Abstract
T follicular helper (TFH) cells are specialized effector CD4+ T cells that help B cells develop germinal centers and memory. However, the transcription factors that regulate TFH differentiation remain incompletely understood. Here we report that selective loss of either Lef1 (LEF-1) or Tcf7 (TCF-1) resulted in TFH defects, while deletion of Lef1 and Tcf7 severely impaired TFH differentiation and germinal centers. Forced expression of LEF-1 enhanced TFH differentiation. LEF-1 and TCF-1 coordinated TFH differentiation by two general mechanisms. First, they established the responsiveness of naïve CD4+ T cells to TFH signals. Second, they promoted early TFH differentiation via the multipronged approach of sustaining expression of IL-6Rα and gp130, enhancing ICOS expression, and promoting expression of Bcl6.
Keywords: Lymphoid enhancer factor (LEF)-1, T cell factor (TCF)-1, IL-6 receptor alpha (IL-6Rα), IL-6 signal transducer (gp130), inducible costimulator (ICOS), B cell lymphoma 6 (Bcl6), Prdm1, Blimp1
T cell help to B cells is a critical component of adaptive humoral immunity1, 2. During viral infections, the formation of germinal centers (GCs) by antigen (Ag)-specific B cells requires key signals provided by T follicular helper (TFH) cells3, resulting in the development of high-affinity long-lived plasma cells and memory B cells4, 5. TFH differentiation begins outside of B cell follicles in a stepwise fashion. Early induction of key molecules of TFH differentiation, such as Bcl6, CXCR5, ICOS, and PD-1, occurs in the T cell zone when CD4+ T cells interact with Ag-presenting dendritic cells (DCs) or other antigen presenting cells (APCs), which then enable the migration of the activated CD4+ T cells towards the border of B cell follicles. Upon recognition of cognate Ag-presenting B cells, the differentiating TFH cells migrate deep inside B cell follicles and further differentiate into GC TFH cells as they direct the generation of GC B cells.
The requirement of repeated interactions with APCs is an important feature of TFH differentiation3, which is presumably connected to the maintenance of the activity of critical transcription factors such as Bcl66, 7, 8, Batf9, STAT310, 11, 12, STAT110, and Ascl213 that support TFH differentiation. Among them, Bcl6 function is absolutely critical. TFH differentiation is completely abrogated in Bcl6−/− CD4+ T cells6, 7, 8 and ectopic Bcl6 expression in CD4+ T cells leads to augmented TFH differentiation6, 9. A number of signaling molecules have been identified that can regulate Bcl6 expression in CD4+ T cells14. However, attempts to polarize CD4+ T cells to TFH in vitro using IL-6 and IL-21 fail to reproducibly induce Bcl6 and CXCR5 expression. Therefore, there are clear gaps in our understanding of the molecular requirements for Bcl6 induction and the factors that support TFH differentiation3.
LEF1-1 and TCF-1 (encoded by Lef1 and Tcf7, respectively) are transcription factors that contain a conserved high mobility group (HMG) DNA binding domain. TCF-1 and LEF-1 are known for their essential roles in early T cell development, including T lineage specification and β-selection during the CD4−CD8− double negative stage15, 16. TCF-1 and LEF-1 critically regulate CD4+ versus CD8+ T cell lineage commitment upon completion of positive selection of CD4+CD8+ double positive thymocytes17, 18. In mature CD8+ T cells, TCF-1 and LEF-1 regulate the generation, maturation and longevity of memory CD8+ T cells in response to viral or bacterial infection19, 20, 21. In mature CD4+ T cells, TCF-1 promotes TH2 differentiation in vitro via positive regulation of GATA-322. TCF-1 restrains expression of interleukin 17 (IL-17A) in developing thymocytes and activated CD4+ T cells23. In addition, TCF-1 can interact with the transcription factor Foxp3 and appears to oppose Foxp3-mediated gene repression in regulatory CD4+ T cells24.
Here we looked for undiscovered regulators of early TFH differentiation and found that LEF-1 and TCF-1 are critical transcriptional regulators of TFH differentiation. Using a knock-in reporter system and RNA-seq analysis we found that these transcription factors were highly expressed in TFH cells upon viral or bacterial infections. Genetic deletion of Lef1, Tcf7, or both factors in CD4+ T cells led to TFH differentiation defects in a dose dependent manner. As a consequence, the magnitude of B cell responses and germinal center reactions was substantially diminished in LEF-1- and TCF-1-deficient mice after infection. Mechanistically, LEF-1 and TCF-1 regulate multiple interacting mechanisms upstream of Bcl6 to preferentially instruct activated CD4+ T cells to undertake TFH differentiation.
RESULTS
Transcriptional profiles of early TFH cells versus TH1 cells
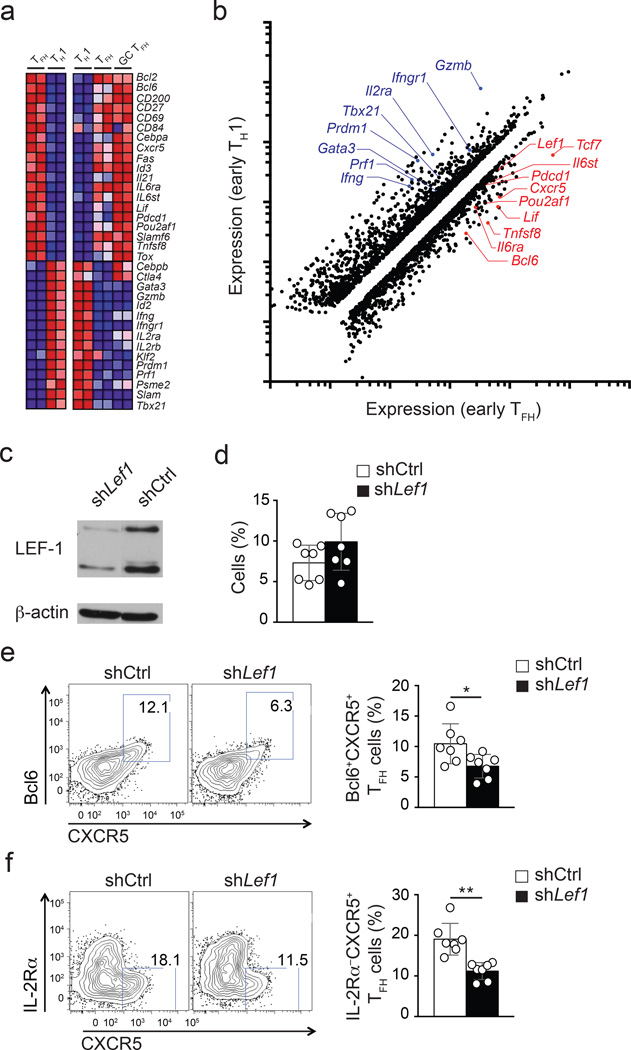
Initial CD4+ T cell contact with APCs in the T cell zone can promote expression of key TFH molecules including Bcl6 and CXCR5. By 72 hours into an acute viral infection, the early TFH and TH1 cells have become fate-committed25, 26. Early TFH cells are IL-2RαloBcl6hiBlimp1−CXCR5hi , while early TH1 cells are IL-2Rα+ and TbethiBcl6−Blimp1hi in the context of acute viral and bacterial infections25, 26, 27, 28. To identify new factors important in the programming of TFH cells we performed gene expression analysis on early TFH and TH1 cells using RNA-seq. Congenically marked CD45.1+ Blimp1-YFP reporter LCMV-specific TCR transgenic CD4+ T cells (SMARTA) were transferred into B6 host mice given an acute infection with the Armstrong strain of LCMV (LCMV-Arm), and early TFH and TH1 cells were isolated three days after infection and purified to homogeneity by sorting IL-2Rα−Blimp1-YFP− and IL-2Rα+Blimp1-YFP+ cells, respectively. RNA-seq was performed on the isolated RNA and early TFH and TH1 transcriptome profiles were obtained (Fig. 1a–b). Analysis revealed that approximately 1,200 genes were upregulated more than 1.5 fold in early TFH cells relative to TH1 cells, and 1,600 genes were downregulated more than 1.5 fold (Fig. 1b). Early TFH cells expressed many genes that are also preferentially expressed by fully differentiated TFH and GC TFH (Bcl6, Cxcr5, Pdcd1, Pou2af1 and Tnfsf8 among others) and had low expression of many genes repressed in fully differentiated TFH and GC TFH (Prdm1, Tbx21, IL2ra, Gzmb and Prf1 among others) (Fig. 1a, b). Thus, major attributes of TFH and TH1 cells are transcriptionally well defined by day 3 of an acute viral infection.
Figure 1. Lef1 expression is associated with TFH cells and regulates early TFH differentiation.
(a) RNA-seq analysis of early TFH (IL-2Rα−Blimp1-YFP−) versus TH1 (IL-2Rα+Blimp1-YFP+) CD45.1+ Blimp1-YFP SMARTA cells isolated from B6 mice 3 d after SMARTA cell transfer and LCMV infection (left panels), and that of TH1 (CXCR5−), TFH (PD-1loCXCR5+), and GC TFH (PD-1hiCXCR5+) sorted 8 d after LCMV from CD45.2+ B6 mice (right panels). Heatmaps of selected genes of interest are shown. (b) Scatter plot of genes showing ≥ |1.5 fold| differential expression in early TFH in comparison to TH1 cells. Select genes of interest are marked. (c) Immunoblot of LEF-1 (two isoforms) and β-actin from shCtrl+ and shLef1+ SMARTA cells. (d-f) Analysis of shCtrl+ or shLef1+ CD45.1+ SMARTA cells (Ametrine+CD45.1+CD4+CD19−), three days after transfer of shRNA-RV-infected SMARTA cells into B6 mice and LCMV infection. (d) shRNA+ SMARTA cell frequency among total CD4+ T cells. (e-f) Phenotyping of shCtrl+ and shLef1+ SMARTA cells. (e) Bcl6+CXCR5+ TFH cells. (f) IL-2Rα−CXCR5+ TFH cells. Quantitation shown as % of SMARTA cells (mean ± s.e.m.). Data are a composite of two independent experiments (n = 7 per group). * P < 0.05, ** P < 0.001 (Student’s t-test).
Lef1 is a transcriptional regulator of TFH differentiation
To further filter the 2,800 gene expression differences between early TFH cells and TH1 cells, we focused on transcription factors. We then performed an additional set of RNA-seq experiments using in vitro activated CD4+ T cells under TH1 polarizing conditions (IL-12 + αIL-4 + αTGF-β) or with IL-6 (IL-6 + αIFN-γ+αIL-12). These screening conditions were used because in vitro stimulation of CD4+ T cells in the presence of IL-6 resulted in some gene expression changes associated with TFH differentiation (Supplementary Fig. 1a–c. Most notably, Il21 was robustly induced by IL-6); however, major aspects of TFH biology were not detected in IL-6-stimulated CD4+ T cells, such as CXCR5 protein expression and sustained Bcl6 expression3, 13, 29, 30 (Supplementary Fig. 1f). This outcome suggested that key transcriptional regulators required for TFH differentiation are not induced under IL-6 conditions in vitro. We next performed a comparative analysis of gene expression differences between the in vivo generated early TFH and the in vitro IL-6 stimulated CD4+ T cells. To reveal critical unidentified early upstream transcriptional regulators of TFH differentiation we focused on genes meeting two conditions: preferential expression by early TFH cells in vivo and lack of differential expression after in vitro stimulation with IL-6. Lef1 satisfied these two conditions (Fig. 1b, Supplementary Fig. 1d, g) and was selected for further analysis in part because LEF-1 is required for the formation of memory CD8+ T cells20 and there are similarities between TFH and memory CD8+ T cell differentiation25, 31.
