Abstract
Background
Ethnic minorities, especially African Americans and Latinos, bear a disproportionate burden of colorectal cancer (CRC) reflected in incidence, cancer stage and mortality statistics. In all ethnic groups first-degree relatives (FDRs) of CRC cases are at elevated disease risk. However, underutilization of CRC screening persists and is particularly evident among minority groups. This study tested a stepped intervention to increase CRC screening among an ethnically diverse sample of FDRs of CRC cases.
Methods
A statewide cancer registry was used to recruit CRC cases and through them their FDRs. Relatives not current on CRC screening were randomized to intervention or usual-care control arms. The stepped intervention consisted of ethnically-targeted and individually-tailored print materials followed by telephone counseling for those unscreened at 6 months.
Results
The sample (N=1280) consisted of 403 Latino, 284 African American, 242 Asian, and 351 White FDRs. Statistically significant effects were observed for the cumulative print+telephone intervention at 12-months (26% intervention vs.18% control) and the print intervention alone at 6 months (15% intervention vs. 10% control). The effect of the print alone versus the cumulative interventions was not significantly different. Stratified analyses indicated that the intervention was effective among Whites, Latinos, and Asians, but not among African-Americans.
Conclusion
Overall, the intervention was effective in increasing in screening rates. Oversampling racial/ethnic minorities allowed for examination of effects within subgroups, revealing no effect among African Americans. This finding illustrates the importance of including sufficient numbers of participants from diverse ethnic sub-groups in intervention research, to enable such stratified analyses.
Introduction
First-degree relatives (FDRs) of colorectal cancer (CRC) cases are at significantly increased risk of developing the disease compared to those with no family history 1. Individuals from families with early onset disease or multiple affected relatives are at even higher risk 2. Most studies that have assessed CRC risk among FDRs have been conducted among Whites.
In the United States, ethnic minorities bear a disproportionate burden of CRC. Incidence rates are highest among African Americans followed by American Indians/Alaskan Natives, Whites, Latinos and Asians 3. Outcomes such as age and stage at diagnosis, survival, and mortality tend to be poorer among minorities 4, and are partially attributable to differences in socioeconomic factors 5. However, the literature consistently documents the persistence of racial disparities in CRC outcomes even after controlling for socioeconomic factors and access 6. Therefore, CRC prevention and control efforts are critically needed for all ethnic groups in the U.S.
Regular receipt of screening has the potential to significantly decrease incidence and mortality and reduce ethnic disparities in CRC 7. Most professional organizations recommend routine CRC screening beginning at age 50 for average risk populations via fecal-occult blood testing (FOBT), fecal immunochemical testing (FIT), sigmoidoscopy, colonoscopy, or CT colonography 8 and some organizations recommend screening initiation before age 50 years for individuals with a family history of CRC 9, 10. Still, only about 60% of the average risk U.S. population is being screened according to current recommendations 11 and screening rates tend to be lower among ethnic minority groups 12. Data from the 2010 Behavioral Risk Factor Surveillance Survey revealed that 62% of non-Latino whites were up-to-date for colorectal cancer screening compared to 31% of Spanish-speaking Latinos, 47% of Asians, and 59% of African Americans 12. A recent study conducted in the VA revealed significantly lower colorectal cancer screening rates among African Americans (42%) compared to non-African American veterans (58%) despite similar access to screening 13. Observational studies, mainly focusing on Whites, suggest that CRC screening rates tend to be only somewhat higher among FDRs compared to average risk individuals 14. Despite the increased risk faced by FDRs, very few intervention studies to increase screening have been conducted in this group 15, 16, and fewer have targeted ethnically-diverse samples.
We conducted a randomized trial to evaluate the effectiveness of a stepped (2-stage) risk notification intervention on CRC screening rates among FDRs of CRC cases. To our knowledge, this is the only randomized trial in the literature to target an ethnically-diverse sample of individuals at elevated for CRC due to family history.
