Abstract
It is the promise of regeneration and therapeutic applications that has sparked an interest in mesenchymal stem cells (MSCs). Following infusion, MSCs migrate to sites of injury or inflammation by virtue of their homing property. To exert optimal clinical benefits, systemically delivered MSCs need to migrate efficiently and in adequate numbers to pathological areas in vivo. However, underlying molecular mechanisms responsible for MSC migration are still not well understood. The Wharton's jelly (WJ) of the umbilical cord is an attractive source of MSCs for stem cell therapy because of its abundant availability and painless collection. In this study, we attempted to identify the role of nonmuscle myosin II (NMII), if any, in the migration of WJ-derived MSCs (WJ-MSCs). Expression of NMII isoforms, NMIIA, and NMIIB was observed both at RNA and protein levels in WJ-MSCs. Inhibition of NMII or its regulator ROCK, by pharmacological inhibitors, resulted in significant reduction in the migration of WJ-MSCs as confirmed by the scratch migration assay and time-lapse microscopy. Next, trying to dissect the role of each NMII isoform in migration of WJ-MSCs, we found that siRNA-mediated downregulation of NMIIA, but not NMIIB expression, led to cells failing to retract their trailing edge and losing cell–cell cohesiveness, while exhibiting a nondirectional migratory pathway. Migration, moreover, is also dependent on optimal affinity adhesion, which would allow rapid attachment and release of cells and, hence, can be influenced by extracellular matrix (ECM) and adhesion molecules. We demonstrated that inhibition of NMII and more specifically NMIIA resulted in increased gene expression of ECM and adhesion molecules, which possibly led to stronger adhesions and, hence, decreased migration. Therefore, these data suggest that NMII acts as a regulator of cell migration and adhesion in WJ-MSCs.
Introduction
Mesenchymal stem cells or multipotent stromal cells (MSCs) are multipotent precursors, which have been harvested from different tissue sources (bone marrow (BM), umbilical cord, dental pulp, adipose tissue, etc.), and are currently being evaluated for their applications in clinical and preclinical studies [1]. Due to their self-renewal and differentiation capacity, homing property, and ability to secrete paracrine factors that can modulate microenvironments, MSCs are now considered candidates with tremendous potential for biomedical research, regenerative medicine, and stem cell-based therapies [2].
Friedenstein, in the 1970s, first proposed the existence of MSCs from BM as BM stromal cells [3] and, since then, a significant amount of work in the MSC field has been attempted with BM-derived MSCs. However, there are limitations associated with the BM-MSCs [4] and a convenient alternative source of MSCs is the umbilical cord, which being a discarded fetus-derived tissue is noncontroversial, abundantly available, and can be easily processed. Wharton's jelly (WJ) is the connective tissue between the umbilical cord vessels and MSCs derived from WJ, shares certain properties both with embryonic and MSCs [5].
A big hurdle in the area of stem cell transplantations is timely delivery of the cells in sufficient numbers to the site of injury. Direct transplantation at the site of injury might be helpful, but not always feasible due to associated problems such as invasive procedure, tissue damage, and difficulty in administering multiple doses. It is by the virtue of their homing property that MSCs, following systemic infusion, can migrate to the area of injury. From a basic research perspective, it is important to understand migration of stem cells at a molecular level to maximize the therapeutic benefits of MSCs. Migration, in general, is a tightly regulated process, which involves changes in the cytoskeleton, cell–substrate adhesions, and extracellular matrix (ECM). It is a well-defined multistep process, which includes front-to-back polarization, extension by protrusion, adhesion formation, cell body translocation, adhesion disassembly, and rear retraction [6].
Nonmuscle myosin II (NMII) is an actin-binding molecular motor that plays a fundamental role in biological processes, which require cellular reshaping and movement such as cell migration, cell adhesion, cell division, and differentiation [7]. The hexameric NMII molecule comprises a pair of heavy chains (NMHC), one pair of essential light chains that stabilizes the NMHC, and one pair of regulatory light chains (RLC) that regulates the NMII activity [7]. Regulation of Mg2+-ATPase activity of NMII depends on reversible phosphorylation of RLC through kinases such as the Rho-associated kinase (ROCK) or MLCK [8].
There are three different isoforms of NMII in vertebrates, NMIIA, NMIIB, and NMIIC, with distinct subcellular localizations and enzymatic properties [8]. In cell migration, while polymerization of actin filaments drives leading edge protrusion, NMII filaments generate contractile forces, which lead to maturation of adhesion sites and retraction of the cell rear. Isoform-specific functions of NMIIA and NMIIB have been described in some migrating cells [8]. Although one previous study reported the role of NMII isoforms during crawling of BM-MSCs from the soft to stiff matrix [9], not much is known about the involvement of NMII in migration or adhesion of MSCs.
In this study, we have investigated the role of NMII and the individual isoforms, NMIIA and NMIIB, in migration of WJ-derived MSCs (WJ-MSCs). We find that inhibiting either NMII or ROCK leads to strong downregulation of migration in WJ-MSCs, as evident from significant reduction in migration speed. Specifically, depletion of NMIIA from WJ-MSCs, but not NMIIB, results in trailing edges. NMIIA-depleted WJ-MSCs also exhibit cell–cell detachment and a less directed migration pathway. Moreover, we present evidence indicating that inhibition of NMII, and more specifically isoform A, leads to increased gene expression of ECM and adhesion molecules in WJ-MSCs. Hence, our data highlight, for the first time to our knowledge, a role of NMII in migration and adhesion of MSCs.
Materials and Methods
Isolation and culture of WJ-MSCs
Fresh human umbilical cords (n=6) were collected after full-term births (cesarean section or normal vaginal delivery) with informed consent using the guidelines approved by the Institutional Ethics Committee and Institutional Committee for Stem Cell Research and Therapy (IC-SCRT) at the Indian Institute of Science Education and Research (IISER)-Kolkata, India. WJ-MSCs from umbilical cords were isolated by the explant culture method as previously described [10]. Briefly, umbilical cord vessels were removed manually, and the exposed perivascular tissue was cut into 1–2 mm pieces or explants. These were next placed in tissue culture dishes in KnockOut Dulbecco's Modified Eagle's Medium (KnockOut DMEM; Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Victoria, Australia), 2 mM L-glutamine (Life Technologies), and 0.5×antibiotic–antimycotic (Life Technologies).
All cultures were incubated at 37°C with 5% humidified CO2. After 7–10 days, when cells started to appear, explants were removed and cells were passaged when 70%–80% confluence was obtained. In most of the experiments, WJ-MSCs at passage 4–7 were used and cells were plated at a density of 5,000 cells/cm2. For an experiment using blebbistatin, WJ-MSCs were plated with 5 μM blebbistatin (Sigma-Aldrich, St. Louis, MO). Y-27632 (Sigma) was used at 7.5 or 10 μM. Corresponding control cultures were treated with 0.2% DMSO (vehicle; Sigma).
Scratch wound healing assay
To investigate the effect of blebbistatin on wound-induced migration, WJ-MSCs were cultured for two passages in the presence and absence of 5 μM blebbistatin, and in the second passage, on reaching complete confluence, a sterile pipette tip was used to scratch the monolayer. Migration of WJ-MSCs into the wound area was evaluated by images recorded using the 10× objective of Olympus Phase Contrast Microscope (Olympus IX-81; Olympus, Tokyo, Japan) at 2-h intervals till wound closure. A similar procedure was applied to WJ-MSCs treated with the ROCK inhibitor Y-27632.
