Abstract
Encouragingly, global rates of new tuberculosis (TB) cases have been falling since 2005, in line with the Millennium Development Goal targets; however, cases of multidrug-resistant (MDR-) and extensively drug-resistant TB (XDR-TB) have been increasing. Fifteen of the world's 27 high MDR- and XDR-TB burden countries are in the World Health Organization (WHO) European Region, of which 10 are in Eastern Europe (including Baltic and Caucasus countries). To address the MDR- and XDR-TB situation in the WHO European Region, a Consolidated Action Plan to Prevent and Combat M/XDR-TB (2011–2015) was developed for all 53 Member States and implemented in 2011. Since the implementation of the Action Plan, the proportion of MDR-TB appears largely to have levelled off among bacteriologically confirmed TB cases in high-burden countries with universal or near universal (>95%) first-line drug susceptibility testing (DST). The treatment success rate, however, continues to decrease. A contributing factor is the substantial proportion of MDR-TB cases that are additionally resistant to either a fluoroquinolone, a second-line injectable agent or both (XDR-TB); high-burden country proportions range from 12.6% to 80.4%. Proportions of XDR-TB range from 5% to 24.8%. Despite much progress in Eastern Europe, critical challenges remain as regards access to appropriate treatment regimens; patient hospitalisation; scale-up of laboratory capacity, including the use of rapid diagnostics and second-line DST; vulnerable populations; human resources; and financing. Solutions to these challenges are aligned with the Post-2015 Global TB strategy. As a first step, the global strategy should be adapted at regional and country levels to serve as a framework for immediate actions as well as longer-term ways forward.
Keywords: MDR-TB, XDR-TB, epidemiology, review, European Region, WHO
More than 50 years after the first anti-tuberculosis chemotherapeutic drugs were introduced, tuberculosis (TB) remains a leading cause of death and life-threatening illness, disproportionately affecting low- and middle-income countries. In 2012, there were approximately 8.6 million new cases of TB worldwide, and 1.3 million people died from the disease.1 In addition, treatment success rates have been severely compromised in recent years due to the increasing prevalence of multidrug-resistant (MDR-) and extensively drug-resistant TB (XDR-TB).1,2 Although, encouragingly, global rates of new TB cases have been falling since 2005, in line with Millennium Development Goal (MDG) targets,3 MDR- and XDR-TB cases have been increasing, with an estimated 450 000 new cases in 2012.1 This review will focus on drug-resistant TB in the eastern European sub-region (including Baltic and Caucasus countries), which has one of the highest rates of MDR- and XDR-TB in the world; the challenges to MDR- and XDR-TB control; and potential ways forward.
COUNTRIES INCLUDED IN THIS REVIEW
The high MDR-TB burden countries in Eastern Europe (including Baltic and Caucasus countries) that have been included in this review and which are within the World Health Organization (WHO) European Region, are Armenia, Azerbaijan, Belarus, Estonia, Georgia, Latvia, Lithuania, the Republic of Moldova, the Russian Federation and Ukraine.1 We limited the scope of this review geographically to countries from this sub-region, as they were eligible to participate in the Structured Operational Research and Training Initiative (SORT IT) Eastern European Programme 2012–2014,4 through which the research presented in this supplement of Public Health Action was conducted.
BACKGROUND TO THE MDR- AND XDR-TB SITUATION IN THE WHO EUROPEAN REGION
MDR-TB is caused by Mycobacterium tuberculosis that is resistant to at least isoniazid (INH) and rifampicin (RMP), the two most potent anti-tuberculosis drugs. XDR-TB is defined as MDR-TB plus any fluoroquinolone and at least one injectable second-line drug (i.e., amikacin, kanamycin or capreomycin). Drug-resistant TB can occur due either to transmission of already resistant strains of M. tuberculosis5 or to suboptimal treatment of susceptible strains, which can develop resistance.6 The duration of treatment is longer for MDR-TB (up to 2 years) than for drug-susceptible TB (6–9 months), with a significantly higher risk of adverse drug reactions7,8 and unsuccessful treatment outcomes, particularly death.9–12 These risks are even higher for XDR-TB.13,14
Fifteen of the world's 27 countries with a high MDR- and XDR-TB burden are in the WHO European Region (Figure 1).15,16 With the dissolution of the Union of Soviet Socialist Republics in the early 1990s, TB and MDR-TB case rates began to increase in the newly independent states, largely due to the ensuing socio-economic crisis and deterioration of the health care system (Figure 2).17,18 Currently, all high-burden MDR-TB countries in the WHO European Region are in the east, and 99% of the region's MDR-TB cases occur in these countries.15,16
FIGURE 1.

Notification rates of MDR-TB cases/100 000 population, European Region, 2012 (reproduced with permission from Tuberculosis Surveillance and Monitoring in Europe 201411). MDR-TB = multidrug-resistant tuberculosis.
FIGURE 2.
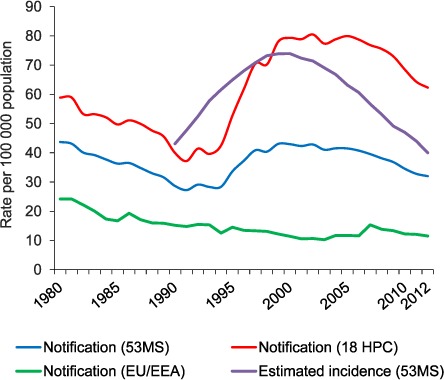
TB notification rate and estimated incidence in the WHO European Region, 1980–2012. MS = Member States (53 in the WHO European Region); HPC = high-priority countries (i.e., high MDR- and XDR-TB burden countries in the WHO European Region, plus Bulgaria, Romania, Turkey and Turkmenistan); EU = European Union; EEA = European Economic Area; MDR-TB = multidrug-resistant tuberculosis; XDR-TB = extensively drug-resistant TB.