Method
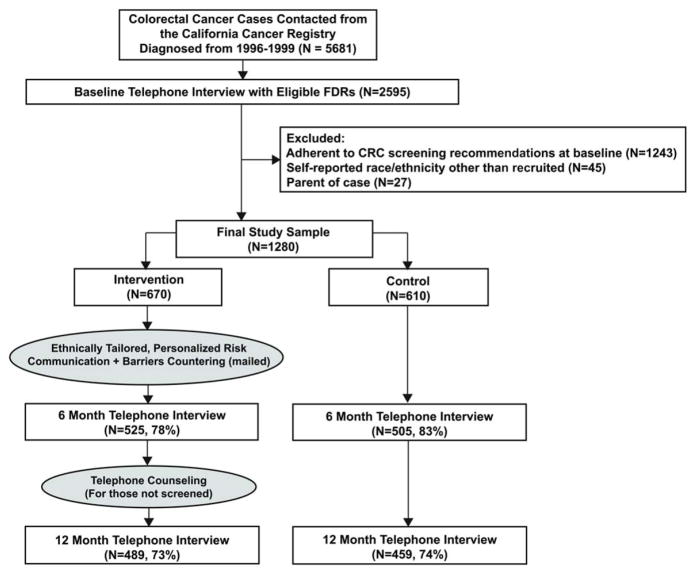
Overview of Study Design (Figure 1)
Figure 1.
Overview of the study design. (CRC indicates colorectal cancer; FDRs, first-degree relatives)
CRC cases identified through the statewide California Cancer Registry (CCR) were contacted and asked to provide information about their FDRs. FDRs not adherent to CRC screening guidelines at baseline were randomized (by family and within race/ethnic strata) to intervention or control arms. The intervention group received an ethnically-targeted and individually-tailored print intervention within 2 weeks after baseline. Both study arms were re-contacted 6 months after baseline. Intervention participants not adherent to screening at 6 months received telephone counseling immediately following the interview. Twelve month telephone interviews were conducted with all participants to assess CRC screening receipt. The control group received the ethnically-targeted intervention booklet after trial completion, but no intervention during the study period. The study protocol was approved by the UCLA Human Subjects Protection Committee. The study was conducted between 1999–2008.
Recruitment of First-Degree Relatives (Table 1)
Table 1.
Recruitment of FDRs of Colorectal Cancer Cases
| White | Latino | Afr. Am. | Asian | Total | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Cases contacted | 1397 | 1652 | 1078 | 1554 | 5681 | |
Attrition due to:
|
|
|
|
|
|
|
| Cases providing relatives | 450 (32%) | 519 (31%) | 276 (26%) | 400 (26%) | 1645 (29%) | |
|
|
||||||
| By mail | 48% | 33% | 39% | 39% | 40% | |
| By phone | 52% | 67% | 61% | 61% | 60% | |
| Relatives provided by cases | 1291 | 1783 | 887 | 1112 | 5073 | |
| 2.9/case | 3.4/case | 3.2/case | 2.8/case | 3.1/case | ||
|
|
||||||
Attrition due to:
|
|
|
|
|
|
|
| Relatives eligible for baseline survey | 925 (72%) | 1294 (73%) | 723 (82%) | 725 (65%) | 3667 (73%) | |
|
|
||||||
Attrition due to:
|
|
|
|
|
|
|
|
|
||||||
| Eligible and completed baseline (based on case ethnicity) | 689 (74%) | 887 (69%) | 494 (68%) | 525 (72%) | 2595 (71%) | |
|
| ||||||
| Met all study eligibility criteria* | 351 | 403 | 284 | 242 | 1280 | |
|
|
||||||
| completed 6 mo. Interview | 304 (87%) | 305 (76%) | 217 (76%) | 204 (84%) | 1030 (80%) | |
|
|
||||||
| completed 12 mo. Interview | 273 (78%) | 279 (69%) | 204 (72%) | 192 (79%) | 948 (74%) | |
Not current on CRC screening at baseline, Abbreviations: FDRs, first-degree relatives; CRC, colorectal cancer
We obtained contact information for all available Asian, Latino, and African-American cases and a random sample of White cases diagnosed in California from 1996–1999 from the statewide California Cancer Registry (CCR). Cases (n = 5681) were mailed ethnically-targeted recruitment materials, with telephone follow-up for those not responding to the mailing. Of the cases contacted, 1645 (29%) provided information on 5073 relatives. Of these, 73% met criteria for inclusion in the baseline survey and 71% of eligible relatives completed the baseline survey. At baseline, 48% of relatives were current on CRC screening (FOBT in past 12 months or sigmoidoscopy in past 5years or colonoscopy in past 10 years), leaving 1280 (52%) fully eligible for study participation. Study eligibility criteria included: age 40–80 years, residing in U.S. or Canada, English/Spanish speaking, sibling/child of case, no personal history of CRC, no presence of CRC high-risk syndromes such as familial adenomatous polyposis, and not current on CRC screening at baseline.
