Abstract
Five new lupane triterpene coumaroyl esters (1–5), together with betulin (6) and a known Buxus alkaloid, N-3-benzoyldihydrocyclomicrophylline F (7), were isolated from a CHCl3-soluble partition of a methanol extract of Buxus cochinchinensis Pierre ex Gagnep. (Buxaceae) collected in Vietnam. Isolation work was monitored using human colon cancer cells (HT-29). The structures of the new compounds (1–5) were determined on the basis of spectroscopic data interpretation. In addition to their cytotoxicity against HT-29 cells and NF-κB (p65) inhibitory activity in an ELISA assay, all isolates, as well as two semi-synthetic compounds derived from betulin and 5, respectively, were also evaluated for their in vitro antiplasmodial activities against the drug-resistant Dd2 strain of Plasmodium falciparum and antifungal effects on the growth of the pathogenic yeast Candida albicans. The new lupane triterpene coumaroyl esters (1–5), along with a betulin derivative and the known Buxus alkaloid, were found to show significant in vitro antimalarial activities, with IC50 values ranging from 0.26 to 2.07 µM.
Keywords: lupane triterpenes, coumaroyl esters, Buxus cochinchinensis, Buxaceae, cytotoxicity, antimalarial activity
Introduction
Buxus, commonly known as “boxwood”, is the largest genus of the plant family Buxaceae, which comprises over 70 species, and is distributed mainly in southern Europe, southern Asia, Africa, Madagascar, and North and Central America [1]. In addition to being widely used for landscaping purposes, plants of the genus Buxus have also been employed in traditional medicine for the treatment of epilepsy, leprosy, malaria, rheumatism, skin infections, toothache, and venereal disease [2]. Phytochemical studies on plants of the this genus have resulted in the isolation and structural characterization of over 200 Buxus alkaloids, which biosynthetically are considered as degraded triterpenoid alkaloids based on a cycloartenol skeleton. As the major biological active agents from Buxus species, these alkaloids have shown a wide variety of activities, including cytotoxicity, antibacterial, antimalarial, and cholinesterase inhibitory properties [3–5].
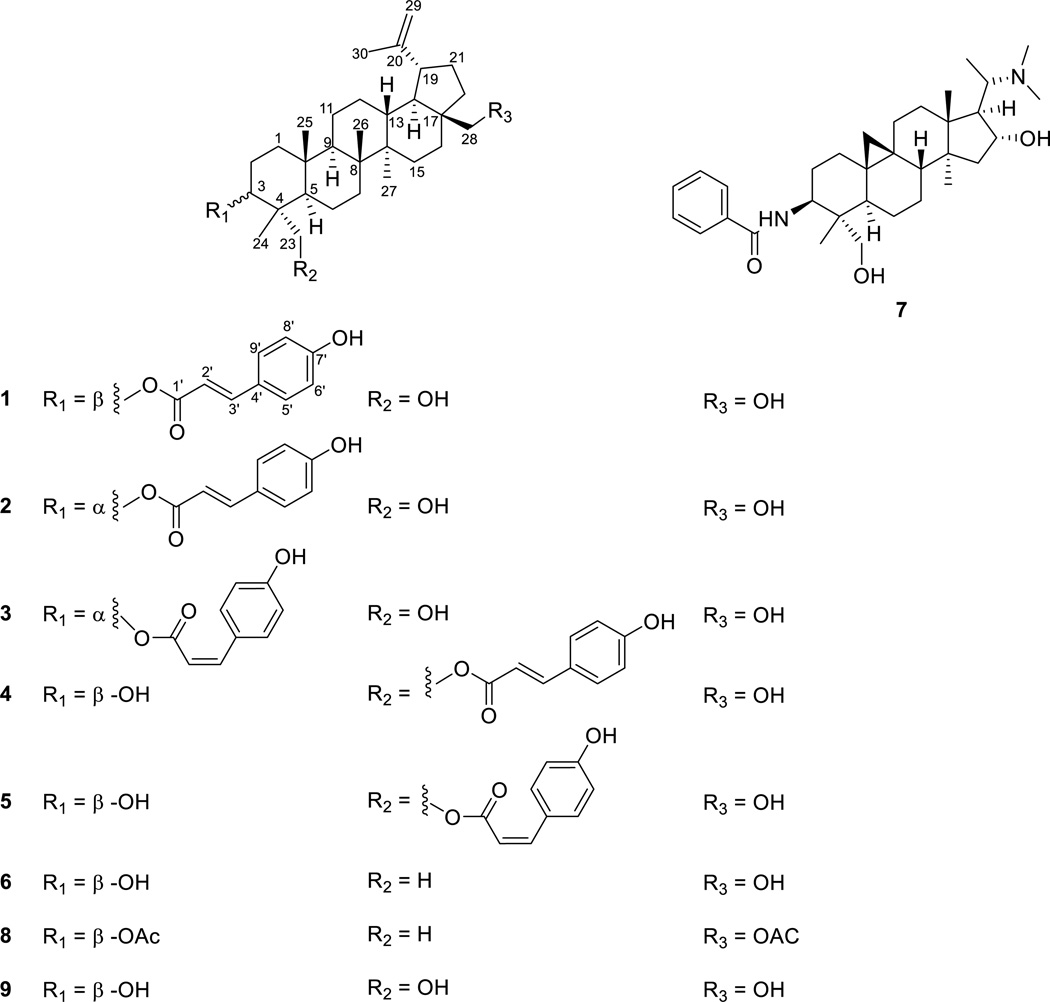
Buxus cochinchinensis Pierre ex Gagnep., a large shrub, 1–2 m high, with gray-white stems and dark green fruits, is a boxwood species native to southeastern Asia [6]. To the best of our knowledge, no phytochemical investigation has been carried out on this plant to date. As part of our continuing efforts to discover naturally occurring biologically active agents from plants, a chloroform-soluble partition of a methanol extract from a combination of leaves, twigs and fruits of B. cochinchinensis collected in Vietnam was found to show cytotoxicity against the HT-29 human colon cancer line, with an ED50 value of 7.8 µg/mL, and thus was fractionated by bioactivity-guided isolation using this assay. Seven lupane-type triterpenes, including five new lupane triterpene coumaroyl esters (1–5), together with two previously known compounds, betulin (6) [7] and N-benzoyldihydrocyclomicrophylline F (7) [8, 9] were isolated and identified in present study. The structures of the new compounds (1–5) were determined as 3-O-(E)-p-coumaroyl-23-hydroxybetulin (1), 3-O-(E)-p-coumaroyl-23-hydroxy-3-epi-betulin (2), 3-O-(Z)-p-coumaroyl-23-hydroxy-3-epi-betulin (3), 23-O-(E)-p-coumaroyl-23-hydroxybetulin (4), and 23-O-(Z)-p-coumaroyl-23-hydroxybetulin (5), on the basis of spectroscopic data interpretation.