In 2010, to address the MDR- and XDR-TB situation in the Region, a Consolidated Action Plan to Prevent and Combat M/XDR-TB (2011–2015) was developed for all 53 member states.16 The goal of the plan is to contain the spread of drug-resistant TB by achieving universal access to prevention, diagnosis and treatment of MDR- and XDR-TB in all member states in the Region by 2015.16 The plan, which has six strategic directions and seven areas of intervention, is aligned with the Global Plan to Stop TB 2011–2015,19 with the following specific targets to be met by the end of 2015: reduce by 20% the proportion of MDR-TB among retreatment patients, diagnose at least 85% of all estimated MDR-TB patients, and successfully treat at least 75% of all patients notified as having MDR-TB. The plan is also designed to address causal determinants of and barriers to TB control, including the use of operational research to inform policy guidance and models of care to reach the targets set forth. Endorsement and implementation of the plan began in 2011.
THE BURDEN OF MDR- AND XDR-TB IN THE WHO EUROPEAN REGION
Currently, while only approximately 4% of the global burden of TB is found in the WHO European Region, a total of 25% of the world's burden of MDR-TB is also found here, indicating the crucial importance of MDR-TB for this region.1 The current estimated annual incidence and proportion of MDR-TB among new and previously treated TB cases in the high-burden MDR-TB countries by subregion are shown in Table 1. MDR-TB rates among new and previously treated cases are significantly higher in these countries than in the top three high MDR-TB burden countries in other WHO regions. In 2012, the highest rates were in Belarus, where respectively 35% and 69% of new and previously treated cases were estimated to have MDR-TB.15 A drug resistance survey conducted in 2010–2011 in Belarus found that respectively 32.3% and 75.6% of new and previously treated patients had MDR-TB, of which 12% had XDR-TB.20
TABLE 1.
Estimated annual incidence of MDR-TB and estimated proportion of new and previously treated TB cases with MDR-TB among all notified TB cases in the 15 high MDR- and XDR-TB burden countries and high-priority countries in the WHO European Region, compared to the top three high MDR- and XDR-TB burden countries in other WHO Regions in 2012
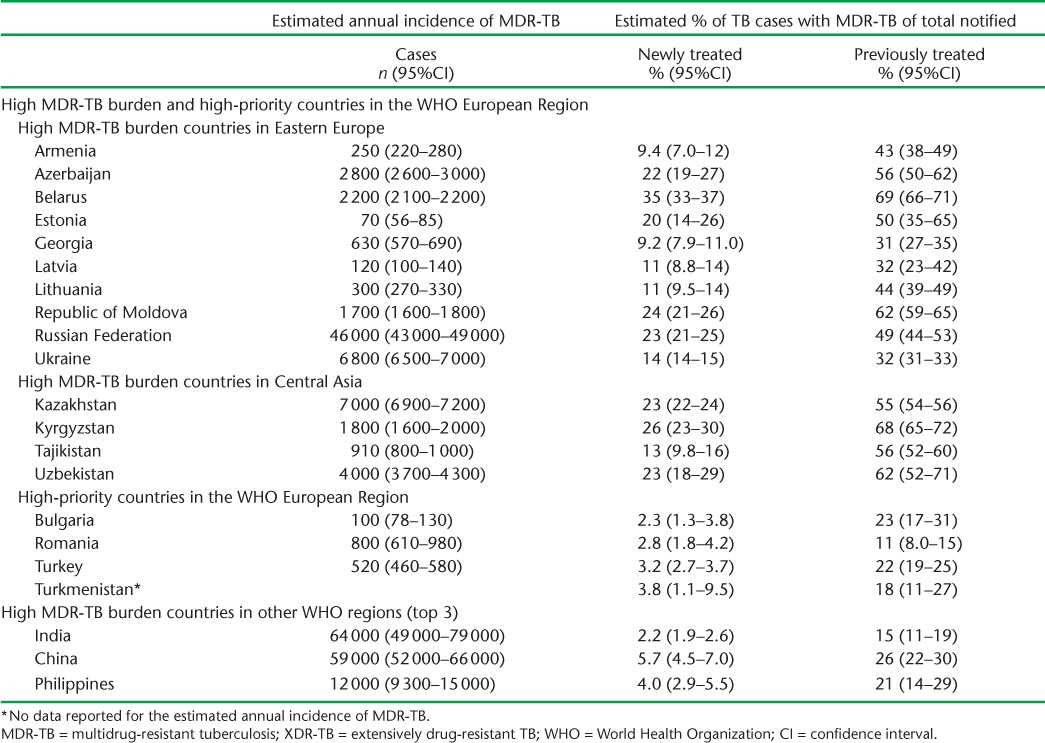
XDR-TB accounts for approximately 9% of drug-resistant cases, with the majority also occurring in the region's 15 high-burden countries.15 However, the added burden of pre-XDR-TB, defined as MDR-TB plus resistance to either a fluoroquinolone or a second-line injectable, is much higher. A recent study utilising regional data found that of those MDR-TB patients who underwent second-line drug susceptibility testing (DST), a total of 41.1% (95% confidence interval [CI] 32.3–50.0) had resistance to either a fluoroquinolone or a second-line injectable agent or both (i.e., either pre-XDR or XDR-TB).21 Concurrently, among new and previously treated cases, MDR-TB treatment success rates have decreased from respectively 72.5% and 50% in 2005 to 66.1% and 46.5% in 2012.15,22 In total, only 49% of people diagnosed with MDR-TB had a successful treatment outcome, well below the 75% target.15,16 Despite these trends in treatment success, there has been a steady decline in mortality rates from TB since 2002, also in line with the MDG targets. The estimated mortality for 2012 was 3.9 per 100 000 population (95%CI 3.8–4.0) in the region, corresponding to approximately 35 000 deaths.15
THE BURDEN OF MDR- AND XDR-TB IN EASTERN EUROPEAN COUNTRIES
Ten of the European Region's high MDR-TB burden countries are in Eastern Europe (Figure 1, Table 1). The TB incidence rate has been falling in most Eastern European countries, except for Azerbaijan and Ukraine.15 Utilising national data routinely reported to the WHO,15 trends in the proportion of MDR-TB among all notified TB cases (new and previously treated) with first-line DST plotted against treatment success rates for each of these countries are shown in Figure 3. It should be noted that year-to-year trend assessments may not be reliable in some cases, given overlapping CIs; however, observations from reported surveillance data are nonetheless described.
FIGURE 3.
