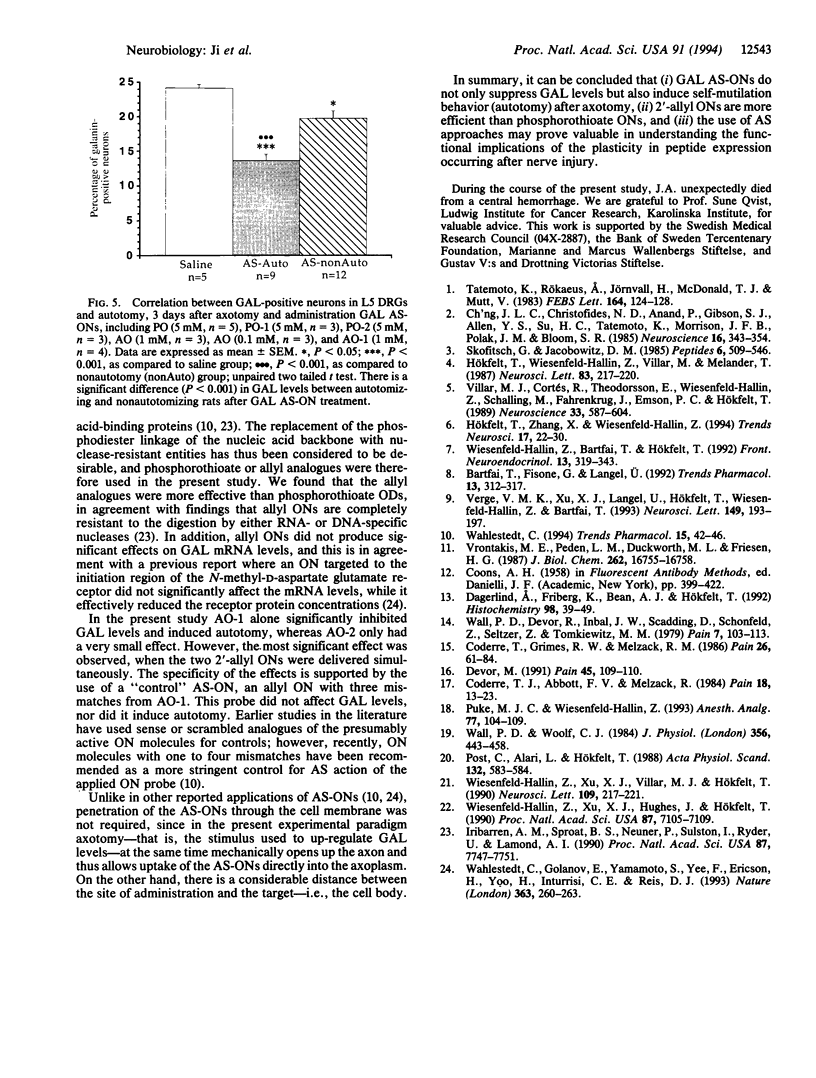
Abstract
Antisense (AS) oligonucleotides (ONs) to galanin (GAL) were applied to the proximal end of a transected sciatic nerve, allowing their cellular uptake and transport into injured axons. GAL expression in dorsal root ganglia and self-mutilation behavior (autotomy) were then studied. AS-ONs with phosphorothioate or allyl modifications significantly suppressed the axotomy-induced increase in GAL levels, as demonstrated by immunohistochemistry and exaggerated autotomy behavior, whereas no significant effect on GAL mRNA levels could be demonstrated with in situ hybridization. Allyl-ONs were more effective than phosphorothioate-ONs. An AS-ON with three base mismatches did not induce any of the above effects. These results support the view that the inhibition of axotomy-induced GAL up-regulation is related to autotomy.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartfai T., Fisone G., Langel U. Galanin and galanin antagonists: molecular and biochemical perspectives. Trends Pharmacol Sci. 1992 Aug;13(8):312–317. doi: 10.1016/0165-6147(92)90098-q. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Ch'ng J. L., Christofides N. D., Anand P., Gibson S. J., Allen Y. S., Su H. C., Tatemoto K., Morrison J. F., Polak J. M., Bloom S. R. Distribution of galanin immunoreactivity in the central nervous system and the responses of galanin-containing neuronal pathways to injury. Neuroscience. 1985 Oct;16(2):343–354. doi: 10.1016/0306-4522(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Abbott F. V., Melzack R. Effects of peripheral antisympathetic treatments in the tail-flick, formalin and autotomy tests. Pain. 1984 Jan;18(1):13–23. doi: 10.1016/0304-3959(84)90122-2. [DOI] [PubMed] [Google Scholar]
- Coderre T. J., Grimes R. W., Melzack R. Deafferentation and chronic pain in animals: an evaluation of evidence suggesting autotomy is related to pain. Pain. 1986 Jul;26(1):61–84. doi: 10.1016/0304-3959(86)90174-0. [DOI] [PubMed] [Google Scholar]
- Dagerlind A., Friberg K., Bean A. J., Hökfelt T. Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry. 1992 Aug;98(1):39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- Devor M. Sensory basis of autotomy in rats. Pain. 1991 May;45(2):109–110. doi: 10.1016/0304-3959(91)90174-V. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Wiesenfeld-Hallin Z., Villar M., Melander T. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci Lett. 1987 Dec 29;83(3):217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Zhang X., Wiesenfeld-Hallin Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 1994 Jan;17(1):22–30. doi: 10.1016/0166-2236(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Iribarren A. M., Sproat B. S., Neuner P., Sulston I., Ryder U., Lamond A. I. 2'-O-alkyl oligoribonucleotides as antisense probes. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7747–7751. doi: 10.1073/pnas.87.19.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post C., Alari L., Hökfelt T. Intrathecal galanin increases the latency in the tail-flick and hot-plate test in mouse. Acta Physiol Scand. 1988 Apr;132(4):583–584. doi: 10.1111/j.1748-1716.1988.tb08369.x. [DOI] [PubMed] [Google Scholar]
