Abstract
Objective
Information on polymorphous low-grade adenocarcinoma (PLGA) consists primarily of case reports and small institutional series with varying recurrence rates. In this report, we describe our institutional experience and conduct a review of the literature to assess the overall incidence of PLGA among oral salivary gland tumors and determine recurrence rates.
Study Design
A retrospective case series and literature based review was performed.
Methods
Retrospective case series at an academic tertiary referral center. Review of clinical records and pathological analysis of tissue specimens from 20 patients treated for PLGA from July 1, 1990 to July 1, 2011. A literature-based review on PLGA was also performed.
Results
Twenty patients (mean age 54 years, 8 males) with PLGA based on pathologic diagnosis were included. The most common initial presentation was an asymptomatic mass (45%) and the most frequent site was the palate (60%). Our literature review identified 54 case reports, 8 case series, and 17 large series. In total 456 cases of PLGA were identified with an overall recurrence rate of 19%. Half of the recurrences occurred by 36 months; however, recurrences were reported up to 24 years after initial resection.
Conclusion
PLGA arises from minor salivary glands and is characteristically slow growing and indolent. While these tumors may be histologically low-grade, our review highlights the high rates of recurrence of these tumors as well as the ability to metastasis to local lymph nodes and distant organs. The mainstay of treatment should be wide surgical excision with long-term oncologic follow up.
Keywords: Polymorphous low-grade adenocarcinoma (PGLA), minor salivary gland tumor
INTRODUCTION
In 1984, Evans and Batsakis characterized a previously unrecognized subset of minor salivary gland adenocarcinoma1 and termed this cytologically bland tumor “polymorphous low-grade adenocarcinoma” (PLGA) because of the histological diversity that was observed. Previous to the clinicopathologic description of PLGA, this tumor had been non-uniformly referred to as low-grade papillary adenocarcinoma, terminal duct carcinoma, and lobular carcinoma.2–4 The World Health Organization recognized the diagnosis in 19905 and some series report PLGA to be one of the most common malignant salivary gland tumors.6,7
While the diagnosis is based on histological characteristics of PLGA, attempts have been made to identify immunohistochemical markers to help distinguish PLGA from other salivary gland tumors, specifically adenoid cystic carcinoma. We refer interested readers to the review by Darling, et al. for comprehensive discussion.8 Briefly, no standard immunohistochemical markers are in routine use, with most diagnoses made based on the hematoxylin and eosin morphology. Smooth muscle actin (SMA) is expected to be expressed in adenoid cystic carcinoma, but not in PLGA, and vimentin, glial fibrillary acidic protein, and c-Kit and other markers may have diagnostic utility.9–12 It is important to distinguish PLGA from high-grade tumors (e.g., adenoid cystic carcinoma) because PLGA tends to have lower local recurrence rates and have lower metastatic potential.13 Although PLGA is considered to behave in a low-grade fashion, PLGA has been documented to spread distantly to the lungs and liver.14,15 Long-term follow-up surveillance is necessary because recurrences have been reported to occur more than 10 years after initial treatment.16 While PLGAs represent a significant proportion of intraoral salivary gland malignancies, large series primarily describe pathology and most case series contain 10–20 patients at a single institution. In this report, we present our institutional experience and report a review of the literature to better understand overall prevalence, and to determine recurrence rates of PLGA of the head and neck.
METHODS
Case Series
Approval for this study was obtained from the University of North Carolina (UNC) Biomedical Institutional Review Board. Patients were identified in the UNC Pathology Database that had a pathologic diagnosis of PLGA from January 1, 1990 to January 1, 2010. All slides were reexamined by a single pathologist (WKF) and assessed for growth pattern, papillary architecture, perineural invasion, vascular invasion and margin status.
The length of patient follow-up, documented recurrences, and last known disease status were also obtained for each patient. Data was tabulated using Excel 2008 (Microsoft Corporation, Redmond, WA), and statistical analysis was performed using GraphPad Prism version 5 for Macintosh (GraphPad Software, San Diego, CA).
