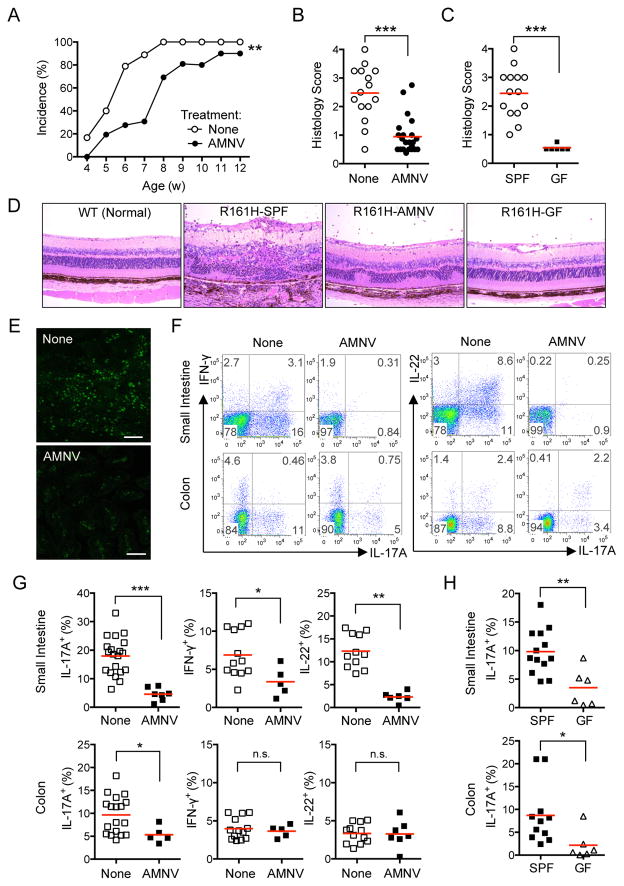
Th17 cells are a major pathogenic effector phenotype in the EAU model, and an elevated Th17 cell-mediated response was reported to correlate with disease in uveitis patients (Chi et al., 2008; Luger et al., 2008; Wang et al., 2012). Several reports have shown the importance of gut commensal microbiota for development of Th17 cells (see Hooper et al., 2012 for review). In view of the high frequency of activated T cells producing IL-17A in the intestinal LP of R161H mice shown in Figure 1, it was logical to ask whether gut commensals might play a role in disease in the spontaneous uveitis model. R161H mice were treated with a broad-spectrum antibiotic cocktail (AMNV: ampicillin, metronidazole, neomycin and vancomycin) in drinking water (Rakoff-Nahoum et al., 2004). Since R161H mice can begin to show signs of disease around weaning age, treatment was given to the breeders and the offspring were continued on the treatment after weaning. In line with results published by others, analysis of fecal material from AMNV-treated mice showed a reduction in total bacterial mass by several orders of magnitude and a reduced community complexity (Figure S2A, B). Weekly fundus examination showed that development of spontaneous uveitis was significantly delayed in AMNV-treated R161H mice compared to the untreated group (Figure 2A). Although ultimately most AMNV-treated R161H mice developed some clinical signs, their disease scores were drastically attenuated, as seen by histopathology of eyes collected at 9–12 wks of age, when disease in untreated controls reaches its peak. This was recapitulated in R161H mice reared under germ-free (GF) conditions (Figure 2B–D). AMNV treatment also decreased the GFP signal in the freshly isolated intestine of R161H Nr4a1GFP mice (Figure 2E), confirming that gut microbiota are required for stimulation. In parallel, frequency of IL-17A producing T cells in the small intestine and colonic lamina propria (LP) of AMNV-treated R161H mice was reduced, whereas IFN-γ and IL-22 were reduced in the small intestine, but not in the colon (Figure 2F and G). The frequency of IL-17A+ T cells was also strongly attenuated in small intestine and colon of GF R161H mice (Figure 2G). There was no expansion of Foxp3+ cells as a result of AMNV treatment (Figure S2C, D).
Figure 2. Elimination of commensal microbiota in R161H mice attenuates spontaneous uveitis and reduces the Th17 cells in the gut.
(A) Delayed onset of spontaneous uveitis in AMNV-treated vs. untreated R161H mice by weekly fundoscopic analysis. **p< 0.005 by 2-way ANOVA.
(B) Histology scores of untreated (None) and AMNV-treated R161H mice (9–12 wks old).
(C) Histology scores of age-matched untreated SPF and germ-free (GF) R161H mice (combined data from 7 and 11 wk-old). ***p<0.0001 by Mann-Whitney U test.
(D) Representative histopathology of SPF, AMNV-treated and GF R161H mice (original magnification, 200x).
(E) Expression of GFP in fresh ileum samples of R161H Nr4a1GFP mice treated or not with AMNV. Images are representative single plane of the Z stack. Scale represents 25 μm.
(F) Representative plots of cytokine-producing LP CD4+ T cells from R161H mice treated, or not, with AMNV.
(G) Compiled data for (F) from at least 5 experiments (4–16 wks old, 2–3 mice were pooled per group in each plot). ***p<0.0001, **p<0.005, *p<0.05, n.s., not significant.
(H) Frequencies of IL-17A-producing LP CD4+ T cells from SPF R161H and GF R161H mice. Data compiled from 2 experiments. **p<0.01, *p<0.05. Also see Figures S2 and S3.
Even though the consequences of microbiota depletion in our R161H mice were compatible with the results of others, it was nevertheless important to consider whether prolonged treatment with antibiotics, or deficient development of the immune system in a GF environment could account for the observed attenuation of disease. As evidence against this notion, neither frequencies of IRBP-specific T cells, nor their proliferative responses, were altered between untreated and AMNV-treated R161H mice, or between SPF and GF-R161H mice (Figure S3A–D). Finally, we examined the ability of the AMNV-treated WT littermates (R161H mice are bred heterozygously) to develop uveitis after active immunization with IRBP161–180. Disease scores 2 wks after immunization of AMNV-treated compared to untreated WT littermates were not reduced (Figure S3E). In addition, lymphocytes from these WT mice responded to antigen-specific or non-specific stimuli similarly, regardless of the treatment (Figure S3F, G). In the aggregate, these results confirm that attenuation of spontaneous uveitis in antibiotic-treated and GF R161H mice was due to depletion of commensal microbiota and not to any alterations in immune function.
