Abstract
Background
Insufficient data exist on population-based trends in morbidity and mortality to determine the success of prevention strategies and improvements in health care delivery in stroke. The aim of this study was to determine trends in incidence and outcome (1-year mortality, 28-day case-fatality) in relation to management and risk factors for stroke in the multi-ethnic population of Auckland, New Zealand (NZ) over 30-years.
Methods
Four stroke incidence population-based register studies were undertaken in adult residents (aged ≥15 years) of Auckland NZ in 1981–1982, 1991–1992, 2002–2003 and 2011–2012. All used standard World Health Organization (WHO) diagnostic criteria and multiple overlapping sources of case-ascertainment for hospitalised and non-hospitalised, fatal and non-fatal, new stroke events. Ethnicity was consistently self-identified into four major groups. Crude and age-adjusted (WHO world population standard) annual incidence and mortality with corresponding 95% confidence intervals (CI) were calculated per 100,000 people, assuming a Poisson distribution.
Results
5400 new stroke patients were registered in four 12 month recruitment phases over the 30-year study period; 79% were NZ/European, 6% Māori, 8% Pacific people, and 7% were of Asian or other origin. Overall stroke incidence and 1-year mortality decreased by 23% (95% CI 5%-31%) and 62% (95% CI 36%-86%), respectively, from 1981 to 2012. Whilst stroke incidence and mortality declined across all groups in NZ from 1991, Māori and Pacific groups had the slowest rate of decline and continue to experience stroke at a significantly younger age (mean ages 60 and 62 years, respectively) compared with NZ/Europeans (mean age 75 years). There was also a decline in 28-day stroke case fatality (overall by 14%, 95% CI 11%-17%) across all ethnic groups from 1981 to 2012. However, there were significant increases in the frequencies of pre-morbid hypertension, myocardial infarction, and diabetes mellitus, but a reduction in frequency of current smoking among stroke patients.
Conclusions
In this unique temporal series of studies spanning 30 years, stroke incidence, early case-fatality and 1-year mortality have declined, but ethnic disparities in risk and outcome for stroke persisted suggesting that primary stroke prevention remains crucial to reducing the burden of this disease.
Introduction
The burden of stroke is large and increasing worldwide, with notable ethnic/racial disparities.[1–7] Effective primary stroke prevention strategies are therefore critical in ageing populations[1,8] and where there is increasing numbers of people surviving with stroke-related disability and ongoing risk.[9] However, because of the considerable challenges to determining temporal trends in incidence and outcome of stroke, there is limited information on the impact of declines (or increases) in rates and case fatality in whole populations. This leaves uncertainties regarding the impact of public health policies and improvements in health service delivery for this important disease.[10–13]
As a large proportion of the burden of stroke is borne outside the hospital sector, it is crucial that incident cases are ascertained and studied in a population-wide context.[10,11,13] Over the last two decades new primary and secondary preventative strategies have been implemented but the effect of the strategies on stroke burden have not been reliably assessed.[14] Accurate population-based data on population trends in stroke incidence and risk factors reflect the success or otherwise of prevention strategies, while case-fatality and mortality trends reflect management and natural history of stroke. All these indices are crucial for evidence-based planning and resource allocation for stroke services and preventive strategies. Yet population-based stroke incidence studies are complex,[10,13] and are limited in number when compared to those using hospital-based registers, mortality data, or studies limited to certain age groups. To address this gap, we used population-based data from the four Auckland Regional Community Stroke (ARCOS I—IV) studies (1981–1982, 1991–1992, 2002–2003 and 2011–2012) to determine 30-year trends in stroke incidence, case-fatality and mortality in the four major ethnic groups (NZ European, Asian, Māori and Pacific Islanders) in Auckland, New Zealand (NZ).
Materials and Methods
Study populations, case ascertainment and diagnostic criteria
The study population and methods of case ascertainment in the four ARCOS studies are described in detail elsewhere.[12,15–18] In brief, these studies utilised harmonised population-based registers of all new cases of stroke in the greater Auckland region over consistent 12 month calendar periods (total resident population aged ≥15 grew from 596,580 in 1981–1982 to 1,119,192 in 2011–2012). Multiple overlapping methods of case ascertainment, and standard WHO clinical diagnostic criteria for stroke were utilised.[19] For the 1991–1992 ARCOS study, all hospital-managed stroke events in the region, and a cluster survey of 25% of all primary care general practitioners (GPs) records, were used to estimate the total number of non-hospitalised, non-fatal stroke events.[12] For the 1981–1982 ARCOS study, a cluster sample of 50% of all registered GPs was used to identify a representative sample of stroke events in the study population. All deceased cases were identified through hospital admissions and discharge reports, systematic searches of post-mortem reports and death certificates in national registries. Data for fatal non-hospitalised SAH cases were collected from medical records only. In the two most recent studies (2002–2003 and 2011–2012)[12,15] no cluster sampling was used and instead the whole study population was monitored for new stroke events. To ensure a complete prospective case-ascertainment in 2002–2003 and 2011–2012 studies we undertook daily searches of hospital presentation data, where a diagnosis suggesting stroke or transient ischaemic attack (TIA) was recorded for all public hospitals and emergency departments, CT/MRI records and hospital discharge registers; weekly checks of all private hospitals, rest homes, and community health services (general practices, hospital outpatient clinics and rehabilitation centres); quarterly checks of coroner/autopsy records, death certificates (from Registrar of Births, Deaths and Marriages) to identify people who had died with any mention of stroke, and NZ Health Information Service data of all fatal and non-fatal stroke/TIA cases in the study population. In 2002–2003 and 2011–2012 studies strokes were subdivided into pathological types (ischaemic stroke [IS], primary intracerebral haemorrhage [PICH], subarachnoid haemorrhage [SAH]) according to neuroimaging (CT, MRI, or necropsy) findings. SAH was defined as “an abrupt onset of a severe headache and/or impaired consciousness or focal neurological signs associated with at least one of the following findings: uniform blood staining of the cerebrospinal fluid; CT evidence of blood in the subarachnoid space; cerebral angiographic identification of an aneurysm or arteriovenous malformation, or identification of SAH at surgery or at autopsy. This definition excludes PICH with extension into the subarachnoid space and subarachnoid bleeding due to trauma, neoplasms, or infections”.[20] All new stroke cases, including suspected strokes, were ascertained by study researchers across all four studies. Consistent diagnostic criteria were used across all studies to allow valid comparisons. Cases without imaging or pathological necropsy confirmation of stroke type were classified as stroke of undetermined type (undetermined). Cardiovascular risk factors and medication use were ascertained via medical records across all studies. All study participants were followed up for 1 year for fatal/non-fatal outcomes. Ethnicity was identified by self-report across all four studies. Regional Ethics Committees approved all four studies.
Statistical analyses
For the 1981–1982 and 2002–2003 ARCOS studies participants selected one self-identified ethnicity to be used for the analyses. For the 1991–1992 and 2011–2012 ARCOS studies, multiple self-identified ethnicities were recorded and ethnicity was prioritised corresponding to the allocation process and using NZ census definitions for Māori, Pacific, Asian/other (“other” included ethnic groups such as other European, Middle Eastern, Latin American and African ethnicities) and NZ Europeans as per the NZ Census.[21] When multiple ethnicities were self-identified, ethnicity was prioritized as a single ethnic group for the purposes of analysis in the following order: Māori, Pacific, Asian/other, NZ European. For example if a person self-identified as NZ European and Māori, they were classified as Māori, and if they self-identified as Pacific and Chinese they were classified as Pacific. Ethnicity information was not available for 2 of the ARCOS 2011–2012 study participants and 46 of the ARCOS 2002–2003 study participants, who were excluded from the ethnicity analyses. Age, sex and ethnic structure of the corresponding Auckland census data were used as the denominator in calculating incidence.
Crude annual age-, sex-, and ethnic-specific stroke incidence (first-ever-in-a-lifetime events) and attack rates (all events, including recurrent strokes), 28-day case fatality (proportion [%] of people with stroke who died within 28 days of stroke onset among the total number of people with incident stroke) and mortality rates (number of people with incident stroke who died in the study population over one-year follow-up period [nominator] divided by the study population at risk [denominator]), with 95% confidence intervals (CI) per 100,000 people were calculated assuming a Poisson distribution. Sampling procedure differences between ARCOS 1981–1982 and 1991–1992 studies were taken into account when computing CI and standard error (SE) of all estimated rates, as described elsewhere.[12] Standardised rates were calculated using the direct method and age-standardised to WHO ‘World’ standard population.[22] Rate ratios (RR) were calculated to evaluate differences in age-adjusted rates between the 1981–1982 and 2011–2012 study periods, and the Wald statistic test of heterogeneity across age groups were performed.[12] For categorical variables, the Cochrane-Armitage test was used to assess whether there was evidence of a statistically significant trend. For continuous variables, analysis of variance or the Kruskal-Wallis test was used.
Completeness of case ascertainment based on the sources of notification was determined using capture-recapture techniques.[23] This involved conducting log-linear modelling assuming a Poisson distribution (unadjusted for sample procedures),[24] and used the four main sources of notification: hospital, general practitioner, death certificate and other sources. The final model with the least deviance, (deviance is an indicator of ‘goodness of fit’ and a lower deviance suggests a better fitting model), for all studies included the main effects of the four sources and the 3-way interaction between hospital, general practitioner and death certificate. A Bonferroni-corrected threshold of p = 0.00013 (based on 380 tests conducted 0.05/380) was used to indicate statistical significance after adjusting for multiple testing.
To compare our findings with similar population-based studies carried out in other countries we searched Medline with the terms”stroke”, “epidemiology”, “incidence”, “ethnic/racial”, “trend(s)”, and “population or community based” for population-based studies of stroke carried out between 1980 and 2014 and published in English. Only population-based studies from high-income countries that reported age-specific raw numbers of first-ever-in-a-lifetime strokes (numerator and populations at risk (denominator) sufficient to calculate incidence rates age-standardised to WHO ‘World’ standard population[22] were included in the analysis. We also compared trends in stroke incidence and mortality rates from our series of Auckland Regional Community Stroke (ARCOS) studies (1981–1982, 1991–1992, 2002–2003, 2011–2012) with those found in the Global Burden of Disease (GBD) 2010 study.[1,25]
Results and Discussion
Results
There were 5400 new stroke patients (47% men) registered across the four studies (Tables 1 and 2). Over the 30-year study period, the proportion of patients presenting with recurrent strokes decreased by 2.95% (95% CI 0.60%-5.83%) from 24.3% to 21.4%. The mean age of individuals with stroke increased (on average by 3 years) across ethnic groups, except for Asian/other ethnic group where age decreased by 4.6 years (95% CI 3.8–14.1). However, the 15-year gap between in the mean age at stroke onset in Māori and Pacific (youngest age group) compared with the mean age of stroke in NZ European (oldest age group) persisted over the 30 years. Although NZ European patients constituted the largest proportion of strokes, their proportional frequency reduced by 23% (95% CI 21.0%-26.0%) over the same period, while the proportional frequency of stroke patients from all other ethnic groups increased, with the largest increase being 8-fold for the Asian/other ethnic groups (Table 1).