Review of the Literature
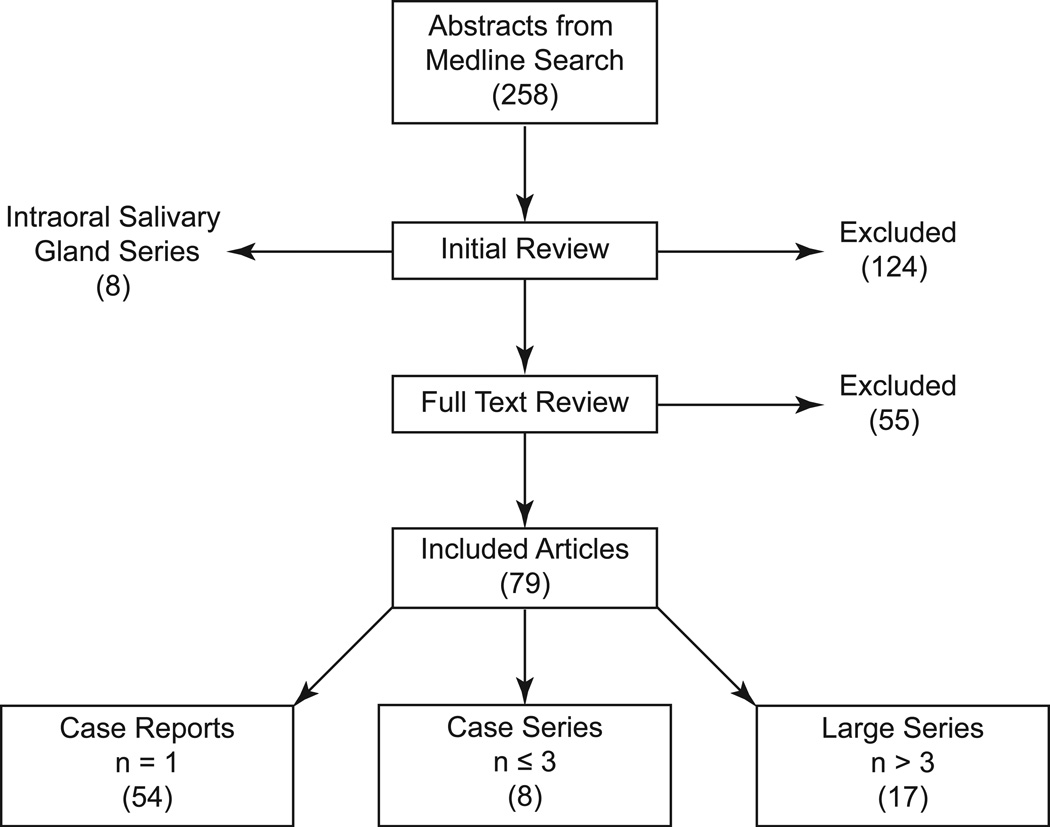
PubMed and MEDLINE databases were queried for “polymorphous low-grade adenocarcinoma” and “polymorphous low grade adenocarcinoma” from January 1984 to January 2012. A total of 258 English articles were identified. Two independent reviewers (AJK & CMW) reviewed abstracts and excluded 124 studies that were not on PLGA, did not present new data (review, editorials and commentaries), focused on histology without presenting new cases, testing new diagnostic methods, or focused on expression or mutational analysis of tumors. Of the included articles, subgroups of case reports (n = 1), case series (n ≤ 3) and “large series” (n > 3) were generated (Figure 1).
Figure 1. Schematic of Literature Review.
RESULTS
Patient Characteristics
In our institutional series, 20 patients were identified who had PLGA tumors verified by a pathologist. The average age at presentation was 53 years old with a range of 32–85 years old. The cohort had a female predominance (1:1.5). Most common initial presentation was an asymptomatic mass (45%). The average follow-up period was 47 months. The palate was the most common initial site for the tumor (60%); however, in our series, 45% of patients presented with extrapalatal tumors including the retromolar trigone, gingiva, nasal septum and maxillary sinus (Table 1).
Table 1.
Demographics and Outcomes of patients with PLGA at UNC.
| Patient | Age | Sex | Site | Initial Stage |
Primary Treatment |
Outcome | Size (cm) | Growth Pattern |
|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | palate | T1N0M0 | WLE | NED | Indeterminate | NTS |
| 2 | 46 | F | palate | T2N0M0 | WLE | NED | Indeterminate | NTS |
| 3 | 44 | M | retromolar trigone | T4aN1M0 | WLE/post-op RT | Distant metastasis | Indeterminate | tubular, solid |
| 4 | 32 | M | palate | T1N0M0 | WLE | NED | 1.4 | cribriform, tubular |
| 5 | 54 | F | palate | T1N0M0 | LE | LR | Indeterminate | tubular, cribriform |
| 6 | 39 | M | retromolar trigone | T1N0M0 | WLE | NED | Indeterminate | NTS |
| 7 | 60 | F | gingiva | T1N0M0 | WLE | NED | 0.3 | tubular |
| 8 | 48 | M | nasal septum, palate | T3N0M0 | WLE | NED | 4.5 | solid, papillary |
| 9 | 59 | M | maxillary sinus | T4aN0M0 | Primary RT/salvage surgery | NED | 5.5 | solid, cribriform |
| 10 | 61 | F | palate | T1N0M0 | WLE | NED | 1.9 | tubular, solid |
| 11 | 85 | F | nasal cavity | T1N0M0 | LE | NED | 1.8 | cribriform, tubular |
