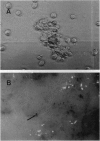
Abstract
We propose that nodule formation is mediated by eicosanoids in insects. Nodulation is the temporally and quantitatively predominant cellular defense response to bacterial infection in insects and other invertebrates. Inhibition of eicosanoid biosynthesis in larvae of the tobacco hornworm Manduca sexta immediately prior to intrahemocoelic infections with the bacterium Serratia marcescens strongly reduced the nodulation response. Inhibition of eicosanoid biosynthesis also reduced formation of cellular aggregates at 1 hr postinfection, which indicates that eicosanoids mediate early stages of nodulation. Separate treatments with specific inhibitors of phospholipase A2, cyclooxygenase, and lipoxygenase reduced nodulation, which supports the view that nodule formation is a complex process involving prostaglandins and lipoxygenase products. The inhibitory effects of the phospholipase A2 inhibitor dexamethasone on nodulation were apparent by 1 hr after infection, and the effects increased, relative to controls, over 24 hr. The dexamethasone effects were expressed in a dose-dependent manner, and they were reversed by treating infected insects with eicosanoid-precursor polyunsaturated fatty acids. Treatments with the saturated fatty acid 16:0, which is not an eicosanoid precursor, did not reverse the dexamethasone effects on nodulation. These findings strongly support the identification of nodulation as a specific insect cellular defense mechanism that is mediated by eicosanoids.
Full text
PDF
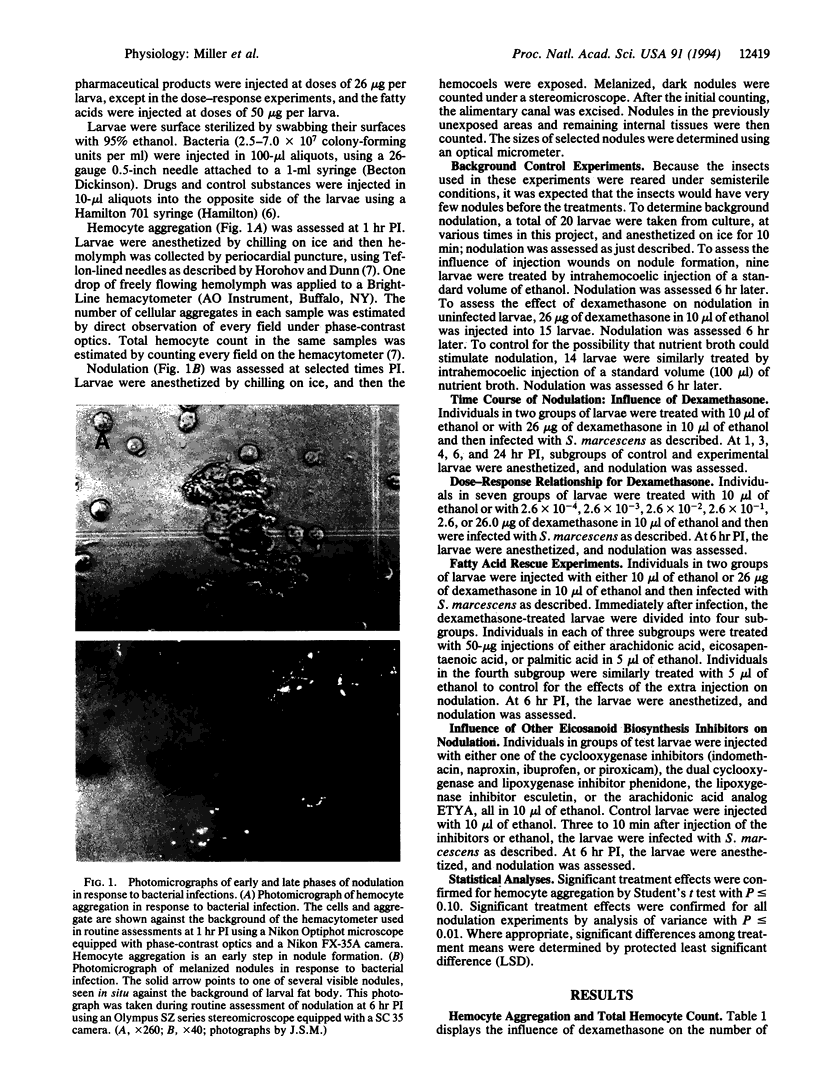
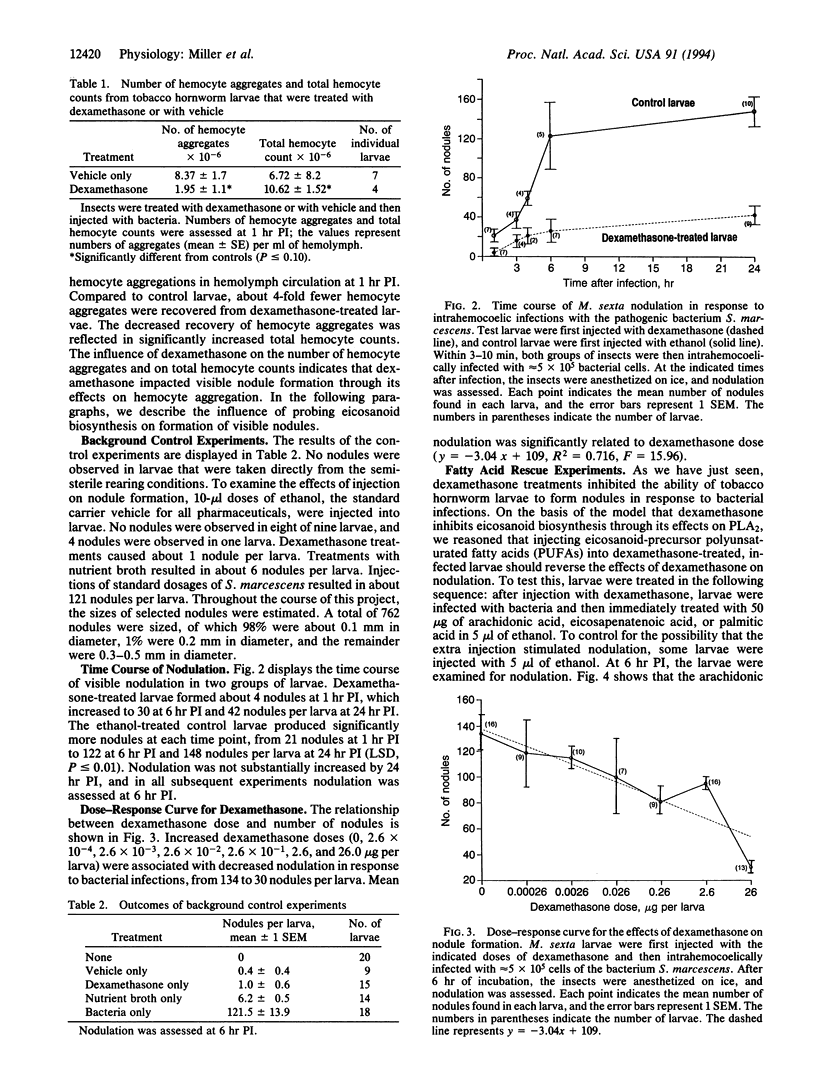
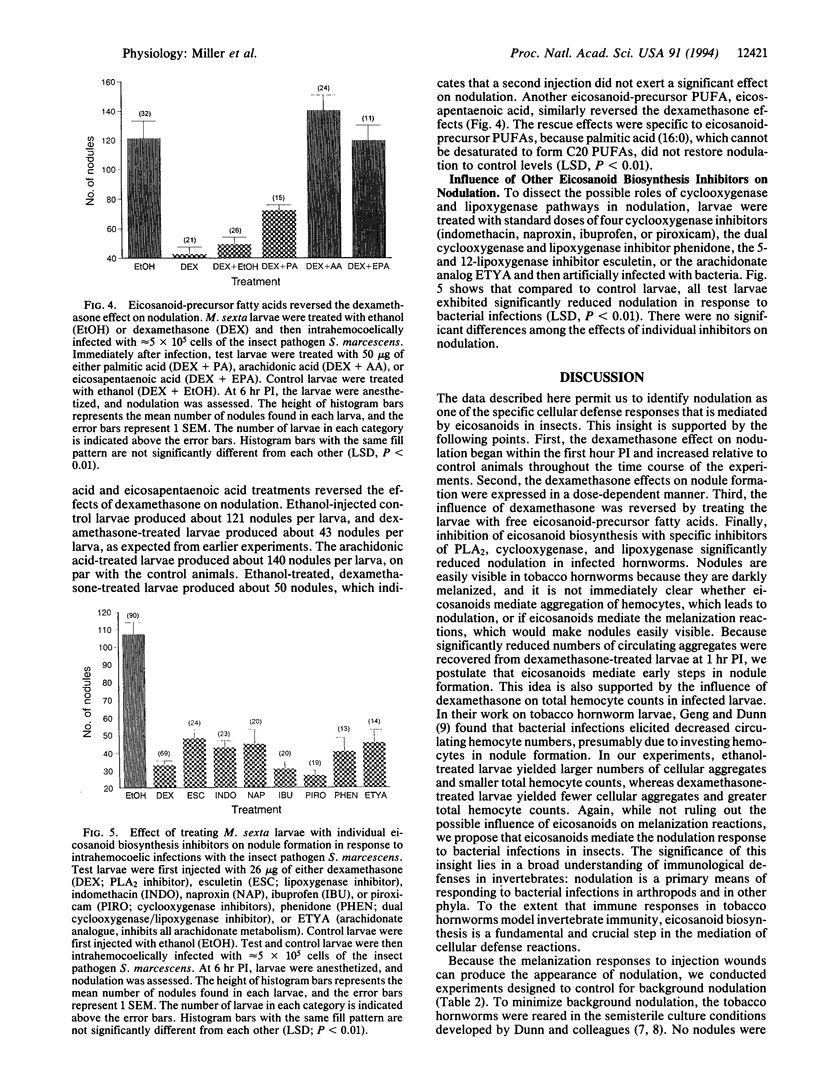

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boman H. G., Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Bonta I. L., Parnham M. J. Immunomodulatory-antiinflammatory functions of E-type prostaglandins. Minireview with emphasis on macrophage-mediated effects. Int J Immunopharmacol. 1982;4(2):103–109. doi: 10.1016/0192-0561(82)90057-1. [DOI] [PubMed] [Google Scholar]