As mentioned above, plants of the genus Buxus have been reported with antimalarial and antifungal related properties. Thus, besides the human colon cancer cell line HT-29 and the NF-κB inhibitory bioassays, all the isolates obtained in the current study, together with two semi-synthetic compounds, 3,28-O-diacetylbetulin [10] and 23-hydroxybetulin [11], which were derived from betulin and 5 respectively, were further screened for their antiplasmodial activities against the drug-resistant Dd2 strain of Plasmodium falciparum and inhibitory effects on the growth of the pathogenic yeast Candida albicans.
Results and Discussion
Compounds 1–5 were found to share a common molecular formula of C39H56O5 as determined from the sodium adduct ion peak [M + Na]+ in the HRESIMS of each compound. Based on their spectroscopic data interpretation, the structures of compounds 1–5 could be determined as coumaroyl esters of lupane-type triterpenoid (Fig. 1.), being stereoisomers or positional isomers to each other, as discussed in detail below.
Fig. 1.
Chemical structures of new compounds 1–9.
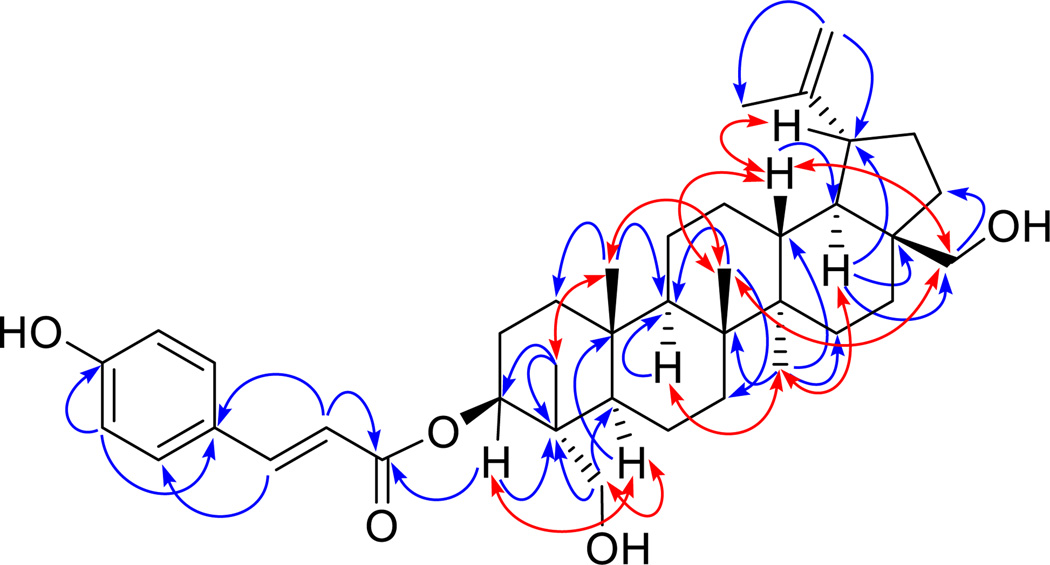
The HRESIMS of 1 afforded a sodiated molecular ion peak at m/z 627.4028, corresponding to an elemental formula of C39H56O5Na (calcd for m/z 627.4025). The IR spectrum of compound 1 showed characteristic absorptions of hydroxy group (3400 cm−1) and conjugated ester carbonyl group (1681 cm−1), as well as those for alkene and/or phenyl ring (1604 and 1514 cm−1) [12, 13]. The UV absorption maxima at 312 and 227 nm suggested the presence of a phenolic acid residue [14]. The 1H NMR spectrum displayed signals attributable to a trans-p-coumaroyl group, with an AA'BB' spin system of a 1,4-disubstituted benzene ring at δH 7.52 (2H, d, J = 8.5 Hz, H-5' and 9') and 6.79 (2H, d, J = 8.5 Hz, H-6' and 8'), as well as a double bond with a trans configuration at δH 6.32 (1H, d, J = 16.0 Hz, H-2') and 7.49 (1H, d, J = 16.0 Hz, H-3'). Also observed in the 1H NMR spectrum were signals of five tertiary methyl groups at δH 0.72 (3H, s, H3-24), 0.85 (3H, s, H3-25), 0.96 (3H, s, H3-27), 1.10 (3H, s, H3-26), and 1.65 (3H, s, H3-30), while resonances at δH 4.84 (1H, dd, J = 11.0 and 5.0 Hz, H-3), δH 2.96 and 3.16 (each 1H, d, J = 10.5 Hz, H-23a and H-23b), as well as δH 3.09 and 3.53 (each 1H, d, J = 10.1 Hz, H-28a and H-28b), were attributed to proton signals attached to an oxygenated methine group and two oxygenated methylene groups, respectively. In addition, the presence of an olefinic methylene was recognized based on the proton resonance at δH 4.68 and 4.55 (each1H, brs, H-29a and H-29b) (Table 1). In the 13C NMR spectrum of 1, besides the nine carbon resonances ascribed to the p-coumaroyl moiety at δC 166.1 (C, C-1'), 114.9 (CH, C-2'), 144.1 (CH, C-3'), 125.1 (C, C-4'), 130.7 (2 × CH, C-2' and 9'), 115.7 (2 × CH, C-6' and 8') and 159.6 (C, C-7'), the remaining 30 carbon signals were classified from DEPT and HSQC analysis into five methyls, ten alkyl methylenes, five alkyl methines, five alkyl quaternary carbons, three oxygen-bearing carbons (including two primary and one secondary) and two alkene carbons belonging to a disubstituted terminal double bond (Table 2). These NMR data suggested that 1 is an (E)-p-coumaroyl ester derived from the pentacyclic lupane-type triterpene, with the skeleton similar to those of the known oxygenated betulin analogues [7, 12, 13, 15].In the HMBC spectrum, besides the correlations with C-4, C-23 and CH3-24, the low-field oxygenated methine proton at δH 4.84 was also found to show a key correlation with C-1', the carbonyl carbon of the coumaroyl moiety. Consequently, it could be deduced that the esterification by a coumaric acid unit occurs at C-3. Furthermore, the oxygenated methylene protons at δH 2.96 and 3.16, were found to show HMBC correlations with C-5 and C-3, respectively, while signals from another set of oxygenated methylene protons at δH 3.09 and 3.53, displayed long range correlations with C-16 and C-22, respectively. Therefore, the positions of the free hydroxy groups were concluded as occurring at C-23 and C-28. In addition, HMBC correlations of the terminal methylene protons of H-29 with CH3-30 and C-19 confirmed the presence of a characteristic isopropene moiety on ring E, which is a typical structural feature of betulin analogues (Fig. 2.). In the NOESY spectrum, key correlation of H-3 with H-5, indicated the β orientation of the coumaryol substituent on C-3. The NOE effects observed from H3-24 to H3-25, as well as from H-23 to H-5, suggested the hydroxyethylene group (C-23) on C-4 to be an α-position. In the same way, the hydroxyethylene group (C-28) on C-17 was inferred as being β oriented based on the NOE resonances of H-28a/H-13 and H3-26. Furthermore, key NOE effects were also observed from H3-25 to H3-26, H3-26 to H-13, H3-27 to H-9 and H-18, as well as H-19 to H-13, which revealed that the relative configuration of compound 1 is consistent with those of known betulin analogues (Fig. 2.). Accordingly, the structure of 1 was elucidated as 3-O-(E)-p-coumaroyl-23-hydroxybetulin.