| 12 | 39 | F | base of tongue | T4aN0M0 | Neutron Beam | Persistent disease | Indeterminate | cribriform, tubular |
| 13 | 49 | F | palate | T1N0M0 | WLE | NED | 0.9 | tubular |
| 14 | 46 | F | nasal cavity | T1N0M0 | WLE | LR, degeneration intermediate grade | 1.5 | solid |
| 15 | 69 | F | palate | T2N0M0 | WLE | NED | 2.1 | solid and cribriform, with bone invasion |
| 16 | 56 | F | palate | T1N0M0 | WLE | NED | 1.6 | solid, trabecular |
| 17 | 61 | M | maxillary sinus, orbit | T3N0M0 | WLE | No follow-up | 6.5 | glandular |
| 18 | 76 | F | palate | T2N0M0 | WLE | NED | 3.2 | solid, tubular, angiomatoid |
| 19 | 46 | M | palate | T2N0M0 | WLE | NED | 2.4 | trabecular, tubular |
| 20 | 46 | M | palate | T2N0M0 | WLE | NED | 2.3 | solid, tubular, cribriform |
WLE= wide local excision; LE= limited excision; RT= radiation therapy; NED= no evidence of disease; LR= local recurrence; NTS= no tumor seen on pathology slide
Histology
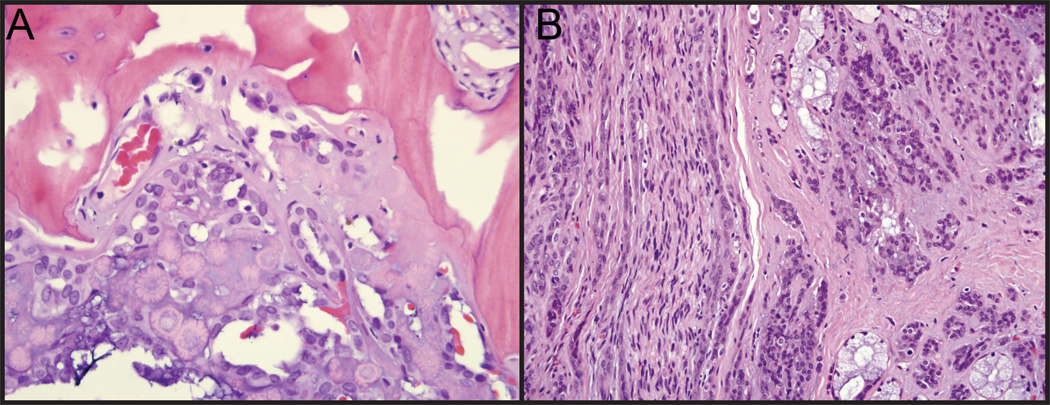
Diagnosis was made based on pathological features. PLGA tumors are composed of uniform, cytologically bland cells that vary from epitheliod, cuboidal, columnar to spindled; they grow in solid, glandular, cribiform, trabecular, cystic, fascicular, or papillary arrangements (Figure 2). Nuclei are ovoid with pale chromatin; nucleoli are inconspicuous and mitotic figures are infrequent. Stroma varies from hyaline to mucoid to fibrovascular; the tumor is non-encapsulated, and typically infiltrates into adjacent stroma. Of the 20 tumors in our institutional series, 13 demonstrated multiple growth patterns (Table 1), 11 tumors demonstrated tubular growth patterns, 9 tumors demonstrated solid growth patterns, 7 tumors had cribriform growth patterns, and 1 tumor demonstrated both papillary and angiomatoid growth patterns. In series we did not identify a correlation between histological patterns and recurrence rates.
Figure 2. Pathologic Diagnosis of PLGA.
(A) PLGA with evidence of bony invasion. (B) PLGA demonstrating infiltration into the stroma.
Treatment and Outcomes
In our institutional series, 18 patients were treated primarily by wide local surgical excision, 1 patient was treated with primary radiation therapy, and 1 patient received primary neutron beam therapy. Average follow up time was 2 years (range 5 months to 13 years). Three patients had recurrence and 1 patient had persistent disease. One patient died due to lung metastases 2.5 years after diagnosis.
