Abstract
Low frequency ultrasound in the 20 to 60 kHz range is a novel physical modality by which to induce selective cell lysis and death in neoplastic cells. In addition, this method can be used in combination with specialized agents known as sonosensitizers to increase the extent of preferential damage exerted by ultrasound against neoplastic cells, an approach referred to as sonodynamic therapy (SDT). The methodology for generating and applying low frequency ultrasound in a preclinical in vitro setting is presented to demonstrate that reproducible cell destruction can be attained in order to examine and compare the effects of sonication on neoplastic and normal cells. This offers a means by which to reliably sonicate neoplastic cells at a level of consistency required for preclinical therapeutic assessment. In addition, the effects of cholesterol-depleting and cytoskeletal-directed agents on potentiating ultrasonic sensitivity in neoplastic cells are discussed in order to elaborate on mechanisms of action conducive to sonochemotherapeutic approaches.
Keywords: Medicine, Issue 101, Sonodynamic therapy, Low frequency ultrasound, Cancer, Leukemia, Sonosensitizers
Introduction
Ultrasound refers to any oscillating sound pressure wave with a frequency greater than the upper limit of human auditory capacities (~ 20 kHz)1. Low frequency ultrasound in the 20-60 kHz range has been utilized in the laboratory as a means of generating emulsions, preparing cellular samples for nucleic acid extraction, for tissue disruption, and for a variety of other tests. The utility of low frequency ultrasound has also been extended to the industrial setting for welding, cleaning various materials, and in materials processing. Commercially available ultrasound generators come in frequencies ranging from 18-60 kHz, and full-scale wattages from 100-1,200 W.
Although ultrasound has long been used in the clinical setting for diagnostic imaging, it has been applied as a therapeutic modality only recently. Ultrasound ≥1 MHz is capable of safely disrupting urinary calculi (kidney stones) and biliary calculi (stones in the gallbladder or in the liver) in patients to reduce symptoms2,3. This approach known as extracorporeal shockwave lithotripsy (ESWL) is now widely applied in the clinic (more than one million patients are treated annually with ESWL in the United States alone4), and offers a modality by which to non-invasively break up calculi with minimal collateral damage through the use of externally applied, focused, high intensity acoustic pulses2-4.
Due to the unique direct shearing forces, as well as cavitation bubbles generated by high intensity ultrasound, these methodologies have been examined in cancer therapy for the treatment of castration-resistant prostate carcinoma and pancreatic adenocarcinoma in an approach known as high intensity focused ultrasound (HIFU)5-8. In a manner very similar to ESWL, HIFU uses multiple ultrasound beams and focuses them on a selected focal area to generate temperatures of 60 °C or higher through the use of acoustic energy, inducing coagulative necrosis in the targeted tissue5. Although other modalities of thermal ablation currently exist (radiofrequency ablation and microwave ablation), HIFU offers a distinct advantage over these methods in that it is the only non-invasive hyperthermic modality5. HIFU has attained mixed results in the clinic and is currently only available in clinical trials8-11. Nevertheless, the limited success it has achieved, and the very promising in vivo data acquired from preclinical mammalian models have demonstrated the potential of ultrasound in cancer therapy.
In an effort to improve HIFU, researchers have attempted to combine ultrasound with appropriate antineoplastic agents to generate a form of sonochemotherapy. Sonodynamic therapy (SDT) is a promising novel treatment modality that has demonstrated impressive antineoplastic activity in both in vitro and in vivo studies1. It has been shown that ultrasound preferentially damages malignant cells based on the size differential between such cells and those of normal histology1,5. SDT incorporates specialized agents known as sonosensitizers to increase the extent of preferential damage exerted by ultrasound against neoplastic cells. While therapeutic applications of SDT have been previously examined, ultrasonic systems used typically employ higher frequency ultrasound (≥1 MHz), and the effects of low kHz frequency ultrasound has yet to be fully explored. Lower frequencies of ultrasound are often more proficient at producing inertial cavitation, a phenomenon that results in the destruction of cells due to the rapid collapsing of microbubbles, inducing physicochemical damage12-14. This difference in the generation of inertial cavitation between MHz and low kHz ultrasound has been attributed to the fact that lower wave frequencies enable microbubbles more time to grow by rectified diffusion in the expansion half cycle, consequently producing more violent collapses during the following compression half cycle12.
We have previously shown that U937 human monocytic leukemia cells are sensitive to low frequency ultrasound (23.5 kHz), and that this sensitivity can be markedly increased through the application of antineoplastic agents that perturb the cytoskeleton15. Further, we have demonstrated that cells are preferentially damaged based on size, with larger cells exhibiting higher ultrasonic sensitivity. In addition, normal human hematopoietic stem cells (hHSCs) and leukocytes at comparable cell sizes are much more resistant to sonication than their neoplastic counterparts15, tentatively suggesting that low frequency ultrasound may be used to preferentially damage malignant cells in the presence of normal tissue.