When expressed in SMARTA CD4+ T cells, an shRNAmir expression vector targeting Lef1 (shLef1-RV) inhibited expression of both LEF-1 protein isoforms (Fig. 1c). To test whether early TFH differentiation in vivo is dependent on LEF-1, SMARTA CD45.1+ CD4+ T cells expressing a control shRNA (shCtrl) or shLef1+ SMARTA CD4+ T cells were transferred into B6 mice. Three days after LMCV infection of recipient mice, shLef1+ SMARTA CD4+ T cells produced approximately half the number of early TFH cells compared to shCtrl+ SMARTA CD4+ T cells as assessed by flow cytometry using either Bcl6+CXCR5+ (Fig. 1e) or IL2Rα−CXCR5+ (Fig. 1f) phenotyping. The impact of LEF-1 knock-down was selective to TFH differentiation, as SMARTA CD4+ T cell activation (CD44 upregulation, not shown) and proliferation (Fig. 1d) were comparable between shCtrl+ and shLef1+ CD4+ T cells. The reduced TFH differentiation by shLef1+ CD4+ T cells indicated that LEF-1 may be an important and dose limiting contributor to this process.
Lef1 controls TFH differentiation and germinal center formation
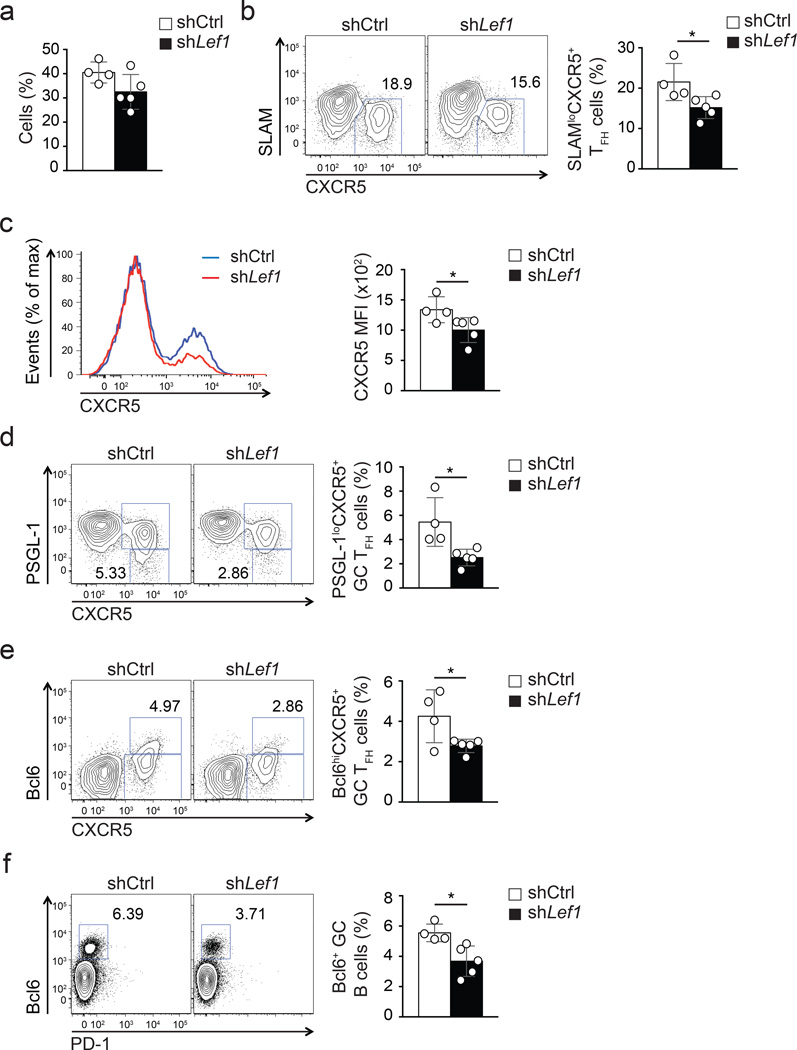
We next examined whether LEF-1 function in CD4+ T cells was important for GC TFH differentiation and germinal center reactions. shLef1+ or shCtrl+ SMARTA CD4+ T cells were transferred into B6 mice and analyzed 8 days after acute LCMV infection of the recipient mice. Activation and proliferation of CD4+ T cells were not affected by reduced Lef1 expression compared to shCtrl (Fig. 2a), but TFH differentiation of shLef1+ cells was impaired (Fig. 2b–c). The shLef1+ TFH defect was less severe than that observed on day 3, potentially due to the fact that sustained gene knock-down in CD4+ T cells in vivo is difficult to accomplish under conditions of rapid proliferation. We note that we have observed milder TFH differentiation defects for most shRNAmir-RVs at peak proliferation time points compared to early time points after infection, including shRNAmir against Bcl6 (data not shown). Nevertheless, shLef1+ SMARTA CD4+ T cells showed defective differentiation into GC TFH, identified here as PSGL-1loCXCR5+ T cells (Fig. 2d) or Bcl6+CXCR5+ T cells (Fig. 2e), compared to shCtrl+ SMARTA CD4+ T cells. As a result, the development of GC B cells (Bcl6+CD19+) was moderately impaired in the presence of shLef1+ SMARTA CD4+ T cells as compared to shCtrl+ cells (Fig. 2f). Taken together, reduction of LEF-1 expression in CD4+ T resulted in loss of TFH and GC TFH cells and a proportional loss of GC B cells during an immune response to LCMV.
Figure 2. LEF-1-dependent TFH differentiation supports germinal center responses.
(a-e) Frequencies and phenotypes of shCtrl+ or shLef1+ CD45.1+ SMARTA cells assessed by flow cytometry at 8 d after SMARTA cell transfer into B6 mice and LCMV infection. (a) Abundance of shRNA+ SMARTA cells (Ametrine+CD45.1+CD4+CD19−) among total CD4+ T cells. (b) Frequency of SLAMloCXCR5+ TFH cells. Quantitation shown as % of SMARTA cells. (c) Expression of CXCR5 on shCtrl+ (blue) and shLef1+ (red) SMARTA cells. (d-e) Frequencies of shCtrl+ and shLef1+ SMARTA GC TFH cells among SMARTA cells phenotyped as PSGL-1loCXCR5+(d) and Bcl6hiCXCR5+ (e). (f) Abundance of GC B cells (Bcl6+CD19+) among total B cells. Data are representative of two independent experiments (n = 4–5 per group, mean ± s.e.m.). * P < 0.05 (Student’s t-test).
Ablation of Lef1 diminishes GC TFH differentiation
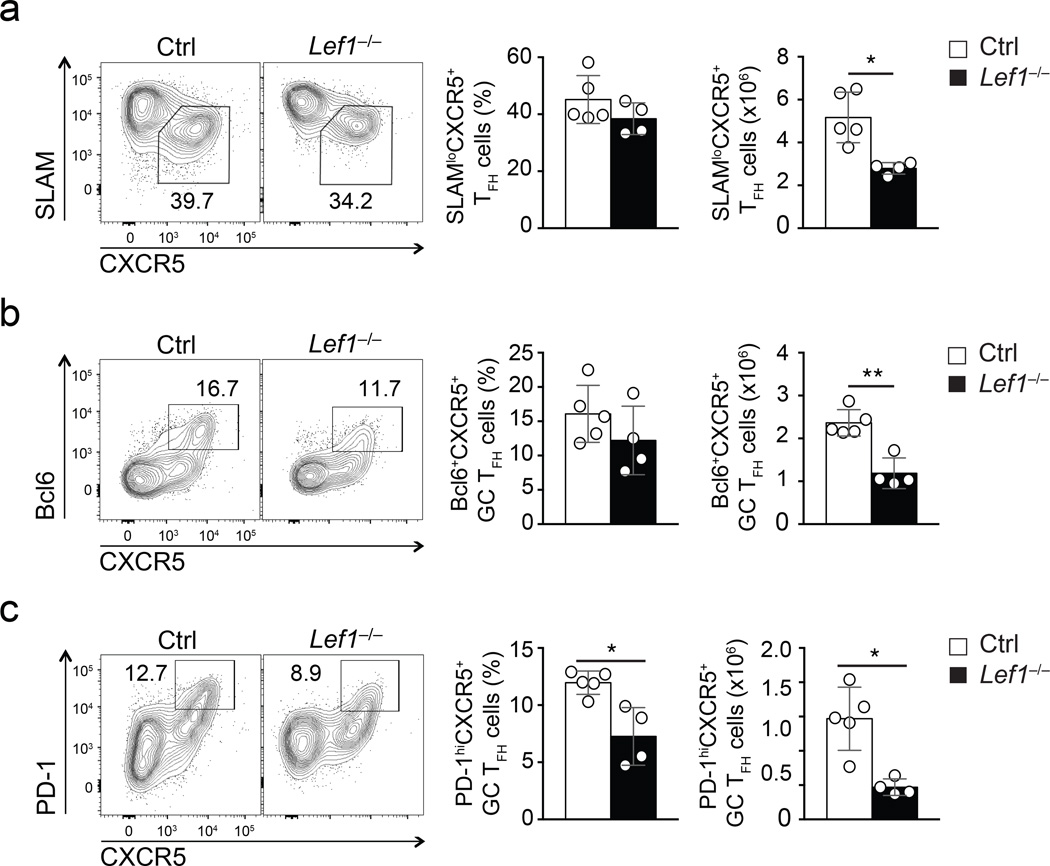
We next investigated the role of LEF-1 in TFH differentiation using conditional gene-targeted Lef1fl/fl mice. Lineage-specific deletion of Lef1 in thymocytes with Cd4-Cre impairs CD4+ T cell lineage choice and diminishes CD4+ T cell output18. To avoid this, we used human CD2 promoter-driven Cre recombinase transgenic mice (hCD2-Cre), which achieve gene ablation in mature T cells32. Mice were also crossed to the Rosa26-STOP-GFP (Rosa26GFP) allele. As marked by GFP expression due to excision of the floxed transcription-translation STOP sequence from Rosa26GFP allele, over 70% of splenic CD4+ T cells in hCD2-Cre Rosa26GFP mice were GFP+, whereas less than 15% of CD4+ thymocytes were GFP+ (Supplementary Fig. 2a). We crossed hCD2-Cre Rosa26GFP to the Lef1fl/fl strain to generate hCD2-Cre Rosa26GFPLef1fl/fl mice (called Lef1−/− hereafter). Both isoforms of LEF-1 were completed ablated in GFP+ CD4+ T cells from Lef1−/− mice (Supplementary Fig. 2b). Late deletion of LEF-1 did not detectably affect thymocyte development or cause aberrant activation of mature T cells (Supplementary Fig. 2d, f, h, i) but reduced total thymic cellularity by approximately 15% and mature CD4+ T cells by approximately 25% (Supplementary Fig. 2e, g). To determine the impact of LEF-1 deficiency in CD4+ T cells on TFH differentiation, we infected Lef1−/− mice and littermate controls (hCD2-Cre−Lef1+/fl or hCD2-Cre+Lef1+/+) with vaccinia virus and assessed the presence of CD44hiCD62L− activated GFP+ CD4+ splenic T cells on day 8 post infection. The frequency of TH1 cells (SLAMhiCXCR5−) were similar in Lef1−/− mice and littermate control mice, though the absolute numbers of SLAMhiCXCR5− TH1 cell were modestly decreased compared to control (p = 0.51, Supplementary Fig. 3), consistent with a modestly reduced CD4+ T cell compartment in uninfected Lef1−/− mice (Supplementary Fig. 2g). SLAM−CXCR5+ TFH cell numbers were more markedly decreased in the vaccinia virus-immunized Lef1−/− mice compared to littermate controls (p = 0.006, Fig. 3a). In particular, the number of GC TFH cells were diminished to a much greater extent in Lef1−/− mice compared to littermate controls (Bcl6+CXCR5+ and PD-1hiCXCR5+ phenotyping Fig. 3b,c). These data further corroborate a role of LEF-1 in directing TFH differentiation.