Review of the Literature
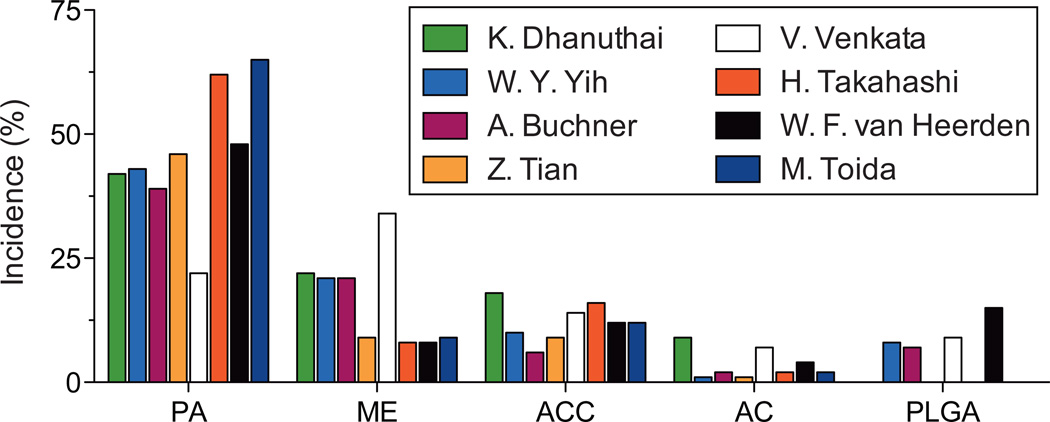
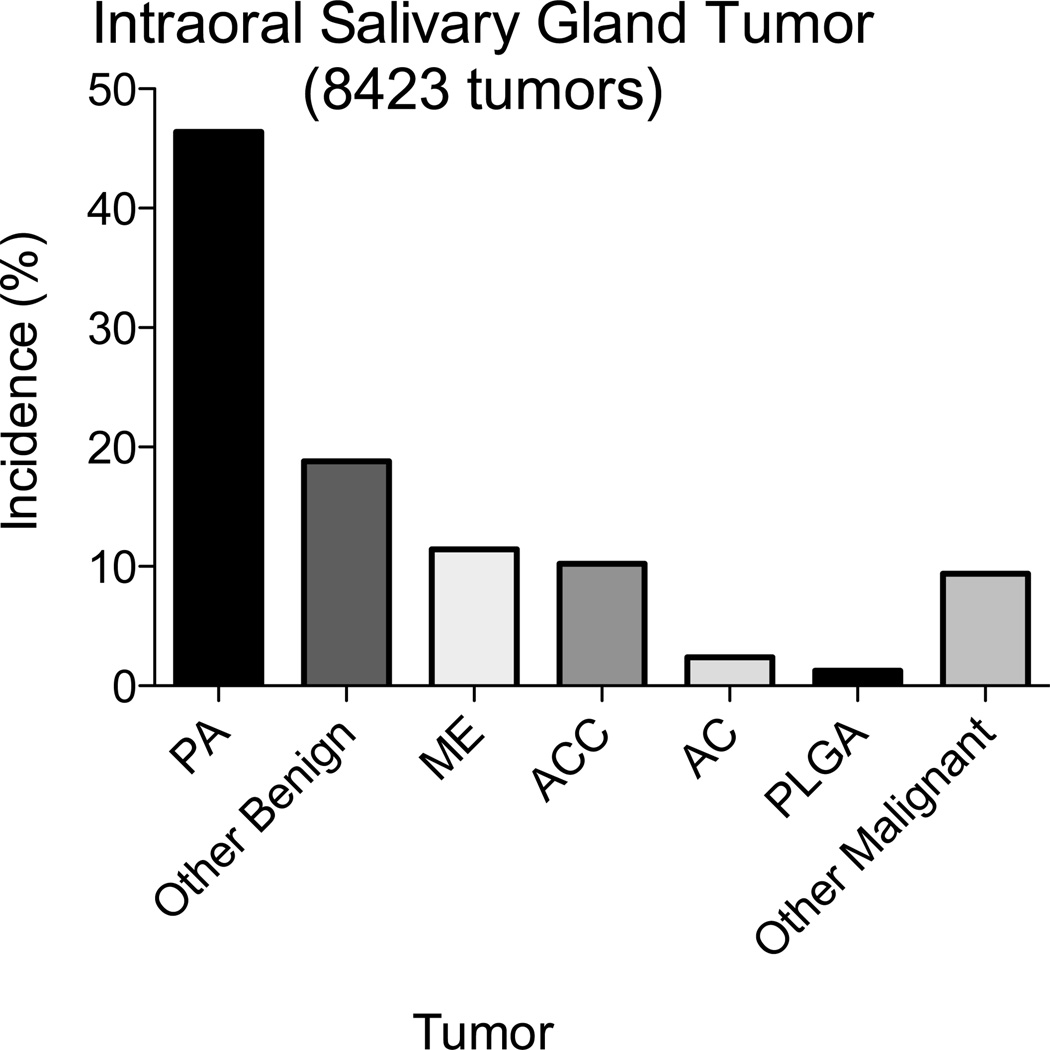
In the literature search, we identified 8 studies that documented the incidence of intraoral salivary gland tumors, specifically pleomorphic adenoma (PA), mucoepidermoid (ME), adenoid cystic carcinoma (ACC), adenocarcinoma (AC) and PLGA (Figures 1 and 3).6,7,17–22 In these 8 studies, the mean incidence of PLGA was 5.3% (range 0.0 to 15.7%). Combining data of the 8 studies resulted in a total of 8,423 intraoral salivary gland tumors and the overall incidence was determined: PA (46.4%), ME (11.46%), ACC (10.2%), AC (2.4%) and PLGA (1.2%) (Figure 4). Other benign (8.7%) and malignant (5.0%) salivary tumors included but are not limited to sialadenoma papilliferum, myoepithelioma, basal cell adenoma, oncocytoma, canalicular adenoma, sialadenoma, salivary duct carcinoma, myoepithelial carcinoma and carcinoma ex-pleomorphic adenoma, and acinic carcinoma. Excluding the large series by Tian et al.20 a total of 628 tumors were identified, 5.3% of which were diagnosed as PLGA.
Figure 3. Intraoral Salivary Gland Tumors.