R161H T cells in the gut preferentially exhibit a Th17 phenotype compared to polyclonal T cells
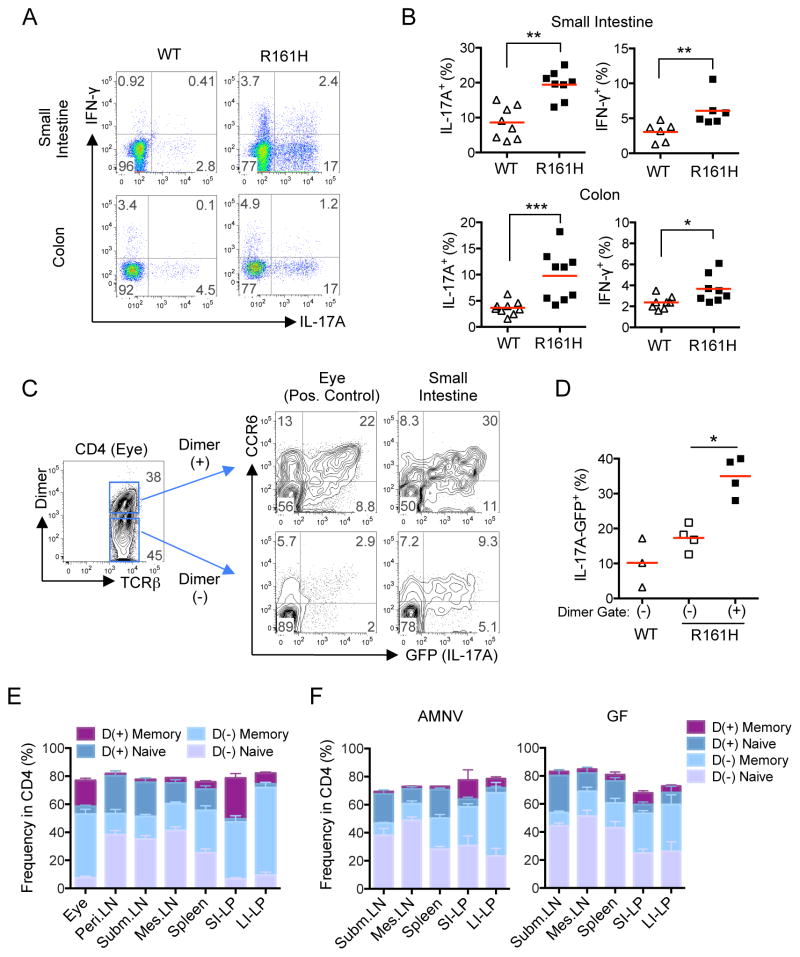
To elucidate a possible connection between increased frequency of IL-17A-producing cells in the intestine of R161H and spontaneous uveitis, we compared frequencies of IL-17A-producing cells in intestine of R161H mice with their healthy WT littermates. Compared to the WT mice, CD4+ T cells isolated from either small or large intestine LP of R161H mice displayed much higher frequencies of IL-17A-producing cells after a short PMA and ionomycin pulse (Figure 3A, B). Essentially identical results were seen directly ex vivo using Il17a-GFP reporter knock-in mice crossed onto the R161H background. In the next step, we compared the frequency of IL-17A-producing cells in the IRBP-specific vs. the non-IRBP-specific CD4+ T cells isolated from intestinal LP of the same R161H mouse. The mice co-expressed the Il17aGFP reporter and T cells bearing the IRBP-specific R161H TCR were detected with an IRBP161–180-MHCII-IgG dimer (p161 dimer) (Karabekian et al., 2005). The p161 dimer-positive population contained a much higher proportion of Th17 cells, as defined by dual IL-17A-GFP and CCR6 positivity, than the dimer-negative population, a pattern highly reminiscent of cells isolated from uveitic eyes of the same mice (Figure 3C, D). Notably, since the Il17aGFP reporter obviates the need for PMA and ionomycin stimulation ex vivo, the observed difference is considered to reflect the actual in vivo situation. Furthermore, while the proportion of p161 dimer+ T cells in the total CD4+ population remains fairly constant in untreated, AMNV-treated and GF mice (Figure S3), the proportion of antigen-experienced cells within the dimer+ population (CD44hi CD62Llo) was markedly more frequent in the gut than in peripheral lymphoid tissues, and that proportion was reduced by AMNV treatment or GF conditions (Figure 3E, F). Together, these results indicate that the clonotypic R161H T cells express a Th17 phenotype in the gut environment more readily than do non-IRBP-specific T cells, similarly to their status in the eye where their cognate antigen is found.
Figure 3. Retina-specific T cells preferentially exhibit an antigen-experienced, Th17 phenotype in the intestine of R161H mice.
(A) The frequency of Th17 cells is enhanced in the intestine of R161H mice compared to their WT littermates. Representative plots of small intestine and colonic LP CD4+ cells from 8-wk old mice for intracellular IL-17A and IFN- γ staining following 4 h ex vivo stimulation with PMA and ionomycin in the presence of Brefeldin A.
(B) Compiled data from several experiments for small intestine and colon (2 mice from each genotype were pooled as a sample). *p<0.05, **p<0.01, ***p<0.001.
(C) IL-17A (GFP) and CCR6 expression of freshly isolated cells from intestinal LP and uveitic eyes in CD4+ p161 Dimer (+) and Dimer (−) populations. One representative experiment of two (in which 2 mice pooled per sample) is shown.
(D) Compiled data from individual small intestines of 3 additional experiments. *p<0.05 by Mann-Whitney U test. Red horizontal lines are group means.
