Summary
The Tet family of methylcytosine dioxygenases (Tet1, Tet2 and Tet3) convert 5-methylcytosine to 5-hydroxymethylcytosine. To date, functional overlap among Tet family members has not been examined systematically in the context of embryonic development. To clarify the potential for overlap among Tet enzymes during development, we mutated the zebrafish orthologs of Tet1, Tet2 and Tet3, and examined single, double and triple mutant genotypes. Here, we identify Tet2 and Tet3 as the major 5-methylcytosine dioxygenases in the zebrafish embryo and uncover a combined requirement for Tet2 and Tet3 in hematopoietic stem cell (HSC) emergence. We demonstrate that Notch signaling in the hemogenic endothelium is regulated by Tet2/3 prior to HSC emergence and show that restoring expression of the downstream gata2b/scl/runx1 transcriptional network can rescue HSCs in tet2/3 double mutant larvae. Our results reveal essential, overlapping functions for tet genes during embryonic development and uncover a requirement for 5hmC in regulating HSC production.
Introduction
In vertebrate species, the epigenetically modified base 5-methylcytosine (5mC) is associated with transcriptional repression and is essential for normal development (Goll and Bestor, 2005). The mechanisms that establish and maintain 5mC are well defined; but less is known about how 5mC is removed (Wu and Zhang, 2011). The ten eleven translocation proteins (Tet1, Tet2 and Tet3) comprise a family of 2-oxoglutarate and Fe(II) dependent dioxygenases that convert 5mC to 5-hydroxymethylcytosine (5hmC) and its oxidative derivatives 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) (He et al., 2011; Tahiliani et al., 2009). Growing evidence suggests that conversion of 5mC to 5hmC and its derivatives can provide a first step toward DNA demethylation through active base excision or passive dilution of oxidized bases (Kohli and Zhang, 2013). Consistent with a role in regulating gene expression through DNA demethylation, 5hmC levels are most abundant in euchromatic regions including transcription start sites, enhancers and exons (Pastor et al., 2011; Stroud et al., 2011; Williams et al., 2011).
Individually, homozygous mutation of Tet1, Tet2 or Tet3 is compatible with mouse embryonic development, although Tet3 mutant mice die perinatally (Kohli and Zhang, 2013). Conditional deletion of Tet3 in oocytes results in delayed demethylation of the paternal genome and increased developmental failure, but viable pups can be recovered (Gu et al., 2011; Inoue et al., 2015). In contrast to single mutants, embryonic stem cells (ESCs) mutated for all three Tet genes contribute poorly to chimeras, suggesting that Tet family members have overlapping functions in promoting embryonic development (Dawlaty et al., 2014). Tet1/2 double homozygous mutant mice can survive into adulthood; but other Tet mutant combinations have yet to be described (Dawlaty et al., 2013).
Tet regulation appears to be of particular importance in the hematopoietic lineage. TET1 was first identified as a fusion partner of the mixed lineage leukemia (MLL) gene in acute myeloid leukemia and has essential oncogenic roles in MLL rearranged leukemias (Huang et al., 2013; Lorsbach et al., 2003). Moreover, Tet1 mutations promote increased self-renewal of progenitor B cells and susceptibility to B cell lymphoma in mice (Cimmino et al., 2015). Mutations in TET2 are common in human myeloid malignancies and Tet2 mutation promotes myeloid transformation in mice and zebrafish (Gjini et al., 2014; Ko et al., 2011; Kunimoto et al., 2012; Li et al., 2011b; Moran-Crusio et al., 2011; Quivoron et al., 2011; Shide et al., 2012; Solary et al., 2014). Consistent with a role in promoting myeloid malignancy, Tet2 mutation causes increased numbers of hematopoietic progenitor cells in the bone marrow and skewed differentiation toward the myelomonocytic lineage in mice (Ko et al., 2015). Similarly, human cord blood cells depleted for TET2 and cells isolated from leukemia patients bearing TET2 mutations exhibit an increase in myeloid-lineage differentiation at the expense of the erythroid-lineage (Madzo et al., 2014; Pronier et al., 2011). Recently, mutation of Tet3 was shown to cause minor decreases in the absolute number of hematopoietic stem cells (HSCs) in the mouse bone marrow, while numbers of myeloid, erythroid and B lymphoid cells were unaffected (Ko et al., 2015).
While the importance of Tet regulation in the adult hematopoietic system is clear, less is known about requirements for Tet genes during early stages of hematopoietic development. Reports using antisense morpholino depletion in zebrafish and shRNA depletion in human ESCs have implicated Tet2 in the regulation of primitive hematopoiesis; but, these results are at odds with the normal primitive hematopoiesis observed in Tet2 mutant mice and zebrafish (Ge et al., 2014; Gjini et al., 2014; Ko et al., 2015; Langlois et al., 2014). The de novo generation of HSCs during the definitive wave of hematopoiesis also appears normal in Tet2 mutant mouse and zebrafish embryos; however, the potential for additional Tet enzymes to contribute to HSC emergence during embryonic development has not been experimentally addressed in mutant animals (Gjini et al., 2014; Ko et al., 2015).
The zebrafish genome encodes single well-conserved orthologs of Tet1, Tet2 and Tet3 (Almeida et al., 2012). To define the requirements for these genes during development, we generated stable lines carrying mutations in each of the zebrafish tet orthologs and we derived single, double and triple homozygous mutant larvae representing all genetic combinations. In this study, we demonstrate that Tet2 and Tet3 are the major 5mC dioxygenases in the zebrafish embryo and that they have overlapping functions in promoting normal development. In addition, we describe a combined requirement for tet2 and tet3 in the de novo generation of HSCs in the embryo, and demonstrate the importance of tet2/3 for Notch signaling and downstream expression of the gata2b/scl/runx1 transcriptional program in the hemogenic endothelium. Our results provide a comprehensive analysis of tet mutants in the developing zebrafish embryo and identify requirements for Tet regulation in the early function of the hemogenic endothelium. These results underscore the importance of epigenetic regulation for the generation of HSCs and identify regulation of 5hmC as an additional variable to be considered in therapeutic applications such as the in vitro differentiation of HSCs from pluripotent precursors.
Results
TALEN induced mutations reveal overlapping requirements for tet2 and tet3 in the zebrafish embryo
To systematically define requirements for 5hmC during development, we introduced mutations into the zebrafish orthologs of Tet1, Tet2 and Tet3 using TAL effector nucleases (TALENs) (Li et al., 2011a; Sander et al., 2011). RNAs encoding TALENs that targeted each gene were separately injected into one-cell stage embryos and we recovered individuals harboring germline transmissible mutations in each of the three genes. The recovered tet2mk17 allele deletes 4 base pairs in exon 8. This deletion results in a frame shift, causing early termination one amino acid 3’ of an essential iron-binding residue (Figure 1A and 1B, Figure S1D-S1F). The tet1mk16 and tet3mk18 alleles harbor 4 and 14 base pair deletions, respectively, in the last coding exon. In addition to causing frame shift and premature termination, these deletions eliminate sequence encoding a C-terminal arginine residue that is required for 2-oxoglutarate binding (Hu et al., 2013) (Figure 1A and 1B, Figure S1A-C and S1G-I). Loss of specific residues involved in cofactor binding and catalysis is predicted to similarly compromise the dioxygenase activity of all three enzymes.
