Abstract
INTRODUCTION: Wnt/β-catenin signaling activation has been reported only during the late steps of Barrett’s esophagus (BE) neoplastic progression, but not in BE metaplasia, based on the absence of nuclear β-catenin. However, β-catenin transcriptional activity has been recorded in absence of robust nuclear accumulation. Thus, we aimed to investigate the Wnt/β-catenin signaling in nondysplastic BE. METHODS: Esophageal tissues from healthy and BE patients without dysplasia were analyzed for Wnt target gene expression by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and immunohistochemistry. Esophageal squamous (EPC1-& EPC2-hTERT), BE metaplastic (CP-A), and adenocarcinoma (OE33) cell lines were characterized for Wnt activation by qRT-PCR, Western blot, and luciferase assay. Wnt activity regulation was examined by using recombinant Wnt3a and Dickkopf-1 (Dkk1) as well as Dkk1 short interfering RNA. RESULTS: Wnt target genes (AXIN2, c-MYC, Cyclin D1, Dkk1) and Wnt3a were significantly upregulated in nondysplastic BE compared with squamous mucosa. Elevated levels of dephosphorylated β-catenin were detected in nondysplastic BE. Nuclear active β-catenin and TOPflash activity were increased in CP-A and OE33 cells compared with squamous cells. Wnt3a-mediated β-catenin signaling activation was abolished by Dkk1 in CP-A cells. TOPFlash activity was elevated following Dkk1 silencing in CP-A but not in OE33 cells. Dysplastic and esophageal adenocarcinoma tissues demonstrated further Dkk1 and AXIN2 overexpression. CONCLUSIONS: Despite the absence of robust nuclear accumulation, β-catenin is transcriptionally active in nondysplastic BE. Dkk1 overexpression regulates β-catenin signaling in BE metaplastic but not in adenocarcinoma cells, suggesting that early perturbation of Dkk1-mediated signaling suppression may contribute to BE malignant transformation.
Introduction
Barrett’s esophagus (BE) is the metaplastic replacement of the normal esophageal squamous epithelium with an intestinal-type columnar epithelium and is clinically important, as it predisposes to esophageal adenocarcinoma (EAC), a malignancy with rapidly increasing incidence and dismal 5-year survival rate [1,2]. The molecular mechanisms underlying the pathogenesis of BE formation remain elusive. The current paradigm of BE formation requires gastric acid and bile reflux to activate signaling pathways, transcriptional factors, and inflammatory processes, which, in turn, regulate proliferation and differentiation and may facilitate the replacement of reflux-damaged squamous cells through columnar metaplasia [3]. In particular, reflux-mediated activation of the CDX2 transcriptional factor, a master regulator of intestinal cell phenotype, is currently believed to drive the BE formation, yet CDX2 alone is not sufficient to induce metaplasia [3,4]. Thus, activation of alternative pathways involved in the development or maintenance of intestinal-type mucosa may predispose to Barrett’s metaplasia.
Wnt/β-catenin signaling pathway is required for early gut morphogenesis and maintenance of intestinal phenotype [5]. Activation of the canonical Wnt/β-catenin signaling is marked by nuclear translocation of dephosphorylated β-catenin in the presence of secreted Wnt glycoproteins or different mitotic signals. β-Catenin is involved in two independent processes: cell–cell adhesion and signal transduction [6]. In the absence of Wnt signal, β-catenin is sequestered in a "destruction complex," consisting of glycogen synthase kinase-3β, casein kinase 1, adenomatous polyposis coli (APC), and Axin [7]. This complex forces β-catenin’s phosphorylation, which then is subjected to subsequent degradation by the ubiquitin-proteasome system [7]. Upon Wnt signal, β-catenin is dephosphorylated and accumulates in the cytoplasm and subsequently to the nucleus, where it binds to members of TCF/LEF-1 transcription factors and initiates transcription of target genes (Cyclin D1, Axin2, c-MYC, Dkk1, etc.) [8].
Aberrant activation of the Wnt/β-catenin signaling has been reported during the late steps of BE neoplastic progression but not in BE metaplasia [9–11]. However, in BE dysplasia and EAC, the common mutations of the pathway’s components found in other cancers, such as β-catenin and APC, are not frequently detected [12]. Instead, the progression of BE dysplasia to EAC is marked by changes, such as loss of the negative regulators of the pathway, Wnt inhibitory factor 1 and secreted frizzled receptor proteins, as well as induction of Wnt-2 expression, which are expected to increase signaling along the Wnt axis [10,13,14]. In contrast to dysplasia and EAC, in nondysplastic BE, the Wnt/β-catenin signaling appears inactivated because of absence of robust nuclear β-catenin accumulation [9]. However, β-catenin transcriptional activity has been reported also in the absence of robust nuclear accumulation [15], whereas the notion that Wnt/β-catenin signaling may contribute to BE formation has been supported already by various observations. Increased levels of the β-catenin target genes, Cyclin D1 and c-myc, have been found in BE before dysplastic progression [16,17], whereas β-catenin signaling has been associated with transcription of several intestinal markers in BE, such as Sox9 and CDX1 [18,19]. Recently, activation of Wnt signaling has been reported efficient for intestinalization of human esophageal keratinocytes in vitro [20]. Therefore, whether Wnt/β-catenin signaling is activated during the early onset of Barrett’s metaplasia is an attractive hypothesis that has not been fully addressed yet.
Dickkopf-1 (Dkk1) is a secreted glycoprotein that blocks Wnt signaling by binding to the extracellular domain of the Wnt co-receptor LRP5/6, thus preventing the Wnt-induced stabilization of β-catenin [21]. Furthermore, Dkk1 has been reported to be a downstream target gene of β-catenin signaling and to exaggerate its inhibition in a negative feedback loop mechanism [22,23]. Intriguingly, unlike other Wnt suppressors, Dkk1 is found to be upregulated in BE dysplasia and EAC compared with BE metaplasia [24]. Previous studies have reported Dkk1 overexpression in human esophageal reflux esophagitis compared with healthy mucosa [25,26]. Taken together, these observations suggest a crucial role for Dkk1 in regulating esophageal biology.
In the present study, we investigated the levels of Wnt/β-catenin signaling activation in nondysplastic BE. By analyzing esophageal tissues and esophageal cell lines, we report a modest level of Wnt/β-catenin signaling activation in BE metaplasia beyond robust nuclear β-catenin accumulation. In addition, we demonstrate a high sensitivity of BE metaplastic cells to increase β-catenin signaling upon Wnt signals in vitro. We further attribute the moderate level of signaling activation in BE to the subsequent increased levels of the Wnt target gene, Dkk1, which regulates the signaling in a negative feedback loop. Finally, we demonstrate the inability of endogenous Dkk1 to regulate β-catenin signaling in adenocarcinoma cells, signifying that early perturbation of Dkk1-mediated signaling suppression may lead to esophageal tumorigenesis.
Materials and Methods
Human Esophageal Specimens
Human esophageal biopsy specimens were collected from patients 18 years or older undergoing endoscopy for BE and healthy individuals undergoing endoscopy for nonesophageal indications. Biopsies from patients with BE were obtained from the site of Barrett’s lesion and the proximal squamous mucosa. Following pathological assessment, samples from patients with Barrett’s dysplasia, EAC, or active esophagitis were excluded. Patients with previous history of eosinophilic esophagitis, Barrett’s associated dysplasia, EAC, or any other malignancy were also excluded. Full esophageal thickness specimens were obtained after surgical esophagectomy from patients with EAC. Studies were approved by the Human Research Review Committee of the Medical College of Wisconsin, and study participants gave written informed consent prior to their studies.
Cell Culture
Human squamous esophageal telomerase-immortalized cells EPC1-hTERT (EPC1) and EPC2-hTERT (EPC2) were the generous gifts of Dr. Hiroshi Nakagawa, Gastroenterology Division, University of Pennsylvania (Philadelphia, PA), and were grown as previously described [26]. A nondysplastic columnar cell line derived from BE (CP-A) was obtained from the American Type Culture Collection (Rockville, MD) and was adapted to MCDB-153 growth medium (Sigma) supplemented according to the manufacturer’s protocol. Poorly differentiated esophageal adenocarcinoma cancer cells (OE33) were obtained from Sigma Aldrich and were maintained in complete growth medium (RPMI-1640 supplemented with 2 mM l-glutamine, 10% bovine serum, and 1% gentamycin). All cell lines were maintained in monolayer culture at 37°C in humidified air with 5% CO2 in growth medium until they were 80% to 90% confluent.
Reagents and Antibodies
Recombinant human Wnt3a (rhWnt3a/200 ng/ml), recombinant human Dkk1 (rhDkk1/500 ng/ml), and neutralizing anti-Dkk1 monoclonal antibody (anti-Dkk1 Ab/10 μg/ml) were obtained from R&D Systems Inc. (Minneapolis, MN). Human IgG (10 μg/ml) was from Innovative Research (Novi, MI). Antibodies against Dkk1 and AXIN2 were obtained from Abcam (Cambridge, MA); active β-catenin (ABC) was from Millipore (Billerica, MA), total β-catenin, cytokeratin 4, cytokeratin 8/18, and GAPDH were obtained from Cell Signaling Technology (Danvers, MA); and horseradish peroxidase secondary antibodies were obtained from Santa Cruz. β-Actin antibody, protease inhibitor cocktail, and all other reagents/chemicals were obtained from Sigma (St. Louis, MO) unless otherwise specified.
RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (PCR) Analysis
RNA was isolated from biopsies and cell cultures using Arcturus PicoPure RNA (Life Technologies, Grand Island, NY) and Qiagen RNeasy Kit (Qiagen, Valencia, CA), respectively. One microgram of total RNA was used for cDNA synthesis using the Bio-Rad’s iScript cDNA synthesis Kit according to the manufacturer’s protocol (Bio-Rad, Hercules, CA). Real-time PCR was performed with Bio-Rad’s SsoFast EvaGreen Supermix, with 250-nM primer concentration, and 1 μl of cDNA per 20-μl reaction. Normalized gene expression was analyzed with Bio-Rad’s CFX software. The primers used for AXIN2, Cyclin D1, Dkk1, C-myc, Wnt3a, CDX2, BMP4, and SOX9 are listed in Table 1.
Table 1.
Sequence of Primers Used for RT-PCR Analysis
| Gene Name | Official Symbol | Gene ID | Sequence |
|---|---|---|---|
| β-actin | ACTB | NM_001101.3 | Forward: 5'-CAC TCT TCC AGC CTT CCT TC-3' |
| Reverse: 5'-GGT GTA ACG CAA CTA AGT CAT AG-3' | |||
| Cyclin D1 | CCND1 | NM_053056.2 | Forward: 5'-CAT CTA CAC CGA CAA CTC CAT C-3' |
| Reverse: 5'-TCT GGC ATT TTG GAG AGG AGG-3' | |||
| Axin2 | AXIN2 | NM_004655.3 | Forward: 5'-TAC CGG AGG ATG CTG AAG GC-3’ |
| Reverse: 5'-CCA CTG GCC GAT TCT TCC TT-3' | |||
| Dickkopf-1 | DKK1 | NM_012242.2 | Forward: 5'-AGC GTT GTT ACT GTG GAG AAG-3' |
| Reverse: 5'-GTG TGA AGC CTA GAA GAA TTA CTG-3' | |||
| c-MYC | MYC | NM_002467 | Forward: 5’-ACC AGA GAA ACC TAA CAG TGC-3’ |
| Reverse: 5’-CTC TTT CAT TTC GGC CAG TTC-3’ | |||
| Wnt3a | WNT3A | NM_033131 | Forward: 5'-CCA TCC TCT GCC TCA AAT TC-3’ |
| Reverse: 5’-TGG ACA GTG GAT ATA GCA GCA-3’ | |||
| CDX-2 | CDX2 | NM_001265 | Forward: 5’-GGA GCT GGA GAA GGA GTT TC-3’ |
| Reverse: 5’-TTT CCT CTC CTT TGC TCT GC-3’ | |||
| SOX-9 | SOX9 | NM_000346 | Forward: 5’-GAC CAG TAC CCG CAC TT-3’ |
| Reverse: 5’-TTC ACC GAC TTC CTC CG-3’ | |||
| BMP-4 | BMP4 | NM_001202 | Forward: 5’-TTG AGT ATC CTG AGC GCC CG-3’ |
| Reverse: 5’-GTT TAT ACG GTG GAA GCC CC-3’ |
Western Blot Analysis
Total cell extracts were prepared by scraping the cells into RIPA buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.25% sodium deoxycholate, 1% NP-40, 10 mM Na3VO4, 40 mM 5-glycerophosphate, 1 mM PMS, 20 mM NaF, protease inhibitor cocktail]. Equal amounts of protein were separated by SDS-PAGE and transferred to nitrocellulose membranes as described previously [26]. The membranes were blocked for 1 hour at room temperature in 3% (w/v) BSA and 3% (w/v) nonfat dry milk in Tris-buffered saline (50 mM Tris-HCl, 150 mM NaCl, pH 7.4) containing 0.1% (v/v) Tween 20, and then they were incubated with a specific primary antibody at 4°C overnight as specified. Detection of bound antibody was performed using a peroxidase-conjugated secondary goat anti-rabbit antibody or goat anti-mouse antibody and ECL chemiluminescence detection kit [Super Signal West Pico (Thermo Scientific)].
Immunohistochemistry and immunofluoresence staining was performed as described previously [26].
Luciferase Reporter Assay
Cells in 60% to 70% confluence were transfected for 48 hours with DNA plasmids of β-catenin-LEF/TCF-sensitive (TOP-flash) or β-catenin-LEF/TCF insensitive (FOP-flash) reporter vectors (Addgene, Cambridge, MA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The following amounts of plasmids were used per well of a 24-well plate: 1 μg and 0.5 μg of TOPflash as well as 1 μg and 0.5 μg of FOPflash for CP-A and OE33 cells, respectively. phRL-TK plasmid (Promega, Madison, WI) was co-transfected as control for transfection efficiency. Forty-eight hours posttransfection, experimentation followed as indicated. Reporter assay was performed using dual luciferase reporter system (Promega). Luciferase activity was measured via GLOMAX 20/20 Luminometer (Promega). Values for each reporter were normalized to phRL-TK values.
Dkk1 Gene Silencing
CP-A cells (60%-70% confluence) were transfected with predesigned short interfering RNA (siRNA), targeting human Dkk-1 [ON-TARGETplus SMARTpool, Human DKK1 (22943), Dharmacon, Lafayette, CO], and a control negative siRNA, targeting a sequence not sharing homology with the human genome (ON-TARGETplus Non-targeting siRNA, Dharmacon), using DharmaFECT transfection reagent as per manufacturer’s protocol (Dharmacon). The ratio of siRNA to the DharmaFECT reagent was 25 nM siRNA to 1.5 μl of transfection reagent.
Statistical Analysis
IBM SPSS-Statistics-19 statistical analysis software was used. All experiments were performed in triplicates, and data were expressed as mean ± SEM. Significant differences were evaluated by Student’s t tests or Mann-Whitney, as noted. P values less than .05 were considered statistically significant.
Results
Expression of Wnt-Signaling Target Genes in Barrett’s Metaplasia
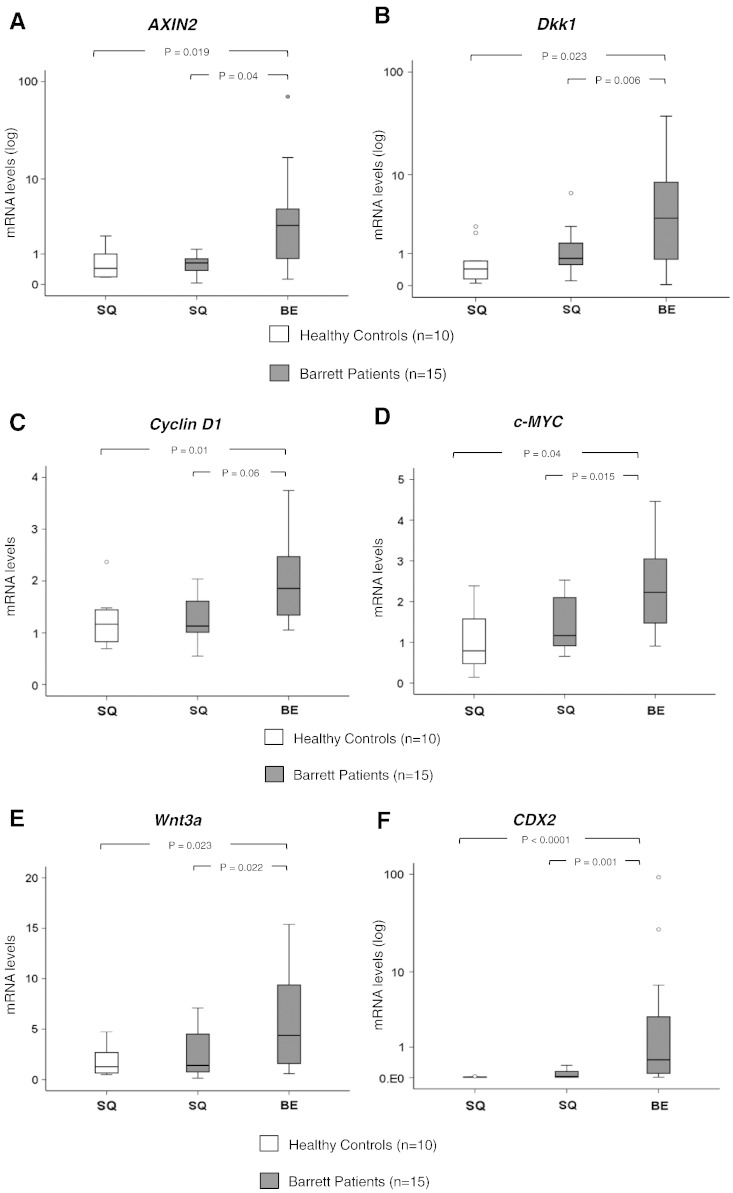
We quantitatively compared mRNA expression of the major Wnt-signaling target genes, AXIN2, Dkk1, Cyclin D1, and c-Myc, between squamous esophageal mucosa and nondysplastic Barrett’s mucosa in patients with BE and healthy controls. Real-time PCR analysis demonstrated that all Wnt target genes were significantly overexpressed in Barrett’s metaplastic mucosa compared with the corresponding paired squamous mucosa in Barrett’s patients and healthy controls (P < .05, Mann-Whitney test) (Figure 1, A–D). No significant differences were observed in the gene expression between squamous mucosa of healthy controls and squamous mucosa of Barrett’s patients. Gene expression of Wnt3a, the major canonical Wnt signal, was higher in Barrett’s metaplasia compared with the squamous epithelium (Figure 1E). Confirmatory of BE lesion, CDX-2 expression was significantly higher in Barrett’s metaplasia tissues as opposed to corresponding squamous tissues (Fig. 1F).
Figure 1.