(E, F) Proportions of Memory (CD44hiCD62Llo) and Naïve (CD44loCD62Lhi) within the Dimer (+) or Dimer (−) populations of CD4+ T cells from LP compared to peripheral lymphoid tissues: (E) SPF R161H mice. (F) AMNV-treated and GF R161H mice (CD44loCD62Llo and CD44hiCD62Lhi populations are not displayed). Mean +/− SEM of 4–12 mice is shown.
R161H T cell activation in the intestine is independent of endogenous IRBP expression
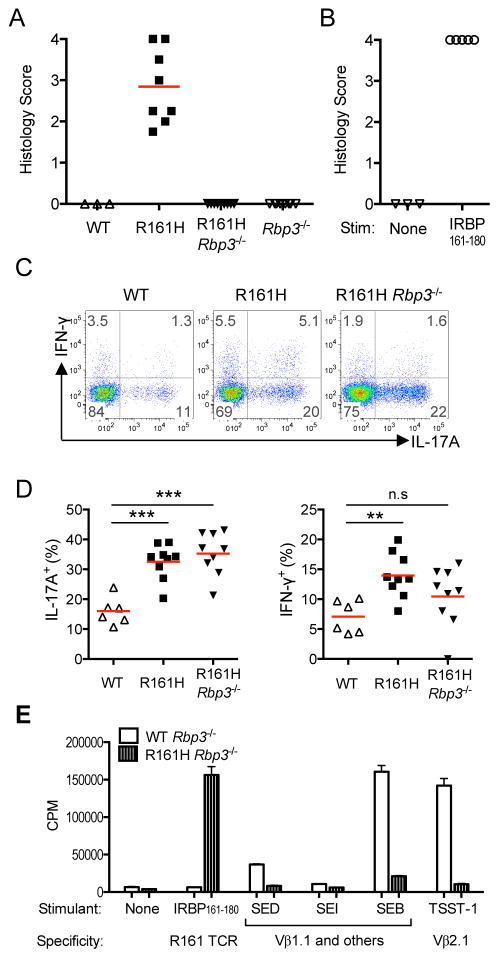
We next asked if the high frequency of Th17 cells in the gut LP of R161H mice is dependent on endogenous IRBP. Towards that end, we crossed R161H mice to Rbp3−/− mice, which lack expression of IRBP (Liou et al., 1998). R161H-Rbp3−/− mice do not develop uveitis, due to lack of the target antigen in their eyes (Figure 4A), but IRBP-specific T cells are present, are functionally responsive to IRBP (Figure S4A), and transfer of the activated cells induces severe uveitis in naïve WT recipient mice (Figure 4B). These mice still had an increased frequency of IL-17A+ T cells in their small intestine and colon LP, comparable to the IRBP-sufficient Rbp+/+ mice and much higher than in WT mice (Figure 4C, D) indicating that endogenous IRBP is not required for activation of IRBP-specific T cells in the gut of R161H mice.
Figure 4. R161H T cell activation in the intestine is not dependent on endogenous IRBP or bacterial superantigen.
(A) Rbp3−/− R161H mice do not develop spontaneous uveitis, due to lack of the target retinal Ag.
(B) Activated R161H Rbp3−/− T cells induce uveitis in WT naïve recipients.
(C) The high proportion of IL-17A-producing CD4+ T cells in the LP compared to WT is maintained in R161H Rbp3−/− mice. Data shown is one of four experiments with similar results.
(D) Compiled data from individual small intestines in 4 additional experiments. **p<0.005, ***p<0.001 by Mann-Whitney U test. Red lines indicate the mean of the group. (E) IRBP-specific T cells do not proliferate in response to commercially available purified enterotoxins with known Vβ specificities. CD4+ T cells isolated from lymph nodes of WT or R161H mice on the Rbp3−/− background were stimulated with 100 ng/ml of the indicated superantigen. SEB: Staphylococcal Enterotoxin B; SEI: Staphylococcal Enterotoxin I; SED: Staphylococcal Enterotoxin D; TSST-1: Toxic Shock Syndrome Toxin-1. Positive control: IRBP161–180 (1 μg/ml). Proliferation was assayed as 3H-thymidine uptake. Also see Figure S4.
In addition to the cognate antigen, T cells can be stimulated through their TCR also by superantigens, which activate broad classes of TCRs by binding to the TCR Vβ chain outside of the antigen-binding region (Fraser and Proft, 2008). We therefore examined a number of commercially available superantigens for ability to stimulate R161H cells. The R161H TCR is Vβ1/Vα17 (Horai et al., 2013). Staphylococcal enterotoxins B (SEB), SED and SEI, which are all reported to bind Vβ1, did not induce proliferation of R161H T cells, although they induced vigorous proliferation of WT T cells (Figure 4E). While we cannot categorically exclude involvement of an as yet uncharacterized superantigen, the evidence speaks against activation of the R161 clonotypic TCR by a superantigen.
