Abstract
Background
Schmallenberg virus (SBV), an arboviral pathogen of ruminants, emerged in northern Europe during 2011 and has subsequently spread across a vast geographic area. While Culicoides biting midges (Diptera: Ceratopogonidae) have been identified as a biological transmission agent of SBV, the role of mosquitoes (Diptera: Culicidae) as potential vectors has not been defined beyond small-scale field collections in affected areas. Culex pipiens L. are one of the most widespread mosquitoes in northern Europe; they are present on farms across the region and have previously been implicated as vectors of several other arboviruses. We assessed the ability of three colony lines of Cx. pipiens, originating from geographically diverse field populations, to become fully infected by SBV using semi-quantitative real-time RT-PCR (sqPCR).
Findings
Two colony lines of Cx. pipiens were created in the UK (‘Brookwood’ and ‘Caldbeck’) from field collections of larvae and pupae and characterised using genetic markers. A third strain of Cx. pipiens from CVI Wageningen, The Netherlands, was also screened during experiments. Intrathoracic inoculation of the Brookwood line resulted in infections after 14 days that were characterised by high levels of RNA throughout individuals, but which demonstrated indirect evidence of salivary gland barriers. Feeding of 322 individuals across the three colony lines on a membrane based infection system resulted in no evidence of full dissemination of SBV, although infections did occur in a small proportion of Cx. pipiens from each line.
Conclusions/Significance
This study established two novel lines of Cx. pipiens mosquitoes of UK origin in the laboratory and subsequently tested their competence for SBV. Schmallenberg virus replication and dissemination was restricted, demonstrating that Cx. pipiens is unlikely to be an epidemiologically important vector of the virus in northern Europe.
Introduction
In 2011 a novel Orthobunyavirus of ruminants, provisionally named Schmallenberg virus (SBV) was identified through metagenomic analysis in Germany [1]. Culicoides biting midges (Diptera, Ceratopogonidae) were rapidly identified by several groups as being the primary biological vector of SBV through field studies [2,3]. These investigations were aided by the standardization of diagnostic tools for detection of SBV RNA in putative vector Culicoides species using colony lines [4]. Since this identification, the vector competence of alternative groups, including mosquitoes and ticks, has not been assessed in detail in the laboratory using colony derived lines, although several studies have failed to find SBV in field collections of mosquitoes [5,6]. This is despite the demonstration that at least one other member of the Orthobunyavirus genus, Oropouche virus, possesses the ability to infect and replicate to full dissemination in both Culicoides and mosquitoes under laboratory conditions [7,8].
In Europe, Culex pipiens L. is the most common and widespread mosquito inhabitant of small water bodies (including containers and ponds within urban areas) [9], although conflation with the morphologically similar Cx. torrentium Martini remains an issue in mapping distribution [10]. The phylogenetics of the Pipiens complex (Cx. pipiens, Cx. quinquefasciatus Say, Cx. australicus Dobrotworsky and Drummond, Cx. globocoxitus (Dobrotworsky and Drummond) and Cx. pallens (Coquillett)), has been the subject of investigation worldwide due to their role in the biological transmission of arboviruses [11,12]. There is still continued debate, however, regarding the specific status and relationship between the members of this complex [12]. In Europe, Cx. pipiens is the sole representative of the Pipiens complex and is observed in two ecological forms; Cx. pipiens form pipiens and Cx. pipiens form molestus. These ecological forms are morphologically inseparable and also found in other regions of the world [11]. Recently, hybrids between these two forms in Europe and North Africa have also been identified [13,14] and these have been shown to be present and sympatric with both forms recorded in the United Kingdom [15]. It has been suggested that hybridization in Cx. pipiens may, alongside differences in host preference [16], drive transmission of West Nile virus (WNV; Flaviviridae, Flavivirus) through hybrids possessing a greater vector competence [17]. The full characterization of individuals as specific forms or hybrids is therefore becoming an increasing important factor in studies [15].
In this paper we provide an account of the colonization and subsequent molecular characterization of two strains of Culex pipiens L. derived from field material collected in the UK. We then investigate vector competence for SBV using these two colony lines and an additional line from The Netherlands, through a combination of intrathoracic inoculation and oral feeding of blood/SBV suspensions through a membrane-based system. In doing so we provide baseline data for further studies of SBV in field-collected mosquitoes and a direct comparison with levels of infection in Culicoides under laboratory conditions, which have been conducted in previous laboratory studies [4].
Methods
Colonization of mosquitoes
Two colony lines of Cx. pipiens designated ‘Caldbeck’ and ‘Brookwood’ were established from field populations located in the UK during May 2011. Several hundred mosquito larvae and pupae were collected from a suburban freshwater pond in Surrey, United Kingdom (51.3809N, -0.2390W) to initiate the Caldbeck line. A similar number of mosquito larvae and pupae were also collected from a container habitat in an allotment area in Surrey (51.3065N, -0.6328W). Mosquito larvae and pupae collected from the field sites were transferred in water from their original habitat to The Pirbright Institute and colonization was conducted at 25 ±1°C with a photo period of 16:8 (light: dark) hours in a contained insectary room. Mosquitoes were allowed to emerge from the container and mate freely in a 32.5 x 32.5 x32.5 cm white net cage (MegaView Science Co. Ltd, Taichung, Taiwan).
Blood feeding of mosquitoes of mixed age was then conducted overnight (exposure from approximately 16.00–09.00h) using a Hemotek system (Hemotek Ltd, UK), with a stretched Parafilm M (Bermis Company, INC., USA) membrane and defibrinated horse blood (TCS Biosciences, UK) heated to 37°C. One black plastic cup (425 ml capacity) containing 125 ml of tap water, which had been allowed to stand for at least 24 hours to allow any chlorine to evaporate, was provided for oviposition from day four post-feeding and egg rafts were collected for up to two weeks after the initial blood-meal. This cycle of blood-feeding and egg collection was repeated on average six times per generation (range 5–12).