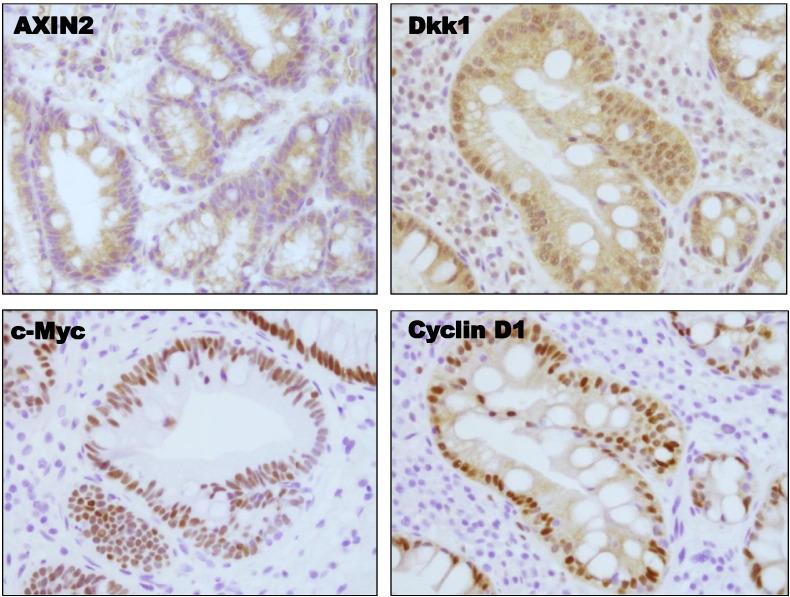
Wnt-signaling target genes expression in Barrett’s metaplasia: (A-D) Box plots of qRT-PCR data for mRNA expression of the Wnt target genes AXIN2, Dkk1, Cyclin D1, and c-MYC in squamous esophageal mucosa (SQ) and metaplastic mucosa (BE) of healthy individuals (n = 10) and Barrett’s patients (n = 15) (normalized with β-actin). Box plots of (E) Wnt3a and (F) CDX2 mRNA expression in the same samples as described above. (G) Immunohistochemistry for AXIN2, Dkk1, c-Myc, and Cyclin D1 protein expression in Barrett’s metaplasia as opposed to squamous mucosa (Supplementary Figure 1). (P values are provided, Mann-Whitney test).
Wnt-signaling target genes expression in Barrett’s metaplasia: (A-D) Box plots of qRT-PCR data for mRNA expression of the Wnt target genes AXIN2, Dkk1, Cyclin D1, and c-MYC in squamous esophageal mucosa (SQ) and metaplastic mucosa (BE) of healthy individuals (n = 10) and Barrett’s patients (n = 15) (normalized with β-actin). Box plots of (E) Wnt3a and (F) CDX2 mRNA expression in the same samples as described above. (G) Immunohistochemistry for AXIN2, Dkk1, c-Myc, and Cyclin D1 protein expression in Barrett’s metaplasia as opposed to squamous mucosa (Supplementary Figure 1). (P values are provided, Mann-Whitney test).
Immunohistochemistry further confirmed high levels of AXIN2, Dkk1, c-Myc, and Cyclin D1 protein expression in the metaplastic columnar epithelial cells of Barrett’s mucosa (Figure 1G). AXIN2 was expressed in cytoplasm, whereas Dkk1, c-Myc, and Cyclin D1 were detected in nucleus. Contrary to BE, expression of AXIN2 and Dkk1 proteins in squamous mucosa was limited to the basal mucosal layer, whereas gastric mucosa demonstrated a weak expression of AXIN2 (Supplementary Figure 1). In all, this positive expression of Wnt target genes suggests activation of Wnt/β-catenin signaling in Barrett metaplasia.
Expression of ABC in Barrett’s Metaplasia
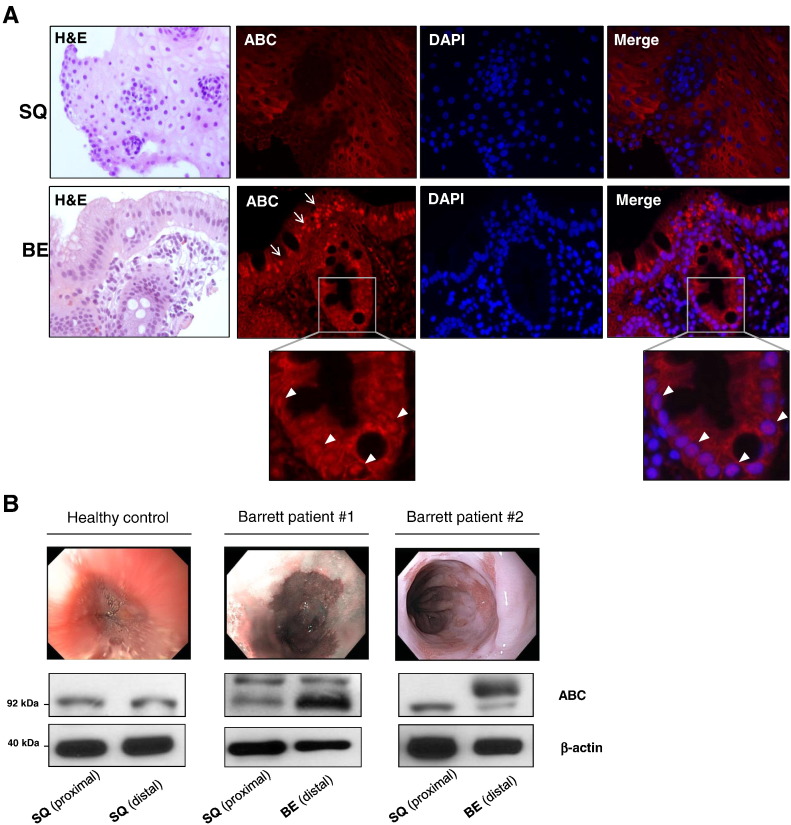
To further evaluate the activation of Wnt/β-catenin signaling in BE, we analyzed the expression levels and the intracellular localization of the transcriptionally ABC in BE metaplasia and compared with those of squamous esophageal epithelium. We emphasized on the amounts of the active form of β-catenin (ABC) rather than total amounts of β-catenin. ABC is the signaling isoform of β-catenin, which is dephosphorylated at Ser37 or Thr4, and is not susceptible to either ubiquitination or degradation [27,28]. Using a specific antibody against the ABC, immunofluoresence staining revealed elevated levels of nuclear (arrowheads) and perinuclear (arrows) ABC in nondysplastic BE tissue, as opposed to squamous epithelium, in which ABC was exclusively localized at cellular junctions (Figure 2A). Further quantification of ABC via Western blotting in biopsies from BE patients and healthy controls demonstrated markedly elevated ABC expression in Barrett’s metaplastic mucosa compared with the corresponding paired squamous mucosa (Figure 2B). Of note, Barrett’s tissues demonstrated also a mobility shift of the protein in SDS-PAGE at a slightly higher molecular weight of 92 kDa, possibly as a result of an extra phosphorylation. The increased amounts of ABC along with its nuclear localization cells are strong indicators of β-catenin signaling transcriptional activity in Barrett’s metaplasia.
Figure 2.
ABC expression in Barrett’s metaplasia: (A) Immunofluorescence microscopy for expression and localization of ABC protein in squamous esophageal mucosa (SQ) and nondysplastic Barrett’s metaplasia (BE). Arrowheads depict nuclear localization, whereas arrows show perinuclear cytoplasmic localization. Representative tissue staining of at least three different tissues is presented. (B) Western blot analysis of ABC protein expression in representative human esophageal biopsies. Significantly elevated amounts of ABC were demonstrated in Barrett’s metaplastic mucosa compared with the corresponding paired squamous mucosa.
Wnt/β-Catenin Signaling in Esophageal Cell Lines
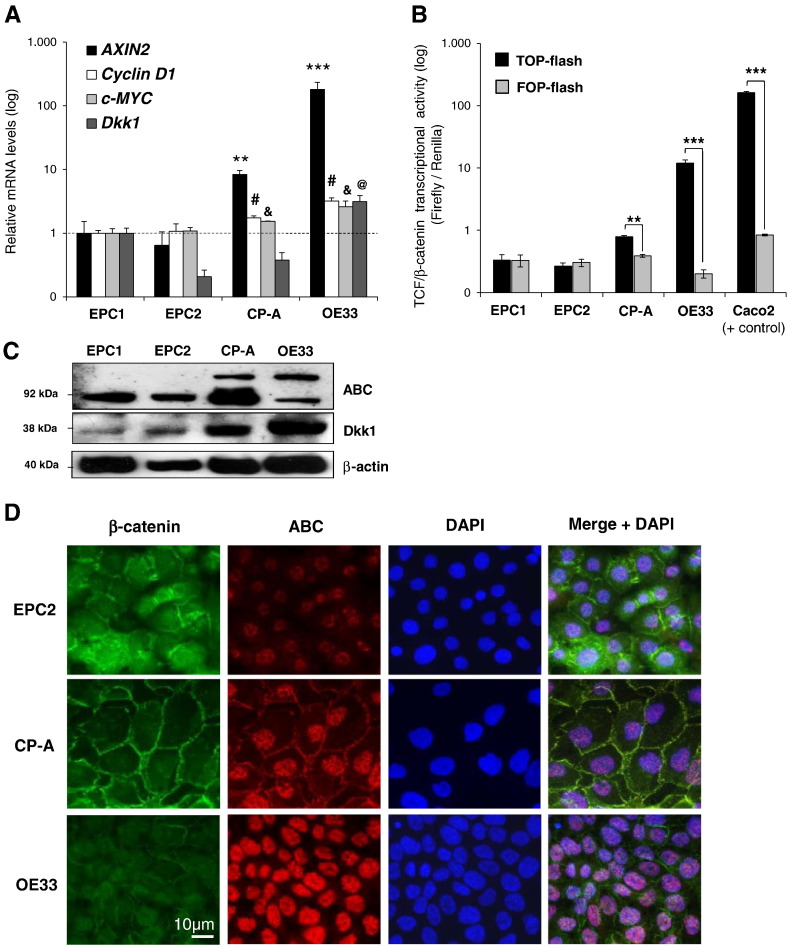
Next, we determined the level of Wnt/β-catenin signaling activation in a panel of resting esophageal cell lines representing squamous esophageal epithelium (EPC1/EPC2), BE metaplasia (CP-A), and esophageal adenocarcinoma (OE33). All cell lines were validated by expression of cytokeratins and typical markers of BE intestinal metaplasia (Supplementary Figure 2, A and B). Resting CP-A and OE33 cells demonstrated increased expression of Wnt target genes (Figure 3A) along with elevated levels of TOP-flash activity (Figure 3B), compared with EPC1 and EPC2 squamous epithelial cells, as shown by real-time PCR and luciferase assay, respectively. Notably, there was no appreciable β-catenin transcriptional activity detected in EPC1 and EPC2 cells. In contrast, the colorectal cancer cell line Caco2, which served as positive control, demonstrated high TOP-flash activity.