Table 1. Baseline characteristics and 28-day outcome of stroke events in each ARCOS register.
| 1981–1982 | 1991–1992 | 2002–2003 | 2011–2012 | P for trend §§ | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Demographics | |||||
| Male | 662 (48.7) | 817 (46.4) | 892 (46.0) | 1012 (48.3) | 0.977 |
| Age, mean (±SD), years*** | |||||
| NZ/European | 72.2 (12.8) | 73.5 (12.1) | 75.6 (12.5) | 75.3 (13.4) | <0.0001 § |
| Māori | 56.7 (14.2) | 55.0 (16.1) | 60.7 (14.3) | 59.6 (15.5) | 0.04 |
| Pacific | 55.8 (9.0) | 59.7 (15.0) | 64.5 (13.6) | 61.6 (14.9) | 0.002 |
| Asian/other | 72.1 (12.8) | 65.6 (13.2) | 65.9 (13.9) | 67.5 (13.3) | 0.199 |
| Overall | 71.2 (13.3) | 71.6 (13.5) | 73.0 (13.8) | 71.6 (14.9) | 0.001 |
| Ethnicity | |||||
| NZ/European | 1248 (91.8) | 1532 (87.0) | 1431 (75.6) | 1434 (68.5) | <0.0001** § |
| Māori | 60 (4.4) | 82 (4.7) | 102 (5.4) | 138 (6.6) | |
| Pacific | 32 (2.4) | 111 (6.3) | 197 (10.4) | 270 (12.9) | |
| Asian/other | 20 (1.5) | 36 (2.0) | 162 (8.6) | 252 (12.0) | |
| Source of notification | <0.0001** § | ||||
| Hospital | 1082 (80.0) | 1092 (62.0) | 1361 (70.2) | 1733 (82.7) | |
| General practitioner | 30 (2.2) | 368 (20.9) | 117 (6.0) | 2 (0.1) | |
| Death certificate | 104 (7.7) | 161 (9.1) | 95 (4.9) | 15 (0.7) | |
| Other sources | 136 (10.1) | 140 (8.0) | 365 (18.8) | 346 (16.5) | |
| Premorbid risk factors (from medical notes) | |||||
| Current smoking | |||||
| NZ/European | 330 (26.7) | 330 (21.7) | 162 (12.6) | 178 (12.8) | <0.0001 § |
| Māori | 32 (53.3) | 41 (50.6) | 35 (38.9) | 55 (40.4) | 0.159 |
| Pacific | 12 (37.5) | 31 (28.7) | 23 (13.1) | 65 (24.4) | 0.001 |
| Asian/other | 0 | 9 (25.0) | 15 (10.3) | 23 (9.3) | 0.013 |
| Overall | 374 (27.7) | 411 (23.5) | 241 (14.0) | 322 (15.8) | <0.0001 § |
| High blood pressure | |||||
| NZ/European | 632 (51.1) | 802 (52.7) | 783 (57.7) | 947 (66.0) | <0.0001 § |
| Māori | 38 (63.3) | 41 (52.6) | 63 (62.4) | 85 (61.6) | 0.690 |
| Pacific | 22 (68.8) | 49 (45.0) | 124 (65.6) | 178 (65.9) | 0.022 |
| Asian/other | 8 (40.0) | 18 (50.0) | 88 (58.7) | 184 (73.0) | <0.0001 § |
| Overall | 700 (51.5) | 910 (52.1) | 1079 (59.0) | 1394 (66.5) | <0.0001 § |
| Myocardial infarction | |||||
| NZ/European | 146 (11.8) | 273 (17.9) | 190 (13.5) | 401 (28.0) | <0.0001 § |
| Māori | 8 (13.8) | 9 (11.4) | 12 (11.9) | 25 (18.1) | 0.266 |
| Pacific | 2 (6.7) | 3 (2.8) | 15 (7.9) | 34 (12.6) | 0.004 |
| Asian/other | 0 | 3 (8.3) | 17 (11.0) | 47 (18.7) | 0.003 |
| Overall | 156 (11.5) | 288 (16.5) | 240 (12.7) | 507 (24.2) | <0.0001 § |
| Previous stroke | |||||
| NZ/European | 314 (25.3) | 404 (26.4) | 361 (25.5) | 308 (21.5) | 0.015 |
| Māori | 14 (23.3) | 21 (25.6) | 12 (11.9) | 21 (15.2) | 0.041 |
| Pacific | 2 (6.3) | 25 (22.5) | 54 (27.8) | 62 (23.2) | 0.234 |
| Asian/other | 0 | 6 (16.7) | 36 (23.1) | 56 (22.3) | 0.067 |
| Overall | 330 (24.3) | 456 (25.9) | 477 (25.1) | 448 (21.4) | 0.016 |
| Diabetes mellitus | |||||
| NZ/European | 98 (7.9) | 193 (12.6) | 179 (12.7) | 236 (16.5) | <0.0001 § |
| Māori | 20 (33.3) | 19 (24.4) | 35 (34.7) | 41 (29.7) | 0.975 |
| Pacific | 12 (46.2) | 16 (15.0) | 69 (36.1) | 117 (43.3) | 0.0003 |
| Asian/other | 4 (20.0) | 8 (22.2) | 40 (26.1) | 77 (30.6) | 0.131 |
| Overall | 134 (10.0) | 236 (13.6) | 329 (17.4) | 471 (22.5) | <0.0001 § |
| Atrial fibrillation | |||||
| NZ/European | NA | NA | 328 (23.7) | 460 (32.1) | <0.0001 § |
| Māori | NA | NA | 29 (28.7) | 42 (30.4) | 0.774 |
| Pacific | NA | NA | 41 (21.5) | 62 (23.0) | 0.704 |
| Asian/other | NA | NA | 18 (11.9) | 47 (18.7) | 0.075 |
| Overall | NA | NA | 416 (22.0) | 611 (29.2) | <0.0001 § |
| Premorbid medication | |||||
| Blood pressure lowering meds | |||||
| NZ/European | 428 (35.2) | 539 (35.2) | 712 (50.8) | 910 (63.5) | <0.0001 § |
| Māori | 26 (43.3) | 21 (25.6) | 49 (48.5) | 79 (57.2) | 0.001 |
| Pacific | 16 (50.0) | 30 (27.0) | 94 (49.2) | 168 (62.2) | <0.0001 § |
| Asian/other | 6 (30.0) | 14 (38.9) | 73 (47.1) | 163 (64.7) | <0.0001 § |
| Overall | 476 (35.0) | 604 (34.3) | 942 (49.0) | 1321 (63.0) | <0.0001 § |
| Antiplatelet agents | |||||
| NZ/European | 314 (27.4) | 358 (23.4) | 665 (48.2) | 707 (49.3) | <0.0001 § |
| Māori | 12 (21.4) | 7 (8.5) | 29 (29.6) | 60 (43.5) | <0.0001 § |
| Pacific | 8 (28.6) | 14 (12.6) | 63 (33.5) | 115 (42.6) | <0.0001 § |
| Asian/other | 4 (25.0) | 8 (22.2) | 56 (36.6) | 117 (46.4) | 0.001 |
| Overall | 338 (24.8) | 387 (22.0) | 832 (42.9) | 999 (47.7) | <0.0001 § |
| Anticoagulants | |||||
| NZ/European | NA | 33 (2.2) | 137 (9.9) | 117 (8.2) | <0.0001 § |
| Māori | NA | 10 (12.2) | 12 (12.2) | 7 (5.1) | 0.052 |
| Pacific | NA | 2 (1.8) | 27 (14.1) | 24 (8.9) | 0.172 |
| Asian/other | NA | 0 | 8 (5.2) | 14 (5.6) | 0.269 |
| Overall | NA | 45 (2.6) | 185 (9.9) | 162 (7.7) | <0.0001 § |
| Lipid lowering drugs | |||||
| NZ/European | NA | NA | 213 (15.6) | 567 (39.5) | <0.0001 § |
| Māori | NA | NA | 13 (13.4) | 58 (42.0) | <0.0001 § |
| Pacific | NA | NA | 19 (10.4) | 117 (43.3) | <0.0001 § |
| Asian/other | NA | NA | 27 (18.0) | 116 (46.0) | <0.0001 § |
| Overall | NA | NA | 272 (14.4) | 858 (40.9) | <0.0001 § |
| Management | |||||
| Admission to hospital within 28 days of stroke onset | |||||
| NZ/European | 768 (61.5) | 1088 (71.0) | 1283 (89.7) | 1291 (90.0) | <0.0001 § |
| Māori | 46 (76.7) | 73 (89.0) | 99 (97.1) | 124 (89.9) | 0.021 |
| Pacific | 22 (68.8) | 87 (78.4) | 188 (95.4) | 259 (95.9) | <0.0001 § |
| Asian/other | 14 (70.0) | 28 (77.8) | 157 (96.9) | 230 (91.3) | 0.010 |
| Overall | 850 (62.5) | 1276 (72.5) | 1727 (91.3) | 1904 (90.9) | <0.0001 § |
| Admission to acute stroke unit | |||||
| NZ/European | NA | NA | 140 (9.8) | 709 (50.8) | <0.0001 § |
| Māori | NA | NA | 32 (31.4) | 61 (46.2) | 0.021 |
| Pacific | NA | NA | 39 (19.8) | 140 (54.1) | <0.0001 § |
| Asian/other | NA | NA | 24 (14.8) | 131 (54.1) | <0.0001 § |
| Overall | NA | NA | 238 (12.3) | 1041 (51.3) | <0.0001 § |
| Neuroimaging, CT/MRI | |||||
| NZ/European | 134 (18.9) | 429 (38.9) | 1236 (86.4) | 1389 (97.1) | <0.0001 § |
| Māori | 18 (50.0) | 50 (67.6) | 99 (97.1) | 134 (97.8) | <0.0001 § |
| Pacific | 6 (42.9) | 50 (57.5) | 180 (91.4) | 261 (97.4) | <0.0001 § |
| Asian/other | 4 (40.0) | 12 (42.9) | 153 (95.0) | 244 (96.8) | <0.0001± |
| Overall | 162 (11.9) | 541 (41.9) | 1694 (87.6) | 2030 (97.2) | <0.0001 § |
| 28-day case-fatality | |||||
| NZ/European | 408 (32.7) | 362 (23.6) | 304 (21.2) | 279 (19.5) | <0.0001 § |
| Māori | 18 (30.0) | 20 (24.4) | 24 (23.5) | 23 (16.7) | 0.033 |
| Pacific | 14 (43.8) | 32 (28.8) | 39 (19.8) | 43 (15.9) | <0.0001 § |
| Asian/other | 10 (50.0) | 7 (19.4) | 24 (14.8) | 47 (18.7) | 0.056 |
| Overall | 450 (33.1) | 421 (23.9) | 407 (20.7) | 393 (18.8) | <0.0001 § |
| Pathological type of stroke | |||||
| Ischaemic stroke | NA | NA | 1380 (71.2) | 1694 (80.8) | <0.0001 § |
| Primary intracerebral haemorrhage | NA | NA | 236 (12.2) | 275 (13.1) | 0.368 |
| Subarachnoid haemorrhage | 90 (6.6) | 76 (4.3) | 96 (5.0) | 87 (4.2) | 0.008 |
| Undetermined | NA | NA | 226 (11.7) | 40 (1.9) | <0.0001 § |
| Time from stroke onset to study assessment (days), median (interquartile range) * | |||||
| NZ/European | 19 (6–38) | 17 (7–64) | 27 (5–129) | 6 (3–27) | <0.0001 § |
| Māori | 31 (5–42) | 18 (6–77) | 25 (4–112) | 7 (3–91) | 0.097 |
| Pacific | 21 (9–58) | 13 (6–57) | 15 (4–132) | 5 (2–27) | <0.0001 § |
| Asian/other | 29 (7–44) | 12 (5–67) | 18 (5–115) | 6 (3–28) | <0.0001 § |
| Overall | 20 (6–38) | 16 (6–64) | 27 (5–139) | 6 (3–29) | <0.0001 § |
| Capture-recapture, missing **** | 131/677 (19.3) | 185/1449 (12.8) | 144/1938 (7.4) | 30/2096 (1.4) | <0.0001 § |
§§ P value calculated using Cochrane-Armitage trend test;
*P value calculated using Kruskal-Wallis test;
**P value calculated using Chi-squared test,
***P value calculated using analysis of variance test,
**** Using log-linear model containing main effects and interaction for hospital x general practitioner x death certificate. Information about medication was collected in a month prior to stroke.
§ P-values that remained significant after applying Bonferroni-correction to adjust for multiple testing for 380 tests (19 variables [age, source of notification, high blood pressure, myocardial infarction, previous stroke, diabetes mellitus, atrial fibrillation, current smoking, blood pressure lowering medication, antiplatelet agents, anticoagulants, lipid lowering drugs, admission to hospital within 28 days of stroke onset, admission to acute stroke unit, neuroimaging/CT/MRI, 28-day case fatality, pathological type of stroke, time from stroke onset, capture-recapture) by 5 ethnic grouping (New Zealand European, Maori, Pacific, Asian/Other and Overall) by four points (ARCOS I 1981/1982, ARCOS II 1991/1992, ARCOS III 2002/2003, ARCOS IV 2011/2012), giving a Bonferroni-corrected p-value threshold of = 0.05/380 tests = 0.00013
Table 2. Crude, age-specific and age-standardised annual stroke incidence rates (first-ever strokes) per 100,000 people-years in Auckland, New Zealand across four study periods (1981–1982, 1991–1992, 2002–2003 and 2011–2012) by sex and ethnicity.