Egg rafts and first instar larvae were transferred to a larval development bowl which contained approximately 1.5L of water and ¼ teaspoon of ground Mr Johnson’s Advance Guinea Pig Food (Mr Johnson’s, UK) added each Monday, Wednesday and Friday. Ten to twelve egg rafts were introduced into each bowl (resulting in approximately 500–800 emerging 1st instar larvae) and the rearing bowl was covered with netting to prevent the release of emerging adults. Larvae required approximately 10–14 days to develop, pupate and emerge as adults and both pupae and adults were collected and placed in clean cages at a density of up to 500 individuals/cage. Sucrose solution (10% w/v) was provided to adults via a cotton wool pad placed in a 6cm diameter sample pot lid.
An additional established line of Cx. pipiens complex mosquitoes was provided from The Netherlands, designated ‘Wageningen’. This line was created in August 2010 from egg rafts collected from a metal water-holding tub in a domestic garden. The colony was maintained on fresh chicken blood using Hemotek blood feeders as described above. Prior to shipping the colony line was maintained at 23 ±1°C and a photoperiod of 16:8 light: dark. Egg rafts were shipped to Pirbright in March 2012 and then maintained under the conditions described for colonies maintained at Pirbright.
Molecular characterization of mosquitoes
Mosquitoes were initially characterized using morphology of emerging adults which restricted candidate species to Cx. pipiens and Cx. torrentium. A series of molecular assays were then used to characterize colony lines to species and ecological form in the original field material (n = 5 ♀ for both lines), first generation (n = 5 ♀ for both lines) and the 19th (Caldbeck) or 20th generation (Brookwood) (n = 40 (20 ♂, 20♀) for both lines). In the individuals from the 19th (Caldbeck) and 20th (Brookwood) generations, extraction of DNA was carried out using three legs from each specimen and the remaining bodies were subsequently prepared as pinned morphological voucher specimens. In field and first generation specimens that had been stored at -20°C in 70% ethanol, DNA was extracted from decapitated whole mosquitoes.
DNA extraction was carried out using specimens placed in 1.5ml microcentrifuge tubes with 180μl PBS and homogenized using a disposable pellet pestle (Sigma-Aldrich, Gillingham, UK) for 30 seconds. Twenty μl of proteinase K and 180μl Qiagen Buffer AL (Qiagen, Crawley, UK) were added to the resulting homogenate and incubated at 56°C for 10 minutes using a dry block heater (Techne Dri-Block DB-2P: Bibby Scientific Ltd, Stone, UK), 200μl of 100% ethanol was then added to each sample and mixed thoroughly by vortexing (Genie 2 Vortex: Scientific Industries Inc., Bohemia, NY, USA). Total DNA was extracted and purified from the resulting homogenate using a DNeasy Blood & Tissue Kit (Qiagen).
Assays used for characterization of mosquitoes are described in Table 1 and conducted in the order given. All polymerase chain reactions (PCR’s) were conducted using a GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) and negative controls for the amplification reactions were carried out at every PCR round. Reactions were stored at 4°C prior to visualization. Amplification success for the COI and ace-2 assays was assessed using 2% (w/v) E-Gel 96 gels containing SYBR Safe (Invitrogen, Paisley, UK) run for 8 minutes on program EP. Results of the CQ11 microsatellite assay were visualized using 2% agarose gels containing SYBR Safe DNA Gel Stain (Invitrogen, Paisley, UK) run for 30 minutes at 100 volts. Bands were identified by comparison with E-Gel Low Range Quantitative DNA Ladder (100–2000bp: Invitrogen). For lanes containing the duplex ace-2 PCR products fragment sizes identified Cx. pipiens (610bp fragment) or Cx. torrentium (416bp fragment). For lanes containing the duplex CQ11 microsatellite locus PCR products fragment sizes were identified as Cx. pipiens f. pipiens (180bp fragment), Cx. pipiens f. molestus (250bp fragment) or Cx. pipiens f. pipiens x f. molestus (presence of both 180bp and 250bp fragments).
Table 1. Polymerase chain reaction (PCR)-based assays used for genetic characterization of Brookwood and Caldbeck Culex pipiens lines.
| Assay | PCR Reaction Mix | PCR Conditions |
|---|---|---|
| COI Barcode assay [41] | Reaction volume of 25μl: 2.5μl nuclease-free water (Qiagen, UK); 12.5μl Qiagen TopTaq Master Mix (Qiagen, UK); 2.5μl CoralLoad Concentrate (Qiagen, UK); 1.25μl of the 10μM forward primer LCO1490 (5’-GGTCAACAAATCATAAAGATATTGG-3’); 1.25μl of the 10μM reverse primer HCO2198 (5’-TAAACTTCAGGGTGACCAAAAAATCA-3’); 5.0μl of template DNA per reaction. | Denaturation at 94°C for three minutes; 35 cycles 94°C for 30 seconds; 46°C for 30 seconds; 72°C for one minute. Final extension step 72°C for ten minutes. |
| Eurasia ace-2 multiplex assay [42] | Reaction volume of 10μl: 0.4μl nuclease-free water (Qiagen, UK); 5.0μl Qiagen TopTaq Master Mix (Qiagen, UK); 0.2μl 25mM Magnesium Chloride (MgCL2) (Qiagen, UK); 1.0μl CoralLoad Concentrate (Qiagen, UK); 0.1μl of 10μM forward primer ACEtorr (5’- TGCCTGTGCTACCAGTGATGTT -3’; 0.1μl of 10μM forward primer ACEpip (5’- GGAAACAACGACGTATGTACT -3’; 0.2μl of 10μM reverse primer B1246s (5’-TGGAGCCTCCTCTTCACGG-3’; 3.0μl of template DNA per reaction. | Denaturation at 94°C for three minutes; 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, 72°C for one minute. Final extension step 72°C for ten minutes. |
| CQ11 microsatellite assay [22] | Reaction volume of 20μl: 0.80μl nuclease-free water (Qiagen, UK); 10.0μl Qiagen TopTaq Master Mix (Qiagen, UK); 0.4μl 25mM Magnesium Chloride (MgCL2) (Qiagen, UK); 0.15μl 20mg/ml Bovine Serum Albumin (Sigma-Aldrich Company Ltd, UK); 2.0μl CoralLoad Concentrate (Qiagen, UK); 0.15μl of 10μM forward primer CQ11F (5’- GATCCTAGCAAGCGAGAAC-3’; 0.20μl of 10μM reverse primer pipCQ11R (5’- CATGTTGAGCTTCGGTGAA -3’; 0.30μl of 10μM reverse primer molCQ11R (5’-CCCTCCAGTAAGGTATCAAC-3’; 6.0μl of template DNA per reaction. | Denaturation 95°C for three minutes; 40 cycles of 94°C for 30 seconds, 55°C for 30 seconds, 72°C for one minute. Final extension step at 72°C for ten minutes. |
Sequencing of the COI Barcode region [18] was performed on PCR product purified using a MinElute PCR purification kit (Qiagen). DNA concentration following purification was estimated using a NanoDrop 2000 UV-Vis Spectrophotometer (Fisher Scientific UK, UK), then diluted using 10 mM Tris-HCL pH 8.0 (Buffer EB: Qiagen) to 1ng per μl per 100 bases (~7ng/μl) and sent for bi-directional sequencing at a commercial facility (Source BioScience, UK) using primers HCO2198 and LCO1490 (3.2μM). Sequences were edited and assembled using CodonCode Aligner (CodonCode Aligner, Centerville, MA, USA) and consensus sequences aligned using MUSCLE [19]. These sequences were compared to previously published sequences using BLAST in MEGA version 5.2 [20].