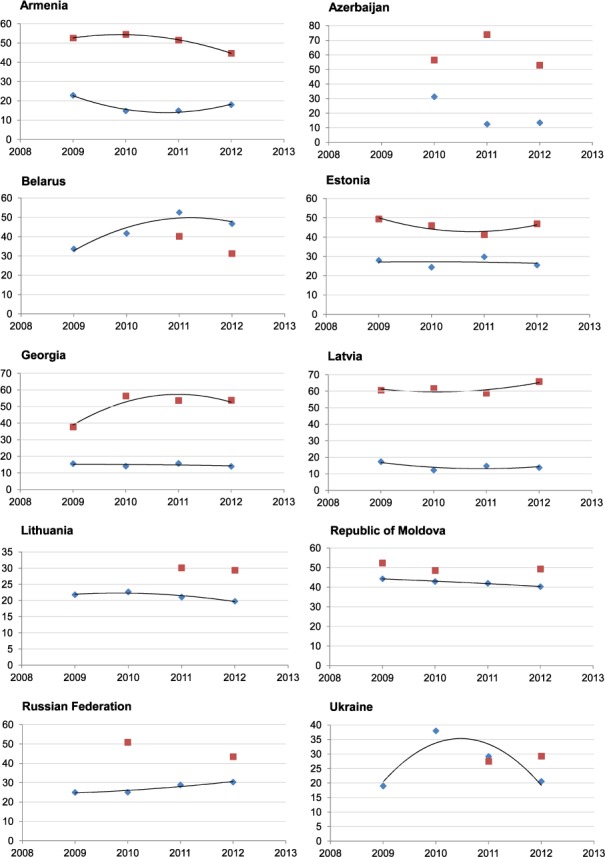
Proportion of MDR-TB among all notified TB cases with first-line DST and treatment success rates in countries in Eastern Europe, 2009–2012. Note: Trend-line not drawn where fewer than 4 data points are available. x-axis = year; y-axis = proportion; ♦ = proportion of MDR-TB among all notified TB cases with first-line DST; ▪ = proportion of all notified MDR-TB cases with a successful treatment outcome for that year. MDR-TB = multidrug-resistant tuberculosis; DST = drug susceptibility testing.
Of the countries that have universal or near universal (>95%) first-line DST coverage among bacteriologically confirmed TB cases (Armenia, Belarus, Estonia, Georgia, Latvia, Lithuania and the Russian Federation), the proportion of MDR-TB appears to have largely levelled off, with the exception of Belarus. The Russian Federation has also experienced a slight but steady increase in MDR-TB since 2009. However, given the large population size of the country, and therefore the number of TB cases (Table 1), even a slight increase in the proportion of MDR-TB represents a large absolute number of cases. Data from the country are, however, only available and reported on a subnational level from certain geographic areas; the currently known burden of MDR-TB may therefore not be representative of the entire country.21
In some countries, as the absolute number of TB cases continues to decrease, so too has the number of MDR-TB cases. Countries that have experienced a levelling-off or a reduction in the proportion of MDR-TB (Armenia, Estonia, Georgia, Latvia and Lithuania) may represent a success in the control of MDR-TB, given the decreasing TB incidence rate and universal DST coverage in these countries; however, data on early deaths that may occur before notification are not available. While the proportion of MDR-TB in the Republic of Moldova and Ukraine also appears to have decreased, first-line DST coverage in these countries is below 80%; the true rates of MDR-TB may therefore be greater than currently reported. Trends in Azerbaijan are also difficult to interpret, as DST coverage is below 80% in the country and no data were reported for 2009.
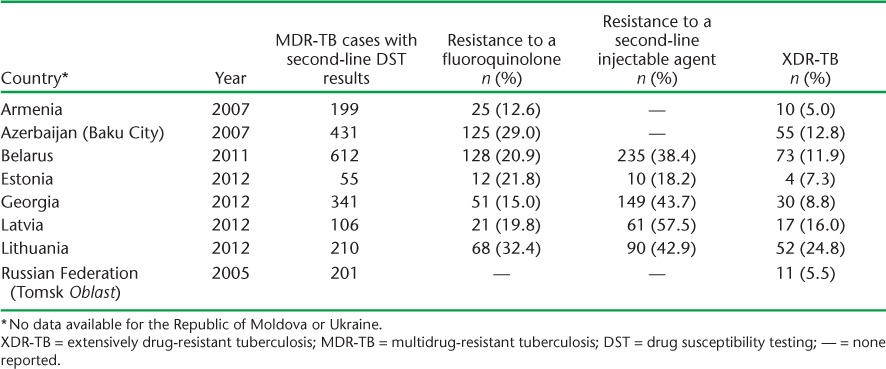
The decreasing treatment success in MDR-TB patients is a continuing problem. Georgia experienced an increase in treatment success rates from 2009 to 2010, which has since levelled off. The rate in Latvia has remained relatively stable, with an increase in 2012 over the previous year. Estonia also experienced an increase in 2012 over the previous year; however, this is after a downward trend since 2009. Critically, apart from a few subsettings, including penitentiary services in Azerbaijan, none of the 10 Eastern European countries have reached the WHO target of a 75% treatment success rate. Contributing to this problem are substantial levels of additional drug resistance and XDR-TB (Table 2).21,23,24
TABLE 2.
Proportions of pre-XDR-TB and XDR-TB among countries in Eastern Europe17
The countries with the highest mortality rates due to TB in the WHO European Region (and the eastern European subregion) are the Republic of Moldova (18 cases/100 000), the Russian Federation (13/100 000) and Ukraine (also 13/100 000).15 These rates are well above both the estimated mortality rate for the region and the mortality rate for the European Union/European Economic Area, which is below 1 case/100 000.
PROGRESS IN MDR-TB CONTROL IN EASTERN EUROPE
Much progress has been achieved in efforts to control TB in Eastern Europe since the implementation of the Action Plan in 2011. A complete review of national programmatic advances as a result of the Action Plan is beyond the scope of this review. However, several large initiatives have been implemented at regional level, including in Eastern Europe, in response to the MDR- and XDR-TB crisis.