Figure 3. Genetic ablation of LEF-1 impairs GC TFH differentiation.
(a-c) Flow cytometry of TFH and GC TFH cells in spleens of Lef1−/− mice and littermate controls infected with vaccinia virus for 8 days. Plots are gated on CD44hiCD62LloGFP+CD4+ T cells. (a) SLAMloCXCR5+ TFH cells. (b-c) Abundance of GC TFH cells phenotyped as Bcl6+CXCR5+ (b) and PD-1hiCXCR5+(c). Cumulative data from four independent experiments are shown (mean ± s.d.). * P < 0.01, ** P < 0.001 (Student’s t-test).
TCF-1 expression is retained in TFH but not TH1 cells
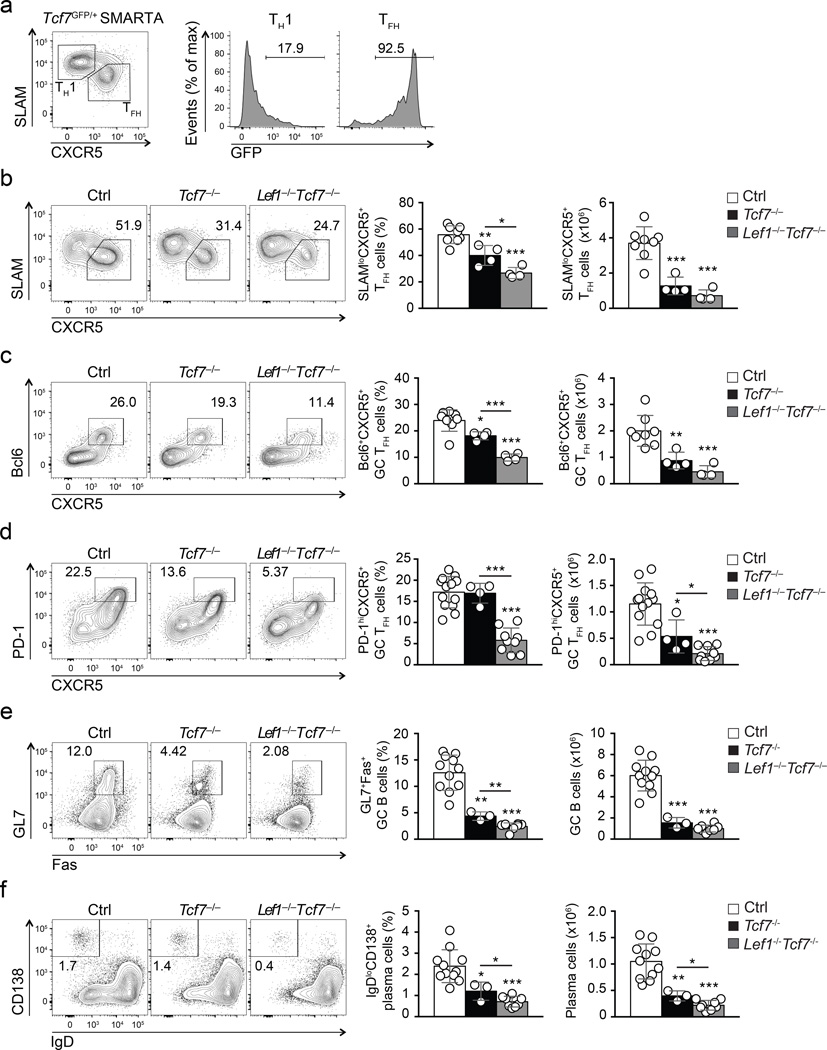
RNA-seq analysis of early TFH and TH1 cells isolated from B6 mice revealed that Tcf7 was also strongly expressed by early TFH cells, but Tcf7 was not induced by in vitro stimulation of CD4+ T cells with IL-6 (Fig. 1b, Supplementary Fig. 1e, g). Given that LEF-1 and TCF-1 are related transcription factors, we investigated whether TCF-1 was also an early regulator of TFH differentiation. For this purpose, we generated Tcf7-GFP knock-in mice (called Tcf7GFP here, Supplementary Fig. 4a). The Tcf7-GFP reporter was abundantly expressed in CD4+ and CD8+ T cells and CD4+CD25+ regulatory T cells but was absent in B220+ cells, demonstrating the reporter fidelity (Supplementary Fig. 4b–d). The expression of Tcf7-GFP was highest in CD44loCD62L+ naïve T cells but was low in antigen-experienced T cell subsets such as CD44hiCD62L+ memory-phenotype T cells, and particularly CD44hiCD62L− effector-phenotype T cells (Supplementary Fig. 4b, c). To analyze TCF-1 expression kinetics in antigen-specific CD4+ T cells we generated Tcf7GFP/+ SMARTA mice and adoptively transferred naïve CD44loCD62L+ SMARTA CD45.2+ CD4+ T cells into CD45.1+ congenic recipients. Following LCMV infection, Tcf7-GFP expression was greatly diminished in SLAMhi CXCR5− TH1 cells by day 8 post-infection compared to naive CD4 T cells, while Tcf7-GFP expression was maintained at a high level by most SLAMloCXCR5+ TFH cells (Fig. 4a).
Figure 4. Both TCF-1 and LEF-1 contribute to regulation of TFH differentiation and B cell responses.
(a) Flow cytometry of Tcf7-GFP expression in SMARTA CD4+ T cells (CD45.2+CD4+) at 8 d after Tcf7GFP/+ SMARTA cell transfer into CD45.1+ recipients and LCMV infection. Gated populations of TH1 (CXCR5−SLAMhi) and TFH (CXCR5+SLAMlo) cells were analyzed for Tcf7-GFP expression. Numbers indicate percent Tcf7-GFP+ cells. Data are representative of ≥ 2 experiments. (b-f) Flow cytometry of Tcf7−/−Lef1−/−Tcf7−/−, and littermate controls 8 d after infection i.v. with vaccinia virus. (b-d) Abundance of SLAMloCXCR5+ TFH cells (b), Bcl6+CXCR5+ GC TFH cells (c), and PD-1hiCXCR5+ GC TFH cells (d) gated on CD44hiCD62L−GFP+CD4+ T cells in spleen. (e-f) Abundance of GL7+Fas+ GC B cells (e) and IgDloCD138+ plasma cells (f) in the same animals (mean ± s.d.). Cumulative data from ≥ 3 experiments are shown. * P < 0.05, ** P < 0.01, *** P < 0.001 (Student’s t-test).
We next investigated if the retention of TCF-1 expression was associated with the TFH differentiation program in response to other in vivo stimuli. Following adoptive transfer of Tcf7GFP SMARTA CD4+ T cells, we infected recipient mice with Listeria monocytogenes expressing the GP61 epitope of LCMV. In other experiments, we directly infected Tcf7GFP/+ mice with vaccinia virus, as a second viral infection model. Whereas SLAMhiCXCR5− TH1 cells that developed in both systems preferentially downregulated Tcf7-GFP expression, SLAMloCXCR5+ TFH cells generated in response to both the bacterial and viral infections highly retained Tcf7-GFP expression (Supplementary Fig. 4e, f). Considering that TCF-1 is known to be markedly downregulated in effector CD8+ T cells33, these observations indicate that retention of TCF-1 expression at the effector phase of a T cell response is unique to TFH cells, and further suggest a possible requirement for TCF-1 in TFH differentiation.
Both LEF-1 and TCF-1 are essential for TFH responses
To address the role of TCF-1 in TFH cells, we generated hCD2-Cre Rosa26GFPTcf7fl/fl mice (called Tcf7−/− hereafter), where all isoforms of TCF-1 were ablated from GFP+ CD4+ T cells (Supplementary Fig. 2c). To investigate the functional redundancy between LEF-1 and TCF-1 we also crossed Tcf7−/− with Lef1−/− mice to generate Lef1−/−Tcf7−/− mice (hCD2-Cre Rosa26GFP Lef1fl/flTcf7fl/fl). Similar to the Lef1−/− mice, we did not observe T cell development defects or aberrant activation of mature T cells in Tcf7−/− mice or Lef1−/−Tcf7−/− mice (Supplementary Fig. 2). Although a modest reduction in thymic and splenic cellularity was observed in Tcf7−/− mice, this was not evident in Lef1−/−Tcf7−/− mice compared to littermate controls (hCD2-Cre−Lef1+/flTcf7+/fl or hCD2-Cre+Lef1+/+Tcf7+/+) (Supplementary Fig. 2d, f, h, i). We examined the CD4+ T cell responses of the Lef1−/−Tcf7−/− mice in response to vaccinia virus infection. On day 8 after infection, analysis of CD44hiCD62L− activated GFP+ CD4+ T cells revealed that the frequencies and numbers of SLAMloCXCR5+ TFH cells were diminished in Tcf7−/− mice compared to control mice (Fig. 4b), with comparable reduction of GC TFH cells (Bcl6+CXCR5+ and PD-1hiCXCR5+ phenotyping, Fig. 4c,d). We found stronger defects in Lef1−/−Tcf7−/− mice compared to Tcf7−/− mice (Fig. 4b-4d), indicating that both LEF-1 and TCF-1 contribute to regulation of TFH differentiation.
Consistent with the observations above, Tcf7−/− and Lef1−/−Tcf7−/− mice exhibited significantly diminished frequency and numbers of GL7+Fas+ GC B cells compared to control mice (Fig. 4e), with the most severe GC B cell defect in Lef1−/−Tcf7−/− mice (Fig. 4e). The number of IgDloCD138+ plasma cells was moderately reduced in Tcf7−/− mice, but severely compromised in Lef1−/−Tcf7−/− mice (Fig. 4f). As a result, vaccinia virus-specific Ab production was significantly impaired in Lef1−/−Tcf7−/− mice compared to controls (Supplementary Fig. 5). In summary, our data indicate that LEF-1 and TCF-1 play critical roles in TFH differentiation and, consequently, B cell help functions in a CD4+ T cell intrinsic manner.