Live imaging of migration
WJ-MSCs were treated with 5 μM blebbistatin for two passages. In the second passage, control and blebbistatin-treated cells were plated at 4,000 cells/cm2, and after overnight incubation, time-lapse videos were recorded every minute over a period of ∼4 h using the time-lapse fluorescence microscope (Axio Observer Z1, Carl Zeiss, Germany) equipped with an environmental chamber set at 37°C and 5% CO2. The movie was made taking 30 frames/s for up to 4 h. Average migration speed expressed as micrometers per hour (μm/h) was determined for individual cells by tracking the total distance covered by the center of a cell nucleus in 4 h using the ZEN 2010 software (Zeiss). A similar procedure was also followed in the experiments where WJ-MSCs were treated with Y-27632.
Immunofluorescence staining
WJ-MSCs were plated, allowed to grow on untreated 8-well chamber slides (Genetix Biotech Asia Pvt Ltd, New Delhi, India), and fixed with 4% paraformaldehyde (Sigma) in PBS for 30 min at room temperature. Next, the cells were permeabilized in 0.3% Triton X-100 (Sigma) and blocked with 10% normal goat serum (KPL, Gaithersburg, MD) in 0.1% bovine serum albumin in PBS. The primary and secondary antibodies used were rabbit anti-NMIIA, rabbit anti-NMIIB, rabbit anti-vimentin (Cell Signaling Technology, Danvers, MA), Alexa Fluor 488 phalloidin (Life Technologies), goat anti-rabbit IgG (H+L) Alexa Fluor 568 conjugate (Life Technologies), and goat anti-rabbit IgG (H+L), F(ab′)2 Fragment Alexa Fluor 488 conjugate (Cell Signaling Technology). Fluorescently labeled WJ-MSCs were counterstained and mounted with the ProLong Gold Antifade Reagent with DAPI (Invitrogen, Eugene, OR). Confocal images were captured on a Zeiss CLSM 710 confocal microscope.
Complementary DNA synthesis and human ECM and adhesion molecules polymerase chain reaction array
Total RNA was isolated using the RNeasy Mini Kit (Qiagen Sciences, Germantown, MD,) or mirVana miRNA isolation (Ambion, Austin, TX) according to the manufacturer's instruction. The RNA yield was quantified using NanoDrop ND-1000 (Thermo Scientific, Waltham, MA), and an equal amount of RNA samples were pooled from three different donors. Complementary DNA (cDNA) of the pooled sample was synthesized using an RT2 First-Strand Kit (Qiagen Sciences) according to the manufacturer's instructions. cDNA was mixed with SYBR Green/ROX qPCR Master Mix (Qiagen Sciences), and 25-μL aliquots were loaded into each well of the Human Extracellular Matrix and Adhesion Molecules RT2 Profiler PCR Array plate (Qiagen Sciences).
Array run was performed on an ABI Biosystems StepOnePlus (Applied Biosystems, Carlsbad, CA), and StepOnePlus version v.2.2 software (Applied Biosystems) was used to obtain the data. The complete analysis was done using the web-based RT2 Profiler PCR Array data analysis software available at www.sabiosciences.com. Samples with a cycle threshold of ≤35 were taken for calculating the fold change in expression. The arithmetic mean of five housekeeping genes was used to normalize the data (Ct=Ct gene − mean Ct housekeeping).
Reverse transcription-polymerase chain reaction confirmation of array data
To confirm the gene expression profile obtained by the polymerase chain reaction (PCR) array, a few select genes were subjected to semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis using pooled cDNAs from the three different samples used for the PCR array experiment. For the NMII PCRs, cDNA was prepared from passaged WJ-MSCs using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). PCR amplification was done using Taq DNA polymerase (Sigma-Aldrich). 18s ribosomal RNA was used as an internal control. The primer sequences used in the reverse transcriptase PCR analysis and product size are listed in Table 1.
Table 1.
Primer Sequences Used for Semiquantitative Reverse Transcription-Polymerase Chain Reaction Analysis
| S. no. | Gene name | Forward primer | Reverse primer | Size (bp) |
|---|---|---|---|---|
| 1 | ITGA5 | 5′ ACTAGGAAATCCATTCACAGTTC 3′ | 5′ GCATAGTTAGTGTTCTTTGTTGG 3′ | 200 |
| 2 | COL12A1 | 5′ ACCCACCTTCCGACTTGAATT 3′ | 5′ TAGGCCCATCTGTTGTTGTAGGG 3′ | 122 |
| 3 | MMP 3 | 5′ GGCTTTCCCAAGCAAATAGC 3′ | 5′ GTGCCCATATTGTGCCTTCT 3′ | 205 |
| 4 | MMP 10 | 5′ CCAGTCTGCTCTGCCTATCC 3′ | 5′ CATCTCAGATCCCGAAGGAA 3′ | 119 |
| 5 | MYH9 (NMIIA) | 5′ GGCCGAAGAGGAGGCCCAG 3′ | 5′ CGGCAGGTTTGGCCTCAG 3′ | 235 |
| 6 | MYH10 (NMIIB) | 5′ CGACGCGTGCCAACGCATC 3′ | 5′ GACACAGTTGATCTTTCAGGAAGG 3′ | 371 |
| 7 | MYH14 (NMIIC) | 5′ CTCCTCTAGTCGGAAGACCTGGC 3′ | 5′ CTGCCCTTGAGTCTAAGTTGG 3′ | 394 |
| 8 | TNC | 5′ AGCATCACCCTGGAATGGAGGA 3′ | 5′ TGTGGCTTGTTGGCTCTTTGGA 3′ | 119 |
| 9 | VTN | 5′ CGAGGAGAAAAACAATGCCAC 3′ | 5′ GAAGCCGTCAGAGATATTTCG 3′ | 498 |
| 10 | 18S | 5′ CGGCTACCACATCCAAGGAA 3′ | 5′ GCTGGAATTACCGCGGCT 3′ | 186 |
Transfection of Si RNA
WJ-MSCs were seeded at a density of 4,000–5,000 cells/cm2 into 12-well plates or 35-mm dishes, and after incubation for 24 h, cells were transfected using Lipofectamine 3000 in the Opti-MEM I medium (both from Life Technologies) according to the manufacturer's protocol. Isoform-specific individual siRNA duplexes against NMIIA and NMIIB (Sigma) were used at a final concentration of 20 nM, and the MISSION siRNA Universal Negative Control (Sigma) was used at the same concentration in the control experiments. Seventy-two hours post-transfection WJ-MSCs were then used for the scratch assay and live-cell imaging or lysed for RT-PCR analysis.