To further examine the unique properties of low frequency ultrasound for potential therapeutic use, we have developed cleaning and stabilization procedures to increase the efficacy and reliability of one of our current sonication systems, the Branson Model SLPe 150 W, 40 kHz Cell Disrupter, equipped with a 20 mm horn fitted into a 7.62 cm cup. In addition, we have been able to determine accurate sample cavitation energies, as well as consistent waveforms and amplitude within the 40 kHz range using a cavitation meter and oscilloscope with hydrophone. By refining and systematizing our protocols, we have been able to establish consistency in our experimental sonications, allowing us to quantitatively compare the sonic sensitivities of neoplastic and normal cells of different histogenetic lineages. Our protocol for the 40 kHz system is presented in extensive detail in order for interested laboratories to be capable of performing comparable experiments, and to evaluate our findings of the antineoplastic effects elicited by low frequency ultrasound. In addition, we examine the dose dependent effects of methyl-β-cyclodextrin (MeβCD; Figure 1), a cholesterol-depleting agent, on increasing the ultrasonic sensitivity of U937 and THP1 human monocytic leukemia cells.
Protocol
1. Cleaning Cell Sonifier System
Clean the union between the horn and converter using chloroform and a swab of cotton. Apply a light oil or mineral oil to the threads of the horn and converter to further remove buildup of metal shavings, rust, or oxidation.
Use chloroform to remove the oil and any other contaminants from the union after wiping the threads with oil.
2. Reassembling the Cell Sonifier and Setting up the System
Reattach the horn to the converter. To properly torque the system, remove the assembled converter and horn from the cup assembly by pulling the horn out of the bottom of the cup (Figure 2A). Place a slot wrench inside one of the three small holes near the top of the converter (Figure 2B). Then, position a torque-wrench fitted with a ½ inch crow’s foot on the slotted part of the horn (Figure 2B). Tighten in standard clockwise direction until the torque meter reads 54.23 N.m (Figure 2C), the torque recommended by the manufacturer16. Fit the tightened converter and horn back into the cup, and mount the horn and converter to a ring stand in an upright position. Reattach the converter to the power supply before setting the system for experimental pulse dosing.
Set the system for pulse dosing using 1 sec of sonication with 1 sec between pulses. Different dosing models may be used for other experimental conditions. Many variables, such as water volume and horn diameter would potentially require changes to this dosing. Using the aforementioned cell sample types, volume, and concentration, empirical data and observation have led to the formulation of this dosing regimen.
Adjust the system to the desired amplitude. In this protocol, use 33% and 50% amplitude to prevent complete cell destruction, which would render the efficacy of sonosensitizers difficult or impossible to gauge. NOTE: In this study the amplitudes are compared.
3. Assessing System Intensity and Function
Hold the cavitation meter at 1.5 cm, which is the level of the sample sonication. Note the readings to assess that the system is running at an expected intensity. Using the current system and water levels, expect between 100-110 W/cm2 for 33% amplitude and 115-125 W/cm2 for 50% amplitude. Note the cavitation meter readings from the meter display which represents cavitation energy in W/cm2 directly. Take these readings at the surface of the water, and ensure no bubbles are present at the face of the meters probe. NOTE: It is important to note that these procedures are done without the cap for access above the horn. They need not be repeated after every sonication. Rather, they should be performed only after cleaning, reassembling, and setting up the system.
Place the hydrophone in a sample vial containing 12.5 ml of water in order to fully immerse the hydrophone sensor. Suspend the hydrophone using the ring stand.
Attach the hydrophone to the input of the oscilloscope in order to assess waveform characteristics. Examine the oscilloscope for a clear sine wave, as aberrant waves can alter the frequency of the system from the specifications of the manufacturer. Both the cavitation meter and oscilloscope give a frequency reading, but only the oscilloscope will show aberrant waves that are indicative of a faulty system or improper assembly.
4. Preparation of Cells and Medium
Prepare the medium by using 240 ml Iscove’s Modified Dubecco’s Medium (IMDM) and 50 ml fetal bovine serum. Do not thaw fetal bovine serum in a hot water bath, as rapid increases in temperature can lead to a compromise in serum stability. Combine 240 µl gentamicin 50 mg/ml and 5 ml penicillin/ streptomycin 100x in a standard culture flask, and add to the medium. Store the medium at 4 °C.
Seed U937 cells at ~4 x 104 cells/ml in a 25 cm2 flask using the prepared medium. Assess cells for concentration and viability using a TC20 cell counter and trypan blue exclusion by placing 15-20 µl of cells and 15-20 µl trypan blue into a micro-centrifuge tube and mixing the contents. Pipette15-20 µl of this mixture into a TC20 sampling slide, and initiate counts by placing the slide into the TC20 cell counter.
Incubate cells at 37 °C and 5% CO2, and check for cell viability using trypan blue exclusion and the TC20 cell counter. When the cells are at an appropriate concentration and/or have been treated with sonosensitizers for an appropriate time frame, transfer 3 ml of cells from the culture flask to a 20 ml glass scintillation vial. In this protocol, grow U937 cells for 48 hr, and then treat with 0.5, 1, or 5 mM MeβCD for 30 min to sufficiently deplete the cholesterol of cells prior to sonication.
5. Cell Sonication
Degas deionized, distilled water using a vacuum and Buchner flask. Transfer the water to the cup horn, and fill to a level of 15 mm above the top of the horn. The sonication process causes further degassing of the water for a period of a few minutes, and can lead to inconsistencies over the course of the experiment if the system is not run prior to sample sonication. Therefore, run the system for ~7 min prior to sample sonication.
