Abstract
The C-family (cbb3) of heme-copper oxygen reductases are proton-pumping enzymes terminating the aerobic respiratory chains of many bacteria, including a number of human pathogens. The most common form of these enzymes contains one copy each of 4 subunits encoded by the ccoNOQP operon. In the cbb3 from Rhodobacter capsulatus, the enzyme is assembled in a stepwise manner, with an essential role played by an assembly protein CcoH. Importantly, it has been proposed that a transient interaction between the transmembrane domains of CcoP and CcoH is essential for assembly. Here, we test this proposal by showing that a genetically engineered form of cbb3 from Vibrio cholerae (CcoNOQPX) that lacks the hydrophilic domain of CcoP, where the two heme c moieties are present, is fully assembled and stable. Single-turnover kinetics of the reaction between the fully reduced CcoNOQPX and O2 are essentially the same as the wild type enzyme in oxidizing the 4 remaining redox-active sites. The enzyme retains approximately 10% of the steady state oxidase activity using the artificial electron donor TMPD, but has no activity using the physiological electron donor cytochrome c4, since the docking site for this cytochrome is presumably located on the absent domain of CcoP. Residue E49 in the hydrophobic domain of CcoP is the entrance of the KC-channel for proton input, and the E49A mutation in the truncated enzyme further reduces the steady state activity to less than 3%. Hence, the same proton channel is used by both the wild type and truncated enzymes.
Keywords: oxygen reductase, membrane protein assembly, bioenergetics, Vibrio cholerae, cbb3
Introduction
The vast majority of aerobic respiration by both prokaryotic and eukaryotic organisms is catalyzed by enzymes that are part of the superfamily of heme-copper oxidoreductases. This superfamily includes both heme-copper O2 reductases (HCOs) as well as NO reductases (NORs). The HCOs are the terminal enzymes in the aerobic respiratory chains of most aerobic prokaryotes and virtually all eukaryotes. Of particular importance is that the HCOs are proton pumps that generate a proton motive force, thus conserving the substantial free energy available from the reaction in which O2 is converted to water.
The HCOs have been categorized to 3 major subfamilies based on structural and phylogenetic analysis (A-, B- and C-families)(1). The C-family HCOs, also known as cytochrome cbb3, are most often expressed in bacteria growing under conditions of low aeration. The bacteria in which cytochrome cbb3 plays a significant physiological role include a number of human pathogens, some of which rely solely on cytochrome cbb3 for aerobic respiration. These enzymes, although capable of pumping 4 H+/O2 (2), under some circumstances pump protons less efficiently in vivo. (3).
The C-family HCOs share one homologous subunit with other heme-copper O2 reductases (1, 4), often referred to as subunit I or, in the case of cytochrome cbb3, CcoN. As in all the HCOs, subunit I contains a low spin heme as well as the heme-copper binuclear active site where O2 binds and is reduced to water. In the case of the cbb3-type oxygen reductases (C-family enzymes), the low spin and high spin hemes are both heme B (protoheme IX). The active-site heme in all other HCOs (A-family and B-family) appears to be variants of either heme O or heme A. Subunit I in all HCOs contains channels leading to the heme-copper active site for the delivery of O2 and for protons (3). There is only one proton input channel for the C-family HCOs (the KC-channel)(4), whereas the A-family HCOs, which include the mitochondrial cytochrome aa3, have two proton-delivery channels (K-channel and D-channel) (3).
With one exception, all the biochemically characterized C-family HCOs have four subunits, CcoN, CcoO, CcoP and CcoQ, encoded by the ccoNOQP operon. These include the enzymes isolated from P. stutzeri (4, 5), R. sphaeroides (6), R. capsulatus (7), P. denitrificans (8), B. japonicum (9), R. marinus (10) and V. cholerae (11). The exception is one of the two cbb3-type HCOs from P. stutzeri (5), in which the CcoQ subunit is absent. The role of the CcoQ subunit is not clear, but it may be important for enzyme stability in at least some cases (12, 13). The X-ray structure of one of the variants of cytochrome cbb3 from P. stutzeri has been determined (4), showing how these subunits are arranged in the complex (Figure 1).