Western blotting
At 72 h post-transfection, WJ-MSCs were washed once with PBS, directly lysed with 2× Laemmli's sample buffer (100 mM Tris pH 6.8, 20% glycerol, 4% SDS, 0.02% bromophenol blue), and boiled. Polyacrylamide gel electrophoresis and western blotting were carried out as per standard protocols. The primary and secondary antibodies used were rabbit anti-NMIIA, rabbit anti-NMIIB, rabbit anti-NMIIC (1:2,000; all from Sigma), mouse anti-GAPDH (1:4,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), HRP-conjugated goat anti-rabbit (1: 2,000; Thermo Scientific, Waltham, MA), and HRP-conjugated goat anti-mouse (1: 5,000; Sigma). Relative band intensity was quantified using ImageJ software (National Institute of Health, Bethesda, MD).
Statistical analysis
Data are presented as mean±standard error of the mean. Data analysis and graphical representations were performed using GraphPad Prism 5 software (GraphPad, La Jolla, CA). Statistical comparisons were made using the two-tailed Student t-test. Significance was accepted at P≤0.05.
Results
WJ-MSCs used in the experiments were routinely characterized for surface marker expression (positive for CD73 and CD90 and negative for CD34) by flow cytometry and in vitro differentiation potential toward adipogenic and chondrogenic/osteogenic lineages (data not shown).
Expression and distribution of NMII isoforms in WJ-MSCs
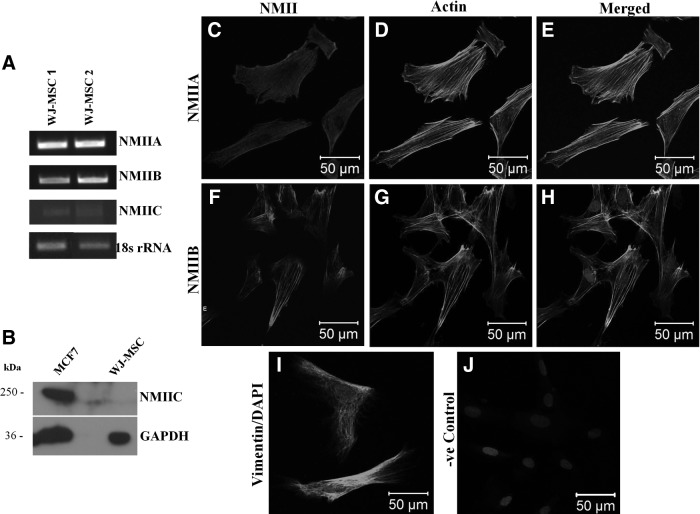
To detect the presence and level of expression of NMII isoforms in WJ-MSCs, RT-PCR analysis using isoform-specific primers was performed with passage 4 cells. While abundant expression of two of the isoforms, NMIIA and NMIIB, was detected at the mRNA level in the WJ-MSC samples tested, NMIIC was found to be very weakly expressed (Fig. 1A). Since, NMIIC was undetectable even at the protein level, as confirmed by western blotting, we decided to proceed further only with NMIIA and NMIIB (Fig. 1B). Next, we wanted to examine the localization of NMII isoforms in cultured WJ-MSCs using antibodies specifically against NMIIA and NMIIB. Immunofluorescence staining demonstrated that both NMIIA and NMIIB were distributed in the cytoplasm (Fig. 1C, F). While NMIIA was more evenly distributed in the whole cell, NMIIB was prominently localized along the stress fibers. Double staining with NMIIA or NMIIB and phalloidin-labeled F-actin revealed colocalization of NMII and actin (Fig. 1E, H). Staining for the expression of vimentin, an intermediate filament protein, was used as a mesenchymal marker (Fig. 1I).
FIG. 1.
mRNA expression of NMIIA, NMIIB, and NMIIC in two different samples of WJ-MSCs, as analyzed by semiquantitative RT-PCR using isoform-specific primers. 18s rRNA was used as an internal control (A). Protein expression of NMIIC was analyzed in WJ-MSCs. The MCF-7 cell line was used as positive control. The same blot was reprobed with the anti-GAPDH antibody to demonstrate equal loading (B). Localization of NMIIA and NMIIB in cultured WJ-MSCs. Representative confocal images of cultured WJ-MSCs immunostained for NMII A and NMIIB (C, F, respectively), phalloidin-stained F-actin (D, G), merged (E, H) and mesenchymal marker vimentin (I) are presented. Nuclei are stained with DAPI. A negative control with the omission of incubation with primary antibody is shown (J). Scale bar: 50 μm. Results are representative of at least two independent biological or donor samples. MSCs, mesenchymal stem cells; NMII, nonmuscle myosin II; RT-PCR, reverse transcription-polymerase chain reaction; WJ, Wharton's jelly; WJ-MSCs, WJ-derived MSCs.
Effect of NMII inhibition on wound-induced migration of WJ-MSCs
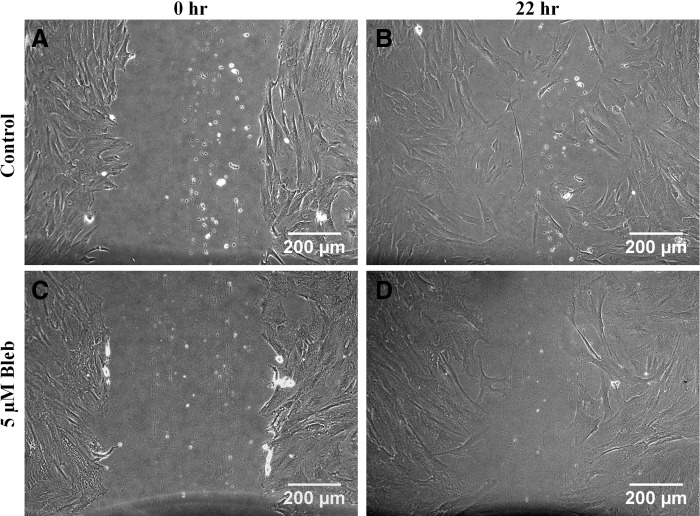
To investigate if NMII plays any role in in vitro migration of WJ-MSCs, we performed scratch assays using passage 4–6 WJ-MSCs in the presence and absence of a small-molecule inhibitor of NMII ATPase activity, blebbistatin [11]. Cells were scraped from an area of a confluent monolayer culture of WJ-MSCs, treated with and without blebbistatin, and their migration was studied during wound closure over a period of 24 h. While the cell-free area got covered completely by untreated WJ-MSCs in 22 h (Fig. 2B), WJ-MSCs treated with blebbistatin were slower to migrate and took longer to heal the wound and cover a similar area (Fig. 2D).
FIG. 2.
Comparison of wound-induced migration between control and blebbistatin-treated WJ-MSCs. Confluent monolayer cultures of vehicle-treated (control) and blebbistatin-treated WJ-MSCs were wounded with a sterile pipette tip at 0 h (A, C). Representative images of migration postscratch assay were obtained at 22 h (B, D). Scale bar: 200 μm. Results are representative of at least three independent biological or donor samples.