Table 1.
1H NMR Chemical Shifts of Compounds 1–5a
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1α | 0.91, m | 1.21 b | 1.34 b | 0.93, m | 0.85, m |
| 1β | 1.61 b | 1.48 b | 1.44 b | 1.69 b | 1.65 b |
| 2α | 1.61 b | 1.92 b | 1.90 b | 1.67 b | 1.63 b |
| 2β | 1.68 b | 1.69 b | 1.67 b | 1.67 b | 1.63 b |
| 3 | 4.84, dd (11.0, 5.0) | 4.94, s | 4.85, s | 3.45, t (8.0) | 3.35 b |
| 5 | 1.27 b | 1.37 b | 1.29 b | 1.03 b | 0.91 b |
| 6α | 1.45b | 1.46b | 1.41b | 1.49b | 1.39b |
| 6β | 1.31b | 1.30b | 1.29b | 1.43b | 1.39b |
| 7a | 1.31b | 1.39 b | 1.34 b | 1.43b | 1.31b |
| 7b | 1.49b | 1.48 b | 1.44 b | 1.43b | 1.31b |
| 9 | 1.35b | 1.46 b | 1.38 b | 1.32 b | 1.25 b |
| 11a | 1.38b | 1.46b | 1.44b | 1.42b | 1.39b |
| 11b | 1.19b | 1.22b | 1.20b | 1.21b | 1.20b |
| 12α | 1.04b | 1.11b | 1.10b | 1.05b | 1.04b |
| 12β | 1.59b | 1.66b | 1.65b | 1.65b | 1.63b |
| 13 | 1.59 b | 1.64 b | 1.63 b | 1.65b | 1.62b |
| 15a | 1.69b | 1.71b | 1.71b | 1.70b | 1.68b |
| 15b | 0.91b | 1.08b | 1.05b | 1.08b | 1.04b |
| 16a | 1.93 b | 1.93b | 1.94b | 1.94b | 1.94b |
| 16b | 1.10 b | 1.24b | 1.22b | 1.22b | 1.21b |
| 18 | 1.50 b | 1.60 b | 1.60 b | 1.57 b | 1.57 b |
| 19 | 2.39, m | 2.40, m | 2.39, m | 2.38, td (10.0, 5.8) | 2.38, td (10.3, 5.7) |
| 21α | 1.28b | 1.41b | 1.41b | 1.41b | 1.41b |
| 21β | 1.87b | 1.96b | 1.94b | 1.94b | 1.94b |
| 22α | 0.88b | 1.06b | 1.05b | 1.05b | 1.05b |
| 22β | 1.87b | 1.88b | 1.86b | 1.86b | 1.86b |
| 23a | 2.96, d, (10.5) | 3.34b | 3.35b | 3.88, d, (11.6) | 3.83, d, (12.2) |
| 23b | 3.16, d, (10.5) | 3.40, d, (11.2) | 3.35b | 4.38, d, (11.6) | 4.28, d, (11.4) |
| 24 | 0.72, s | 0.95, s | 0.92, s | 0.81, s | 0.76,s |
| 25 | 0.85, s | 0.92, s | 0.89, s | 0.87, s | 0.84, s |
| 26 | 1.10, s | 1.05 b | 1.02 b | 1.03, s | 1.00, s |
| 27 | 0.96, s | 1.05 b | 1.03 b | 0.98, s | 0.93, s |
| 28a | 3.09, d, (10.1) | 3.34b | 3.35b | 3.34, d, (10.9) | 3.34 b |
| 28b | 3.53, d, (10.1) | 3.82, d, (10.3) | 3.82, d, (10.3) | 3.80, d, (10.9) | 3.80, d, (11.2) |
| 29a | 4.68, brs | 4.70, brs | 4.70, brs | 4.68, brs | 4.67, brs |
| 29b | 4.55, brs | 4.59, brs | 4.59, brs | 4.57, brs | 4.58, brs |
| 30 | 1.65, s | 1.69, s | 1.70, s | 1.67, s | 1.68, s |
| Coumaric acid | |||||
| 1' | |||||
| 2' | 6.32, d, (16.0) | 6.33, d, (15.9) | 5.88, d, (12.6) | 6.27, d, (15.9) | 5.84, d, (12.6) |
| 3' | 7.49, d, (16.0) | 7.66, d, (15.8) | 6.89, d, (12.8) | 7.63, d, (15.9) | 6.89, d, (12.7) |
| 5', 9' | 7.52 (8.5) | 7.44 (8.5) | 7.64 (8.5) | 7.41 (8.6) | 7.61 (8.4) |
| 6', 8' | 6.79, d, (8.5) | 6.84, d, (8.5) | 6.79, d, (8.6) | 6.83, d, (8.6) | 6.80, d, (8.6) |
Measured at 400 MHz; NMR data of compound 1 was obtained in DMSO-d6; NMR data of compounds 2–5 were obtained in CDCl3 with TMS as internal standard; J values (Hz) are given in parentheses. Assignments are based on 1H-1H COSY, HSQC, and HMBC spectroscopic data.
Multiplicity patterns unclear due to signal overlapping.
Table 2.