Ectopic Lef1 expression augments TFH differentiation
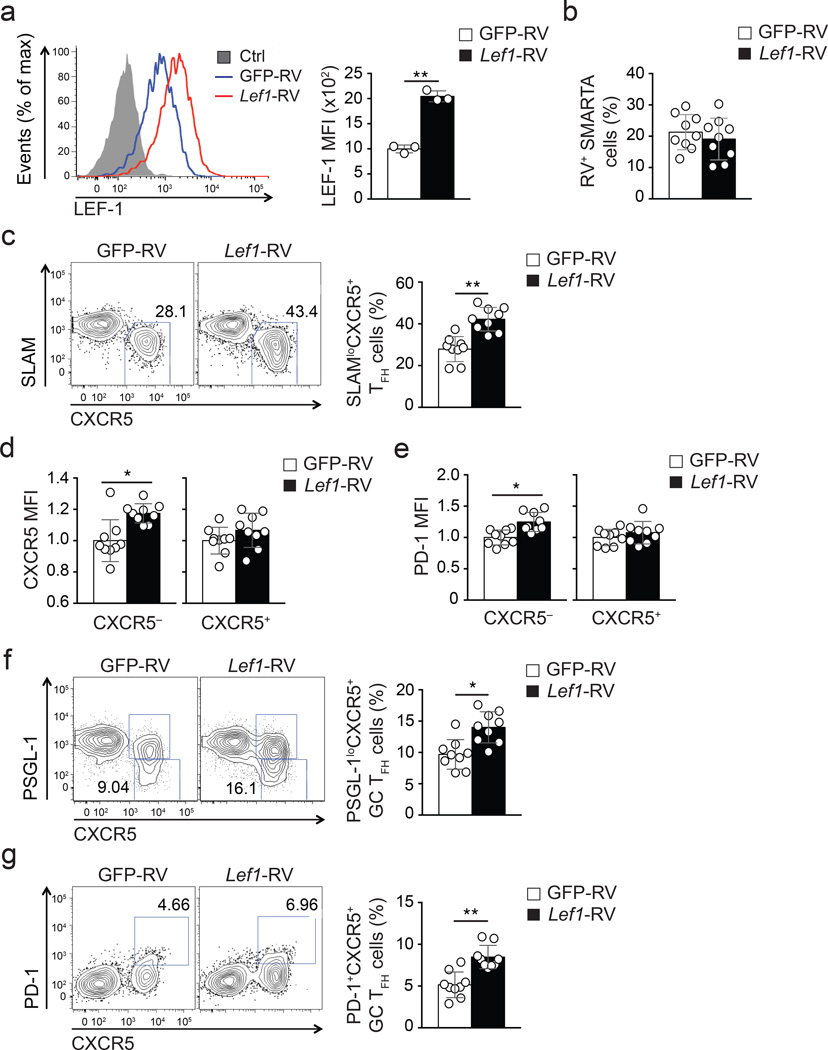
We next tested whether enhanced expression of one of these transcription factors could augment TFH differentiation of antigen-specific CD4+ T cells. Given that LEF-1 and TCF-1 exhibited overlapping activities instructing TFH differentiation, we examined TFH differentiation of CD4+ T cells after ectopic expression of LEF-1. LEF-1 can be expressed as two isoforms in CD4+ T cells due to differential promoter usage (Fig. 1c), with the full-length isoform containing a unique N-terminal β-catenin-binding domain. We used a retrovirus expressing the full-length Lef1 (Lef1-RV+) and confirmed increased expression of LEF-1 protein in Lef1-RV+ SMARTA CD45.1+ CD4+ T cells by flow cytometry (Fig. 5a) and immunoblot analysis (data not shown). GFP-RV+ or Lef1-RV+ CD45.1+ SMARTA CD4+ T cells were transferred into B6 mice, which were then infected with LCMV. The overall activation and proliferation of Lef1-RV+ CD4+ T cells was normal compared to GFP-RV+ CD4+ T cells (Fig. 5b and data not shown). Ectopic LEF-1 expression resulted in enhanced TFH development by Lef1-RV+ cells compared to GFP-RV+ cells 8 days post-infection (Fig. 5c). Moreover, we found that Lef1-RV+ TH1 cells (SLAMhiCXCR5−) exhibited unexpectedly increased expression of canonical TFH molecules CXCR5 (Fig. 5d) and PD-1 (Fig. 5e) compared to GFP-RV+ cells. Most importantly, GC TFH cells (phenotyped as either PSGL-1loCXCR5+ or PD-1hiCXCR5+ cells Fig. 5f, g) developed at significantly higher frequencies among Lef1-RV+ SMARTA CD4+ T cells compared to GFP-RV+ control cells.
Figure 5. Enhanced Lef1 expression leads to augmented TFH differentiation.
(a) Expression of LEF-1 in GFP-RV+ (blue) and Lef1-RV+ (red) SMARTA cells assessed by flow cytometry. (b-g) Frequencies and phenotypes of GFP-RV+ or Lef1-RV+ SMARTA cells (CD45.1+CD4+CD19−) assessed by flow cytometry at 8 d after SMARTA cell transfer into B6 mice (CD45.2+) and LCMV infection. (b) Abundance of RV+ SMARTA cell (GFP+CD45.1+CD4+CD19−) among total CD4+ T cells. (c) Abundance of SLAMloCXCR5+ TFH cells among RV+ SMARTA cells. (d-e) Expression of canonical TFH markers CXCR5 (d) and PD-1 (e) on CXCR5− TH1 and CXCR5+ TFH cells by Lef1-RV+ and GFP-RV+ cells, normalized to the mean MFI per group (mean ± s.e.m.). (f-g) Abundance of GC TFH cells phenotyped as PSGL-1loCXCR5+(f) and PD-1hiCXCR5+(e) of RV+ SMARTA cells. Data are a composite of two independent experiments (n = 9 per group). * P < 0.01, ** P < 0.001 (Student’s t-test).
LEF-1 enhances expression of IL-6 receptors and ICOS
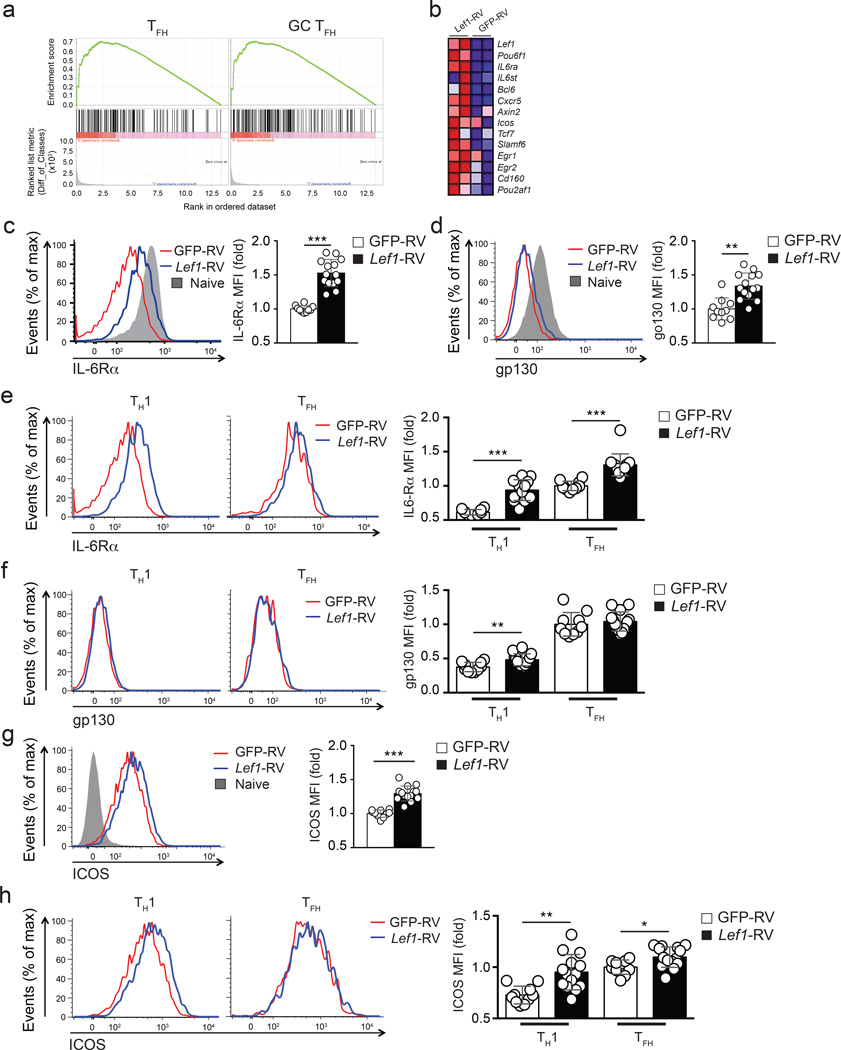
To gain insights into how LEF-1 regulates TFH differentiation, we performed RNA-seq on GFP-RV+ or Lef1-RV+ CXCR5lo TH1 and CXCR5hi TFH SMARTA CD4+ T cells. We next used transcriptional signatures of TFH and GC TFH cells (see Methods) and Gene Set Enrichment Analysis (GSEA) to investigate whether these gene expression signatures were enriched in Lef1-RV+ TH1 cells in comparison to control TH1 cells (GFP-RV+). We found substantial enrichment of the TFH and GC TFH gene signatures (Supplementary Table 1) in TH1 cells constitutively expressing Lef1 (NES = 1.21, TFH GSEA; NES = 1.29, GC TFH GSEA, Fig. 6a) compared to control TH1 cells. Detailed examination revealed that differential expression of Il6rα, Il6st, Bcl6, Cxcr5, Slamf6, and Pou2af1 were particularly notable in the Lef1-RV+ TH1 cells compared to GFP-RV+ TH1 cells (Fig. 6b).
Figure 6. LEF-1 regulates expression of IL-6 receptor chains and ICOS.
(a-b) RNA-seq analysis of GFP-RV+ and Lef1-RV+ SMARTA cells (CD45.1+CD4+CD19−) sorted into CXCR5+ TFH or CXCR5− TH1 populations isolated from B6 mice 4 d after SMARTA cell transfer and LCMV infection. (a) GSEA enrichment analysis showing enrichment of TFH and GC TFH gene signatures in Lef1-RV+ TH1 cells compared to GFP-RV+ TH1 cells. (b) Heat map of selected genes upregulated in Lef1-RV+ TH1 cells compared to GFP-RV+ TH1 cells. (c-h) Flow cytometry of GFP-RV+ or Lef1-RV+ SMARTA cells (CD45.1+CD4+CD19) assessed at 3 d after SMARTA cell transfer into B6 mice (CD45.2+) and LCMV infection. (c-d) Expression of IL6Rα(c) and gp130 (d) on RV+ SMARTA cells. (e-f) Comparative expression of IL-6Rα (e) and gp130 (f) GFP-RV+ (red) and Lef1-RV+ (blue) TH1 and TFH cells. (g-h) Abundance of ICOS on total RV+ SMARTA cells (g), CXCR5+ TFH and CXCR5− TH1 cell subpopulations (h) in the same animal (mean ± s.e.m.). Data are a composite of three independent experiments (n = 10–14 per group). * P < 0.05, ** P < 0.01, *** P < 0.001 (Student’s t-test).
Considering the induction of both IL-6 receptor genes Il6rα and Il6st in Lef1-RV+ TH1 cells and the fact that IL-6 receptor signaling is one of the earliest signals that instructs TFH cell differentiation3, we tested whether LEF-1-augmented TFH differentiation may be mediated through enhanced surface expression of IL-6Rα and gp130 (also known as IL-6Rβ, encoded by Il6st). We analyzed the expression of IL-6Rα and gp130 on the surface of Lef1-RV+ and GFP-RV+ SMARTA CD4+ T cells at day 3 after infection with LCMV, a time when IL-6 receptor signaling is known to be critical for TFH differentiation10. Ectopic expression of LEF-1 in Lef1-RV+ SMARTA CD4+ T cells resulted in increased expression of IL-6Rα compared to GFP-RV+ SMARTA CD4+ T cells (Fig. 6c). When comparing IL-6Rα expression between naïve CD4+ T cells and activated Lef1-RV+ and GFP-RV+ SMARTA CD4+ T cells, LEF-1 overexpression reduced the downregulation of IL-6Rα observed in activated GFP-RV+ CD4+ T cells (Fig. 6c). LEF-1 overexpression had similar effects on gp130, reducing the downregulation of gp130 observed in activated GFP-RV+ CD4+ T cells (Fig. 6d). We then examined the expression of IL-6Rα and gp130 on TFH and TH1 subpopulations. A modest increase in IL-6Rα expression was observed on TFH cells, whereas Lef1-RV+ TH1 cells expressed >150% more IL-6Rα compared to GFP-RV+ TH1 cells (Fig. 6e). While the expression of gp130 was only moderately increased in total Lef1-RV+ SMARTA CD4+ T cells compared to GFP-RV+ SMARTA CD4+ T cells (Fig. 6d), gp130 was preferentially upregulated on Lef1-RV+ TH1 cells compared to GFP-RV+ TH1 cells (Fig. 6f).