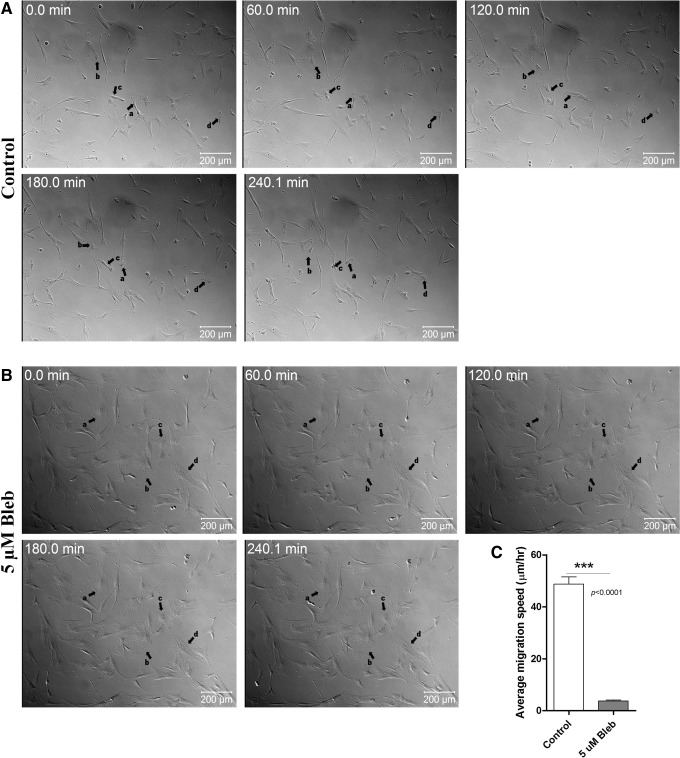
Effect of NMII inhibition on migration of WJ-MSCs in real time
To further understand the role of NMII in WJ-MSC migration in real time, WJ-MSC cultures were exposed to 5 μM blebbistatin for two passages, and in the second passage, the time-lapse video was captured over a time period of ∼4 h. On treating with blebbistatin for two passages, morphology changed and about 80% of the treated cells had a flattened appearance and were bigger in size, which is consistent with our previous report [12]. There was a complete halt in the movement of these flattened big cells (Fig. 3B and Supplementary Video S2; Supplementary Data are available online at www.liebertpub.com/scd). WJ-MSCs, in the vehicle-treated control cultures (Fig. 3A and Supplementary Video S1), exhibited robust migration. The migration speed of control WJ-MSCs was calculated to be 48.7±2.8 μm/h, while that of blebbistatin-treated WJ-MSCs was 3.7±0.38 μm/h (P<0.0001) (Fig. 3C).
FIG. 3.
Time-lapse images from WJ-MSC culture exposed to 5 μM blebbistatin. Time-lapse videos (uploaded as Supplementary Videos S1 and S2) of WJ-MSCs treated without and with 5 μM blebbistatin were captured. Representative DIC still images taken from the time-lapse videos of control (A) and blebbistatin-treated (B) WJ-MSCs at different time points are shown. Black arrows show the migration of a few randomly selected cells (a, b, c and d). Numbers on the images indicate time in hours. Results are representative of at least two independent biological or donor samples. Scale bar: 200 μm. Comparison of migration speed between control and blebbistatin-treated WJ-MSCs (C). Sample size used was 10 cells per sample, and two different biological or donor samples were used. Bars represent mean±SEM. (Student's t-test, two tailed, *** represents P<0.0001).
Effect of ROCK inhibition on wound-induced migration of WJ-MSCs
ROCK plays a role in various fundamental cellular activities such as cell proliferation, survival, and migration [13,14]. It regulates NMII function through RLC phosphorylation, which is either by phosphorylating RLCs directly or by inhibiting myosin light chain phosphatase, which is responsible for dephosphorylation of RLCs [7]. In the meantime, a previous study on WJ-MSCs reported that the ROCK inhibitor increased thaw survival rates and retained the stemness and differentiation potential following cryopreservation [15].
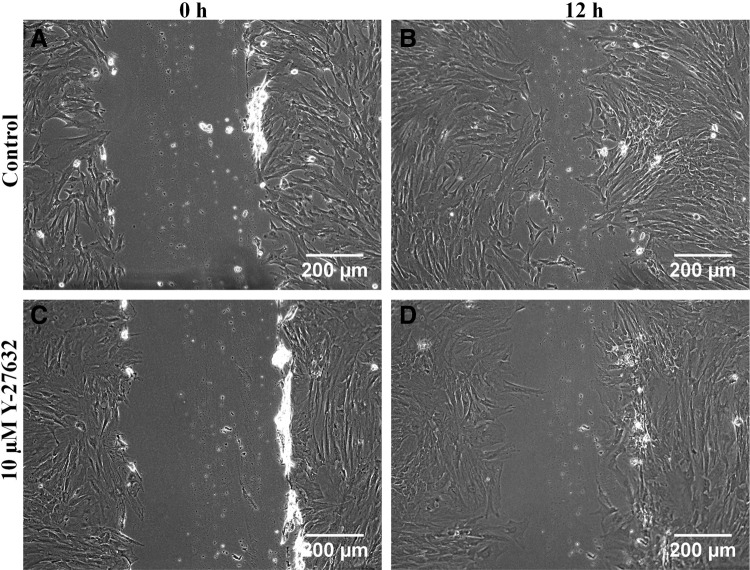
To test if ROCK plays a role in migration of WJ-MSCs by regulating the NMII function, WJ-MSCs were cultured for two passages in the absence and presence of the 7.5 μM ROCK inhibitor Y-27632, and in the second passage, we compared the migration of the two populations in a standard scratch wound assay. After 12 h, there was a distinct difference in the number of cells that had migrated into the scratch between the control (Fig. 4B) and the Y-27632-treated WJ-MSC samples (Fig. 4D). Wound closure was observed to be delayed in Y-27632-treated WJ-MSCs.
FIG. 4.
Comparison of wound-induced migration between control and Y-27632-treated WJ-MSCs. Confluent monolayer cultures of control and Y-27632-treated WJ-MSC cultures were wounded with a sterile pipette tip at 0 h (A, C). Representative images of migration are shown for control and treated cells at 12 h after wounding (B, D). Scale bar: 200 μm. Results are representative of at least three independent biological or donor samples.
Effect of ROCK inhibition on migration of WJ-MSCs in real time
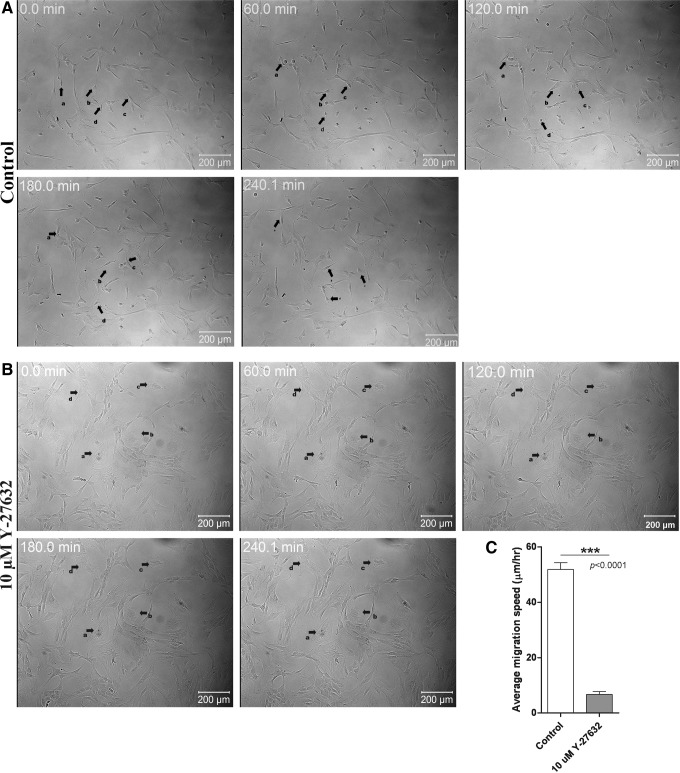
Next, WJ-MSCs were plated in the presence and absence of 7.5 μM Y-27632 and cultured for two passages. In the second passage, we used the time-lapse video microscopy to examine and compare migration between control and treated WJ-MSCs. Control cells displayed a typical migration pattern with an average speed of migration of 51.8±2.4 μm/h (Fig. 5A, Supplementary Video S3). In contrast, in the presence of Y-27632, WJ-MSCs had a flattened appearance and their migration ability was distinctly impaired and the movement of cells was restricted (Fig. 5B and Supplemental Video S4). There was a significant difference in the migration speed between the control and treated WJ-MSCs (P<0.0001) (Fig. 5C).