The overall incidence of PLGA varies dramatically between different studies from 0.0% to 15.5% of IOSGT.
Figure 4. Overall Pooled Rates of Various Intraoral Salivary Gland Tumors.
In a total of 8423 pooled IOSGT cases the overall incidence of PA, other benign tumors, ME, ACC, AC, PLGA and other malignant tumors was 46.4%, 18.8%, 11.46%, 10.2%, 2.4%, 1.2%, and 9.4% respectively.
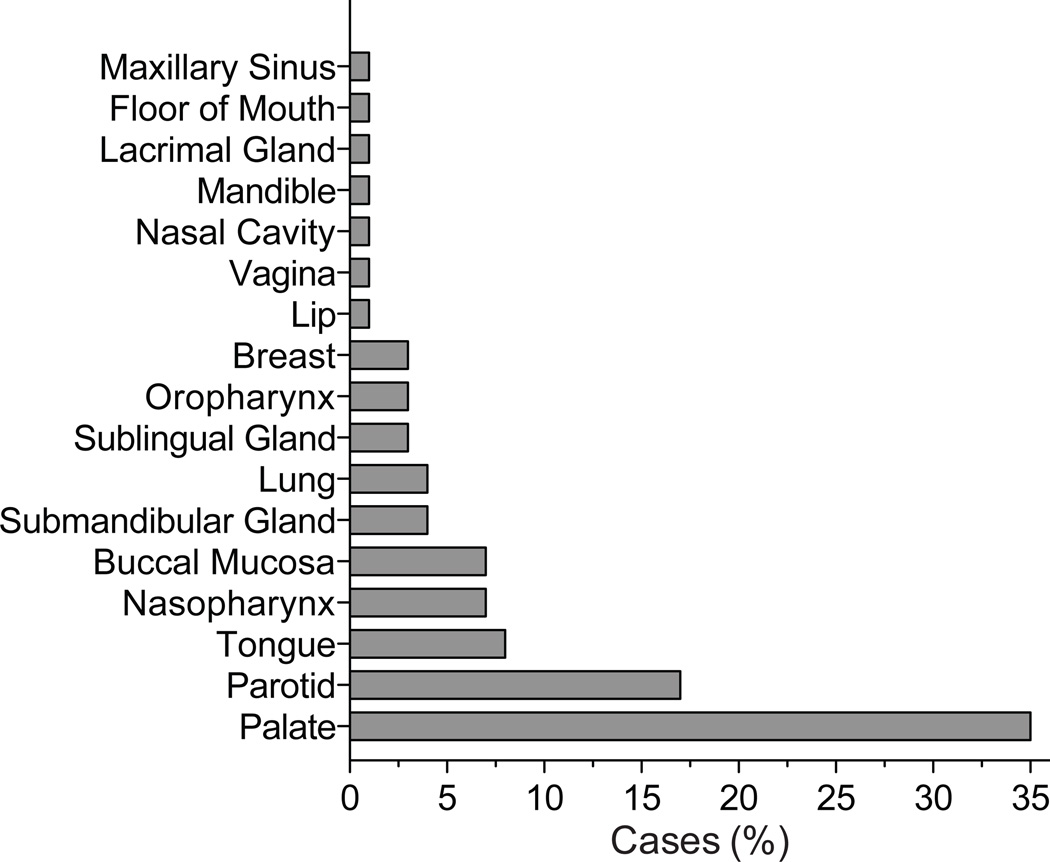
Overall, the literature search resulted in 54 case reports, eight case series (n ≤ 3), and 17 PLGA series (Figure 1). The case reports (n = 1) and case series (n ≤ 3) that included patient follow-up were pooled to produce a “cohort of cases” that included 72 patients (Supplemental Table 1). The age range in the cohort of cases was 12 to 84 years old with an average presenting age of 54. The gender distribution was nearly equal between male (49%) and female (51%). While the palate was the most common site of presentation in the case cohort with 25 cases (35%), additional sites were identified including the lips, nasal cavity, mandible, lacrimal gland, maxillary sinus, and floor of mouth (Figure 5, Supplemental Table 2).
Figure 5. Location of PLGA tumors.
PLGA tumors are most commonly identified on the palate but PLGA can present in a variety of different locations.