Retina specific T cells in the intestine receive a signal through their clonotypic TCR
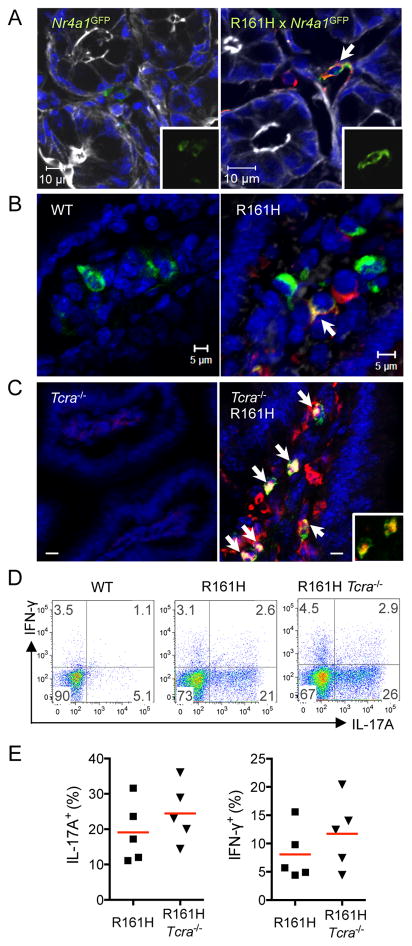
The data thus far strongly implied involvement of the R161 TCR in activation of the uveitogenic T cells in the intestine by gut commensals. To directly demonstrate presence of TCR signaling in the IRBP-specific T cells within the gut environment, we examined co-localization of p161 dimer with TCR signaling indicators in ileal tissue sections. By immunofluorescent staining, p161 dimer co-localized with the GFP signal in R161H-Nr4a1GFP mice (Figure 5A), or with tyrosine-phosphorylated Zap-70, a proximal signaling molecule downstream of the TCR, in R161H mice (Figure 5B). These results confirmed TCR signaling by activation of 2 different downstream molecules. Nevertheless, because T cells (normal or TCR Tg) can express a 2nd TCR due to incomplete exclusion of the TCRα chain (Hardardottir et al., 1995; He et al., 2002) we had to exclude the possibility that the R161H cells were being activated through a putative 2nd TCR. Towards that end, we crossed the R161H mice to Tcra−/− mice, which lack endogenous TCRαβ-expressing CD4+ T cells (Yoshitomi et al., 2005). R161H-Tcra−/− mice develop uveitis that is at least as severe as that developed by their R161H- Tcra+/+ littermates (Figure S5A). Flow cytometric analysis confirmed that all CD4+ T cells in these mice stained with p161 dimer (Figure S5B, C). Co-localization of phospho-Zap-70 with CD4 was clearly detectable in CD4+ cells in the small intestine of R161H-Tcra−/− animals (Figure 5C) and these T cells appeared to be high producers of IL-17A (Figure 5D and E, S5D and E). These results demonstrate that CD4+ T cells in the gut signal through their clonotypic retina-specific R161H TCR.
Figure 5. Retina-specific T cells in the intestine receive a TCR signal through their clonotypic TCR.
(A) Nr4a1GFP and R161H Nr4a1GFP LP samples were co-stained with the p161 dimer (red). GFP alone is shown in the inset (blue = Hoechst 33342, white = phalloidin).
(B) Detection of phospho-Zap70 (pZap70, green) and p161 dimer (red) in the gut LP of WT or R161H mice. Arrow shows a double-positive cell in the overlay (blue = Hoechst 33342).
(C) pZap70 (green) co-localizes with CD4 staining (red) in gut LP of R161H-Tcra−/− mice. Sections of ileum. Double-positive cells are yellow (arrows and inset). Scale bar, 5 μm.
(D) Small intestine LP of R161H Tcra−/− mice. The high proportion of IL-17A-producing CD4+ T cells compared to WT is maintained. Colon showed similar data.
(E) Compiled data from individual small intestines in 2 repeat experiments. Also see Figure S5.
Signals derived from intestinal microbiota activate retina-specific T cells for pathogenicity
To examine whether signals derived from gut microbiota are able to activate retina-specific T cells to cause uveitis, we first asked whether transfer of GF mice to SPF housing conditions would restore normal development of uveitis. R161H-GF animals at 4 wks of age were co-housed with R161H-SPF animals and disease development was monitored by weekly fundus examination. Ex-GF R161H mice transferred to SPF conditions developed uveitis with similar kinetics and similar final histology scores to R161H-SPF controls (Figure 6A, B).
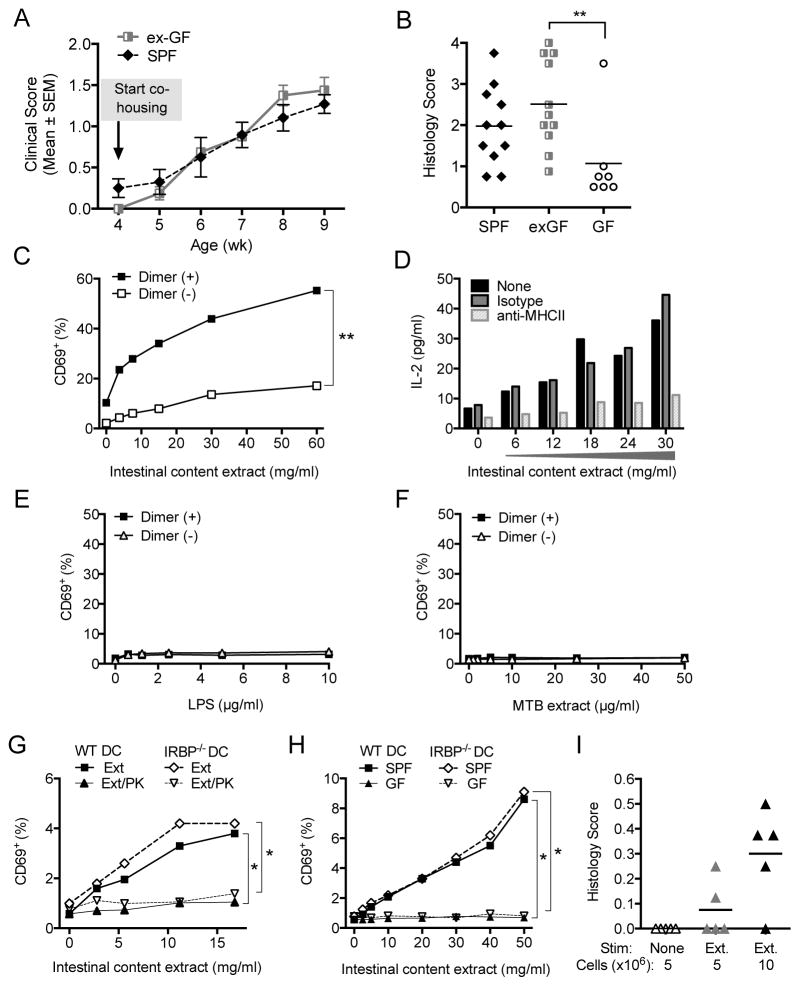
Figure 6. Microbial signal(s) from intestinal contents are sufficient to activate retina- specific T cells for pathogenicity.