- Puke M. J., Wiesenfeld-Hallin Z. The differential effects of morphine and the alpha 2-adrenoceptor agonists clonidine and dexmedetomidine on the prevention and treatment of experimental neuropathic pain. Anesth Analg. 1993 Jul;77(1):104–109. doi: 10.1213/00000539-199307000-00021. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Immunohistochemical mapping of galanin-like neurons in the rat central nervous system. Peptides. 1985 May-Jun;6(3):509–546. doi: 10.1016/0196-9781(85)90118-4. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Rökaeus A., Jörnvall H., McDonald T. J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983 Nov 28;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- Verge V. M., Xu X. J., Langel U., Hökfelt T., Wiesenfeld-Hallin Z., Bartfai T. Evidence for endogenous inhibition of autotomy by galanin in the rat after sciatic nerve section: demonstrated by chronic intrathecal infusion of a high affinity galanin receptor antagonist. Neurosci Lett. 1993 Jan 12;149(2):193–197. doi: 10.1016/0304-3940(93)90769-h. [DOI] [PubMed] [Google Scholar]
- Villar M. J., Cortés R., Theodorsson E., Wiesenfeld-Hallin Z., Schalling M., Fahrenkrug J., Emson P. C., Hökfelt T. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience. 1989;33(3):587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- Vrontakis M. E., Peden L. M., Duckworth M. L., Friesen H. G. Isolation and characterization of a complementary DNA (galanin) clone from estrogen-induced pituitary tumor messenger RNA. J Biol Chem. 1987 Dec 15;262(35):16755–16758. [PubMed] [Google Scholar]
- Wahlestedt C. Antisense oligonucleotide strategies in neuropharmacology. Trends Pharmacol Sci. 1994 Feb;15(2):42–46. doi: 10.1016/0165-6147(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Wahlestedt C., Golanov E., Yamamoto S., Yee F., Ericson H., Yoo H., Inturrisi C. E., Reis D. J. Antisense oligodeoxynucleotides to NMDA-R1 receptor channel protect cortical neurons from excitotoxicity and reduce focal ischaemic infarctions. Nature. 1993 May 20;363(6426):260–263. doi: 10.1038/363260a0. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Devor M., Inbal R., Scadding J. W., Schonfeld D., Seltzer Z., Tomkiewicz M. M. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979 Oct;7(2):103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Woolf C. J. Muscle but not cutaneous C-afferent input produces prolonged increases in the excitability of the flexion reflex in the rat. J Physiol. 1984 Nov;356:443–458. doi: 10.1113/jphysiol.1984.sp015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z., Bartfai T., Hökfelt T. Galanin in sensory neurons in the spinal cord. Front Neuroendocrinol. 1992 Oct;13(4):319–343. [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z., Xu X. J., Hughes J., Horwell D. C., Hökfelt T. PD134308, a selective antagonist of cholecystokinin type B receptor, enhances the analgesic effect of morphine and synergistically interacts with intrathecal galanin to depress spinal nociceptive reflexes. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7105–7109. doi: 10.1073/pnas.87.18.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z., Xu X. J., Villar M. J., Hökfelt T. Intrathecal galanin potentiates the spinal analgesic effect of morphine: electrophysiological and behavioural studies. Neurosci Lett. 1990 Feb 5;109(1-2):217–221. doi: 10.1016/0304-3940(90)90566-r. [DOI] [PubMed] [Google Scholar]