Morphological specimens were vouchered by pinning using the double mounted method [21]. Prior to pinning, the last three segments of the male abdomens were removed using a microscapel and cleared by incubating individually in 200μl of 10% w/v KOH for 20 minutes at 6°C using a dry block heater (Techne Dri-Block DB-2P). Following incubation phallosomes were removed and transferred to acetic acid for 5 minutes to neutralize any remaining KOH. The phallosomes were then dehydrated in 100% ethanol for 20 minutes and transferred to soften in 1:1 clove oil: 100% ethanol mix for 20 minutes and transferred to 100% clove oil for at least 20 minutes prior to mounting. The genitalia were then slide mounted in Euparal with the ventral surface facing upwards with the dististyles extended. Digital images were taken using a QICAM Fast 1394 digital camera (QImaging, Surrey, BC, Canada) and Image-Pro Insight (MediaCybernetics, Rockville, MD, USA) mounted on a Leica M80 stereo light microscope (Leica Microsystems, Milton Keynes, UK) for pin-mounted specimens and on a Nikon Alphaphot-2 compound light microscope (Nikon UK Ltd, Kingston Upon Thames, Surrey, UK) for slide-mounted specimens.
Culex pipiens specimens from the Wageningen line were also characterized using morphological identification to separate them from specimens of Cx. torrentium [22]. Egg rafts had originally collected from an above-ground location (typical for Cx. pipiens f. pipiens), but were not morphologically vouchered. Two years following initial colonization, a total of 64 individuals were processed using the CQ11 microsatellite assay to define Cx. pipiens forms present in the line [22].
Infection studies with SBV
The SBV strain used for studies of infection of mosquitoes has been previously described in detail and was originally provided by the Friedrich-Loeffler-Institute, Isle of Riems, Germany [4]. All infection studies were carried out using batches of approximately 40–50, 2–3 day old adult Cx. pipiens from the 11th generation of the two UK colony lines (Brookwood and Caldbeck) and an unknown generation of the Wageningen line.
Intrathoracic Inoculation
Approximately 200 individuals of the Brookwood line were inoculated intrathoracically (IT) using 0.4μl of SBV suspension and a micro-injector equipped with a foot driver (Drummond Scientific Nanoject II: Drummond Scientific, USA). The SBV strain had been passaged once through a Culicoides sonorensis Wirth and Jones cell line and four times through a Baby Hamster Kidney (BHK-21) cell line (Cq value 10–12; infectivity on BHK-21 cells of 5–5.5 log10TCID50). Ten of the IT inoculated Brookwood line were processed immediately by sqPCR using an identical assay to a previous study [4], and the remainder incubated for 14 days at 25 ± 1°C with access to 10% sucrose solution.
Following incubation, 35 Brookwood line mosquitoes were processed as whole insects using sqPCR and a further 8 were used to determine if SBV infected the salivary glands and was secreted through eliciting salivation and dissection. In these individuals, a drop of pillocarpine (parasympathomimetic alkaloid: Sigma Aldrich, UK) solution (1:4 diluted) was applied to the ventral surface of each mosquito and saliva collected into a 1 μl microcapillary glass tube containing 10% fetal bovine serum (FBS). The collected media was then expelled into individual Eppendorf tubes containing 0.5ml of Schneider’s Drosophila Media (Gibco: Life Technologies, Paisley, UK) and 10% FBS supplemented with 1000 IU/ml Penicillin/Streptomycin and 4μg/ml Amphotericin B, before processing in duplicate using sqPCR. Each of these eight individuals was then dissected into head and abdomen/thorax and processed using sqPCR. The Cq values resulting from testing were then used to infer SBV RNA quantity in samples using means of the two Cq values. Where one of the duplicated samples did not give Cq value while the other did, the single value was used.
Membrane-based Infections
Culex pipiens mosquitoes from all three lines were then fed on defibrinated sheep-blood (TCS Biosciences, UK) / SBV suspensions via the Hemotek system (Hemotek Ltd, UK) and a Parafilm M membrane (Bermis Company, INC., USA). A 1:1 mixture of blood and virus was used throughout the experiments and resulted in blood meals of Cq 14–15. Five membrane fed Brookwood line and three Caldbeck line mosquitoes were processed immediately for SBV RNA and the remainder incubated for 14 days at 25 ±1°C with access to 10% sucrose solution. Following incubation, 322 individuals were processed for SBV RNA as whole insects (114 from the Brookwood line; 85 from the Caldbeck line and 123 from the Wageningen line). The remaining 18 from the Caldbeck line were treated as for the dissemination experiment in the earlier IT inoculation study.