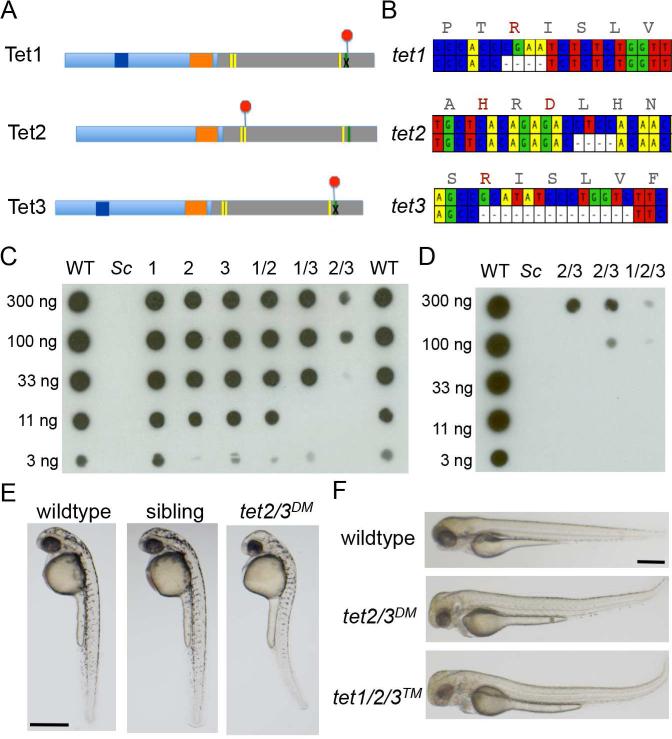
Figure 1. Mutation of zebrafish tet1, tet2 and tet3.
(A) Schematic illustrating early termination caused by TALEN mutations in zebrafish tet1, tet2 and tet3. Red octagons indicate the position of early termination signals. Yellow bars indicate conserved iron binding residues, the green bar indicates the arginine required for 2-oxoglutarate binding.
(B) Schematic depicting deleted bases in zebrafish tet1, tet2 and tet3. Corresponding amino acid sequences for the wild-type allele are included, with residues required for cofactor binding or catalysis indicated in red.
(C) Dot blot for 5hmC on genomic DNA isolated from larvae at 5 dpf. Numbers indicate the mutated tet gene(s) in each sample. Sc indicates DNA isolated from Saccharomyces cerevisiae. Horizontal rows depict 3-fold serial dilutions of DNA.
(D) Dot blot for 5hmC on genomic DNA isolated from larvae at 5 dpf including DNA isolated from tet1/2/3™ larvae.
(E) Lateral views of a representative wild-type larva, a tet2/3DM larva and a sibling larva derived from a tet2mk17/mk17, tet3mk18/+ intercross at 36 hpf.
(F) Lateral views of a representative wild-type larva, a tet2/3DM larva and tet1/2/3™ larva at 3 dpf. All scale bars indicate 500 μM. See also Figure S1.
Modest (less than 3-fold) reductions in total 5hmC were observed in larvae that were homozygous mutant for tet1, tet2, or tet3 (Figure 1C). More considerable reductions in 5hmC were observed in double mutants, with the tet2mk17/mk17, tet3mk18/mk18 double mutant (tet2/3DM) combination producing the most dramatic decrease (>30-fold) (Figure 1C). 5hmC levels were further reduced in tet1/2/3 triple mutant (tet1/2/3™) larvae; indicating that all three mutated genes encode proteins that are compromised for catalytic activity (Figure 1D). The enhanced loss of 5hmC in tet2/3DM larvae compared to other double mutant combinations argues that Tet2 and Tet3 are the predominant 5mC oxidases in the zebrafish embryo and that they function redundantly to promote the formation of 5hmC during development.
Zebrafish that were homozygous for mutations in tet1, tet2 or tet3 were viable to adulthood, as were tet1/2 and tet1/3 double homozygous mutants. In contrast, combined mutation of tet2 and tet3 was not compatible with survival beyond the larval period. The tet2/3DM larvae were morphologically indistinguishable from wild-type controls during the first 24 hours post fertilization (hpf), but subtle abnormalities in brain development emerged on the second day post fertilization (dpf). By 36 hpf, smaller eyes, abnormal brain morphology, altered pigmentation and a modest curvature of the trunk were apparent in 25% of larvae derived from intercrosses between tet2mk17/mk17, tet3mk18/+ adults (Figure 1E). Genotyping (n=20) confirmed that the morphologically abnormal embryos all carried homozygous mutations in both tet2 and tet3, demonstrating a combined requirement for the two genes in zebrafish development. Despite the additional reduction in 5hmC, tet1/2/3™ larvae were morphologically indistinguishable from tet2/3 double mutants at all stages examined (Fig 1F and data not shown).
Overlapping requirements for Tet2 and Tet3 in definitive but not primitive hematopoiesis
Given the combinatorial effects of tet2 and tet3 mutation on overall development, and the known roles of Tets in later stages of hematopoiesis, we tested whether tet2 and tet3 had overlapping roles in regulating hematopoiesis during embryonic development. Development of non-HSC derived primitive erythroid and myeloid cells was normal in tet2/3DM larvae and tet2mk17/mk17 siblings as assessed by expression of gata1, scl and pu.1 at 25 hpf by whole mount in situ hybridization (WISH) (Figure 2A-2I). In contrast, expression of HSC dependent definitive blood cell markers was dramatically reduced in tet2/3DM larvae compared to stage matched wild-type controls. While mature rag1 positive T-cells were readily observed in the thymus of wild-type larvae at 5 dpf, rag1 positive cells were absent from the corresponding region of tet2/3DM larvae (Figure 3A-3B). Similarly, the Tg(lysC:GFP) transgenic line, which labels definitive granulocytes (Hall et al., 2007), produced a clear fluorescent signal in blood from wild-type larvae at 6 dpf, but fluorescent cells were not detected in tet2/3DM larvae at this stage (Figure 3C-3D).
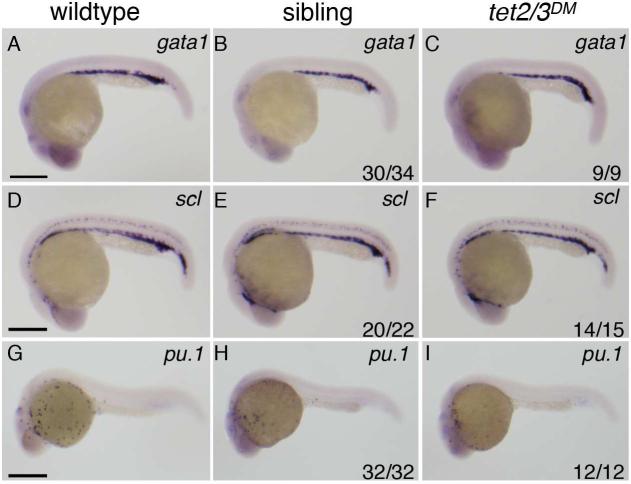
Figure 2. Markers of primitive hematopoiesis are similarly expressed in wildtype, tet2mk17/mk17 and tet2/3DM larvae.