(A) Sequential fundoscopy scores of ex-GF R161H mice. GF R161H mice (N=4) co-housed with SPF mice (N=6) after weaning. One representative kinetic of 2 repeat experiments is shown.
(B) Histological evaluation of ex-GF R161H mice at the end of co-housing. Data from 2 experiments (9 wk-old and 12-wk old) are combined. Control GF R161H mice were obtained at 12-wks of age. **p<0.005 by Mann-Whitney test.
(C) Induction of CD69 expression in CD4+ Dimer (+) vs. Dimer (−) T cells from LN of R161H mice after 20 h of stimulation with intestinal content extracts. **p<0.005 by 2-way ANOVA.
(D) IL-2 production by R161H lymphocytes in response to the extracts in presence or absence of anti-MHC class II Ab.
(E–F) Induction of CD69 expression in R161H lymphocytes after 20 h of stimulation with LPS (E) or with M. tuberculosis (MTB) extract (F).
(G) Naïve R161H Rbp3 −/− Rag2−/− cells stimulated with intestinal content extracts (Ext) treated or not with Proteinase K (PK) in presence of APC (DC) from WT or Rbp3−/− mice.
(H) Cultures as in G, stimulated with intestinal content extract from GF or SPF R161H donors. *p<0.05. (Note related data shown in Figure S6.)
(I) R161H lymphocytes were cultured with the protein-rich extracts of intestinal contents (Ext) for 3 days and indicated numbers of the cells were injected into WT recipients. Shown are histology scores in recipients on day 11. One representative experiment of four is shown.
Next, we examined whether bacteria-rich protein extracts from gut contents would directly activate IRBP-specific T cells in vitro and trigger their pathogenic potential. Extracts were prepared from bacteria-rich intestinal contents of R161H mice and were used to activate T cells from peripheral lymph nodes of R161H mice in vitro. The IRBP-specific (dimer-positive) T cells upregulated expression of the activation marker CD69 much more vigorously than did the nonspecific (dimer-negative) population from the same mouse, in a dose-dependent fashion (Figure 6C). IL-2 production, generally considered to indicate a T cell response to antigen, was inhibited by an anti-MHC class II antibody (Figure 6D), suggesting that the activation signal was received via TCR-MHC interactions. In contrast, innate stimuli alone (LPS and extract of M. tuberculosis) did not cause activation (Figure 6E). To further strengthen the notion of priming in the gut by non-cognate antigen, we cultured R161H Rbp3−/− lymph node cells with the extract. As before, the clonotypic T cells responded with CD69 upregulation and IL-2 production to the intestinal extract (Figure S6A, B), but not to innate stimuli alone (Figure S6B, C). Next, to confirm that a protein component in the extracts is necessary for activation of retina-specific T cells, the extracts were subjected to Proteinase K treatment or heat denaturation, and presented to naïve CD44loCD62LhiCD69−CD25− T cells from R161H Rbp3−/− Rag2−/− mice (only R161 TCR) by splenic CD11c+ APC from WT or Rbp3−/− mice (Figure 6G and S6E–F). Naïve R161H T cells responded to the untreated extract highly significantly, though less vigorously than total clonotype-positive T cells. Importantly, activation did not occur to LPS alone, and was significantly reduced by proteinase K treatment as well as by heat denaturation. Finally, to confirm requirement for bacteria, we examined intestinal content extracts from GF compared to SPF R161H mice for their ability to activate the naïve clonotypic T cells from Rbp3−/− donors. Extracts from GF mice, normalized per gram contents, were much less stimulatory than those of SPF mice (Figure 6H and S6G). In all cases, APC isolated from WT and Rbp3−/− mice, which lack the cognate antigen, provided equivalent stimulation. Because intestinal contents of GF mice contained much less protein than those of SPF mice (1.0 ± 0.14 vs. 14.3 ± 1.3 mg/ml, likely due to lack of microbial content), we also normalized the amount of extracts in the cultures by protein concentration, with the same outcome (Figure S6H). These results strongly support the conclusion that R161 T cells respond to protein component(s) from gut commensal bacteria, and not from cognate or unknown endogenous Ag.
Finally, to connect intestinal stimuli to the disease process, we examined whether exposure to extracts of bacteria-rich intestinal contents could make R161H cells pathogenic. R161H T cells infused into naïve WT recipients do not trigger disease unless they are activated before transfer (Horai et al., 2013). Notably, R161H lymph node cells cultured with extracts of intestinal contents induced EAU in 40–86% of WT recipient mice within 6–10 days of transfer, whereas no disease was observed within this timeframe in recipients of non-activated R161H cells (either freshly isolated or cultured without intestinal extracts) (Figure 6I). Thus, gut microbe-rich protein extracts contained the necessary and sufficient stimuli to make R161H T cells capable of breaching the intact blood-retinal barrier and causing uveitis.
DISCUSSION
A number of studies have documented the ability of gut commensals to affect the expression of autoimmunity at proximal as well as distal sites. The effects can be inhibitory (diabetes in NOD mice Markle et al., 2013; Wen et al., 2008), or more often enhancing (several models of arthritis, EAE and colitis Berer et al., 2011; Chappert et al., 2013; Garrett et al., 2010; Lee et al., 2011; Morton et al., 2014; Wu et al., 2010; Yoshitomi et al., 2005). The mechanisms are complex and may involve several unrelated microorganisms in concert (Garrett et al., 2010). Induction of Th17 responses in the gut by organisms such as SFB has been demonstrated in association with autoimmunity (Ivanov et al., 2009), but it is unclear how the responses observed in the intestinal tract translate to antigen-specific immunity in the target tissue, especially in view of the recent report that SFB-induced Th17 cells in the gut are SFB-specific (Goto et al., 2014; Yang et al., 2014). In the current study, we present compelling evidence that autopathogenic T cells that cause disease in a distal site receive a microbiota-dependent activation signal in the gut through their clonotypic TCR that is not dependent on an endogenous source of the cognate antigen, and precedes the clinical onset of pathology.