13C NMR Chemical Shifts of Compounds 1–5a
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 37.6 | 33.8 | 33.7 | 38.6 | 38.4 |
| 2 | 22.9 | 23.0 | 23.0 | 26.0 | 26.0 |
| 3 | 73.6 | 76.1 | 76.4 | 72.8 | 72.7 |
| 4 | 41.3 | 41.2 | 41.1 | 42.5 | 42.5 |
| 5 | 46.2 | 46.0 | 45.8 | 48.5 | 48.3 |
| 6 | 17.1 | 18.2 | 18.3 | 18.2 | 18.2 |
| 7 | 33.2 | 33.7 | 33.7 | 34.0 | 33.9 |
| 8 | 40.4 | 40.9 | 40.9 | 40.9 | 40.9 |
| 9 | 49.7 | 50.3 | 50.3 | 50.6 | 50.5 |
| 10 | 36.2 | 37.1 | 37.0 | 37.1 | 37.0 |
| 11 | 20.4 | 20.7 | 20.7 | 20.9 | 20.8 |
| 12 | 24.8 | 25.1 | 25.2 | 25.2 | 25.2 |
| 13 | 36.7 | 37.2 | 37.3 | 37.3 | 37.3 |
| 14 | 42.2 | 42.8 | 42.8 | 42.7 | 42.7 |
| 15 | 26.7 | 27.0 | 27.0 | 27.0 | 27.0 |
| 16 | 29.0 | 29.2 | 29.2 | 29.2 | 29.2 |
| 17 | 47.4 | 47.7 | 47.8 | 47.8 | 47.8 |
| 18 | 48.2 | 48.8 | 48.8 | 48.8 | 48.8 |
| 19 | 47.3 | 47.8 | 47.8 | 47.9 | 47.8 |
| 20 | 150.3 | 150.4 | 150.5 | 150.4 | 150.4 |
| 21 | 29.3 | 29.7 | 29.8 | 29.7 | 29.8 |
| 22 | 33.8 | 34.0 | 34.0 | 34.0 | 34.0 |
| 23 | 62.8 | 68.9 | 69.0 | 67.0 | 67.0 |
| 24 | 13.2 | 16.8 | 16.9 | 11.9 | 11.9 |
| 25 | 16.2 | 16.4 | 16.4 | 16.7 | 16.6 |
| 26 | 15.7 | 16.0 | 16.0 | 16.0 | 16.0 |
| 27 | 14.5 | 15.0 | 15.0 | 14.8 | 14.7 |
| 28 | 57.9 | 60.6 | 60.6 | 60.6 | 60.6 |
| 29 | 109.6 | 109.7 | 109.7 | 109.8 | 109.7 |
| 30 | 18.8 | 19.1 | 19.1 | 19.1 | 19.1 |
| Coumaric acid | |||||
| 1' | 166.1 | 167.8 | 167.0 | 168.0 | 167.2 |
| 2' | 114.9 | 115.0 | 116.5 | 114.7 | 116.5 |
| 3' | 144.1 | 145.7 | 144.8 | 145.4 | 144.3 |
| 4' | 125.1 | 126.7 | 126.8 | 126.6 | 127.2 |
| 5', 9' | 130.7 | 130.2 | 132.6 | 130.1 | 132.4 |
| 6', 8' | 115.7 | 115.9 | 115.2 | 116.0 | 115.1 |
| 7' | 159.6 | 158.3 | 157.6 | 158.6 | 157.3 |
Measured at 100 MHz; NMR data of compound 1 was obtained in DMSO-d6; NMR data of compounds 2–5 were obtained in CDCl3 with TMS as internal standard. Assignments are based on HSQC, and HMBC NMR spectra.
Fig. 2.
Selected HMBC ( ) and NOESY (
) and NOESY ( ) correlations observed for compound 1.
) correlations observed for compound 1.
The HRESIMS of compound 2 showed a sodiated molecular ion peak at m/z 627.4048, corresponding to a molecular formula of C39H56O5Na, the same as that of compound 1. The NMR spectra of compound 2 were quite comparable with that of compound 1, with the major differences focused on ring A. In the 1H NMR of 1, H-3 appeared at δH 4.94 as a broad singlet, with the coupling pattern very different from that of compound 1, where it was observed as a double doublet with coupling constants of 11.0 and 5.0 Hz (Table 1). This implied that rather than an equatorial β-position in compound 1, the coumaroyl substituent on C-3 in compound 2 adopted an axial α-substitution. Due to this change, a gauche γ-effect on C-1 of compound 1 was caused by the axially oriented coumaroyl group at C-3 [16], which led to an upfield shift of 3.8 ppm for C-1 in the 13C NMR spectrum, when compared with this same signal in compound 1 (Table 2). Furthermore, in the NOESY spectrum, H-3 was observed to show strong correlations with H-24 and H-2β, as well as relatively weak correlations with H-23 and H-2α, which was consistent with its presumed equatorial β-configuration. Thus, the structure of compound 2 was elucidated as 3-O-(E)-p-coumaroyl-23-hydroxy-3-epi-betulin (2), or the C-3 epimer of compound 1.
The molecular formula of compound 3 was determined to be C39H56O5Na, the same as that of compounds 1 and 2, from the sodiated molecular ion peak at m/z 627.4060 in the HRESIMS. A close inspection of the NMR data of compounds 2 and 3 revealed that the major differences were in the coumaroyl residue on C-3. By comparison of the 1H NMR spectrum of 3 with that of 2, the olefinic doublets of H-2' and H-3' of the coumaroyl group were upfield shifted from δH 6.33 and 7.66 to δH 5.88 and 6.89, respectively, with the coupling constant being ~12.7 Hz, smaller than ~16.0 Hz, which was observed for that of compound 2 (Table 1). These notable differences suggested that in compound 3, the double bond of the p-coumaroyl group adopted a Z configuration rather than an E configuration. Further analysis of the 2D NMR of 3 revealed the presence of the comparable HMBC effects and NOE correlations with that of compound 2. Accordingly, the structure of compound 3 was determined as 3-O-(Z)-p-coumaroyl-23-hydroxy-3-epi-betulin (3), a configurational stereoisomer of compound 2.
Compound 4 gave a molecular formula of C39H56O5, the same as that of compounds 1–3, based on the analysis of the HRESIMS. When the 1H NMR data of compound 4 were compared with those of compound 1, the oxygenated methine proton signal assigned to H-3, was shifted upfield from δH 4.84 ppm to δH 3.45 ppm, while the protons of one oxygenated methylene group appeared at relatively lowfield region with chemical shifts of δH 3.88 (1H, d, J = 11.6 Hz) and 4.38 (1H, d, J = 11.6 Hz), respectively (Table 1). In the HMBC spectrum, these two oxygenated methylene protons were observed to have correlations with C-3, C-4, C-24 and the C-1', the carbon of the carbonyl group of the coumaroyl moiety. All this evidence suggested that the esterification with the coumaroyl residue occurred at C-23 in compound 4, instead of on C-3 in compound 1. In addition, the equatorial β position of the free hydroxy group on C-3 was concluded from the coupling pattern of H-3 (t, J = 8.0 Hz), and confirmed by the observed NOE correlations of H-3 with H-23, H-1α as well as H-5. Thus, the structure of compound 4 was elucidated as 23-O-(E)-p-coumaroyl-23-hydroxybetulin (4), a positional isomer of compound 1.
Compound 5 showed comparable NMR spectra to those of 4. The cis configuration of the coumaroyl group was recognized based on the notable upfield shifted olefinic protons (H-2' and H-3') at δH 5.84 and 6.89 ppm, as well as a relatively smaller coupling constant of ~12.7 Hz ascribed to these two characteristic doublets when compared with compound 4 (Table 1). Further HMBC analysis revealed that the (Z)-p-coumaroyl functionality was also located at C-23, the same as in compound 4. Moreover, key NOE effects observed for 5 were similar to those observed for 4. Consequently, compound 5 was designated as 23-O-(Z)-p-coumaroyl-23-hydroxybetulin (5), a configurational stereoisomer of compound 4.
