Abstract
Background
Drug dependence and adverse childhood experiences (ACE) are commonly reflected by dysregulation of the hypothalamic-pituitary-adrenal axis (HPA). Accumulating research indicates that the neuropeptide oxytocin may regulate HPA function, resulting in reductions in neuroendocrine reactivity to social stress among individuals with drug dependence. However, emerging literature suggests that individual differences may differentially impact intranasal oxytocin’s effects on human social behaviors.
Methods
This study employed a double-blind, placebo-controlled design to examine the extent to which ACE influenced the effects of intranasal oxytocin (40 IU) on neuroendocrine reactivity to a laboratory social stress paradigm in a sample of 31 cocaine-dependent individuals.
Results
ACE scores modified the relationship between intranasal oxytocin and cortisol reactivity. While ACE modified the relationship between intranasal oxytocin and DHEA response in a similar direction to what was seen in cortisol, it did not reach statistical significance.
Conclusions
Findings are congruent with the emerging hypothesis that intranasal oxytocin may differentially attenuate social stress reactivity among individuals with specific vulnerabilities. Future research examining the nuances of intranasal oxytocin’s therapeutic potential is necessary.
Keywords: oxytocin, drug dependence, social stress reactivity, HPA
1. Introduction
Abundant research has demonstrated an association between substance dependence and dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Clarke et al., 2008; Koob, 2008; Sinha, 2008). The effects of adverse childhood experiences (ACE; e.g. physical, psychological, or environmental threats to stable and functional development) on drug dependence (Benjet et al., 2013; Enoch, 2011; Mersky et al., 2013) and HPA axis function are also salient (Carpenter et al., 2011; Heim et al., 2002; MacMillan et al., 2009). Indeed, recent research illustrates the combined negative effects of ACE and substance dependence on stress reactivity in the HPA axis (Doan et al., in press; Gerra et al., 2014; Schäfer et al., 2010).
Epidemiological studies have found that ACE increases the risk for the development of substance use disorders (SUD)(Anda et al., 2002; Dube et al., 2003). Previous studies indicate that childhood maltreatment is associated with earlier initiation of illicit drug use (Nomura et al., 2012) and that child abuse and maltreatment is more prevalent among cocaine-dependent individuals compared to the general population (Medrano et al., 2002). Studies of cocaine-dependent individuals have found a significant association between exposure to childhood maltreatment and psychological distress in response to aversive psychosocial stimuli. For example, one study found that the severity of childhood maltreatment was positively associated with greater perceived threat and harm-avoidance coping strategies in cocaine-dependent individuals (Hyman et al., 2007). These data are particularly alarming as stress is a significant acute and long-term risk factor for drug craving and relapse (Back et al., 2010; McKay et al., 1995).
Perturbations in the HPA axis as a result of ACE play an important role in the development and maintenance of SUDs. Glucocorticoid receptors are ubiquitous in corticolimbic brain regions that regulate emotion (Aronsson et al., 1988). A growing literature suggests that chronic stress produces a persistent elevation in glucocorticoid levels and subsequently accelerates neuronal loss, delays myelination, and attenuates neuronal growth in corticolimbic brain regions which may be manifested as maladaptive coping strategies such as drug use (De Bellis, 2002; De Bellis et al., 2002; Dunlop et al., 1997; Gould et al., 1997; Sapolsky et al., 1990). Of note, one study of treatment seeking cocaine-dependent individuals found that changes in HPA hormones to a laboratory stress task were associated with higher amounts of cocaine use during relapse episodes (Sinha et al., 2006). Preclinical studies have found that postnatal stressors increase the reinforcing properties of drugs of abuse, an effect that appears to be related to HPA hormones (Daskalakis and Yehuda, 2015). Thus, the HPA axis may be an important mechanistic link between childhood maltreatment and substance use disorders.
The neuropeptide oxytocin appears to mitigate the effects of stress in individuals with SUDs. For example, compared with placebo, intranasal oxytocin produced significantly lower symptoms of alcohol withdrawal and anxiety in alcohol-dependent subjects (Pedersen et al., 2013). Others have found that intranasal oxytocin produces significantly less craving and anxiety to a social stress task than placebo in marijuana-dependent subjects (Carson et al., 2013; McRae-Clark et al., 2013; Pedersen et al., 2013). Intranasal oxytocin is also known to enhance prosocial and affiliative behaviors in rodents (Ferguson et al., 2001; Witt et al., 1992) and non-human primates (Chang and Platt, 2013). Indeed, some researchers attribute intranasal oxytocin’s therapeutic potential to its capacity to enhance reward pathways in response to non drug-related stimuli (e.g. interpersonal relationships, mitigating stress responses) which are commonly eroded by drug abuse over time (see MacGregor and Bowen, 2012, for review).