Data Collection
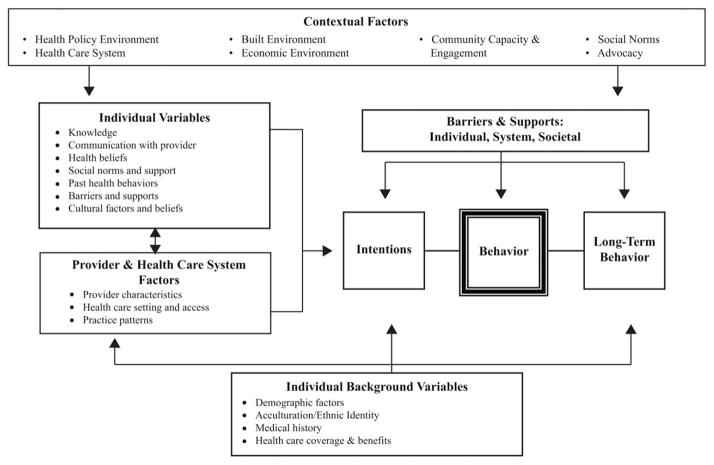
Interview items were conceptually guided by the Health Behavior Framework (See Figure 2) and drawn from prior work by the investigators 17–19. Demographic variables assessed included age, marital status, income, insurance status, race/ethnicity, country of birth, and years in the United States among those foreign born. Health history variables included previous cancer diagnosis, cancer in blood relatives other than index case, and other CRC risk factors (i.e., polyps, inflammatory bowel disease).
Figure 2.
Health Behavior Framework.
Baseline and follow-up interviews also assessed knowledge of CRC risk factors (13 items) and screening recommendations (4 items), cancer-related distress (5 items, Cronbach’s alpha = .79), perceived risk (1 item), barriers to screening (13 items assessed practical barriers to screening such as transportation, cost, and time constraints as well as psychological barriers such as fear of finding cancer, embarrassment, concern about pain, and perception that screening is unpleasant), and patient provider interactions related to CRC (3 items).
Study outcome
Self-reported receipt of any CRC screening test (FOBT, sigmoidoscopy, colonoscopy) at 6 and 12 month follow-ups. A description of each test was provided before assessing receipt. Self-reported screening has been found in previous studies to have reasonable sensitivity (Endoscopy = .79, FOBT = .82) and specificity (Endoscopy = .90, FOBT = .78) 20.
Description of the Intervention
An ethnically-targeted and individually-tailored (utilizing information from baseline interview) print intervention was mailed to all intervention group participants two weeks after baseline. Participants unscreened at the 6-month telephone interview received a brief barriers counseling session, delivered during the same telephone call, after completion of the interview. A major component of the print and telephone interventions was a personalized CRC risk assessment. Risk labels for intervention purposes were based on participant preferences expressed during qualitative pilot work. The “very high-risk” group was defined as having at least one of the following: two or more FDRs with CRC, at least one FDR with CRC before 50 years of age, two or more blood relatives with CRC before age 50, personal history of colorectal polyps, or inflammatory bowel disease. All other participants had one FDR with CRC, and fell into the “high-risk” category.
The print intervention was a six page booklet titled “If my relative had colon cancer does it mean I will get it?” It included information about CRC, risk factors for CRC including family history, screening tests and recommendations, statements to encourage screening and counterarguments to common barriers to screening. Separate ethnically-targeted (mainly through appropriate photographs and culturally relevant graphics) versions of the materials were created for African Americans, Whites, Latinos (English and Spanish), and Asians. All versions of materials included the same basic text. Three individually-tailored inserts were included in each booklet to address participant-specific readiness to change, barriers to screening, and CRC risk. The latter utilized a thermometer graphic to convey the participant’s risk category and his/her specific personal risk factors that contributed to the risk category.