Figure 3.
Wnt/β-catenin signaling in esophageal cell lines: (A) qRT-PCR analysis of AXIN2, Dkk1, Cyclin D1, and c-MYC mRNA expression in two squamous esophageal cell lines (EPC1/EPC2), one BE metaplasia cell line (CP-A), and esophageal adenocarcinoma cell line (OE33) in resting conditions. (B) Luciferase assay in all cell lines in resting conditions. TOP-flash, and not FOP-flash, is responsive to co-activation of TCF/LEF by β-catenin. The colorectal cancer cell line Caco2 was used as positive control. Relative mean gene expression to that of EPC1 cells is presented. (C) Western blot analysis of ABC and Dkk1 protein expression in all cell lines at resting conditions. β-Actin serves as a loading control. (D) Immunofluorescence microscopy for colocalization of total β-catenin (Ab against C-terminus) and ABC protein in all cell lines at resting conditions. ABC was predominantly nuclear, whereas total β-catenin was localized in the membrane and adherent junctions. Representative tissue staining of at least three different tissues is presented [comparisons between cell lines for Cyclin D1#, c-MYC&, and Dkk1@; #, &, @P < .05; **P < .01;***P < .001; t test).
As shown by Western blotting in resting cells, the levels of ABC were significantly higher in CP-A and OE33 cell lines compared with squamous cell lines (Figure 3C). Of note, CP-A and OE33 cells also demonstrated a mobility shift of the protein in SDS-PAGE at a slightly higher molecular weight of 92 kDa. The intracellular localizations of the total β-catenin and ABC were further evaluated by immunofluorescence microscopy. In all cell lines, ABC was stained predominantly in nucleus, whereas polyclonal antibody against the C-terminus detected the total β-catenin in cellular membranes and adherent junctions (Figure 3D). However, in CP-A cells, the amount of ABC was markedly increased in the nucleus compared with squamous cell lines. In OE33 cells, the ABC was exclusively detected in the nucleus.
Taken together, these findings support an increased β-catenin transcriptional activity in resting BE metaplastic epithelial cells in vitro as opposed to squamous epithelial cells, whereas BE-associated adenocarcinoma cells demonstrate even higher level of signaling activation.
Wnt Signal Activates β-Catenin Signaling in CP-A Cells
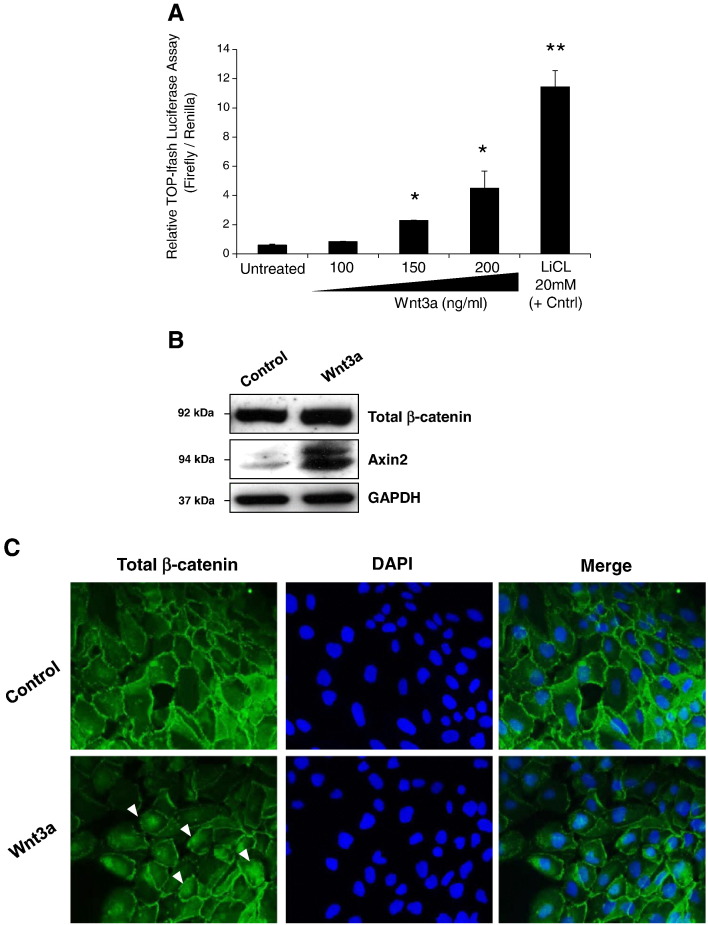
The overexpression of Wnt3a mRNA in BE metaplasia tissues as shown above prompted us to examine next whether BE metaplastic cells respond to canonical Wnt signals by activating β-catenin mediated transcription. Using different concentrations of rhWnt3a, we stimulated CP-A cells for 24 hours. As shown by luciferase assay, rhWnt3a significantly increased TOP-flash activity in a concentration-dependent manner (Figure 4A). Lithium chloride, the chemical activator of β-catenin signaling, could significantly increase TOP-flash activity in CP-A cells and served as positive control. Western blotting showed that continuous stimulation of CP-A cells with rhWnt3a 200 ng/ml for 24 hours resulted in stabilization of total β-catenin and increased AXIN2 protein expression (Figure 4B). Immunofluorescence microscopy further verified the nuclear translocation of β-catenin in CP-A cells after rhWnt3a stimulation (arrows; Figure 4C). These results underscore the sensitivity of BE metaplastic cells to activate β-catenin signaling upon Wnt3a signal.
Figure 4.
Dose-dependent activation of β-catenin transcriptional activity in CP-A cells via Wnt3a: (A) Luciferase assay in CP-A cells following treatment with different concentrations of rhWnt3a for 24 hours. Lithium chloride served as positive control (*P < .05; **P < .01, t test). (B) Western blot analysis of total β-catenin and AXIN2 protein expression in CP-A cells following treatment with 200 ng/ml of rhWnt3a for 24 hours. GAPDH served as a loading control. (C) Immunofluorescence staining for total β-catenin in CP-A cells following rhWnt3a treatment as described above verified nuclear localization of β-catenin in CP-A cells (arrows).
Rapid and Transient β-Catenin Activation by Wnt3a in CP-A Cells
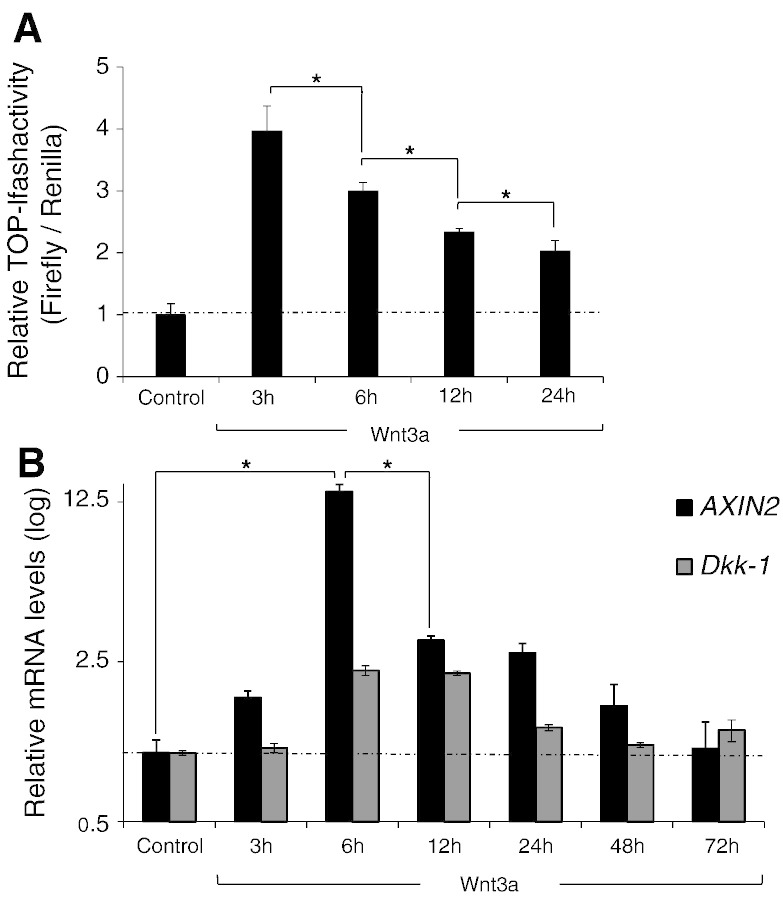
We questioned next whether Wnt3a activates β-catenin transcription in a time-dependent fashion. As shown by luciferase assay of CP-A cells, TOP-flash activation reached a peak 3 hours after the incubation with rhWnt3a (200 ng/ml) and then gradually decreased after 6, 12, and 24 hours (Figure 5A). This transient fashion of TOPflash activation in CP-A cells upon Wnt3a stimulation was further confirmed by real-time PCR for the Wnt target genes AXIN2 and Dkk1. As shown in Figure 5B, transcription of AXIN2 and Dkk1 mRNA reached a peak 6 hours after Wnt3a incubation and markedly decreased after 12, 24, and 48 hours. Notably, after 48 hours of continuous Wnt3a stimulation, the gene levels of both target genes returned back to the base levels. These findings support the notion that canonical Wnt3a signal could rapidly and in a transient fashion activate β-catenin signaling in BE metaplastic epithelial cells.