(A-I) Representative lateral views of wild-type larvae compared to tet2/3DM and tet2mk17/mk17 sibling larvae derived from tet2mk17/mk17, tet3mk18/+ intercrosses.
(A-C) WISH for the primitive erythroid marker gata1 at 25 hpf.
(D-F) WISH for the primitive erythroid marker scl at 25 hpf.
(G-I) WISH for the primitive myeloid marker pu.1 at 25 hpf.
Numbers in the lower right hand corner indicate the fraction of embryos exhibiting WISH labeling similar to the representative image. Scale bars indicate 500 μM.
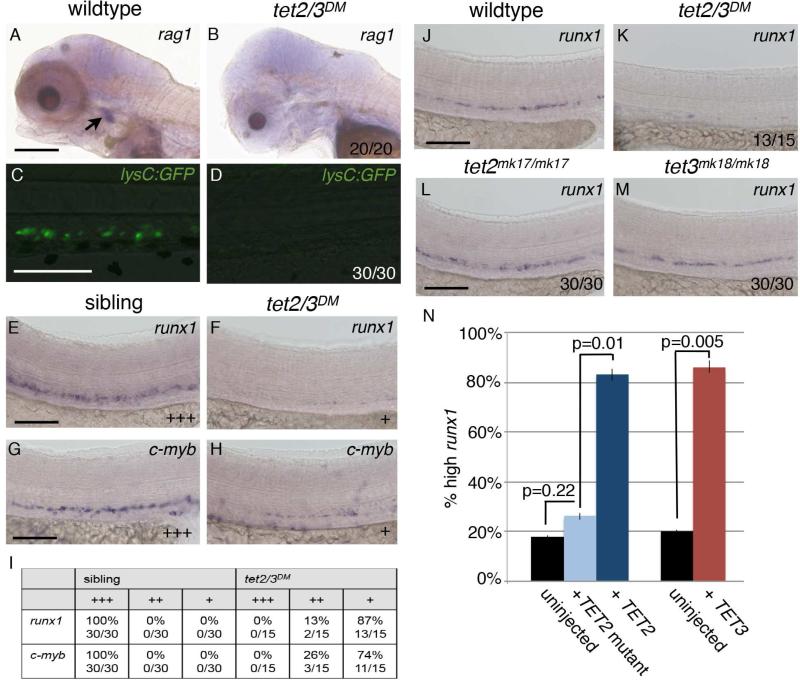
Figure 3. tet2 and tet3 have overlapping functions in HSC development.
(A and B) WISH for rag1 at 5 dpf. Arrow indicates thymic T-cells in wild-type larvae.
(C and D) GFP labeled macrophages and neutrophils in 6 dpf larvae carrying the Tg(lysC:GFP) transgene.
(E and F) WISH for the HSC marker runx1 in the DA at 36 hpf.
(G and H) WISH for the HSC marker c-myb in the DA at 36 hpf.
(I) Number of sibling and tet2/3DM embryos with wild type (+++), reduced (++), or nearly absent (+) runx1 labeling in the DA. Numbers are representative of three independent crosses.
(J-M) WISH for runx1 in the DA at 32 hpf.
(N) Graph indicating the percent of tet2/3DM larvae exhibiting high runx1 staining in the DA in uninjected controls or following injection with 100 pg of mRNA encoding TET2, TET2 H1382Y.D1384A (TET2 mutant) or TET3. Numerical data is presented as the mean ± standard error of the mean (SEM).
Numbers in the lower right corner of images indicate the fraction of larvae with WISH labeling similar to the representative image. All scale bars indicate 100 μM. See also Figure S2.
The loss of differentiated definitive blood cells in tet2/3DM larvae can be attributed to a defect in HSC development. During normal embryonic development, HSCs emerge from the hemogenic endothelium in the ventral wall of the dorsal aorta (DA). In tet2/3DM larvae, we found that expression of the HSC-associated genes, runx1 and c-myb, was reduced in the DA at 36 hpf, whereas runx1 expression in tet2 and tet3 single homozygous mutant larvae was indistinguishable from wildtype (Figure 3E-3M). Moreover, in tet2/3DM larvae, c-myb positive hematopoietic stem and progenitor cells (HSPCs) could not be detected in the caudal hematopoietic tissue (CHT) niche, a secondary, transient site for HSC amplification (Figure S2A-S2F). Importantly, runx1 expression was rescued by injecting mRNA encoding either human TET2 or TET3 into one-cell stage embryos derived from tet2mk17/mk17, tet3mk18/+ intercrosses (Figure 3N). Injection of mRNA encoding a catalytically dead version of TET2 failed to rescue wild-type levels of runx1 expression, directly implicating the 5mC dioxygenase activity of TET2 in regulating HSC development (Figure 3N).
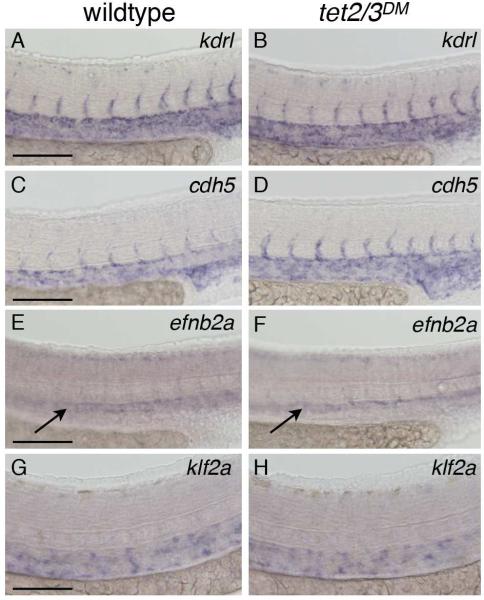
Normal vascular development, arterial specification, and blood flow-induced Nitric Oxide (NO) signaling are known prerequisites for HSC development (Adamo et al., 2009; Jagannathan-Bogdan and Zon, 2013; North et al., 2009). WISH for the vascular markers kdrl and cdh5, and the arterial marker efnb2a revealed similar expression in wildtype and tet2/3DM larvae, demonstrating the presence of an overtly intact vasculature (Figure 4A-4F). Visual inspection of tet2/3DM larvae by bright field microscopy showed that blood flow was grossly normal during the first two days of development, and klf2a, an immediate early responder to blood flow, was expressed at similar levels in wild-type and tet2/3DM larvae (Figure 4G-4H). Moreover, while exposure to the NO agonist S-nitroso-N-acetyl-penicillamine (SNAP) was sufficient to rescue runx1 expression in silent heart (sih) morpholino injected embryos lacking blood circulation, SNAP exposure was unable to rescue runx1 expression in tet2/3DM larvae (Figure S3A-S3E). Taken together, these results suggest that the HSC defects observed in tet2/3DM larvae are not secondary to defects in vascular development or aberrant blood flow.
Figure 4. Normal vasculature and blood flow in tet2/3DM larvae.
(A and B) WISH for the vascular marker kdrl at 31 hpf.
(C and D) WISH for vascular marker cdh5 at 31 hpf.
(E and F) WISH for the arterial marker efnb2a at 31 hpf.