Attach the scintillation vial containing the cells to the holding device. Then, attach the holding device to the top of the cup horn and set to an elevation of 15 mm from the top of the horn (at the water surface using the sliding mechanism).
The cells are typically sonicated using three 1 sec pulses of ultrasound, with 1 sec spacing in between each of these pulses. For this protocol, sonicate cells at 33% and 50% amplitude using the abovementioned pulse dosing.
6. Assessing Cells for Damage Post-sonication
Assess the suspended cells for damage and viability using trypan blue and the TC20 cell counter. In addition, analyze the sample using a Z2 counter. For Z2 counter analysis, pipette a 100 µl cell suspension into 20 ml isotonic saline (200:1 ratio). Prior to analysis, flush the aperture of the Z2 particle analyzer at least twice using isotonic saline. Place the Z2 sample in the counter holder, and raise the platform to the aperture before initiating counts.
Acquire the data from the TC20 and Z2 counters using software provided by the manufacturer. Analyze these data for cell damage, viability, and the creation of cellular debris from sonication.
7. XTT Assay to Determine Cell Viability and Mitochondrial Activity of Treated U937 and THP1 Cells
Prior to the XTT assays, grow U937 and THP1 cells under the same conditions. Pretreat cells with the same concentration range of MeβCD for 30 min prior to sonication. Use the same ultrasound parameters as before except that cells are now sonicated using a range of 1-3 one sec pulses spaced one sec apart to develop a range of damage for the XTT kit to assess. Note: Many of these steps are taken directly from the XTT kit manual and are only repeated for the convenience of interested laboratories.
Collect cells by centrifugation at 200 x g for 10 min. Resuspend cell pellet in the growth medium previously described. Resuspend cells at ~1 x 105 cells/ml. Seed U937 cells at 100 µl per well into a flat-bottom 96-well microtiter plate in triplicate, and then repeat for THP1 cells using a separate 96-well plate. Include 3 control wells containing 100 µl of complete growth medium alone as blank absorbance readings. Then, incubate the inoculated plates for 24 hr.
Defrost two aliquots of the XTT reagent and the activation reagent at 37 °C prior to use. Swirl aliquots gently until clear solutions are obtained. Add 0.1 ml of the activation reagent to 5.0 ml of the XTT reagent, which forms enough activated XTT solution for one 96-well microtiter plate assay. Repeat the process for the other plate.
Add 50 µl of the activated-XTT solution to each well. Return the plate to the cell culture CO2 incubator for 2 hr. Shake the plate gently following the incubation period to evenly distribute the orange color in the positive wells. Measure the absorbance of the assay wells containing the cells and the blank background control wells at a wavelength between 450-500 nm wavelength using a microtiter plate reader.
Either zero the microtiter plate reader using the blank control wells or subtract their average value from the specific results. Measure the absorbance of all the assay wells again at a wavelength between 630-690 nm and subtract the values from the 450-500 nm values obtained. Note: This second absorbance determination helps eliminate non-specific readings from the assay results. The XTT assay was repeated four times for both cell lines.
Representative Results
System stability is paramount in obtaining repeatable and reliable results. The proper torque specification of 54.23 N.m achieved proper coupling of the transducer and sonotrode2, with the consistency of the waveform being assessed through the use of an oscilloscope and hydrophone (Figure 3A). Figure 3B depicts measurements inside the sample vial, and Figure 3C assesses stability within the cup horn. As expected, the amplitude was slightly reduced due to some energy being absorbed by the glass. However, the waveform was not perturbed or distorted in either panel B or C, which is necessary for reliable delivery of sonic energy to the sample. A waveform that does not appear as a clear singular sine wave indicates a faulty transducer or improper setup. This is expressed in Figure 3D which shows multiple aberrant waves, a clear absence of a sine wave, and a frequency reading not expected with a properly functioning system. The cavitation meter used to measure the cavitation energy generated (Figure 4) found the sound intensity to be 100-110 W/cm2 at 33% amplitude, and 125-130 W/cm2 at 50% amplitude.
Once the 40 kHz system was appropriately calibrated and assembled in its entirety (Figure 5), reliable cell destruction was readily attained, as determined by both the TC20 and Z2 counters (Figure 6). As expected, more extensive cell damage was observed in cell populations sonicated at 50%, rather than at 33% amplitude. Nevertheless, 33% amplitude is useful as a starting point to detect potential sonic sensitizing effects of administered agents, as it produces noticeable damage, but leaves room for more damage to be detected when agents are applied to assess their potentiation of ultrasonic sensitivity. This is demonstrated in Figure 7A, where the dose dependent effects of MeβCD on potentiating the ultrasonic sensitivity of U937 cells are presented. Although non-sonicated MeβCD-treated cells were very similar in viability to non-sonicated, untreated cells, the viability markedly dropped after three pulses of 1 sec 40 kHz ultrasound was applied. In addition, the decrease in viability after sonication appears to have been dose dependent, as 5 mM MeβCD produced the largest drop in cell count, followed by 1 mM, and then 0.5 mM MeβCD.