However, recent literature suggests that individual and contextual differences may impact the effects of intranasal oxytocin on social stress reactivity (Bartels, 2012; Bertsch et al., 2012; Campbell and Hausmann, 2013; DeWall et al., 2014). For example, in a study of non-human primates, oxytocin increased social contact in subordinate males but not in dominant males (Winslow and Insel, 1991). Collectively, these findings have illuminated critical nuances regarding intranasal oxytocin’s therapeutic potential (Bartz et al., 2011; Olff et al., 2013). As a result, one new hypothesis regarding intranasal oxytocin’s effects on human behavior suggests that intranasal oxytocin attenuates stress responses more effectively for those with vulnerabilities such as poor coping and emotion regulation skills compared to those equipped with more adaptive stress responses (Bartz et al., 2010; Cardoso et al., 2012; Quirin et al., 2011). Interestingly, alterations in the oxytocin system have been found in adults with a history of childhood maltreatment. For example, women with a history of child abuse exhibit lower levels of oxytocin as compared to women without abuse histories (Heim et al., 2009). Men with a history of maternal neglect exhibit lower oxytocin regulation of HPA axis hormones than men without a history of maternal neglect (Meinlschmidt and Heim, 2007). Thus, in light of the association between ACE and substance use disorders (Douglas et al., 2010; Kessler et al., 1997), it is essential to investigate intranasal oxytocin’s potential therapeutic benefits while also taking into consideration the influence of ACE on intranasal oxytocin’s effects.
Translational research focusing on oxytocin is currently limited by the scarcity of studies aimed at identifying populations for whom intranasal oxytocin may be beneficial. One critical gap in this literature is that studies examining the effects of intranasal oxytocin on social stress reactivity in the context of commonly co-occurring vulnerabilities, such as substance dependence and ACE, are scant. Addressing this question is essential to better understand potential therapeutic applications of intranasal oxytocin and pathways to treatment for this high risk population. This exploratory study addressed this gap in the literature by examining whether ACE severity influenced the effects of intranasal oxytocin on measures of HPA axis function (e.g. salivary cortisol and Dehydroepiandrosterone [DHEA]) to a social stress laboratory paradigm among cocaine-dependent individuals.
2. Material and Methods
2.1 Participants
Our sample was comprised of 31 cocaine-dependent individuals who responded to local media advertisements. Inclusion criteria included current cocaine dependence consistent with DSM-IV diagnostic criteria (Sheehan et al., 1998a). Exclusion criteria included (1) pregnancy, nursing, or ineffective means of birth control; (2) premenstrual dysphoric disorder; (3) history of or current significant hematological, endocrine, cardiovascular, pulmonary, renal, gastrointestinal, or neurological diseases; (4) history of or current psychotic, panic, eating, or bipolar affective disorders; (5) current major depressive disorder or PTSD; (6) history of or current medical conditions that might affect HPA axis activity; (7) synthetic glucocorticoid or exogenous steroid therapy within one month of testing; (8) psychotropic medications, opiates or opiate antagonists, benzodiazepines, antipsychotics, beta-blockers and other medications that might interfere with HPA axis activity; (9) acute illness or fever; (10) body mass index ≥ 35; (11) DSM-IV criteria for other substance dependence except caffeine, nicotine or marijuana within the past 60 days or (12) unwillingness or inability to maintain abstinence from alcohol and other drugs of abuse (except nicotine) for three days prior to the laboratory sessions.
2.2 Measures
Inclusion and exclusion criteria were determined by The Mini-International Neuropsychiatric Interview (MINI; Sheehan et al., 1998b) and the substance use module of the Structured Clinical Interview for DSM-IV (SCID-IV; First et al., 1994).
The Adverse Childhood Experiences (ACE) Survey (Felitti et al., 1998) is a 10-item self-report survey used to assess ten domains including childhood abuse (emotional, sexual and physical), neglect (emotional and physical) and household dysfunction (domestic violence, substance abuse, mental illness, criminal household member and parental divorce/separation). Responses to each item have 1 (yes) and 0 (no) options. Total scores were obtained by summing all items (Cronbach’s α=.65). While no specific cutoff scores exist for the ACE, higher are associated with increased risk for physical and mental health problems and functional impairment in adulthood (Anda et al., 2002; Chapman et al., 2004; Dube et al., 2002).
2.3 Procedures
All procedures received Institutional Review Board (IRB) approval. To minimize the impact of recent drug/alcohol use on stress reactivity and minimize potential for interaction between study medication and cocaine or alcohol, participants were asked to remain abstinent from cocaine and other drugs for a minimum of three days prior to the study procedures. Abstinence was assessed using self-reports, urine drug screens (Roche Diagnostics, Indianapolis, Indiana), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc., St. Louis, Missouri). Participants were assessed at the same time of day (beginning at 11:00 am) to control for diurnal variations in HPA function. Female participants performed a urine pregnancy test prior to study procedures. THC positive urine drug screens were allowed if the participant denied use in the past three days due to the extended period of detection of THC in urine. Smokers were provided with a nicotine patch.