At the 6-month follow-up, trained counselors delivered the brief telephone intervention to unscreened participants in the intervention condition. Upon completion of the interview (during the same telephone encounter), the computer algorithm indicated if a participant needed counseling and provided participant-specific prompts to counselors, who were blinded to participant group assignment prior to this point. The brief counseling session (9 minutes on average) consisted of standardized scripts explaining the mailed risk assessment, countering barriers, reminding participants of their intentions to get screened, eliciting and addressing other relevant concerns, and provided information about local clinics offering CRC screening services.
Statistical Analysis
Assuming an intraclass correlation of 0.50, 500 participants/condition (250 families, 2 participants/family) provided 80% power to detect a 0.1 risk difference (alpha=0.05) for the stepped cumulative intervention.
Outcome analyses used logistic regression models fit with generalized estimating equations and exchangeable correlation to account for clustering on family. The outcome measure to assess the stepped cumulative intervention (print and telephone) was any CRC screening test obtained at any time during the study period (i.e., at 6 or 12 months). A priori secondary analyses estimated the effect of the print intervention alone (receipt of any test at 6 months), and intervention effects stratified by ethnicity. We also compared the incremental increase in test receipt from 6 (print only) to 12 (print + telephone) months using adjusted confidence interval overlap 21. All analyses were intent-to-treat and included all randomized participants. Participants with missing outcomes were assumed to be unscreened. Individuals reporting test receipt due to symptoms (N=15) were classified as unscreened. Analyses excluding this group yielded similar results. Multivariable analyses were conducted to examine the effects of demographic and other factors, independent of the intervention. All demographic and HBF variables (Tables 2 and 3) were included in each multivariable model (total sample and ethnic specific) except for income (due to high proportion of missing data) and country of birth (due to high correlation with ethnicity). Diagnostics confirmed adequate fit for all models.
Table 2.
Demographic Characteristics of the Sample
| Characteristic | White (N=351) | Latino (N=403) | Afr. Am. (N=284) | Asian (N=242) | Total (N=1280) |
|---|---|---|---|---|---|
|
| |||||
| % | % | % | % | % | |
| Age (years)* | |||||
| Mean (sd) | 52 (9) | 51 (9) | 53 (10) | 51 (10) | 51 (9) |
|
| |||||
| 40–49 | 49 | 56 | 48 | 57 | 53 |
| 50–64 | 40 | 35 | 35 | 29 | 35 |
| ≥ 65 | 11 | 9 | 17 | 14 | 12 |
|
| |||||
| Gender | |||||
| Male | 46 | 41 | 41 | 48 | 44 |
|
| |||||
| Relationship to Case* | |||||
|
| |||||
| Sibling | 26 | 35 | 38 | 33 | 33 |
| Child | 74 | 65 | 62 | 67 | 67 |
|
| |||||
| Marital Status* | |||||
|
| |||||
| Married/ living as married | 70 | 68 | 53 | 75 | 67 |
|
| |||||
| Education* | |||||
|
| |||||
| Some College | 72 | 39 | 57 | 85 | 61 |
|
| |||||
| Household Income* | |||||
|
| |||||
| ≥ $50,000 | 57 | 37 | 31 | 67 | 47 |
|
| |||||
| Health Insurance* | |||||
|
| |||||
| Yes | 89 | 84 | 87 | 94 | 88 |
|
| |||||
| Born in the U.S.+ | |||||
|
| |||||
| Yes | 93 | 73 | 98 | 48 | 63 |
|
| |||||
| Years in the U.S.* | |||||
|
| |||||
| Mean (sd) yrs in U.S. | -- | 30 (13) | -- | 26 (13) | 28 (13) |
p ≤ 0.05 for differences by race/ethnicity, chi-square test for categorical variables, ANOVA for continuous variables;
p ≤0.05 between Latino and Asian
Table 3.