FIG. 5.
Time-lapse images from WJ-MSC culture exposed to 10 μM Y-27632.Time-lapse videos (uploaded as Supplementary Videos S3 and S4) of WJ-MSCs treated without and with 10 μM Y-27632 were obtained. Representative DIC still images taken from the time-lapse videos of control (A) and Y-27632-treated (B) WJ-MSCs at different time points are shown. Black arrows show the migration of a few randomly selected cells (a, b, c and d). Numbers on the images indicate time in hours. Results are representative of at least two independent biological or donor samples. Scale bar: 200 μm. Comparison of migration speed between control and Y-27632-treated WJ-MSCs (C). Sample size used was 10 cells per sample, and two different biological or donor samples were used. Bars represent mean±SEM. (Student's t-test, two tailed, *** represents P<0.0001).
Therefore, the above data indicate the involvement of NMII activity in the migration of WJ-MSCs.
Effect of NMII inhibition on ECM and adhesion molecule gene expression
Cell migration is also dependent on optimal levels of adhesion. Since the inhibition of NMII activity substantially reduced the migration of WJ-MSCs, we next wanted to investigate how this affected the ECM and adhesion molecule expression in WJ-MSCs. The Human Extracellular Matrix and Adhesion Molecules RT2 Profiler PCR Array were used to assess the effect of NMII inhibition on the gene expression profile of WJ-MSCs. This array profiles the expression of 84 genes important for cell–cell and cell–matrix interactions (Table 2).
Table 2.
Human Extracellular Matrix and Adhesion Molecule Genes Screened Using the RT2 Profiler™ PCR Array
| ADAMTS1 | ADAMTS13 | ADAMTS8 | CD44 | CDH1 | CLEC3B | CNTN1 | COL11A1 | COL12A1 | COL14A1 | COL15A1 | COL16A1 |
| COL1A1 | COL4A2 | COL5A1 | COL6A1 | COL6A2 | COL7A1 | COL8A1 | CTGF | CTNNA1 | CTNNB1 | CTNND1 | CTNND2 |
| ECM1 | FN1 | HAS1 | ICAM1 | ITGA1 | ITGA2 | ITGA3 | ITGA4 | ITGA5 | ITGA6 | ITGA7 | ITGA8 |
| ITGAL | ITGAM | ITGAV | ITGB1 | ITGB2 | ITGB3 | ITGB4 | ITGB5 | KAL1 | LAMA1 | LAMA2 | LAMA3 |
| LAMB1 | LAMB3 | LAMC1 | MMP1 | MMP10 | MMP11 | MMP12 | MMP13 | MMP14 | MMP15 | MMP16 | MMP2 |
| MMP3 | MMP7 | MMP8 | MMP9 | NCAM1 | PECAM1 | SELE | SELL | SELP | SGCE | SPARC | SPG7 |
| SPP1 | TGFBI | THBS1 | THBS2 | THBS3 | TIMP1 | TIMP2 | TIMP3 | TNC | VCAM1 | VCAN | VTN |
| ACTBa | B2Ma | GAPDHa | HPRT1a | RPLP0a | HGDCb | RTCc | RTCc | RTCc | PPCd | PPCd | PPCd |
Housekeeping genes.
Human genomic DNA contamination control.
Reverse transcription control.
Positive polymerase chain reaction control.
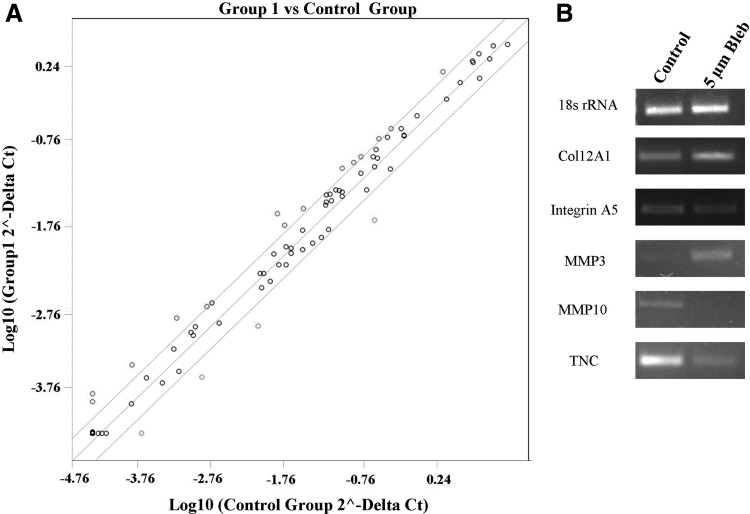
Out of the 84 genes screened, 14 were found to be upregulated by blebbistatin treatment, while 4 genes were downregulated, as shown in Table 3. We focused on genes with a greater than twofold change between WJ-MSCs treated with and without blebbistatin. A scatterplot of the data is shown in Fig. 6A. Some of the genes, which were upregulated, belonged to the collagen and laminin family of genes like COL11A1, COL12A1, COL16A1, COL8A, LAMB1, and LAMA2, respectively. Other genes like VTN, MMP3, MMP12, and TIMP3 were also upregulated. The genes that were downregulated in blebbistatin-treated WJ-MSCs, such as TNC, SPP 1, MMP 10, and COL7A1, have been reported to play a role in cell migration, wound healing, and so on. The remaining genes of the array were not significantly affected by blebbistatin treatment.
Table 3.