Betulin derived lupane-type triterpenes are widely distributed throughout the plant kingdom. A variety of biological activities, including cytotoxicity, antiviral and antibacterial activities, anti-inflammatory activity, and in vitro antimalarial effect, have been ascribed to certain betulin derivatives [17]. The natural occurring of 3-epi-betulin derived triterpenes is much less common than the normal ones, and plant family Celastraceae seems to be one of the major resource containing 3-epi-betulin derivatives [18, 19]. When considering the natural occurring esterification of the hydroxy groups of these betulin-type triterpenes, cinnamon acid derived substitution is not very common and which is more often happened on C-3 and C-28 rather than on other positions of the skeleton. The co-occurrence of E- and Z-isomers of the coumaryl moieties in present study may raise the question that if the E/Z-isomerization was induced by light in vitro. During the isolation procedure, the purification of these compounds always lasted for several days with the samples exposed to natural light at room temperature, but no notable change has been detected for the ratio of E/Z- coumaryl isomers by HPLC analysis. In addition, for pure compounds 1–5, which dissolved in deuterium DMSO or CDCl3 and stored in room temperature without light prevention for over 72 hours, no alteration were observed between E/Z- coumaryl isomers. Thus, in present study, the co-occurrence of the E/Z-isomers of the coumaryl moieties of the triterpens in Buxus cochinchinensis is more likely derived from a light-independent isomerization, as same as that reported for plants Perrottetia arisanensis and Strychnos vanprukii Craib [18, 20]. All pure compounds obtained in the present investigation were evaluated for their cytotoxic activity against the HT-29 human colon cancer cell line. The known Buxus alkaloid N-3-benzoyldihydrocyclomicrophylline F (7) and the new compound, 3-O-(Z)-p-coumaroyl-23-hydroxy-3-epi-betulin (3), were found to be the most cytotoxic agents against HT-29 cells in present study, with ED50 values of 1.9 µM and 3.3 µM, respectively. In addition, marginal cytotoxic effects were observed for 3-O-(E)-p-coumaroyl-23-hydroxy-3-epi-betulin (2) and 23-O-(Z)-p-coumaroyl-23-hydroxybetulin (5). In this bioassay, triterpenes with a (Z)-p-coumaroyl group exhibited more potent activity than the (E)-p-coumaroyl triterpene esters (Table 3).
Table 3.
| Compound | HT-29c | NF-κB (p65)d | P. falciparume |
|---|---|---|---|
| 1 | > 20 | > 20 | 0.65 ± 0.06 |
| 2 | 13.2 | > 20 | 1.28 ± 0.13 |
| 3 | 3.3 | > 20 | 1.02 ± 0.09 |
| 4 | > 20 | > 20 | 0.26 ± 0.01 |
| 5 | 18.4 | > 20 | 0.63 ± 0.02 |
| 6 | > 20 | > 20 | > 10 |
| 7 | 1.9 | > 20 | 2.07 ± 0.13 |
| 8 | > 20 | > 20 | > 10 |
| 9 | > 20 | 4.0 | 1.33 ± 0.37 |
| Paclitaxelf | 0.001 | - | - |
| Rocaglamideg | - | 0.08 | - |
| Artemisininh | - | - | 0.0007 |
No obvious growth inhibition of C. albicans cells were observed for the test compounds at a concentration of 40 mg/mL.
Results are expressed as ED50 or IC50 values (µM).
Compounds with ED50 values of > 20 µM in HT-29 cells are considered inactive.
Compounds with IC50 values of > 20 µM on an enzyme-based ELISA NF-κB (p65) assay are considered inactive.
Compounds with IC50 values of > 10 µM in P. falciparum assay are considered inactive.
Used as a positive control substance for the cytotoxicity assay.
Used as a positive control substance for the cytotoxicity assay.
Used as a positive control substance for the P. falciparum assay.
An ELISA NF-κB assay was also employed to evaluate the p65 (Rel A) inhibitory activity of all the isolates, most of which were considered inactive with their IC50 values of >20 µM in this assay, except for the semi-synthetic compound 23-hydroxybetulin (IC50 = 4.0 µM). This result implied that the occurrence of a free hydroxy group at C-3, C-23 and C-28 in the molecule might be important for the observed NF-κB inhibitory activity ascribed to 23-hydroxybetulin.
Furthermore, the drug-resistant Dd2 strain of Plasmodium falciparum and a pathogenic strain of Candida albicans (ATCC® 18804TM) was used to evaluate the antiplasmodial and antifungal effects of all the isolates, respectively. No obvious growth inhibition of C. albicans cells were observed for the test compounds at a concentration of 40 µg/mL.
In the P. falciparum assay, all the new compounds (1–5), along with the known Buxus alkaloid, N-benzoyldihydrocyclomicrophylline F (7), were found to show significant in vitro antimalarial activities, with IC50 values ranging from 0.26 to 2.07 µM (Table 3). To gain some information on the structure-activity relationships, two semi-synthetic compounds, 3,28-O-diacetylbetulin and 23-hydroxybetulin, which were derived from betulin and compound 5 respectively, were also subjected to the same bioassay. The test results showed that 3,28-O-diacetylbetulin, the diacetate derivative of betulin was inactive (IC50 >20 µM), while 23-hydroxybetulin, the hydrolysis product of compound 5, retained antimalarial potency, with an IC50 value of 1.33 ± 0.37 µM. Although belutin triterpenes have been reported to possess antimalarial activities, the in vitro tests of these analogues against the parasitic strains to date are not quite significant. According to previously studies, betulinic acid exhibited in vitro antiplasmodial activities against Plasmodium falciparum strains K1, T9–96 and 3D7, with IC50 values ranging from 19.0 µM to 56.8 µM, and its methylate showed in vitro antimalarial activity agains P. falciparum strain 3D7 with an IC50 value of 7.0 µm [21–23]. All this evidence suggested that the substitutution of a hydroxy group or a coumaroyl ester group at C-23 plays an important role for the observed antiplasmodial activities ascribed to these betulin derivatives. Moreover, esterification at C-3 or C-23 by coumaroyl group seems to slightly increase the resultant activity, while 3-epi betulin analogues were found be less potent than the normal 3β hydroxy derivatives.
Although many Buxus plants have documented ethnomedicinal uses for the treatment of malaria [3, 4], and some plant extracts of in this genus have also been reported to show antimalarial activities in in vitro and/or in vivo studies [24, 25], the chemical types of the active principles that are responsible for the antiplasmodial effects are incompletely understood. As the major constituents of boxwood species, Buxus alkaloids have been proposed as the most likely active constituents for the antimalarial activity ascribed to these plants. However, to the best of our knowledge, thus far, only the known cycloartane alkaloid O-tigloylcyclovirobuxeine-B, isolated from B. sempervirens, has been reported to show in vitro antiplasmodial activity against Plasmodium falciparum, with an IC50 value of 0.92 µM (0.46 µg/mL) [26]. The present investigation has revealed that besides the well-known Buxus alkaloids, lupane triterpenoids based on 23-hydroxybetulin, are another class of constituents potentially responsible for the antimalarial properties exhibited by plants of the genus Buxus.