(G and H) WISH for the blood flow dependent marker klf2a at 36 hpf.
Scale bars indicate 100 μM. See also Figure S3.
HSC emergence is compromised in tet2/3DM larvae
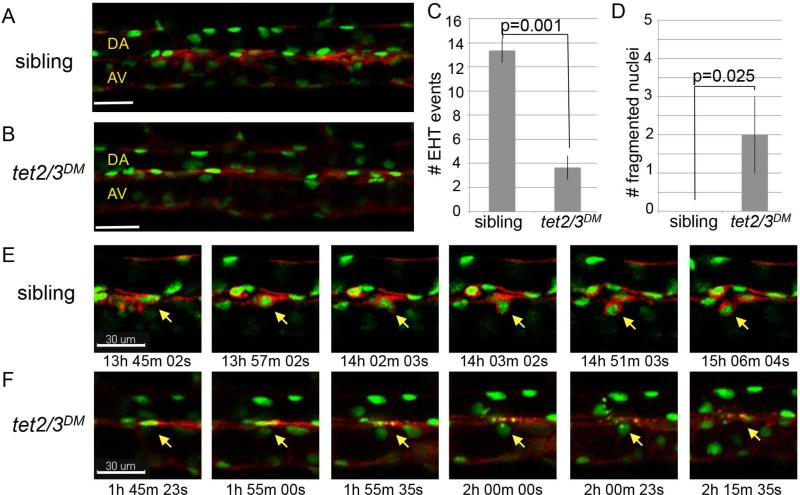
Beginning around 32 hpf, nascent HSCs emerge from the ventral aortic endothelium of the zebrafish embryo through a process termed the endothelial to hematopoietic transition (EHT) (Bertrand et al., 2010; Kissa and Herbomel, 2010). To test whether EHT was impacted by mutation of tet2/3, the tet2 and tet3 mutant alleles were introduced into a Tg(kdrl:Ras-mCherry)S896, Tg(kdrl:H2B-EGFP)mu122 transgenic background (Chi et al., 2008; Kochhan et al., 2013). In this background, membrane mCherry and nuclear GFP are expressed in the vascular endothelium and emergent HSCs, allowing EHT events to be identified based on stereotypical changes in cell morphology (Bertrand et al., 2010; Kissa and Herbomel, 2010). Prior to the onset of EHT, tet2/3DM larvae and siblings from tet2mk17/mk17, tet3mk18/+ intercrosses exhibited similar fluorescent labeling with the two transgenes (Figure 5A-5B). A defined region of the DA was then monitored in tet2/3DM larvae and sibling controls (n=3 each) between 30 and 46 hpf by time-lapse confocal microscopy. Analysis of image sets revealed a four-fold reduction in the number of EHT events detected in tet2/3DM larvae compared to siblings, indicating that EHT is compromised in the double mutants (average of 3 vs 13 EHT events, p=0.001; Figure 5C and 5E, Movie S1).
Figure 5. Tet2/3 are required for HSC emergence through the endothelial to hematopoietic transition.
(A and B) Merged images depicting GFP and mCherry labeling of the vasculature in Tg(kdrl:Ras-mCherry), Tg(kdrl:H2B-EGFP) transgenic larvae at 30 hpf.
(C) Number of EHT events detected between 30 and 46 hpf in tet2/3DM larvae and siblings. Numerical data is presented as the mean ± SEM.
(D) Number of fragmented nuclei observed in the DA of tet2/3DM larvae and their siblings between 30 and 46 hpf. Numerical data is presented as the mean ± SEM.
(E) Sequences from Supplemental Movie S1 documenting the stepwise emergence of an HSC from the the DA of a sibling larva. For each time point, merged GFP and mCherry images are shown. The yellow arrow indicates the cell undergoing EHT.
(F) Sequences from Supplemental Movie S2 documenting a cell undergoing nuclear fragmentation in the DA of a tet2/3DM larva. For each time point, merged GFP and mCherry images are shown. The yellow arrow indicates the cell with nuclear fragmentation.
In each time-lapse sequence from tet2/3DM larvae, we also observed between one and three cells within the DA undergoing nuclear fragmentation (Figure 5D and 5F, Movie S2). In contrast, fragmentation was never detected in time-lapse sequences from siblings (Figure 5D). The nuclear fragmentation phenotype we observed in tet2/3DM larvae is reminiscent of that described for zebrafish embryos depleted for runx1 by morpholino injection, and suggests that a fraction of cells undergoing EHT also die via apoptosis in the absence of tet2/3 (Kissa and Herbomel, 2010). Elevated levels of TUNEL positive cells were not observed in the DA or surrounding tissues of fixed tet2/3DM larvae (n=10, data not shown). However, apoptotic events restricted to the few cells undergoing EHT would be difficult to capture in fixed embryos.
Tet2/3 are required for Notch signaling and expression of key hematopoietic transcription factors in the hemogenic endothelium
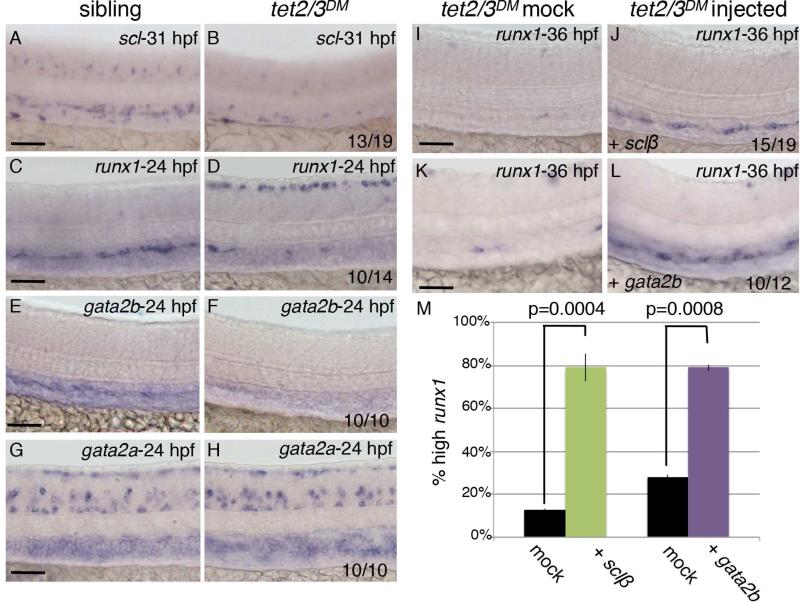
The reduction in EHT events in tet2/3DM larvae suggested a requirement for Tet2/3 in the function or specification of the hemogenic endothelium, which gives rise to nascent HSCs. The hematopoietic transcription factors runx1 and scl are both expressed in the hemogenic endothelium prior to the initiation of EHT and are required for this process (Kissa and Herbomel, 2010; Zhen et al., 2013). By WISH, we found that expression of both runx1 and scl was reduced in the DA of tet2/3DM larvae prior to HSC emergence, indicating that Tet2 and Tet3 are required to promote the hemogenic potential of the vascular endothelium (Figure 6A-6D). Expression of scl and runx1 is controlled in part by the transcription factor Gata2 in mouse, and the zebrafish genome encodes for two Gata2 paralogs that have undergone subfunctionalization (Butko et al., 2015; Gao et al., 2013; Gottgens et al., 2002; Pimanda et al., 2007). Zebrafish gata2a is broadly expressed throughout the hematopoietic system, and is important for vascular morphogenesis (Zhu et al., 2011). In contrast, zebrafish gata2b is specifically detected in the hemogenic endothelium and is required for runx1 expression within this tissue (Butko et al., 2015). Consistent with the HSC specific phenotypes observed in tet2/3DM larvae, we found that tet2/3 mutation compromised expression of gata2b, while gata2a expression was unaffected at similar stages (Figure 6E-6H). Collectively, these observations identify Tet2/3 as essential regulators of the gata2b/scl/runx1 transcriptional network in the hemogenic endothelium.