| Age, sex and ethnicity group | 1981–1982 | 1991–1992 | 2002–2003 | 2011–2012 | P for | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | Rate (95% CI) | N | n | Rate (95% CI) | N | n | Rate (95% CI) | N | n | Rate (95% CI) | trend | |
| Total | |||||||||||||
| 15–64* | 518112 | 286 | 55 (46; 64) | 624828 | 347 | 56 (48; 63) | 788106 | 391 | 50 (45; 55) | 956037 | 528 | 55 (51; 60) | |
| 65–74 | 49812 | 260 | 522 (432; 612) | 56388 | 373 | 661 (564; 759) | 59454 | 336 | 565 (505; 626) | 95190 | 363 | 381 (342; 421) | |
| 75–84 | 22965 | 350 | 1524 (1298; 1750) | 31701 | 412 | 1300 (1139; 1460) | 37815 | 438 | 1158 (1050; 1267) | 48387 | 442 | 913 (828; 999) | |
| 85+ | 5691 | 134 | 2355 (1791; 2918) | 8541 | 173 | 2026 (1684; 2367) | 12507 | 258 | 2063 (1811; 2315) | 19578 | 310 | 1583 (1407; 1760) | |
| Total | 596580 | 1030 | 173 (158; 188) | 721458 | 1305 | 181 (168; 194) | 897882 | 1423 | 158 (150; 167) | 1119192 | 1643 | 147 (140; 154) | <0.0001 |
| Standardised § | 156 (143; 170) | 156 (145; 167) | 139 (132; 147) | 119 (114; 125) | <0.0001 | ||||||||
| Male | |||||||||||||
| 15–64* | 256500 | 164 | 64 (50; 78) | 308997 | 197 | 64 (52; 75) | 380139 | 216 | 57 (49; 64) | 461418 | 264 | 57 (50; 64) | |
| 65–74 | 22251 | 158 | 710 (553; 867) | 25452 | 201 | 790 (628; 951) | 28173 | 198 | 703 (605; 801) | 45678 | 211 | 462 (400; 524) | |
| 75–84 | 8742 | 150 | 1716 (1328; 2104) | 11946 | 155 | 1298 (1038; 1557) | 15210 | 189 | 1243 (1065; 1420) | 21759 | 223 | 1025 (890; 1159) | |
| 85+ | 1509 | 38 | 2518 (1386; 3651) | 2421 | 34 | 1404 (932; 1876) | 3633 | 64 | 1762 (1330; 2193) | 6807 | 93 | 1366 (1089; 1644) | |
| Total | 289002 | 510 | 176 (155; 198) | 348816 | 587 | 168 (150; 186) | 427155 | 667 | 156 (144; 168) | 535662 | 791 | 148 (137; 158) | 0.0006 |
| Standardised | 184 (163; 209) | 167 (150; 185) | 156 (144; 168) | 129 (120; 138) | <0.0001 | ||||||||
| Female | |||||||||||||
| 15–64* | 261612 | 122 | 47 (35; 58) | 315831 | 150 | 47 (38; 57) | 407967 | 175 | 43 (37; 49) | 494631 | 264 | 53 (47; 60) | |
| 65–74 | 27561 | 102 | 370 (269; 472) | 30936 | 172 | 556 (437; 675) | 31281 | 138 | 441 (368; 515) | 49509 | 152 | 307 (258; 356) | |
| 75–84 | 14223 | 200 | 1406 (1131; 1682) | 19755 | 257 | 1301 (1096; 1505) | 22605 | 249 | 1102 (965; 1238) | 26634 | 219 | 822 (713; 931) | |
| 85+ | 4182 | 96 | 2296 (1646; 2945) | 6120 | 139 | 2271 (1833; 2709) | 8874 | 194 | 2186 (1879; 2494) | 12771 | 217 | 1699 (1473; 1925) | |
| Total | 307578 | 520 | 169 (149; 190) | 372642 | 718 | 193 (175; 211) | 470727 | 756 | 161 (149; 172) | 583545 | 852 | 146 (136; 156) | <0.0001 |
| Standardised § | 133 (118; 151) | 143 (130; 158) | 124 (115; 133) | 110 (103; 119) | <0.0001 | ||||||||
| European | |||||||||||||
| 15–64* | 422202 | 224 | 53 (43; 63) | 459267 | 233 | 51 (42; 59) | 501426 | 222 | 44 (38; 50) | 450759 | 252 | 56 (49; 63) | |
| 65–74 | 47481 | 238 | 501 (411; 591) | 52125 | 341 | 654 (552; 756) | 48633 | 219 | 450 (391; 510) | 64806 | 239 | 369 (322; 416) | |
| 75–84 | 22209 | 342 | 1540 (1309; 1771) | 30303 | 387 | 1277 (1114; 1441) | 34332 | 378 | 1101 (990; 1212) | 35916 | 354 | 986 (883; 1088) | |
| 85+ | 5577 | 130 | 2331 (1764; 2898) | 8253 | 167 | 2024 (1675; 2372) | 11790 | 233 | 1976 (1722; 2230) | 16776 | 279 | 1663 (1468; 1858) | |
| Total | 497469 | 934 | 188 (171; 205) | 549948 | 1128 | 205 (189; 221) | 596181 | 1052 | 176 (166; 187) | 568257 | 1124 | 198 (186; 209) | 0.992 |
| Standardised § | 153 (139–167) | 150 (139; 163) | 124 (116; 132) | 122 (114; 130) | <0.0001 | ||||||||
| Māori | |||||||||||||
| 15–64* | 52179 | 36 | 69 (37; 101) | 63762 | 48 | 75 (49; 101) | 77742 | 53 | 68 (50; 87) | 88470 | 74 | 84 (65; 103) | |
| 65–74 | 1266 | 6 | 474 (-62; 1010) | 1344 | 3 | 223 (-29; 476) | 2292 | 22 | 960 (559; 1361) | 4452 | 22 | 494 (288; 701) | |
| 75–84 | 336 | 4 | 1190 (-459; 2840) | 429 | 8 | 1865 (573; 3157) | 654 | 10 | 1529 (581; 2477) | 1572 | 19 | 1209 (665; 1752) | |
| 85+ | 51 | 0 | 0 | 72 | 2 | 2778 (-1072; 6628) | 144 | 4 | 2778 (56; 5500) | 243 | 2 | 823 (-318; 1964) | |
| Total | 53832 | 46 | 85 (51; 120) | 65607 | 61 | 93 (65; 121) | 80832 | 89 | 110 (87; 133) | 94737 | 117 | 123 (101; 146) | 0.014 |
| Standardised § | 134 (78; 229) | 168 (116; 241) | 202 (157; 259) | 156 (128; 189) | 0.757 | ||||||||
| Pacific | |||||||||||||
| 15–64* | 33672 | 20 | 59 (23; 96) | 64506 | 51 | 79 (55; 103) | 89724 | 66 | 74 (56; 91) | 107688 | 126 | 117 (97; 137) | |
| 65–74 | 741 | 10 | 1350 (167; 2532) | 2025 | 21 | 1037 (481; 1593) | 3840 | 47 | 1224 (874; 1574) | 6417 | 43 | 670 (470; 870) | |
| 75–84 | 213 | 0 | 0 | 597 | 12 | 2010 (402; 3618) | 1392 | 24 | 1724 (1034; 2414) | 2679 | 29 | 1082 (689; 1476) | |
| 85+ | 33 | 0 | 0 | 108 | 2 | 1852 (-715; 4418) | 246 | 3 | 1220 (-160; 2600) | 582 | 7 | 1203 (312; 2094) | |
| Total | 34659 | 30 | 87 (43; 130) | 67236 | 86 | 128 (96; 160) | 95202 | 140 | 147 (123; 171) | 117366 | 205 | 175 (151; 199) | <0.0001 |
| Standardised § | 147 (80; 269) | 225 (163; 310) | 218 (183; 261) | 197 (171; 226) | 0.374 | ||||||||
| Asian & Other combined | |||||||||||||
| 15–64* | 10059 | 6 | 60 (-8; 127) | 37293 | 15 | 40 (13; 68) | 119214 | 50 | 42 (30; 54) | 309123 | 76 | 25 (19; 30) | |
| 65–74 | 324 | 6 | 1852 (-244; 3947) | 894 | 8 | 895 (-86; 1875) | 4689 | 42 | 896 (625; 1167) | 19515 | 58 | 297 (221; 374) | |
| 75–84 | 207 | 4 | 1932 (-746; 4610) | 372 | 5 | 1344 (166; 2522) | 1437 | 21 | 1461 (836; 2086) | 8220 | 40 | 487 (336; 637) | |
| 85+ | 30 | 4 | 13333 (-5146; 31812) | 108 | 2 | 1852 (-715; 4418) | 327 | 7 | 2141 (555; 3727) | 1971 | 22 | 1116 (650; 1583) | |
| Total | 10620 | 20 | 188 (72; 305) | 38667 | 30 | 78 (40; 115) | 125667 | 120 | 95 (78; 113) | 338829 | 196 | 58 (50; 66) | <0.0001 |
| Standardised § | 360 (185; 701) | 158 (92; 271) | 166 (137; 202) | 68 (59; 79) | <0.0001 | ||||||||
N is the estimated population size and n is the number of events.
* 16–64 in 2011–2012,
§ Age-standardised to WHO world population
Between the 1981–1982 and 2011–2012 studies there were significant increases across all ethnic groups in the proportion of patients admitted to hospital within 28 days of stroke onset (28% [95% CI 26%-31%]), and in patients having neuroimaging verification of stroke pathological types (84.9% [95% CI 62.8%-100.0%]). There were also increases in patients receiving blood pressure lowering (27.0% [95% CI 24.0%-31.0%])) and antiplatelet (aspirin, clopidogrel, dipyridamole: 21.0% [95% CI 17.0%-24.0%]) medications pre-stroke (Table 1). However, there was a significant decrease in the proportion of stroke patients receiving anticoagulants pre-stroke from 2002–2003 to 2011–2012 (2.1% [95% CI 0.66%-3.5%]).
We observed that risk factors prevalence increased significantly over 30 years (Table 1), with the proportion of patients with a history of high blood pressure (≥160/95 mmHg; including people on antihypertensive medication) increasing between the first and last survey by 15.0% (95% CI 11.7%-18.4%), myocardial infarction by 12.6% (95% CI 12.8%-15.1%), and diabetes mellitus by 12.5% (95% CI 10.1%-14.9%), across all ethnic groups except Māori for high blood pressure, myocardial infarction and Type 2 diabetes mellitus, and Asian/other people for diabetes mellitus. However, there was a statistically significant decrease in the frequency of current smokers among stroke patients by 11.9% (95% CI 9.1%-14.8%), except for Pacific people in whom the smoking rate over the last decade increased by almost two times. There was also a noticeable reduction in the proportional frequency of strokes due to SAH (2.5% [95% CI 2.3%-2.6%]), and a noticeable decline in 28-day stroke case-fatality of 14.8% [95% CI 9.7%-20.0%] from 1981–1982 in 2011–2012 across all ethnic groups. Māori and Pacific people with stroke had a greater prevalence of smoking (40.4% and 24.4%) and diabetes mellitus (29.7% and 43.3%) compared with NZ Europeans (12.8% and 16.5%, respectively), but lower prevalence of MI (18.1% and 12.6% vs 28.0%) (Table 1).
Between 2002–2003 and 2011–2012, there were significant increases in the proportion of stroke patients identified as having atrial fibrillation (AF) (7.2% [95% CI 2.4%-12.0%], only significant for NZ Europeans), patients receiving lipid lowering medications (26.6% [15.0%-38.2%], patients admitted to acute stroke unit (37.3% [95% CI 18.1–56.5%]). There was a significant decrease of patients with undetermined stroke (from 11.7% to 1.9%), across all ethnic groups.
Age-standardised stroke mortality rates (Table 3) reduced by almost 3 times between 1981–1982 and 2011–2012, declining from 98/100,000 (95% CI 88/100,000–110/100,000) person-years in 1981–1982 to 37/100,000 (95% CI 34/100,000–40/100,000) person-years in 2011–2012 (test for trend p < 0.0001 [which remained significant after adjustment for multiple-testing]). Decreases in age-adjusted 1-year stroke mortality rates were seen in NZ Europeans (2% per year), Māori (1.4% per year) and Asian/other ethnic groups (7% per year) over the last 30 years. Age-adjusted stroke mortality rates (per 100,000 person-years) in Pacific people reduced from 127 (95% CI 81–198) in 1991–1992 to 124 (95% CI 96–162) in 2002–2003 and 74 (95% CI 58–95) in 2011–2012 (p for trend = 0.0002).