Processing of samples for sqPCR
Whole Cx. pipiens, decapitated bodies and heads were homogenized individually for 1 min at 25hz in 100μl of Schneider’s Drosophila Media (Gibco: Life Technologies, Paisley, UK) containing 10% FBS using a TissueLyser (Qiagen, UK) and 3mm stainless steel beads (Dejay Distribution Ltd., UK). Nucleic acid extraction of SBV from samples was subsequently carried out using a Universal Biorobot (Qiagen, UK) in a 96-well format using a QIAamp All Nucleic Acid MDx Kit (Qiagen, UK). Schmallenberg virus RNA in Cx. pipiens samples was quantified using a sqPCR that targeted the S segment of the genome [23]. Comparisons of RNA quantities were made using Cq values generated from samples.
Statistical analyses
Statistical differences in Cq values were assessed using comparison of confidence intervals and non-parametric Wilcoxon rank-sum and Kruskal-Wallis tests.
Ethics statement
All three Cx. pipiens colony lines were created from material taken from privately owned land related to the authors and further collections should be requested from the corresponding author (SC). Blood used during experiments and in routine colony maintenance was supplied by TCS biosciences for the Brookwood and Caldbeck lines in the UK and chicken blood was taken from the commercial Kemperkip slaughterhouse in The Netherlands. Use of blood from these sources does not require ethical permission or licensing in either the UK or in The Netherlands.
Results
Colonization and molecular characterisation of UK Cx. pipiens lines
Both UK lines of Cx. pipiens were colonized with what anecdotally appeared to be a relatively limited bottleneck in the F1 and F2 generations. Results of the molecular assays used to characterize the Brookwood and Caldbeck Cx. pipiens lines are summarized in Table 2. Indels, frame shifts and stop codons were absent among COI sequences and their translations, suggesting pseudogenes were not present in the alignment. In contrast to a previous study [24], the nucleotide substitution of a G for an A at the 3rd position of the 68th codon of the COI gene fragment in Cx. pipiens f. molestus when compared to Cx. pipiens f. pipiens specimens did not occur. Morphological images and DNA sequences including electropherograms have been made publically available via the Barcode of Life Data System (BOLD) [25] under project PIRCX (dx.doi.org/10.5883/DS-PIRCX) and DNA sequences are also available on GenBank (accession numbers Brookwood: F0 - KM439036-KM439038, KM439054, KM439055, F1-KM439041-KM439045 and F20-KM438957-KM438995, KM439040; Caldbeck, F0 - KM439046-KM439050, F1- KM439039, KM439051-KM439053 and F19-KM438996-KM439035). The exact generation of the Wageningen Cx. pipiens line was unknown, but the line had been in rearing for > 2 years at the time of analysis.
Table 2. Species and form identification of mosquito lines used during infection studies determined using COI sequence identity, and CQ11 microsatellite and ace-2 multiplex assays (* carried out through CQ11 assay only on individuals of an unrecorded generation > 2 years following colonization).
| Colony line | Generation | n | Culex torrentium | Cx. pipiens form pipiens | Cx. pipiens form molestus | Cx. pipiens hybrid |
|---|---|---|---|---|---|---|
| Brookwood | Field | 5 | 2 | 2 | 0 | 1 |
| F 1 | 5 | 0 | 4 | 0 | 1 | |
| F 20 | 40 | 0 | 10 | 5 | 25 | |
| Caldbeck | Field | 5 | 0 | 5 | 0 | 0 |
| F 1 | 5 | 0 | 5 | 0 | 0 | |
| F 19 | 40 | 0 | 40 | 0 | 0 | |
| Wageningen | F* | 64 | - | 5 | 47 | 12 |
Infection studies with SBV
Intrathoracic Inoculation
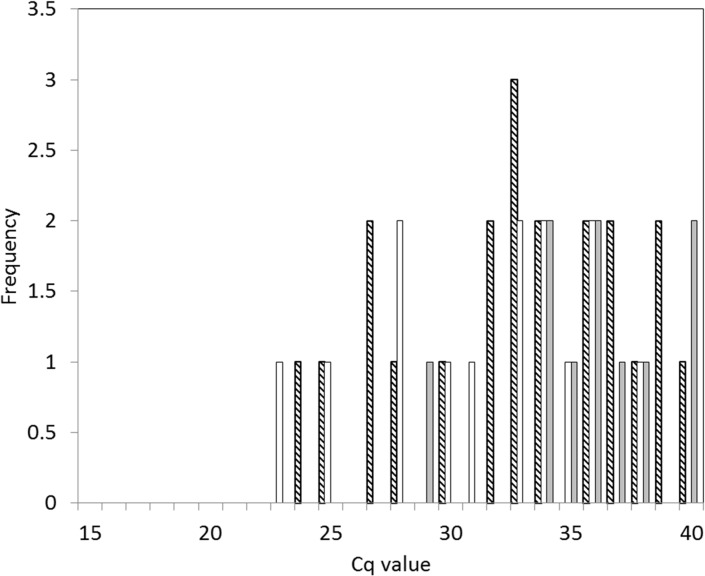
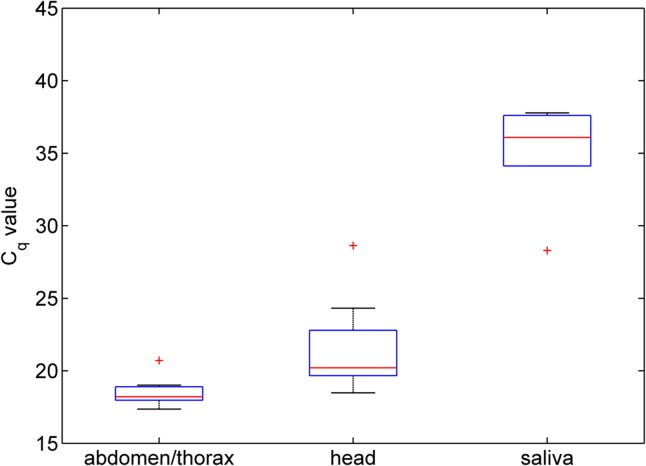
All IT inoculated Brookwood line Cx. pipiens processed were found to contain SBV RNA. Individuals processed immediately following IT infection (n = 10) contained a median Cq of 21.6 (Range = 21.0–22.1), which was significantly less than those incubated for 14 days (n = 35) which contained a median Cq of 17.3 (Range = 16.4–30.1; Wilcoxon rank-sum test: W = 320, P<0.001) (Fig 1). In dissected, IT inoculated individuals processed at day 14 (n = 8) (Fig 2), the abdomen and thorax contained the greatest quantity of SBV RNA (median Cq = 18.2, Range = 17.4–20.7); followed by the head (median Cq = 20.2, Range = 18.5–28.6). Duplicated detection of SBV RNA was recorded in saliva of six Brookwood line mosquitoes processed, while two gave positive but unduplicated detection (median Cq = 36.1, Range = 28.3–37.8). Differences in SBV RNA load between body parts and saliva were statistically significant (χ2 = 15.86, P<0.001) and pairwise comparison identified significantly less SBV RNA in saliva than in the abdomen/thorax and head (χ2 = 18.14; P<0.001).