Materials and Methods
General experimental procedures
Optical rotations were recorded on a Perkin-Elmer 343 automatic polarimeter (PerkinElmer). UV spectra were measured on a Hitachi U-2910 spectrophotometer (Hitachi). NMR spectroscopic data were obtained on a Bruker Avance DRX-400 spectrometer (Bruker), using standard Bruker pulse sequences (room temperature), and processed with Topspin™ 3.1 (Bruker BioSpin). High-resolution electrospray ionization mass spectra (HRESIMS) were performed on a Micromass Q-Tof™ (Micromass) mass spectrometer (calibration with sodium iodide). Analytical TLC was carried out with precoated 250 µm thickness silica gel UV254 aluminum-backed plates (Sorbent Technologies), and preparative TLC was carried out with precoated 500 µm thickness silica gel UV254 glass-backed plates (Sorbent Technologies). Column chromatography was conducted on silica gel (230–400 mesh; Sorbent Technologies). The HPLC was carried out on a Waters system comprised of a 600 controller, a 717 Plus autosampler, and a 2487 dual wavelength absorbance detector. Waters Xbridge® (4.6 × 150 mm), semi-preparative (10 × 150 mm), and preparative (19 × 150 mm) C18 (5 µm) columns were used for analytical, semi-preparative and preparative HPLC, respectively.
Plant material
A combination of the leaves, twigs and fruits of Buxus cochinchinensis Pierre ex Gagnep. (Buxaceae) was collected in Nui Chua National Park, Ninh Thuan Province, Vietnam (11°41.128' N; 109°09.694' E) by DDS, TNN, and Bui Van Thanh, on 24 July, 2011, who also identified this plant. A voucher specimen (original collection Soejarto et al. 14857) has been deposited in the John G. Searle Herbarium of the Field Museum of Natural History (under accession number FM-2300811), Chicago, Illinois.
Extraction and isolation
A combination of the dried and milled leaves, twigs and fruits of Buxus cochinchinensis (5 kg) was macerated overnight using 95% MeOH at room temperature (3 × 6 L). This crude extract was concentrated under reduced pressure to yield 750 g of thick dark brown syrup, which was partitioned sequentially with hexane (3 × 2 L) and chloroform (3 × 2 L). The chloroform partition was washed with 1% saline solution (3 L) to yield 150 g of a partially detannified chloroform-soluble extract, which was found to be active against the HT-29 cell line (ED50 = 12.0 µg/mL). Accordingly, part of this fraction (140 g) was subjected to separation over a Si gel column (7 × 50 cm), with a gradient elution system CH2Cl2–acetone (20:1, 15:1, 10:1, 8:1, 6:1, 4:1, 3:1, 2:1, 1:1 and pure acetone, 4L per gradient), to yield 17 fractions (F01–F17). A substantial amount of a white precipitate formed from a non-cytoxic subfraction F03 (15:1, 23.0 g) when it was dissolved in a mixture of CHCl3–methanol (3:1). Betulin (6) (1.0 g, purity > 95%) was obtained after the recrystallization of this precipitate from the same solvent. Fractions F06 (10:1) and F12 (3:1) were found to be the most active against the HT-29 cells (ED50 = 8.0 and 6.3 µg/ml, respectively) among these sub-fractions obtained. Fraction F06 (2.5 g) was chromatographed over an open C18-reversed phase column (2 × 20 cm) with a MeOH-H2O gradient solvent system (50:50, 60:40, 70:30, 80:20, and 90:10, 200 mL per gradient), to yield 17 pooled subfractions (F601– F617). Subfractions F610 (80:20, 100 mg) and F611 (80:20, 110 mg) were detected by TLC as terpene-rich subfractions, and were combined and subjected to separation by preparative TLC (20 × 20 cm, 500 µm × 2), developed by CHCl3–acetone (5:1, 200 mL for each plate), to yield compound 4 (Rf = 0.7, 12.0 mg, purity > 98%), and three subfractions designated as F6(10–11)A–D. Subfraction F6(10–11)C (30 mg) was chromatographed on a semi-preparative RP-18 column (150 mm × 10 mm i.d.) by HPLC, using a gradient CH3CN-H2O solvent system [50:50 to 100% CH3CN (both solvent with 0.1 formic acid) in 20 min; flow rate 6.0 mL/min], to afford compound 5 (15 mg, tR = 11.9 min, purity > 95%). Subfraction F6(10–11)D (33 mg) was purified on the same semi-preparative RP-18 column by HPLC, using a gradient CH3CN-H2O solvent system [60:40 to 100% CH3CN in 17 min; flow rate 5.0 mL/min], to afford compounds 2 (6.0 mg, tR = 15.2 min, purity > 95%) and 3 (7.0 mg; tR = 15.8 min, purity > 95%), respectively. Compound 1 (15 mg, purity > 97%) was precipitated from the parent solution of subfraction F615 (90:10, 35 mg) and recrystallized from a mixture of MeOH-H2O.
Fraction F12 (1.2 g) was subjected to passage over a Sephadex LH-20 (2 × 35 cm) and eluted with 100% MeOH (600 mL) to furnish five pooled subfractions (F1201– F1205). Subfraction F1204 (200 mg) which gave a positive test for Dragendorff’s reagent, was subjected to separation over a Si gel column (1 × 40 cm) with gradient elution system CHCl3–MeOH (20:1, 10:1, 5:1and 2:1, 200 mL per gradient), to afford the known alkaloid N-benzoyldihydrocyclomicrophylline F (7) (2:1, 5.0 mg).
3-O-(E)-p-coumaroyl-23-hydroxybetulin (1): white powder; m.p. 199–200°C; [α]20D +32.0 (c 0.06, MeOH); UV (MeOH) λmax (log ε) 203 (4.23), 227 (4.08), 312 (4.34) nm; IR (film) νmax 3400, 2942, 2859, 1681, 1632, 1604, 1514, 1450, 1369, 1168, 1012, 831, 756 cm−1; 1H NMR (400 MHz, DMSO) and 13C NMR (150 MHz, DMSO) data, see Tables 1 and 2; HRESIMS m/z 627.4028 [M + Na]+ (calcd for C39H56O5Na, 627.4025).
3-O-(E)-p-coumaroyl-23-hydroxy-3-epi-betulin (2): white powder; m.p. 180–182°C; [α]20D +5.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 205 (4.23), 226 (4.10), 312 (4.34) nm; IR (film) νmax 3350, 2945, 2854, 1681, 1631, 1604, 1514, 1450, 1368, 1167, 1025, 831, 756 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables 1 and 2; HRESIMS m/z 627.4048 [M + Na]+ (calcd for C39H56O5Na, 627.4025).