The R161H mouse model recapitulates the spontaneous nature of human uveitis, which makes it suitable for the study of natural triggers of the disease (Horai et al., 2013). Moreover, the target antigen is one of several known conserved retinal proteins that elicit memory responses in uveitis patients, and are likely to be involved in disease pathology (Gery et al., 1996). The spontaneous and highly penetrant nature of uveitis in R161H mice appears to be due to their enhanced frequency of retina-specific T cells (Horai et al., 2013), which amplify the naturally low penetrance of uveitis to a frequency that can be studied in the laboratory. To our knowledge, this is the first report that directly links autoimmunity to the neuroretina and the breakdown of the immune privilege of the eye to normal commensal microbes. Furthermore, our data offer a plausible explanation of the paradox that underlies uveitis, where autoreactive T cells must be primed by an antigen that is inaccessible to them in order to elicit pathology, by identifying the commensal microbiome as source of non-cognate antigen that can activate them in the periphery.
While our data showing that autoreactive T cells receive a signal in the gut through their clonotypic TCR appear compelling, by themselves they do not prove that this is the initial site where their activation occurs. Although at the present state of technology we are unable to demonstrate that IRBP-specific R161H Th17 cells detected in the gut have not ‘visited’ and been exposed to their cognate antigen in the eye, three lines of evidence speak for activation in the gut as a primary triggering event for pathogenicity: (i) high frequency of activated T cells in the gut or R161H mice (as early as 17 days, the earliest point examined) preceded their appearance in the eye and onset of clinical disease, (ii) absence of endogenous antigen did not change the high frequency of R161H T cell activation in the gut; (iii) our previous data (Zhou et al., 2012) showed that priming of retina-specific T cells within the ocular microenvironment results in acquisition of tolerance rather than effector function. Similarly, the tools currently do not exist to demonstrate that IRBP-specific T cells, which have been to the gut, end up in the eye. Although recently a method to endoscopically photolabel T cells within the gut has been described (Morton et al., 2014), several years of backcrossing would be required to apply this technology to the R161H model.
While it appeared that the activation of T cells in the gut is driven at least in part by an interaction with the IRBP-specific TCR, independent of endogenous IRBP, this does not exclude a role for innate microbial stimuli. Our data showed that IRBP-specific T cells are activated by bacteria-rich intestinal contents extracts more rigorously than polyclonal non-IRBP specific T cells and that their activation is MHC class II dependent, microbes have their own “built in” adjuvant effect, and it is likely that this contributes to activation for pathogenicity under in vivo conditions, similarly to the innate signals provided by mycobacteria in complete Freund’s adjuvant. In fact, previous studies may have confused this with specific TCR effects. For example, mimicry was proposed (but not supported by data) in the case of spontaneous EAE. In contrast to the IRBP-specific TCR, the endogenous myelin oligodendrocyte glycoprotein autoantigen was in fact needed to cooperate with the microbiota for autoreactive T and B cell activation (Berer et al., 2011). We therefore believe that ours is the first direct demonstration of the involvement of an autoreactive TCR, rather than an innate response to conserved microbial components, in commensal microbiota-dependent autoimmune disease expressed at a distant site.
Identification of the causative microorganism(s) and dissection of the microbial-derived antigen(s) that can trigger uveitis would have far-reaching implications for therapy of uveitis. While our data provide strong evidence for a necessary role of the TCR, they do not identify the bacterial component(s) that could serve as that non-cognate antigen(s). Our attempts to identify such an organism have thus far proved difficult. Treatment of R161H mice with individual antibiotics composing the AMNV cocktail, which in some cases were helpful to narrow down the bacterial species involved (Ramanan et al., 2014), did not prove fruitful, as the protective effect of each single antibiotics was minimal (data not shown). We also examined a possible role for SFB, which is present in our colony. Previous studies showed that monocolonization of GF mice with SFB reconstituted their ability to develop spontaneous arthritis (Wu et al., 2010) or immunization-induced EAE (Lee et al., 2011). Depletion of SFB from R161H mice by vancomycin treatment did not abrogate spontaneous uveitis (data not shown). We would interpret these data to mean that (a) the non-cognate antigen(s) that engage the TCR in the gut can be expressed by multiple bacterial species, and (b) the “deterministic” signal for T helper cell differentiation whereby the innate milieu drives the direction of antigen-specific T cell differentiation (also proposed by Goto et al., 2014; Yang et al., 2014) can be provided not only by SFB, but also by other commensal microorganisms. Monocolonization of GF R161H mice with candidate microorganism(s) would be needed to identify the causative species. However, due to the complexity of the commensal microbiota, and the likely dependence of the effects on a combination of species (as has already been documented even in the absence of an antigen-specific component in the TRUC model of colitis, Garrett et al., 2010), such an approach is currently beyond the scope of this study.
Collectively, the data reported here provide strong evidence that commensal microorganisms constitute a trigger and/or amplification signal to autoreactive T cells that drive spontaneous uveitis. While our data do not exclude a role for innate signals, which are always present in a microbial milieu and may provide an adjuvant effect, they support a critical role for signaling through the retina-specific clonotypic R161 TCR by non-cognate antigen(s) derived from commensals or their products. Our findings have implications for pathogenesis of human uveitis, as well as broader implications for autoimmunity in general. Given the huge diversity of the endogenous microbiota, it is conceivable that activation of autoreactive TCRs by crossreactive products derived from commensals is not restricted to IRBP. Based on our data, we propose that triggering of autoreactive TCRs by commensals may represent an underappreciated mechanism in the etiology of autoimmune diseases.
EXPERIMENTAL PROCEDURES
Mice
IRBP-specific TCR transgenic (R161H) mice were generated on the B10.RIII background as previously described (Horai et al., 2013). Rbp3−/− mice (Liou et al., 1998) (a kind gift from Dr. Gregory Liou, Medical College of Georgia), Tcra−/− mice (Mombaerts et al., 1992) (Taconic), Il17aGFP knock-in reporter mice (Biocytogen, BCG-0001; Jackson #018472) and Nr4a1GFP reporter mice (Moran et al., 2011) (a kind gift from Dr. Kristin Hogquist at University of Minnesota) were backcrossed onto B10.RIII background and crossed to R161H mice. Mice were maintained under SPF conditions at the National Eye Institute, NIH. Germ-free R161H mice were rederived at Central Institute for Experimental Animals (Kanagawa, Japan) and were maintained at Sankyo Labo Service, Co. Ltd. (Ibaraki, Japan).