RNA-seq also revealed that Icos expression was upregulated on Lef1-RV+ TH1 cells compared to GFP-RV+ TH1 cells (Fig. 6b). Because ICOS plays essential roles during both early and late stages of TFH differentiation26, we further examined ICOS expression. ICOS protein was increased in Lef1-RV+ T cells compared to GFP-RV+ cells (Fig. 6g), and its upregulation occurred predominantly on Lef1-RV+ TH1 cells (Fig. 6h), to levels comparable to GFP-RV+ TFH cells. These observations indicate that LEF-1 functions to help CD4+ T cells retain surface expression of IL-6 receptors and upregulate ICOS expression to enhance responsiveness of activated CD4+ T cells to IL-6 and ICOS-L signals, two essential signals for early TFH differentiation.
We then asked whether overexpression of LEF-1 could rescue TFH differentiation in the absence of Bcl6. Cd4-Cre Bcl6fl/fl CD4+ T cells fail to differentiate into TFH cells during acute viral infections or protein immunizations34. Lef1-RV+ or GFP-RV+ Cd4-Cre Bcl6fl/fl SMARTA CD4+ T cells transferred into B6 mice failed to differentiate into TFH cells in vivo at day 8 after LCMV infection of the recipient mice (Supplementary Fig. 6). These results indicate that LEF-1-mediated regulation of IL-6 receptor complex and ICOS expression act upstream of Bcl6 expression early in TFH differentiation.
Lef1−/− Tcf7−/− GC TFH cells have extensive gene regulation defects
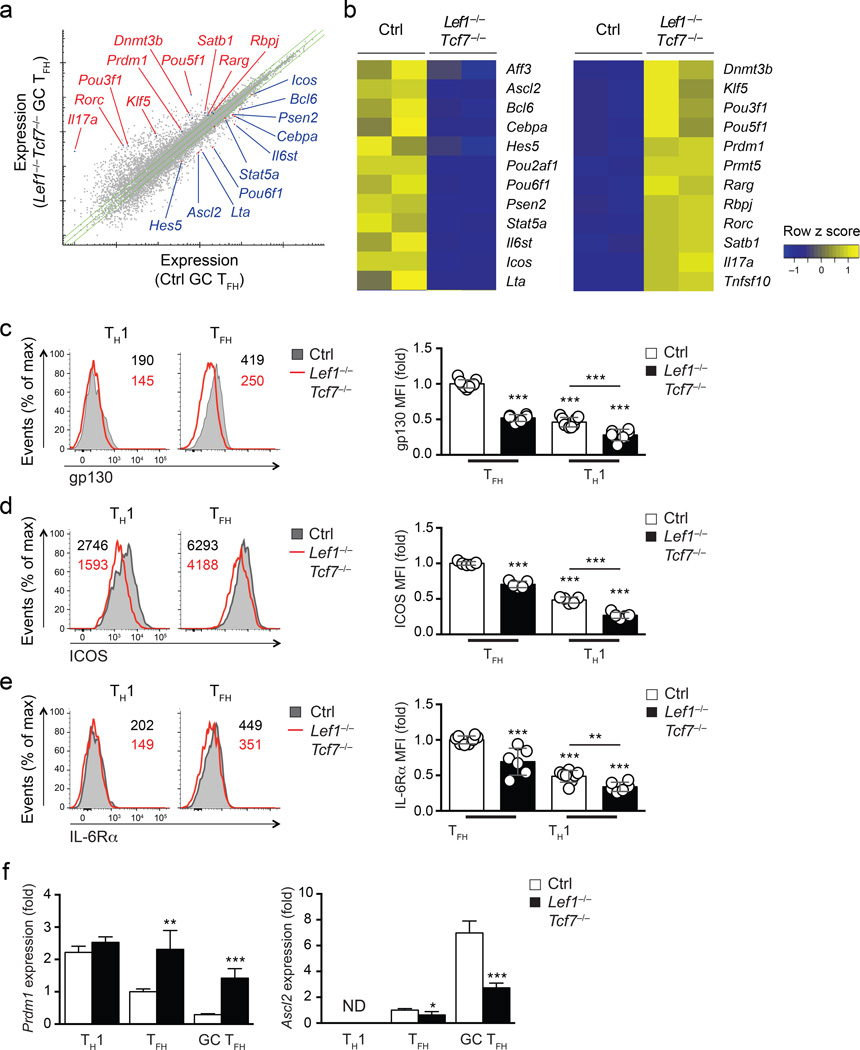
We further assessed the requirements of LEF-1 and TCF-1 for expression of key TFH molecules by transcriptomic analysis of Lef1−/−Tcf7−/− GC TFH cells. RNA-seq was performed using total RNA extracted from GC TFH cells (sorted as PD-1hiCXCR5+ of CD44hiCD62LloGFP+CD4+ T cells) isolated from Lef1−/−Tcf7−/− and control mice (hCD2-Cre−Lef1+/flTcf7+/fl or hCD2-Cre+Lef1+/+Tcf7+/+) on day 8 after vaccinia virus infection. 306 genes were downregulated and 668 genes upregulated in Lef1−/−Tcf7−/− GC TFH cells in comparison to control GC TFH cells (FDR < 0.01 and fold change ≥ 1.5, Fig. 7a). In line with the enhanced Il6st and Icos expression induced by overexpression of LEF-1, Lef1−/−Tcf7−/− GC TFH cells had greatly reduced Il6st and Icos transcripts compared to control cells (Fig. 7b). Flow cytometry showed decreased expression of gp130 and ICOS protein on Lef1−/−Tcf7−/− CXCR5+ TFH cells compared to TFH cells from controls (Fig. 7c and 7d). Although the decrease of Il6ra mRNA in Lef1−/−Tcf7−/− GC TFH cells did not reach statistical significance in the transcriptomic analysis, IL-6Rα protein expression was consistently reduced on Lef1−/−Tcf7−/− CXCR5+ TFH cells compared to TFH cells from control mice (P < 0.001, Fig. 7e). These observations indicate essential and overlapping roles of both LEF-1 and TCF-1 in supporting IL-6 receptor and ICOS expression during TFH differentiation.
Figure 7. LEF-1 and TCF-1-dependent transcriptional regulation of TFH-related genes.
(a) RNA-seq analysis of PD-1hiCXCR5+ GC TFH cells sorted from spleens of Lef1−/−Tcf7−/− and littermate controls 8 d after vaccinia virus infection. Green lines mark mean gene expression of ≥ |1.5 fold| differences. Select genes of interest are marked. (b) Heatmap of selected differentially regulated genes between control and Lef1−/−Tcf7−/− GC TFH cells. (c-e) Flow cytometry of Lef1−/−Tcf7−/− and littermate controls 8 d after infection i.v. with vaccinia virus. CXCR5+ TFH and CXCR5− TH1 cells were analyzed for gp130 (c), ICOS (d), and IL-6Rα (e) expression (mean ± s.d.). Bar graphs are normalized to the mean MFI on control TFH cells. Data are a composite of 4 independent experiments (n = 5–9 per group). (f) Gene expression of Ascl2 and Prdm1 was determined by quantitative RT-PCR in CXCR5− TH1, PD-1loCXCR5+ TFH, and PD-1hiCXCR5+ GC TFH cells sorted from Lef1−/−Tcf7−/− and littermate controls 8 d after infection i.v. with vaccinia virus. Data are from 2 experiments with each sample measured in duplicate and normalized to control TFH cells. ND, not reliably detected. * P < 0.05, ** P < 0.01, *** P < 0.001 (Student’s t-test).
The amount of Bcl6 transcripts was diminished in PD-1hiCXCR5+ GC TFH cells from Lef1−/−Tcf7−/− mice compared to those from control mice (Fig. 7b), while the expression of Prdm1, which encodes the transcription factor Blimp1, was substantially elevated in Lef1−/−Tcf7−/− GC TFH cells (Fig. 7b). Bcl6 and Blimp1 are known to have mutually antagonistic roles during differentiation of TFH cells6. Blimp1 directly inhibits Bcl6 expression and is a potent inhibitor of TFH differentiation6, 28, 30. We validated the increased expression of Prdm1 in Lef1−/−Tcf7−/− PD-1hiCXCR5+ GC TFH by qPCR (Fig. 7f). This increase was specific to GC TFH (PD-1hiCXCR5+) and TFH (PD-1loCXCR5+) cells because TH1 cells (CXCR5−) from both Lef1−/−Tcf7−/− and control mice expressed comparable levels of Prdm1 (Fig. 7f). The transcription factor Ascl2 was shown to be important in TFH differentiation13. Ascl2 expression was reduced in Lef1−/−Tcf7−/− GC TFH cells, but the reduction was less pronounced in PD-1loCXCR5+ TFH cells compared to controls (Fig. 7f). Rorc (encoding RORγt) and Il17a were virtually absent in control GC TFH cells, but these genes were expressed in Lef1−/−Tcf7−/− GC TFH cells (Fig. 7b). Although Th17 gene expression is not normally observed in vaccinia virus infection, these observations are in line with the known role of TCF-1 in restraining Th17 differentiation23 and indicate that LEF-1 and TCF-1 could suppress alternative TH cell fates during TFH differentiation, perhaps in conjunction with Bcl6, which is also known to suppress alternative cell fates3. Other transcriptional changes observed in Lef1−/−Tcf7−/− GC TFH cells compared to control GC TFH cells included the differential expression of POU family transcription factors (decreased expression of Pou2af1 and Pou6f1, and increased expression of Pou3f1 and Pou5f1) and key molecules in the Notch pathway (decreased expression of Hes5 and Psen2, and increased expression of Rbpj). The role of these factors in TFH cells remains to be investigated. Overall, these observations suggest that LEF-1 and TCF-1 contribute to regulation of many genes in activated, antigen-specific CD4+ T cells in vivo, including the positive regulation of Bcl6 and repression of Blimp1 to induce TFH differentiation.