Health Behavior Framework Constructs at Baseline
| Health Behavior Framework Constructs | White (N=351) | Latino (N=403) | Afr. Am. (N=284) | Asian (N=242) | Total (N = 1280) |
|---|---|---|---|---|---|
| Risk Factor Knowledge * | |||||
|
| |||||
| Mean (sd) Continuous (Range = 0–13) | 8.42 (1.94) | 7.86 (1.83) | 7.93 (1.92) | 8.33 (2.01) | 8.12 (1.93) |
|
| |||||
| Knowledge of Screening Guidelines | |||||
|
| |||||
| Mean (sd) Continuous (Range = 0–4) | 1.33 (1.03) | 1.38 (1.06) | 1.31 (1.01) | 1.37 (1.12) | 1.35 (1.05) |
|
| |||||
| Cancer Distress* | |||||
|
| |||||
| Mean (sd) Continuous (Range = 0–3) | 1.61 (0.42) | 1.73 (0.47) | 1.61 (0.47) | 1.61 (0.43) | 1.65 (0.45) |
|
| |||||
| Barriers | |||||
|
| |||||
| Mean (sd) Continuous (Range = 0–13) | 3.97 (2.00) | 4.20 (2.19) | 3.79 (2.32) | 4.08 (2.35) | 4.02 (2.20) |
|
| |||||
| Patient Provider Interaction* | |||||
|
| |||||
| Mean (sd) Continuous (Range = 0–3) | 0.83 (0.95) | 0.58 (0.79) | 0.56 (0.77) | 0.59 (0.79) | 0.65 (0.84) |
|
| |||||
| Assessed Cancer Risk | |||||
|
| |||||
| Very high risk (%) | 17 | 20 | 17 | 19 | 18 |
|
| |||||
| Perceived Risk* | |||||
|
| |||||
| Very likely (%) | 4 | 8 | 11 | 7 | 7 |
p ≤ 0.05 for differences by race/ethnicity, chi-square tests for categorical variables, ANOVA for continuous variables
Results
Participants
Table 2 provides demographic characteristics, by ethnicity. Education levels overall were fairly high (61% had at least some college), and most (88%) reported having some form of public (i.e., Medicare, Medicaid) or private insurance. Significant variability was observed among ethnic groups for education, income, and insurance, with Whites and Asian Americans showing greater socioeconomic advantage.
Health Behavior Framework Factors at Baseline
Descriptive information on Health Behavior Framework factors assessed at baseline is included in Table 3. On average participants correctly answered 8 out of 13 risk factor knowledge items, with minor ethnic differences. Knowledge of screening guidelines was low, with an average of only 1.35 of the 4 items correctly answered. Perceived risk for CRC was not high, with only 7% of the sample indicating that they were “very likely” to develop CRC in their lifetime, 59% “somewhat likely” and 35% “not likely”. Overall, participants endorsed 4 barriers out of 13 assessed. Whites scored highest on the composite measure of patient provider interactions regarding CRC screening. Approximately 18% of participants were categorized as “Very High Risk” for intervention purposes. In this category, 58% had an FDR with CRC diagnosed before age 50 years, 35% had two or more FDRs with CRC, and 11% had a history of polyps.
Randomization Check
No significant differences were observed between Intervention and Control groups at baseline (data not shown). Attrition was not different between Intervention and Control groups at 6 month (22% vs 17%) or 12 month (27% vs. 26%) follow-ups.
Intervention Effect
As indicated in Table 4, the print intervention alone (OR=1.6) and the cumulative stepped intervention (OR=1.6) both demonstrated statistically significant effects in the total sample. Intervention effects were modest with print alone demonstrating a 5 percentage point advantage (50% relative advantage) and the stepped cumulative intervention an 8 percentage point advantage (44% relative advantage) over the control conditions. There was no significant effect size difference between the print alone versus the cumulative (print+telephone) interventions (p>.05); however, the study was not powered for this contrast. In stratified analyses, the combined intervention was effective in all ethnic groups except African Americans while the print intervention alone did not achieve statistical significance in any ethnic group.
Table 4.
Intervention Effect: Colorectal Cancer Screening Rates at 6 and 12 Month Follow-up
| 6 Month Follow-Up Print Alone n=1280 |
12 Month Follow-Up Cumulative Print + Phone n=1280 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Intervention % | Control % | OR | p-value | Intervention % | Control % | OR | p-value | |
| TOTAL SAMPLE (n=1280) | 15 | 10 | 1.6 | .006 | 26 | 18 | 1.6 | .001 |
| White (n=351) | 15 | 10 | 1.5 | .182 | 30 | 20 | 1.7 | .045 |
| Latino (n=403) | 14 | 8 | 1.7 | .117 | 24 | 14 | 1.9 | .027 |
| African American (n=284) | 12 | 10 | 1.3 | .684 | 23 | 22 | 1.1 | .906 |
| Asian (n=242) | 18 | 10 | 2.0 | .073 | 28 | 17 | 1.9 | .039 |
OR, odds ratio. Results from GEE logistic regression models accounting for clustering within families.