Similar effects on cell viability and mitochondrial activity were observed in both U937 and THP1 cells that were treated with varying concentrations of MeβCD prior to sonication, as assessed with the XTT kit (Figure 7B). Although THP1 cells appear to be slightly more sonic resistant than U937 cells, both human leukemia lines are damaged in a dose dependent manner. Higher concentrations of MeβCD and more pulses of ultrasound produced the lowest reduction of XTT to a soluble, brightly colored orange derivative for both U937 and THP1 cells, corresponding to lower cell viability and mitochondrial activity.
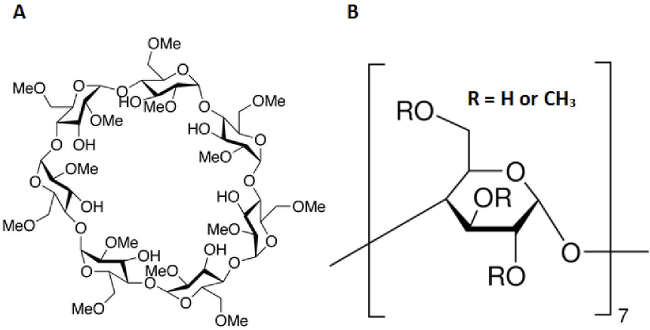 Figure 1. Molecular structure of methyl-β-cyclodextrin. (A) Complete structure of methyl-β-cyclodextrin (MeβCD). (B) MeβCD is a polymer of seven repeating units with a R group of either H or CH3. Molecular Formula: C56H98O35. Molecular Weight = 1331.36.
Figure 1. Molecular structure of methyl-β-cyclodextrin. (A) Complete structure of methyl-β-cyclodextrin (MeβCD). (B) MeβCD is a polymer of seven repeating units with a R group of either H or CH3. Molecular Formula: C56H98O35. Molecular Weight = 1331.36.
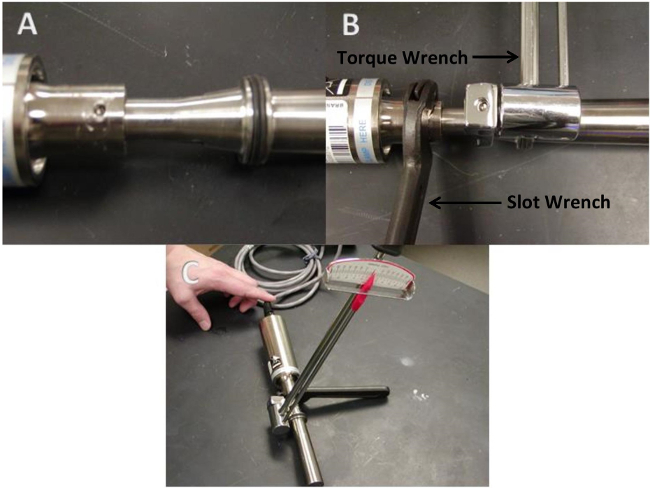 Figure 2. Properly torqueing the 40 kHz ultrasound system. (A) The horn and converter are removed from the cup prior to torqueing. (B) Position the slot wrench and torque wrench at their respective positions. (C) Apply 54.23 N.m of torque in a clockwise direction before reattaching the horn and converter to the cup.
Figure 2. Properly torqueing the 40 kHz ultrasound system. (A) The horn and converter are removed from the cup prior to torqueing. (B) Position the slot wrench and torque wrench at their respective positions. (C) Apply 54.23 N.m of torque in a clockwise direction before reattaching the horn and converter to the cup.
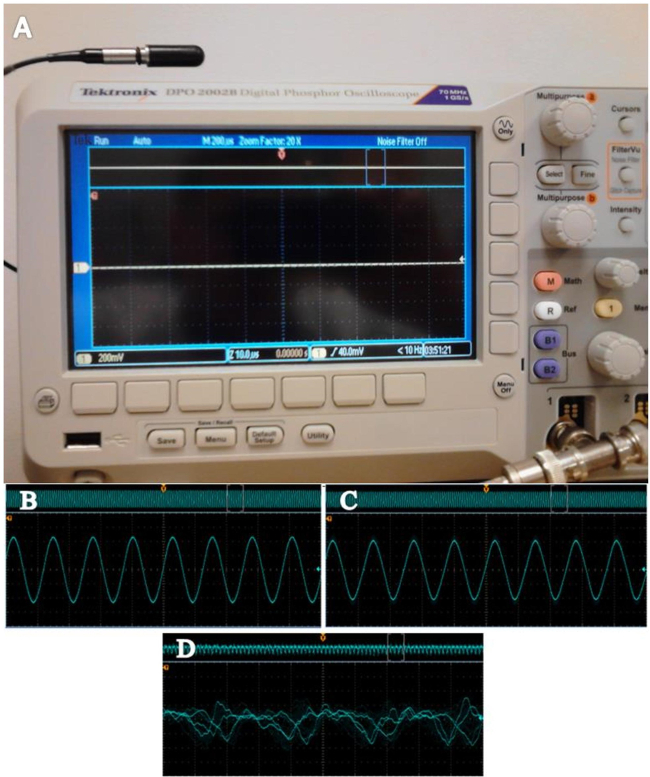 Figure 3. Use of an oscilloscope to characterize the waveform of the ultrasonic system. (A) An oscilloscope with hydrophone can be used to evaluate amplitude and waveform consistency. (B) A recording taken with the cup directly above the horn at 1.5 cm and set at 33% amplitude. (C) The waveform pictured here is from a reading acquired when the hydrophone is placed within the 20 ml glass scintillation vial. The vial is positioned at 1.5 cm above the horn and again set at 33% amplitude. (D) An oscilloscope screenshot of a system with aberrant frequencies as would be expected with a faulty system or improper coupling of the horn converter union. Please click here to view a larger version of this figure.