Figure 6. Tet2/3 regulate expression of the gata2b/scl/runx1 transcriptional network in the hemogenic endothelium.
(A-B) WISH for scl at 31 hpf.
(C-D) WISH for runx1 at 24 hpf.
(E-F) WISH for gata2b at 24 hpf.
(G-H) WISH for gata2a at 24 hpf.
(I-J) WISH for runx1 in the DA of mock injected and sclβ mRNA-injected tet2/3DM embryos at 36 hpf.
(K-L) WISH for runx1 in the DA of mock injected and gata2b mRNA-injected sibling embryos at 36 hpf.
(M) Percent of tet2/3DM embryos with high runx1 expression following mock injection, injection with mRNA encoding Sclβ (50pg) or Gata2b (200pg). Numerical data is presented as the mean ± SEM.
Numbers in the lower right corner of images indicate the fraction of larvae with WISH labeling similar to the representative image. All scale bars indicate 50 μM. See also Figure S4.
To clarify whether disruption of gata2b/scl/runx1 transcriptional program could account for the HSC defects observed in tet2/3 double mutant larvae, we next tested whether reintroducing mRNA encoding scl or gata2b into tet2/3DM embryos could rescue HSC production. In vitro transcribed mRNA encoding Scl or Gata2b was injected into one cell-stage embryos derived from tet2mk17/mk17, tet3mk18/+ intercrosses and runx1 expression was subsequently examined in the DA of tet2/3 double mutants by WISH at 36 hpf. Injection of either mRNA was sufficient to rescue wild-type levels of runx1 expression in the DA of tet2/3DM larvae, identifying the gata2b/scl/runx1 network as the primary hematopoietic program regulated by Tet2/3 during HSC emergence (scl: p=0.0004, gata2b: p=0.0008, Figure 6I-6M).
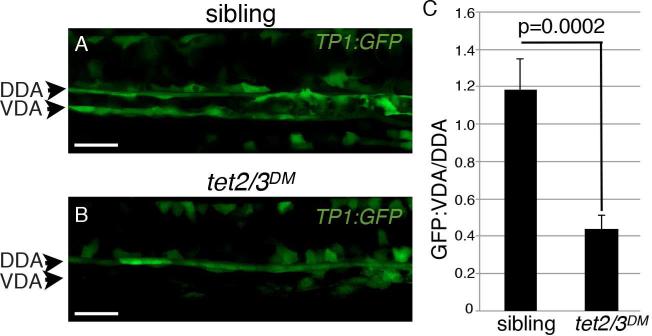
Analysis of genomic DNA from sorted vascular endothelial cells revealed that the promoters of gata2b, runx1 and scl were similarly unmethylated in wildtype and tet2/3DM larvae, suggesting that Tet2/3 do not directly regulate expression of these genes via promoter demethylation (Figure S4). This observation raised the possibility that Tet2/3 act upstream of gata2b in regulating HSC emergence. Notch signaling is required for the expression of gata2b in the zebrafish hemogenic endothelium and Gata2 is a direct target of Notch signaling in the mouse dorsal aorta, making this pathway a strong candidate for Tet2/3 regulation during HSC emergence (Butko et al., 2015; Robert-Moreno et al., 2005). To test whether mutation of tet2/3 disrupted Notch signaling in the hemogenic endothelium, we introduced the tet2 and tet3 mutant alleles into a Notch reporter line, Tp1:GFP, which expresses GFP under the control of tandem Notch responsive elements (Parsons et al., 2009). At the whole embryo level, wildtype and tet2/3DM larvae exhibited similar patterns of GFP expression from the Tp1:GFP transgene, suggesting that Notch signaling was not globally compromised by mutation of tet2/3 (Figure S5A-S5D). At higher resolution, morphologically wild-type embryos carrying the Tp1:GFP transgene exhibited the expected strong expression of Tp1:GFP along both the dorsal and ventral walls of the DA (Figure 7A). In tet2/3DM larvae, Tp1:GFP expression on the dorsal side of the DA appeared similar to wild type, however, expression along the ventral wall appeared weaker and discontinuous (n=10/11, Figure 7B). Quantification of mean GFP fluorescent intensity in each region revealed a 3-fold reduction in the ratio of GFP fluorescence in the ventral DA compared to the dorsal DA in tet2/3 double mutants (p=0.0002, Figure 7C). This specific reduction in GFP in the ventral DA reveals a requirement for Tet2/3 in regulating Notch signaling in the hemogenic endothelium and provides a mechanistic explanation for the down regulation of gata2b observed in tet2/3DM larvae. Taken together these results uncover a requirement for Tet2/3 in the early function of the hemogenic endothelium and identify Notch signaling and the downstream expression of the gata2b/scl/runx1 transcriptional network as key targets of Tet2/3 regulation during HSC emergence.
Figure 7. Tet2/3 are required for Notch signaling in the hemogenic endothelium.
(A-B) Confocal images of Tp1:GFP expression in the dorsal aorta of sibling and tet2/3DM larvae at 28 hpf. DDA indicates the dorsal wall of the dorsal aorta. VDA indicates the ventral wall of the dorsal aorta.
(C) Ratio of GFP fluorescence intensity in the VDA/DAA in sibling and tet2/3DM larvae (n=11 per genotype). Numerical data is presented as the mean ± SEM. Scale bars indicate 100 μM.
Discussion
Our systematic analysis of tet mutant phenotypes revealed Tet2 and Tet3 to be the major 5mC dioxygenases in the zebrafish embryo. To date, a number of studies in mouse have focused on Tet1 and Tet2, likely due to the fact that these are the only Tet orthologs expressed in ESCs (Wu and Zhang, 2011). Nonetheless, Tet3 is up regulated upon differentiation of ESCs and is highly expressed in many differentiated primary tissues (Dawlaty et al., 2013; Li et al., 2015). Our analysis of zebrafish tet mutants, combined with the survival of Tet1/2 mutant mice and the poor differentiation capacity of Tet1/2/3 mutant ESCs, suggest that Tet2 and Tet3 may also have important overlapping requirements in promoting mammalian development (Dawlaty et al., 2014; Dawlaty et al., 2013). Intriguingly, while at least Tet3 is maternally deposited in mouse, few, if any, tet transcripts are detected in RNA-seq data from 2-cell stage zebrafish embryos and 5hmC is not detected in the zebrafish embryo by immunofluorescence until the bud stage (Almeida et al., 2012; Gu et al., 2011). These observations imply that mRNAs encoding the Tet enzymes are not maternally deposited in zebrafish and suggest that, in contrast to mammals, the zebrafish genome does not contain significant amounts of 5hmC prior to segmentation. Zebrafish do not undergo the same Tet dependent erasure and reestablishment of global 5mC patterns observed during mammalian preimplantation development, providing one potential explanation for this distinction (Jiang et al., 2013; Potok et al., 2013). The lack of maternal deposition and limited dependency on Tet enzymes during the first 24 hours post fertilization make zebrafish a powerful system for examining Tet requirements in later developmental processes, including those associated with tissue specific development and differentiation.