Figure 1. The structure of modified versions of cytochrome cbb3 from Vibrio cholerae.
(A) The ccoNOQP operon of V. cholerae and engineered variants used in this work. (B) The structures corresponding to the operons shown in Panel (A) based on the structure of the enzyme from P. stutzeri (PDB ID: 3MK7) (4). Subunit I (CcoN), subunit II (CcoO), subunit III (CcoP) and subunit IV (CcoQ) are shown in pink, green, blue and orange, respectively. CcoPX and CcoPSol in ribbon structure represent the transmembrane helices of the CcoP and the soluble domain of the CcoP subunit, respectively. It is assumed that CcoQ is an integral part of the complex, though this has not yet been demonstrated.
Whereas CcoN, with 12 transmembrane helices, is homologous to subunit I in the A- and B-family HCOs, the CcoO, CcoP and CcoQ subunits share no similarity with subunits found in A- or B-family HCOs. Subunits CcoO, CcoP and CcoQ each contain at least one but no more than two transmembrane helices. CcoO contains a hydrophilic domain with one heme c, which is the immediate electron donor to the low spin heme b. CcoP in most cases contains two hemes c, but in some enzymes one or three, which provide the “electron wire” directing electrons from periplasmic electron donors to the heme c in CcoO. CcoQ is not required for either assembly or catalytic activity of the cytochromes cbb3 from either R. sphaeroides (14) or from B. japonicum (15). However, CcoQ has been shown to be important for stabilizing the interaction between CcoP and the core complex CcoNO in the enzyme from R. capsulatus (12).
A number of bacterial genomes encode putative variants of cytochrome cbb3, which include only CcoN and CcoO, presumably representing a minimal 2-subunit core required for function (16). This is consistent with observations that subcomplexes of the cytochromes cbb3 from B. japonicum and from P. denitrificans that contain only CcoN and CcoO retain at least a small amount of TMPD oxidase activity (15, 17). On the other hand, the assembly of the CcoNOQP complex as an active enzyme requires the CcoP subunit (15, 18, 19).
Just downstream of the ccoNOQP operon in many bacterial genomes is a second gene cluster, ccoGHIS, which encodes proteins required for the assembly of the cytochrome cbb3 (7, 12, 15, 18, 20, 21). CcoG, CcoI and CcoS all play roles in the maturation of the CcoN subunit of cytochrome cbb3 (22), and are proposed to be either necessary for or assist in the insertion of the two hemes b (CcoS) (20) or CuB (CcoI, CcoG) (20–22). CcoH appears to be necessary for the assembly of the final active CcoNOQP complex from two pre-assembled complexes, CcoNO and CcoQP (18, 22). Crosslinking and immunoprecipitation studies show that CcoH interacts with CcoP primarily via interactions with the single transmembrane span of CcoH (23). A second interaction of CcoH with the CcoNO complex also has been shown (23) as well as an interaction with the mature CcoNOQP complex (5). Hence, it is possible that CcoH is an authentic subunit of the mature enzyme, but is readily lost upon purification (23). In the absence of CcoH, neither the active cytochrome cbb3 nor the CcoNO or CcoQP subcomplexes can be detected in the membranes of R. capsulatus (23).
The current work tests the model that the assembly of cytochrome cbb3 might only require interactions between CcoH with the transmembrane domain of CcoP by truncating the C-terminal hydrophilic domain of CcoP from the cytochrome cbb3 from Vibrio cholerae. The results confirm that the truncated CcoNOQPX is assembled and is stable, and retains about 10% of the TMPD oxidase activity of the wild type enzyme.