Figure 3. Use of an oscilloscope to characterize the waveform of the ultrasonic system. (A) An oscilloscope with hydrophone can be used to evaluate amplitude and waveform consistency. (B) A recording taken with the cup directly above the horn at 1.5 cm and set at 33% amplitude. (C) The waveform pictured here is from a reading acquired when the hydrophone is placed within the 20 ml glass scintillation vial. The vial is positioned at 1.5 cm above the horn and again set at 33% amplitude. (D) An oscilloscope screenshot of a system with aberrant frequencies as would be expected with a faulty system or improper coupling of the horn converter union. Please click here to view a larger version of this figure.
 Figure 4. Use of a cavitation meter to characterize the sound intensity of the ultrasonic system. The cavitation meter seen here is used to represent cavitation energy in W/cm2. This is a very important instrument for assessing consistency of energy with respect to the sample.
Figure 4. Use of a cavitation meter to characterize the sound intensity of the ultrasonic system. The cavitation meter seen here is used to represent cavitation energy in W/cm2. This is a very important instrument for assessing consistency of energy with respect to the sample.
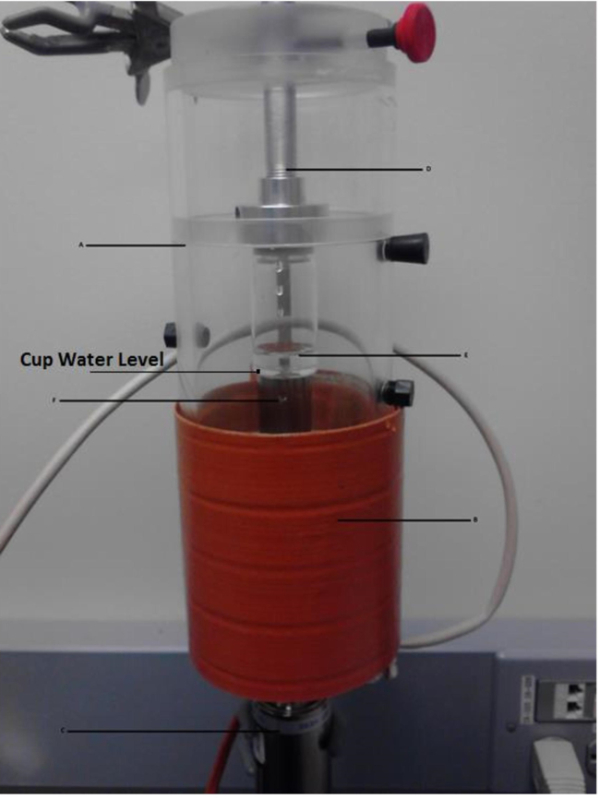 Figure 5. Key components of the 40 kHz ultrasound system. The ultrasonic system is shown complete with cup horn (A), heating apparatus (B), the converter (C), and sample positioning mechanism (D). This design allows for easy positioning of the sample (E) above the horn (F).
Figure 5. Key components of the 40 kHz ultrasound system. The ultrasonic system is shown complete with cup horn (A), heating apparatus (B), the converter (C), and sample positioning mechanism (D). This design allows for easy positioning of the sample (E) above the horn (F).
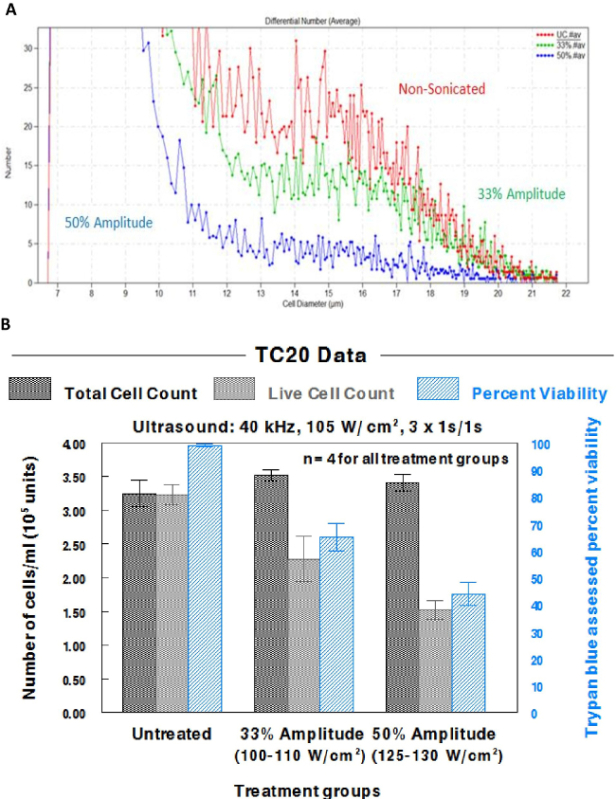 Figure 6. Effects of different levels of amplitude on the ultrasonic lysis of U937 cells using the TC20 and Z2 counters. Cells were sonicated with the 40 kHz cell dismembrator at 33% amplitude (100-110 W/cm2) or 50% amplitude (125-130 W/cm2) using three 1 sec pulses of ultrasound, with 1 sec spacing in between each of these pulses. (A) A screenshot from the Z2 counter software demonstrates the effect of sonication on U937 cells. (B) Data from the TC20 counter were compiled in a graph to demonstrate the reproducibility of the results. These data include the total and live cell counts, as well as the trypan blue assessed cell viability. Bars reflect the standard error of the mean (SEM) for 4 independent cell populations. Please click here to view a larger version of this figure.