Figure 5.
Wnt3a activates β-catenin transcriptional activity time dependently in CP-A cells: (A) Luciferase assay and (B) qRT-PCR analysis of AXIN2 and Dkk1 mRNA expression in CP-A cells following treatment with 200 ng/ml of rhWnt3a for 3, 6, 12, and 24 hours (*P < .05; t test).
Dkk1 Suppresses Wnt3a-Induced β-Catenin Signaling in CP-A Cells
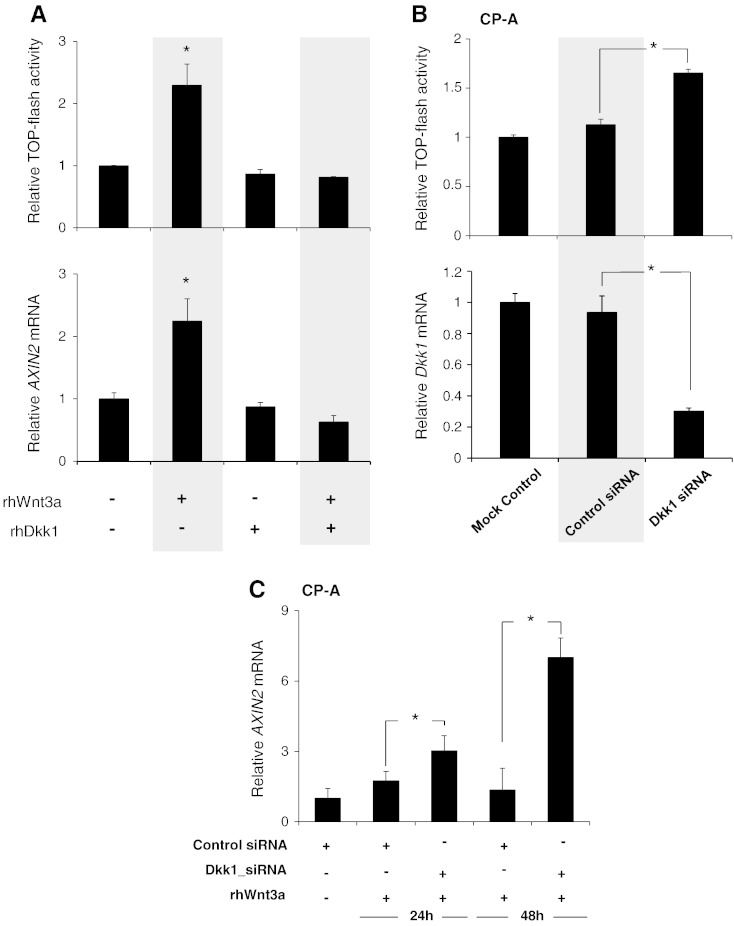
Given that Dkk1 counteracts the Wnt3a binding to LRP5/6 receptor [22], we questioned next whether the subsequent Dkk1 upregulation following Wnt3a-induced β-catenin signaling activation could explain its transient character. Thus, CP-A cells were incubated with rhWnt3a in the presence or absence of rhDkk1. As shown in Figure 6A, rhDkk1 abolished the ability of rhWnt3a to increase TOPflash activity and AXIN2 transcription, suggesting that excess amounts of extracellular Dkk1 suppress the Wnt-axis in BE.
Figure 6.
Dkk1 suppresses Wnt3a-mediated β-catenin transcriptional activation in CP-A cells: (A) Luciferase assay (top) and qRT-PCR analysis of AXIN2 gene expression (bottom) in CP-A cells following stimulation for 24 hours with rhWnt3a (200 ng/ml) and rhDkk-1 (500 ng/ml) for 24 hours as indicated. (B) Luciferase assay (top) and qRT-PCR analysis of Dkk1 gene expression (bottom) in CP-A cells following siRNA-mediated Dkk1 gene silencing. (C) qRT-PCR analysis of AXIN2 gene expression in CP-A cells following siRNA-mediated Dkk1 gene silencing and stimulation for 24 hours with rhWnt3a (200 ng/ml), as indicated. The histograms demonstrate mean AXIN2 gene expression (normalized to β-actin) and TOP flash relative to unstimulated cells of three independent experiments (*P < .05; t test).
Furthermore, following Dkk1 siRNA-mediated gene knockdown in resting CP-A cells (over 75% knockdown), a significant increase in TOPflash activity was observed (Figure 6B). This observation implies that even the endogenous baseline levels of Dkk1 suppress Wnt/β-catenin signaling activation in CP-A cells. To further address whether Dkk1 transcriptional activation as the result of Wnt3a-induced signaling could suppress further activation, we applied rhWnt3a to CP-A cells after sufficient siRNA-mediated Dkk1 gene silencing. As shown by real-time PCR, the AXIN2 mRNA levels were significantly increased following Wnt3a incubation for 24 and 48 hours in the CP-A cells, which underwent Dkk1 silencing compared with the wild-type cells (Figure 6C). These findings suggest that Dkk1 upregulation follows in response to β-catenin signaling activation to suppress signaling in an autocrine negative feedback loop. BE metaplastic cells, which are unable to respond to Wnt3a-induced signaling activation by upregulating Dkk1, may be subject to constitutive β-catenin transcriptional activation.
Dkk1 Overexpression in BE-Associated Adenocarcinoma
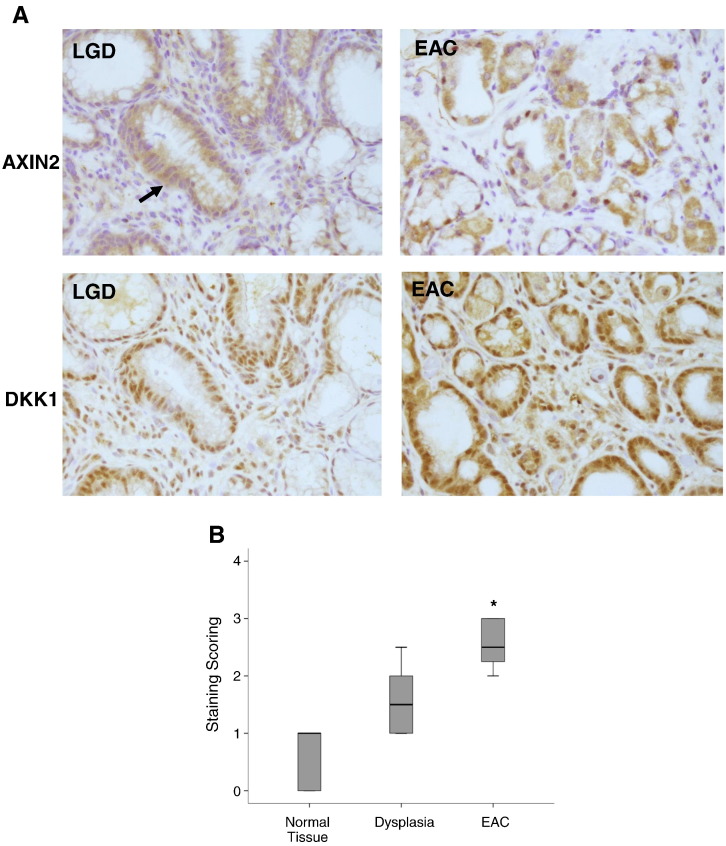
To address the significance of these observations in BE neoplastic progression, we investigated next the AXIN2 and Dkk1 expression in esophageal tissues with BE-associated dysplasia and carcinoma. Immunohistochemistry in dysplastic and carcinoma tissues, which were obtained from patients with EAC undergoing esophagectomy, demonstrated markedly elevated protein expression of both major Wnt targets AXIN2 and Dkk1 (Figure 7, A and B). These expression patterns support further Wnt signaling in those tissues and come in agreement with the already reported activation of Wnt/β-catenin signaling during the neoplastic progression of Barrett’s metaplasia [9].
Figure 7.
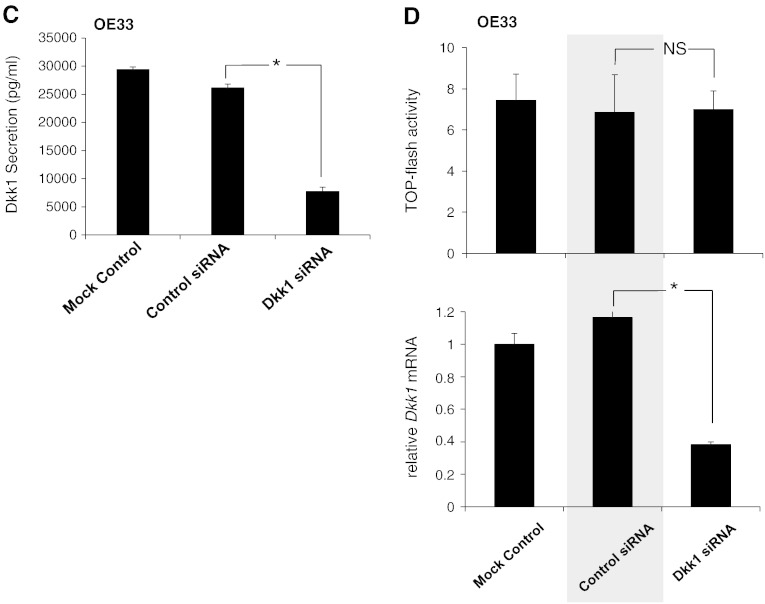
Dkk1 in BE-associated adenocarcinoma: (A) Representative immunohistochemistry for Axin2 and Dkk1 protein expression in esophageal tissues with BE-associated low-grade dysplasia (LGD) and EAC. The arrow indicates the area of the LGD, depicting large and irregular nuclei displaced from their normal position near the basement membrane. (B) Box plot analysis of immunohistochemical experiments for Dkk1. A simple grading system of 0 to 3 was used. Overall staining was evaluated in SQ (n = 8), LGD (n = 5), and EAC (n = 8). Median values are shown as thick lines (*P < .05; Mann-Whitney test). (C) ELISA of Dkk1 secretion in culture medium of OE33 cells following transfection with Dkk1-siRNA. Histograms depict the mean of three independent biological replicates (± SEM) (*P < .05 compared with control siRNA; t test). (D) Luciferase assay (top) and qRT-PCR analysis of Dkk1 gene expression (bottom) in OE33 cells following siRNA-mediated Dkk1 gene silencing. NS: nonsignificant (*P < .05; t test).