Fig 1. Frequency histogram of responses of Cx. pipiens lines to attempted infection with Schmallenberg virus.
Black = Intrathoracically inoculated Brookwood line; Cross-hatch = Orally fed Brookwood line; White = Orally fed Caldbeck line; Grey = Orally fed Netherlands line. Samples of >40 Cq are excluded.
Fig 2. Observed Cq values for Schmallenberg virus in intrathoracically inoculated Cx. pipiens of the Brookwood line dissected at day 14 post-infection.
The box-and-whisker plot shows the median (horizontal line), interquartile range (box), 1.5 times the interquartile range (whiskers) and any outliers (crosses).
Membrane-based Infections
All eight orally fed Cx. pipiens (five from the Brookwood line and three from the Caldbeck line) processed immediately following oral feeding on SBV/sheep blood suspension tested positive for SBV RNA (median Cq = 21.6, Range = 20.0–24.5). SBV RNA was detected in 27 of 322 Cx. pipiens from all lines incubated for 14 days following oral feeding (median Cq = 33.6, Range = 22.6–40.0). Using Fishers exact tests there no significant difference was found between the proportions of each line containing detectable SBV RNA. Median Cq values from individuals containing detectable SBV RNA were significantly different between the IT inoculated Brookwood line incubated for 14 days and the membrane fed Brookwood (W = 6; P<0.001), Caldbeck (W = 6; P<0.001) and Netherlands (W = 1; P<0.001) lines incubated for the same period following exposure. The maximum quantity of SBV RNA in any membrane-fed individual (Cq = 22.6) remained below the median quantity developed by Cx. pipiens during IT inoculation (Cq = 17.3) and peak RNA quantities in the Brookwood, Caldbeck and Netherlands lines fell outside the 95th percentile of IT inoculated values for the Brookwood line. The highest SBV RNA Cq value within IT inoculated individuals was 22.6, which was identical to the lowest value returned by any membrane-fed individual (Table 3). Dissection of 18 Cx. pipiens of the Caldbeck line produced no evidence of replicating SBV in the abdomen/thorax and head with all Cq values recorded being >34 (Table H in S1 File).
Table 3. SBV RNA quantity detected in Culex pipiens from three colony lines fed a mixture of blood and tissue culture passaged virus or intrathoracically (IT) inoculated and then incubated for 14 days prior to processing.
| Cq | Culex pipiens colony line / Treatment | |||
|---|---|---|---|---|
| Brookwood (Membrane fed) | Caldbeck (Membrane fed) | Wageningen (Membrane fed) | Brookwood (IT inoculated) | |
| ≥40 | 92 | 71 | 113 | 0 |
| 40–35 | 9 | 3 | 6 | 0 |
| 35–30 | 7 | 6 | 3 | 1 |
| 30–25 | 4 | 3 | 1 | 0 |
| 25–20 | 2 | 2 | 0 | 5 |
| 20–15 | 0 | 0 | 0 | 29 |
| Total | 114 | 85 | 123 | 35 |
| Median C q | 33.3 | 32.6 | 35.4 | 17.3 |
| Range C q | 39–23.3 | 37.7–22.6 | 40–28.8 | 30–15.7 |
| RNA detection rate (%) | 19.3 | 16.5 | 8.1 | 100 |
Discussion
This study demonstrated that three separate colony lines of Cx. pipiens originating from northern Europe were refractory to dissemination of SBV to the salivary glands following oral feeding on blood-virus mixtures through an artificial membrane. While 8.1–19.3% of Cx. pipiens lines tested at 14 days post-feeding contained detectable quantities of SBV RNA, the Cq values recorded were significantly higher than those recorded for IT inoculated individuals. These IT inoculated mosquitoes from the Brookwood line demonstrated a significant increase in SBV RNA detected from day 0 to day 14 post-inoculation and at day 14 had SBV RNA in their saliva. The smaller quantity of SBV RNA developed in membrane fed individuals in comparison to IT inoculation can be explained by the presence of midgut infection and/or escape barriers to infection and/or more general immune responses within the haemoceol, as documented in over vector-arbovirus relationships [26]. These barriers are either bypassed or overwhelmed during IT inoculation with the exception of those associated with the salivary glands. In addition, at least some of the higher Cq values generated (>35) may have occurred through the presence of inactivated SBV from the original blood meal remaining within the mosquito, which has been discussed previously [4]. The low proportion of infections in Cx. pipiens lines tested adds weight to recent studies that failed to detect SBV in field populations of mosquitoes [5,27,28], although other aspects of vectorial capacity known to vary among lines of this species (e.g. host preference and autogeny) may have a significant impact in the field. Culicoides therefore remain the only confirmed vector group for SBV to date [3,4,28,29].