Differentially Expressed Extracellular Matrix and Adhesion Molecule-Related Genes, Between Control and Blebbistatin-Treated Wharton's Jelly-Derived Mesenchymal Stem Cells, as Determined by Quantitative Analysis Using Polymerase Chain Reaction Array
| No | Gene symbol | Gene description | Fold difference |
|---|---|---|---|
| Genes upregulated in blebbistatin-treated WJ-MSCs compared to DMSO-treated WJ-MSCs | |||
| 1. | COL11A1 | Collagen, type XI, alpha 1 | 2.1163 |
| 2. | COL12A1 | Collagen, type XII, alpha 1 | 2.631 |
| 3. | COL16A1 | Collagen, type XVI, alpha 1 | 2.5417 |
| 4. | COL8A1 | Collagen, type VIII, alpha 1 | 2.0893 |
| 5. | ITGB2 | Integrin, beta 2 | 4.3317 |
| 6. | LAMA2 | Laminin, alpha 2 | 2.4196 |
| 7. | LAMB1 | Laminin, beta 1 | 2.1631 |
| 8. | MMP3 | Matrix metallopeptidase 3 (stromelysin 1, progelatinase) | 3.2155 |
| 9. | MMP12 | Matrix metallopeptidase 12 (macrophage elastase) | 3.6105 |
| 10. | SELE | Selectin E | 3.3808 |
| 11. | THBS1 | Thrombospondin 1 | 2.0145 |
| 12. | TIMP3 | TIMP metallopeptidase inhibitor 3 | 2.4945 |
| 13. | VCAN | Versican | 2.2274 |
| 14. | VTN | Vitronectin | 4.4588 |
| Genes downregulated in blebbistatin-treated WJ-MSCs compared to DMSO-treated WJ-MSCs | |||
| 1. | COL7A1 | Collagen, type VII, alpha 1 | −3.6865 |
| 2. | MMP 10 | Matrix metallopeptidase 10 (stromelysin 2) | −3.4167 |
| 3. | SPP1 | Secreted phosphoprotein 1 | −4.0363 |
| 4. | TNC | Tenascin C | −5.0796 |
MSCs, mesenchymal stem cells; WJ, Wharton's jelly; WJ-MSCs, WJ-derived MSCs.
FIG. 6.
Scatterplot comparing ECM and adhesion molecule gene expression profile between control and blebbistatin-treated WJ-MSCs (A). RT-PCR validation of a few randomly selected genes from the ECM and Adhesion Molecules RT2 Profiler PCR Array. PCR was performed using complementary DNA pooled from three different samples, each of blebbistatin-treated and their corresponding control WJ-MSCs. 18s rRNA was used as an internal control (B). ECM, extracellular matrix.
RT-PCR validation of PCR array data
Semiquantitative RT-PCR of select genes, with pooled cDNA samples used in the array, was performed to validate the array data (Fig. 6B). The differential expression pattern observed between the blebbistatin-treated and control populations of WJ-MSCs in the array was consistent with the RT-PCR data for the select genes tested. PCRs with individual samples (comprising the pool) of blebbistatin-treated and corresponding control WJ-MSCs were also performed to confirm the pooled sample data (data not shown).
Wound-induced migration of WJ-MSCs following siRNA-mediated downregulation of NMII isoforms
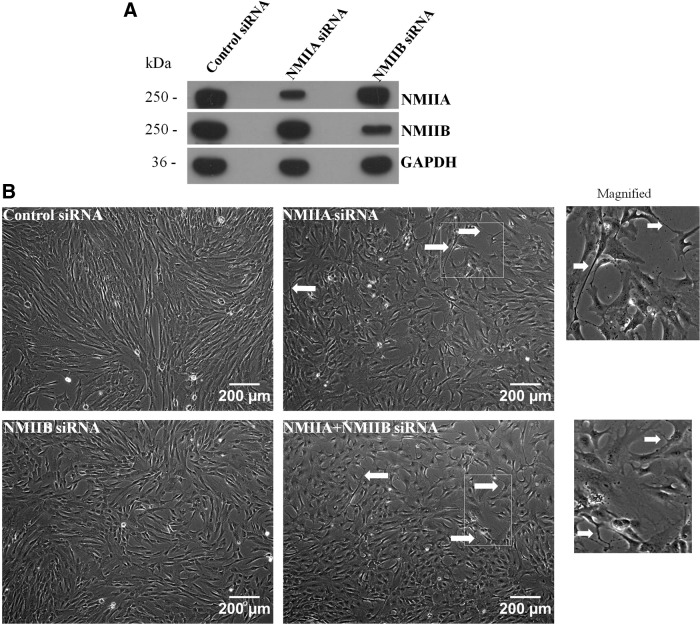
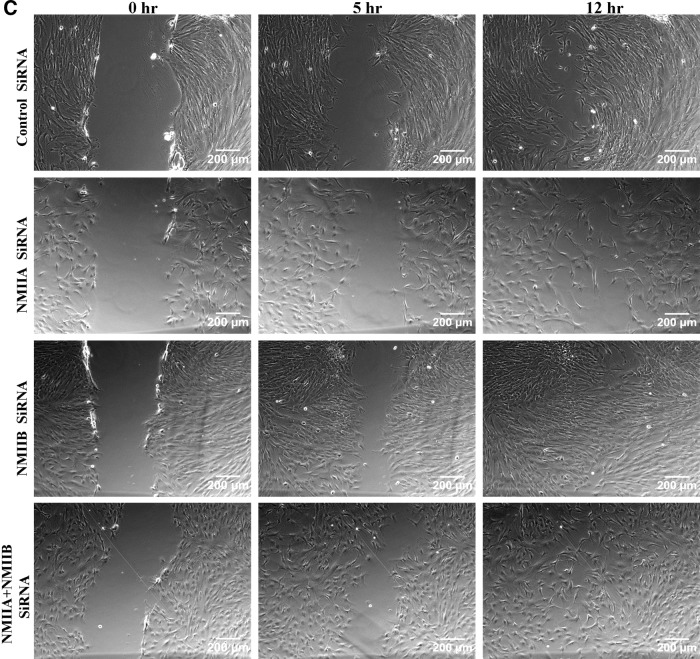
Although the three NMII isoforms identified till date, NMIIA, NMIIB, and NMIIC, have 64%–80% amino acid homology, they are not functionally redundant and play different roles in cell migration, cytokinesis, regulation of cell shape, and so on. [16]. Blebbistatin inhibits all the three isoforms of NMII. Hence, to directly assess which isoform of NMII is responsible for controlling migration in WJ-MSCs, siRNA-mediated knockdown of NMIIA or NMIIB was carried out. WJ-MSCs grown at 60%–70% density were transfected with NMIIA- or NMIIB-specific siRNA. Using siRNA sequences specific for each target, we could significantly decrease the expression of NMIIA or NMIIB without affecting the expression of the other isoform (Fig. 7A), as confirmed by western blotting. Densitometric assessment of western blots indicated that transfection with siRNA reduced protein levels of NMIIA and NMIIB by ∼60% and 80%, respectively. First, siRNA-mediated knockdown of NMIIA, but not NMIIB, caused a change in the morphology of WJ-MSCs as the cells could not retract their trailing edge and, therefore, they had extended tails (Fig. 7B, arrowheads). A similar change in cell morphology was also seen when WJ-MSCs were cotransfected with NMIIA and NMIIB siRNAs to achieve dual knockdown (Fig. 7B, arrowheads). Next, migration was studied and compared in a scratch wound assay 96 h post-transfection (Fig. 7C–F). A difference was noted in the manner by which the NMIIA-depleted WJ-MSCs migrated to heal the wound. While the control and NMIIB-depleted cells moved as a cohesive monolayer in a directional manner to close the gap (Fig. 7C, E), NMIIA-depleted WJ-MSCs demonstrated cell–cell detachment and appeared to migrate in a nondirectional and random manner (Fig. 7D). A similar phenomenon was observed in the cells depleted of both NMIIA and NMIIB (Fig. 7F).