Figure 6. Effects of different levels of amplitude on the ultrasonic lysis of U937 cells using the TC20 and Z2 counters. Cells were sonicated with the 40 kHz cell dismembrator at 33% amplitude (100-110 W/cm2) or 50% amplitude (125-130 W/cm2) using three 1 sec pulses of ultrasound, with 1 sec spacing in between each of these pulses. (A) A screenshot from the Z2 counter software demonstrates the effect of sonication on U937 cells. (B) Data from the TC20 counter were compiled in a graph to demonstrate the reproducibility of the results. These data include the total and live cell counts, as well as the trypan blue assessed cell viability. Bars reflect the standard error of the mean (SEM) for 4 independent cell populations. Please click here to view a larger version of this figure.
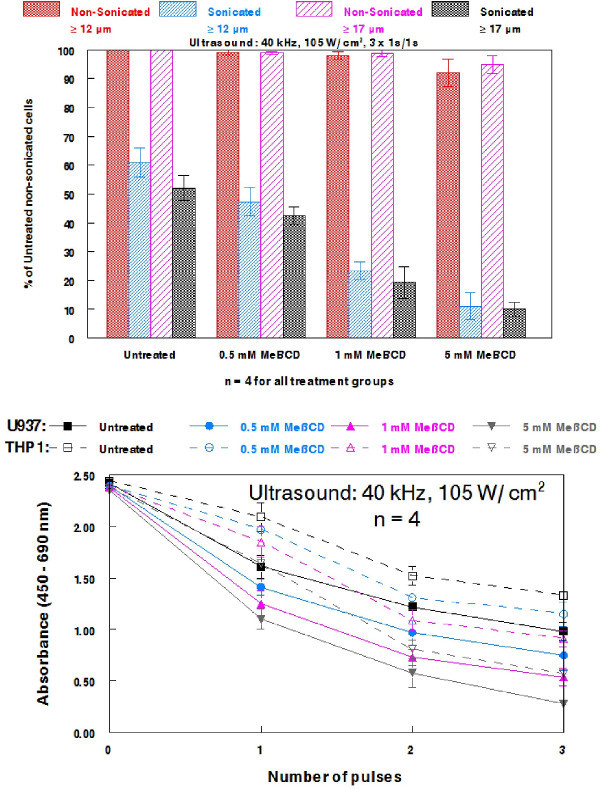 Figure 7. Effects of methyl-β-cyclodextrin on potentiating the ultrasonic sensitivity of U937 and THP1 cells. (A) U937 cells were treated with varying concentration of MeβCD for 30 min prior to being sonicated with 40 kHz ultrasound (105 W/cm2) using three 1 sec pulses spaced 1 sec apart. Cell populations were grouped into ≥12 µm and ≥17 µm. (B) U937 or THP1 cells were again treated with varying concentrations of MeβCD for 30 min prior to being sonicated with the 40 kHz system using 1-3 pulses of 1 sec ultrasound spaced 1 sec apart. Cell viability and mitochondrial activity were assessed with the XTT kit. 100 µl of cells were seeded per well into a flat-bottom 96-well microtiter plate. The plate was incubated for 24 hr prior to the addition of XTT solution. Cells were then incubated for an additional 2 hr before the wavelength was read. Bars reflect SEM for 4 independent cell populations. Please click here to view a larger version of this figure.
Figure 7. Effects of methyl-β-cyclodextrin on potentiating the ultrasonic sensitivity of U937 and THP1 cells. (A) U937 cells were treated with varying concentration of MeβCD for 30 min prior to being sonicated with 40 kHz ultrasound (105 W/cm2) using three 1 sec pulses spaced 1 sec apart. Cell populations were grouped into ≥12 µm and ≥17 µm. (B) U937 or THP1 cells were again treated with varying concentrations of MeβCD for 30 min prior to being sonicated with the 40 kHz system using 1-3 pulses of 1 sec ultrasound spaced 1 sec apart. Cell viability and mitochondrial activity were assessed with the XTT kit. 100 µl of cells were seeded per well into a flat-bottom 96-well microtiter plate. The plate was incubated for 24 hr prior to the addition of XTT solution. Cells were then incubated for an additional 2 hr before the wavelength was read. Bars reflect SEM for 4 independent cell populations. Please click here to view a larger version of this figure.