Multivariable Analyses
In multivariable analyses, we tested the independent effects of the cumulative stepped intervention and other factors that could influence CRC screening, in the overall sample and in separate regression models for each ethnic group (Table 5). A significant intervention effect (OR=1.9) was observed in the total sample and in stratified analyses among Whites (OR=1.7), Latinos (OR=3.9), and Asians (OR=2.6), but not African Americans. An interaction term (ethnicity x intervention) added to the total sample model was not significant (results not shown). In the total sample, in addition to the intervention, older age and having insurance, fewer barriers, and higher patient provider communication predicted increased likelihood of screening. Barriers emerged as a significant predictor among Latinos and African Americans only. Insurance was a significant and strong predictor (OR = 18.6) among Latinos only, although there was a positive trend among African Americans (p = .058). Patient provider interaction was a significant predictor only among Whites and Latinos.
Table 5.
Multivariable Logistic Regression Models for Colorectal Cancer Screening Receipt at 12 month Follow-Up, Overall and Stratified by Ethnicity
| Variable | Categories | Total (N=866) OR |
White (N=257) OR |
Latino (N=254) OR |
Afr. Am. (N=185) OR |
Asian (N=170) OR |
|---|---|---|---|---|---|---|
| Treatment |
Control Intervention |
1.9* | 1.7* | 3.9* | 1.2 | 2.6* |
| Marital status |
Other Married |
1.2 | 0.9 | 0.8 | 1.4 | 4.9* |
| Education |
<College >Some college |
0.9 | 0.5* | 1.0 | 0.7 | 4.4* |
| Insurance |
Uninsured Insured |
3.6* | 2.2 | 18.6* | 5.2+ | 2.3 |
| Sex |
Male Female |
1.0 | 0.8 | 0.6 | 1.7 | 1.7 |
| Age | (10-year increase) | 1.3* | 1.1 | 2.8* | 1.3 | 1.5 |
| Relation to Case |
Child Sibling |
0.9 | 1.0 | 0.8 | 0.7 | 1.2 |
| Cancer History |
No Yes |
0.8 | 0.6 | 1.7 | 0.4 | 0.5 |
| Actual Cancer Risk Category |
High Risk Very High Risk |
1.5 | 1.3 | 2.0 | 1.4 | 1.9 |
| Perceived Risk to develop CRC |
Not/Somewhat Very Likely |
1.5 | 4.5 | 1.0 | 2.7 | 0.6 |
| Patient-Provider Interaction | (continuous) | 1.4* | 1.4* | 1.7* | 1.4 | 1.3 |
| Barriers | (continuous) | 0.9* | 0.9 | 0.8* | 0.8* | 0.9 |
| Risk Factor Knowledge | (continuous) | 1.1 | 1.1 | 1.1 | 0.9 | 1.2 |
| Screening Guidelines Knowledge | (continuous) | 1.0 | 1.0 | 1.1 | 0.9 | 1.0 |
| Cancer Distress | (continuous) | 1.2 | 1.0 | 1.7 | 0.8 | 1.9 |
| Intraclass correlation | −.01 | −.09 | .25 | −.14 | −.01 | |
OR, odds ratio. Estimates from GEE logistic regression models accounting for clustering by family. Sample sizes reduced due to missing data for some predictors.
p<.05,
p=.058
Discussion
Overall, our stepped, nested intervention (mail + telephone) achieved a statistically significant, though modest, increase in CRC screening rates (OR=1.6) in our ethnically-diverse sample of FDRs of CRC cases. This effect was robust in that it emerged in both the unadjusted as well as in the multivariable analysis (OR=1.9) that controlled for demographic and other factors. The print intervention alone also yielded a statistically significant effect (OR=1.6) that was not significantly different from that observed for the cumulative intervention. This suggests the need for careful consideration of the value of investing in more resource-intense interventions at the population level given the modest yield in added benefit. The effect size we observed is comparable to those reported in the literature for interventions targeting those with a family history of CRC. Kinney and colleagues (2014) reported an OR of 2.8 for a high intensity intervention delivered by certified genetic counselors compared to a mailed brochure 22. Lowery and colleagues (2014) observed an OR of 1.32 for tailored telephone education compared to a mailing among a sample with a family history of colorectal cancer 23.