All countries in Eastern Europe have developed national MDR- and XDR-TB response plans in consultation with the WHO. These plans are based on country TB drug resistance surveys, resource availability, human immunodeficiency virus (HIV) burden and other national contexts.25 Several technical advisory mechanisms have also been established in the region to achieve the comprehensive goals of the Action Plan and national MDR- and XDR-TB response plans. These mechanisms include the Green Light Committee/Europe (GLC/Europe), an independent technical advisory body to support countries with state-of-the-art clinical advice and to scale up programme management of MDR- and XDR-TB;26 the European Respiratory Society-WHO Electronic Consilium (consilia are multidisciplinary teams of specialists organised to give expert clinical consultation for MDR- and XDR-TB and other difficult-to-treat TB cases, such as TB-HIV and paediatric cases);27 the European Tuberculosis Laboratory Initiative (ELI) to improve and expand second-line DST and scale up diagnostic capacity, including the use of rapid molecular tests such as Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA);28 and the Regional Interagency Collaborating Committee on TB Control (RCC-TB), to improve partnerships and strengthen coordination among partners.29
Ongoing monitoring and evaluation will reveal the full impact of the Action Plan including these initiatives and others on the control of MDR-TB in the Region. However, several indicators of overall progress are already evident, such as the reduction in TB incidence rates and stabilisation of MDR-TB rates in many of the countries in the Eastern European subregion. In addition, treatment coverage for MDR-TB patients increased from 63% of estimated MDR-TB patients in 2011 to 96% in 2013.25 Extensive progress has also been made in the coverage and quality of TB surveillance. Since 2010, nationwide representative data on levels of MDR-TB in Belarus, and now Azerbaijan (in addition to other countries in the WHO European Region), have been made available through nationwide drug resistance surveys.20,21,30
CHALLENGES REMAINING
Despite progress and good impetus in efforts to control MDR-TB rates in Eastern Europe in recent years, critical challenges remain. These challenges are most often interconnected and multifaceted; in addition, some are highly specific to the country context, while others are apparent throughout Eastern Europe and all high-burden countries in the WHO European Region. While not intending to be an exhaustive list, some of the most critical of these latter challenges are discussed below.
MDR-TB with additional resistance
The high levels of pre-XDR-TB and XDR-TB in Eastern Europe (Table 2) are of great concern. Despite widespread coverage of second-line drugs, there is still inadequate treatment and insufficient patient support mechanisms in some Eastern European countries, including some member states of the European Union.25 Evidence of this is seen in projects that are not supported internationally by technical agencies such as the WHO or the GLC, where treatment success among MDR-TB patients is extremely low (28% in some settings).31 This is mainly due to incomplete treatment regimens and lack of full access to all necessary second-line anti-tuberculosis drugs. Adverse drug events leading to poor treatment adherence during the long course of MDR- and XDR-TB treatment also severely compromise treatment success.32 Despite the recent conditional approval of two new medicines (bedaquiline and de-lamanid) by the international drug regulatory authorities, their full-scale use is not in place, as there is a need for the establishment of strong pharmacovigilence systems.
Patient hospitalisation
Contributing to the spread of drug-resistant forms of TB, some countries hospitalise patients unnecessarily while awaiting DST results or during the intensive phase of drug-susceptible anti-tuberculosis treatment.33–38 Ambulatory services and other models of care, including home-based treatment, are not fully functional in these countries.25 In the absence of adequate airborne infection control, hospitalisation can lead to nosocomial transmission to health care workers, ancillary staff and other patients, and secondary infection with MDR- or XDR-TB strains.39 A recent meta-analysis found no difference in treatment outcomes of patients treated in ambulatory vs. hospitalised settings, and the WHO currently recommends minimising unnecessary hospitalisations and using ambulatory rather than hospital-based models of care for MDR-TB treatment.11 This is also likely to be much more acceptable to patients in the longer term.
Rapid diagnostics for first-line DST
New rapid molecular tests for MDR-TB, such as Xpert, play a vital role in the rapid identification and control of drug-resistant TB:40 in theory, the quicker the detection of drug-resistant strains, the faster patients can be initiated on appropriate treatment regimens, thereby minimising the window of transmission, although this also depends on good linkages and referral systems to care. Rapid diagnostic technologies, however, are not yet universally available in all Eastern European countries.16,32,41 Reduced funding due to the financial crisis in some countries has also exacerbated the difficulties in scaling up diagnostic capacities, including the use of molecular tests and improving biosafety.
Second-line DST and surveillance
Data on second-line DST are still limited, and electronic data management is lacking in many countries in Eastern Europe, adding to difficulties in analysing programme performance.25 Some countries in Eastern Europe collect second-line anti-tuberculosis drug resistance data only during subnational surveys, which are not repeated, while other countries have limited or no data.21 Rapid second-line DST is essential so that treatment can be adapted to resistance patterns in a timely manner. However, only Armenia, Georgia and Latvia currently have universal or near universal coverage of second-line DST.15
Vulnerable populations
Another serious challenge to MDR- and XDR-TB control is reaching vulnerable populations such as children, migrants, prisoners and people living with HIV, who are at greater risk for contracting and developing MDR- or XDR-TB.42 Considerable efforts have been made on this front since 2011, with the development of a minimum package for cross-border TB control and care in 2012,43 and in 2013 the International Union Against Tuberculosis and Lung Disease (Paris, France), the WHO and other international stakeholders issued an official statement of 12 action points to improve TB prevention and control in prisons.44 However, many countries in Eastern Europe have yet to implement the recommendations put forth in these statements.
Children
Children are often a neglected and vulnerable group with regard to MDR- and XDR-TB. This is due to the low number of bacilli in sputum among children,45 which makes TB, MDR- and XDR-TB harder to diagnose with sputum smear microscopy, culture and molecular tests.46 In 2011, only approximately 4% of the estimated cases of childhood TB in the 10 high-burden Eastern European countries were detected and reported,45 and reporting on paediatric MDR- and XDR-TB is currently very limited. WHO guidelines on childhood TB, including paediatric diagnostics and drug formulations, have recently been updated;47 however, these need to be adopted into national strategic TB plans and practice in Eastern Europe.