- Geng C. X., Dunn P. E. Plasmatocyte depletion in larvae of Manduca sexta following injection of bacteria. Dev Comp Immunol. 1989 Winter;13(1):17–23. doi: 10.1016/0145-305x(89)90012-8. [DOI] [PubMed] [Google Scholar]
- Hampson A. J., Rowley A. F., Barrow S. E., Steadman R. Biosynthesis of eicosanoids by blood cells of the crab, Carcinus maenas. Biochim Biophys Acta. 1992 Mar 4;1124(2):143–150. doi: 10.1016/0005-2760(92)90090-i. [DOI] [PubMed] [Google Scholar]
- Russell V. W., Dunn P. E. Lysozyme in the midgut of Manduca sexta during metamorphosis. Arch Insect Biochem Physiol. 1991;17(2-3):67–80. doi: 10.1002/arch.940170202. [DOI] [PubMed] [Google Scholar]
- Stanley-Samuelson D. W., Jensen E., Nickerson K. W., Tiebel K., Ogg C. L., Howard R. W. Insect immune response to bacterial infection is mediated by eicosanoids. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1064–1068. doi: 10.1073/pnas.88.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley-Samuelson D. W., Ogg C. L. Prostaglandin biosynthesis by fat body from the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 1994 May;24(5):481–491. doi: 10.1016/0965-1748(94)90043-4. [DOI] [PubMed] [Google Scholar]