Abstract
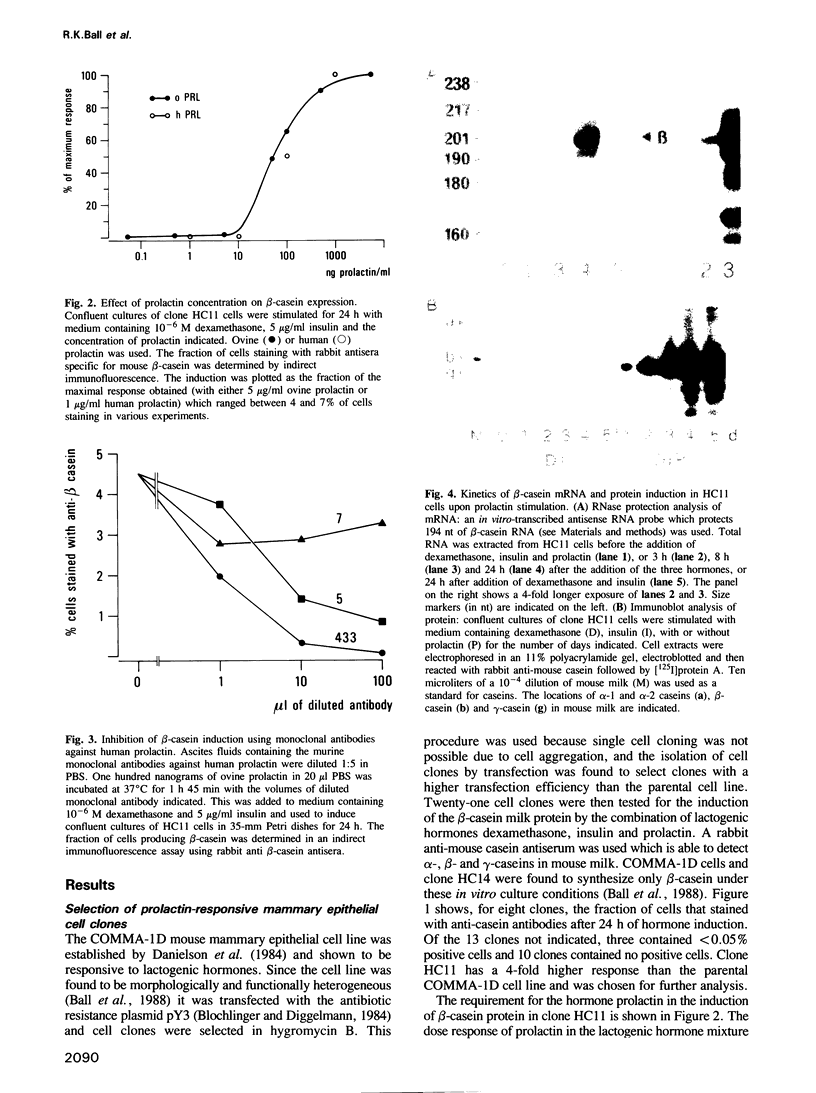
In order to study the hormonal regulation of gene expression in mammary epithelial cells, we isolated a prolactin-responsive cell clone, HC11, from the COMMA-1D mouse mammary epithelial cell line. Clone HC11 was selected as a unique example of a cloned mouse mammary epithelial cell which has no requirement for complex, exogenously added, extracellular matrix or co-cultivation with other cell types for the prolactin-dependent in vitro induction of the endogenous beta-casein gene by lactogenic hormones. Induction of beta-casein mRNA is rapid and was detected 3 h after hormone stimulation. A prolactin-dependent increase in the rate of transcription of the beta-casein gene was shown in an in vitro nuclear transcription assay. beta-Casein protein was detected in an immunoblot assay after 24 h, and further accumulated during 5 days of hormone treatment. To identify low-abundance proteins induced directly after prolactin stimulation, mRNA was accumulated during 5 h of stimulation of HC11 cells with prolactin in the presence of cycloheximide. Following cycloheximide removal, the mRNA was translated into protein during a 60-min [35S]methionine pulse and the proteins were resolved by DEAE ion exchange HPLC and SDS-PAGE. A strong induction of a 120-kd cytosolic protein was detected which was maximally expressed within 6 h of hormone stimulation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. K., Ziemiecki A., Schönenberger C. A., Reichmann E., Redmond S. M., Groner B. v-myc alters the response of a cloned mouse mammary epithelial cell line to lactogenic hormones. Mol Endocrinol. 1988 Feb;2(2):133–142. doi: 10.1210/mend-2-2-133. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D. F., Harrelson A. L., Darnell J. E., Jr Dependence of liver-specific transcription on tissue organization. Mol Cell Biol. 1985 Oct;5(10):2623–2632. doi: 10.1128/mcb.5.10.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Danielson K. G., Oborn C. J., Durban E. M., Butel J. S., Medina D. Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djiane J., Kelly P. A., Katoh M., Dusanter-Fourt I. Prolactin receptor: identification of the binding unit by affinity labelling and characterization of poly- and monoclonal antibodies. Horm Res. 1985;22(3):179–188. doi: 10.1159/000180092. [DOI] [PubMed] [Google Scholar]
- Durban E. M., Medina D., Butel J. S. Comparative analysis of casein synthesis during mammary cell differentiation in collagen and mammary gland development in vivo. Dev Biol. 1985 Jun;109(2):288–298. doi: 10.1016/0012-1606(85)90456-7. [DOI] [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Haldosén L. A., Gustafsson J. A. Characterization of hepatic lactogen receptor. Subunit composition and hydrodynamic properties. J Biol Chem. 1987 May 25;262(15):7404–7411. [PubMed] [Google Scholar]