Material and Methods
Site-directed Mutagenesis and Purification of cbb3 Oxidase
The mutations were constructed using QuikChange site-directed mutagenesis kits from Stratagene. DNA oligonucleotides were synthesized at Integrated DNA Technologies. Sequence verification of the mutagenesis product was performed at the Biotechnology Center at the University of Illinois at Urbana-Champaign. The expression, purification, and characterization of the V. cholerae cbb3 wild type and mutants was performed as previously described (11, 33). Briefly, V. cholerae (0395N1) cells were grown at 37 °C and 220 rpm in LB media with 100 µg/L ampicillin (Fisher Biotech) and 100 µg/L streptomycin (Sigma). The expression of protein was induced with 0.2% L-(+)-arabinose (Sigma). The membranes were collected at 40000 rpm by ultracentrifugation from cell lysate and solubilized with 0.5% dodecyl β-d-maltoside (Anatrace). The protein was then purified using a Ni-NTA affinity column (Qiagen, CA).
Thermal stability measurements
Dynamic light scattering (DLS) techniques were applied to measure the size distribution (Z-average size) of enzymes while varying temperatures (DLS, Malvern Zetasizer Nano ZS90, Malvern instruments Ltd., U.K.). The Z-average size is a parameter also known as the cumulants mean as the harmonic intensity averaged particle diameter. Increase in Z-average size with DLS exhibits increase in the size of enzymes resulting from the formation of aggregate enzymes due to protein denaturation. 3 individual DLS Data points were collected at each 2.5°C increment between 4°C and 65°C, and the experiments were repeated twice for each sample. For the measurement, 5 μM of the cbb3 variants were prepared in 50 mM Sodium phosphate buffer, 100 mM NaCl and 0.05% dodecyl β-d-maltoside(DDM) at pH 8.0.
>Reconstitution of the V. cholerae cbb3 enzyme into phospholipid vesicles
Cytochrome c oxidase vesicles were produced by reconstituting the V. cholerae cbb3 into small unilamellar phospholipid vesicles, and detergent was removed by Bio-beads (BioRad) essentially as in (34). Asolectin (Sigma) (80mg/ml) was mixed with 2% cholic acid in 100 mM Hepes at pH 7.4 and then sonicated using a model W-375 sonicator (Heat Systems-Ultrasonics, Inc.) on ice under a stream of Argon gas. The cbb3 oxidase was added to the sonicated lipid/cholate mixture to a final concentration of 1 µM. Bio-beads (66 mg/mL) were added to the enzyme mixture every 30min for 4 hours at 4 °C with agitation. After adding 100mM Hepes buffer (0.5 mL per mL of mix), bio-beads were added every 30 minutes for another 3 hours (133mg/mL during the first two hours and 266 mg/mL during the last hour) at room temperature. The proteoliposomes were dialyzed overnight against 60mM KCl.
Isolation and Characterization of soluble CcoP (CcoPsol)
To amplify the periplasmic domain of ccoP gene (141–326 aa), forward and reverse primers were designed to introduce HindIII and BamHI sites, respectively, which facilitated cloning into the expression vector pET-17b to yield a new construct, pETsolCcoP(Apr): 5’-CCCAAGCTTCAGACCACTAACTTACGCGAAATCC-3’ (forward primer) and 5’-CGGGATCCTTACTTATTCTCTGAGTTGCTTAAGCTC-3’ (reverse primer). The modified signal peptide of cytochrome c550 from Thiobacillus versutus was introduced before N-terminal of the operon ccoPsol and expressed successfully in E. coli BL21(DE3), which also contains pEC86(Cmr), yielding a soluble CcoP. DNA sequencing (Biotechnology Center at the University of Illinois at Urbana Champaign) confirmed that the insert was identical in sequence to the periplasmic domain of CcoP. Condition of cell growth and enzyme expression were previously described (26). The cells were grown in 1L of LB medium, supplemented with 100 μg/ml ampicillin and 30 μg/ml chloramphenicol, in a 2.8 L Fernbach flask at 37 °C and 100 rpm, and the gene expression is induced with 1mM ITPG when OD600 is about 0.6–1.0. The cells were harvested at 4 °C by centrifugation at 8,000×g for 10 min. The soluble extract was prepared as previously described in (26). Crude extract was loaded onto CM-52 cellulose column (Whatman) preequilibrated with 25mM Tris-HCl buffer (pH 7.5) and the adsorbed proteins from column were eluted with linear gradient of NaCl from 0 to 1 M. Partially purified protein fractions were purified further by the DEAE-Sepharose column as described above. Purified fractions of the CcoPsol were concentrated (200–300 uM) using concentrator (Amicon) with YM-10 membranes and stored at −80°C after flash-frozen in liquid nitrogen.