At 11:30 a.m., baseline salivary cortisol and dehydroepiandrosterone (DHEA) data were collected. Subjects were served lunch and remained in the testing room where they remained until 1:15 p.m. subjects received study intranasal oxytocin or placebo. Study participants were randomly assigned to intranasal oxytocin (40 IU) or placebo treatment. All medication was dispensed in a double-blind manner. Subjects self-administered 40 IUs of oxytocin nasal spray or matching placebo at 1:15pm. This dose and timing of administration was selected based on previous studies that have used similar doses of intranasal oxytocin (Ditzen et al., 2009; McRae-Clark et al., 2013). Intranasal oxytocin and matching placebo were compounded by Pitt Street Pharmacy Custom Compounding (Mt. Pleasant, South Carolina).
2.4 Social Stress Paradigm Procedures
Participants performed the Trier Social Stress Task (TSST; Kirschbaum et al., 1993) and were told that at 2:00 (s)he would soon perform to an audience a speech and arithmetic task. The topic of the speech was why (s)he should be hired for a particular job (the individual’s “dream job”). The participant was asked to deliver the speech as though speaking to a group of hiring managers. The participant was told that the team is trained to observe your behavior. The experimenter then gave the participant 5 min to prepare the speech. At 2:05, three individuals unfamiliar to the participant (the audience) entered the room and were seated; the individual was instructed by one audience member (the spokesperson) to stand and begin his/her prepared speech (without notes) for five minutes. If the individual paused, (s)he was instructed by the spokesperson to continue. At the end of the speech task (2:10), the individual was instructed to serially subtract 13 from 1,022 as quickly and accurately as possible. The mental math recitation continued for 5 minutes. At the end, the spokesperson instructed the participant to stop and to be seated, and the audience left the procedure room. Data were collected from participants at 2:15 (immediately after the task) and at 5-, 30- and 60-minutes post-task.
2.5 Endocrine Assays
Enzyme-linked immunosorbent assays were used to measure salivary cortisol and DHEA levels (Salimetrics, LLC, State College, PA). The cortisol kit has a lower sensitivity limit of <0.003 µg/dL. The DHEA assay has a lower sensitivity limit of 5 pg/ml.
2.6 Data Processing and Statistical Analysis
Prior to the analysis, standard descriptive statistics were used to summarize the demographic and clinical characteristics of the study population. Demographic, clinical, and use characteristics were examined for univariate predictive relationships with the stated outcomes as well as possible confounding effects with treatment with outcomes. Residual normality was tested using a combination of graphical (Q-Q plots) and statistical tests (Shapiro-Wilk test). When model residuals were found to violate assumptions of normality or homoscedasticity, appropriate transformations were used. In order to gauge the effect of intranasal oxytocin or placebo on the outcomes response to the administration of oxytocin or placebo (prior to the stress task), linear mixed effects models containing the baseline and post intranasal oxytocin or placebo administration were assessed. To estimate the effect of administration of oxytocin or placebo on the response to the social stress task; linear mixed effects models that use all serially measured time points following administration of the study drug tested the neuroendocrine responses to intranasal oxytocin compared to placebo as well as the modification of the oxytocin effect by increased ACE scores (treatment × ACE interaction). Model based means and standard errors were used to test group level comparisons at each post medication time point. Included model factors were randomized oxytocin treatment assignment, ACE scores, time, pre-medication cortisol/DHEA levels, the treatment assignment × time interaction, and the treatment assignment × ACE interaction. The effects of the administration of intranasal oxytocin versus placebo are measured as the pre-TSST levels, adjusted for pre-medication levels, and are noted as mean group differences and associated standard errors (Δ±SEM). Additionally, a summary measure of area under each neuroendocrine response curve following medication administration was calculated using the trapezoidal rule and compared between groups adjusting for pre-medication levels (adjusted for baseline levels). Spearman correlations between baseline ACE scores and neuroendocrine responses immediately following TSST are noted as the relationship (rho) between the variables and the associated significance level. All statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC). Significance for all planned pairwise comparisons was set at a two-sided p value of 0.05 and no correction for multiple testing was applied to reported p values.