Discussion
To attain optimal results, special care should be taken to carefully position the sample and clean the converter-horn union. The placement of the sample in the horn is important for obtaining consistent cell destruction, as changing the distance from the horn will alter the acoustic foci, and therefore alter the energy the sample is exposed to. The acoustic energy within the cup horn can be mapped using the cavitation meter to find the position of maximum cavitation. In addition, the cavitation meter, along with the oscilloscope are vital for determining the sound intensity the cells are being exposed to, as well as the homogeneity of the waveform. Therefore, these instruments should be used to detect problems with the system, and help determine what troubleshooting may be needed to correct system instability.
As previously mentioned, the low frequency system may act to further degas the water throughout the experiment if not run for several minutes prior to sample sonication. This initial run must be performed to yield a relatively degassed sonication medium and thus consistent results during experiments. In addition, cells should not be sonicated at or near the maximum amplitude when assessing the efficacy of sonosensitizers, as the true extent of sensitization would be difficult to assess. Using 33% amplitude on the 40 kHz system is an ideal setting, as it produces notable damage, but provides sonosensitizers ample room to demonstrate their efficacy, as demonstrated with MeβCD against U937 and THP1 cells (Figure 7). These data also confirm that MeβCD sensitizes multiple leukemia lines to low frequency ultrasound in a dose dependent manner.
There have been a number of experiments done with higher frequency in the range of 0.75 MHz to 8 MHz showing evidence of intramembrane cavitation bubbles being generated through sonication17-19. However, questions still remain in regards to the exact mechanism of ultrasound-induced cell lysis18. We have shown a link between fluidizing the cytoskeleton and increased sonic sensitivity using low frequency ultrasound15, a phenomenon demonstrated by other laboratories20, 21. In addition, we have found that microfilament-disrupting agents such as cytochalasin B potentiate ultrasonic sensitivity in multiple leukemia lines, but not hHSCs or leukocytes22, suggesting that inhibition of actin polymerization may be a sonosensitizing mechanism of particular interest. We have also observed that vincristine, a microtubule-disrupting agent that inhibits tubulin polymerization23, 24, markedly increases the ultrasonic sensitivity of different leukemia types in vitro including acute myeloid leukemia, chronic myeloid leukemia, and acute lymphoid leukemia. By contrast, cytoskeletal-directed agents that stabilize cytoskeletal components (paclitaxel and jasplakinolide) appear to make cells resistant to sonication, reflected by lower rates of cell lysis22. Taken together, these data support the hypothesis that fluidizing the cytoskeletal components of neoplastic cells is indeed an important factor in increasing the efficacy of SDT25. The present study also demonstrates that cholesterol depletion may be another method by which to further potentiate the ultrasonic sensitivity of neoplastic cells, as MeβCD-treated U937 cells are markedly sensitized to 40 kHz ultrasound.
While our sonication protocols have demonstrated marked antineoplastic activity in vitro, the current methodology is limited to work in culture and small vertebrate models that are able to fit in the vials used for sonication. We have shown that zebrafish can be safely sonicated using pulsed low frequency ultrasound (20 kHz), and that their tolerance to chemotherapeutic agents is quantitatively comparable to doses tolerated by murine models26, suggesting that tumor-bearing zebrafish may be used in preliminary investigations to assess the in vivo antineoplastic activity of these protocols. Nevertheless, administering chemotherapeutic agents prior to sonication of mammalian models has been reported in the MHz range1, and such protocols can likely be extended to incorporate low frequency ultrasound, as well as cholesterol-depleting and cytoskeletal-directed agents.
Potential clinical applications of this form of SDT may involve extracorporeal blood sonication in which antineoplastic agents are administered intravenously (i.v.) prior to the blood being removed for sonication25. This method removes potential sound barriers posed by human anatomy, and may be an effective way to damage leukemic blasts, as well as metastases from solid tumors. It is also possible that cholesterol-depleting and cytoskeletal-directed agents could be used in HIFU protocols that are already being examined in the clinic in an attempt to improve the efficacy of this treatment modality.
The methods described in the present study are capable of assessing the value of potential sonosensitizers, and further system refinement may enhance this utility. However, there are many variables to be considered when using such ultrasonic devises, including power supply quality, acoustic foci, and individual variation among converters. Therefore, future research will focus on visualizing the sonic waves and understanding their influence on results. SDT has shown to enhance cell lysis in vitro and may prove to be clinically viable if more in vivo data in mammalian models are acquired. Experiments examining other potentially exploitable characteristics of malignant cells, as well as various combined modalities involving multiple agents and ultrasound continue in our laboratory.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
The authors would like to thank the staff of the Syracuse University Department of Physics workshop for their innovative assistance in matters relating to our system design.