Steady-state Activity
Steady-state activity was measured with a YSI model 53 oxygen monitor. For the TMPD oxidase activity, the reaction mixture contained buffer (50 mM Sodium phosphate, 100 mM NaCl and 0.05% DDM at pH 6.5), 10 mM ascorbate and 500 μM TMPD as previously described (25). The pH dependence of the TMPD enzyme activity displays only data between pH 6.5 and pH 9.0 since below pH 6.5 the enzyme activity was decreased. For the measurement of cytochrome c enzyme activity, the cytochrome cbb3 wild type and the NOQPX were tested respectively using 20 mM ascorbate in combination with either 50 μM of the V. cholerae cytochrome c4 or 50 μM of the CcoPsol under the same buffer conditions as for the TMPD enzyme activity assay.
Resonance Raman spectroscopy
The resonance Raman spectra were obtained as previously described (35). Briefly, the 413.1 nm excitation from a Kr ion laser (Spectra-Physics, Mountain View, CA) was focused to a ∼30 μm spot on the spinning quartz cell rotating at ∼ 1000 rpm. The scattered light, collected at a right angle to the incident laser beam, was focused on the 100 μm-wide entrance slit of a 1.25 m Spex spectrometer equipped with a 1200 grooves/mm grating (Bausch & Lomb, Analytical Systems Division, Rochester, NY), where it was dispersed and then detected by a liquid nitrogen-cooled CCD detector (Princeton Instruments, Trenton, NJ). A holographic notch filter (Kaiser Optical Systems, Ann Arbor, MI) was used to remove the laser line. The Raman shifts were calibrated with indene. The concentration of enzyme was 50 μM. The laser powers used for all Raman measurements are indicated in the captions.
CO-Flash-Photolysis
The enzyme samples were prepared as previously described (25). Briefly, the enzyme at a concentration of ∼5 μM was transferred to a modified anaerobic cuvette and the atmosphere was exchanged to N2 on a vacuum line. The enzyme was completely reduced by addition of 2 mM ascorbate and 2 μM phenazine methosulphate (PMS). Then, N2 was exchanged for CO so that anaerobic incubation in CO results in formation of fully reduced CO-bound cbb3 oxidase. The CO-ligand was photolyzed by a 10 ns laser flash at 532 nm (Brilliant B, Quantel), followed by detection of absorbance changes by an apparatus from Applied Photophysics (36).
Flow-Flash measurements
Flow-flash Measurements were performed using a modified stopped-flow apparatus (Applied Photophysics, Surrey, U.K.) and data analyzed as described in (24, 38). The enzyme solution (50 mM Hepes, 50 mM KCl, 0.03% DDM and 50 μM EDTA at pH 7.4) was rapidly mixed in a 1:5 ratio with an O2-saturated buffer, and the reaction with O2 was initiated by flash photolysis of the CO-enzyme complex approximately 200 ms after mixing. The kinetics was monitored optically as absorbance differences at single wavelengths.