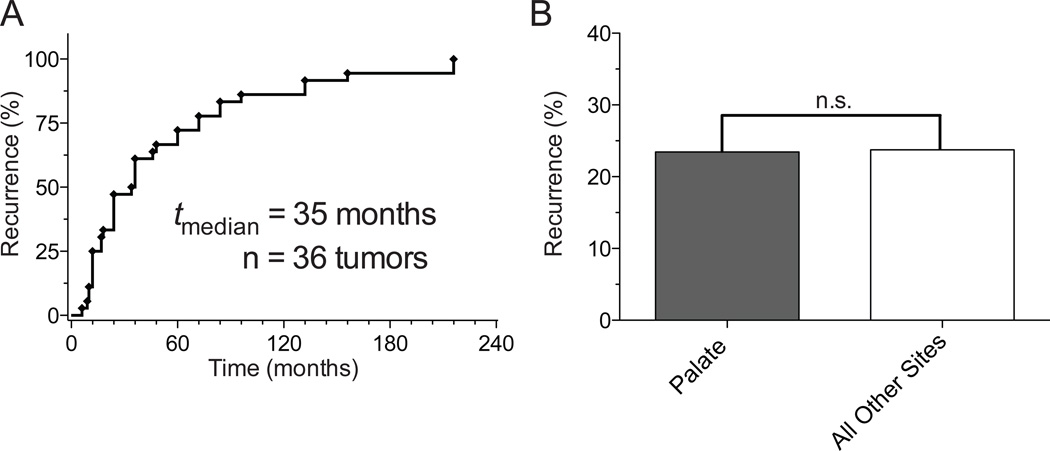
Seventeen series identified a total of 384 patients diagnosed with PLGA who had follow-up and recurrence data (Supplemental Table 3). The average age of presentation was 57 years with a slight female predominance (53%). Recurrence rates varied dramatically between studies, from 0% in three studies23–25 to 33.3% in three studies.13,26,27 Of the 384 patients, there were 70 reported recurrences resulting in an overall recurrence rate of 18.2% (Supplemental Table 3). The average time to recurrence in this group was 70 months (range between 5 to 288 months). The recurrence rate in the 72 patient “cohort of cases” was 23.6%, which was not statistically different from the recurrence rate of the 384 patient series (p = 0.33). The overall recurrence rate in the 456 identified cases was 19.1%. Of reports that detailed patient specific data in regards to latency between initial diagnosis and recurrence, 50% of recurrences occurred by 35 months (Figure 6A). The longest latency between treatment and recurrence was found to be 24 years.16 Of the recurrences, 54% were local, 32% were nodal and 8% were distant. Overall recurrence rate with of palatal tumors and pooled samples of all other sites was similar 23.5% and 27.7% respectively (p= 0.59, Figure 6B). Eleven tumors with recurrence data were reported to have originated in major salivary glands. The rate of recurrence rate of tumors presenting in parotid, submandibular or sublingual glands was 36.4% and was not statistically significant (p = 0.41) compared to the recurrence rates at all other sites of 24.2%. Distant metastasis were identified in the lungs and the liver.15,28,29
Figure 6.
A) Time to Recurrence. Half of PLGA tumors recur within 35 months; however PLGA tumors have the ability to recur decades after the initial presentation. B) Recurrence by Site. Location of initial tumor does not significantly affect the rate of recurrence. Recurrence rate of palatal tumor was 23.5% compared to 27.4 in a pooled samples of all other sites (p= 0.59).
The primary treatment modality was surgical resection of the tumor; however, 2.1% of patients received primary radiation therapy and 33.1% received adjuvant radiation therapy after surgical resection. Rates of recurrence for surgical excision without adjuvant radiation was 24.4% compared to 26.1% for surgical excision with adjuvant radiation therapy (p = 0.83).
DISCUSSION
Because PLGA and adenoid cystic carcinoma have different prognoses with respect to risk of local recurrence, distant metastasis, and death due to the neoplasm, it is important to distinguish these two neoplasms. This can be difficult if sampling is limited to core or incisional biopsy of a large lesion. Both can show similar architectural patterns, e.g. cribriform architecture. Fortunately, most adenoid cystic carcinomas show admixture of the cribriform pattern with tubular or solid patterns, regularly show extra-cellular gray matrix in pseudo-lumena, and routinely show a more basaloid cytology in the ductal component. Smooth muscle actin (SMA) immunoreactivity is expected in the majority of adenoid cystic carcinoma, but in a minority of PLGA. Both neoplasms need to be resected with negative margins, such that a diagnostic error on the initial biopsy should be recognized at the time of diagnosis of the resection specimen.
The prevalence of PLGA among oral salivary gland tumors varies greatly by series from 0 to 15.7%.6,21 In a pooled sample of over 8,000 intraoral salivary gland tumors identified in the literature, the reported incidence of PLGA was 1.2%; however, this number was significantly influenced by the low incidence of PLGA in the series that identified 31 PLGA tumors out of 6,982 oral salivary gland tumors (0.44%).20 This low rate of PLGA tumors observed by Tian and colleagues may represent a difference in geographic or racial rates of PLGA in an Eastern Chinese population or could also result from under diagnosis.
PLGA is classically a neoplasm of the minor salivary glands that most commonly presents with involvement of the palate; however, cases have been documented throughout the oral cavity and in the sublingual gland, submandibular gland, and parotid gland. In the published case reports 24% of tumors were identified in major salivary glands (i.e. parotid, submandibular or sublingual gland). Primary tumors have also been reported in locations outside of the head and neck including the vagina and breast.15,30
While surgical excision is the main stay of treatment, in our institutional series, 2 patients elected to undergo primary radiation because of the magnitude of surgical excision required at presentation. A third patient in our institutional series underwent adjuvant radiation therapy because of positive margins (Table 1). In the literature review, one-third of patient underwent adjuvant radiation therapy. While we are unable to ascertain the reason in all cases, close and positive margins were frequently denoted. The recurrence rate with adjuvant radiation was not significantly different than with excision alone.