Evaluation of spontaneous and induced ocular disease
Development of uveitis was monitored by weekly fundoscopic observation using a binocular microscope. For histopathology, eyes were enucleated and fixed in 4% glutaraldehyde for 1 hour and transferred to 10% formaldehyde for at least 24 h, embedded in methacrylate, and processed for H&E staining. Scores were assigned by a masked observer on a scale of 0–4, according to the criteria for EAU scoring described in detail elsewhere, based on the number, size and type of lesions (Horai and Caspi, 2010).
Isolation of lamina propria lymphocytes
LP lymphocytes were isolated essentially as described, with modifications (Atarashi et al., 2011; Scheiffele and Fuss, 2002), and are described in detail in the Supplemental Procedures.
Flow cytometric analysis
Eyes were minced and treated with 1 mg/ml collagenase D for 30–45 min at 37°C. Eye-infiltrating cells and lymphocytes isolated from tissues were resuspended in media for ex vivo stimulation, or directly in FACS buffer. Abs and detailed methods are in the Supplemental Procedures. For intracellular staining, cells were stimulated in complete RPMI + 10% FBS with 10 ng/ml PMA, 500 ng/ml Ionomycin and Brefeldin A for 4 h, fixed in 4% paraformaldehyde and permeabilized with Triton buffer (0.5% Triton X-100 + 0.1% BSA in PBS).
Depletion of intestinal commensals
Mice were treated with a broad-spectrum antibiotic cocktail of ampicillin, metronidazole, neomycin and vancomycin (AMNV) as described (Rakoff-Nahoum et al., 2004). Treatment was given to pregnant dams and continued after weaning. Control animals were housed in conventional set-ups on the same rack.
Preparation of intestinal bacteria-rich protein extracts
Intestines were flushed with cold PBS, contents were centrifuged at 2000g at 4°C for 10 min and supernatant was discarded. Pellets were resuspended to make 2 g fecal matter in 1 ml of PBS (2 g/ml) containing Aprotinin (10 μg/ml), Leupeptin (10 μg/ml) and PMSF (0.5 mM) (Sigma), followed by 3 freeze-thaw cycles at −80°C and room temperature for 20 min to disrupt the bacterial cells. Cell lysis was completed by using a sonicator (Heat Systems) for five high intensity cycles of 30 sec each on ice. Samples were then centrifuged at 14000g at 4°C for 30 min and the supernatant was collected and sterilized by filtration through 0.2 μm filter. Proteinase treatment was by incubating the extracts with Proteinase K (100 μg/ml; Fisher Scientific) at 55°C overnight, followed by 65°C for 1 hour to inactivate the enzyme. Heat denaturation of the extracts was at 95°C for 15 min. Protein was quantitated by DC Protein Assay (BioRad) per manufacturer’s instructions.
Proliferation assays
For lymphocyte activation assays, 5×105 total lymph node cells, or 2×105 FACS-sorted naïve CD62LhiCD44loCD69−CD25− CD4+ T cells and MACS-purified 105 splenic CD11c+ dendritic cells, were placed in round-bottom 96-well plates in complete RPMI-medium with 10% FBS, and stimulated with serial dilutions of protein-rich extracts from intestinal contents or with IRBP161–180 for control. In some experiments, 10 μg/ml of anti-mouse I-A/I-E mAb (clone M5/114.15.2) or rat IgG2b isotype control (BioLegend) was added to the culture. Cells were harvested 20–24 h after stimulation for flow cytometry analysis and supernatants were collected for quantitation of IL-2 by ELISA (R&D). For lymphocyte proliferation assays, 5×105 lymph node cells were cultured in triplicate in round-bottom 96-well plates in complete RPMI with 10% FBS and stimulated with IRBP161–180 or superantigens SEB, SED, SEI or TSST-1 at the indicated concentrations. After 48 h, 1 μCi/well of [3H] thymidine was added for another 16–18 h. Plates were harvested (Brandel) and counted by liquid scintillation (PerkinElmer).
Adoptive transfer
R161H lymph node cells were activated with intestinal contents (1:30 dilution) in DMEM-10% FBS in the presence of recombinant mouse IL-12, recombinant human IL-2 (Proleukin) and anti-IL-4 mAb (clone 11B11) for 3 days. After removal of dead cells by centrifugation over Lympholyte-M (Cedarlane, NC), 5 to 10 million lymphocytes were injected i.p. into naïve WT recipient mice. Fundoscopic evaluation of disease was performed between days 4–12. Eyes were collected on day 10–13 for histological examination and disease scoring, as described above.
Ex vivo visualization of GFP-expressing cells in intestinal tissues and immunofluorescent staining of intestinal sections
Ex vivo imaging of GFP expressing cells of Nr4a1GFP reporter mice was done on freshly collected tissues. All samples were kept on ice in PBS and placed onto a glass bottomed dish (MatTek Corporation) with mounting media Fluoromount-G (Southern Biotech) or Vectashield H-1000 (Vector Laboratories). Intestines were opened longitudinally and placed with the lumen down. The initial focal plane was chosen at 10 and 15 μm from the surface of the tissue and Z stack data for movies was collected from 5 to 60 μm. Images were acquired using 40x objective in inverted Zeiss LSM 700 or 780 confocal microscope. Bright field contrast adjustments were applied and movies were captured using Zen 2012 Lite software (Carl Zeiss). For immunofluorescence (IF) staining, cleaned intestines were fixed in paraformaldehyde, passed through 10% and 20% sucrose and snap-frozen. Staining was performed and images were acquired as described in detail in Supplemental Procedures.
