Abstract
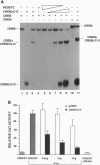
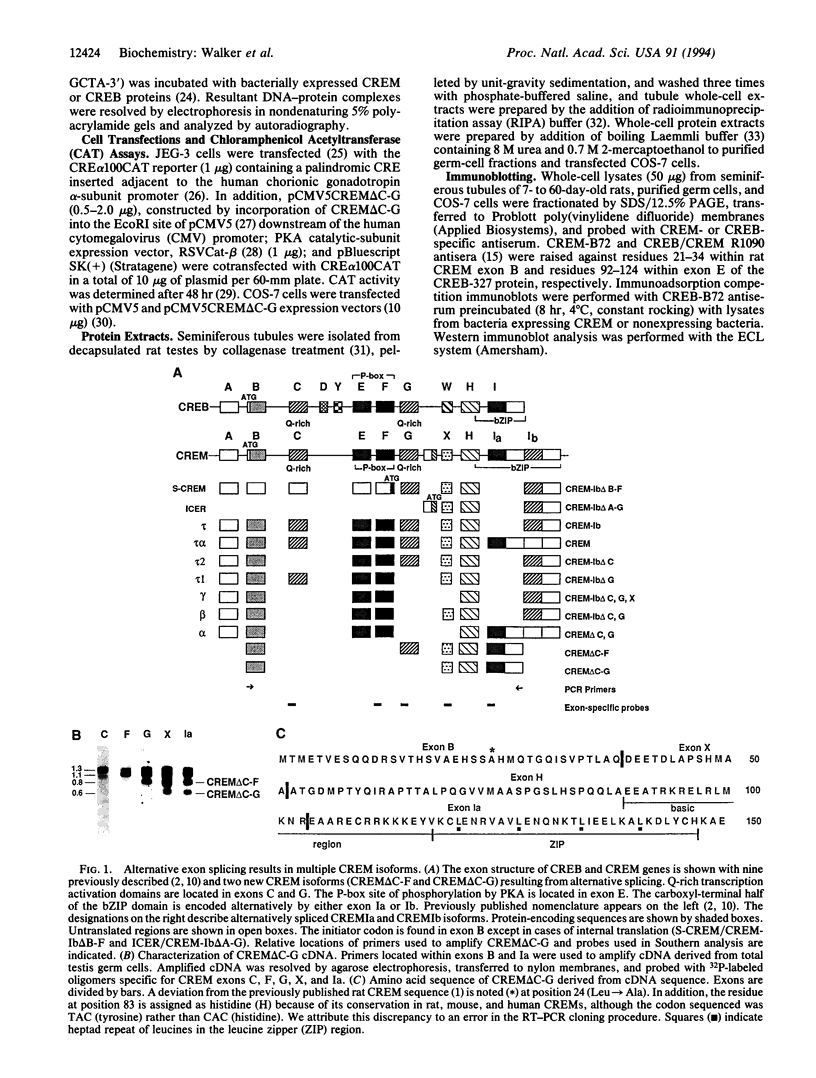
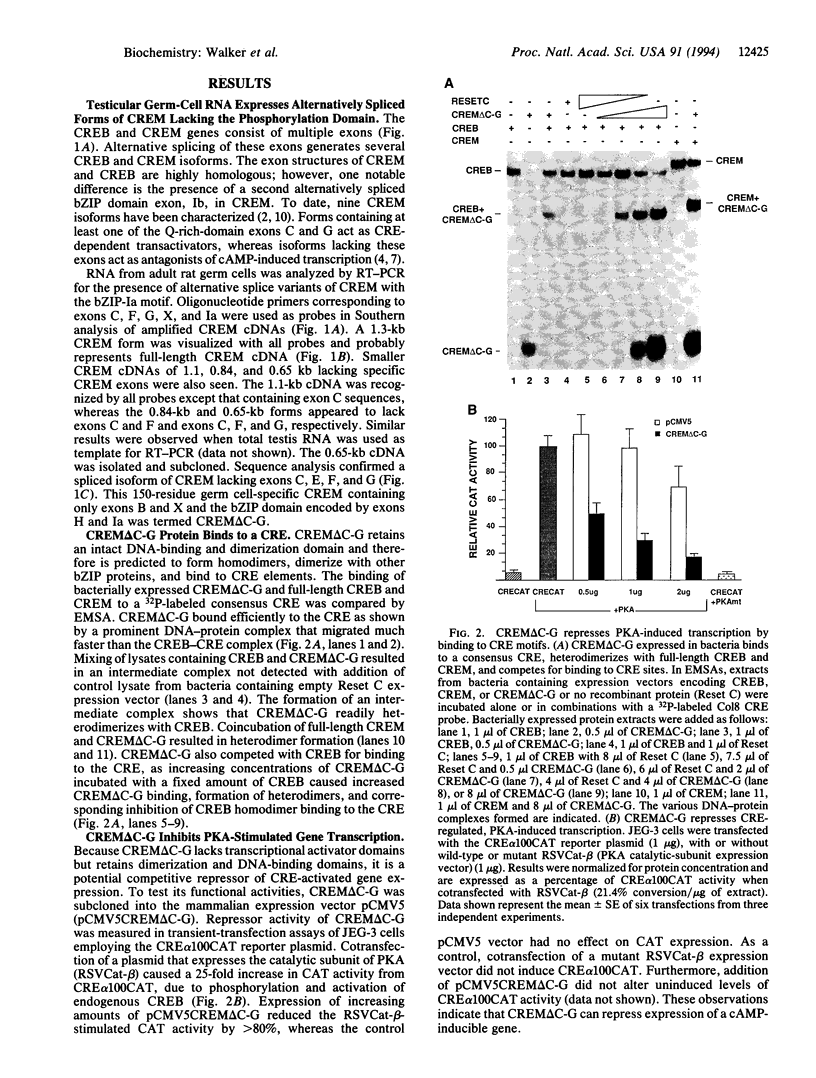
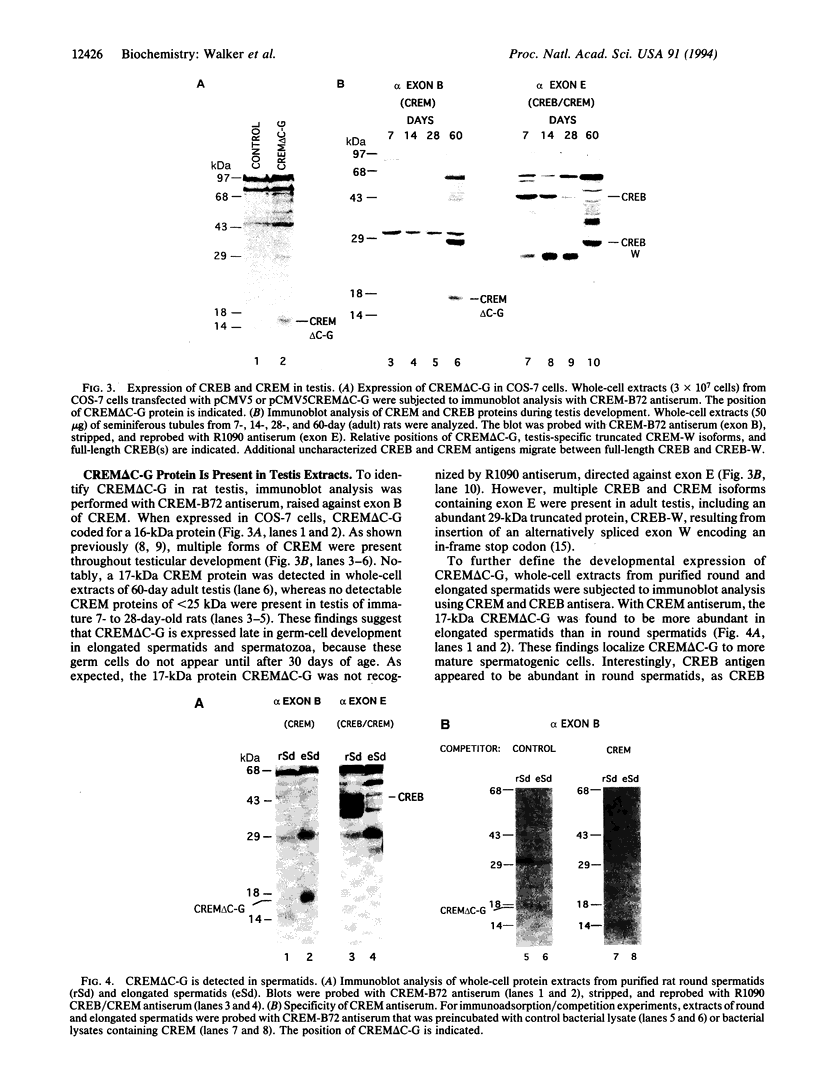
cAMP response element-binding protein (CREB) and modulator protein (CREM) regulate the transcription of cAMP-responsive genes via phosphorylation by cAMP-dependent protein kinase A. Reverse transcription and polymerase chain amplification of RNA from male germ cells identify an alternatively spliced CREM isoform, CREM delta C-G, lacking four exons including those encoding the protein kinase A-regulated phosphorylation domain and the flanking glutamine-rich transcriptional activation domains. CREM delta C-G retains exons that encode the basic-leucine zipper (bZIP) DNA-binding domain, binds to cAMP response elements (CREs), and competitively inhibits binding of CREB and CREM to CREs. Expression of CREM delta C-G inhibits transcription of a CRE-containing chloramphenicol acetyltransferase reporter plasmid induced by endogenous CREB. Antiserum to CREM detects CREM delta C-G in elongated spermatids from rat testis. These observations indicate that CREM delta C-G is a unique form of a competitive negative regulator of CREB-mediated gene transcription expressed in a maturation-dependent manner in haploid germ cells. The developmental specificity of CREM delta C-G suggests that it may play a role in transcriptional regulation during spermatogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellvé A. R., Millette C. F., Bhatnagar Y. M., O'Brien D. A. Dissociation of the mouse testis and characterization of isolated spermatogenic cells. J Histochem Cytochem. 1977 Jul;25(7):480–494. doi: 10.1177/25.7.893996. [DOI] [PubMed] [Google Scholar]
- Brindle P., Linke S., Montminy M. Protein-kinase-A-dependent activator in transcription factor CREB reveals new role for CREM repressors. Nature. 1993 Aug 26;364(6440):821–824. doi: 10.1038/364821a0. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Andres D. A., Goldstein J. L., Russell D. W., Brown M. S. cDNA cloning and expression of the peptide-binding beta subunit of rat p21ras farnesyltransferase, the counterpart of yeast DPR1/RAM1. Cell. 1991 Jul 26;66(2):327–334. doi: 10.1016/0092-8674(91)90622-6. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Delmas V., Laoide B. M., Masquilier D., de Groot R. P., Foulkes N. S., Sassone-Corsi P. Alternative usage of initiation codons in mRNA encoding the cAMP-responsive-element modulator generates regulators with opposite functions. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4226–4230. doi: 10.1073/pnas.89.10.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas V., van der Hoorn F., Mellström B., Jégou B., Sassone-Corsi P. Induction of CREM activator proteins in spermatids: down-stream targets and implications for haploid germ cell differentiation. Mol Endocrinol. 1993 Nov;7(11):1502–1514. doi: 10.1210/mend.7.11.8114765. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Erickson R. P. Post-meiotic gene expression. Trends Genet. 1990 Aug;6(8):264–269. doi: 10.1016/0168-9525(90)90209-o. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Mellström B., Benusiglio E., Sassone-Corsi P. Developmental switch of CREM function during spermatogenesis: from antagonist to activator. Nature. 1992 Jan 2;355(6355):80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Menzel P., Leonard J., Fischer W. H., Montminy M. R. Characterization of motifs which are critical for activity of the cyclic AMP-responsive transcription factor CREB. Mol Cell Biol. 1991 Mar;11(3):1306–1312. doi: 10.1128/mcb.11.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992 Jul 10;70(1):105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Deutsch P. J., Lin J., Habener J. F. Distinct adenosine 3',5'-monophosphate and phorbol ester-responsive signal transduction pathways converge at the level of transcriptional activation by the interactions of DNA-binding proteins. Mol Endocrinol. 1989 May;3(5):868–880. doi: 10.1210/mend-3-5-868. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Waeber G., Habener J. F. Multiple adenosine 3',5'-cyclic [corrected] monophosphate response element DNA-binding proteins generated by gene diversification and alternative exon splicing. Mol Endocrinol. 1990 Jun;4(6):920–930. doi: 10.1210/mend-4-6-920. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., CLERMONT Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952 Nov 20;55(4):548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laoide B. M., Foulkes N. S., Schlotter F., Sassone-Corsi P. The functional versatility of CREM is determined by its modular structure. EMBO J. 1993 Mar;12(3):1179–1191. doi: 10.1002/j.1460-2075.1993.tb05759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A. Both isoforms of the cAMP-dependent protein kinase catalytic subunit can activate transcription of the prolactin gene. J Biol Chem. 1989 Apr 25;264(12):6870–6873. [PubMed] [Google Scholar]
- Meistrich M. L., Longtin J., Brock W. A., Grimes S. R., Jr, Mace M. L. Purification of rat spermatogenic cells and preliminary biochemical analysis of these cells. Biol Reprod. 1981 Dec;25(5):1065–1077. doi: 10.1095/biolreprod25.5.1065. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Habener J. F. Cyclic adenosine 3',5'-monophosphate response element binding protein (CREB) and related transcription-activating deoxyribonucleic acid-binding proteins. Endocr Rev. 1993 Jun;14(3):269–290. doi: 10.1210/edrv-14-3-269. [DOI] [PubMed] [Google Scholar]
- Molina C. A., Foulkes N. S., Lalli E., Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993 Dec 3;75(5):875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982 Fall;3(4):404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Quinn P. G. Distinct activation domains within cAMP response element-binding protein (CREB) mediate basal and cAMP-stimulated transcription. J Biol Chem. 1993 Aug 15;268(23):16999–17009. [PubMed] [Google Scholar]