Table 3. Crude, age-specific and age-standardised annual stroke mortality rates per 100,000 people in Auckland, New Zealand across four study periods (1981–1982, 1991–1992, 2002–2003 and 2011–2012) by sex and ethnicity.
| Age and ethnic group | 1981–1982 | 1991–1992 | 2002–2003 | 2011–2012 | Trend | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | Rate (95% CI) | N | n | Rate (95% CI) | N | n | Rate (95% CI) | N | n | Rate (95% CI) | P value | |
| Total | |||||||||||||
| 15–64* | 518112 | 104 | 20 (15; 26) | 624828 | 122 | 20 (16; 23) | 788106 | 89 | 11 (9; 14) | 956037 | 84 | 9 (7; 11) | |
| 65–74 | 49812 | 168 | 337 (265; 409) | 56388 | 136 | 241 (196; 287) | 59454 | 99 | 167 (134; 199) | 95190 | 90 | 95 (75; 114) | |
| 75–84 | 22965 | 266 | 1158 (961; 1355) | 31701 | 226 | 713 (615; 811) | 37815 | 232 | 614 (535; 692) | 48387 | 184 | 380 (325; 435) | |
| 85+ | 5691 | 126 | 2214 (1667; 2761) | 8541 | 148 | 1733 (1422; 2044) | 12507 | 227 | 1815 (1579; 2051) | 19578 | 237 | 1211 (1056; 1365) | |
| Total | 596580 | 664 | 111 (99; 123) | 721458 | 632 | 88 (80; 95) | 897882 | 647 | 72 (67; 78) | 1119192 | 595 | 53 (49; 57) | <0.0001 |
| Standardise § | 98 (88; 110) | 72 (67; 79) | 57 (53; 62) | 37 (34; 40) | <0.0001 | ||||||||
| Male | |||||||||||||
| 15–64* | 256500 | 58 | 23 (14; 31) | 308997 | 61 | 20 (15; 25) | 380139 | 48 | 13 (9; 16) | 461418 | 36 | 8 (5; 10) | |
| 65–74 | 22251 | 98 | 440 (317; 564) | 25452 | 83 | 326 (242; 410) | 28173 | 59 | 209 (156; 263) | 45678 | 55 | 120 (89; 152) | |
| 75–84 | 8742 | 100 | 1144 (827; 1461) | 11946 | 102 | 854 (670; 1038) | 15210 | 88 | 579 (458; 699) | 21759 | 96 | 441 (353; 529) | |
| 85+ | 1509 | 28 | 1856 (884; 2828) | 2421 | 22 | 909 (529; 1288) | 3633 | 58 | 1596 (1186; 2007) | 6807 | 73 | 1072 (826; 1318) | |
| Total | 289002 | 284 | 98 (82; 114) | 348816 | 268 | 77 (67; 87) | 427155 | 253 | 59 (52; 67) | 535662 | 260 | 49 (43; 54) | <0.0001 |
| Standardise § | 104 (88; 123) | 76 (67; 87) | 59 (52; 66) | 39 (35; 44) | <0.0001 | ||||||||
| Female | |||||||||||||
| 15–64* | 261612 | 46 | 18 (10; 25) | 315831 | 61 | 19 (14; 25) | 407967 | 41 | 10 (7; 13) | 494631 | 48 | 10 (7; 12) | |
| 65–74 | 27561 | 70 | 254 (170; 338) | 30936 | 53 | 171 (125; 217) | 31281 | 40 | 128 (88; 168) | 49509 | 35 | 71 (47; 94) | |
| 75–84 | 14223 | 166 | 1187 (916; 1418) | 19755 | 124 | 628 (517; 738) | 22605 | 144 | 637 (533; 741) | 26634 | 88 | 330 (261; 399) | |
| 85+ | 4182 | 98 | 2343 (1687; 3000) | 6120 | 126 | 2059 (1651; 2466) | 8874 | 169 | 1904 (1617; 2192) | 12771 | 164 | 1284 (1088; 1481) | |
| Total | 307578 | 380 | 124 (106; 141) | 372642 | 364 | 98 (87; 108) | 470727 | 394 | 84 (75; 92) | 583545 | 335 | 57 (51; 64) | <0.0001 |
| Standardise § | 92 (79; 106) | 67 (60; 75) | 55 (50; 61) | 35 (32; 40) | <0.0001 | ||||||||
| NZ European | |||||||||||||
| 15–64* | 422202 | 76 | 18 (12; 24) | 459267 | 72 | 16 (12; 20) | 501426 | 44 | 9 (6; 11) | 450759 | 35 | 8 (5; 10) | |
| 65–74 | 47481 | 156 | 329 (256; 401) | 52125 | 120 | 230 (183; 277) | 48633 | 60 | 123 (92; 155) | 64806 | 49 | 76 (54; 97) | |
| 75–84 | 22209 | 258 | 1162 (961; 1362) | 30303 | 204 | 673 (578; 768) | 34332 | 193 | 562 (483; 641) | 35916 | 135 | 376 (312; 439) | |
| 85+ | 5577 | 122 | 2188 (1639; 2737) | 8253 | 145 | 1757 (1437; 2076) | 11790 | 195 | 1654 (1422; 1886) | 16776 | 204 | 1216 (1049; 1383) | |
| Total | 497469 | 612 | 123 (109; 137) | 549948 | 541 | 98 (89; 107) | 596181 | 492 | 83 (75; 90) | 568257 | 423 | 74 (67; 82) | <0.0001 |
| Standardise § | 96 (86; 107) | 67 (61; 74) | 49 (45; 54) | 35 (31; 39) | <0.0001 | ||||||||
| Māori | |||||||||||||
| 15–64* | 52179 | 14 | 27 (7; 47) | 63762 | 18 | 28 (15; 41) | 77742 | 16 | 21 (10; 31) | 88470 | 17 | 19 (10; 28) | |
| 65–74 | 1266 | 6 | 474 (-62; 1010) | 1344 | 3 | 223 (-29; 476) | 2282 | 7 | 305 (79; 532) | 4452 | 4 | 90 (2; 178) | |
| 75–84 | 336 | 4 | 1190 (-459; 2840) | 429 | 9 | 2098 (727; 3469) | 654 | 5 | 765 (94; 1435) | 1572 | 9 | 573 (198; 947) | |
| 85+ | 51 | 0 | 0 | 72 | 2 | 2778 (-1072; 6628) | 144 | 2 | 1389 (-536; 3314) | 243 | 3 | 1235 (-162; 2632) | |
| Total | 53832 | 24 | 45 (19; 70) | 65607 | 32 | 49 (32; 66) | 80832 | 30 | 37 (24; 50) | 94737 | 33 | 35 (23; 47) | 0.203 |
| Standardise § | 96 (47; 196) | 133 (85; 209) | 77 (50; 118) | 53 (36; 77) | 0.015 | ||||||||
| Pacific | |||||||||||||
| 15–64* | 33672 | 12 | 36 (7; 64) | 64506 | 27 | 42 (26; 58) | 89724 | 17 | 19 (10; 28) | 107688 | 22 | 20 (12; 29) | |
| 65–74 | 741 | 4 | 540 (-208; 1288) | 2025 | 10 | 494 (188; 800) | 3840 | 21 | 547 (313; 781) | 6417 | 20 | 312 (175; 448) | |
| 75–84 | 213 | 0 | 0 | 597 | 10 | 1675 (135; 3215) | 1392 | 19 | 1365 (751; 1979) | 2679 | 16 | 597 (305; 890) | |
| 85+ | 33 | 0 | 0 | 108 | 0 | 0 | 246 | 7 | 2846 (738; 4954) | 582 | 10 | 1718 (653; 2783) | |
| Total | 33 | 16 | 46 (14; 78) | 67236 | 47 | 70 (48; 92) | 95202 | 64 | 67 (51; 84) | 117366 | 68 | 58 (44; 72) | 0.949 |
| Standardise § | 69 (30; 160) | 127 (81; 198) | 124 (96; 162) | 74 (58; 95) | 0.005 | ||||||||
| Asian/other | |||||||||||||
| 15–64* | 10059 | 2 | 20 (-19; 59) | 37293 | 5 | 13 (2; 25) | 119214 | 11 | 9 (4; 15) | 309123 | 10 | 3 (1; 5) | |
| 65–74 | 324 | 2 | 617 (-593; 1827) | 894 | 3 | 336 (-44; 715) | 4689 | 8 | 171 (52; 289) | 19515 | 16 | 82 (42; 122) | |
| 75–84 | 207 | 4 | 1932 (-746; 4610) | 372 | 3 | 806 (-106; 1719) | 1437 | 10 | 696 (265; 1127) | 8220 | 24 | 292 (175; 409) | |
| 85+ | 30 | 4 | 13333 (-5146; 31812) | 108 | 1 | 926 (-889; 2741) | 327 | 8 | 2446 (751; 4142) | 1971 | 20 | 1015 (570; 1459) | |
| Total | 10620 | 12 | 113 (23; 203) | 38667 | 12 | 31 (13; 49) | 125667 | 37 | 29 (20; 39) | 338829 | 70 | 21 (16; 25) | <0.0001 |
| Standardise § | 238 (102; 557) | 70 (37; 132) | 64 (45; 91) | 27 (21; 34) | <0.0001 | ||||||||
* 16–64 in 2011–2012,
§ Age-standardised to WHO world population
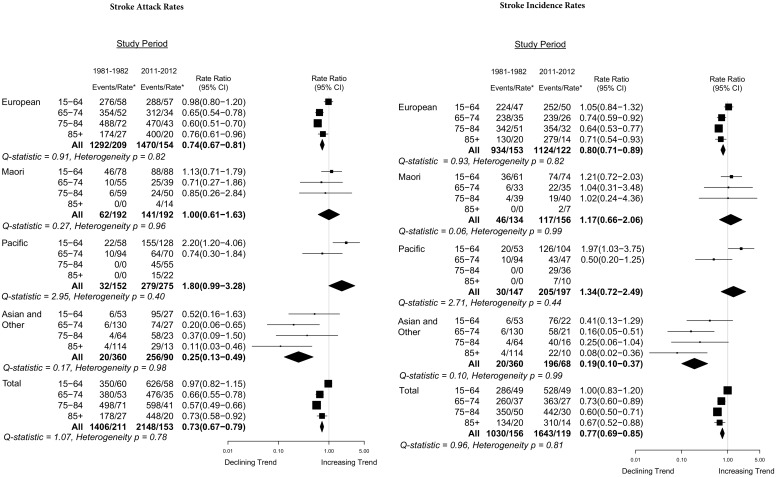
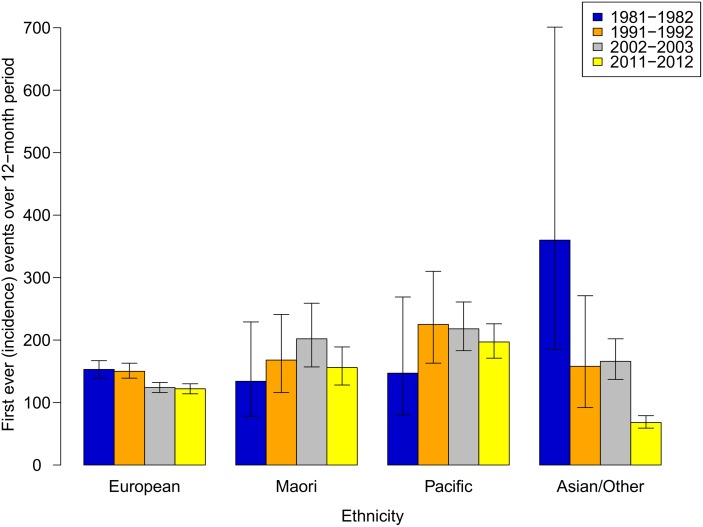
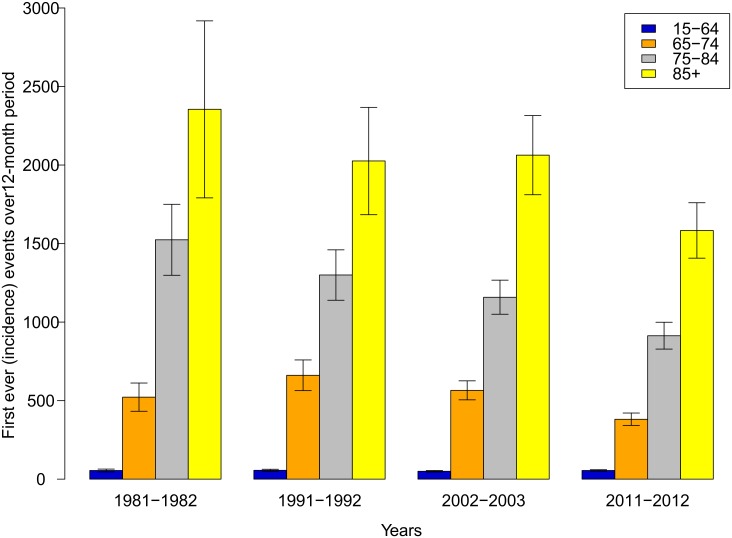
Over the 30-year study period, there was a 23% (95% CI 15%-31%) reduction in stroke incidence (Fig 1) and 27% (95% CI 21%-33%) reduction in stroke attack rate overall (Table 4 and Fig 1). These reductions were largely due to decreases in NZ Europeans (decrease 20% in incidence, 26% attack rates) and Asian/others (decrease 81% [95% CI 63%-90%] in incidence, 75% [95% CI 51%-87%] in attack rates) (Figs 2 and 3). However, in Māori and Pacific people, there were non-significant increases in stroke incidence (first-ever strokes) and attack rates (incident and recurrent strokes combined) between 1981–1982 and 2011–2012 study periods. Overall, decreases in stroke incidence and attack rates were limited to people 65 years or older (Fig 3). In people aged 15–64 years, there were no significant changes in stroke incidence or attack rates, except for Pacific people where stroke incidence and attack rates doubled (Fig 1).
Fig 1. Forest plot of stroke incidence and attack rate ratios (2011–2012 compared with 1981–1982), with rates age-adjusted to the WHO world population.