Migrants
Migrants often face a myriad of challenges such as discrimination, economic adversity, language barriers, stigma and fear of deportation.48 These challenges, combined with the migratory nature of the population, pose enormous barriers and difficulties in accessing diagnosis and continuous anti-tuberculosis treatment services.48 Both internal and cross-border migration enhance TB transmission. Many migrants also live in close proximity with family members or other individuals, as is the case with refugees and seasonal migratory workers living in temporary housing. All of these factors increase the risk of developing, contracting and transmitting drug-resistant forms of TB. Complicating the situation, some countries in both Eastern and Western Europe deport migrants with TB without considering the public health and human rights issues involved, or without taking adequate infection control measures, thereby increasing the risk of cross-border transmission.43,49 Levels of migration vary substantially across Eastern Europe, as do reported rates of TB among migrants; in 2010, 2.4% of notified TB cases in Lithuania were foreign-born, compared to 17.6% in Estonia.50 In the Russian Federation in 2011, less than 5% of notified cases were reported as foreign-born through routine surveillance; however, an earlier study from 2005 found that 26.9% of detected TB cases in Moscow were among migrants.51
Prisoners
Similar to migrants, incarcerated patients have a much higher risk for developing or contracting drug-resistant TB compared to the general population. In 2011, the pooled rate of TB in prisons (from all reporting sites) in the Russian Federation was 14 times that of the general population. Rates in Azerbaijan and Georgia were respectively 23 and 26 times higher in prisons than in the general population.15 Prisons in Eastern Europe are often poorly ventilated and crowded, and incarcerated patients spend long periods of time in these environments.52 Other determinants are high rates of HIV infection, injecting drug use and poor nutritional status.44 Human and financial resources for TB and MDR-TB prevention and control in prison are often scarce in Eastern Europe, and there are still gaps in coordination between civilian and penitentiary TB services.25 There are, however, recent best practice examples of TB and MDR- and XDR-TB control in the Azerbaijan prison sector, and effective continuity of TB care for released prisoners in Azerbaijan and the Republic of Moldova,53 which should be scaled up in the region.
Persons living with HIV
Individuals living with HIV are highly susceptible to TB,54 and Eastern Europe has one of the fastest growing HIV epidemics in the world.55 Approximately 65% of new HIV infections in the region in 2010 occurred in the Russian Federation and Ukraine.56 These countries also had the highest rates in the region in 2011, with respectively 44 and 36 cases per 100 000 population.56 Most countries, however, lack a functioning TB-HIV coordinating mechanism to facilitate the delivery of integrated TB and HIV services, including those related to narcology services for those with drug or alcohol dependency.25
Human resources
Lack of human resources is an important challenge that affects all levels of MDR- and XDR-TB prevention control and care in Eastern Europe. There is particular need for specialised human resources to manage cases of drug-resistant TB in both children and adults, deliver adequate services for case detection and scale up diagnostic and laboratory capacity.25,57
Funding
In 2011, there was a considerable projected funding gap of over 60% to fully implement the Action Plan for M/XDR-TB in the European Region.16 This funding gap has still not been met. In countries financially supported by the Global Fund to Fight AIDS, Tuberculosis and Malaria (The Global Fund, Geneva, Switzerland), the treatment success rate among MDR- and XDR-TB patients is 78% compared to 20% in other settings without Global Fund support.58 This is strong evidence of the need for funding from The Global Fund and other international donor agencies to address the current challenges to MDR- and XDR-TB control. A critical challenge in Eastern Europe will be the gradual shift in funding to national mechanisms, which is a requirement under The Global Fund's New Funding Model, and to ensure that progress is not lost due to financial gaps.
WAYS FORWARD
The 2015 MDG deadline is fast approaching, as are the deadlines for the Global Plan to Stop TB and the Consolidated Action Plan for M/XDR-TB in the European Region. In response to this, and the continuing challenges facing the control of TB and MDR- and XDR-TB, an ambitious Post-2015 Global TB Strategy has recently been developed by the WHO and approved by the World Health Assembly.59 The strategy, which has several milestones for 2025 and 2035, comprises three main pillars summarised in Table 3. It is imperative that the Post-2015 Global TB Strategy be adapted to regional and country-specific contexts so that there is a framework for continued efforts to prevent and combat TB and MDR- and XDR-TB, and that there is a seamless transition between pre- and post-2015 plans. This is particularly the case in Eastern Europe and the WHO European Region as a whole, which has the world's largest proportion of high MDR- and XDR-TB burden countries.
TABLE 3.
Post-2015 global tuberculosis strategy framework (reproduced with permission from the draft global strategy and targets for tuberculosis prevention, care and control after 2015 (A67/11)59

In Eastern Europe, as M. tuberculosis strains resistant to first- and second-line drugs increasingly replace drug-susceptible strains, TB cases will become increasingly difficult to treat. This may already explain the falling treatment success rates seen across the region. In line with the current regional Action Plan and the post-2015 Global Strategy, several immediate actions are needed to address the challenges to MDR- and XDR-TB control in Eastern Europe. First, to address the spread of primary infection with drug-resistant strains of M. tuberculosis, improved infection control is needed. In line with this, it is essential that hospital financing mechanisms be revised to promote free ambulatory care instead of a ‘fee-per-bed’ policy, which promotes hospitalisation and ongoing transmission of drug-resistant TB.60,61 A new vaccine is also needed. Second, to address acquired resistance, prompt and improved treatment is necessary. Full access to all necessary second-line anti-tuberculosis drugs needs to be ensured. In addition, emphasis should be placed on shorter and more effective treatment regimens and psychosocial support mechanisms to improve adherence in the face of adverse side effects. Countries should make particular effort to address populations and projects that are not supported by technical agencies such as the WHO and GLC. Bedaquiline and other new TB medicines also need to continue to be developed urgently and introduced under specific conditions, such as compassionate use and with special attention to pharmacovigilance. To guard against resistance to these new drugs, it is critical that international regulations on their use be strengthened, and that availability of drug regimens be ensured so that drug stockouts and subsequent misuse of new drugs be prevented. Third, and vital to curbing both routes of infection with drug-resistant TB, is early and rapid detection. Central to this is strengthening laboratory capacity to improve the use of current rapid diagnostics and second-line DST. In addition, there is a need for improved, easily applicable and affordable molecular tests in high-burden areas. Fourth, vulnerable populations are at highest risk for contracting or developing MDR- and XDR-TB, and also pose a risk for transmitting and sustaining disease transmission if not properly treated. If an end to the TB epidemic is to be realised, MDR- and XDR-TB among vulnerable populations should be addressed urgently.