Polymorphous low-grade adenocarcinoma is a low-grade and indolent neoplasm, with 10–20% risk of local recurrence, and low risk for distant metastasis and death due to PLGA. In the review of the literature the recurrence rates ranged from 0 to 33% with an average recurrence rate in nearly 500 tumors of 19.1%. While 50% of tumors recurred by 35 months, PLGA has the ability to recur decades after the initial treatment with one recurrence documented 24 years after initial treatment. In our study there was no difference in recurrence rates from the palate or major salivary glands compared to a pooled subset of all other tumors. The high rates of recurrence and the long latency between initial diagnosis and recurrence make it essential that the clinician remain vigilant for decades after the tumor is initially treated.
CONCLUSIONS
PLGA has a recurrence rate that approaches 20%; therefore, while histologically low grade this tumor does have a significant potential for recurrence and even regional and distant metastasis. Wide surgical resection is the mainstay of PLGA treatment and the role of radiation is unclear. It is essential to maintain long-term follow up, as local recurrence or metastasis can occur several years later.
Supplementary Material
Acknowledgments
This work was supported by the UNC MD/PhD program, T32GM008719 (AJK), the National Institute of Mental Health, F30 MH074266 (AJK), the National Heart, Lung, and Blood Institute, F30 HL094020 (CMW), and the National Institute on Deafness and other Communicative Disorders, T32DC005360 (GA).
Footnotes
Presentation: Poster at the 2014 Triological Combined Sections Meeting Spring Meeting in Miami, FL. Abst #202
Financial Disclosures: The authors have no other funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- 1.Evans HL, Batsakis JG. Polymorphous low-grade adenocarcinoma of minor salivary glands. A study of 14 cases of a distinctive neoplasm. Cancer. 1984;53:935–942. doi: 10.1002/1097-0142(19840215)53:4<935::aid-cncr2820530420>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Allen MS, Jr, Fitz-Hugh GS, Marsh WL., Jr Low-grade papillary adenocarcinoma of the palate. Cancer. 1974;33:153–158. doi: 10.1002/1097-0142(197401)33:1<153::aid-cncr2820330123>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Batsakis JG, Pinkston GR, Luna MA, Byers RM, Sciubba JJ, Tillery GW. Adenocarcinomas of the oral cavity: a clinicopathologic study of terminal duct carcinomas. J Laryngol Otol. 1983;97:825–835. doi: 10.1017/s0022215100095062. [DOI] [PubMed] [Google Scholar]
- 4.Freedman PD, Lumerman H. Lobular carcinoma of intraoral minor salivary gland origin. Report of twelve cases. Oral Surg Oral Med Oral Pathol. 1983;56:157–166. doi: 10.1016/0030-4220(83)90282-7. [DOI] [PubMed] [Google Scholar]
- 5.Seifert G, Brocheriou C, Cardesa A, Eveson JW. WHO International Histological Classification of Tumours. Tentative Histological Classification of Salivary Gland Tumours. Pathol Res Pract. 1990;186:555–581. doi: 10.1016/S0344-0338(11)80220-7. [DOI] [PubMed] [Google Scholar]
- 6.van Heerden WF, Raubenheimer EJ. Intraoral salivary gland neoplasms: a retrospective study of seventy cases in an African population. Oral Surg Oral Med Oral Pathol. 1991;71:579–582. doi: 10.1016/0030-4220(91)90366-k. [DOI] [PubMed] [Google Scholar]
- 7.Yih WY, Kratochvil FJ, Stewart JC. Intraoral minor salivary gland neoplasms: review of 213 cases. J Oral Maxillofac Surg. 2005;63:805–810. doi: 10.1016/j.joms.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Darling MR, Schneider JW, Phillips VM. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: a review and comparison of immunohistochemical markers. Oral oncology. 2002;38:641–645. doi: 10.1016/s1368-8375(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 9.Turk AT, Wenig BM. Pitfalls in the biopsy diagnosis of intraoral minor salivary gland neoplasms: diagnostic considerations and recommended approach. Advances in anatomic pathology. 2014;21:1–11. doi: 10.1097/PAP.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 10.Edwards PC, Bhuiya T, Kelsch RD. C-kit expression in the salivary gland neoplasms adenoid cystic carcinoma, polymorphous low-grade adenocarcinoma, and monomorphic adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:586–593. doi: 10.1067/moe.2003.31. [DOI] [PubMed] [Google Scholar]
- 11.Curran AE, Allen CM, Beck FM, Damm DD, Murrah VA. Distinctive pattern of glial fibrillary acidic protein immunoreactivity useful in distinguishing fragmented pleomorphic adenoma, canalicular adenoma and polymorphous low grade adenocarcinoma of minor salivary glands. Head Neck Pathol. 2007;1:27–32. doi: 10.1007/s12105-007-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson C, Krutchkoff D, Pedersen C, Cartun R, Berman M. Polymorphous low grade adenocarcinoma of minor salivary gland: a clinicopathologic and comparative immunohistochemical study. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1990;3:76–82. [PubMed] [Google Scholar]