Table 4. Crude, age-specific and age-standardised annual attack (first-ever and recurrent strokes combined) rates per 100,000 people-years in Auckland, New Zealand across four study periods (1981–1982, 1991–1992, 2002–2003 and 2011–2012) by sex and ethnicity.
| Age, sex and ethnicity group | 1981–1982 | 1991–1992 | 2002–2003 | 2011–2012 | P value for trend | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | Rate (95% CI) | N | n | Rate (95% CI) | N | n | Rate (95% CI) | N | n | Rate (95% CI) | ||
| Total | |||||||||||||
| 15–64* | 518112 | 350 | 68 (58;78) | 624828 | 433 | 69 (61; 77) | 788106 | 484 | 61 (56; 67) | 956037 | 626 | 65 (60; 71) | |
| 65–74 | 49812 | 380 | 763 (654; 871) | 56388 | 512 | 908 (795; 1021) | 59454 | 460 | 774 (703; 844) | 95190 | 476 | 500 (455; 545) | |
| 75–84 | 22965 | 498 | 2169 (1899; 2438) | 31701 | 611 | 1927 (1730; 2125) | 37815 | 667 | 1764 (1630; 1898) | 48387 | 598 | 1236 (1137; 1335) | |
| 85+ | 5691 | 178 | 3128 (2478; 3778) | 8541 | 247 | 2892 (2460; 3324) | 12507 | 390 | 3118 (2809; 3428) | 19578 | 448 | 2288 (2076; 2500) | |
| Total | 596580 | 1406 | 236 (218; 253) | 721458 | 1803 | 250 (235; 265) | 897882 | 2001 | 223 (213; 233) | 1119192 | 2148 | 192 (184; 200) | <0.0001 |
| Standardised § | 211 (196; 228) | 213 (201; 227) | 193 (185; 202) | 153 (147; 160) | <0.0001 | ||||||||
| Male | |||||||||||||
| 15–64* | 256500 | 204 | 80 (64; 95) | 308997 | 252 | 82 (69; 94) | 380139 | 273 | 72 (63; 80) | 461418 | 323 | 70 (62; 78) | |
| 65–74 | 22251 | 224 | 1007 (820; 1193) | 25452 | 290 | 1139 (947; 1332) | 28173 | 273 | 969 (854; 1084) | 45678 | 272 | 595 (525; 666) | |
| 75–84 | 8742 | 216 | 2471 (2005; 2937) | 11946 | 252 | 2109 (1768; 2451) | 15210 | 277 | 1821 (1607; 2036) | 21759 | 305 | 1402 (1244; 1559) | |
| 85+ | 1509 | 46 | 3048 (1803; 4294) | 2421 | 41 | 1694 (1175; 2212) | 3633 | 95 | 2615 (2089; 3141) | 6807 | 138 | 2027 (1689; 2366) | |
| Total | 289002 | 690 | 239 (214; 264) | 348816 | 835 | 239 (218; 261) | 427155 | 918 | 215 (201; 229) | 535662 | 1038 | 194 (182; 206) | <0.0001 |
| Standardised § | 248 (223; 276) | 236 (216; 258) | 214 (200; 228) | 167 (157; 178) | <0.0001 | ||||||||
| Female | |||||||||||||
| 15–64* | 261612 | 146 | 56 (43; 69) | 315831 | 181 | 57 (47; 67) | 407967 | 211 | 52 (45; 59) | 494631 | 303 | 61 (54; 68) | |
| 65–74 | 27561 | 156 | 566 (440; 692) | 30936 | 222 | 718 (587; 848) | 31281 | 187 | 598 (512; 683) | 49509 | 204 | 412 (356; 469) | |
| 75–84 | 14223 | 282 | 1983 (1655; 2310) | 19755 | 359 | 1817 (1577; 2058) | 22605 | 390 | 1725 (1554; 1897) | 26634 | 293 | 1100 (974; 1226) | |
| 85+ | 4182 | 132 | 3156 (2395; 3918) | 6120 | 206 | 3366 (2799; 3934) | 8874 | 295 | 3324 (2945; 3704) | 12771 | 310 | 2427 (2157; 2698) | |
| Total | 307578 | 716 | 233 (209; 257) | 372642 | 968 | 260 (239; 281) | 470727 | 1083 | 230 (216; 244) | 583545 | 1110 | 190 (179; 201) | <0.0001 |
| Standardised § | 181 (163; 201) | 190 (175; 206) | 173 (162; 184) | 140 (132; 149) | <0.0001 | ||||||||
| NZ European | |||||||||||||
| 15–64* | 422202 | 276 | 65 (54; 76) | 459267 | 293 | 64 (55; 73) | 501426 | 269 | 54 (47; 60) | 450759 | 288 | 64 (57; 71) | |
| 65–74 | 47481 | 354 | 746 (636; 855) | 52125 | 459 | 881 (764; 997) | 48633 | 303 | 623 (553; 693) | 64806 | 312 | 481 (428; 535) | |
| 75–84 | 22209 | 488 | 2197 (1922; 2473) | 30303 | 579 | 1911 (1709; 2113) | 34332 | 568 | 1654 (1518; 1790) | 35916 | 470 | 1309 (1190; 1427) | |
| 85+ | 5577 | 174 | 3120 (2464; 3776) | 8253 | 241 | 2920 (2476; 3364) | 11790 | 345 | 2926 (2617; 3235) | 16776 | 400 | 2384 (2151; 2618) | |
| Total | 497469 | 1292 | 260 (240; 280) | 549948 | 1572 | 286 (267; 305) | 596181 | 1485 | 249 (236; 262) | 568257 | 1470 | 259 (245; 272) | 0.165 |
| Standardised § | 209 (193; 226) | 206 (193; 220) | 171 (162; 180) | 154 (145; 163) | <0.0001 | ||||||||
| Māori | |||||||||||||
| 15–64* | 52179 | 46 | 88 (52; 124) | 63762 | 58 | 91 (63; 119) | 77742 | 60 | 77 (58; 97) | 88470 | 88 | 99 (79; 120) | |
| 65–74 | 1266 | 10 | 790 (98; 1482) | 1344 | 8 | 595 (183; 1008) | 2282 | 24 | 1047 (628; 1466) | 4452 | 25 | 562 (341; 782) | |
| 75–84 | 336 | 6 | 1786 (-235; 3806) | 429 | 14 | 3263 (1554; 4973) | 654 | 15 | 2294 (1133; 3454) | 1572 | 24 | 1527 (916; 2138) | |
| 85+ | 51 | 0 | 0 | 72 | 2 | 2778 (-1072; 6628) | 144 | 5 | 3472 (429; 6516) | 243 | 4 | 1646 (33; 3259) | |
| Total | 53832 | 62 | 115 (75; 156) | 65607 | 104 | 129 (104; 153) | 80832 | 104 | 129 (104; 153) | 94737 | 141 | 149 (124; 173) | 0.073 |
| Standardised § | 192 (122; 304) | 254 (188; 341) | 247 (196; 311) | 192 (161; 229) | 0.867 | ||||||||
| Pacific | |||||||||||||
| 15–64* | 33672 | 22 | 65 (27; 104) | 64506 | 65 | 101 (74; 127) | 89724 | 88 | 98 (78; 119) | 107688 | 155 | 144 (121; 167) | |
| 65–74 | 741 | 10 | 1350 (167; 2532) | 2025 | 33 | 1630 (899; 2360) | 3840 | 67 | 1745 (1327; 2163) | 6417 | 64 | 997 (753; 1242) | |
| 75–84 | 213 | 0 | 0 | 597 | 13 | 2178 (536; 3819) | 1392 | 39 | 2802 (1922; 3661) | 2679 | 45 | 1680 (1189; 2171) | |
| 85+ | 33 | 0 | 0 | 108 | 2 | 1852 (-715; 4418) | 246 | 8 | 3252 (998; 5506) | 582 | 15 | 2577 (1273; 3882) | |
| Total | 34659 | 32 | 92 (47; 138) | 67236 | 113 | 168 (131; 205) | 95202 | 202 | 212 (183; 241) | 117366 | 279 | 238 (210; 266) | <0.0001 |
| Standardised § | 152 (85; 274) | 291 (220; 384) | 329 (284; 382) | 275 (244; 310) | 0.012 | ||||||||
| Asian/other | |||||||||||||
| 15–64* | 10059 | 6 | 60 (-8; 127) | 37293 | 17 | 46 (17; 74) | 119214 | 64 | 54 (41; 67) | 309123 | 95 | 31 (25; 37) | |
| 65–74 | 324 | 6 | 1852 (-244; 3947) | 894 | 12 | 1342 (268; 2416) | 4689 | 55 | 1173 (863; 1483) | 19515 | 74 | 379 (293; 466) | |
| 75–84 | 207 | 4 | 1932 (-746; 4610) | 372 | 5 | 1344 (166; 2522) | 1437 | 34 | 2366 (406; 3161) | 8220 | 58 | 706 (524; 887) | |
| 85+ | 30 | 4 | 13333 (-5146; 31812) | 108 | 2 | 1852 (-715; 4418) | 327 | 10 | 3058 (1163; 4954) | 1971 | 29 | 1471 (936; 2007) | |
| Total | 10620 | 20 | 188 (72; 305) | 38667 | 36 | 93 (54; 132) | 125667 | 163 | 130 (110; 150) | 338829 | 256 | 76 (66; 85) | <0.0001 |
| Standardised § | 360 (185; 701) | 194 (122; 310) | 234 (197; 277) | 90 (79; 101) | <0.0001 | ||||||||
* 16–64 in 2011–2012,
§ Age-standardised to WHO world population
Fig 2. Stroke incidence rates over 3 decades by ethnicity.
Fig 3. Stroke incidence rates over 3 decades by 4 age groups.
In 2011–2012, 54% (95% CI 48%-61%) of first ever incident and 51% (95% CI 46%-57%) of recurrent strokes occurred in people younger than 75 years (Table 2). The proportion of incident and recurrent strokes in Māori and Pacific people younger than 75 years was twice that of NZ Europeans (44% [95% CI 40%-48%] and 41% [95% CI 35%-47%] incident and recurrent stroke in NZ Europeans aged 15–74 years compared to 82% [95% CI 74%-90%] and 80% [95% CI 72%-89%] in Māori and 82% [95% CI 73%-93%] and 78% [95% CI 69%-89%] in Pacific people aged 15–74 years, respectively). The temporal trends in declining stroke incidence and attack rates were consistent in both males and females aged 65 years and older (Tables 2 and 4), with the most noticeable decline observed in males (Tables 2 and 4) and NZ Europeans (Tables 2 and 4, Fig 1).
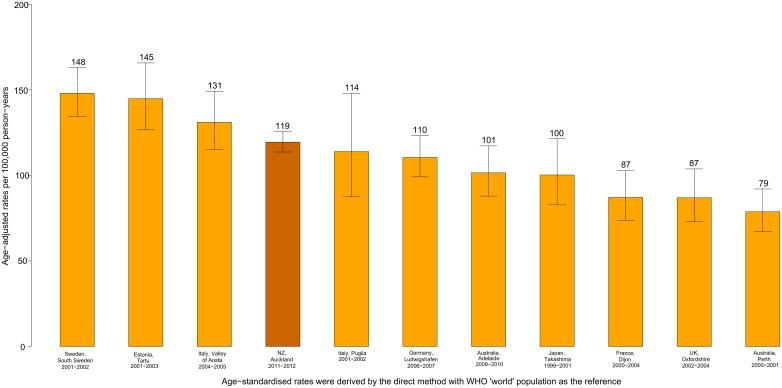
Stroke mortality rates increased with age across all study periods. In 2011–2012, there were no significant differences in age-adjusted stroke mortality rates (per 100,000 person-years) between males and females (39 [95% CI 35–44] and 35 [95% CI 32–40], respectively). In 2011–2012, age-adjusted stroke mortality rates were highest in Pacific and Māori people (74 [95% CI 58–95] and 53 [95% CI 36–77], respectively), and lowest in Asian/other and NZ/European people (27 [95% CI 21–34] and 35 [95% CI 31–39] respectively). Māori and Pacific people had a test for trend of p<0.0001, which reflected both an increase in stroke incidence and mortality rates from 1981–1992 and a decline in stroke incidence and mortality rates from 2002–2012. All other ethnicities (NZ/Europeans, Asian/other), total, males, females all had test for trend p<0.0001 which reflected the continuous decline in stroke incidence and mortality rates across the study period. The overall current level of stroke incidence rates in NZ remains high compared to other developed countries where comparable population-based stroke incidence studies were carried out in 2000–2014 period[26–32] (Fig 4).
Fig 4. Annual age-adjusted stroke incidence rates in population-based studies[14,26–32,48–51] carried out in high-income countries in 2000–2014.
Discussion
The main findings of this study include: (a) a clear trend towards reducing stroke incidence, 28-day case-fatality and 1-year mortality rates; (b) an increase in the frequency of elevated blood pressure, myocardial infarction, Type 2 diabetes mellitus and AF among stroke patients, but decrease in the frequency of current smoking; (c) better survival of acute stroke patients across all ethnic groups; and (d) persistent differences in stroke incidence, mortality rates and risk factor prevalence across the four major ethnic groups in NZ. Overall, the ethnic disparities have lessened overtime, especially over the last study decade. Our findings of decrease in stroke incidence and mortality rates overall in the study population but an increase, although not significant, in stroke rates in people younger than 64 years old (particularly females, Māori and Pacific Island people) are concordant with those of the GBD 2010 Study which identified a continuous decline in stroke incidence and mortality rates in high-income countries,[1] and a trend towards a stable or increasing stroke burden in people younger than 65 years.[1] The latter is of particular concern as our data showed an increased frequency of several major vascular risk factors in people with stroke that was also observed in the general NZ population and other countries.[14,33–35] This includes an epidemic of obesity and Type 2 diabetes mellitus in children and young adults,[36,37] as well as behavioural and dietary risk factors.[38–40] An increase (or no reduction) in the incidence of stroke in middle-age populations has been observed in other studies,[41,42] thus re-emphasising the need for intensification of appropriate primary stroke prevention strategies, especially on at a population level.