3.Results
3.1 Patient Demographics and Clinical Characteristic
A total of 42 individuals were assessed for eligibility; 35 met requirements and were enrolled and randomized into the study. Of the randomized participants, 32 had valid ACE scores; two participants had missing ACE scores and one participant was excluded due to questionable validity of neuroendocrine values. Our analysis sample consisted of 31 participants with valid ACE and neuroendocrine data. The mean age of the study participants was 42.6 years (SD, 9.2), 83.9% were males and 16.1% were Caucasian (Table 1). There were no differences for any baseline demographic or clinical characteristics. Both cortisol and DHEA mixed model errors were in violation of model assumptions (residual normality) and were transformed using the natural logarithm.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristics | Overall n=31 |
Treatment Assignment | P Value | |
|---|---|---|---|---|
| Placebo n=16 |
Oxytocin n=15 |
|||
| Demographics | ||||
| Age (yrs) | 42.6 ± 9.2 | 43.7 ± 7.6 | 41.4 ± 10.7 | 0.652 |
| Male % (n) | 83.9 (26) | 93.7 (15) | 73.3 (11) | 0.172 |
| Caucasian % (n) | 16.1 (5) | 18.8 (3) | 13.3 (2) | 0.999 |
| Smoke Cigs % (n) | 71.0 (22) | 68.8 (11) | 73.3 (11) | 0.999 |
| ACE Score | 1.68 ± 1.42 | 1.63 ± 1.15 | 1.73 ± 1.71 | 0.887 |
| Clinical Measures | ||||
| DHEA (ng/ml) | 2.33 ± 1.14 | 2.13 ± 0.70 | 2.57 ± 1.48 | 0.789 |
| Cortisol (ng/ml) | 0.20 ± 0.14 | 0.17 ± 0.08 | 0.23 ± 0.18 | 0.494 |
| Use Characteristics | ||||
| Currently Enrolled in Tx | 3.2 (1) | 6.3 (1) | 0.0 (0) | -- |
| Past Enrolled in Tx | 38.7 (12) | 37.5 (6) | 40.0 (6) | 0.886 |
| Age at First Use | 24.7 ± 6.8 | 24.7 ± 5.8 | 24.7 ± 8.0 | 0.638 |
| Age at Dependence | 29.2 ± 8.7 | 28.6 ± 7.5 | 29.7 ± 10.0 | 0.984 |
| Total Years of Use | 16.1 ± 8.1 | 16.1 ± 7.6 | 16.1 ± 8.9 | 0.969 |
| Using Days per Month | 16.2 ± 6.5 | 17.0 ± 7.4 | 15.3 ± 5.3 | 0.782 |
| Days abstinent | 10.6 ±12.0 | 11.8 ± 15.3 | 9.5 ± 8.0 | 0.592 |
| $ per Using Day | 75.6 ± 53.7 | 87.2 ± 68.1 | 63.2 ± 30.0 | 0.468 |
Note. Standard descriptive statistics were used to summarize the general demographic and clinical data. Cortisol and DHEA means and standard deviations are untransformed scores. Group differences in continuous characteristics were assessed using a Wilcoxon Rank-Sum test and differences in categorical characteristics were assessed using normal (Pearson’s) chi-square tests (Fisher’s Exact test used when appropriate).
3.2 Neuroendocrine Response to Intranasal Oxytocin and TSST
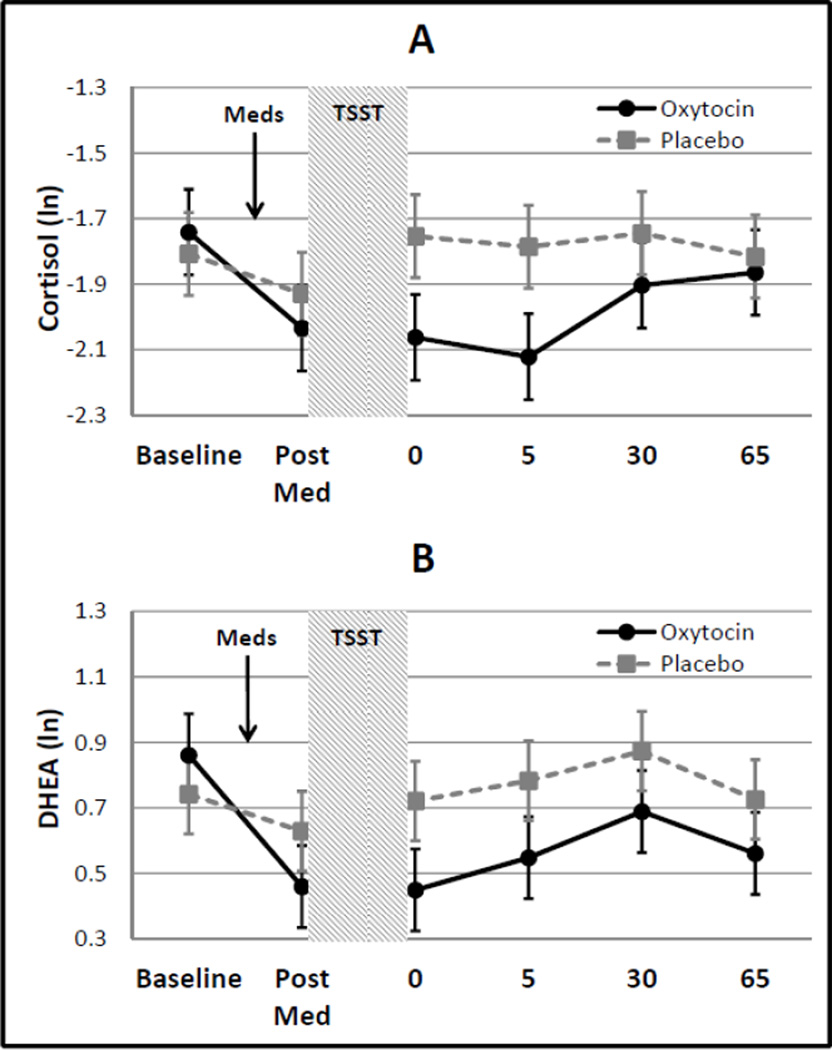
In response to the administration of the study drug (pre-TSST), there was a significant decrease in measured neuroendocrine levels in the study cohort (Cortisol: Δ=−0.21±0.09, t29=−2.3, p=0.032 and DHEA: Δ=−0.26±0.09, t29=−2.9, p=0.007). These changes in response to the medication/placebo were not statistically different from the null hypothesis of ‘no change’ in the placebo group (Cortisol: Δ=−0.12±0.13, t29=−1.0, p=0.348 and DHEA: Δ=−0.11±0.12, t29=−0.9, p=0.368), whereas the administration of intranasal oxytocin induced a significant decrease in both cortisol and DHEA levels (Cortisol: Δ=−0.29±0.13, t29=−2.2, p=0.035 and DHEA: Δ=−0.40±0.13, t29=−3.2, p=0.004). Although measured neuroendocrine level decreases were greater in the oxytocin group, the difference was not significantly reduced as compared to the placebo group (Cortisol: Δ=−0.17±0.18, t29=−0.9, p=0.361 and DHEA: Δ=−0.29±0.18, t29=−1.6, p=0.114).