In the current study, we examine requirements for Tet2/3 during embryonic stages of hematopoietic development. Defects in primitive hematopoiesis following antisense morpholino depletion of tet2 in zebrafish and impaired differentiation of primitive embryonic/yolk sac progenitors following shRNA depletion of TET2 in human ESCs have been reported (Ge et al., 2014; Langlois et al., 2014). However, these results are difficult to reconcile with the normal primitive hematopoiesis observed in published mouse and zebrafish models of Tet2 mutation (Gjini et al., 2014; Ko et al., 2011; Kunimoto et al., 2012; Li et al., 2011b; Moran-Crusio et al., 2011; Quivoron et al., 2011; Shide et al., 2012; Solary et al., 2014). While it is difficult to definitively address the discrepancies between these studies, both shRNA and antisense morpholino technologies can be susceptible to off target effects (Kok et al., 2015; Scherer and Rossi, 2003). Similar to other published studies of Tet2 mutants, we find that primitive hematopoiesis proceeds normally in the Tet2 homozygous mutant zebrafish generated by our laboratory, and we demonstrate that combinatorial elimination of Tet2 and Tet3 catalytic functions does not further impact primitive hematopoiesis. It is important to note that because the truncation mutations used in this study leave N-terminal coding sequence intact, we cannot rule out the possibility that Tet2/3 have combinatorial dioxygenase independent functions in regulating primitive hematopoiesis.
The earliest stages of definitive hematopoiesis also appear unaffected in Tet2 single mutant mice and zebrafish, although diminished expression of c-myb, but not runx1, was observed in the DA of tet2 morpholino injected zebrafish embryos (Ge et al., 2014; Gjini et al., 2014; Ko et al., 2011; Kunimoto et al., 2012; Li et al., 2011b; Moran-Crusio et al., 2011; Quivoron et al., 2011; Shide et al., 2012; Solary et al., 2014). In genetic models, loss of Tet2 eventually leads to an expansion of hematopoietic progenitor cells in the bone marrow and skewed myeloid differentiation (Gjini et al., 2014; Ko et al., 2011; Li et al., 2011b; Moran-Crusio et al., 2011). However, the long latency that precedes these phenotypes suggests secondary somatic mutations may be a contributing factor. In contrast to these later hematopoietic phenotypes, we find that combined mutation of tet2/3 causes an early loss of definitive blood cells, resulting from compromised HSC production. Importantly, abnormalities in HSC development occurred in embryos that were morphologically quite normal, and had normal expression of vascular markers. The relatively normal development of tet2/3DM larvae supports a specific role for tet2 and tet3 in regulating transcription of the embryonic HSC developmental program rather than a more generalized role in regulating global transcription.
Compared to tet1, both tet2 and tet3 transcripts are relatively enriched in the DA of the developing zebrafish embryo (Ge et al., 2014). This expression pattern provides a potential explanation for the specific overlapping Tet2/3 requirements in HSC production. Analysis of double mutant larvae revealed a combined requirement for tet2/3 in regulating Notch signaling in the hemogenic endothelium, suggesting a role for 5hmC in the specification or early function of this tissue. Enrichment of 5hmC has been reported at Notch receptor and ligand genes in other tissues, but the functional significance of these changes has not been determined (Terragni et al., 2014). Intriguingly, we find Notch signaling to be relatively intact in other tissues of tet2/3DM larvae, indicating that Tet2/3 are likely be involved in fine-tuning the activation of this pathway in select cell types. Notch signaling is essential for HSC development in vertebrates, and has been implicated in both specification of the dorsal aorta and downstream HSC production (Jagannathan-Bogdan and Zon, 2013; Robert-Moreno et al., 2005). Tet2/3 appear to be dispensable for Notch regulation of arterial specification, as we observe normal expression of the arterial marker ephrinb2 in tet2/3DM larvae. Instead, disruption in Notch signaling in the hemogenic endothelium favors a select requirement for Tet2/3 in the regulation of HSC specification. This model is consistent with the downstream disruption of the gata2b/scl/runx1 transcriptional network observed in tet2/3DM larvae and our observation that reintroducing scl or gata2b mRNA can rescue HSC production in double mutants. Notably, Notch regulation is dispensable for scl expression during the primitive wave of hematopoiesis, which is consistent with the normal scl expression observed in tet2/3DM primitive erythrocytes (Burns et al., 2005; Kim et al., 2013).
Collectively, these results uncover a requirement for Tet regulation of 5hmC in the early function of the zebrafish hemogenic endothelium. A deeper understanding of how HSC generation is regulated in vivo, is expected to facilitate the in vitro production of HSCs for therapeutic purposes. Importantly, our results identify regulation of 5hmC as an additional variable to be considered in the optimization of protocols for HSC differentiation from pluripotent progenitors. This observation may be of particular relevance given recent data highlighting the impact of cell culture conditions on global 5hmC levels (Blaschke et al., 2013; Nestor et al., 2015; Yin et al., 2013).
Experimental Procedures
Zebrafish husbandry
Zebrafish maintenance and breeding were conducted under full animal use and care guidelines with approval by the institutional animal care and use committee. Zebrafish were raised under standard conditions at 28° C.
TALEN mutagenesis
TALEN sequences were selected using Targeter 2.0 software (Doyle et al., 2012). TAL repeat assembly was achieved using the Golden Gate assembly method and assembled repeats were integrated into the GoldyTALEN scaffold (Bedell et al., 2012; Cermak et al., 2011). Assembled vectors served as templates for in vitro mRNA transcription using the T3 mMessage mMachine kit (Ambion) according to manufacturer's instructions. 50-100 pg of mRNA was injected into wild-type embryos at the one-cell stage. Details of mutation recovery and genotyping can be found in Supplemental Experimental Procedures.
5hmC dot blot
Genomic DNA was isolated from larvae at 5 dpf by phenol-chloroform extraction and ethanol precipitation. Following RNAse treatment and denaturation, serially diluted DNA was spotted onto nitrocellulose membranes. Cross-linked membranes were incubated with 0.02% Methylene Blue to validate uniform DNA loading. Membranes were blocked with 5% bovine serum albumin and incubated with anti-5hmC antibody (1:10,000; Active Motif) followed by a horseradish peroxidase-conjugated antibody (1:15,000; Active Motif). Signal was detected using the ECL Prime Detection Kit (GE).
WISH
WISH was performed as described (Thisse and Thisse, 2008). For all probes except gata2b, 10% dextran sulfate was added to the hybridization buffer.