3-O-(Z)-p-coumaroyl-23-hydroxy-3-epi-betulin (3): white powder; m.p. 165–167°C; [α]20D −26.5 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (4.22), 310 (4.19) nm; IR (film) νmax 3352, 2944, 2859, 1688, 1604, 1514, 1451, 1376, 1167, 1026, 831, 756 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables 1 and 2; HRESIMS m/z 627.4060 [M + Na]+ (calcd for C39H56O5Na, 627.4025).
23-O-(E)-p-coumaroyl-23-hydroxybetulin (4): white powder; m.p. 165–167°C; [α]20D +3.0 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (4.07), 228 (3.95), 313 (4.24) nm; IR (film) νmax 3370, 2940, 2864, 1687, 1632, 1604, 1514, 1450, 1381, 1167, 1022, 831, 755 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables 1 and 2; HRESIMS m/z 627.4023 [M + Na]+ (calcd for C39H56O5Na, 627.4025).
23-O-(Z)-p-coumaroyl-23-hydroxybetulin (5): white powder; m.p. 162–163°C; [α]20D +51 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 204 (4.10), 311 (4.08) nm; IR (film) νmax 3374, 2942, 2870, 1691, 1604, 1514, 1451, 1376, 1166, 1023, 832, 756 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (150 MHz, CDCl3) data, see Tables 1 and 2; HRESIMS m/z 627.4054 [M + Na]+ (calcd for C39H56O5Na, 627.4025).
Preparation of 3,28-O-diacetylbetulin (8) from betulin (6)
Betulin (6) (18 mg) was dissolved in a mixture of pyridine (1.0 mL) and acetic anhydride (1.0 mL). After being stirred overnight at room temperature, the excess pyridine and acetic anhydride were evaporated to yield nearly 19 mg of 3,28-O-diacetylbetulin (8) (putity > 98%). This compound was obtained as white powder [m.p. 222–223°C (lit. m.p. 223–224°C)], and spectroscopic data (NMR and MS) were consistent with published values [10].
Preparation of 23-hydroxybetulin (9) from 23-O-(Z)-p-coumaroyl-23-hydroxybetulin (5)
23-O-(Z)-p-coumaroyl-23-hydroxybetulin (5) (2.0 mg) was dissolved in 0.3 mL MeOH, then 0.3 mL of LiOH·H2O (5 mg LiOH dissolved in 0.3 mL of H2O) was added. After being stirred overnight at room temperature, 3 mL of saturated aqueous solution of NaCl were added to this mixture, and which was further partitioned with CHCl3. The organic phase was evaporated under reduced pressure after washing with water to give 23-hydroxybetulin (9) (1.4 mg, purity > 95%). This compound was obtained as white powder [m.p. 258–260°C (lit. m.p. 260–262°C)], and spectroscopic data (NMR and MS) were consistent with published values [11].
Cytotoxicity assay
The cytotoxic activity of extracts, chromatographic fractions of extracts, and all pure compounds were evaluated against human colon cancer (HT-29) cell line, according to a previously described protocol [27]. Paclitaxel (Sigma-Aldrich, ≥ 97%) was used as positive control substance.
Enzyme-based ELISA NF-κB assay
The NF-κB p65 subunit inhibitory activity of pure compounds was tested in an ELISA NF-κB assay, which was carried out according to a published protocol [28, 29]. Rocaglamide (Enzo Life Sciences, ≥ 97%) was used as a positive control substance.
Antimalarial bioassay
The effects of compounds 1–6 on Plasmodium falciparum Dd2 parasite growth was measured in a 72 h growth assay, as described previously with minor modifications [30–32]. Artemisinin (Sigma-Aldrich, ~ 98%) was used as positive control substance.
Antifungal bioassay
A pathogenic strain of Candida albicans (ATCC® 18804™) was used to evaluate the antifungal effects of all the isolates, according to a published protocol [33]. The antibiotic, amphotericin B (Sigma-Aldrich, ~ 80%), was used as a positive control substance in this bioassay, which showed 100% growth inhibition of C. albicans cells at a concentration of 0.2 mg/mL.
Supplementary Material
Acknowledgment
This study was supported by grant P01 CA125066 (awarded to A.D. Kinghorn) from NCI, NIH. We are grateful to Dr. Craig McElroy, College of Pharmacy, The Ohio State University, for facilitating the acquisition of the NMR spectroscopic and mass spectrometric data measurements. The plant material was collected under the terms and conditions of a Memorandum of Agreement between the University of Illinois at Chicago and the Institute of Ecology and Biological Resources (IEBR) of the Vietnam Academy of Science and Technology, Hanoi, Vietnam. Thanks are expressed to the Director of Nui Chua National Park for permission, and to the Director of IEBR for overseeing the field operation in the collection of the plant.
Footnotes
The 1H, 13C and 2D NMR spectra of the new compounds 1–5, as well as the 1H NMR spectra of two semisynthetic compounds, are available in the Supporting Information.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Batdorf LR. Boxwood: an Illustrated Encyclopedia. Boyce, VA: American Boxwood Society; 2004. [Accessed February 19, 2015]. pp. 1–343. Hawaii Book Library website, available online at http://www.hawaiilibrary.net/article/whebn0000334850/buxus. [Google Scholar]
- 2. [Accessed July 23 2014]; Available on line at https://www.botanical.com/botanical/mgmh/b/box---67.html. [Google Scholar]
- 3.Atta-ur-Rahman, Choudhary MI. Chemistry and biology of steroidal alkaloids. In: Cordell GA, editor. The Alkaloids. Vol. 50. San Diego: Academic Press; 1998. pp. 61–108. [Google Scholar]
- 4.Ata A, Andersh BJ. Buxus steroidal alkaloids: chemistry and biology. Alkaloids Chem Biol. 2008;66:191–213. doi: 10.1016/s1099-4831(08)00203-4. [DOI] [PubMed] [Google Scholar]
- 5.Yan YX, Sun Y, Li ZR, Zhou L, Qiu MH. Chemistry and biological activities of Buxus alkaloids. Curr Bioact Compd. 2011;7:47–64. [Google Scholar]
- 6.Julius A. Buxus holttumiana of Peninsular Malaysia and Thailand is a variety of B. cochinchinensis (Buxaceae) Phyotaxa. 2014;167:201–204. [Google Scholar]
- 7.Mahato SB, Kundu AP. Review article number 98: 13C NMR spectra of pentacyclic triterpenoids - a compilation and some salient features. Phytochemistry. 1994;37:1517–1575. [Google Scholar]
- 8.Kupchan SM, Kennedy RM, Schleigh WR, Ohta G. Buxus alkaloids. XII. Benzamide alkaloids from Boxus sempervirens. Tetrahedron. 1967;23:4563–4586. doi: 10.1016/s0040-4020(01)92557-8. [DOI] [PubMed] [Google Scholar]