A longer-term approach to addressing MDR- and XDR-TB among vulnerable populations, and in line with Pillars 1 and 2 of the Post-15 Global TB Plan, is strengthening the health systems.62 This implies a move away from vertical TB service delivery mechanisms, which are often difficult for vulnerable populations such as migrants and persons living with HIV to access or to receive appropriate care, to a coordinated/integrated health care system adapted to client needs. This approach would need intensified collaboration and joint action by HIV and TB control programmes. In addition, such a model would enable greater emphasis to be placed on social determinants of health, such as poor living and working conditions, HIV infection, malnutrition, smoking, diabetes and drug and alcohol use disorders, which are major drivers of the TB and MDR- and XDR-TB epidemic.42,63,64 A health systems approach could also alleviate the challenge of specific human resources and financing currently needed for TB services. Great care must be taken, however, for health systems strengthening to be implemented cautiously, systematically and with appropriate financial backing to avoid suboptimal hastily introduced health care reforms, which could have a negative effect on TB programmes.57
Pillar 3 of the Post-15 Global TB Plan will also play a critical role in the future of MDR- and XDR-TB control. One third of the population is estimated to be latently infected with TB; as long as there is latent tuberculous infection, there is the possibility of the development and transmission of TB disease. If the vision of the Post-15 Global TB Strategy is to be met, there is an urgent need for research and development on vaccines and new medicines for TB, MDR- and XDR-TB. In addition to basic sciences research, there is also a need for operational research. The role of operational research has been successfully and extensively demonstrated in numerous settings.4,65–67 Through surveillance and routine data collection, it is currently possible to understand the burden and trends in TB and MDR- and XDR-TB, although improvements in reporting are needed. It is envisaged that the operational research agenda will be greatly strengthened and expanded under Pillar 3; this should have an enormous impact on improving programme performance and serve as an evidence base for policy and practice.
CONCLUSION
The burden of MDR- and XDR-TB in Eastern Europe is high. While much progress has been made in controlling drug-resistant TB since the launch of the Consolidated Action Plan in 2011, the prevalence of second-line drug resistance has severely threatened treatment success and continued progress in controlling MDR- and XDR-TB in Eastern Europe. While mortality rates do not appear to have been affected yet, vigilance and intensified efforts are needed as we head into 2015 if decreasing mortality rates are to be kept on track. There are several current challenges in MDR- and XDR-TB control in Eastern Europe as regards access to appropriate treatment regimens, patient hospitalisation, scale-up of laboratory capacity, including the use of rapid diagnostics and second-line DST, vulnerable populations, human resources and TB financing.
Solutions to these challenges are aligned with the Post-2015 Global TB strategy. As a first step, the global TB strategy must be adapted at regional and country levels to serve as a framework for immediate actions as well as ways forward in the longer term. Longer-term solutions include strengthening health systems as a way to ensure adequate TB care for vulnerable populations and to address social determinants of health, which are drivers of the TB epidemic.42 In addition to initiatives and continued efforts under Pillars 1 and 2 of the Post-2015 Global TB Strategy, Pillar 3, research, will play a critical role in achieving a vaccine and new medicines for MDR- and XDR-TB, improving TB programme performance and creating an evidence base for effective policy as we head into a new era of TB control.
Acknowledgments
The authors alone are responsible for the content of this paper, which may not necessarily represent the policies, decisions or views of the World Health Organization (WHO). In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution intergovernmental organisation licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited. The analysis of WHO surveillance data and writing of this review paper was led by the WHO Regional Office for Europe, Copenhagen, Denmark.
The authors thank AD Harries, R Zachariah and AMV Kumar for their valuable review of the manuscript.
Footnotes
Conflict of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Accessed September 2014. [Google Scholar]
- 2.Abubakar I, Zignol M, Falzon D et al. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013;13:529–539. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 3.Murray C J L, Ortblad K F, Guinovart C et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsay A, Harries A D, Zachariah R, Bissell K. The Structured Operational Research and Training Initiative for public health programmes. Public Health Action. 2014;4:79–84. doi: 10.5588/pha.14.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB) Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2010.3. http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf. Accessed September 2014. [PubMed] [Google Scholar]
- 6.Zalutskaya A, Wijkander M, Jureen P. Multidrug-resistant Myobacterium tuberculosis caused by the Beijing genotype and a specific T1 genotype clone (SIT No. 266) is widely transmitted in Minsk. Int J Mycobacteriol. 2013;2:194–198. doi: 10.1016/j.ijmyco.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Nathanson E, Gupta R, Huamani P et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8:1382–1384. [PubMed] [Google Scholar]
- 8.Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–1477. doi: 10.1164/rccm.200206-626OC. [DOI] [PubMed] [Google Scholar]
- 9.Coninx R, Mathieu C, Debacker M et al. First-line tuberculosis therapy and drug-resistant Mycobacterium tuberculosis in prisons. Lancet. 1999;353:969–973. doi: 10.1016/s0140-6736(98)08341-x. [DOI] [PubMed] [Google Scholar]