- 13.Seethala RR, Johnson JT, Barnes EL, Myers EN. Polymorphous low-grade adenocarcinoma: the University of Pittsburgh experience. Arch Otolaryngol Head Neck Surg. 2010;136:385–392. doi: 10.1001/archoto.2010.39. [DOI] [PubMed] [Google Scholar]
- 14.Pogodzinski MS, Sabri AN, Lewis JE, Olsen KD. Retrospective study and review of polymorphous low-grade adenocarcinoma. Laryngoscope. 2006;116:2145–2149. doi: 10.1097/01.mlg.0000243200.35033.2b. [DOI] [PubMed] [Google Scholar]
- 15.Asioli S, Marucci G, Ficarra Get al. Polymorphous adenocarcinoma of the breast. Report of three cases. Virchows Arch. 2006;448:29–34. doi: 10.1007/s00428-005-0084-2. [DOI] [PubMed] [Google Scholar]
- 16.Evans HL, Luna MA. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24:1319–1328. doi: 10.1097/00000478-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Buchner A, Merrell PW, Carpenter WM. Relative frequency of intra-oral minor salivary gland tumors: a study of 380 cases from northern California and comparison to reports from other parts of the world. J Oral Pathol Med. 2007;36:207–214. doi: 10.1111/j.1600-0714.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- 18.Dhanuthai K, Boonadulyarat M, Jaengjongdee T, Jiruedee K. A clinico-pathologic study of 311 intra-oral salivary gland tumors in Thais. J Oral Pathol Med. 2009;38:495–500. doi: 10.1111/j.1600-0714.2009.00791.x. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi H, Fujita S, Tsuda N, Tezuka F, Okabe H. Intraoral minor salivary gland tumors: a demographic and histologic study of 200 cases. Tohoku J Exp Med. 1990;161:111–128. doi: 10.1620/tjem.161.111. [DOI] [PubMed] [Google Scholar]
- 20.Tian Z, Li L, Wang L, Hu Y, Li J. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg. 2010;39:235–242. doi: 10.1016/j.ijom.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Toida M, Shimokawa K, Makita Het al. Intraoral minor salivary gland tumors: a clinicopathological study of 82 cases. Int J Oral Maxillofac Surg. 2005;34:528–532. doi: 10.1016/j.ijom.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Venkata V, Irulandy P. The frequency and distribution pattern of minor salivary gland tumors in a government dental teaching hospital, Chennai, India. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:e32–e39. doi: 10.1016/j.tripleo.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Moore BA, Burkey BB, Netterville JL, Butcher RB, 2nd, Amedee RG. Surgical management of minor salivary gland neoplasms of the palate. Ochsner J. 2008;8:172–180. [PMC free article] [PubMed] [Google Scholar]
- 24.Gnepp DR, Chen JC, Warren C. Polymorphous low-grade adenocarcinoma of minor salivary gland. An immunohistochemical and clinicopathologic study. Am J Surg Pathol. 1988;12:461–468. doi: 10.1097/00000478-198806000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Crean SJ, Bryant C, Bennett J, Harris M. Four cases of polymorphous low-grade adenocarcinoma. Int J Oral Maxillofac Surg. 1996;25:40–44. doi: 10.1016/s0901-5027(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 26.Slootweg PJ, Muller H. Low-grade adenocarcinoma of the oral cavity. A comparison between the terminal duct and the papillary type. J Craniomaxillofac Surg. 1987;15:359–364. doi: 10.1016/s1010-5182(87)80083-5. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Garcia R, Rodriguez-Campo FJ, Munoz-Guerra MF, Nam-Cha SH, Sastre-Perez J, Naval-Gias L. Polymorphous low-grade adenocarcinoma of the palate: report of cases. Auris Nasus Larynx. 2005;32:275–280. doi: 10.1016/j.anl.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Hannen EJ, Bulten J, Festen J, Wienk SM, de Wilde PC. Polymorphous low grade adenocarcinoma with distant metastases and deletions on chromosome 6q23-qter and 11q23-qter: a case report. J Clin Pathol. 2000;53:942–945. doi: 10.1136/jcp.53.12.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monaco SE, Khalbuss WE, Ustinova E, Liang A, Cai G. The cytomorphologic spectrum of salivary gland type tumors in the lung and mediastinum: A report of 16 patients. Diagn Cytopathol. 2011 doi: 10.1002/dc.21733. [DOI] [PubMed] [Google Scholar]
- 30.Young S, Leon M, Talerman A, Teresi M, Emmadi R. Polymorphous low-grade adenocarcinoma of the vulva and vagina: a tumor resembling adenoid cystic carcinoma. Int J Surg Pathol. 2003;11:43–49. doi: 10.1177/106689690301100113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.