Additional studies to confirm refractory status in Cx. pipiens populations could include attempts to isolate SBV from saliva of infected individuals. While SBV RNA was detected in IT inoculated individuals during the current study, this was relatively difficult to interpret in some cases due to the values being close to the cut-off value chosen for the assay. Isolation of virus would therefore provide additional security that what is being detected is live virus rather than trace inactivated virus from the original blood-virus meal. In addition, feeding of field mosquito populations on viraemic hosts would also provide a more natural system. One challenge in accomplishing this aim is the relatively short period of viraemia in ruminant hosts [30]. When paired with variation in field availability of mosquitoes and their blood-feeding responses, this makes studies extremely challenging to perform under biosecure conditions. In addition, a wide variety of virus, environmental and co-infection factors could all significantly influence transmission in the field as has been reviewed for both arboviruses and other pathogens [26,31]. This includes the potential for largely refractory populations to become co-infected with microfilariae which have the potential to disrupt the midgut integrity and lead to a far higher rate of competence for arboviruses [32,33].
This study also highlights the ongoing issues surrounding the classification of Cx. pipiens. Individuals of the pipiens, molestus and hybrid forms of Cx. pipiens have recently been recorded sharing the same habitat within Europe [13,15]. However, the fact that this genetic diversity was not substantially reduced within the colony lines and that these forms could be consistently supported within the Brookwood and Wageningen laboratory lines after initial colonization was unexpected. Interestingly, Cx. torrentium, which was inadvertently included in the original material for colonization from the Brookwood line, did not establish successfully and was not detected in subsequent screening, which may be due to a lack of stenogamy in this population. The different forms of Cx. pipiens have previously been characterized by ecological phenotypic characteristics such as autogeny, host preference and stenogamy that would be expected to provide selective advantages or disadvantages under colony conditions [34]. Further study is therefore required to both elucidate the taxonomic relationship and specific status of these two Cx. pipiens forms and define their status within the Brookwood and Wageningen colony lines. These investigations could include the characterization of phenotypic variation in relation to genotype.
While a recent study has highlighted an issue of cross-reaction when Cx. torrentium is processed alongside Cx. pipiens individuals using the CQ11 assay [15], we avoided this issue in the case of the Brookwood and Caldbeck lines by using a multi-stage screening of identity, with separation at species-level prior to definition of Cx. pipiens form identity. The Wageningen line used the alternative route of morphological identification to species level and then application of the CQ11 assay. A major consideration is whether vector competence studies are better served by isotype colonies that are often created to ensure phenotypic identity, as opposed to more natural lines created from taxonomically diverse field material, as used in the current study. The influence of hybridization on vector competence is challenging to assess, not least because the vector competence assays in the current study were not linked directly to species diagnostics for logistical reasons. Hence, while the Caldbeck line (which contained only Cx. pipiens f. pipiens, even in the original field material) is highly likely to be representative of this form, results from the Brookwood line are more difficult to interpret. The fact that these lines and the additional Wageningen line originating from The Netherlands failed to support full SBV dissemination, however, would suggest that the presence of hybrids in tested populations does not substantially influence vector competence for SBV, in contrast to WNV [17]. Further studies to examine this issue directly with zoonotic pathogens would be useful in elucidating the potential role that these hybrids might play in arbovirus outbreaks in northern Europe.
Until relatively recently, testing the vector competence of UK mosquitoes for arboviruses has been a relatively low priority. Recent emergence of Culicoides-borne pathogens [6,35], incursions of exotic mosquitoes in northern Europe [36] and outbreaks of arboviruses in the southern Mediterranean [37], have heightened awareness of this area and led to dedicated risk assessments of mosquito-borne arboviruses for the UK [9,38] and most recently the demonstration that Ochlerotatus detritus (Haliday) can support full replication of Japanese encephalitis virus following membrane-based feeding [39]. This highlights that testing a range of potential vector species is of significant importance in risk assessment studies for arbovirus transmission. The establishment of UK-derived colony lines of Cx. pipiens within this study contributes to providing fundamental data regarding not only vector competence, but a wide-array of other phenotypic variation that may influence vectorial capacity. Colonies allow the standardization of populations tested according to age, rearing conditions and a wide-variety of other factors that can otherwise influence studies [40]. The Brookwood and Caldbeck colony lines can be provided to global workers for such studies under the Biotechnology and Biological Sciences Research Council National Capability initiative, through contact with the corresponding author.
Supporting Information
Duplicated Cq values from Cx. pipiens mosquitoes intrathoracically (Brookwood line) inoculated with Schmallenberg virus and then processed immediately using sqPCR (Table A in S1 File). Duplicated Cq values from Cx. pipiens mosquitoes (Brookwood line) intrathoracically inoculated with Schmallenberg virus and then processed following a 14 day incubation period using sqPCR (Table B in S1 File). Duplicated Cq values from Cx. pipiens mosquitoes (Brookwood line) intrathoracically inoculated with Schmallenberg virus and then processed following a 14 day incubation period. Saliva from each mosquito was collected using a glass capillary tube and insecticidal treatment and the body was then dissected into head and abdomen/thorax before processing using sqPCR (Table C in S1 File). Cq values from Cx. pipiens mosquitoes (Brookwood line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed immediately using sqPCR (Table D in S1 File). Cq values from Cx. pipiens mosquitoes (Brookwood line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed after a 14 day incubation period using sqPCR. A total of 92 samples returned no Cq value or a value >40 (Table E in S1 File). Cq values from Cx. pipiens mosquitoes (Caldbeck line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed immediately using sqPCR (Table F in S1 File). Cq values from Cx. pipiens mosquitoes (Caldbeck line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed after a 14 day incubation period using sqPCR. A total of 71 samples returned no Cq value or a value >40 (Table G in S1 File). Cq values from Cx. pipiens mosquitoes (Caldbeck line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed following a 14 day incubation period. Saliva from each mosquito was collected using a glass capillary tube and insecticidal treatment and the body was then dissected into head and abdomen/thorax before processing using sqPCR (Table H in S1 File). Cq values from Cx. pipiens mosquitoes (Wageningen line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed after a 14 day incubation period using sqPCR. A total of 113 samples returned no Cq value or a value >40 (Table I in S1 File).
(DOCX)
Acknowledgments
We would like to acknowledge the assistance of M.A. Carpenter and E.M. Carpenter in initially identifying the source habitat for the Caldbeck line and in their collection. We also thank the referees for their detailed comments on the manuscript.