TCF-1 binds directly to key TFH-associated gene loci
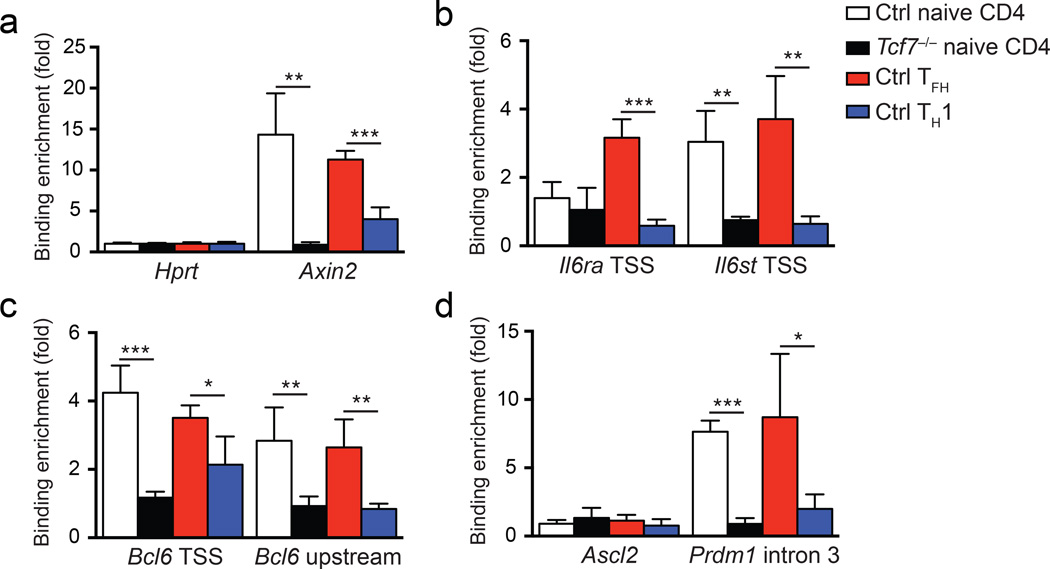
We used chromatin immunoprecipitation (ChIP) to assess if LEF-1 and TCF-1 directly regulate the differentially expressed genes identified above. Both TCF-1 and LEF-1 have a highly homologous HMG DNA binding domain which recognizes the same DNA consensus motif. Because TCF-1 ChIP reagents are of substantially higher quality than currently available LEF-1 reagents, we focused on identifying TCF-1 bound genes in TFH cells. Because most TFH cells retained TCF-1 expression similar to naïve CD4+ T cells (Fig. 4a), we used our TCF-1 ChIP-seq data from naïve wild-type CD4+ T cells (unpublished) as a reference for selection of potential TCF-1 DNA binding sites. TCF-1 enrichment was observed at the transcription start site (TSS) of IL6st, the TSS of Bcl6, a −2.8kb region upstream of Bcl6 TSS and intron 3 of Prdm1 in naïve CD4+ T cells, but was not associated with the Il6ra and Ascl2 genes (Supplementary Fig. 7a). We then performed TCF-1 ChIP using wild-type and Tcf7−/− naïve CD4+ T cells to ensure binding specificity. As a positive control, TCF-1 bound to the TSS of Axin2, a well characterized TCF-1 responsive gene15, in wild-type naïve CD4+ T cells, and this was completely abrogated in Tcf7−/− naïve CD4+ T cells (Fig. 8a). In addition, TCF-1 binding to Axin2 was enriched in TFH cells (CXCR5+) over TH1 cells (CXCR5−) from B6 mice infected with vaccinia virus (Fig. 8a), consistent with increased expression of TCF-1 protein in TFH cells. TCF-1 bound to the Il6st gene in wild-type naïve CD4+ T cells (Fig. 8b, right), and this was also enriched in TFH cells (Fig. 8b). Although TCF-1 did not bind the Il6ra gene in wild-type naïve CD4+ T cells, it was recruited to the Il6ra TSS in wild-type TFH cells (Fig. 8b, left), suggesting TCF-1 recruitment to this site is part of the TFH differentiation program. Compared to naïve CD4+ T cells, TCF-1 did not exhibit enriched binding at Il6st or Il6ra locus in wild-type TH1 cells, in line with diminished expression of both IL-6Rα and gp130 in TH1 cells (Fig. 7c, e). TCF-1 binding to the transcription start site of Icos was not detected (Supplementary Fig. 7b) These data suggest that TCF-1 directly regulates the induction of IL-6 receptor chains to sustain IL-6 receptor complex expression by activated CD4+ T cells in vivo, allowing for TFH differentiation (Supplementary Fig. 8).
Figure 8. TCF-1 binds to key TFH-associated genes in TFH cells.
(a-d) ChIP assays using anti-TCF-1 antibody or control IgG were performed on naive control CD4+ T cells (CD44loCD62L+CD4+); naive Tcf7−/− CD4+ T cells (GFP+CD44loCD62L+CD4+); WT TFH cells (CXCR5+CD44hiCD62L−CD4+); and WT TH1 cells (CXCR5−CD44hiCD62L−CD4+). TFH and TH1 cells were sorted from B6 mice 8 d after vaccinia virus infection. Quantitation of enriched TCF-1 binding was done at the positive control Axin2 gene (a), the TSS of the Il6ra and Il6st genes (b), the TSS and a −2.8 kb upstream regulatory region of the Bcl6 gene (c), and the TSS of Ascl2 and intron 3 of Prdm1 (d), and data are means ± s.d. from 3 independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001 (Student’s t-test).
We next examined ChIP association between TCF-1 and key transcription factor genes for TFH differentiation. TCF-1 bound to intron 3 of the Prdm1 gene, the major regulatory site of Prdm1 expression35, in both naïve CD4+ T cells and CXCR5+ TFH cells (Fig. 8d), suggesting a direct involvement of TCF-1 and its homologue LEF-1 in Blimp1 suppression in TFH cells. Given that Prdm1 is not expressed by naïve CD4+ T cells, binding of TCF-1 at this site suggests that TCF-1 may antagonize Prdm1 expression upon T cell activation. In addition, we observed specific binding of TCF-1 to the TSS of Bcl6 and an upstream regulatory region of Bcl6 in naive CD4+ T cells (Fig. 8c) and this binding pattern was maintained in TFH cells (Fig. 8c). Robust enrichment of TCF-1 was observed in the Prdm1, Bcl6, Il6ra, and Il6st gene loci in wild-type TFH cells compared to Tcf7−/− TFH cells (Supplementary Fig. 7b). We did not observe enriched TCF-1 binding in the Ascl2 TSS (Fig. 8c–d), albeit we could not exclude the possibility that Ascl2 is regulated by LEF-1 and TCF-1 through more distal regulatory regions. TCF-1 binding to the Bcl6 upstream region and Prdm1 intron was abrogated in TH1 cells as compared to TFH (Fig. 8d), in line with the greatly reduced expression of TCF-1 in TH1 cells. These observations suggest that downregulation of TCF-1 in TH1 cells is important for upregulation of Blimp1 and Blimp1-mediated repression of Bcl6 in TH1 cells, while retention of TCF-1 in early TFH cells ensures proper upregulation of Bcl6 and subsequent suppression of Blimp1 during TFH differentiation (Supplementary Fig. 8).
DISCUSSION
TFH differentiation can be initiated at an early time point during T cell activation, but the regulators of this important decision process are still being defined. Here we initiated an investigation to identify novel pathways in TFH differentiation by characterizing genes differentially expressed in early TFH in vivo but not modulated by supplementing IL-6 in vitro. We show that the pair of transcription factors LEF-1 and TCF-1 influence TFH differentiation by regulating circuits upstream of Bcl6. We found that LEF-1 and TCF-1 coordinate TFH differentiation by two general mechanisms. First, they establish the responsiveness of naïve CD4+ T cells to TFH signals by promoting expression of IL-6 receptor chains and binding to Prdm1 and Bcl6. Second, they promote early TFH differentiation of activated CD4+ T cells via multipronged activities sustaining expression of IL-6Rα and gp130, enhancing ICOS expression and promoting expression of Bcl6 while inhibiting Blimp1 expression.
IL-6 is a critical early regulator of TFH differentiation, as Il6−/− mice fail to have any TFH cell differentiation during the DC priming phase of an acute antiviral immune response10. In mice whose DCs constitutively overexpress IL-6, the major phenotype observed is a dramatic increase in TFH cell and germinal centers36. Therefore, regulation of IL-6 receptor expression on naive CD4+ T cells and early activated CD4+ T cells is a clear mechanism by which LEF-1 and TCF-1 will influence TFH differentiation.
Bcl6 is essential for TFH differentiation, while Blimp1 is a powerful antagonist of TFH differentiation. Our observations that expression of LEF-1 results in aberrant expression of Bcl6 in TH1 cells, Blimp1 expression is aberrantly upregulated in Lef1−/−Tcf7−/− GC TFH cells, and both Bcl6 and Prdm1 are directly bound TCF-1 targets indicate that LEF-1 and TCF-1 likely dually regulate both of these critical transcription factors. While we cannot rule out the possibility that the de-repression of Prdm1 results from reduced Bcl6 expression in Lef1−/−Tcf7−/− TFH and GC TFH cells, we speculate that LEF-1 and TCF-1 directly repress Prdm1 expression. LEF-1 and TCF-1 are known to positively and negatively regulate gene expression, depending on the interacting factors. For examples, both proteins can interact with β-catenin coactivator and TLE corepressors, and LEF-1 and TCF-1 repress Cd4 in CD8+ T cells18. Future analysis of molecular mechanisms by which LEF-1 and TCF-1 regulate Prdm1 and Bcl6 genes will be important, as will analysis of how LEF-1 and TCF-1 interact with other regulators of Bcl6 and Prdm1, such as STAT1, STAT3, STAT5, Foxo1, and Klf23, 10, 11, 28, 37, 38. Nevertheless, our data provide proof that LEF-1 and TCF-1 regulate the balance between Bcl6 and Blimp1 expression.
ICOS expression was selectively impaired on Lef1−/−Tcf7−/− TFH cells, and ICOS expression was enhanced on Lef1-RV+ cells. In multiple models, moderate ICOS changes have been observed to enhance TFH differentiation38, 39, 40, 41 or function42. ICOS appears not to be a direct target of LEF-1 and TCF-1, though distal cis-elements were not explored. Alternatively, ICOS may be indirectly regulated by LEF-1 and TCF-1. Future studies will further elucidate LEF-1 and TCF-1 signaling axes modulating ICOS expression. Overall, the combined influence of LEF-1 and TCF-1 on IL-6Rα, gp130, Bcl6, Blimp1, and ICOS makes a dense network of interactions that create a strong pro-TFH signaling environment in a cell sustaining LEF-1 and/or TCF-1 expression.
LEF-1 and TCF-1 functions likely continue to be important in fully differentiated TFH and GC TFH cells. LEF-1 and TCF-1 both continue to be expressed in GC TFH cells. Bcl6 expression is essential in GC TFH cells3 and continued regulation of both Bcl6 and Prdm1 are central aspects of GC TFH biology. ICOS is also a major regulator of GC TFH biology26, 40. IL-6 receptor signaling is not usually essential in GC TFH cells due to compensatory capabilities of IL-21 or IL-27 at later time points29, 43, 44. Nevertheless, IL-6 receptor likely plays a major role in sustaining GC TFH under normal physiological conditions. IL-6 is required for sustaining TFH and GC responses in chronic LCMV infection in mice45, and IL-6 is positively associated with TFH cells and GCs in SIV+ macaques46.
The activities of LEF-1 and TCF-1 appear to pre-program the responsiveness of a given naive CD4+ T cell to TFH signals, prior to any exposure of the cell to antigen. Therefore, we speculate that transient or sustained inflammatory or pathogenic conditions that alter LEF-1 or TCF-1 expression in naive T cells may have a global impact altering the capacity of naive CD4+ T cells to respond to TFH induction signals in the presence of pathogens or autoimmunity triggers. Ultimately, it will be useful to determine how homeostatic signals act in concert with LEF-1 and TCF-1 to modulate the expression or poised status of TFH-associated genes in naïve CD4+ T cells to properly orchestrate the development progression from naive cell to TFH or non-TFH cell fates.
LEF-1 and TCF-1 are highly expressed in resting naive CD4+ and CD8+ T cells, but TCF-1 and LEF-1 are downregulated in effector CD8+ T cells and TH1 cells, suggesting Lef1 and Tcf7 are regulated by T cell activation. TCR dwell time influences TFH versus non-TFH differentiation in a TCR signal strength intrinsic manner47. We speculate these processes may be interrelated.