- 9.Sangare M, Khuong-Huu F, Herlem D, Millie A, Septe B, Berenger G, Lukacs G. Revision of the configuration of the C-4 hydroxymethylene group in Buxus alkaloids by 13C NMR spectroscopy. Tetrahedron Lett. 1975;22/23:1791–1794. [Google Scholar]
- 10.Symon AV, Veselova NN, Kaplun AP, Vlasenkova NK, Fedorova GA, Lyutik AI, Gerasimova GK, Shvets VI. Synthesis and antitumor activity of cyclopropane derivatives of betulinic and betulonic acids. Russ J Bioorg Chem. 2005;31:286–291. doi: 10.1007/s11171-005-0039-z. [DOI] [PubMed] [Google Scholar]
- 11.Dracinsky M, Richtr V, Krecek V, Sejbal J, Klinot J, Budesinsky M. Triterpenes. Part CXIII. Preparation and conformational study of 19β,28-epoxy-18α-olean-5-ene derivatives. Collect Czech Chem Commun. 2006;71:387–410. [Google Scholar]
- 12.Chang CI, Kou YH. Two new lupane-type triterpenes from Diospyros maritima. J Nat Prod. 1999;62:309–310. doi: 10.1021/np980217v. [DOI] [PubMed] [Google Scholar]
- 13.Chumkaew P, Kato S, Chantrapromma K. A new triterpenoid ester from the fruits of Bruguiera parviflora. Chem Pharm Bull. 2005;53:95–96. doi: 10.1248/cpb.53.95. [DOI] [PubMed] [Google Scholar]
- 14.Robbins RJ. Phenolic acids in foods: an overview of analytical methodology. J Agric. Food Chem. 2003;51:2866–2887. doi: 10.1021/jf026182t. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero-Analco JA, Martineau L, Saleem A, Madiraju P, Muhammad A, Durst T, Haddad P, Arnason JT. Bioassay-guided isolation of the antidiabetic principle from Sorbus decora (Rosaceae) used traditionally by the Eeyou Istchee Cree First Nations. J Nat Prod. 2010;73:1519–1523. doi: 10.1021/np1003005. [DOI] [PubMed] [Google Scholar]
- 16.Schneider HJ, Volker H. Carbon-13 nuclear magnetic resonance substituent-induced shieldings and conformational equilibria in cyclohexanes. J Org Chem. 1978;43:3866–3873. [Google Scholar]
- 17.Alakurtti S, Maekelae T, Koskimies S, Yli-Kauhaluoma J. Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci. 2006;29:1–13. doi: 10.1016/j.ejps.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Du YC, Lin AS, Wu CC, Hsieh PW, Chen YH, Chen IH, Chen SL, Yen HF, Lubken T, Chang FR, Wu YC. New cytotoxic lupane triterpenes from Perrottetia arisanensis. Planta Med. 2009;75:848–855. doi: 10.1055/s-0029-1185438. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Li CJ, Yang JZ, Ma J, Li Y, Bao XQ, Chen XG, Zhang D, Zhang DM. Lupane triterpenoids from the stems of Euonymus carnosus. J Nat Prod. 2014;77:276–284. doi: 10.1021/np400851k. [DOI] [PubMed] [Google Scholar]
- 20.Chien NQ, Hung NV, Santarsiero BD, Mesecar AD, Cuong NM, Soejarto DD, Pezzuto JM, Fong HHS, Tan GT. New 3-O-acyl betulinic acids from Strychnos vanprukii Craib. J Nat Prod. 2004;67:994–998. doi: 10.1021/np030469i. [DOI] [PubMed] [Google Scholar]
- 21.Steele JCP, Warhurst DC, Kirby GC, Simmonds MSJ. In vitro and in vivo evaluation of betulinic acid as an antimalarial. Phytother Res. 1999;13:115–119. doi: 10.1002/(SICI)1099-1573(199903)13:2<115::AID-PTR404>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler HL, Franzyk H, Sairafianpour M, Tabatabai M, Tehrani MD, Bagherzadeh K, Hagerstrand H, Staerk D, Jaroszewski JW. Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: structure-activity relationships for betulinic acid analogues. Bioorg Med Chem. 2004;12:119–127. doi: 10.1016/j.bmc.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Duker-Eshun G, Jaroszewski JW, Asomaning WA, Oppong-Boachie F, Christensen SB. Antiplasmodial constituents of Cajanus cajan. Phytother Res. 2004;18:128–130. doi: 10.1002/ptr.1375. [DOI] [PubMed] [Google Scholar]
- 24.Esmaeili S, Naghibi F, Mosaddegh M, Sahranavard S, Ghafari S, Abdullah NR. Screening of antiplasmodial properties among some traditionally used Iranian plants. J Ethnopharmcol. 2009;121:400–404. doi: 10.1016/j.jep.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Orhan IE, Erdem SA, Senol FS, Kartal M, Sener B. Exploration of cholinesterase and tyrosinase inhibitory, antiprotozoal and antioxidant effects of Buxus sempervirens L. (boxwood) Ind Crops Prod. 2012;40:116–121. [Google Scholar]
- 26.Althaus J, Jerz G, Winterhalter P, Kaiser M, Brun R, Schmidt TJ. Antiprotozoal activity of Buxus sempervirens and activity-guided isolation of O-tigloylcyclovirobuxeine-B as the main constituent active against Plasmodium falciparum. Molecules. 2014;19:6184–6201. doi: 10.3390/molecules19056184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan L, Kardono LBS, Riswan S, Chai HB, Carcache de Blanco EJ, Pannell CM, Soejarto DD, McCloud TG, Newman DJ, Kinghorn AD. Isolation and characterization of minor analogues of silvestrol and other constituents from a large-scale recollection of Aglaia foveolata. J. Nat. Prod. 2010;73:1873–1878. doi: 10.1021/np100503q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFκ:B. Nucleic Acids Res. 2001;29:e21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng Y, Balunas MJ, Kim JA, Lantvit DD, Chin YW, Chai HB, Sugiarso S, Kardono LBS, Fong HHS, Pezzuto JM, Swanson SM, Carcache-Blanco EJ, Kinghorn AD. Bioactive 5,6-dihydro-alpha-pyrone derivatives from Hyptis brevipes. J Nat Prod. 2009;72:1165–1169. doi: 10.1021/np9001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett TN, Paguio M, Gligorijevic B, Seudieu C, Kosar AD, Davidson E, Roepe PD. Novel, rapid, and inexpensive cell-based quantification of antimalarial drug efficacy. Antimicrob Agents Chemother. 2004;48:1807–1810. doi: 10.1128/AAC.48.5.1807-1810.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai Y, Harinantenaina L, Bowman JD, Da Fonseca IO, Brodie PJ, Goetz M, Cassera MB, Kingston DGI. Isolation of antiplasmodial anthraquinones from Kniphofia ensifolia and synthesis and structure–activity relationships of related compounds. Bioorg Med Chem. 2014;22:269–276. doi: 10.1016/j.bmc.2013.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard. Vol. 17. Wayne, PA: NCCLS document M27-A; 1997. pp. 1–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.