Data Availability
All relevant data are available via the BOLD database (dx.doi.org/10.5883/DS-PIRCX).
Funding Statement
Studies were financially supported by Defra grant SE: 0542 and BBSRC grant: BBS/E/I/00001445.
References
- 1. Hoffmann B, Bauer B, Bauer C, Batza HJ, Beer M, Clausen PH et al. (2009) Monitoring of Putative Vectors of Bluetongue Virus Serotype 8, Germany. Emerging Infectious Diseases 15: 1481–1484. 10.3201/eid1509.090562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Elbers ARW, Meiswinkel R, van Weezep E, Sloet van Oldruitenborgh-Oosterbaan MM, Kooi EA (2013) Schmallenberg virus in Culicoides spp. biting midges, the Netherlands, 2011. Emerging Infectious Diseases 19: 106–109. 10.3201/eid1901.121054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Regge N, Deblauwe I, De Deken R, Vantieghem P, Madder M, et al. (2012) Detection of Schmallenberg virus in different Culicoides spp. by real-time RT-PCR. Transboundary and Emerging Diseases 59: 471–475. 10.1111/tbed.12000 [DOI] [PubMed] [Google Scholar]
- 4. Veronesi E, Henstock M, Gubbins S, Batten C, Manley R, Barber J, et al. (2013) Implicating Culicoides Biting Midges as Vectors of Schmallenberg Virus Using Semi-Quantitative RT-PCR. Plos One 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scholte EJ, Mars MH, Braks M, Den Hartog W, Ibanez-Justicia A, Koopmans M, et al. (2014) No evidence for the persistence of Schmallenberg virus in overwintering mosquitoes. Medical and Veterinary Entomology 28: 110–115. 10.1111/mve.12010 [DOI] [PubMed] [Google Scholar]
- 6. Koenraadt CJM, Balenghien T, Carpenter S, Ducheyne E, Elbers ARW, Fife M, et al. (2014) Bluetongue, Schmallenberg—what is next? Culicoides-borne viral diseases in the 21st Century. Bmc Veterinary Research 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinheiro FP, Darosa A, Gomes MLC (1982) Transmission of Oropouche virus from man to hamster by the midge Culicoides paraensis . Science 215: 1251–1253. [DOI] [PubMed] [Google Scholar]
- 8. Hoch AL, Pinheiro FP, Roberts DR, Gomes ML (1987) Laboratory transmission of Oropouche virus by Culex Quinquefasciatus Say. Bulletin of the Pan American Health Organization 21: 55–61. [PubMed] [Google Scholar]
- 9. Medlock JM, Snow KR, Leach S (2005) Potential transmission of West Nile virus in the British Isles: an ecological review of candidate mosquito bridge vectors. Medical and Veterinary Entomology 19: 2–21. [DOI] [PubMed] [Google Scholar]
- 10. Hesson JC, Ostman O, Schafer M, Lundstrom JO (2011) Geographic Distribution and Relative Abundance of the Sibling Vector Species Culex torrentium and Culex pipiens in Sweden. Vector-Borne and Zoonotic Diseases 11: 1383–1389. 10.1089/vbz.2011.0630 [DOI] [PubMed] [Google Scholar]
- 11. Farajollahi A, Fonseca DM, Kramer LD, Kilpatrick AM (2011) "Bird biting" mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infection Genetics and Evolution 11: 1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harbach RE (2012) Culex pipiens: species versus species complex—taxonomic history and perspective. Journal of the American Mosquito Control Association 28: 10–23. [DOI] [PubMed] [Google Scholar]
- 13. Gomes B, Kioulos E, Papa A, Almeida APG, Vontas J, Pinto J (2013) Distribution and hybridization of Culex pipiens forms in Greece during the West Nile virus outbreak of 2010. Infection Genetics and Evolution 16: 218–225. [DOI] [PubMed] [Google Scholar]
- 14. Gomes B, Parreira R, Sousa CA, Novo MT, Almeida APG, Donnelly MJ, et al. (2012) The Culex pipiens complex in continental Portugal: distribution and genetic structure. Journal of the American Mosquito Control Association 28: 75–80. [DOI] [PubMed] [Google Scholar]
- 15. Danabalan R, Ponsonby DJ, Linton Y-M (2012) A critical assessment of available molecular identification tools for determining the status of Culex pipiens SL in the United Kingdom. Journal of the American Mosquito Control Association 28: 68–74. [DOI] [PubMed] [Google Scholar]
- 16. Fritz ML, Walker ED, Miller JR, Severson DW, Dworkin I (2015) Divergent host preferences of above- and below-ground Culex pipiens mosquitoes and their hybrid offspring. Medical and Veterinary Entomology 29: 115–123. 10.1111/mve.12096 [DOI] [PubMed] [Google Scholar]
- 17. Ciota AT, Chin PA, Kramer LD (2013) The effect of hybridization of Culex pipiens complex mosquitoes on transmission of West Nile virus. Parasites & Vectors 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003) Biological identifications through DNA barcodes. Proceedings of the Royal Society B-Biological Sciences 270: 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tamuram K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Becker N, Dušan P, Zgomba M, Boase C, Madon M, Dahl C, et al. (2010) Mosquito Research Techniques In: Becker N, Dušan P, Zgomba M, Boase C, Madon M, Dahl C, et al. editors. Mosquitoes and their Control, 2nd Edition Berlin, Germany: Springer Berlin Heidelberg; pp. 43–61. [Google Scholar]
- 22. Bahnck CM, Fonseca DM (2006) Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: Culicidae) and hybrid populations. American Journal of Tropical Medicine and Hygiene 75: 251–255. [PubMed] [Google Scholar]
- 23. Bilk S, Schulze C, Fischer M, Beer M, Hlinak A, Hoffmann B, et al. (2012) Organ distribution of Schmallenberg virus RNA in malformed newborns. Veterinary Microbiology 159: 236–238. 10.1016/j.vetmic.2012.03.035 [DOI] [PubMed] [Google Scholar]
- 24. Shaikevich EV (2007) PCR-RFLP of the COI gene reliably differentiates Cx. pipiens, Cx. pipiens f. molestus and Cx. torrentium of the pipiens complex. European Mosquito Bulletin: 25–30. [Google Scholar]
- 25. Ratnasingham S, Hebert PDN (2007) BOLD: The Barcode of Life Data System (www.barcodinglife.org). Molecular Ecology Notes 7: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mellor PS (2000) Replication of arboviruses in insect vectors. Journal of Comparative Pathology 123: 231–247. [DOI] [PubMed] [Google Scholar]