Statistical analysis
Statistical data analysis was done using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA). Differences between groups were determined using Mann-Whitney test or two-way ANOVA, as appropriate for the experiment. p values of <0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors thank Drs. Koji Atarashi (RIKEN) and Ivan Fuss (NIAID, NIH) for sharing protocols, Dr. Kristin Hogquist (University of Minnesota) for Nr4a1GFP reporter mice, Dr. Gregory Liou (Medical College of Georgia) for Rbp3−/− mice, all members of the Caspi lab for help and advice, NEI Flow Cytometry Core for assistance in cell sorting and analysis, NEI Genetic Engineering Core for the assistance in antibiotics treatment and mouse colony maintenance, NEI Histology Core for processing of histology specimens and NIH-DVR Pathology and Bacteriology labs for bacteriological analysis. We thank Drs. Silvio Gutkind (NIDCR), William E. Paul (NIAID), Katsuko Sudo (Tokyo Medical University) and Kikuji Itoh (Japan SLC, Inc.) for support and useful discussions. R.R.C is supported by NEI intramural funding, project # EY000184 and K.H. is supported by CREST, Japan Science and Technology Agency (Tokyo, Japan).
Footnotes
AUTHOR CONTRIBUTIONS
R.H. and C.R.Z.-B. designed and performed the experiments, and analyzed the data together. P.D.-P. designed imaging and intestinal content extraction protocol. Both P.D.-P. and J.L.K. carried out imaging experiments. P.B.S., J.C. and Y.J. provided experimental assistances including fundus examination and genotyping. C-C.C. evaluated histology slides. H.Y. and K.H. provided expertise, access to the GF facility, and helped to interpret the data. R.R.C. conceptualized and supervised the study. R.H., C.R.Z.-B. and R.R.C wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nature Reviews Immunology. 2010;10:735–744. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- Chappert P, Bouladoux N, Naik S, Schwartz RH. Specific gut commensal flora locally alters T cell tuning to endogenous ligands. Immunity. 2013;38:1198–1210. doi: 10.1016/j.immuni.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Zhu X, Yang P, Liu X, Lin X, Zhou H, Huang X, Kijlstra A. Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Invest Ophthalmol Vis Sci. 2008;49:3058–3064. doi: 10.1167/iovs.07-1390. [DOI] [PubMed] [Google Scholar]
- Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–236. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–243. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I, Mochizuki M, Nussenblatt RB. Retinal specific antigens and immunopathogenic processes they provoke. Progress in Retinal Research. 1996;5:75–109. [Google Scholar]
- Gonzalez-Fernandez F, Van Niel E, Edmonds C, Beaver H, Nickerson JM, Garcia-Fernandez JM, Campohiaro PA, Foster RG. Differential expression of interphotoreceptor retinoid-binding protein, opsin, cellular retinaldehyde-binding protein, and basic fibroblastic growth factor. Exp Eye Res. 1993;56:411–427. doi: 10.1006/exer.1993.1055. [DOI] [PubMed] [Google Scholar]
- Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- Hardardottir F, Baron JL, Janeway CA., Jr T cells with two functional antigen-specific receptors. Proc Natl Acad Sci U S A. 1995;92:354–358. doi: 10.1073/pnas.92.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Janeway CA, Jr, Levine M, Robinson E, Preston-Hurlburt P, Viret C, Bottomly K. Dual receptor T cells extend the immune repertoire for foreign antigens. Nat Immunol. 2002;3:127–134. doi: 10.1038/ni751. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai R, Caspi RR. Neuromethods. SpringerLink; 2010. Retinal Inflammation: Uveitis/Uveoretinitis. Animal Models for Retinal Diseases; pp. 207–225. [Google Scholar]
- Horai R, Silver PB, Chen J, Agarwal RK, Chong WP, Jittayasothorn Y, Mattapallil MJ, Nguyen S, Natarajan K, Villasmil R, et al. Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J Autoimmun. 2013;44:21–33. doi: 10.1016/j.jaut.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabekian Z, Lytton SD, Silver PB, Sergeev YV, Schneck JP, Caspi RR. Antigen/MHC class II/Ig dimers for study of uveitogenic T cells: IRBP p161–180 presented by both IA and IE molecules. Invest Ophthalmol Vis Sci. 2005;46:3769–3776. doi: 10.1167/iovs.05-0187. [DOI] [PubMed] [Google Scholar]
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GI, Fei Y, Peachey NS, Matragoon S, Wei S, Blaner WS, Wang Y, Liu C, Gottesman ME, Ripps H. Early onset photoreceptor abnormalities induced by targeted disruption of the interphotoreceptor retinoid-binding protein gene. The Journal of Neuroscience. 1998;18:4511–4520. doi: 10.1523/JNEUROSCI.18-12-04511.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton AM, Sefik E, Upadhyay R, Weissleder R, Benoist C, Mathis D. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc Natl Acad Sci U S A. 2014;111:6696–6701. doi: 10.1073/pnas.1405634111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proal AD, Albert PJ, Marshall T. Autoimmune disease in the era of the metagenome. Autoimmun Rev. 2009;8:677–681. doi: 10.1016/j.autrev.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41:311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele F, Fuss IJ. Induction of TNBS colitis in mice. Curr Protoc Immunol. 2002;Chapter 15(Unit 15):19. doi: 10.1002/0471142735.im1519s49. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Tian Y, Lei B, Xiao X, Ye Z, Li F, Kijlstra A, Yang P. Decreased IL-27 expression in association with an increased Th17 response in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci. 2012;53:4668–4675. doi: 10.1167/iovs.12-9863. [DOI] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi H, Sakaguchi N, Kobayashi K, Brown GD, Tagami T, Sakihama T, Hirota K, Tanaka S, Nomura T, Miki I, et al. A role for fungal {beta}-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J Exp Med. 2005;201:949–960. doi: 10.1084/jem.20041758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Horai R, Silver PB, Mattapallil MJ, Zarate-Blades CR, Chong WP, Chen J, Rigden RC, Villasmil R, Caspi RR. The living eye “disarms” uncommitted autoreactive T cells by converting them to Foxp3(+) regulatory cells following local antigen recognition. J Immunol. 2012;188:1742–1750. doi: 10.4049/jimmunol.1102415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.