FIG. 7.
SiRNA-mediated downregulation of NMII isoforms. Western blots of WJ-MSC lysates, prepared 72 h post-transfection, showed selective downregulation of NMIIA and NMIIB protein expression by siRNA specific for NMIIA or NMIIB isoforms, respectively. The same blot was reprobed with anti-GAPDH antibody to demonstrate equal loading (A). Morphology of WJ-MSCs 72 h after transfection with negative control siRNA, NMIIA siRNA, NMIIB SiRNA, or cotransfection with NMIIA plus NMIIB siRNA. SiRNA-mediated downregulation of NMIIA, but not NMIIB, resulted in extended tails as shown by white arrows. Boxed regions are zoomed. Scale bar: 200 μm (B). Scratch migration assay of siRNA-transfected WJ-MSCs. WJ-MSCs were seeded in 12-well plates and transfected with negative control siRNA or siRNAs against NMIIA, NMIIB, and both NMIIA and NMIIB using Lipofectamine 3000. Seventy-two hours after siRNA transfection, confluent monolayer cultures were wounded with a sterile pipette tip. Images of the wound healing were captured at 0 h, 5, and 12 h (C). Scale bar: 200 μm. Results are representative of at least three independent biological or donor samples.
Discussion
MSCs are currently being assessed in the clinical setting. One persistent challenge in the field of stem cell-based transplantation therapies is successful delivery of the cells to the site of injured tissue or organ. Since the property of migration and homing is one of the defining features of MSCs, understanding the underlying mechanisms of MSC trafficking is of clinical relevance and general scientific interest. In this context, in our study, we investigated the role of NMII in migration of WJ-MSCs. NMIIA and NMIIB expression was detected by RT-PCR and western blotting in cultured WJ-MSCs, while NMIIC, although detected faintly by RT-PCR, was undetectable by western blotting. Pharmacological inhibition using specific inhibitors, blebbistatin for NMII and Y-27632 for ROCK, resulted in WJ-MSCs with a flattened appearance, which showed decreased migration as evident by both the scratch assay and live-cell imaging. Furthermore, assessment and comparison of accumulated distance and migration speed between the individually tracked control and blebbistatin- or Y-27632-treated WJ-MSCs revealed significant differences.
Since blebbistatin inhibits all NMII isoforms, next, to understand the role of individual isoforms, we selectively downregulated the expression of NMIIA and NMIIB in WJ-MSCs using RNA interference technology and evaluated migration. SiRNA-mediated knockdown of NMIIA, but not NMIIB, expression in WJ-MSCs resulted in cells with extended tails suggesting a defect in the rear retraction. NMIIA-depleted WJ-MSCs also had altered migration properties where they exhibited loss of cell–cell contact and directionality during migration. Furthermore, the presence of NMIIB could not compensate for the absence of NMIIA and restore this defect in migration. WJ-MSCs depleted of both NMIIA and NMIIB also demonstrated similar defects in rear retraction and migration property. Therefore, specifically, the NMIIA isoform appears to be responsible for this since the control and NMIIB-depleted cells moved like a sheet and their migration was directional.
Consistently, previous studies on NMIIA-deficient embryonic stem (ES) cells, CHO.K1, and human foreskin fibroblasts (HFF) reported elongated trailing tails and decreased directional persistence in the NMIIA-ablated cells [17,18]. However, in contrast to our data, a substantial increase in cell migration was observed both in blebbistatin-treated and NMIIA-ablated ES and HFF cells [18]. Although categorized as stem cells, there are fundamental differences between ES cells and MSCs with respect to their immortality and plasticity, and also, these two populations of stem cells express different nuclear proteomes [19]. In the light of the above, findings in ES cells cannot always be extended to MSCs and, hence, this study in WJ-MSCs assumes importance. In fact, despite certain similarities in basic characteristics such as morphology and immunophenotype, MSCs even from distinct tissue sources exhibit different biological properties.
Cell migration requires low-affinity adhesion to facilitate rapid attachment and release of the cell. ECM proteins are known to influence cell adhesion and migration and, in fact, many ECM and adhesion molecules like integrins, selectins, and cadherins are regulated during migration.
Data from our ECM and adhesion molecule quantitative PCR array revealed an upregulation of COL, VCAN, and LAM gene expression in NMII-inhibited WJ-MSCs. These are components of ECM and their upregulation indicates that they could be playing a role in stronger adherence of blebbistatin-treated cells to the substratum [20,21]. Similarly, VTN, a serum-spreading protein responsible for spreading of cells and adhesion [22], was found to be upregulated in our blebbistatin-treated WJ-MSCs, which by morphology were flattened and more spread out than the control WJ-MSCs. MMPs play a role in the turnover and degradation of ECM components and basement membranes, which influences many biological processes such as proliferation, migration, and angiogenesis [23]. A higher expression of MMP3 and MMP12 was noted in blebbistatin-treated WJ-MSCs compared to control WJ-MSCs. Although this increase in expression cannot be directly linked, it could be in an attempt to overcome adherence and allow migration. TIMPs inhibit active forms of MMPs, and TIMP3 has been shown to affect cell migration and reduce cancer cell migration and invasion [24]. Accordingly, we see an upregulation of TIMP3 expression in blebbistatin-treated WJ-MSCs, which also have limited migratory capacity.
In addition, blebbistatin treatment of WJ-MSCs led to downregulation of the following genes: SPP1, TNC, COL7A1, and MMP10. SPP1 or osteopontin is a glycoprotein of the ECM, which has been shown to promote cellular migration in MSCs [25] and in several types of cancer and normal cells [26,27]. TNC is an ECM glycoprotein, which has been shown to promote migration in different cell types like fibroblasts, astrocytes, and endothelial cells [28,29]. MMP10 has been shown to enhance cellular migration and invasion in macrophages, cultured keratinocytes, HeLa cells, and so on. [30,31]. Therefore, taken together, the array-based gene expression profile indicates that inhibition of NMII activity in WJ-MSCs affects the expression of certain ECM and cell adhesion molecules, which supports the decreased migration trend observed.
To further assess the isoform-specific role of NMII in the regulation of adhesion and ECM gene expression, WJ-MSCs were treated with negative control, NMIIA, or NMIIB siRNA and the gene expression profile for a few select genes was compared. WJ-MSCs depleted of NMIIA by siRNA treatment demonstrated a similar expression profile for COL12A, MMP3, ITG5A, and VTN as blebbistatin-treated WJ-MSCs, while in contrast, depletion of NMIIB did not cause much change in the expression from control cells, suggesting a role of NMIIA in the regulation of these genes (Supplementary Fig. S1). For TNC and MMP10, the RT-PCR profile of NMIIA-depleted cells did not quite match with blebbistatin-treated cells and this difference could be either due to blebbistatin achieving a more complete inhibition than individual siRNAs or human sample-to-sample variations.
To conclude, our results clearly demonstrate that WJ-MSC migration is dependent on the NMII activity, and inhibition of NMII activity in these cells strongly suppresses migration. Moreover, the isoforms, NMIIA and NMIIB, have differential effects on migration of WJ-MSCs. NMII was also shown to influence the adhesion properties of WJ-MSCs. This is the first report to our knowledge highlighting the involvement of NMII in migration and adhesion of MSCs. Our future work would concentrate on understanding the in-depth mechanistic role of NMIIA in WJ-MSC migration.