Following the stressor (TSST), there was no significant increase in either cortisol or DHEA from the pre-TSST time point (Cortisol: Δ =−0.07±0.07, t115=−1.0, p=0.315 and DHEA: Δ=−0.04±0.06, t115=−0.7, p=0.515; Figure 1). Additionally, there was no modifying effect of the administration of intranasal oxytocin on cortisol or DHEA response levels immediately following the TSST (Cortisol: Δ =0.20±0.15, t115=1.4, p=0.166 and DHEA: Δ=0.10±0.13, t115=0.8, p=0.419).
Figure 1.
Cortisol and DHEA response to administration of oxytocin/placebo and the TSST. Data are shown as natural logarithm transformed means and associated standard errors.
3.3 Effect of ACE on Neuroendocrine Response to the TSST
Although there was not a significant effect of intranasal oxytocin on response to the TSST (Figure 1), it was hypothesized that the correlation between neuroendocrine levels and ACE could modify the effect of intranasal oxytocin. There was a significant modifying effect of ACE scores on the relationship between intranasal oxytocin administration and cortisol in response to the TSST (β = 0.30±0.12, t115=2.5, p=0.013). Results indicate that there is a significant positive association between baseline ACE scores and cortisol response levels in the untreated placebo group (β = 0.24±0.09, t115=2.5, p=0.014) that were attenuated in those that received intranasal oxytocin (β = 0.09±0.06, t115=1.6, p=0.121). Similarly, the analysis of the area under the cortisol response curve (following administration of medication/placebo) was significantly increased in placebo randomized participants (β = 2.7±1.2, t13=2.3, p=0.039) and not in those receiving intranasal oxytocin (β = −0.3±0.9, t12=−0.3, p=0.748). Although the modifying effect of ACE scores on the relationship between intranasal oxytocin and the DHEA response was similar in direction to what was seen in cortisol, it failed to reach statistical significance (β = 0.19±0.11, t115=1.8, p=0.072). The positive association between baseline ACE scores and DHEA response to the TSST in the placebo group was not as robust as was seen in the cortisol response (β = 0.14±0.09, t115=1.6, p=0.110). However, the group receiving intranasal oxytocin was attenuated in a similar manner (β = 0.04±0.05, t115=0.8, p=0.419). Unlike the cortisol response, the area under the DHEA response curve was not significantly associated with ACE scores in either the placebo (β = 33.9±22.1, t13=1.5, p=0.149) or oxytocin groups (β = −7.2±10.1, t12=−0.7, p=0.492). These relationships are shown in Figure 2 for the time point immediately following the TSST. Similar to the results from the longitudinal analysis, there was a strong correlation between baseline ACE scores and cortisol in the group that received placebo (ρ=0.60; p=0.015) that was attenuated in the group that received oxytocin (ρ=−0.18; p=0.515); the correlations between baseline ACE scores and DHEA measured following TSST were insignificant in both treatment groups. The remaining time points show similar relationships between ACE scores and cortisol/DHEA levels across treatment groups (Data not shown).
Figure 2.
Linear correlation between ACE scores and neuroendocrine levels by oxytocin treatment group. Data are shown at neuroendocrine marker taken immediately following TSST and correlations are rank order correlations with associated p-values. Cortisol: Intranasal oxytocin ρ=−0.18 (p=0.515), Placebo ρ=0.60 (p=0.015); DHEA: Intranasal oxytocin ρ=−0.10 (p=0.719), Placebo ρ=0.32 (p=0.233).