Given the inhibitory effects of Dkk1 on β-catenin signaling activity in BE metaplasia, we questioned whether Dkk1 exerts similar signaling suppression in BE-associated adenocarcinoma. Therefore, we analyzed next the levels of β-catenin signaling in OE33 cells following Dkk1 gene silencing (Figure 7C). As shown by luciferase assay and real-time PCR, the Dkk1 knockdown had no effect on the baseline TOPflash activity in OE33 (Figure 7D), suggesting the inability of Dkk1 to autoregulate the levels of β-catenin signaling in OE33 cells as it did in CP-A cells. Taken together, these findings suggest that the upregulation of Dkk1 in EAC is the result of the aberrant activation of the Wnt/β-catenin signaling, without, however, being able to regulate the signaling levels like in its precursor lesion of Barrett metaplasia.
Discussion
In this study, we report a moderate level of Wnt/β-catenin signaling activation, beyond robust nuclear β-catenin, in nondysplastic BE and provide evidence that Dkk1 overexpression suppresses Wnt axis in BE metaplastic cells but not in adenocarcinoma cells. By analyzing human esophageal tissues, we showed significant upregulation of the major Wnt target genes along with elevated levels of perinuclear and nuclear localization of ABC in nondysplastic BE as opposed to squamous esophageal mucosa. In vitro, Wnt/β-catenin signaling activation was confirmed in BE metaplastic (CP-A) and adenocarcinoma (OE33) cell lines by increased TOPflash activity, elevated levels of nuclear ABC, and overexpression of Wnt target genes compared with squamous epithelial cells. Recombinant Wnt3a activated β-catenin signaling in CP-A cells, an effect that was significantly attenuated by recombinant Dkk1. Reversely, Dkk1 knockdown increased TOPflash activity and AXIN2 gene expression in these cells, underlying the Dkk1-mediated suppression of Wnt axis. Furthermore, we have shown elevated AXIN2 and Dkk1 protein expression in BE-associated dysplastic and cancerous tissues, ratifying the aberrant Wnt activation during BE carcinogenesis [9–11]. However, contrary to CP-A cells, Dkk1 knockdown in OE33 cells did not alter TOPflash activity, underscoring the possible inability of Dkk1 to suppress Wnt axis in cancer cells. Together, these findings support the role of Wnt signaling in the development of BE metaplasia and define Dkk1 as a potential key suppressor toward neoplastic progression.
To date, the canonical Wnt/β-catenin signaling is believed to be inactivated in BE metaplasia based on the absence of robust nuclear β-catenin in patient biopsies [9]. However, our study clearly points to an elevated β-catenin activity in BE metaplasia, compared with normal squamous epithelium, as confirmed by Wnt target gene overexpression and increased levels of dephosphorylated ABC. This discrepancy can partly be explained by the latest developments in our understanding on how β-catenin functions. Undoubtedly, β-catenin nuclear accumulation is a clear hallmark of canonical Wnt signaling activation [29]. However, β-catenin accumulation alone and nuclear localization are not indicative of transcriptional activity [30]. It is now appreciated that β-catenin signaling can be observed in the absence of robust nuclear accumulation and that the signaling form of β-catenin can be a minor, nuclear-enriched population of the total cytosolic pool of β-catenin [15]. As such, β-catenin levels alone appear to be insufficient to explain possible Wnt signal, whereas the intracellular localization and the posttranslational modifications (phosphorylations) of β-catenin appear to be more important in dictating signaling function. Characteristically, β-catenin, which remains unphosphorylated at glycogen synthase kinase residues 33, 37, and 41, is intrinsically more active than β-catenin, which gets phosphorylated at these residues [28]. Thus, using a specific antibody detecting the dephosphorylated (Ser 37002FThr 41) ABC, we verified higher β-catenin activity in BE compared with squamous mucosa. Furthermore, the Wnt target gene overexpression, such as AXIN2, as reported here, is a more sensitive indicator of even low levels of signaling activation [31]. Our approach in detecting β-catenin activity as opposed to immunohistochemistry alone revealed a modest and not a high signaling activation in metaplasia. This modest activity goes along with previous speculations driven by the upregulation of β-catenin transcriptional regulators in BE despite the absence of robust nuclear β-catenin [11,32]. On the other hand, in dysplastic and cancerous tissues, elevated levels of nuclear β-catenin correlated with higher Wnt signaling activation [11], signifying that robust nuclear β-catenin may be addressed only in aberrant signaling activation.
The trigger of the β-catenin activation in BE remains unclear. Given the signaling inactivation in squamous epithelium of the adult esophagus [9,33] and the fact that BE develops in response to severe gastroesophageal reflux disease, the trigger should be sought in the reflux-associated mucosal injury. Indeed, refluxate components and inflammatory mediators, such as tumor necrosis factor–α, have been reported to activate Wnt signaling in vitro [32,34]. Another possible scenario is that the enhanced β-catenin activity in BE is dictated by Wnt-independent phosphorylation of β-catenin. In fact, β-catenin tyrosine (Y) phosphorylation by active receptor tyrosine kinases affects Wnt signaling [35–37], and enhanced AKT-mediated β-catenin activation has already been reported in BE-associated carcinogenesis [36]. In favor of this scenario, both BE biopsies and CP-A cells demonstrated a mobility shift of the ABC protein in SDS-PAGE at slightly higher molecular weight of 92 kDa, indicating that a portion of the dephosphorylated ABC can carry extra activating phosphorylation. Nevertheless, our current findings mainly support activation via Wnts. The Wnt3a gene overexpression in metaplastic BE along with the high sensitivity of CP-A cells to increase β-catenin transcriptional activity upon Wnt3a stimulation allows us to speculate that elevated amounts of canonical Wnts within the microenviroment of BE metaplasia dictate β-catenin activity. In line with this notion, differential expression of Wnt ligands has already been suggested in squamous epithelium [38], whereas the reflux-associated expression of the Wnt-antagonist Dkk1 may prevent canonical Wnt signaling as a response to constitutive increase of Wnt signals during reflux esophagitis [26]. These scenarios summarize a complex mechanism behind the β-catenin activity in BE, which still merits further elucidation.
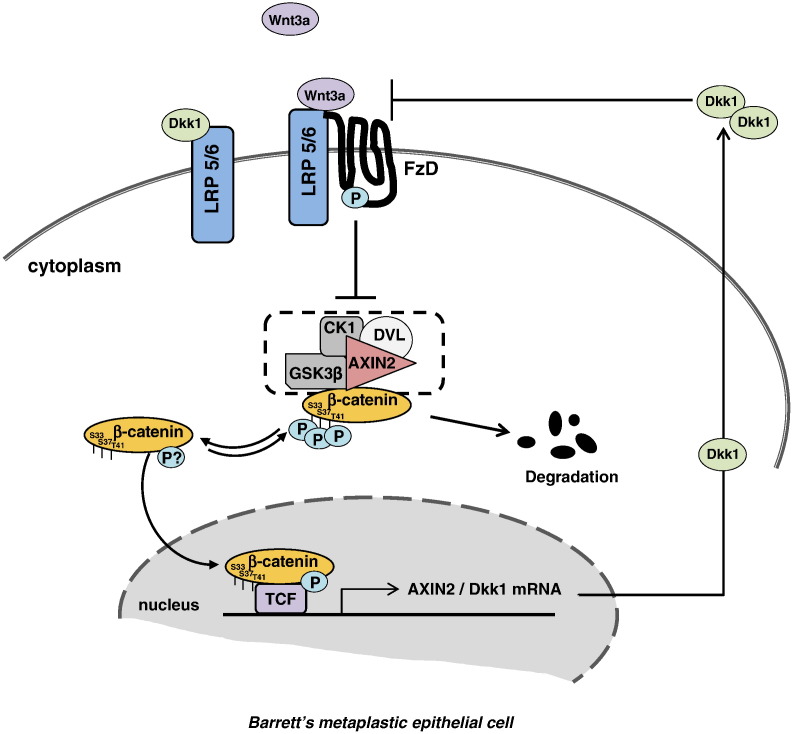
The absence of higher signaling activation in BE implies the existence of a compensative mechanism. We attributed this moderate β-catenin activity in BE to the subsequent overexpression of the Wnt target gene Dkk1. It is known that Dkk1 protein suppresses the Wnt signaling by antagonizing the binding of canonical Wnts to LRP5/6 receptors in a negative feedback loop [22]. The increased signaling activation of β-catenin by Wnt3a was blocked by Dkk1, whereas Dkk1 knockdown in CP-A cells resulted in increased β-catenin transcriptional activity along with a higher sensitization of the cells to Wnt3a. Taken together, these observations suggest a Dkk1-mediated suppression of Wnt-induced β-catenin activation in BE and allow us to propose a model of a closed circuit of Wnt signaling regulation in BE metaplasia, which aims to sustain the β-catenin activity to certain low levels. The secreted endogenous Wnt3a activates the canonical signaling, resulting downstream to Dkk1 gene increased transcription and subsequently to elevated Dkk1 protein secretion, which in turn counteracts an excessive Wnt3a receptor binding (Figure 8). This proposed model explains the positive AXIN2 and Dkk1 expression observed in the basal high proliferative layer of the normal squamous mucosa, suggesting that similar expression patterns, which indicate Wnt signaling activation, are associated with increased proliferation as seen in BE. Intriguingly, despite its overexpression, Dkk1 failed to show similar suppression of β-catenin activity in BE-associated adenocarcinoma cells, indicating that early perturbation of Dkk1-mediated signaling suppression might be responsible for esophageal tumorigenesis.