In conclusion, our study discovers novel roles for LEF-1 and TCF-1 in TFH differentiation. Thus, a better understanding of the signals regulating LEF-1 and TCF-1 and their downstream targets in activated CD4+ T cells will have implications for understanding how to enhance TFH differentiation, as well as for understanding non-TFH CD4+ T cell differentiation processes.
METHODS
Mice and viral infections
C57BL/6J (B6), B6.SJL, Cd4-Cre, and Rosa26GFP mice were purchased from the Jackson Laboratory. Mouse strains described below were obtained from in-house breeders of either LJI or University of Iowa animal facility. SMARTA (LCMV gp66-77-IAb specific) TCR transgenic mice48, Tcf7fl/fl and Lef1fl/fl mice were described16, 18. Bcl6fl/fl mice and hCD2-Cre mice were from Drs. Toshitada Takemori49 and Paul Love32, respectively. Blimp1-YFP BAC transgenic mice were crossed to the SMARTA strain to generate Blimp1-YFP SMARTA mice26. Tcf7-GFP reporter mice were generated in house, and detailed targeting strategy and characterization will be published elsewhere (manuscript in review). All animals were analyzed at 6–12 weeks of age, and both genders included without randomization or “blinding”. All mouse experiments were performed under protocols approved by the Institutional Animal Use and Care Committees of LJI and the University of Iowa. For acute viral infection, 2.5 – 5.0 × 105 plaque-forming units (PFU) and 2.5 × 105 PFU was used for LCMV Armstrong (LCMV-Arm) and vaccinia virus, respectively. Virus was prepared in plain DMEM and injected intraperitoneally or intravenously.
Flow cytometry
Single cell suspension was prepared from the spleen of mice infected with LCMV or vaccinia virus, and surface-stained as previously described16, 26. The fluorochrome-conjugated antibodies and their clone numbers are CD4 (RM4-5), CD44 (IM7), CD62L (MEL-14), PD-1 (J43), IL-6Rα (D7715A7), gp130 (KGP130), ICOS (C398.4A), Fas (15A7), GL7 (GL7), IgD (11–26), CD138 (281-2), Bcl6 (K112-91), and all were from eBiosciences. SLAM (TC15-12F12.2) was from BioLegend. PSGL-1 (2PH1) was from BD Biosciences. For detection of CXCR5, a two-step26 or three-step6 staining protocol was used with biotinylated anti-CXCR5 or unconjugated anti-CXCR5 Abs, respectively (2G8 from BD Biosciences). For intracellular detection of Bcl6, the surface-stained cells were fixed and permeabilized with the Foxp3/transcription factor staining buffer (eBiosciences), followed by incubation with fluorochrome-conjugated Bcl6 antibody. Data were collected on LSRII and FACSVerse (BD Biosciences) and analyzed with FlowJo software (TreeStar).
Immunoblot
For analysis of knockdown of LEF-1 or targeted deletion of TCF-1 and LEF-1, shCtrl and shLef1 SMARTA cells or CD4+ and CD8+ T cells (5 × 105 each) were sorted, and denatured in SDS Loading Buffer at 100°C for 5 minutes. Cell lysates were probed with anti-TCF-1 (C46C7; Cell Signaling Technology), anti-LEF-1 (C18A7 and C12A5; Cell Signaling Technology) or anti-β-actin (loading control; I-19; Santa Cruz Biotechnology).
Retroviral transductions
Naïve SMARTA CD4+ T cells were purified by negative selection using either magnetic beads (Miltenyi Biotec) or EasySep kit (STEMCELL), and resuspended in D-10 [DMEM + 10% FCS (fetal calf serum) + 2 mM GlutaMax (Life Technologies) + 100 U/ml Penicillin/Streptomycin (Life Technologies) + 50 µM β-mercaptoethanol] with 2 ng/ml hIL-7 or 10 ng/ml hIL-2 (Peprotech). 2 × 106 SMARTA cells were seeded in 24-well plates coated with 8 µg/ml anti-CD3 (clone 17A2, BioXcell) and anti-CD28 (clone 37.51, BioXcell). Retroviral soups were given at 24 and 36 hours after stimulation. After 72 hours in vitro stimulation, SMARTA cells were transferred into 6-well plates in D-10 with 10 ng/ml hIL-2 for two days. One day prior to cell sorting reporter expressing cells (FACSAria, BD Biosciences) for transfer, culture media was replaced with D-10 with 2 ng/ml hIL-7. Detailed information is described elsewhere50.
Cell sorting
All the cell sorting was done on a FACSAria (BD Biosciences). For RNA-seq analyses, early TFH (IL-2Rα−Blimp1-YFP−) or early TH1 (IL-2Rα+Blimp1-YFP+) SMARTA cells, or CXCR5− (TH1), PD1loCXCR5+ (TFH), and PD1hiCXCR5+ (GC TFH) subsets among activated GFP+CD4+ splenic T cells of Lef1−/−Tcf7−/− or control mice were sorted on day 3 after LCMV infection or on day 8 after vaccinia virus infection, respectively. GFP-RV+ or Lef1-RV+ SMARTA cells were sorted as SLAMhiCXCR5lo (TH1) or SLAMloCXCR5hi (TFH) cells on day 4 after LCMV infection. For ChIP analysis, CXCR5− (TH1) and CXCR5+ (TFH) cells were sorted from activated CD4+ splenic T cells on day 8 after vaccinia virus infection. Also, CD44loCD62Lhi naïve CD4+ T cells were sorted from WT or Tcf7−/− (Cd4-CreTcf7fl/fl).
Retrovirus production and cell transfers
Murine Lef1 cDNA (clone ID 6401514, Open Biosystems) was cloned into expressing retroviral vector (pMIG-GFP). Lef1 shRNA sequence (Transomic) was cloned into pLMPd-Ametrine vector, as reported26, 31. Virions were obtained from the Plat-E cells as described50. Briefly, culture supernatants were collected 24 and 48 hours after transfection, filtered through 0.45 µm syringe filter, and saved at 4°C until used for transductions.
Naïve or retrovirally transduced SMARTA cells were transferred into mice intravenously via retro-orbital sinus. When using transduced SMARTA cells, 100% of transferred cells were transduced (Ametrine+CD45.1+). Cell transfer numbers are 4–5 × 105, 2 × 105, and 5 × 103 SMARTA cells were day 3, 4, and 8 experiments, respectively.
In vitro activation of CD4+ T cells
Naïve SMARTA cells were negatively isolated by using CD4+ T cell isolation kit (Miltenyi or StemCell). 2 × 106 SMARTA cells were seeded on 24 well plates coated with 8 µg/mL αCD3 (clone 17A2, BioXcell) and αCD28 (clone 37.51, BioXcell). For TH1polarization, Smarta cells were given with 20 µg/mL of αIL-4 (clone 11B11, BioXcell) and αTGF-β (clone 1D11, BioXcell) and 20 ng/mL of rmIL-12 (Peprotech). For IL-6 condition, 10 µg/mL of αIFN-γ (clone XMG1.2, BioXcell) and αIL-12 (clone R1-5D9, BioXcell) and 20 ng/mL of rmIL-6 (Peprotech) were added in culture media.
Quantitative RT-PCR
Total RNA from the sorted cells was extracted, reverse-transcribed, and quantitative PCR were performed as described16.
RNA-Seq and transcriptome analysis (Xue Group)
Total RNA was extracted from the sorted PD-1+CXCR5+ cells from Tcf7−/−Lef1−/− or control mice, and two biological replicates were obtained for each genotype. cDNA synthesis and amplification were performed using SMARTer Ultra Low Input RNA Kit (Clontech) starting with 10 ng of total RNA per sample following manufacturer’s instructions. cDNA was fragmented with Q800R sonicator (Qsonica) and used as input for NEBNext Ultra DNA Library Preparation Kit (NEB). Libraries were sequenced on Illumina’s HiSeq2000 in single read mode with the read length of 50 nucleotides producing 60–70 million reads per sample. Sequence data in fastq format were generated using CASAVA 1.8.2 processing pipeline from Illumina.
The sequencing quality of RNA-Seq libraries was assessed by FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/, v0.10.1). Because of biased GC content in the 5’ end, the first 12 bases of each read in all 4 samples were trimmed off. RNA-Seq data reproducibility was evaluated by computing Pearson’s correlation of FPKM values for all genes between biological replicates. The Pearson’s correlation coefficient between the two biological replicates was 0.937 for the control samples and 0.986 for the Tcf7−/− Lef1−/− samples, indicating strong reproducibility.
The RNA-Seq libraries were then processed by RSEM (v1.2.19) to estimate expression levels of all genes. The expression level of a gene is expressed as a gene-level FPKM (Fragments Per Kilobase of transcripts per Million mapped reads) value. EBSeq (v1.5.4), as an integral component of RSEM package, was used to identify differentially expressed genes. UCSC genes for mouse mm9 from iGenome (http://support.illumina.com/sequencing/sequencing_software/igenome.html) were used for gene annotation. The RNA-seq data are deposited at the Gene Expression Omnibus under accession number GSE66781.
RNA-Seq and transcriptome analysis (Crotty Group)
Total RNA was extracted from cells stored in Trizol using the miRNeasy Mini Kit (Qiagen 217004). [1] For RNA-seq analysis for early TFH and TH1 cells: poly(A) RNA was isolated using the Poly(A) Purist MAG kit (Ambion AM1922) from 200 ng total RNA of each sample. Resulting poly(A) RNA was then fragmented and prepared, according to the manufacturer’s instructions (ABI 4452437 Rev B), into barcoded, strand-specific libraries using The SOLiD Total RNA-Seq Kit (ABI 4445374). Following library preparation, 15 ng of each library was converted into SOLiD Wildfire compatible fragments using the 5500 W Conversion Primer Kit (Life Technologies) and 5 rounds of PCR. Libraries were then pooled at equimolar concentrations using Quant-iT PicoGreen dsDNA Assay Kit (Life Technologies) and sequenced on a Life Technologies 5500XL W Genetic Analyzer. SOLiD 5500-2 sequencing outcomes were converted from color space to nucleotide space by using Galaxy solid2fastq script. [2] For RNA-seq analysis for GFP-RV+ or Lef1-RV+ SMARTA cells obtained 4 days after LCMV infection: 500 ng of each sample’s total RNA was then prepared into mRNA libraries, according to manufacturer’s instructions (Illumina, RS-122-2103). The resulting libraries were deep sequenced on the Illumina 2500 in Rapid Run Mode, using single-end reads with lengths of 50 nucleotides (> 24 million reads per condition). The single-end reads that passed Illumina filters were filtered for reads aligning to tRNA, rRNA, adapter sequences, and spike-in controls.
The reads were then aligned to UCSC mm9 reference genome using TopHat (v 1.4.1). DUST scores were calculated with PRINSEQ Lite (v 0.20.3) and low-complexity reads (DUST > 4) were removed from the BAM files. The alignment results were parsed via the SAMtools to generate SAM files. Read counts to each genomic feature were obtained with the htseq-count program (v 0.6.0) using the “union” option. After removing absent features (zero counts in all samples), the raw counts were then imported to R/Bioconductor package DESeq2 to identify differentially expressed genes among samples. DESeq2 normalizes counts by dividing each column of the count table (samples) by the size factor of this column. The size factor is calculated by dividing the samples by geometric means of the genes. This brings the count values to a common scale suitable for comparison. P-values for differential expression are calculated using binomial test for differences between the base means of two conditions. These p-values are then adjusted for multiple test correction using Benjamini Hochberg algorithm to control the false discovery rate. We considered genes differentially expressed between two groups of samples when the DESeq2 analysis resulted in an adjusted P-value of <0.05 and the fold-change in gene expression was 1.5-fold. Cluster analyses including principal component analysis (PCA) and hierarchical clustering were performed using standard algorithms and metrics. Hierarchical clustering was performed using complete linkage with Euclidean metric. RNA-seq data are deposited at the Gene Expression Omnibus under accession number GSE67336.