4.Discussion
This pilot study examined the extent to which the effects of a 40 IU dose of intranasal oxytocin on social stress reactivity were influenced by the severity of ACE. Results indicate that ACE scores and cortisol reactivity to the TSST were positively correlated in our sample. This association was attenuated among participants in the oxytocin condition. Additionally, the less robust correlation between ACE scores and DHEA reactivity to the TSST was also attenuated among participants in the oxytocin condition. These preliminary findings suggest that in our sample, ACE scores were correlated with cortisol reactivity among participants who received placebo, but not among those who received intranasal oxytocin.
We found no effect of intranasal oxytocin on cortisol levels in response to the TSST. A significant and positive association between the cortisol responses to the TSST and the number of adverse childhood experiences was found in cocaine-dependent subjects that received placebo. This association was blunted in cocaine-dependent subjects that received intranasal oxytocin. A previous study found no effect of intranasal oxytocin on cortisol levels in response to the TSST in a group of marijuana-dependent subjects (McRae-Clark et al., 2013). Childhood maltreatment was not assessed in the study of McRae-Clark and colleagues, and therefore it remains unclear if the present findings are consistent across other substances of abuse. Cortisol reactivity in response to stress tasks has been shown to predict relapse in cocaine-dependent subjects (Back et al., 2010; Sinha et al., 2006). Thus, the present findings suggest that intranasal oxytocin may be beneficial in reducing risk for relapse among cocaine-dependent individuals with a history of childhood maltreatment. Others have found that the ability to regulate emotions plays a similar moderating role in HPA responses to social stress (Allwood et al., 2011; Daubenmier et al., 2014)
There was no effect of the TSST on DHEA levels in the placebo group. In contrast to the cortisol findings there was no modifying effect of ACE scores on DHEA levels in response to the TSST. Both cortisol and DHEA are released from the adrenal glands in response to adrenocorticotropic hormone (ACTH) and the TSST increases DHEA levels in marijuana-dependent subjects and intranasal oxytocin appears to attenuate DHEA levels in these individuals (McRae-Clark et al., 2013). Thus, we had anticipated to find a significant increase in DHEA following the TSST and that the increase would be attenuated in the group that received intranasal oxytocin. However, data from a previous study demonstrates that there is significant individual variability in the DHEA response to social stress (Lennartsson et al., 2012). In addition, drug abstinence from six to 18 days can affect DHEA levels in cocaine-dependent subjects (Buydens-Branchey et al., 2002). Therefore it is possible that variability in duration of abstinence precluded us from finding a significant effect of the TSST on DHEA reactivity. While previous studies have demonstrated that intranasal oxytocin dosing and the endogenous oxytocin system are associated with both social behaviors and stress reactivity in the HPA axis, no previous studies have examined the effects of intranasal oxytocin’s effects on social stress reactivity in the presence of multiple vulnerabilities such as substance dependence and ACE. Substance dependence and ACE commonly co-occur (Mersky et al., 2013). Because the literature examining the potential for intranasal oxytocin to mitigate various substance use behaviors is growing rapidly (McGregor and Bowen, 2012), there remains a great need for research to identify factors such as ACE that may influence intranasal oxytocin’s clinical applications among substance-dependent populations. Although our study is exploratory, our findings contribute to the scarce literature identifying subpopulations of individuals, namely, those with co-occurring ACE and substance dependence, who may benefit from intranasal oxytocin. Our findings also support the emerging hypothesis that intranasal oxytocin may have more beneficial effects among individuals with existing, or multiple, vulnerabilities. Perhaps our findings, in combination with those from previous studies (Bartz et al., 2010; Cardoso et al., 2012), indicate that intranasal oxytocin is less likely to influence already adaptive stress responses, whereas individuals with more challenging interpersonal histories who are more prone to maladaptive social stress reactivity are more likely to reap intranasal oxytocin’s benefits.
Limitations
While this study utilized a rigorous double-blind, placebo-controlled design, the experiment was exploratory and conducted in a small sample of cocaine-dependent individuals. A previous study found that intranasal oxytocin attenuated the normal diurnal reduction in basal levels of cortisol in healthy controls with a history of childhood adversity (Meinlschmidt and Heim, 2007). The present study did not include groups of healthy controls or a “no-stress” condition so it is unclear if the present findings demonstrate altered sensitivity of the HPA axis to intranasal oxytocin in cocaine-dependent subjects with a history of childhood adversity. Future research should aim to replicate our findings in larger samples of substance-dependent men and women. Findings may have also been influenced by the distribution of ACE scores in our sample. Among the 31 participants retained in our sample, none in the placebo group reported ACE scores greater than three, and none reported ACE scores greater than five. Thus, the generalizability of our findings may be limited. Future studies would also benefit from employing an additional, more thorough measure of childhood trauma and/or posttraumatic stress symptoms to assess the extent to which ACE are associated with current subjective distress and symptomatology. The internal consistency of the ACE scale fell slightly below the traditional cutoff in this sample, which may indicate the need for more sophisticated measures of childhood adversity and trauma to be utilized in future studies. Chronic powder cocaine use may also influence rhinoanatomy and intranasal oxytocin absorption, which may influence the results presented here (Guastella et al., 2013). It is important for future studies to drug use and route of administration. Finally, our procedures required participants to be acutely abstinent from drug use to mitigate the potential for drug interaction and the potential confound of intoxication on study findings. However, we acknowledge that days abstinent can influence DHEA levels, which may have influenced the findings presented here.