References
- Trendowski M. The promise of sonodynamic therapy. Cancer Metastasis Rev. 2014;33(1):143–160. doi: 10.1007/s10555-013-9461-5. [DOI] [PubMed] [Google Scholar]
- Semins MJ, Trock BJ, Matlaga BR. The effect of shock wave rate on the outcome of shock wave lithotripsy: A meta-analysis. J Urol. 2008;179(1):194–197. doi: 10.1016/j.juro.2007.08.173. [DOI] [PubMed] [Google Scholar]
- Pace KT, Ghiculete D, Harju M, Honey RJ. Shock Wave Lithotripsy at 60 or 120 shocks per minute: A randomized, double-blind trial. J Urol. 2005;174(2):595–599. doi: 10.1097/01.ju.0000165156.90011.95. [DOI] [PubMed] [Google Scholar]
- McClain PD, Lange JN, Assimos DG. Optimizing shock wave lithotripsy: a comprehensive review. Rev Urol. 2013;15(2):49–60. [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4(3):255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- Cordeiro ER, Cathelineau X, Thüroff S, Marberger M, Crouzet S, de la Rosette JJ. High-intensity focused ultrasound (HIFU) for definitive treatment of prostate cancer. BJU Int. 2012;110(9):1228–1242. doi: 10.1111/j.1464-410X.2012.11262.x. [DOI] [PubMed] [Google Scholar]
- Li PZ, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2012;11(6):655–660. doi: 10.1016/s1499-3872(12)60241-0. [DOI] [PubMed] [Google Scholar]
- Xiaoping L, Leizhen Z. Advances of high intensity focused ultrasound (HIFU) for pancreatic cancer. Int J Hyperthermia. 2013;29(7):678–682. doi: 10.3109/02656736.2013.837199. [DOI] [PubMed] [Google Scholar]
- Lukka H, et al. High-intensity focused ultrasound for prostate cancer: a systematic review. Clin Oncol (R Coll Radiol) 2011;23(2):117–127. doi: 10.1016/j.clon.2010.09.002. [DOI] [PubMed] [Google Scholar]
- So AI. HIFU ablation is not a proven standard treatment for localized prostate cancer) Can Urol Assoc J. 2011;5(6):424–426. doi: 10.5489/cuaj.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzet S, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: oncologic outcomes and morbidity in 1002 patients. Eur Urol. 2014;65(5):907–914. doi: 10.1016/j.eururo.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Tezel A, Mitragotri S. Interactions of inertial cavitation bubbles with stratum corneum lipid bilayers during low-frequency sonophoresis. Biophys J. 2003;85:3502–3512. doi: 10.1016/S0006-3495(03)74770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Wang CC, Blankschtein D, Langer R. An investigation of the role of cavitation in low-frequency ultrasound-mediated transdermal drug transport. Pharm Res. 2002;19(8):1160–1169. doi: 10.1023/a:1019898109793. [DOI] [PubMed] [Google Scholar]
- Ueda H, Mutoh M, Seki T, Kobayashi D, Morimoto Y. Acoustic cavitation as an enhancing mechanism of low-frequency sonophoresis for transdermal drug delivery. Biol Pharm Bull. 2009;32(5):916–920. doi: 10.1248/bpb.32.916. [DOI] [PubMed] [Google Scholar]
- Trendowski M, Yu G, Wong Y, Aquafondata C, Christen T, Fondy TP. The real deal: Using cytochalasin B in sonodynamic therapy to preferentially damage leukemia cells. Anticancer Res. 2014;34(5):2195–2202. [PubMed] [Google Scholar]
- Sonifier Cell Disrupter Model SLPeEDP. Branson Ultrasonics Corporation; 2010. Available from: http://www.emersonindustrial.com/en-US/documentcenter/BransonUltrasonics/Sonifier_Brochure.pdf. [Google Scholar]
- Lagneaux L, et al. Ultrasonic low-energy treatment: A novel approach to induce apoptosis in human leukemic cells. Exp Hematol. 2002;30(11):1293–1301. doi: 10.1016/s0301-472x(02)00920-7. [DOI] [PubMed] [Google Scholar]
- Krasovitskia B, Frenkelb V, Shohama S, Kimmel K. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc Natl Acad Sci USA. 2011;108(8):3258–3263. doi: 10.1073/pnas.1015771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram J, Mellein BR, Mitragotri S. An experimental and theoretical analysis of ultrasound-induced permeabilization of cell membranes. Biophys J. 2003;84(5):3087–3101. doi: 10.1016/S0006-3495(03)70034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi N, et al. Low intensity ultrasound perturbs cytoskeleton dynamics. Soft Matter. 2012;8(8):2438–2443. doi: 10.1039/C2SM07246G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Leow RS, Hu Y, Wan JM, Yu AC. Single-site sonoporation disrupts actin cytoskeleton organization. J R Soc Interface. 2014;11(95):20140071. doi: 10.1098/rsif.2014.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendowski M, et al. Preferential enlargement of leukemia cells using cytoskeletal-directed agents and cell cycle growth control parameters to induce sensitivity to low frequency ultrasound. Cancer Lett. 2015;In press doi: 10.1016/j.canlet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: Microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999;17(3):1061–1070. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- Verrills NM, Kavallaris M. Improving the targeting of tubulin-binding agents: lessons from drug resistance studies. Curr Pharm Des. 2005;11(13):1719–1733. doi: 10.2174/1381612053764706. [DOI] [PubMed] [Google Scholar]
- Trendowski M. The inherent metastasis of leukaemia and its exploitation by sonodynamic therapy. Crit Rev Oncol Hematol. 2014;In press doi: 10.1016/j.critrevonc.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Trendowski M, Wong V, Wellington K, Hatfield S, Fondy TP. Tolerated doses in zebrafish of cytochalasins and jasplakinolide for comparison with tolerated doses in mice in the evaluation of pre-clinical activity of microfilament-directed agents in tumor model systems in vivo. In Vivo. 2014;28(6):1021–1031. [PubMed] [Google Scholar]