Although the overall decline in stroke incidence rates is in line with some population-based studies,[43–47] the decline in stroke incidence in NZ over the last 30 years is almost 20% less than that of comparable studies for a similar study period (Fig 4).[13,14,26–32,48–51] Further, this study has highlighted that the relatively high level of stroke incidence in NZ is largely attributed to high rates in Māori and Pacific people. Moreover, unlike similar studies,[14,30,44] we found a significant trend towards increase in the prevalence of elevated blood pressure, history of myocardial infarction and diabetes mellitus among stroke patients and this may be a contributing factor to the observed differences in NZ compared to other high-income countries. Indeed, the 2008/2009 NZ Adult Nutrition Survey showed that there was an increase in mean systolic blood pressure in the overall population since 2002, but in particular in Māori men aged 35–74. The prevalence of hypertension in the NZ population in 2008/2009 was estimated at 31%, with only 15% on anti-hypertensive medication.[39] However, the increasing prevalence of elevated blood pressure, MI and Type 2 diabetes in stroke patients may also be related to increased detection of undiagnosed hypertension, diabetes and other health conditions over the study period, therefore these data should be interpreted with caution. The proportion of recurrent strokes has shown a statistically significant decrease over the study period overall and NZ European and Maori people in particularly, although the level of recurrent strokes (21.4%) in NZ remains relatively high compared to some other developed countries.[52] There was no statistically significantly change in the proportion of recurrent strokes in Pacific, Asian and other ethnic groups in NZ from 1981 to 2012 and but showed a trend towards increasing. This and the overall high rate of recurrent strokes in NZ (especially in Maori and Pacific people younger than 75 years) signify the need for better, culturally appropriate secondary stroke prevention strategies.
The younger age of stroke onset in Māori is also reflected in the lower life expectancy at birth of Māori, which in 2006 was at least eight years less than that for non-Māori for both genders.[53] Of note is the increase in the prevalence of atrial fibrillation and low levels of pre-morbid anticoagulation of patients with AF over the last decade. Despite 36.1% of ischaemic stroke patients having AF in the 2011–2012 study, only 26.5% of these patients were being treated with anticoagulation therapy prior to stroke. A similar increase in the prevalence of AF in stroke patients was recently observed in another population-based study.[54] This, together with the global epidemic of AF,[55] suggests that it is now becoming the leading risk factor for stroke.
Similar diverging trends and ethnic disparities in stroke incidence have been shown in other comparable population-based studies in the US[43] and UK,[44] suggesting unfavorable trends in the prevalence of risk factors.[56] In the Atherosclerosis Risk in Communities cohort there was a 40% increase in the proportion of hypertension and diabetes from 1987 to 2011.[46] Ethnic differences in stroke risk have been attributed to differences in socioeconomic status (SES) and exposure to risk factors.[57] Lower SES groups have greater exposure to cardiovascular risk factors, including hypertension, smoking, poor diet, physical inactivity, diabetes and excess alcohol use.[58] Also, lower SES groups may have limited access to, or make less effective use of, services to manage these risk factors (e.g., early hypertension detection and control).[59] However, there is evidence that not all differences among ethnic groups are explained by differences in cardiovascular risk factors, suggesting genetic[60] and other factors such as socioeconomic disparities and the experience of discrimination[61] may be important. There remains uncertainty about the relative importance of stroke risk factor management and other factors in causing these inequalities. The impact of SES, changing family dynamics and cultural values on stroke risk and recovery in Māori and Pacific Island people is uncertain.[62] Ethnic-specific differences in stroke risk factors have also been shown in some other multi-ethnic populations[63–70] suggesting the need for culturally tailored primary and secondary preventative strategies.
If the current temporal trend in the distribution of stroke risk factors in the population continues, we are likely to observe an increase in the incidence of stroke in the near future. Primary stroke prevention is a mainstream strategy to reduce stroke burden in a population.[71–73] However, the relative significance of various determinants of stroke occurrence as well as cultural appropriateness of stroke prevention strategies, especially among Māori and Pacific people,[3] are not known, hindering the development of effective, evidence-based primary stroke prevention strategies on both individual and population levels.[4,74–79] Furthermore, a detailed analysis of recent changes in stroke risk factors is needed to explain the diverging trends in stroke incidence and mortality rates in the different ethnic groups in NZ and to further inform culturally appropriate primary stroke prevention strategies.[80]
Better management (including enhanced admission rates to acute stroke units) and resulting improved 28-day and 1-year survival of acute stroke patients have been observed in other studies.[13,32,81,82] Although hospital admission has improved in this study over the 30 year period, there is still an underutilisation of acute stroke units (only 51% of stroke patients were treated in acute stroke units) and thrombolytic therapy for ischaemic stroke patients (only 5% of ischaemic stroke patients received alteplase [data not shown]) against the recommended corresponding figures in the NZ guidelines.[83] A recent individual-participant data meta-analysis of alteplase[84] demonstrated that irrespective of age or stroke severity, and despite an increased risk of fatal intracranial haemorrhage during the first few days after treatment, alteplase significantly improves the overall odds of a good stroke outcome when delivered within 4·5 h of stroke onset, with earlier treatment associated with bigger proportional benefits. Similar to some previous observations[85,86] but contrary to others[87–89] we found a moderate, though non-statistically significant, decrease in the incidence of subarachnoid haemorrhage that may be related to the reduced smoking rates[90] in NZ.[91]
The observed level and magnitude in stroke mortality decline in Auckland, NZ (3-fold decline [from 98/100,000 person-years in 1981–1982 to 37/100,000 person-years in 2011–2012]) is similar to official NZ statistics on stroke mortality rates,[92] supporting the validity of our data. However, our findings of faster declines in stroke mortality rates than stroke incidence rates, in line with our previous estimates,[93] suggest a further rise of stroke related disability over the next decade due to population growth and aging in NZ. This is of particular importance for Māori and Pacific groups where there has been a substantial increase in stroke in younger age groups, thus emphasizing the importance and priority of primary stroke prevention. A mismatch in temporal trends between stroke incidence and mortality rates has been shown in some other populations.[94]
Novelty and Translation
To the best of our knowledge, this is the first, longest and largest population-based survey to compare long-term trends and 1-year outcomes of stroke. There are several important new findings reported in this paper. The first is the diverging and changing trends in stroke incidence and stroke risk factors in different ethnic groups of the population. The trends in stroke incidence for Māori and Pacific Island people behave are similar to those observed in low to middle-income countries, while in NZ/ Europeans, trends in stroke incidence are like those in high-income countries with ethnically more homogeneous populations. Ethnicity/race is a proxy for disparity[95] and this is relevant internationally because other ethnically diverse societies will need to make provisions for population sub-groups prone to disparity to ensure that they have access to appropriate health care. Second, our findings were obtained before and during contemporary changes in prevention (primary and secondary) and service delivery (acute stroke unit care and thrombolysis) in a whole population context. This provides, for the first time, direct feedback to health-policy decision-makers on the relative success of these changes in medical practice. Specifically, the study demonstrated that current stroke preventative and management strategies are far more effective in reducing stroke risk and mortality on a population level than those used before mid-1990s. This should encourage health systems worldwide to seriously consider implementing modern stroke preventive and treatment strategies if they have not already done so. Third, this is the first documentation, to the best of our knowledge, of stroke mortality rates falling faster than stroke incidence rates. If this trend is also borne out in other populations then health systems will need to adapt in order to accommodate the likely increase in rehabilitation services needs in order to cater for this increase in prevalence and, therefore, make plans to implement more effective and culturally appropriate primary stroke prevention strategies at a population level. The differences in the stroke incidence trends by ethnicity are also important to take into account when projecting stroke burden in multi-ethnic communities on a national and global scale (including Global Burden of Disease estimates) and further reinforce the need for culturally appropriate prevention and health care strategies in multi-ethnic communities. The diverging trends in stroke incidence in the different ethnic groups and different rates of reduction in stroke mortality and incidence rates should be generalizable to other ethnically diverse health-care systems that have achieved similar risk factor modification.
Strength and limitations
The strength of our study is that it includes four large prospective population-based stroke registers (5400 stroke patients; 3,335,112 person-years of observation) across a long timespan (over 30 years: 1981–2012). The major limitation of the study was a relatively low number of strokes in some ethnic groups, especially in the Asian/other ethnic group making it difficult to determine whether trends in incidence, attack and mortality rates were statistically significant. Although the numbers were small, one possible explanation for the apparent large reduction in stroke incidence and mortality rates in Asian/other ethnic group may be related to the large influx of younger, presumably generally healthy, Asian immigrants in NZ that has been particularly marked over the last two decades.[21] The NZ Health and Disability sector uses the NZ Health Statistics definition for ethnicity ‘as a social construct of group affiliation and identity’ where ‘‘ethnicity is the ethnic group or groups that people identify with or feel they belong to’.[96] In the 1981–1982 and 2002–2003 studies, only one ethnicity was collected for participants in each study, preventing us from applying prioritised ethnicity classification in these studies. Although this may have introduced a classification bias by ethnicity, we believe the effect of the bias was not large and was likely to be non-differential as most of the ethnic priority classifications in the 1991–1992 and 2011–2012 studies were also based on the first ethnicity self-identified by the study participants. In addition, less than 10% of the last surveys study participants indicated more than one ethnicity. We lacked statistical power to stratify our analyses by ethnicity to generate specific ethnicity-covariate estimates for risk of stroke onset due to having small numbers of stroke patients per ethnic minority, particularly the Asian subgroup. Given the continuous population increase in Auckland and in New Zealand in general, it is likely that we will have improved statistical power to investigate ethnicity-covariate interactions in a future ARCOS study. Another limitation of our series of studies is that the first two ARCOS studies (1981–1982 and 1991–1992) used a cluster sampling scheme approach for case-ascertainment[97] and, therefore, may have underestimated the number of stroke cases in these study periods. Although we applied the same criteria for cardiovascular risk factors recorded across all studies, we acknowledge that the accuracy of information about these risk factors was prone to some degree of variation and, therefore, should be interpreted with caution. However, the consistency of the assessment procedures, diagnostic criteria, the implementation of quality control procedures during the four studies, together with the decreased estimated number of missing stroke cases (as determined by capture-recapture analyses), reassure us that case-ascertainment was high across all these studies. Finally, due to the lack of reliable verification of pathological types of stroke in the 1981–1982 and 1991–1992 studies, analysis of changes in pathological types of stroke was limited to the 2002–2003 and 2011–2012 ARCOS studies. The larger proportion of ischaemic strokes in 2011–2012 compared to 2002–2003 may be related to a greater use of brain scanning (97% in 2011–2012; 88% in 2002–2003) which shifted classification of some strokes as ‘undetermined’ to the ischaemic group.
Conclusions
A continuous and recently accelerated reduction in total stroke incidence and mortality has been found over the last three decades in the Auckland region of NZ. Despite a trend towards closing the gap in reducing the difference in stroke burden between NZ European and non-European ethnic groups in NZ, the 15-year differences in the mean age of stroke onset in Māori and Pacific compared to NZ/ Europeans has persisted. Some positive changes in the profile of risk factors in people with stroke, such as overall decline in the proportion of current smokers and recurrent strokes, and increased use of antiplatelet, blood pressure and lipid lowering medications, were counterbalanced by increasing prevalence of high blood pressure, myocardial infarction, diabetes mellitus, AF, and low use of anticoagulants in people with AF. This, together with the ongoing discordance between trends in stroke incidence and mortality rates and persistent ethnic disparities in stroke burden, emphasises the need for better understanding of causes of ethnic disparities in stroke burden. Culturally appropriate primary stroke prevention strategies need to be prioritised at a population level.[98] Some of these strategies need government intervention in terms of increases in taxes on cigarettes and alcohol for example, reduction in the levels of salt and sugar in the population,[99,100] the use of advertising campaigns informed by behavioural change methods to reduce smoking, alcohol, increase physical activity, or evidence-based interventions. Government led health interventions should include stroke programmes ideally already at the school age in the regular teaching plan among other health education programs. To inform future stroke prevention strategies and reduce stroke burden, a better understanding (including modelling)[101] of the interplay between trends in stroke (and stroke sub-types) risk factors, demographic (e.g., aging of the population, ethnic/racial disparities) and health care (e.g., introduction of acute stroke units, thrombolysis for ischaemic stroke), morbidity (e.g. incidence and disability) and mortality rate changes in different populations is required.