- 10.Mitnick C, Bayona J, Palacios E et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 11.Falzon D, Jaramillo E, Schünemann H J et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–528. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 12.Falzon D, Jaramillo E, Wares F, Zignol M. Universal access to care for multidrug-resistant tuberculosis: an analysis of surveillance data. Lancet Infect Dis. 2013;3:690–697. doi: 10.1016/S1473-3099(13)70130-0. [DOI] [PubMed] [Google Scholar]
- 13.Shean K, Streicher E, Pieterson E et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLOS ONE. 2013;8:e63057. doi: 10.1371/journal.pone.0063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietersen E, Ignatius E, Streicher E M et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383:1230–1239. doi: 10.1016/S0140-6736(13)62675-6. [DOI] [PubMed] [Google Scholar]
- 15.European Centre for Disease Prevention and Control, WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2014. Stockholm, Sweden: ECDC; 2014. [Google Scholar]
- 16.WHO Regional Office for Europe. Roadmap to prevent and combat drug-resistant tuberculosis. Copenhagen, Denmark: WHO; 2011. http://www.euro.who.int/__data/assets/pdf_file/0014/152015/e95786.pdf. Accessed September 2014". [Google Scholar]
- 17.Raviglione M C. The TB epidemic from 1992 to 2002. Tuberculosis (Edinb) 2003;83:4–14. doi: 10.1016/s1472-9792(02)00071-9. [DOI] [PubMed] [Google Scholar]
- 18.Shilova M V, Dye C. The resurgence of tuberculosis in Russia. Philos Trans R Soc Lond, B, Biol Sci. 2001;356:1069–1075. doi: 10.1098/rstb.2001.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Global tuberculosis control. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB 2010.7. http://www.who.int/tb/publications/global_report/2010/en/ Accessed September 2014. [Google Scholar]
- 20.Skrahina A, Hurevich H, Zalutskaya A et al. Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull World Health Organ. 2013;91:36–45. doi: 10.2471/BLT.12.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zignol M, Dara M, Dean A S Drug-resistant tuberculosis in the WHO European Region: an analysis of surveillance data. Drug Resist Updat 2014. [DOI] [PubMed]
- 22.World Health Organization. Global tuberculosis control: surveillance, planning, financing, WHO report 2002. Geneva, Switzerland: WHO; 2002. WHO/CDS/TB/2002.295. [Google Scholar]
- 23.Falzon D, Gandhi N, Migliori G B et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J. 2013;42:156–168. doi: 10.1183/09031936.00134712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoffels K, Allix-Béguec C, Groenen G, Wanlin M. From multidrug-to extensively drug-resistant tuberculosis: upward trends as seen from a 15-year nationwide study. PLOS ONE. 2013;8:e63128. doi: 10.1371/journal.pone.0063128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Regional Office for Europe. Regional Committee for Europe: Progress Reports. Copenhagen, Denmark: WHO; 2013. [Google Scholar]
- 26.Green Light Committee of the Working Group on MDR-TB STOP TB PARTNERSHIP. Green Light Committee Initiative, Annual Report 2009. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2010.14. http://whqlibdoc.who.int/hq/2010/WHO_HTM_TB_2010.14_eng.pdf. Accessed September 2014. [Google Scholar]
- 27.Blasi F, Dara M, van der Werf M J, Migliori G B. Supporting TB clinicians managing difficult cases: the ERS/WHO Consilium. Eur Respir J. 2013;41:491–494. doi: 10.1183/09031936.00196712. [DOI] [PubMed] [Google Scholar]
- 28.WHO Regional Office for Europe. European Tuberculosis Laboratory Initiative. Copenhagen, Denmark: WHO; 2014. http://www.euro.who.int/en/health-topics/communicable-diseases/tuberculosis/activities/european-tuberculosis-laboratory-initiative Accessed September 2014. [Google Scholar]
- 29.WHO Regional Office for Europe. WHO Regional Office for Europe. Terms of reference for RCC-TB. Copenhagen, Denmark: WHO; 2014. http://www.euro.who.int/en/health-topics/communicable-diseases/tuberculosis/activities/regional-collaborating-committee-on-tuberculosis-control-and-care-rcc-tb/terms-of-reference-for-rcc-tb Accessed September 2014. [Google Scholar]
- 30.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2010.3. http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf. Accessed September 2014. [Google Scholar]
- 31.Cegielski J P, Dalton T, Yagui M et al. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2014 Jul 23 doi: 10.1093/cid/ciu572. pii: ciu572. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lange C, Abubakar I, Alffenaar J-W C et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TB-NET consensus statement. Eur Respir J. 2014;44:23–63. doi: 10.1183/09031936.00188313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dara M, Mkrtchyan Z, Ghukasyan G. Extensive review of TB prevention, care and control services in Armenia. Copenhagen, Denmark: World Health Organization, Regional Office for Europe; 2012. http://www.euro.who.int/__data/assets/pdf_file/0007/160864/e96506.pdf. Accessed September 2014. [Google Scholar]
- 34.Dara M, Gozalov O, Javadli O, Turusbekova N. Review of tuberculosis prevention, control and care in Azerbaijan. Copenhagen, Denmark: World Heath Organization Regional Office for Europe; 2012. http://www.euro.who.int/__data/assets/pdf_file/0005/193703/NTP-AZE_review_report_ENG_final-4.pdf?ua=1. Accessed September 2014. [Google Scholar]
- 35.de Colombani P. Review of the National Tuberculosis Programme in Belarus. Copenhagen, Denmark: World Health Organization, Regional Office for Europe; 2012. http://www.euro.who.int/__data/assets/pdf_file/0003/170337/Review-of-the-National-Tuberculosis-Programme-in-Belarus.pdf. Accessed September 2014. [Google Scholar]
- 36.de Colombani P, Ahmedov S, Blöndal K, Ciobanu S. Review of the National Tuberculosis Programme in the Republic of Moldova, 4–15 February 2013. Copenhagen, Denmark: WHO Regional Office for Europe; 2013. [Google Scholar]
- 37.World Health Organization. Regional Office for Europe. Review of the National Tuberculosis Programme in Ukraine. Copenhagen, Denmark: WHO; 2011. [Google Scholar]
- 38.Nodieva A, Jansone I, Broka L, Pole I, Skenders G, Baumanis V. Recent nosocomial transmission and genotypes of multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2010;14:427–433. [PubMed] [Google Scholar]