- Hennighausen L. G., Sippel A. E. Characterization and cloning of the mRNAs specific for the lactating mouse mammary gland. Eur J Biochem. 1982 Jun 15;125(1):131–141. doi: 10.1111/j.1432-1033.1982.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Jaggi R., Salmons B., Muellener D., Groner B. The v-mos and H-ras oncogene expression represses glucocorticoid hormone-dependent transcription from the mouse mammary tumor virus LTR. EMBO J. 1986 Oct;5(10):2609–2616. doi: 10.1002/j.1460-2075.1986.tb04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. K., Yu-Lee L. Y., Clift S. M., Brown T. L., Rosen J. M. The rat casein multigene family. Fine structure and evolution of the beta-casein gene. J Biol Chem. 1985 Jun 10;260(11):7042–7050. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Katoh M., Ferland L. H., Houdebine L. M., Teyssot B., Dusanter-Fourt I. The interaction of prolactin with its receptors in target tissues and its mechanism of action. Recent Prog Horm Res. 1984;40:379–439. doi: 10.1016/b978-0-12-571140-1.50014-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Spencer S. A., Cachianes G., Hammonds R. G., Collins C., Henzel W. J., Barnard R., Waters M. J., Wood W. I. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987 Dec 10;330(6148):537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L. C., Tsuyuki D., Myal Y., Shiu R. P. Isolation and sequencing of a cDNA clone for a prolactin-inducible protein (PIP). Regulation of PIP gene expression in the human breast cancer cell line, T-47D. J Biol Chem. 1987 Nov 5;262(31):15236–15241. [PubMed] [Google Scholar]
- Nicoll C. S., Mayer G. L., Russell S. M. Structural features of prolactins and growth hormones that can be related to their biological properties. Endocr Rev. 1986 May;7(2):169–203. doi: 10.1210/edrv-7-2-169. [DOI] [PubMed] [Google Scholar]
- Ponta H., Ball R., Steinmetz M., Groner B. Hormonal regulation of cell surface expression of the major histocompatibility antigen H-2Ld in transfected cells. EMBO J. 1985 Dec 16;4(13A):3447–3453. doi: 10.1002/j.1460-2075.1985.tb04103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen J. M., Jones W. K., Rodgers J. R., Compton J. G., Bisbee C. A., David-Inouye Y., Yu-Lee L. Y. Regulatory sequences involved in the hormonal control of casein gene expression. Ann N Y Acad Sci. 1986;464:87–99. doi: 10.1111/j.1749-6632.1986.tb15996.x. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Iwasiow B. M. Prolactin-inducible proteins in human breast cancer cells. J Biol Chem. 1985 Sep 15;260(20):11307–11313. [PubMed] [Google Scholar]
- Staindl B., Berger P., Kofler R., Wick G. Monoclonal antibodies against human, bovine and rat prolactin: epitope mapping of human prolactin and development of a two-site immunoradiometric assay. J Endocrinol. 1987 Aug;114(2):311–318. doi: 10.1677/joe.0.1140311. [DOI] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Wiens D., Park C. S., Stockdale F. E. Milk protein expression and ductal morphogenesis in the mammary gland in vitro: hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction. Dev Biol. 1987 Mar;120(1):245–258. doi: 10.1016/0012-1606(87)90122-9. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Banerjee M. R., Oka T. Nucleotide sequence of a cDNA encoding mouse beta casein. Nucleic Acids Res. 1986 Oct 24;14(20):8224–8224. doi: 10.1093/nar/14.20.8224. [DOI] [PMC free article] [PubMed] [Google Scholar]