4.1 Conclusions
Our findings contribute to the existing literature by demonstrating the modifying effects of ACE on the association between intranasal oxytocin and neuroendocrine stress reactivity in a sample of cocaine-dependent individuals. While preliminary, these findings suggest a need to examine further the nuances of intranasal oxytocin’s efficacy and therapeutic potential in substance-dependent populations.
Highlights.
We examined the influenced of adverse childhood experiences on the effects of oxytocin on social stress reactivity among cocaine-dependent individuals.
There was a significant modifying effect of ACE scores on the relationship between oxytocin and cortisol reactivity.
The modifying effect of ACE on the relationship between oxytocin and DHEA reactivity was similar in direction to what was seen in cortisol, however it failed to reach statistical significance.
Oxytocin may differentially attenuate social stress reactivity among individuals with specific vulnerabilities.
Acknowledgements
This manuscript is the result of work supported, in part, by resources from the National Institute on Child Health and Human Development and the Office of Research on Women’s Health (K12HD055885), the National Institute on Drug Abuse (P50DA016511), and the National Center for Advancing Translational Sciences (UL1TR000062).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Flanagan had primary responsibility for the research question, drafting and editing the manuscript. Dr. Moran-Santa Maria implemented the study, drafted the methods section, and edited the manuscript. Mr. Baker completed data analysis and drafted the data analysis and results sections. Drs. McRae-Clark and Brady are PIs on the grant that funded the study and contributed to conceptualization of the research question, modifying the analytic plan, and editing the manuscript.
References
- Allwood MA, Handwerger K, Kivlighan KT, Granger DA, Stroud LR. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological psychology. 2011;88:57–64. doi: 10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Whitfield CL, Felitti VJ, Chapman D, Edwards VJ, Dube SR, Williamson DF. Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatric services. 2002;53:1001–1009. doi: 10.1176/appi.ps.53.8.1001. [DOI] [PubMed] [Google Scholar]
- Aronsson M, Fuxe K, Dong Y, Agnati LF, Okret S, Gustafsson JA. Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc Natl Acad Sci U S A. 1988;85:9331–9335. doi: 10.1073/pnas.85.23.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A. Oxytocin and the social brain: beware the complexity. Neuropsychopharmacology. 2012;37:1795–1796. doi: 10.1038/npp.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, Ochsner KN. Oxytocin selectively improves empathic accuracy. Psychological Science. 2010;21:1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Benjet C, Borges G, Medina-Mora ME, Méndez E. Chronic childhood adversity and stages of substance use involvement in adolescents. Drug and Alcohol Dependence. 2013;131:85–91. doi: 10.1016/j.drugalcdep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Schmidinger I, Neumann ID, Herpertz SC. Reduced plasma oxytocin levels in female patients with borderline personality disorder. Hormones and behavior. 2012;63:424–429. doi: 10.1016/j.yhbeh.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hudson J, Dorota Majewska M. Perturbations of plasma cortisol and DHEA-S following discontinuation of cocaine use in cocaine addicts. Psychoneuroendocrinology. 2002;27:83–97. doi: 10.1016/s0306-4530(01)00037-3. [DOI] [PubMed] [Google Scholar]
- Campbell A, Hausmann M. Effects of oxytocin on women's aggression depend on state anxiety. Aggressive behavior. 2013;39:316–322. doi: 10.1002/ab.21478. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Linnen A, Joober R, Ellenbogen MA. Coping style moderates the effect of intranasal oxytocin on the mood response to interpersonal stress. Experimental and Clinical Psychopharmacology. 2012;20:84. doi: 10.1037/a0025763. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology. 2011;214:367–375. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Guastella AJ, Taylor ER, McGregor IS. A brief history of oxytocin and its role in modulating psychostimulant effects. Journal of Psychopharmacology. 2013;27:231–247. doi: 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- Chang SW, Platt ML. Oxytocin and social cognition in rhesus macaques: Implications for understanding and treating human psychopathology. Brain Res. 2013 doi: 10.1016/j.brainres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ. Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Clarke T, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. REVIEW: HPA-axis activity in alcoholism: examples for a gene–environment interaction. Addiction biology. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Yehuda R. Early Maternal Influences on Stress Circuitry: Implications for Resilience and Susceptibility to Physical and Mental Disorders. Frontiers in Endocrinology. 2015;5:244. doi: 10.3389/fendo.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Hayden D, Chang V, Epel E. It's not what you think, it's how you relate to it: Dispositional mindfulness moderates the relationship between psychological distress and the cortisol awakening response. Psychoneuroendocrinology. 2014;48:11–18. doi: 10.1016/j.psyneuen.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD. Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology. 2002;27:155–170. doi: 10.1016/s0306-4530(01)00042-7. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- DeWall CN, Gillath O, Pressman SD, Black LL, Bartz JA, Moskovitz J, Stetler DA. When the Love Hormone Leads to Violence Oxytocin Increases Intimate Partner Violence Inclinations Among High Trait Aggressive People. Social Psychological and Personality Science. 2014;5:691–697. [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Doan SN, Dich N, Evans GW. Childhood Cumulative Risk and Later Allostatic Load: Mediating Role of Substance Use. Health Psychology. doi: 10.1037/a0034790. in press. [DOI] [PubMed] [Google Scholar]