Acknowledgments
We are indebted to the research team for their dedication and performance; the support of staff at the Coroner’s Office in Auckland; the assistance of staff of the New Zealand Health Information Service; the help provided by Waitemata DHB, Auckland DHB, Counties Manukau DHB; the support of many doctors, nurses, stroke service providers within and outside Auckland; and of course the ARCOS participants and their families and friends. We would also like to thank our International Advisory Committee members (Prof. Craig Anderson, Prof. Maurice Giroud) for their support throughout the study and comments on the draft of the manuscript. We also thank Helen McDonald for secretarial support for the study. Kathryn McPherson holds the Laura Fergusson Trust Chair.
The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. Other authors who had full access to the data include Rita Krishnamurthi, Kathryn McPherson, Suzanne Barker-Collo, P. Alan Barber, Varsha Parag, Emma Witt, Amy Jones, Priya Parmar, Rohit Bhattacharjee, and Braden Te Ao. All other authors (Bruce Arroll, Elaine Rush, Yogini Rathnasabapathy, Derrick Bennett, Martin Tobias, Craig Anderson, Alice Theadom, Max Abbott, Ruth Bonita) had full access to the aggregated data used in the manuscript.
Contributors
Members of the ARCOS IV Study Group are: Steering Committee: Valery L. Feigin (Chair and Principal Investigator), Rita Krishnamurthi, Kathryn McPherson, Suzanne Barker-Collo, P. Alan Barber, Varsha Parag, Bruce Arroll, Elaine Rush, Yogini Rathnasabapathy, Emma Witt, Amy Jones, Derrick A. Bennett. Diagnostic Adjudication Group: P. Alan Barber, Valery L. Feigin, Yogini Rathnasabapathy. Operations Group: Valery L. Feigin, Rita Krishnamurthi, Emma Witt, Amy Jones
Data Availability
Aggregate data underlying the findings are available in the body of the paper. All original person-specific data cannot be shared publicly due to ethical restrictions but are available upon request to those who meet the criteria for access to confidential data. Please contact Dr Rita Krishnamurthi, National Institute for Stroke & Applied Neurosciences, AUT University to request access.
Funding Statement
Support was provided by grant number 10/458 from the Health Research Council of New Zealand [http://www.hrc.govt.nz]. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. (2014) Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 383: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonita R, Broad JB, Beaglehole R (1997) Ethnic variations in stroke incidence and case fatality: the Auckland Stroke study. Stroke 28: 758–761 [DOI] [PubMed] [Google Scholar]
- 3. Feigin V, Carter K, Hackett M, Barber PA, McNaughton H, Dyall L, et al. (2006) Ethnic disparities in incidence of stroke subtypes: Auckland Regional Community Stroke Study, 2002–2003. Lancet Neurology 5: 130–139. [DOI] [PubMed] [Google Scholar]
- 4. Carter K, Anderson C, Hacket M, Feigin V, Barber PA, Broad J, et al. (2006) Trends in Ethnic Disparities in Stroke Incidence in Auckland, New Zealand, During 1981 to 2003. Stroke 37: 56–62. [DOI] [PubMed] [Google Scholar]
- 5. Dyall L, Feigin V, Brown P, Roberts M (2008) Stroke: A Picture of Health Disparities in New Zealand. Social Policy Journal of New Zealand 33: 178–191. [Google Scholar]
- 6. Feigin VL, McNaughton H, Dyall L, Feigin VL, McNaughton H, Dyall L (2007) Burden of stroke in Maori and Pacific peoples of New Zealand. International Journal of Stroke 2: 208–210. 10.1111/j.1747-4949.2007.00140.x [DOI] [PubMed] [Google Scholar]
- 7. Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, et al. (1998) Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 147: 259–268. [DOI] [PubMed] [Google Scholar]
- 8. Murray CJL (2014) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. The Lancet 385: 117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fisher A, Martin J, Srikusalanukul W, Davis M (2014) Trends in stroke survival incidence rates in older australians in the new millennium and forecasts into the future. Journal of Stroke and Cerebrovascular Diseases 23: 759–770. 10.1016/j.jstrokecerebrovasdis.2013.06.035 [DOI] [PubMed] [Google Scholar]
- 10. Bonita R, Beaglehole R (1995) Monitoring stroke. An international challenge. Stroke 26: 541–542. [DOI] [PubMed] [Google Scholar]
- 11. Sudlow CLM, Warlow CP (1996) Comparing Stroke Incidence Worldwide: What Makes Studies Comparable? Stroke 27: 550–558. [DOI] [PubMed] [Google Scholar]
- 12. Anderson CS, Carter KN, Hackett ML, Feigin V, Barber PA, Broad JB, et al. (2005) Trends in stroke incidence in Auckland, New Zealand, during 1981 to 2003. Stroke 36: 2087–2093. [DOI] [PubMed] [Google Scholar]
- 13. Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. The Lancet Neurology 8: 355–369. 10.1016/S1474-4422(09)70025-0 [DOI] [PubMed] [Google Scholar]
- 14. Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. (2004) Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 363: 1925–1933. [DOI] [PubMed] [Google Scholar]
- 15. Krishnamurthi R, Jones A, Barber A, Barker-Collo S, McPherson K, Bennett D, et al. (2014) Methodology of a Population-Based Stroke and TIA Incidence and Outcomes Study: The Auckland Regional Community Stroke Study (ARCOS IV) 2011–2012. International Journal of Stroke 9: 140–147. 10.1111/ijs.12108 [DOI] [PubMed] [Google Scholar]
- 16. Bonita R, Beaglehole R, North JD (1984) Event, incidence and case fatality rates of cerebrovascular disease in Auckland, New Zealand. Am J Epidemiol 120: 236–243. [DOI] [PubMed] [Google Scholar]
- 17. Bonita R, Broad JB, Anderson NE, Beaglehole R (1995) Approaches to the problems of measuring the incidence of stroke: the Auckland Stroke Study, 1991–1992. International Journal of Epidemiology 24: 535–542. [DOI] [PubMed] [Google Scholar]
- 18. Bonita R, Broad JB, Beaglehole R (1993) Changes in stroke incidence and case-fatality in Auckland, New Zealand, 1981–91. Lancet 342: 1470–1473. [DOI] [PubMed] [Google Scholar]
- 19. Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T (1980) Cerebrovascular disease in the community: results of a WHO collaborative study. Bulletin of the World Health Organization 58: 113–130. [PMC free article] [PubMed] [Google Scholar]
- 20. Bonita R, Beaglehole R, North JD (1983) Subarachnoid hemorrhage in New Zealand: an epidemiological study. Stroke 14: 342–347. [DOI] [PubMed] [Google Scholar]
- 21.Statistics New Zealand (2014) New Zealand Census 2013. Statistics New Zealand.
- 22. Ahmad O, Boschi-Pinto C, Lopez A, Murray C, Lozano R, Inoue M (2000) Age Standardization of Rates: A new WHO Standard GPE Discussion Paper Series: no31. Geneva: World Health Organization. [Google Scholar]
- 23. Hook EB, Regal RR (1995) Capture-recapture methods in epidemiology: Methods and limitations. Epidemiologic Reviews 17: 243–264. [DOI] [PubMed] [Google Scholar]
- 24. Tilling K, Sterne JA, Wolfe CD (2001) Estimation of the incidence of stroke using a capture-recapture model including covariates. IntJ Epidemiol 30: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 25. Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. (2013) Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. The Lancet Global Health 1: e259–e281. 10.1016/S2214-109X(13)70089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corbin DOC, Poddar V, Hennis A, Gaskin A, Rambarat C, Wilks R, et al. (2004) Incidence and case fatality rates of first-ever stroke in a Black Caribbean population: The Barbados register of strokes. Stroke 35: 1254–1258. [DOI] [PubMed] [Google Scholar]
- 27. Hallstrom B, Jonsson AC, Nerbrand C, Norrving B, Lindgren A (2008) Stroke incidence and survival in the beginning of the 21st century in southern Sweden: comparisons with the late 20th century and projections into the future. Stroke 39: 10–15. [DOI] [PubMed] [Google Scholar]
- 28. Vibo R, Korv J, Roose M (2005) The Third Stroke Registry in Tartu, Estonia: decline of stroke incidence and 28-day case-fatality rate since 1991. Stroke 36: 2544–2548. [DOI] [PubMed] [Google Scholar]
- 29. Corso G, Bottacchi E, Giardini G, De La Pierre F, Meloni T, Pesenti Campagnoni M, et al. (2009) Community-based study of stroke incidence in the Valley of Aosta, Italy—CARe-cerebrovascular aosta registry: Years 2004–2005. Neuroepidemiology 32: 186–195. 10.1159/000195688 [DOI] [PubMed] [Google Scholar]
- 30. Islam MS, Anderson CS, Hankey GJ, Hardie K, Carter K, Broadhurst R, et al. (2008) Trends in Incidence and Outcome of Stroke in Perth, Western Australia During 1989 to 2001: The Perth Community Stroke Study. Stroke 39: 776–782. 10.1161/STROKEAHA.107.493643 [DOI] [PubMed] [Google Scholar]
- 31. Leyden JMM, Kleinig TJMP, Newbury JMMD, Castle SMABARN, Cranefield JRN, Anderson CSMP, et al. (2013) Adelaide Stroke Incidence Study: Declining Stroke Rates but Many Preventable Cardioembolic Strokes. Stroke 44: 1226–1231. 10.1161/STROKEAHA.113.675140 [DOI] [PubMed] [Google Scholar]
- 32. Palm F, Urbanek C, Rose S, Buggle F, Bode B, Hennerici MG, et al. (2010) Stroke Incidence and Survival in Ludwigshafen am Rhein, Germany: the Ludwigshafen Stroke Study (LuSSt). Stroke 41: 1865–1870. 10.1161/STROKEAHA.110.592642 [DOI] [PubMed] [Google Scholar]
- 33. Sprafka JM, Virnig BA, Shahar E, McGovern PG (1994) Trends in diabetes prevalence among stroke patients and the effect of diabetes on stroke survival: The Minnesota Heart Survey. Diabetic Medicine 11: 678–684. [DOI] [PubMed] [Google Scholar]
- 34. Gunarathne A, Patel JV, Potluri R, Gill PS, Hughes EA, Lip GYH (2008) Secular trends in the cardiovascular risk profile and mortality of stroke admissions in an inner city, multiethnic population in the United Kingdom (1997–2005). Journal of Human Hypertension 22: 18–23. [DOI] [PubMed] [Google Scholar]
- 35. Ziegler PD, Glotzer TV, Daoud EG, Singer DE, Ezekowitz MD, Hoyt RH, et al. (2012) Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. American Journal of Cardiology 110: 1309–1314. 10.1016/j.amjcard.2012.06.034 [DOI] [PubMed] [Google Scholar]
- 36. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet 384: 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alberti KGMM, Zimmet P (2013) Epidemiology: Global burden of disease-where does diabetes mellitus fit in? Nature Reviews Endocrinology 9: 258–260. 10.1038/nrendo.2013.54 [DOI] [PubMed] [Google Scholar]
- 38. Health Mo (2013) Health Loss in New Zealand: A report from the New Zealand Burden of Diseases, Injuries and Risk Factors Study, 2006–2016. Wellington: Ministry of Health. [Google Scholar]
- 39. McLean RM, Williams S, Mann JI, Miller JC, Parnell WR (2013) Blood pressure and hypertension in New Zealand: Results from the 2008/09 Adult Nutrition Survey. New Zealand Medical Journal 126. [PubMed] [Google Scholar]
- 40. Ezzati M, Riboli E (2013) Behavioral and dietary risk factors for noncommunicable diseases. New England Journal of Medicine 369: 954–964. 10.1056/NEJMra1203528 [DOI] [PubMed] [Google Scholar]
- 41. Rastenyte D, Sopagiene D, Virviciute D, Jureniene K (2006) Diverging trends in the incidence and mortality of stroke during the period 1986–2002: a study from the stroke register in Kaunas, Lithuania. ScandJ Public Health 34: 488–495. [DOI] [PubMed] [Google Scholar]
- 42. Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. (2012) Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology 79: 1781–1787. 10.1212/WNL.0b013e318270401d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. (2010) Stroke incidence is decreasing in whites but not in blacks: A population-based estimate of temporal trends in stroke incidence from the greater cincinnati/northern kentucky stroke study. Stroke 41: 1326–1331. 10.1161/STROKEAHA.109.575043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heuschmann PUMDMPH, Grieve APPD, Toschke AMMDMPHM, Rudd AGF, Wolfe CDAMDF (2008) Ethnic Group Disparities in 10-Year Trends in Stroke Incidence and Vascular Risk Factors: The South London Stroke Register (SLSR). Stroke 39: 2204–2210. 10.1161/STROKEAHA.107.507285 [DOI] [PubMed] [Google Scholar]
- 45. Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. (2005) Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study).[see comment]. Lancet 366: 1773–1783. [DOI] [PubMed] [Google Scholar]
- 46. Koton S, Schneider ALC, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. (2014) Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA—Journal of the American Medical Association 312: 259–268. [DOI] [PubMed] [Google Scholar]