- 39.Bassili A, Fitzpatrick C, Qadeer E, Fatima R, Floyd K, Jaramillo E. A systematic review of the effectiveness of hospital- and ambulatory-based management of multidrug-resistant tuberculosis. Am J Trop Med Hyg. 2013;89:271–280. doi: 10.4269/ajtmh.13-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans C A C. GeneXpert—a game-changer for tuberculosis control? PLOS MED. 2011;8:e1001064. doi: 10.1371/journal.pmed.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drobniewski F A, Hoffner S, Rüsch-Gerdes S, Skenders G, Thomsen V, WHO European Laboratory Strengthening Task Force. Recommended standards for modern tuberculosis laboratory services in Europe. Eur Respir J. 2006;28:903–909. doi: 10.1183/09031936.06.00084906. [DOI] [PubMed] [Google Scholar]
- 42.Lönnroth K, Jaramillo E, Williams B G, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 43.Dara M, de Colombani P, Petrova-Benedict R et al. The minimum package for cross-border TB control and care in the WHO European Region: a Wolfheze Consensus Statement. Eur Respir J. 2012;40:1081–1090. doi: 10.1183/09031936.00053012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dara M, Chadha S S, Melchers N V et al. Time to act to prevent and control tuberculosis among inmates. [Official Statement of The International Union Against Tuberculosis and Lung Disease] Int J Tuberc Lung Dis. 2013;17:4–5. doi: 10.5588/ijtld.12.0909. [DOI] [PubMed] [Google Scholar]
- 45.Acosta C D, Rusovich V, Harries A D, Ahmedov S, van den Boom M, Dara M. A new roadmap for childhood tuberculosis. Lancet Glob Health. 2014;2:e15–17. doi: 10.1016/S2214-109X(13)70153-0. [DOI] [PubMed] [Google Scholar]
- 46.Stop TB Partnership Childhood TB Subgroup, World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Int J Tuberc Lung Dis. 2006;10:1205–1211. [PubMed] [Google Scholar]
- 47.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2nd ed. Geneva, Switzerland: WHO; 2014. WHO/HTM/TB/2014.03. [PubMed] [Google Scholar]
- 48.Bone A. A Human Rights Approach to tuberculosis. Geneva, Switzerland: WHO; 2001. WHO/CDS/STB/2001.9. http://www.who.int/hhr/information/A%20Human%20Rights%20Approach%20to%20Tuberculosis.pdf. Accessed September 2014. [Google Scholar]
- 49.Heldal E, Kuyvenhoven J V, Wares F et al. Diagnosis and treatment of tuberculosis in undocumented migrants in low- or intermediate-incidence countries. [Workshop report] Int J Tuberc Lung Dis. 2008;12:878–888. [PubMed] [Google Scholar]
- 50.van der Werf M J, Hollo V, Noori T. Is tuberculosis crossing borders at the Eastern boundary of the European Union? Eur J Public Health. 2013;23:1058–1063. doi: 10.1093/eurpub/ckt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorbunov A V, Kochetkova E I. [Organization of detection of patients with tuberculosis in Moscow] Probl Tuberk Bolezn Legk. 2005;(8):18–22. [Russian] [PubMed] [Google Scholar]
- 52.Dara M, Acosta C D. Tuberculosis prevention and control in prisons: do we know enough? Int J Tuberc Lung Dis. 2014;18:758–759. doi: 10.5588/ijtld.14.0362. [DOI] [PubMed] [Google Scholar]
- 53.Dara M, Acosta C D. Best practices in prevention, control and care for drug-resistant tuberculosis. Copenhagen, Denmark: World Health Organization, Regional Office for Europe; 2013. http://www.euro.who.int/__data/assets/pdf_file/0020/216650/Best-practices-in-prevention,control-and-care-for-drugresistant-tuberculosis-Eng.pdf?ua=1. Accessed September 2014. [Google Scholar]
- 54.Corbett E L, Watt C J, Walker N et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 55.Joint United Nations Programme on HIV/AIDS. Report on the global AIDS epidemic 2013. Geneva, Switzerland: UNAIDS/WHO; 2013. UNAIDS/JC2209E. http://www.euro.who.int/__data/assets/pdf_file/0015/240045/Progress-report-2011,-HIV-AIDS-in-Europe-and-central-Asia.pdf?ua=1. Accessed September 2014. [Google Scholar]
- 56.European Centre for Disease Prevention and Control, WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2012. Stockholm, Sweden: ECDC; 2013. http://www.euro.who.int/__data/assets/pdf_file/0018/235440/e96953.pdf?ua=1. Accessed September 2014. [Google Scholar]
- 57.Atun R, Weil D E C, Eang M T, Mwakyusa D. Health-system strengthening and tuberculosis control. Lancet. 2010;375:2169–2178. doi: 10.1016/S0140-6736(10)60493-X. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. Regional Office for Europe. Regional GLC (rGLC) report for the WHO European Region for 2012. Copenhagen, Denmark: WHO Regional Office for Europe; 2013. [Google Scholar]
- 59.World Health Organization. Draft global strategy and targets for tuberculosis prevention, care and control after 2015. A67/11. Geneva, Switzerland: WHO; 2014. http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_11-en.pdf?ua=1. Accessed September 2014. [Google Scholar]
- 60.Migliori G B, Khomenko A G, Punga V V et al. Cost-effectiveness analysis of tuberculosis control policies in Ivanovo Oblast, Russian Federation. Ivanovo Tuberculosis Project Study Group. Bull World Health Organ. 1998;76:475–483. [PMC free article] [PubMed] [Google Scholar]
- 61.Gillini L, Davtyan K, Davtyan H et al. TB financing in East Europe promotes unnecessary hospital admissions: the case of Armenia. J Infect Dev Ctries. 2013;7:289–292. doi: 10.3855/jidc.3396. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization. Stop TB policy paper: contributing to health system strengthening: guiding principles for national tuberculosis programmes. Geneva, Switzerland: WHO; 2008. [PubMed] [Google Scholar]
- 63.Lönnroth K, Castro K G, Chakaya J M et al. Tuberculosis control and elimination 2010–50: cure, care, and social development. Lancet. 2010;375:1814–1829. doi: 10.1016/S0140-6736(10)60483-7. [DOI] [PubMed] [Google Scholar]
- 64.Rasanathan K, Kurup A S, Jaramillo E, Lönnroth K. The social determinants of health: key to global tuberculosis control. Int J Tuberc Lung Dis. 2011;15(Suppl 2):S30–S36. doi: 10.5588/ijtld.10.0691. [DOI] [PubMed] [Google Scholar]
- 65.Zachariah R, Harries A D, Ishikawa N et al. Operational research in low-income countries: what, why, and how? Lancet Infect Dis. 2009;9:711–717. doi: 10.1016/S1473-3099(09)70229-4. [DOI] [PubMed] [Google Scholar]
- 66.Zachariah R, Ford N, Maher D et al. Is operational research delivering the goods? The journey to success in low-income countries. Lancet Infect Dis. 2012;12:415–421. doi: 10.1016/S1473-3099(11)70309-7. [DOI] [PubMed] [Google Scholar]
- 67.Raviglione M M, Ben B Marais, Floyd K K et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet. 2012;379:1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]