Heatmaps
Heatmaps were generated with normalized data of RNA-seq analyses for early TFH/TH1 cells and for GFP-RV+/Lef1-RV+ TFH/TH1 cells. Microarray analysis used published TH1, TFH and GC TFH cell sets (GSE21380, ref. 51) using the GenePattern software suite (genepattern.broadinstitute.org).
GSEA analysis
GSEA analysis was performed using the GSEA software from the Broad Institute. TFH and GC TFH gene sets were generated in-house with genes that were expressed in TFH (PD1loCXCR5+) and GC TFH (PD1hiCXCR5+) by more than 2-fold in comparison to TH1 (PD1−CXCR5−) cells, respectively (data obtained from GSE21380). Enrichment of genes, which were upregulated in Lef1-RV+ Th1 cells in comparison to GFP-RV+ TH1 cells by more than 1.2-fold, was then ranked by the Diff_of_Classes.
Chromatin immunoprecipitation (ChIP)
Sorted CD4+ T cells were cross-linked with 1% formaldehyde in medium for 5 minutes, processed using truChIP Chromatin Shearing Reagent Kit (Covaris), and sonicated for 5 minutes on Covaris S2 ultrasonicator. The sheared chromatin was immunoprecipitated with anti-TCF-1 (C46C7, Cell Signaling Technologies) or control rabbit IgG and washed as previously described. The immunoprecipitated DNA segments were used for PCR quantification. For calculation of enriched TCF-1 binding in a given cell type, each TCF-1 ChIP sample was first normalized to corresponding IgG ChIP sample, and the signal at a target region was then normalized to that at the Hprt promoter region.
Statistical analysis
Data sets were analyzed with the Student’s t-test with a two-tailed distribution assuming equal sample variance.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. Yang for helping ex vivo screening of target genes obtained from RNA-seq analysis for early TFH and TH1 cells and Z. Fu for generous help in performing GSEA analysis and for the generation of heatmap. We also thank LJI Flow Cytometry Flow Cytometry Core Facility (C. Kim, K.V. Gunst, and L. Nosworthy) and the University of Iowa Flow Cytometry Core facility (J. Fishbaugh, H. Vignes and G. Rasmussen) for cell sorting. We thank I. Antoshechkin (Millard and Muriel Jacobs Genetics and Genomics Laboratory at the Caltech) for Tcf7−/−Lef1−/− RNA-seq, and J. T. Harty for providing vaccinia virus to the Xue laboratory. This study is supported by grants from the American Cancer Society (RSG-11-161-01-MPC to H.-H.X.), and the NIH (AI105351, AI112579, AI115149, and AI119160 to H.-H.X., AI113806 to W.P., and AI109976, AI063107, and AI072543 to S.C.). J.A.G is supported in part by a Pre-doctoral Immunology Training Grant AI007485. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS
Y.S.C., J.A.G., S.X., F.L. and Q.S. performed the experiments and analyzed the data. Z.H. analyzed the RNA-seq data under supervision of W.P. P.E.L provided critical reagents. Y.S.C., S.C., and H.H.X. conceived the project and wrote the paper. S.C. and H.H.X. supervised the overall study.
The authors declare no conflicts of interest.
REFERENCES
- 1.Crotty S. A brief history of T cell help to B cells. Nature reviews Immunology. 2015;15(3):185–189. doi: 10.1038/nri3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nature Immunology. 2015;16(2):142–152. doi: 10.1038/ni.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunological reviews. 2012;247(1):52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 5.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 6.Johnston RJ, Poholek AC, Ditoro D, Yusuf I, Eto D, Barnett B, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325(5943):1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325(5943):1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31(3):457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Ise W, Kohyama M, Schraml BU, Zhang T, Schwer B, Basu U, et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nature Immunology. 2011;12(6):536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi YS, Eto D, Yang JA, Lao C, Crotty S. Cutting Edge: STAT1 Is Required for IL-6-Mediated Bcl6 Induction for Early Follicular Helper Cell Differentiation. The Journal of Immunology. 2013;190(7):3049–3053. doi: 10.4049/jimmunol.1203032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29(1):138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray JP, Marshall HD, Laidlaw BJ, Staron MM, Kaech SM, Craft J. Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity. 2014;40(3):367–377. doi: 10.1016/j.immuni.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Chen X, Zhong B, Wang X, Chu F, et al. Transcription factor achaete-scute homologue 2 initiates follicular T-helper-cell development. Nature. 2014;507(7493):513–518. doi: 10.1038/nature12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi YS, Yang JA, Crotty S. Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Current opinion in immunology. 2013:1–7. doi: 10.1016/j.coi.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber BN, Chi AW-S, Chavez A, Yashiro-Ohtani Y, Yang Q, Shestova O, et al. A critical role for TCF-1 in T-lineage specification and differentiation. Nature. 2011;476(7358):63–68. doi: 10.1038/nature10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Zhou X, Steinke FC, Liu C, Chen S-C, Zagorodna O, et al. The TCF-1 and LEF-1 transcription factors have cooperative and opposing roles in T cell development and malignancy. Immunity. 2012;37(5):813–826. doi: 10.1016/j.immuni.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinke FC, Xue H-H. From inception to output, Tcf1 and Lef1 safeguard development of T cells and innate immune cells. Immunologic research. 2014;59(1–3):45–55. doi: 10.1007/s12026-014-8545-9. [DOI] [PubMed] [Google Scholar]
- 18.Steinke FC, Yu S, Zhou X, He B, Yang W, Zhou B, et al. TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4(+) T cell fate and interact with Runx3 to silence Cd4 in CD8(+) T cells. Nature Immunology. 2014;15(7):646–656. doi: 10.1038/ni.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeannet G, Boudousquié C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(21):9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou X, Xue H-H. Cutting edge: generation of memory precursors and functional memory CD8+ T cells depends on T cell factor-1 and lymphoid enhancer-binding factor-1. The Journal of Immunology. 2012;189(6):2722–2726. doi: 10.4049/jimmunol.1201150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, Yu S, Zhao D-M, Harty JT, Badovinac VP, Xue H-H. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(2):229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu Q, Sharma A, Oh SY, Moon H-G, Hossain MZ, Salay TM, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nature Immunology. 2009;10(9):992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Q, Sharma A, Ghosh A, Sen JM. T cell factor-1 negatively regulates expression of IL-17 family of cytokines and protects mice from experimental autoimmune encephalomyelitis. The Journal of Immunology. 2011;186(7):3946–3952. doi: 10.4049/jimmunol.1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Loosdregt J, Fleskens V, Tiemessen MM, Mokry M, van Boxtel R, Meerding J, et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity. 2013;39(2):298–310. doi: 10.1016/j.immuni.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, et al. Bcl6 Expressing Follicular Helper CD4 T Cells Are Fate Committed Early and Have the Capacity To Form Memory. The Journal of Immunology. 2013;190(8):4014–4026. doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity. 2011;34(6):932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pepper M, Pagán AJ, Igyártó BZ, Taylor JJ, Jenkins MK. Opposing signals from the bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35(4):583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. Journal of Experimental Medicine. 2012;209(2):243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eto D, Lao C, Ditoro D, Barnett B, Escobar TC, Kageyama R, et al. IL-21 and IL-6 are critical for different aspects of B cell immunity and redundantly induce optimal follicular helper CD4 T cell (Tfh) differentiation. PloS one. 2011;6(3):e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oestreich KJ, Mohn SE, Weinmann AS. Molecular mechanisms that control the expression and activity of Bcl-6 in T(H)1 cells to regulate flexibility with a T(FH)-like gene profile. Nature Immunology. 2012 doi: 10.1038/ni.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen R, Bélanger S, Frederick MA, Li B, Johnston RJ, Xiao N, et al. In vivo RNA interference screens identify regulators of antiviral CD4(+) and CD8(+) T cell differentiation. Immunity. 2014;41(2):325–338. doi: 10.1016/j.immuni.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vacchio MS, Wang L, Bouladoux N, Carpenter AC, Xiong Y, Williams LC, et al. A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells. Nat Immunol. 2014;15(10):947–956. doi: 10.1038/ni.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao D-M, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. The Journal of Immunology. 2010;184(3):1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nance JP, Belanger S, Johnston RJ, Takemori T, Crotty S. Cutting Edge: T Follicular Helper Cell Differentiation Is Defective in the Absence of Bcl6 BTB Repressor Domain Function. Journal of immunology. 2015;194(12):5599–5603. doi: 10.4049/jimmunol.1500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, Calame KL. Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. The Journal of Immunology. 2004;173(2):1158–1165. doi: 10.4049/jimmunol.173.2.1158. [DOI] [PubMed] [Google Scholar]
- 36.Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic function of Blimp-1 in dendritic cells. Journal of Experimental Medicine. 2011;208(11):2193–2199. doi: 10.1084/jem.20110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J-Y, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, et al. The Transcription Factor KLF2 Restrains CD4(+) T Follicular Helper Cell Differentiation. Immunity. 2015;42(2):252–264. doi: 10.1016/j.immuni.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai C-Y, Emslie E, et al. ICOS Coreceptor Signaling Inactivates the Transcription Factor FOXO1 to Promote Tfh Cell Differentiation. Immunity. 2015;42(2):239–251. doi: 10.1016/j.immuni.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratama A, Srivastava M, Williams NJ, Papa I, Lee SK, Dinh XT, et al. MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nature Communications. 2015;6:6436. doi: 10.1038/ncomms7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber JP, Fuhrmann F, Feist RK, Lahmann A, Al Baz MS, Gentz L-J, et al. ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2. Journal of Experimental Medicine. 2015;212(2):217–233. doi: 10.1084/jem.20141432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogel KU, Edelmann SL, Jeltsch KM, Bertossi A, Heger K, Heinz GA, et al. Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity. 2013;38(4):655–668. doi: 10.1016/j.immuni.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, et al. Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature. 2013;496(7446):523–527. doi: 10.1038/nature12058. [DOI] [PubMed] [Google Scholar]
- 43.Batten M, Ramamoorthi N, Kljavin NM, Ma CS, Cox JH, Dengler HS, et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. Journal of Experimental Medicine. 2010;207(13):2895–2906. doi: 10.1084/jem.20100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, et al. In vivo regulation of Bcl6 and T follicular helper cell development. The Journal of Immunology. 2010;185(1):313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harker JA, Lewis GM, Mack L, Zuniga EI. Late Interleukin-6 Escalates T Follicular Helper Cell Responses and Controls a Chronic Viral Infection. Science (New York, NY) 2011;334(6057):825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. The Journal of clinical investigation. 2012 doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tubo NJ, Pagán AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, et al. Single Naive CD4+ T Cells from a Diverse Repertoire Produce Different Effector Cell Types during Infection. Cell. 2013;153(4):785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. European Journal of Immunology. 1998;28(1):390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 49.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. Journal of Experimental Medicine. 2012;209(11):2079–2097. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi YS, Crotty S. Retroviral Vector Expression in TCR Transgenic CD4(+) T Cells. Methods in molecular biology (Clifton, NJ) 2015;1291:49–61. doi: 10.1007/978-1-4939-2498-1_5. [DOI] [PubMed] [Google Scholar]
- 51.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) Journal of immunology. 2010;185(1):190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.