Unlike prior studies conducted within clinical settings, we were not able to provide a direct link to services given participating relatives resided across California, had differing insurance coverage for colorectal cancer screening, and received health care through many different providers and systems. Also, only individuals who were not up to date on screening were included in the study and this group may have been less inclined to get screened compared the general population of FDRs. More intensive interventions may be necessary for this subgroup of unscreened/under-screened FDRs.
Stratified analyses showed that in general the cumulative stepped intervention was effective among Whites, Latinos and Asians but not among African Americans for whom the screening rates, at 12 month follow-up, in the intervention (23%) and control (22%) conditions were essentially identical. Therefore, inadequate sample size is not a likely explanation for the lack of effect. The lack of effect appears to be related to the fact that the control group showed the same rate of improvement from baseline as the intervention group. Ethnic differences in demographic and Health Behavior Framework factors do not offer easy explanations for this finding. While African Americans in our sample were slightly more disadvantaged in education and income compared to Whites and Asians, they were very similar to Latinos among whom there was a significant intervention effect. Similarly, Health Behavior Framework factors such as barriers, patient-provider interaction, or knowledge of CRC risk factors and CRC screening were not substantially different among African Americans compared to other groups.
Consistent with the literature, patient provider interaction emerged as a particularly important influence on screening in multivariable analyses 24, 25. Insurance was an important predictor of screening among Latinos and African Americans. Latinos and African Americans in our sample reported lower incomes than Asians and Whites. Therefore it is possible that Latinos and African Americans without insurance were less likely to have the resources for out of pocket costs of screening and thus less likely to be screened. Although African Americans and Latinos did not report more barriers compared to Whites and Asians, barriers were significant predictors of screening receipt only among these two groups, further supporting the possibility that it is not the mere presence of barriers but rather the ability to overcome barriers that drives screening. Cancer history, actual or perceived cancer risk, knowledge, and cancer distress were not predictive in any of the groups.
Limitations of this study include the relatively low response rate among CRC cases who provided information on their relatives, which compromises generalizability. Reliance on self-reports to assess CRC screening is another limitation. Also, we did not provide risk-specific screening recommendations, which could be viewed as a limitation by some. Clinical guidelines recommend a tailored approach to screening, based on risk-stratification, including among those with a family history of CRC 26. However, given that we recruited ethnically diverse participants from a state cancer registry, had no institutional relationship with them, and did not know whether their insurance or personal finances could/would cover the costs of specific tests, we chose the more conservative option of not being directive regarding specific screening tests. Our intervention included information on all screening tests and encouraged participants to discuss their family history and particular risk factors with their physician to determine which test would be the most appropriate for them.
This study was one of the first to evaluate the effect of a theory-based intervention on CRC screening in an ethnically-diverse sample of FDRs of CRC cases. Due to sample size limitations most studies that include diverse samples are not able to examine intervention effects separately in each ethnic group and therefore assume that the overall intervention effect applies to all sub-groups. Our study was unique in that, due to our deliberate oversampling of ethnic minority cancer cases, we were able to conduct secondary stratified analyses to explore intervention effects in subgroups. These analyses revealed that the intervention effect was strongest among Latinos followed by Asians and Whites and that the intervention was not effective among African Americans. Such nuanced information on interventions effectiveness has important implications for broader implementation and dissemination of successful public health interventions.
In summary, our stepped approach was able to produce a significant intervention effect on CRC screening in a high-risk population. Although the observed effect was modest, such an effect applied at the population level has the potential to have significant public health impact. Future research may need to explore cost-efficient interventions that can produce larger effect sizes as well as interventions that are effective across all ethnic subgroups.
Acknowledgments
This publication was supported by Grant 1R01 CA75367 from the NIH, National Cancer Institute. Catherine Crespi’s involvement in this manuscript was supported by Grant CA16024 from the National Cancer Institute.