- Ruppert S., Cole T. J., Boshart M., Schmid E., Schütz G. Multiple mRNA isoforms of the transcription activator protein CREB: generation by alternative splicing and specific expression in primary spermatocytes. EMBO J. 1992 Apr;11(4):1503–1512. doi: 10.1002/j.1460-2075.1992.tb05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Sheen J. Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988 Jul 30;67(2):271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Stehle J. H., Foulkes N. S., Molina C. A., Simonneaux V., Pévet P., Sassone-Corsi P. Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature. 1993 Sep 23;365(6444):314–320. doi: 10.1038/365314a0. [DOI] [PubMed] [Google Scholar]
- Steinberger A. In vitro techniques for the study of spermatogenesis. Methods Enzymol. 1975;39:283–296. doi: 10.1016/s0076-6879(75)39026-5. [DOI] [PubMed] [Google Scholar]
- Vallejo M., Penchuk L., Habener J. F. Somatostatin gene upstream enhancer element activated by a protein complex consisting of CREB, Isl-1-like, and alpha-CBF-like transcription factors. J Biol Chem. 1992 Jun 25;267(18):12876–12884. [PubMed] [Google Scholar]
- Waeber G., Meyer T. E., LeSieur M., Hermann H. L., Gérard N., Habener J. F. Developmental stage-specific expression of cyclic adenosine 3',5'-monophosphate response element-binding protein CREB during spermatogenesis involves alternative exon splicing. Mol Endocrinol. 1991 Oct;5(10):1418–1430. doi: 10.1210/mend-5-10-1418. [DOI] [PubMed] [Google Scholar]
- Walker W. H., Fitzpatrick S. L., Saunders G. F. Human placental lactogen transcriptional enhancer. Tissue specificity and binding with specific proteins. J Biol Chem. 1990 Aug 5;265(22):12940–12948. [PubMed] [Google Scholar]
- Walker W. H., Stein B., Ganchi P. A., Hoffman J. A., Kaufman P. A., Ballard D. W., Hannink M., Greene W. C. The v-rel oncogene: insights into the mechanism of transcriptional activation, repression, and transformation. J Virol. 1992 Aug;66(8):5018–5029. doi: 10.1128/jvi.66.8.5018-5029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. P., Sassone-Corsi P. Hormonal control of gene expression: multiplicity and versatility of cyclic adenosine 3',5'-monophosphate-responsive nuclear regulators. Mol Endocrinol. 1993 Feb;7(2):145–153. doi: 10.1210/mend.7.2.8385737. [DOI] [PubMed] [Google Scholar]