RNA synthesis and microinjection
The human TET3 vector used for mRNA production has been previously described (Ko et al., 2013). The human TET2 ORF corresponding to Genebank: NM_001127208 was was amplified from cDNA made from SH-SY5Y neuroblastoma cells. Following sub-cloning, the TET2 ORF was introduced into the pEF1/V5-His vector (Invitrogen) to allow for in vitro transcription. Mutant TET2 (H1382Y, D1384A) was generated using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent). pExpress-1-gata2b was purchased from Transomic Technologies. For scl-β, RT-PCR amplified scl-β cDNA with sequence corresponding to Genbank: EF488003 was cloned into pCS2+. Sequences of all clones were confirmed by conventional DNA sequencing. In all cases, capped RNA was synthesized using mMessage mMachine (Ambion) with Sp6 or T7 polymerase as appropriate to the vector. For each experimental condition, mRNA was injected into at least fifty embryos derived from tet2mk17/mk17, tet3mk18/+ intercrosses.
Time-lapse confocal microscopy
Embryos were anesthetized with 0.02% tricaine and embedded in 0.5% low-melt agarose. Embryos were scanned using a SP8 confocal microscope (Leicia) at 28.5°C. Confocal z-stacks were acquired every 7 to 10 minutes between 30 and 46 hpf. Approximately 25 planes were collected per time point at a spacing of 3 μm. Data was analyzed using Imaris software and exported in QuickTime. All confocal planes were sequentially analyzed to identify changes in cell morphology consistent with EHT and to identify nuclear fragmentation events.
Tp1:GFP imaging and quantification
Mounted samples were scanned using an SP8 confocal microscope (Leica) using 40X water immersion objective at 26 hpf. For each image, approximately 60 planes were captured at a spacing of 0.38 μm. Data processed using Imaris software and quantified using ImageJ.
Statistical analysis
The Student unpaired 2-tailed t-test was used for statistical analysis.
Supplementary Material
Highlights.
- Tet2 and Tet3 are the major 5-methylcytosine dioxygenases in the zebrafish embryo
- Tet2 and Tet3 have overlapping requirements in hematopoietic stem cell emergence
- Loss of Tet2/3 compromises expression of the gata2b/scl/runx1 hematopoietic program
- Notch signaling in the hemogenic endothelium is dependent on Tet2/3
Acknowledgments
This work was funded by a Louis V. Gerstner, Jr. Young Investigators Award to MGG, grant #2014-010 from the Tri-Institutional Stem Cell Initiative to MGG and TE, and NCI grant P30 CA008748 to MSKCC. We thank the MSKCC Molecular Cytology and Flow Cytometry cores for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida RD, Loose M, Sottile V, Matsa E, Denning C, Young L, Johnson AD, Gering M, Ruzov A. 5-hydroxymethyl-cytosine enrichment of non-committed cells is not a universal feature of vertebrate development. Epigenetics. 2012;7:383–389. doi: 10.4161/epi.19375. [DOI] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG, 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, et al. In vivo genome editing using a high-efficiency TALEN system. Nature. 2012;491:114–118. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes & development. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butko E, Distel M, Pouget C, Weijts B, Kobayashi I, Ng K, Mosimann C, Poulain FE, McPherson A, Ni CW, et al. Gata2b is a restricted early regulator of hemogenic endothelium in the zebrafish embryo. Development. 2015;142:1050–1061. doi: 10.1242/dev.119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic acids research. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi NC, Shaw RM, De Val S, Kang G, Jan LY, Black BL, Stainier DY. Foxn4 directly regulates tbx2b expression and atrioventricular canal formation. Genes & development. 2008;22:734–739. doi: 10.1101/gad.1629408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino L, Dawlaty MM, Ndiaye-Lobry D, Yap YS, Bakogianni S, Yu Y, Bhattacharyya S, Shaknovich R, Geng H, Lobry C, et al. TET1 is a tumor suppressor of hematopoietic malignancy. Nature immunology. 2015;16:653–662. doi: 10.1038/ni.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawlaty MM, Breiling A, Le T, Barrasa MI, Raddatz G, Gao Q, Powell BE, Cheng AW, Faull KF, Lyko F, et al. Loss of tet enzymes compromises proper differentiation of embryonic stem cells. Dev Cell. 2014;29:102–111. doi: 10.1016/j.devcel.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawlaty MM, Breiling A, Le T, Raddatz G, Barrasa MI, Cheng AW, Gao Q, Powell BE, Li Z, Xu M, et al. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev Cell. 2013;24:310–323. doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle EL, Booher NJ, Standage DS, Voytas DF, Brendel VP, Vandyk JK, Bogdanove AJ. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic acids research. 2012;40:W117–122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Johnson KD, Chang YI, Boyer ME, Dewey CN, Zhang J, Bresnick EH. Gata2 cis-element is required for hematopoietic stem cell generation in the mammalian embryo. The Journal of experimental medicine. 2013;210:2833–2842. doi: 10.1084/jem.20130733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Zhang RP, Wan F, Guo DY, Wang P, Xiang LX, Shao JZ. TET2 plays an essential role in erythropoiesis by regulating lineage-specific genes via DNA oxidative demethylation in a zebrafish model. Molecular and cellular biology. 2014;34:989–1002. doi: 10.1128/MCB.01061-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjini E, Mansour MR, Sander JD, Moritz N, Nguyen AT, Kesarsing M, Gans E, He S, Chen S, Ko M, et al. A Zebrafish Model of Myelodysplastic Syndrome Produced Through tet2 Genomic Editing. Molecular and cellular biology. 2014;35:789–804. doi: 10.1128/MCB.00971-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annual review of biochemistry. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Gottgens B, Nastos A, Kinston S, Piltz S, Delabesse EC, Stanley M, Sanchez MJ, Ciau-Uitz A, Patient R, Green AR. Establishing the transcriptional programme for blood: the SCL stem cell enhancer is regulated by a multiprotein complex containing Ets and GATA factors. The EMBO journal. 2002;21:3039–3050. doi: 10.1093/emboj/cdf286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–610. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol. 2007;7:42. doi: 10.1186/1471-213X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Li Z, Cheng J, Rao Q, Gong W, Liu M, Shi YG, Zhu J, Wang P, Xu Y. Crystal structure of TET2-DNA complex: insight into TET-mediated 5mC oxidation. Cell. 2013;155:1545–1555. doi: 10.1016/j.cell.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Huang H, Jiang X, Li Z, Li Y, Song CX, He C, Sun M, Chen P, Gurbuxani S, Wang J, et al. TET1 plays an essential oncogenic role in MLL-rearranged leukemia. Proc Natl Acad Sci U S A. 2013;110:11994–11999. doi: 10.1073/pnas.1310656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Shen L, Matoba S, Zhang Y. Haploinsufficiency, but not defective paternal 5mC oxidation, accounts for the developmental defects of maternal Tet3 knockouts. Cell reports. 2015;10:463–470. doi: 10.1016/j.celrep.2014.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan-Bogdan M, Zon LI. Hematopoiesis. Development. 2013;140:2463–2467. doi: 10.1242/dev.083147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Zhang J, Wang JJ, Wang L, Zhang L, Li G, Yang X, Ma X, Sun X, Cai J, et al. Sperm, but not oocyte, DNA methylome is inherited by zebrafish early embryos. Cell. 2013;153:773–784. doi: 10.1016/j.cell.