Footnotes
Conflict of interest statement: No financial disclosures were reported by any of the authors.
References
- 1.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42:216–227. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Bleiker EM, Menko FH, Taal BG, et al. Screening behavior of individuals at high risk for colorectal cancer. Gastroenterology. 2005;128:280–287. doi: 10.1053/j.gastro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Colorectal Cancer Facts & Figures. Atlanta, Georgia: 2014. [Google Scholar]
- 4.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 5.Steinbrecher A, Fish K, Clarke CA, West DW, Gomez SL, Cheng I. Examining the association between socioeconomic status and invasive colorectal cancer incidence and mortality in California. Cancer Epidemiol Biomarkers Prev. 2012;21:1814–1822. doi: 10.1158/1055-9965.EPI-12-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White A, Vernon SW, Franzini L, Du XL. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010;116:4622–4631. doi: 10.1002/cncr.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, van Ballegooijen M, Zauber AG, Jemal A. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Biomarkers Prev. 2012;21:728–736. doi: 10.1158/1055-9965.EPI-12-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Available from: http://www.ahrq.gov/clinic/uspstf08/colocancer/colors.htm.
- 9.Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2014;64:30–51. doi: 10.3322/caac.21212. [DOI] [PubMed] [Google Scholar]
- 10.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening test use--United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]
- 12.Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med. 2014;46:228–236. doi: 10.1016/j.amepre.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 13.May FP, Bromley EG, Reid MW, et al. Low uptake of colorectal cancer screening among African Americans in an integrated Veterans Affairs health care network. Gastrointest Endosc. 2014;80:291–298. doi: 10.1016/j.gie.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rees G, Martin PR, Macrae FA. Screening participation in individuals with a family history of colorectal cancer: a review. Eur J Cancer Care (Engl) 2008;17:221–232. doi: 10.1111/j.1365-2354.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 15.Carey M, Sanson-Fisher R, Macrae F, et al. Improving adherence to surveillance and screening recommendations for people with colorectal cancer and their first degree relatives: a randomized controlled trial. BMC Cancer. 2012;12:62. doi: 10.1186/1471-2407-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rawl SM, Champion VL, Scott LL, et al. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Educ Couns. 2008;71:215–227. doi: 10.1016/j.pec.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastani R, Glenn BA, Taylor VM, et al. Integrating theory into community interventions to reduce liver cancer disparities: The Health Behavior Framework. Prev Med. 2010;50:63–67. doi: 10.1016/j.ypmed.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastani R, Maxwell AE, Bradford C, Das IP, Yan KX. Tailored risk notification for women with a family history of breast cancer. Prev Med. 1999;29:355–364. doi: 10.1006/pmed.1999.0556. [DOI] [PubMed] [Google Scholar]
- 19.Maxwell AE, Bastani R, Crespi CM, Danao LL, Cayetano RT. Behavioral mediators of colorectal cancer screening in a randomized controlled intervention trial. Prev Med. 2011;52:167–173. doi: 10.1016/j.ypmed.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- 21.Pestman WR. Two elementary statistical coverage problems. Elemente der Mathematik. 2011;66:30–37. [Google Scholar]
- 22.Kinney AY, Boonyasiriwat W, Walters ST, et al. Telehealth personalized cancer risk communication to motivate colonoscopy in relatives of patients with colorectal cancer: the family CARE Randomized controlled trial. J Clin Oncol. 2014;32:654–662. doi: 10.1200/JCO.2013.51.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowery JT, Horick N, Kinney AY, et al. A randomized trial to increase colonoscopy screening in members of high-risk families in the colorectal cancer family registry and cancer genetics network. Cancer Epidemiol Biomarkers Prev. 2014;23:601–610. doi: 10.1158/1055-9965.EPI-13-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Courtney RJ, Paul CL, Sanson-Fisher RW, et al. Individual- and provider-level factors associated with colorectal cancer screening in accordance with guideline recommendation: a community-level perspective across varying levels of risk. BMC Public Health. 2013;13:248. doi: 10.1186/1471-2458-13-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boonyasiriwat W, Hung M, Hon SD, et al. Intention to undergo colonoscopy screening among relatives of colorectal cancer cases: a theory-based model. Ann Behav Med. 2014;47:280–291. doi: 10.1007/s12160-013-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124(2):544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]