Figure 8.
Schematic illustration of the Dkk1-mediated suppression of the Wnt-axis in BE metaplastic cells.
The implications of our findings presuppose a dual approach, first with respect to BE development and second with respect the BE neoplastic progression. To date, investigation of canonical Wnt/β-catenin signaling in BE metaplasia has been discouraged because of the absence of robust nuclear β-catenin [9]. However, the notion that the signaling plays no role in BE development is remarkable given the role of Wnt signaling in inducing columnar differentiation during foregut morphogenesis [33] and its efficiency to induce esophageal intestinal metaplasia [20,39]. In the light of our current findings, Wnt/β-catenin signaling pathway appears moderately activated in BE metaplasia. One way in which Wnt signaling could contribute to the columnar differentiation is through activation of BMP signaling. BMP4 signaling was reduced in a mouse model following deletion of Wnt ligands [40], suggesting that Wnt signaling may be positioned upstream of BMP pathway in specifying the columnar differentiation. In line with this conclusion, the stimulation of CP-A cells with recombinant Wnt3a upregulated the BMP gene expression (unpublished data, Lyros). However, it needs to be examined whether β-catenin activity at the levels detected in BE is sufficient for the induction and/or maintenance of intestinal BE metaplasia.
The current theories on the origin of BE include a direct origin from the esophageal-stratified squamous epithelium or proximal migration of the gastric cardiac epithelium with subsequent intestinalization. Recent evidence supports that Barrett glands, although unique structures, share many similarities with gastric glands undergoing intestinalization, supporting an origin from stem cells in the cardia [41]. By showing a relatively lower AXIN2 protein expression in gastric mucosa than in BE, we can speculate that activation of Wnt signaling in BE might dictate the intestinal phenotype of the migrating stem cells of the cardia, rather than being a gastric tissue characteristic. However, a more comprehensive study on Wnt gene profiling between BE metaplasia and gastric mucosa is required to define whether Wnt signaling activation characterizes only BE metaplasia.
On the orther hand, Wnt signaling appears to be an important driver of BE malignant transformation. Previous reports implicate a progressive increase of Wnt signaling in the metaplasia–dysplasia–carcinoma sequence of BE [9,11,42]. In an effort to elucidate this activation, changes, such as increased expression of Wnt2 ligand or epigenetic loss of the Wnt inhibitory factor 1 and secreted frizzled receptor proteins negative regulators, have already been reported in EAC [10,13,14]. However, the trigger for the aberrant β-catenin signaling activation in BE toward dysplasia remains largely unknown. Interestingly, loss-of-function mutations in APC, β-catenin, or Axin, which have been associated with high β-catenin activity [43], are not frequently detected in BE or EAC [12]. Thus, the identification of Dkk1 as signaling suppressor in BE metaplasia, but not in EAC, may suggest a significant mechanism of esophageal tumorigenesis. In fact, not all patients with BE will finally develop EAC, indicating that even the metaplastic mucosa reserves host defense in order to reverse signal transduction and prevent carcinogenesis. Thus, Dkk1 could serve as such host defense against further Wnt activation in BE metaplasia and its loss of function to induce malignant transformation. Intriguingly, Dkk1 is found to be overexpressed in EAC [24], while, at the same time, β-catenin activity is also significantly elevated, indicating high possibility of a defect within Dkk1 negative feedback loop caused either by posttranscriptional Dkk1 loss of function or by aberrant signaling activation downstream of the Dkk1 site of action. Further investigations testing these scenarios in BE-associated dysplasia and EAC are currently ongoing.
An acknowledged limitation of this study is that, despite a moderate level of activation, the role of β-catenin signaling in BE initiation remains questionable. In view of our findings, Wnt signaling can play an important role in BE development as opposed to the current belief. Although speculative, several observations favor this hypothesis, including the overexpression of Cyclin D1 and c-MYC before dysplasia even occurs [16,17] and the transcriptional regulation of intestinal markers by β-catenin activity [18,19], along with the efficiency of Wnt signaling to intestinalize human esophageal keratinocytes in vitro [20]. Undoubtedly, our study compliments these observations and brings the issue of Wnt signaling back in the forefront of BE pathogenesis. A second limitation is the doubtful role of AXIN2 overexpression in regulating the Wnt-axis in BE and EAC. Given its suppressive features as a main component of the β-catenin destruction complex [44], AXIN2 could also suppress β-catenin activity downstream of Wnt-receptor binding. This scenario could highlight a multistage suppression of Wntaxis in BE metaplasia and as such further justify a moderate β-catenin activity, whereas, in EAC, it could indicate that the aberrant β-catenin signaling activation either originates downstream of the AXIN2 site of action or is even Wnt axis independent. These attractive scenarios are speculative and merit further investigation.
In conclusion, we define a moderate β-catenin signaling activation in BE metaplasia, compared with squamous esophageal mucosa, capsizing the current view in which β-catenin is transcriptionally inactive in BE metaplasia based on the absence of its robust nuclear accumulation. We attribute this moderate state of signaling activation to the subsequent overexpression of the Wnt target gene Dkk1, which counteracts Wnt signals in a negative feedback loop. Intriguingly, similar Dkk1-mediated signaling suppression was not evident in adenocarcinoma cells, signifying that early perturbation of Dkk1-mediated signaling suppression might be responsible for neoplastic progression. Although it remains unclear if this moderate β-catenin activity is responsible for BE development, our findings point to its significance with respect to BE malignant transformation and emphasize the need of further studies regarding Wnt signaling in esophageal tumorigenesis.
The following are the supplementary data related to this article.
(A) Immunohistochemistry of AXIN2 and Dkk1 protein expression in squamous esophageal mucosa and AXIN2 expression in gastric mucosa. (B) Immunofluorescence microscopy of isotype controls mouse IgG and rabbit IgG in squamous mucosa.
(A) Immunofluorescence microscopy for expression of cytokeratin 4 (squamous epithelium) and cytokeratin 8/18 (columnar epithelium) as well as (B) qRT-PCR analysis of SOX9, CDX2, and BMP4 mRNA expression in all cell lines in resting conditions.
Acknowledgements
This study was supported in part by National Institutes of Health grant R01DK025731, Laura Gralton on behalf of Daniel and Laura Gruber Charitable Lead Trust #3, and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number 8UL1TR000055 and Zablocki Veterans Affairs Medical Center research support.
The authors are thankful for the generous gift of fluorescence microscope from Levy Family 459 (Cerald, Ellin, Douglas, and Patti Levy).
Footnotes
Disclosures: Authors have nothing to disclose.
This study was supported in part by National Institutes of Health grant R01DK025731, Laura Gralton on behalf of Daniel and Laura Gruber Charitable Lead Trust #3, and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number 8UL1TR000055 and Zablocki Veterans Affairs Medical Center research support.