- 27. Wernike K, Jost H, Becker N, Schmidt-Chanasit J, Beer M (2014) Lack of evidence for the presence of Schmallenberg virus in mosquitoes in Germany, 2011. Parasites & Vectors 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balenghien T, Pages N, Goffredo M, Carpenter S, Augot D, Jacquier E, et al. (2014) The emergence of Schmallenberg virus across Culicoides communities and ecosystems in Europe. Preventive Veterinary Medicine 116: 360–369. 10.1016/j.prevetmed.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 29. Elbers ARW, Meiswinkel R, van Weezep E, van Oldruitenborgh-Oosterbaan MMS, Kooi EA (2013) Schmallenberg Virus in Culicoides spp. Biting Midges, the Netherlands, 2011. Emerging Infectious Diseases 19: 106–109. 10.3201/eid1901.121054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoffmann B, Scheuch M, Hoper D, Jungblut R, Holsteg M, Schirrmeier H, et al. (2012) Novel Orthobunyavirus in Cattle, Europe, 2011. Emerging Infectious Diseases 18: 469–472. 10.3201/eid1803.111905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuno G, Chang GJJ (2005) Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clinical Microbiology Reviews 18: 608–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Turell MJ, Rossignol PA, Spielman A, Rossi CA, Bailey CL (1984) Enhanced arboviral transmission by mosquitoes that concurrently ingested microfilariae. Science 225: 1039–1041. [DOI] [PubMed] [Google Scholar]
- 33. Mellor PS, Boorman J (1980) Multiplication of bluetongue virus in Culicoides nubeculosus (Meigen) simultaneously infected with the virus and the microfilariae of Ochocerca cervicalis (Raillet and Henry). Annals of Tropical Medicine and Parasitology 74: 463–469. [DOI] [PubMed] [Google Scholar]
- 34. Becker N, Joest A, Weitzel T (2012) The Culex pipiens complex in Europe. Journal of the American Mosquito Control Association 28: 53–67. [DOI] [PubMed] [Google Scholar]
- 35. Carpenter S, Wilson A, Mellor PS (2009) Culicoides and the emergence of bluetongue virus in northern Europe. Trends in Microbiology 17: 172–178. 10.1016/j.tim.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 36. Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. (2012) A Review of the Invasive Mosquitoes in Europe: Ecology, Public Health Risks, and Control Options. Vector-Borne and Zoonotic Diseases 12: 435–447. 10.1089/vbz.2011.0814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. (2007) Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370: 1840–1846. [DOI] [PubMed] [Google Scholar]
- 38. Medlock JM, Vaux AGC (2011) Assessing the possible implications of wetland expansion and management on mosquitoes in Britain. European Mosquito Bulletin: 38–65. [Google Scholar]
- 39. Mackenzie-Impoinvil L, Impoinvil DE, Galbraith SE, Dillon RJ, Ranson H, Johnson N, et al. (2014) Evaluation of a temperate climate mosquito, Ochlerotatus detritus (Aedes detritus), as a potential vector of Japanese encephalitis virus. Medical and Veterinary Entomology 29: 1–9. 10.1111/mve.12083 [DOI] [PubMed] [Google Scholar]
- 40. Richards SL, Lord CC, Pesko KN, Tabachnick WJ (2010) Environmental and Biological Factors Influencing Culex pipiens quinquefasciatus (Diptera: Culicidae) Vector Competence for West Nile Virus. American Journal of Tropical Medicine and Hygiene 83: 126–134. 10.4269/ajtmh.2010.09-0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294–299. [PubMed] [Google Scholar]
- 42. Smith JL, Fonseca DM (2004) Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae). American Journal of Tropical Medicine and Hygiene 70: 339–345. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Duplicated Cq values from Cx. pipiens mosquitoes intrathoracically (Brookwood line) inoculated with Schmallenberg virus and then processed immediately using sqPCR (Table A in S1 File). Duplicated Cq values from Cx. pipiens mosquitoes (Brookwood line) intrathoracically inoculated with Schmallenberg virus and then processed following a 14 day incubation period using sqPCR (Table B in S1 File). Duplicated Cq values from Cx. pipiens mosquitoes (Brookwood line) intrathoracically inoculated with Schmallenberg virus and then processed following a 14 day incubation period. Saliva from each mosquito was collected using a glass capillary tube and insecticidal treatment and the body was then dissected into head and abdomen/thorax before processing using sqPCR (Table C in S1 File). Cq values from Cx. pipiens mosquitoes (Brookwood line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed immediately using sqPCR (Table D in S1 File). Cq values from Cx. pipiens mosquitoes (Brookwood line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed after a 14 day incubation period using sqPCR. A total of 92 samples returned no Cq value or a value >40 (Table E in S1 File). Cq values from Cx. pipiens mosquitoes (Caldbeck line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed immediately using sqPCR (Table F in S1 File). Cq values from Cx. pipiens mosquitoes (Caldbeck line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed after a 14 day incubation period using sqPCR. A total of 71 samples returned no Cq value or a value >40 (Table G in S1 File). Cq values from Cx. pipiens mosquitoes (Caldbeck line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed following a 14 day incubation period. Saliva from each mosquito was collected using a glass capillary tube and insecticidal treatment and the body was then dissected into head and abdomen/thorax before processing using sqPCR (Table H in S1 File). Cq values from Cx. pipiens mosquitoes (Wageningen line) fed through a membrane on a Schmallenberg virus/blood suspension and then processed after a 14 day incubation period using sqPCR. A total of 113 samples returned no Cq value or a value >40 (Table I in S1 File).
(DOCX)
Data Availability Statement
All relevant data are available via the BOLD database (dx.doi.org/10.5883/DS-PIRCX).