Supplementary Material
Acknowledgments
This work was funded by the Indian Institute of Science Education and Research (IISER), Kolkata, and the Indian Council of Medical Research (ICMR), India. We are grateful to Dr. Jayanta Chatterjee, consultant gynecologist, Astha, and Kalyani, West Bengal, India, for generously providing the umbilical cord samples. We thank Mr. Ritabrata Ghosh for technical assistance with microscopy and live-cell imaging and Dr. Mohit Prasad for providing the secondary antibody. We are also thankful to Mr. Avinash Sharma for his help with scratch wound assays and PCRs.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313 [DOI] [PubMed] [Google Scholar]
- 2.Meirelles LS, Fontes AM, Covas DT. and Caplan AI. (2009). Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 20:419–427 [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhjan RK. and Lalykina KS. (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3:393–403 [DOI] [PubMed] [Google Scholar]
- 4.Stenderup K, Justesen J, Clausen C. and Kassem M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33:919–926 [DOI] [PubMed] [Google Scholar]
- 5.Bongso A, Fong CY. and Gauthaman K. (2008). Taking stem cells to the clinic: major challenges. J Cell Biochem 105:1352–1360 [DOI] [PubMed] [Google Scholar]
- 6.Lauffenburger DA. and Horwitz AF. (1996). Cell migration: a physically integrated molecular process. Cell 84:359–369 [DOI] [PubMed] [Google Scholar]
- 7.Vicente-Manzanares M, Ma X, Adelstein RS. and Horwitz AR. (2009). Non-muscle myosin II takes a centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10:778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heissler SM. and Manstein DJ. (2013). Nonmuscle myosin-2: mix and match. Cell Mol Life Sci 70:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raab M, Swift J, Dingal PC, Shah P, Shin JW. and Discher DE. (2012). Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol 199:669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Meng H, Li C, Hao M, Wang Y, Yu Z, Li Q, Han J, Zhai Q. and Qiu L. (2010). Umbilical cord-derived mesenchymal stem cells isolated by a novel explantation technique can differentiate into functional endothelial cells and promote revascularization. Stem Cell Dev 19:1511–1522 [DOI] [PubMed] [Google Scholar]
- 11.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A. and Sellers JR. (2004). Mechanism of blebbistatin inhibition of Myosin II. J Biol Chem 34:35557–35563 [DOI] [PubMed] [Google Scholar]
- 12.Sharma T, Kumari P, Pincha N, Mutukula N, Saha S, Jana SS. and Ta M. (2014). Inhibition of non-muscle myosin II leads to G0/Garrest of Wharton's jelly-derived mesenchymal stromal cells. Cytotherapy 16:640–652 [DOI] [PubMed] [Google Scholar]
- 13.Mueller BK, Mack H. and Teusch N. (2005). Rho kinase a promising drug target for neurological disorders. Nat Rev Drug Discov 4:387–398 [DOI] [PubMed] [Google Scholar]
- 14.Riento K. and Ridley AJ. (2003). Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4:446–456 [DOI] [PubMed] [Google Scholar]
- 15.Gauthaman K, Fong CY, Subramania A, Biswas A. and Bongso A. (2010). ROCK Inhibitor Y-27632 Increases Thaw-Survival Rates and Preserves Stemness and Differentiation Potential of Human Wharton's Jelly Stem Cells After Cryopreservation. Stem cell rev and Rep 6:665–676 [DOI] [PubMed] [Google Scholar]
- 16.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A. and Parkos CA. (2007). A Unique Role for Nonmuscle Myosin Heavy Chain IIA in Regulation of Epithelial Apical Junctions. PLoS One 2:e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK. and Horwitz AF. (2007). Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol 176:573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS. and Yamada KM. (2007). Myosin IIA regulates cell motility and actomyosin–microtubule crosstalk. Nat Cell Biol 9:299–309 [DOI] [PubMed] [Google Scholar]
- 19.Jaishankar A, Barthelery M, Freeman WM, Salli U, Ritty TM. and Vrana KE. (2009). Human embryonic and mesenchymal stem cells express different nuclear proteomes. Stem Cells Dev 18:793–802 [DOI] [PubMed] [Google Scholar]
- 20.Clyman RI, McDonald KA. and Kramer RH. (1990). Integrin receptors on aortic smooth muscle cells mediate adhesion to fibronectin, laminin, and collagen. Circ Res 67:175–186 [DOI] [PubMed] [Google Scholar]
- 21.Wight TN. (2002). Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol 14:617–623 [DOI] [PubMed] [Google Scholar]
- 22.Stefansson S, Su EJ, Ishigami S, Cale JM, Gao Y, Gorlatova N. and Lawrence DA. (2007). The contributions of integrin affinity and integrin-cytoskeletal engagement in endothelial and smooth muscle cell adhesion to vitronectin. J Biol Chem 282:15679–15689 [DOI] [PubMed] [Google Scholar]
- 23.Dufour A, Zucker S, Sampson NS, Kuscu C. and Cao J. (2010). Role of matrix metalloproteinase-9 dimers in cell migration: designs of inhibitory peptides. J Biol Chem 285:35944–359956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker AH, Edwards DR. and Murphy G. (2002). Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115:3719–3727 [DOI] [PubMed] [Google Scholar]
- 25.Zou C, Luo Q, Qin J, Shi Y, Yang L, Ju B. and Song G. (2013). Osteopontin promotes mesenchymal stem cell migration and lessens cell stiffness via integrin β1, FAK, and ERK pathways. Cell Biochem Biophys 65:455–462 [DOI] [PubMed] [Google Scholar]
- 26.Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ, Tsai CH, Hsu HC, Liu SH. and Tang CH. (2009). Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Physiol 221:98–108 [DOI] [PubMed] [Google Scholar]
- 27.Li JJ, Han M, Wen JK. and Li AY. (2007). Osteopontin stimulates vascular smooth muscle cell migration by inducing FAK phosphorylation and ILK dephosphorylation. Biochem Biophys Res Commun 356:13–19 [DOI] [PubMed] [Google Scholar]
- 28.Trebaul A, Chan EK. and Midwood KS. (2007). Regulation of fibroblast migration by tenascin-C. Biochem Soc Trans 35:695–697 [DOI] [PubMed] [Google Scholar]
- 29.Nishio T, Kawaguchi S, Yamamoto M, Iseda T, Kawasaki T. and Hase T. (2005). Tenascin-C regulates proliferation and migration of cultured astrocytes in a scratch wound assay. Neuroscience 132:87–102 [DOI] [PubMed] [Google Scholar]
- 30.Krampert M, Bloch W, Sasaki T, Bugnon P, Rulicke T, Wolf E, Aumailley M, Parks WC. and Werner S. (2004). Activities of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix degradation and keratinocyte organization in wounded skin. Mol Biol Cell 15:5242–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray MY, Birkland TP, Howe JD, Rowan AD, Fidock M, Parks WC. and Gavrilovic J. (2013). Macrophage migration and invasion is regulated by MMP10 expression. PLoS One 8:e63555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.