Results
Figure 1 shows the gene constructs that were examined in this work. The wild type enzyme from V. cholerae is encoded by the ccoNOQP operon (Figure 1A) which yields the 4-subunit CcoNOQP cytochrome cbb3 (Figure 1B). For the current work, all the constructs included a His-tag at the C-terminus of CcoN to facilitate purification. The CcoP subunit has two transmembrane helices and large hydrophilic domain which lies on the top of the enzyme (facing the periplasm). CcoO has a single transmembrane domain and a hydrophilic domain that is sandwiched between CcoN and CcoP. When the ccoP gene is deleted from the operon, expression of the remaining ccoNOQ does not result in any detectable enzyme in the membranes of V. cholerae (Table 1). The hydrophilic domain of CcoP is connected to the transmembrane helices by a long helical linker from Q89 to A136 (4). Stop codons were inserted either just after Q89 or after Q109, and the operons ccoNOQP89X and ccoNOQP109X were expressed. Each yielded truncated variants of cytochrome cbb3 in yields comparable to the wild type enzyme, and the truncated enzymes, CcoNOQP89X and CcoNOQP109X, were stable and could be purified and examined (Table 1). Since the two truncated versions appeared to be identical, only the version truncated after Q89 was examined in detail. This will be referred to either as the truncated enzyme or CcoNOQPX. It is evident that the hydrophilic domain of CcoP is not required for the assembly or stability of cytochrome cbb3.
Table 1.
Comparison of the properties of the wild type and mutant variants of cytochrome cbb3 from V. cholerae
| Mutants | Turnover % (e−/sec cbb3) |
Assembly |
|---|---|---|
| WT | 100 (200e-/sec) | + |
| E49III A* | 10 | + |
| CcoNOQP89X (his I) | 9 | + |
| CcoNOQP89X (hisIII ) | 15 | + |
| CcoNOQP109X (his I) | 8 | + |
| CcoNOQP89X -E49III A | <3 | + |
| CcoNO | N/D | − |
All residue numbers are for cytochrome cbb3 from V. cholerae. The superscripts indicate mutations in Subunit I (CcoN) or Subunit III (CcoP); “his”, histidine tag; “*”, previously reported in (25). The assembly of the cytochrome cbb3 are indicated as “+”, normal assembly; “-” no enzyme complex obtained after purification; “ND”, not determined.
Subunit and heme analysis
Figure 2 shows the SDS-PAGE analysis of the purified wild type and CcoNOQPX. When stained with Coomassie blue, CcoN, CcoO and CcoP are apparent in the wild type enzyme at 57 kDa, 23 kDa and 34 kDa (Figure 2A). The small subunit CcoQ is not observed. In contrast, the band corresponding to CcoP is missing from the truncated version of the enzyme. Similarly, when the bands are visualized using a stain for the covalently bound heme c, both CcoO and CcoP are evident in the lane with the wild type enzyme, but only CcoO is in the lane with the truncated enzyme (Figure 2B). The remaining transmembrane fragment is expected to be present in the CcoNOQPX enzyme having a molecular weight of 9.7 kDa and is not observed in the gel. In order to verify the presence of the small, hydrophobic fragment of CcoP89X in the preparation of CcoNOQPX, the His-tag was moved from subunit CcoN to the C-terminus of CcoPX, following residue W88. The CcoNOQP89His enzyme was expressed and purified using the Ni-affinity resin (Table 1). The isolated enzyme contained CcoO and CcoN, demonstrating that the transmembrane domain of the CcoPX remains attached to the truncated cytochrome cbb3.
Figure 2. Heme analysis of the wild type cytochrome cbb3 and the truncated CcoNOQPX.
(A) SDS-PAGE gel stained with Coomassie Brilliant Blue (B) SDS PAGE gel stained with 3,3’, 5,5’-tetramethyl benzidine (TMBZ) and H2O2 to identify a covalently bound heme c. Each lane contains ∼10 μg of protein. In each panel, the lane 1, 2 and 3 contains molecular weight markers, the wild type, and the truncated CcoNOQPX enzymes, respectively (C) UV-visible spectra of the truncated CcoNOQPX, showing the air-oxidized, dithionite-reduced and dithionite-reduced plus CO forms. The inset shows the enlarged spectra in the α- and β-bands region. The buffer used was 50 mM sodium phosphate, 100 mM NaCl and 0.05% DDM at pH 8.0. (D) UV-visible spectra of the dithionite-reduced CO-bound minus dithionite-reduced form of the truncated CcoNOQPX compared to the wild type. Experimental conditions: 50 mM Hepes, 50 mM KCl, 0.03% DDM, 3 μM PMS, 3 mM ascorbate, ∼100 μM dithionite and 1 mM CO at pH 7.4 and 298K.