References
- 1.Spechler S.J., Goyal R.K. The columnar-lined esophagus, intestinal metaplasia, and Norman Barrett. Gastroenterology. 1996;110:614–621. doi: 10.1053/gast.1996.v110.agast960614. [DOI] [PubMed] [Google Scholar]
- 2.Yousef F., Cardwell C., Cantwell M.M., Galway K., Johnston B.T., Murray L. The incidence of esophageal cancer and high-grade dysplasia in Barrett's esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237–249. doi: 10.1093/aje/kwn121. [DOI] [PubMed] [Google Scholar]
- 3.Souza R.F., Krishnan K., Spechler S.J. Acid, bile, and CDX: the ABCs of making Barrett's metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211–G218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 4.Kong J., Crissey M.A., Funakoshi S., Kreindler J.L., Lynch J.P. Ectopic Cdx2 expression in murine esophagus models an intermediate stage in the emergence of Barrett's esophagus. PLoS One. 2011;6:e18280. doi: 10.1371/journal.pone.0018280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gregorieff A., Grosschedl R., Clevers H. Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(−/−)/Tcf1(−/−) embryos. EMBO J. 2004;23:1825–1833. doi: 10.1038/sj.emboj.7600191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson W.J., Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kishida S., Yamamoto H., Ikeda S., Kishida M., Sakamoto I., Koyama S., Kikuchi A. Axin, a negative regulator of the wnt signaling pathway, directly interacts with adenomatous polyposis coli and regulates the stabilization of beta-catenin. J Biol Chem. 1998;273:10823–10826. doi: 10.1074/jbc.273.18.10823. [DOI] [PubMed] [Google Scholar]
- 8.Aoki M., Hecht A., Kruse U., Kemler R., Vogt P.K. Nuclear endpoint of Wnt signaling: neoplastic transformation induced by transactivating lymphoid-enhancing factor 1. Proc Natl Acad Sci U S A. 1999;96:139–144. doi: 10.1073/pnas.96.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian Y.S., Osterheld M.C., Bosman F.T., Fontolliet C., Benhattar J. Nuclear accumulation of beta-catenin is a common and early event during neoplastic progression of Barrett esophagus. Am J Clin Pathol. 2000;114:583–590. doi: 10.1309/3QLC-5MF1-JYXU-A5XX. [DOI] [PubMed] [Google Scholar]
- 10.Clement G., Braunschweig R., Pasquier N., Bosman F.T., Benhattar J. Alterations of the Wnt signaling pathway during the neoplastic progression of Barrett's esophagus. Oncogene. 2006;25:3084–3092. doi: 10.1038/sj.onc.1209338. [DOI] [PubMed] [Google Scholar]
- 11.Moyes L.H., McEwan H., Radulescu S., Pawlikowski J., Lamm C.G., Nixon C., Sansom O.J., Going J.J., Fullarton G.M., Adams P.D. Activation of Wnt signalling promotes development of dysplasia in Barrett's oesophagus. J Pathol. 2012;228:99–112. doi: 10.1002/path.4058. [DOI] [PubMed] [Google Scholar]
- 12.Choi Y.W., Heath E.I., Heitmiller R., Forastiere A.A., Wu T.T. Mutations in beta-catenin and APC genes are uncommon in esophageal and esophagogastric junction adenocarcinomas. Mod Pathol. 2000;13:1055–1059. doi: 10.1038/modpathol.3880194. [DOI] [PubMed] [Google Scholar]
- 13.Clement G., Guilleret I., He B., Yagui-Beltran A., Lin Y.C., You L., Xu Z., Shi Y., Okamoto J., Benhattar J. Epigenetic alteration of the Wnt inhibitory factor-1 promoter occurs early in the carcinogenesis of Barrett's esophagus. Cancer Sci. 2008;99:46–53. doi: 10.1111/j.1349-7006.2007.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou H., Molina J.R., Harrington J.J., Osborn N.K., Klatt K.K., Romero Y., Burgart L.J., Ahlquist D.A. Aberrant methylation of secreted frizzled-related protein genes in esophageal adenocarcinoma and Barrett's esophagus. Int J Cancer. 2005;116:584–591. doi: 10.1002/ijc.21045. [DOI] [PubMed] [Google Scholar]
- 15.Maher M.T., Mo R., Flozak A.S., Peled O.N., Gottardi C.J. Beta-catenin phosphorylated at serine 45 is spatially uncoupled from beta-catenin phosphorylated in the GSK3 domain: implications for signaling. PLoS One. 2010;5:e10184. doi: 10.1371/journal.pone.0010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arber N., Lightdale C., Rotterdam H., Han K.H., Sgambato A., Yap E., Ahsan H., Finegold J., Stevens P.D., Green P.H. Increased expression of the cyclin D1 gene in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 1996;5:457–459. [PubMed] [Google Scholar]
- 17.Schmidt M.K., Meurer L., Volkweis B.S., Edelweiss M.I., Schirmer C.C., Kruel C.D., Gurski R.R. c-Myc overexpression is strongly associated with metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Dis Esophagus. 2007;20:212–216. doi: 10.1111/j.1442-2050.2007.00673.x. [DOI] [PubMed] [Google Scholar]
- 18.Blache P., van de Wetering M., Duluc I., Domon C., Berta P., Freund J.N., Clevers H., Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lickert H., Domon C., Huls G., Wehrle C., Duluc I., Clevers H., Meyer B.I., Freund J.N., Kemler R. Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development. 2000;127:3805–3813. doi: 10.1242/dev.127.17.3805. [DOI] [PubMed] [Google Scholar]
- 20.Kong J., Crissey M.A., Stairs D.B., Sepulveda A.R., Lynch J.P. Cox2 and beta-catenin/T-cell factor signaling intestinalize human esophageal keratinocytes when cultured under organotypic conditions. Neoplasia. 2011;13:792–805. doi: 10.1593/neo.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B.M., Delius H., Hoppe D., Stannek P., Walter C. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 22.Niida A., Hiroko T., Kasai M., Furukawa Y., Nakamura Y., Suzuki Y., Sugano S., Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen L., Jensen M.H., Krishna S. Dickkopf1—a new player in modelling the Wnt pathway. PLoS One. 2011;6:e25550. doi: 10.1371/journal.pone.0025550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darlavoix T., Seelentag W., Yan P., Bachmann A., Bosman F.T. Altered expression of CD44 and DKK1 in the progression of Barrett's esophagus to esophageal adenocarcinoma. Virchows Arch. 2009;454:629–637. doi: 10.1007/s00428-009-0769-z. [DOI] [PubMed] [Google Scholar]
- 25.Ali I., Rafiee P., Hogan W.J., Jacob H.J., Komorowski R.A., Haasler G.B., Shaker R. Dickkopf homologs in squamous mucosa of esophagitis patients are overexpressed compared with Barrett's patients and healthy controls. Am J Gastroenterol. 2006;101:1437–1448. doi: 10.1111/j.1572-0241.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 26.Lyros O., Rafiee P., Nie L., Medda R., Jovanovic N., Schmidt J., Mackinnon A., Venu N., Shaker R. Dickkopf-1, the Wnt antagonist, is induced by acidic pH and mediates epithelial cellular senescence in human reflux esophagitis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G557–G574. doi: 10.1152/ajpgi.00153.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cong F., Schweizer L., Chamorro M., Varmus H. Requirement for a nuclear function of beta-catenin in Wnt signaling. Mol Cell Biol. 2003;23:8462–8470. doi: 10.1128/MCB.23.23.8462-8470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staal F.J., Noort Mv M., Strous G.J., Clevers H.C. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Rep. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moon R.T., Kohn A.D., De Ferrari G.V., Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 30.Kiely B., O'Donovan R.T., McKenna S.L., O'Sullivan G.C. Beta-catenin transcriptional activity is inhibited downstream of nuclear localisation and is not influenced by IGF signalling in oesophageal cancer cells. Int J Cancer. 2007;121:1903–1909. doi: 10.1002/ijc.22794. [DOI] [PubMed] [Google Scholar]
- 31.Leung J.Y., Kolligs F.T., Wu R., Zhai Y., Kuick R., Hanash S., Cho K.R., Fearon E.R. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- 32.Chen X., Jiang K., Fan Z., Liu Z., Zhang P., Zheng L., Peng N., Tong J., Ji G. Aberrant expression of Wnt and Notch signal pathways in Barrett's esophagus. Clin Res Hepatol Gastroenterol. 2012;36:473–483. doi: 10.1016/j.clinre.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs I.J., Ku W.Y., Que J. Genetic and cellular mechanisms regulating anterior foregut and esophageal development. Dev Biol. 2012;369:54–64. doi: 10.1016/j.ydbio.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tselepis C., Perry I., Dawson C., Hardy R., Darnton S.J., McConkey C., Stuart R.C., Wright N., Harrison R., Jankowski J.A. Tumour necrosis factor-alpha in Barrett's oesophagus: a potential novel mechanism of action. Oncogene. 2002;21:6071–6081. doi: 10.1038/sj.onc.1205731. [DOI] [PubMed] [Google Scholar]
- 35.Brembeck F.H., Rosario M., Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Fang D., Hawke D., Zheng Y., Xia Y., Meisenhelder J., Nika H., Mills G.B., Kobayashi R., Hunter T., Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Veelen W., Le N.H., Helvensteijn W., Blonden L., Theeuwes M., Bakker E.R., Franken P.F., van Gurp L., Meijlink F., van der Valk M.A. beta-Catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut. 2011;60:1204–1212. doi: 10.1136/gut.2010.233460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali I., Rafiee P., Zheng Y., Johnson C., Banerjee B., Haasler G., Jacob H., Shaker R. Intramucosal distribution of WNT signaling components in human esophagus. J Clin Gastroenterol. 2009;43:327–337. doi: 10.1097/mcg.0b013e31816256ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong J., Nakagawa H., Isariyawongse B.K., Funakoshi S., Silberg D.G., Rustgi A.K., Lynch J.P. Induction of intestinalization in human esophageal keratinocytes is a multistep process. Carcinogenesis. 2009;30:122–130. doi: 10.1093/carcin/bgn227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goss A.M., Tian Y., Tsukiyama T., Cohen E.D., Zhou D., Lu M.M., Yamaguchi T.P., Morrisey E.E. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDonald S.A., Lavery D., Wright N.A., Jansen M. Barrett oesophagus: lessons on its origins from the lesion itself. Nat Rev Gastroenterol Hepatol. 2015;12:50–60. doi: 10.1038/nrgastro.2014.181. [DOI] [PubMed] [Google Scholar]
- 42.Osterheld M.C., Bian Y.S., Bosman F.T., Benhattar J., Fontolliet C. Beta-catenin expression and its association with prognostic factors in adenocarcinoma developed in Barrett esophagus. Am J Clin Pathol. 2002;117:451–456. doi: 10.1309/1db6-gfvh-ra6w-q07y. [DOI] [PubMed] [Google Scholar]
- 43.Polakis P., Hart M., Rubinfeld B. Defects in the regulation of beta-catenin in colorectal cancer. Adv Exp Med Biol. 1999;470:23–32. doi: 10.1007/978-1-4615-4149-3_3. [DOI] [PubMed] [Google Scholar]
- 44.Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P.M., Birchmeier W. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Immunohistochemistry of AXIN2 and Dkk1 protein expression in squamous esophageal mucosa and AXIN2 expression in gastric mucosa. (B) Immunofluorescence microscopy of isotype controls mouse IgG and rabbit IgG in squamous mucosa.
(A) Immunofluorescence microscopy for expression of cytokeratin 4 (squamous epithelium) and cytokeratin 8/18 (columnar epithelium) as well as (B) qRT-PCR analysis of SOX9, CDX2, and BMP4 mRNA expression in all cell lines in resting conditions.