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PG, Albacker CE, Lu YF, Jang IH, Lim Y, Heffner GC, Arora N, Bowman TV, Lin MI, Lensch MW, et al. Signaling axis involving Hedgehog, Notch, and Scl promotes the embryonic endothelial-to-hematopoietic transition. Proc Natl Acad Sci U S A. 2013;110:E141–150. doi: 10.1073/pnas.1214361110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- Ko M, An J, Bandukwala HS, Chavez L, Aijo T, Pastor WA, Segal MF, Li H, Koh KP, Lahdesmaki H, et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature. 2013;497:122–126. doi: 10.1038/nature12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, An J, Pastor WA, Koralov SB, Rajewsky K, Rao A. TET proteins and 5-methylcytosine oxidation in hematological cancers. Immunological reviews. 2015;263:6–21. doi: 10.1111/imr.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Bandukwala HS, An J, Lamperti ED, Thompson EC, Hastie R, Tsangaratou A, Rajewsky K, Koralov SB, Rao A. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci U S A. 2011;108:14566–14571. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhan E, Lenard A, Ellertsdottir E, Herwig L, Affolter M, Belting HG, Siekmann AF. Blood flow changes coincide with cellular rearrangements during blood vessel pruning in zebrafish embryos. PloS one. 2013;8:e75060. doi: 10.1371/journal.pone.0075060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli RM, Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. 2013;502:472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell. 2015;32:97–108. doi: 10.1016/j.devcel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto H, Fukuchi Y, Sakurai M, Sadahira K, Ikeda Y, Okamoto S, Nakajima H. Tet2 disruption leads to enhanced self-renewal and altered differentiation of fetal liver hematopoietic stem cells. Scientific reports. 2012;2:273. doi: 10.1038/srep00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois T, da Costa Reis Monte Mor B, Lenglet G, Droin N, Marty C, Le Couedic JP, Almire C, Auger N, Mercher T, Delhommeau F, et al. TET2 deficiency inhibits mesoderm and hematopoietic differentiation in human embryonic stem cells. Stem Cells. 2014;32:2085–2097. doi: 10.1002/stem.1718. [DOI] [PubMed] [Google Scholar]
- Li T, Huang S, Zhao X, Wright DA, Carpenter S, Spalding MH, Weeks DP, Yang B. Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic acids research. 2011a;39:6315–6325. doi: 10.1093/nar/gkr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Yang D, Li J, Tang Y, Yang J, Le W. Critical role of Tet3 in neural progenitor cell maintenance and terminal differentiation. Molecular neurobiology. 2015;51:142–154. doi: 10.1007/s12035-014-8734-5. [DOI] [PubMed] [Google Scholar]
- Li Z, Cai X, Cai CL, Wang J, Zhang W, Petersen BE, Yang FC, Xu M. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011b;118:4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsbach RB, Moore J, Mathew S, Raimondi SC, Mukatira ST, Downing JR. TET1, a member of a novel protein family, is fused to MLL in acute myeloid leukemia containing the t(10;11)(q22;q23). Leukemia. 2003;17:637–641. doi: 10.1038/sj.leu.2402834. [DOI] [PubMed] [Google Scholar]
- Madzo J, Liu H, Rodriguez A, Vasanthakumar A, Sundaravel S, Caces DB, Looney TJ, Zhang L, Lepore JB, Macrae T, et al. Hydroxymethylation at gene regulatory regions directs stem/early progenitor cell commitment during erythropoiesis. Cell reports. 2014;6:231–244. doi: 10.1016/j.celrep.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor CE, Ottaviano R, Reinhardt D, Cruickshanks HA, Mjoseng HK, McPherson RC, Lentini A, Thomson JP, Dunican DS, Pennings S, et al. Rapid reprogramming of epigenetic and transcriptional profiles in mammalian culture systems. Genome biology. 2015;16:11. doi: 10.1186/s13059-014-0576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mechanisms of development. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, Ko M, McLoughlin EM, Brudno Y, Mahapatra S, Kapranov P, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature. 2011;473:394–397. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimanda JE, Ottersbach K, Knezevic K, Kinston S, Chan WY, Wilson NK, Landry JR, Wood AD, Kolb-Kokocinski A, Green AR, et al. Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci U S A. 2007;104:17692–17697. doi: 10.1073/pnas.0707045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potok ME, Nix DA, Parnell TJ, Cairns BR. Reprogramming the maternal zebrafish genome after fertilization to match the paternal methylation pattern. Cell. 2013;153:759–772. doi: 10.1016/j.cell.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronier E, Almire C, Mokrani H, Vasanthakumar A, Simon A, da Costa Reis Monte Mor B, Masse A, Le Couedic JP, Pendino F, Carbonne B, et al. Inhibition of TET2-mediated conversion of 5-methylcytosine to 5-hydroxymethylcytosine disturbs erythroid and granulomonocytic differentiation of human hematopoietic progenitors. Blood. 2011;118:2551–2555. doi: 10.1182/blood-2010-12-324707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, Do Cruzeiro M, Delhommeau F, Arnulf B, Stern MH, et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer cell. 2011;20:25–38. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JR. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nature biotechnology. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer LJ, Rossi JJ. Approaches for the sequence-specific knockdown of mRNA. Nature biotechnology. 2003;21:1457–1465. doi: 10.1038/nbt915. [DOI] [PubMed] [Google Scholar]
- Shide K, Kameda T, Shimoda H, Yamaji T, Abe H, Kamiunten A, Sekine M, Hidaka T, Katayose K, Kubuki Y, et al. TET2 is essential for survival and hematopoietic stem cell homeostasis. Leukemia. 2012;26:2216–2223. doi: 10.1038/leu.2012.94. [DOI] [PubMed] [Google Scholar]
- Solary E, Bernard OA, Tefferi A, Fuks F, Vainchenker W. The Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases. Leukemia. 2014;28:485–496. doi: 10.1038/leu.2013.337. [DOI] [PubMed] [Google Scholar]
- Stroud H, Feng S, Morey Kinney S, Pradhan S, Jacobsen SE. 5-Hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome biology. 2011;12:R54. doi: 10.1186/gb-2011-12-6-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terragni J, Zhang G, Sun Z, Pradhan S, Song L, Crawford GE, Lacey M, Ehrlich M. Notch signaling genes: myogenic DNA hypomethylation and 5-hydroxymethylcytosine. Epigenetics. 2014;9:842–850. doi: 10.4161/epi.28597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nature protocols. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PA, Rappsilber J, Helin K. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes & development. 2011;25:2436–2452. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. Journal of the American Chemical Society. 2013;135:10396–10403. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- Zhen F, Lan Y, Yan B, Zhang W, Wen Z. Hemogenic endothelium specification and hematopoietic stem cell maintenance employ distinct Scl isoforms. Development. 2013;140:3977–3985. doi: 10.1242/dev.097071. [DOI] [PubMed] [Google Scholar]
- Zhu C, Smith T, McNulty J, Rayla AL, Lakshmanan A, Siekmann AF, Buffardi M, Meng X, Shin J, Padmanabhan A, et al. Evaluation and application of modularly assembled zinc-finger nucleases in zebrafish. Development. 2011;138:4555–4564. doi: 10.1242/dev.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.