- Douglas KR, Chan G, Gelernter J, Arias AJ, Anton RF, Weiss RD, Kranzler HR. Adverse childhood events as risk factors for substance dependence: partial mediation by mood and anxiety disorders. Addictive behaviors. 2010;35:7–13. doi: 10.1016/j.addbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ. Adverse childhood experiences and personal alcohol abuse as an adult. Addictive Behaviors. 2002;27:713–725. doi: 10.1016/s0306-4603(01)00204-0. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Dunlop SA, Archer MA, Quinlivan JA, Beazley LD, Newnham JP. Repeated prenatal corticosteroids delay myelination in the ovine central nervous system. The Journal of maternal-fetal medicine. 1997;6:309–313. doi: 10.1002/(SICI)1520-6661(199711/12)6:6<309::AID-MFM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, Benjamin L. Biometrics Research Department. New York, NY: New York State; 1994. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II) (Version 2.0) [Google Scholar]
- Gerra G, Somaini L, Manfredini M, Raggi MA, Saracino MA, Amore M, Leonardi C, Cortese E, Donnini C. Dysregulated responses to emotions among abstinent heroin users: Correlation with childhood neglect and addiction severity. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;48:220–228. doi: 10.1016/j.pnpbp.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Cameron HA. Adrenal steroids suppress granule cell death in the developing dentate gyrus through an NMDA receptor-dependent mechanism. Brain research. Developmental brain research. 1997;103:91–93. doi: 10.1016/s0165-3806(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Hickie IB, McGuinness MM, Otis M, Woods EA, Disinger HM, Chan HK, Chen TF, Banati RB. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38:612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Wagner D, Wilcox MM, Miller AH, Nemeroff CB. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depression and Anxiety. 2002;15:117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Sinha R. Childhood maltreatment, perceived stress, and stress-related coping in recently abstinent cocaine dependent adults. Psychol Addict Behav. 2007;21:233–238. doi: 10.1037/0893-164X.21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological medicine. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson AK, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biological psychology. 2012;90:143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biological Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: Oxytocin as a potential treatment for drug addiction. Hormones and behavior. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Alterman AI, Cacciola JS, Kaplan MR. An examination of the cocaine relapse process. Drug Alcohol Depend. 1995;38:35–43. doi: 10.1016/0376-8716(95)01098-j. [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Maria MM, Brady KT. Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study. Psychopharmacology. 2013;228:623–631. doi: 10.1007/s00213-013-3062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano MA, Hatch JP, Zule WA, Desmond DP. Psychological distress in childhood trauma survivors who abuse drugs. Am J Drug Alcohol Abuse. 2002;28:1–13. doi: 10.1081/ada-120001278. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biol Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Mersky JP, Topitzes J, Reynolds AJ. Impacts of adverse childhood experiences on health, mental health, and substance use in early adulthood: A cohort study of an urban, minority sample in the US. Child Abuse & Neglect. 2013;37:917–925. doi: 10.1016/j.chiabu.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Hurd YL, Pilowsky DJ. Life-time risk for substance use among offspring of abusive family environment from the community. Subst Use Misuse. 2012;47:1281–1292. doi: 10.3109/10826084.2012.695420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Smedley KL, Leserman J, Jarskog LF, Rau SW, Kampov-Polevoi A, Casey RL, Fender T, Garbutt JC. Intranasal oxytocin blocks alcohol withdrawal in human subjects. Alcoholism, clinical and experimental research. 2013;37:484–489. doi: 10.1111/j.1530-0277.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirin M, Kuhl J, Düsing R. Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology. 2011;36:898–904. doi: 10.1016/j.psyneuen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer I, Teske L, Schulze-Th�sing J, Homann K, Reimer J, Haasen C, Hissbach J, Wiedemann K. Impact of childhood trauma on hypothalamus-pituitary-adrenal axis activity in alcohol-dependent patients. European Addiction Research. 2010;16:108–114. doi: 10.1159/000294362. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998a;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998b;59:22–33. [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Insel TR. Social status in pairs of male squirrel monkeys determines the behavioral response to central oxytocin administration. J Neurosci. 1991;11:2032–2038. doi: 10.1523/JNEUROSCI.11-07-02032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt DM, Winslow JT, Insel TR. Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol Biochem Behav. 1992;43:855–861. doi: 10.1016/0091-3057(92)90418-f. [DOI] [PubMed] [Google Scholar]