- 47. Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MMB (2012) Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. European Journal of Epidemiology 27: 287–295. 10.1007/s10654-012-9673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benatru I, Rouaud O, Durier J, Contegal F, Couvreur G, Bejot Y, et al. (2006) Stable stroke incidence rates but improved case-fatality in Dijon, France, from 1985 to 2004. Stroke 37: 1674–1679. [DOI] [PubMed] [Google Scholar]
- 49. Manobianca G, Zoccolella S, Petruzzellis A, Miccoli A, Logroscino G (2010) The incidence of major stroke subtypes in southern Italy: a population-based study. European Journal of Neurology 17: 1148–1155. 10.1111/j.1468-1331.2010.02983.x [DOI] [PubMed] [Google Scholar]
- 50. Kita Y, Turin TC, Ichikawa M, Sugihara H, Morita Y, Tomioka N, et al. (2009) Trend of stroke incidence in a Japanese population: Takashima stroke registry, 1990–2001. International Journal of Stroke 4: 241–249. 10.1111/j.1747-4949.2009.00293.x [DOI] [PubMed] [Google Scholar]
- 51. Syme PD, Byrne AW, Chen R, Devenny R, Forbes JF (2005) Community-based stroke incidence in a Scottish population: the Scottish Borders Stroke Study. Stroke 36: 1837–1843. [DOI] [PubMed] [Google Scholar]
- 52. Talelli P, Greenwood RJ, Talelli P, Greenwood RJ (2008) Recurrent stroke: where do we stand with the secondary prevention of noncardioembolic ischaemic strokes? Therapeutic Advances in Cardiovascular Disease 2: 387–405. 10.1177/1753944708093411 [DOI] [PubMed] [Google Scholar]
- 53. Health Mo, editor (2010) Tatau Kahukura: Māori Health Chart Book 2010. 2nd ed Wellington: Ministry of Health. [Google Scholar]
- 54. Friberg L, Rosenqvist M, Lindgren A, Terént A, Norrving B, Asplund K (2014) High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke. [DOI] [PubMed] [Google Scholar]
- 55. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. (2014) Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation 129: 837–847. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gulliford MC, Charlton J, Rudd A, Wolfe CD, Toschke AM (2010) Declining 1-year case-fatality of stroke and increasing coverage of vascular risk management: population-based cohort study. Journal of Neurology, Neurosurgery & Psychiatry 81: 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sacco RL, Kargman DE, Zamanillo MC (1995) Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology 45: 659–663. [DOI] [PubMed] [Google Scholar]
- 58. Kaplan GA, Keil JE (1993) Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 88: 1973–1998. [DOI] [PubMed] [Google Scholar]
- 59. Casper M, Wing S, Strogatz D, Davis CE, Tyroler HA (1992) Antihypertensive treatment and US trends in stroke mortality, 1962 to 1980 [see comments]. American Journal of Public Health 82: 1600–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, et al. (2000) Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: the Study of Health Assessment and Risk in Ethnic groups (SHARE)[see comment]. Lancet 356: 279–284. [DOI] [PubMed] [Google Scholar]
- 61. Harris R, Tobias M, Jeffreys M, Waldegrave K, Karlsen S, Nazroo J (2006) Effects of self-reported racial discrimination and deprivation on Māori health and inequalities in New Zealand: cross-sectional study. Lancet 367: 2005–2009. [DOI] [PubMed] [Google Scholar]
- 62. Harwood M, McNaughton H, McPherson K, Weatherall M (2000) Ethnicity and equity: missing the point.[comment]. Stroke 31: 2517–2518. [DOI] [PubMed] [Google Scholar]
- 63. Hajat C, Dundas R, Stewart JA, Lawrence E, Rudd AG, Howard R, et al. (2001) Cerebrovascular risk factors and stroke subtypes: differences between ethnic groups. Stroke 32: 37–42. [DOI] [PubMed] [Google Scholar]
- 64. Dundas R, Morgan M, Redfern J, Lemic-Stojcevic N, Wolfe C (2001) Ethnic differences in behavioural risk factors for stroke: implications for health promotion. Ethnicity & Health 6: 95–103. [DOI] [PubMed] [Google Scholar]
- 65. McGruder HF, Malarcher AM, Antoine TL, Greenlund KJ, Croft JB (2004) Racial and ethnic disparities in cardiovascular risk factors among stroke survivors: United States 1999 to 2001. Stroke 35: 1557–1561. [DOI] [PubMed] [Google Scholar]
- 66. Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, et al. (2001) Race-ethnic disparities in the impact of stroke risk factors the Northern Manhattan stroke study. Stroke 32: 1725–1731. [DOI] [PubMed] [Google Scholar]
- 67. Dundas R, Morgan M, Redfern J, Lemic-Stojcevic N, Wolfe C (2001) Ethnic differences in behavioural risk factors for stroke: Implications for health promotion. Ethnicity and Health 6: 95–103. [DOI] [PubMed] [Google Scholar]
- 68. Diaz KM, Booth Iii JN, Calhoun DA, Irvin MR, Howard G, Safford MM, et al. (2014) Healthy lifestyle factors and risk of cardiovascular events and mortality in treatment-resistant hypertension: The Reasons for Geographic and Racial Differences in Stroke study. Hypertension 64: 465–471. 10.1161/HYPERTENSIONAHA.114.03565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Howard G, Cushman M, Howard VJ, Kissela BM, Kleindorfer DO, Moy CS, et al. (2013) Risk factors for intracerebral hemorrhage: The reasons for geographic and racial differences in stroke (REGARDS) study. Stroke 44: 1282–1287. 10.1161/STROKEAHA.111.000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, et al. (2011) Traditional risk factors as the underlying cause of racial disparities in stroke: Lessons from the half-full (empty?) glass. Stroke 42: 3369–3375. 10.1161/STROKEAHA.111.625277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Strong K, Mathers C, Bonita R (2007) Preventing stroke: saving lives around the world. Lancet Neurology 6: 182–187. [DOI] [PubMed] [Google Scholar]
- 72. Editorial (2007) Stroke—prevention is better than cure. The Lancet 369: 247. [DOI] [PubMed] [Google Scholar]
- 73. Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. (2011) Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association.[Erratum appears in Stroke. 2011 Feb;42(2):e26]. Stroke 42: 517–584. 10.1161/STR.0b013e3181fcb238 [DOI] [PubMed] [Google Scholar]
- 74. Fink J (2006) Ethnic trends in stroke in New Zealand: closing the gaps or widening? The New Zealand medical journal 119: 1–3. [PubMed] [Google Scholar]
- 75. Dyall L, Carter K, Bonita R, Anderson C, Feigin V, Kerse N, et al. (2006) Incidence of stroke in women in Auckland, New Zealand. Ethnic trends over two decades: 1981–2003. New Zealand Medical Journal 119. [PubMed] [Google Scholar]
- 76. Blakely T, Tobias M, Robson B, Ajwani S, Bonne M, Woodward A (2005) Widening ethnic mortality disparities in New Zealand 1981–99. Social Science & Medicine 61: 2233–2251. [DOI] [PubMed] [Google Scholar]
- 77. McPherson KM, Harwood M, McNaughton HK (2003) Ethnicity, equity, and quality: lessons from New Zealand. BMJ 327: 443–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bonita R, Broad JB, Beaglehole R (1997) Ethnic differences in stroke incidence and case fatality in Auckland, New Zealand. Stroke 28: 758–761. [DOI] [PubMed] [Google Scholar]
- 79. Bell C, Swinburn B, Stewart A, Jackson R, Tukuitonga C, Tipene-Leach D (1996) Ethnic differences and recent trends in coronary heart disease incidence in New Zealand. New Zealand Medical Journal 109: 66–68. [PubMed] [Google Scholar]
- 80. Feigin VL, Krishnamurthi R, Barber PA, Arroll B (2014) Stroke prevention in New Zealand: Can we do better? International Journal of Stroke 9: 61–63. [DOI] [PubMed] [Google Scholar]
- 81. Hannon N, Callaly E, Moore A, Ní Chróinín D, Sheehan O, Marnane M, et al. (2011) Improved late survival and disability after stroke with therapeutic anticoagulation for atrial fibrillation: A population study. Stroke 42: 2503–2508. 10.1161/STROKEAHA.110.602235 [DOI] [PubMed] [Google Scholar]
- 82. Chróinín DN, Callaly EL, Duggan J, Merwick A, Hannon N, Sheehan Q, et al. (2011) Association between acute statin therapy, survival, and improved functional outcome after ischemic stroke: The North Dublin population stroke study. Stroke 42: 1021–1029. 10.1161/STROKEAHA.110.596734 [DOI] [PubMed] [Google Scholar]
- 83. Stroke Foundation of New Zealand, New Zealand Guidelines Group (2010) New Zealand Clinical Guidelines for Stroke Management 2010. Wellington: Stroke Foundation of New Zealand and New Zealand Guidelines Group. [Google Scholar]
- 84. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. (2014) Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. The Lancet 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ (2007) Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J NeurolNeurosurgPsychiatry 78: 1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kwon JW, Lee HJ, Hyun MK, Choi JE, Kim JH, Lee NR, et al. (2013) Trends in the incidence of subarachnoid hemorrhage in South Korea from 2006–2009: An ecological study. World Neurosurgery 79: 499–503. 10.1016/j.wneu.2012.07.032 [DOI] [PubMed] [Google Scholar]
- 87. Macpherson KJ, Lewsey JD, Jhund PS, Gillies M, Chalmers JWT, Redpath A, et al. (2011) Trends in incidence and in short term survival following a subarachnoid haemorrhage in Scotland, 1986–2005: A retrospective cohort study. BMC Neurology 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Inagawa T (2001) Trends in incidence and case fatality rates of aneurysmal subarachnoid hemorrhage in Izumo City, Japan, between 1980–1989 and 1990–1998. Stroke 32: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 89. Sakurai Y, Li JH, Komatsu S, Saito H, Abe S, Yamada A, et al. (1995) The secular trend in the incidence of subarachnoid hemorrhage in Miyagi, Japan: 1979–1990. The Tohoku journal of experimental medicine 177: 89–92. [DOI] [PubMed] [Google Scholar]
- 90. Feigin V, Parag V, Lawes CMM, Rodgers A, Suh I, Woodward M, et al. (2005) Smoking and Elevated Blood Pressure Are the Most Important Risk Factors for Subarachnoid Hemorrhage in the Asia-Pacific Region: An Overview of 26 Cohorts Involving 306 620 Participants. Stroke 36: 1360–1365. [DOI] [PubMed] [Google Scholar]
- 91. Health Mo (2012) The Health of New Zealand Adults 2011/12 Key findings of the New Zealand Health Survey. Wellington: Ministry of Health. [Google Scholar]
- 92. Health Mo (2013) Mortality and Demographic Data 2010. Wellington: Ministry of Health. [Google Scholar]
- 93. Tobias M, Cheung J, Carter K, Anderson C, Feigin VL, Tobias M, et al. (2007) Stroke surveillance: population-based estimates and projections for New Zealand. Australian & New Zealand Journal of Public Health 31: 520–525. [DOI] [PubMed] [Google Scholar]
- 94. Vaartjes IP, O'Flaherty MPMD, Capewell SPMD, Kappelle JPMD, Bots MPMD. Remarkable Decline in Ischemic Stroke Mortality Is not Matched by Changes in Incidence. Stroke. [DOI] [PubMed] [Google Scholar]
- 95. Kawachi I, Daniels N, Robinson DE (2005) Health disparities by race and class: Why both matter. Health Affairs 24: 343–352. [DOI] [PubMed] [Google Scholar]
- 96. Ministry of Health (2004) Ethnicity Data Protocols for the Health and Disability Sector. Wellington: Ministry of Health. 0-478-25845-3 0-478-25845-3 [Google Scholar]
- 97. Fraser GE (1978) The estimation of disease frequency using a population sample. International Journal of Epidemiology 7: 277–284. [DOI] [PubMed] [Google Scholar]
- 98. Napier AD, Ancarno C, Butler B, Calabrese J, Chater A, Chatterjee H, et al. Culture and health. The Lancet 384: 1607–1639. [DOI] [PubMed] [Google Scholar]
- 99. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. (2014) Global sodium consumption and death from cardiovascular causes. The New England journal of medicine 371: 624–634. 10.1056/NEJMoa1304127 [DOI] [PubMed] [Google Scholar]
- 100. Waxman A (2004) The WHO global strategy on diet, physical activity and health: The controversy on sugar. Development 47: 75–82. [Google Scholar]
- 101. Briggs ADM, Mytton OT, Kehlbacher A, Tiffin R, Rayner M, Scarborough P (2013) Overall and income specific effect on prevalence of overweight and obesity of 20% sugar sweetened drink tax in UK: Econometric and comparative risk assessment modelling study. BMJ (Online) 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Aggregate data underlying the findings are available in the body of the paper. All original person-specific data cannot be shared publicly due to ethical restrictions but are available upon request to those who meet the criteria for access to confidential data. Please contact Dr Rita Krishnamurthi, National Institute for Stroke & Applied Neurosciences, AUT University to request access.