A pyridine hemochrome analysis was performed on the preparations of both the wild type and truncated enzyme to quantify the heme contents. The results showed the expected heme b:heme c ratio of 2:3 for the wild type enzyme, and a heme b:heme c ratio of 2:1 for the truncated cytochrome cbb3. The data indicate the absence of the hemes c in CcoP due to the truncation, consistent with the lack of CO binding to the hemes c in CcoP shown in the UV-visible heme spectra of the truncated enzyme (see Figure 2C and 2D).
Thermal stability
Dynamic light scattering (DLS) was used to monitor thermal denaturation of the proteins as the temperature was increased from 4 °C to 65 °C. Protein denaturation results in aggregation which is observed by DLS as an increase in the “Z-average” parameter. Figure 3 displays that the Z-average starts to increase at ∼42°C for both the wild type and CcoNOQPX indicating that the thermal stability of the truncated cytochrome cbb3 is similar to that of the wild type.
Figure 3. Thermal stability of the truncated enzyme (CcoNOQPX) shown in red, compared to the wild type cytochrome cbb3, shown in black.
The size distribution profile (Z-average size) of enzymes was determined using Dynamic light scattering (DLS) technique while varying the temperature. The traces have been shown from 25 to 57 °C for better comparison.
Steady state kinetics
The steady state oxygen reductase assay was performed using the artificial electron donor TMPD. The wild type enzyme has a turnover of about 200 e−/sec and the CcoNOQPX enzyme retains about 9% of this activity (18 e−/sec) (Table 1). TMPD is often used as a reductant for cytochrome c-dependent enzymes, and the truncated cytochrome cbb3 still retains a cytochrome c in the remaining CcoO subunit. The low activity is likely due to the fact that the heme c in the CcoO subunit is buried within the subunit and not readily accessed by the TMPD molecules in solution. The activity of the truncated enzyme (CcoNOQP89His) isolated by the His-tag located at the C-terminus of CcoPX has higher specific activity, about 15% of the wild type value (Table 1), suggesting that perhaps there is a fraction of the preparation of the truncated enzyme isolated by the His-tag on CcoN which is inactive due to the loss of the transmembrane helices of CcoP subunit from the truncated CcoNOQPX.
The KC-proton channel that is required to deliver protons to the active site for catalytic function has its entrance, E49, within the remaining hydrophobic domain of CcoPX (24, 25). Mutation of this residue (E49A) in the full-length V. cholerae cytochrome cbb3 reduces the TMPD oxidase activity to 10% of the wild type value (24, 25). The same mutation, E49A, in the CcoNOQPX variant reduces the activity from 9% to less than 3% of the wild type activity (Table 1). The data suggest that the proton input to the active site in the truncated CcoNOQPX is the same used by the wild type enzyme.
The E49A mutant has a shifted pH-dependence of the TMPD oxidase activity (Figure 4), with a maximum at about pH 7.4, whereas the activity of the wild type enzyme increases at lower pH values. The pH-dependence of the activity of the truncated enzyme is similar to that of the wild type, consistent with the truncated enzyme using the same proton delivery channel as the wild type.
Figure 4. pH dependence of the steady-state oxygen reductase activity of the wild type cytochrome cbb3 the truncated CcoNOQPX variant and the E49IIIA mutant of the full length enzyme.
The assays, using TMPD as the electron donor, were performed as described in the text.
The delivery of protons to the active site of the enzyme through the KC-channel leads to the expectation that the truncated cytochrome cbb3 should generate a voltage across the membrane during catalytic turnover. This is due to the fact that electrons are provided from the periplasmic side of the membrane (from TMPD via CcoO for the truncated enzyme) whereas protons are from the opposite side of the membrane, corresponding to the cytoplasm in vivo. This prediction was tested by measuring the TMPD oxidase activity of the wild type and truncated enzymes reconstituted in proteoliposomes. This should generate a transmembrane voltage (proton motive force) that will act to slow the rate of enzyme catalysis. Addition of a protonophore will collapse the proton motive force and, therefore, result in increased enzymatic activity. The activities of the enzymes in proteoliposomes were measured in the absence (controlled activity) or presence (uncontrolled activity) of a protonophore. The steady state activity increased in the presence of a protonophore for both the wild type and truncated enzymes (Table 2). The ratio of uncontrolled/controlled activity, known as the respiratory control ratio (RCR), is about 4 for the wild type enzyme and 2.7 for the CcoNOQPX enzyme. Hence, catalysis by the truncated enzyme generates a charge separation across the bilayer.
Table 2.
Comparison of O2 reduction activities and respiratory control ratio (RCR) of the wild type and mutant enzymes before and after reconstitution into phospholipid vesicles.
| activity (e− sec−1cbb3−1) |
||||
|---|---|---|---|---|
| In vesicles |
||||
| Cbb3 | in detergent | controlled | uncontrolled | RCR |
| WT | 198±8 (100%) | 17 | 62 | 3.9 |
| CcoNOQP89X (hisI) | 18±2 (9%) | 8 | 21 | 2.7 |
| E49IIIA | 20±2 (10%) | 12 | 24 | 2.2 |
Expression of the hydrophilic domain of CcoP and its efficiency as an electron donor to the truncated cytochrome cbb3
Using the same protocol used for the expression of V. cholerae cytochrome c4 (26), the hydrophilic domain of CcoP (denoted CcoPsol) was expressed (Figure 5). CcoPsol was reduced and tested as a reductant for steady state activity with the truncated cytochrome cbb3. No oxygen reductase activity was observed using concentrations of CcoPsol up to 50 μM (Figure 6), suggesting that the binding affinity of the hydrophilic domain of CcoP is very low and the majority of the interaction free energy between CcoP and the CcoNO complex must be mediated through the membrane domains. Furthermore, the truncated enzyme exhibits no activity using the natural electron donor, the reduced cytochrome c4, either in the presence or the absence of CcoPsol (Figure 6). Not surprisingly, the periplasmic domain of CcoP is necessary for the functional interaction of cytochrome c4.
Figure 5. Characteristics of the purified CcoPsol.
(A) SDS-PAGE gel stained with Coomassie Brilliant Blue (B): SDS PAGE gel stained with 3,3’, 5,5’-tetramethyl benzidine (TMBZ) and H2O2 to identify a covalently bound heme c. Lane assignment: M, molecular weight markers; Lane 1, CcoPsol (C) UV-visible spectra of the ferricyanide-oxidized (dotted line) and dithionite-reduced (solid line) forms of CcoPsol from V. cholerae. The buffer used was 50mM sodium phosphate and 100 mM NaCl at pH 8.0.
Figure 6. Comparison of the steady-state oxygen reductase activity of the wild type cytochrome cbb3 and the CcoNOQPX on various substrates.
The enzyme activity of the truncated cbb3 was compared to that of wild type cbb3 on various substrates, 0.5 mM TMPD, 50 μM cytochrome c4 and 50 μM CcoPSol. Experimental conditions: 50mM Sodium phosphate, 100mM NaCl, 10∼20 mM ascorbate and 0.05% DDM at pH 6.5 and 25°C.
Spectroscopic characterization of the hemes
After verifying the normal subunit assembly and stability of the native and truncated enzymes, the hemes of the enzymes were examined by spectroscopic analysis. Figure 2C shows the UV-visible spectra of the air-oxidized, the dithionite-reduced and CO-bound forms of the truncated enzyme. There is a small but reproducible red shift in the Soret band of the oxidized form of the truncated enzyme from 411 nm to 413 nm compared to the wild type. This is likely due to the absence of the two heme c’s from CcoP. The reduced form of the truncated enzyme shows smaller 550/560 ratio compared to the wild type, also consistent with the lack of the heme c components in CcoP (Figure 2C). Figure 2D shows the UV-visible spectra of CO-bound minus dithionite-reduced form of the truncated enzyme along with wild type. There are distinct differences between the wild type and the truncated enzyme in the absorption of the reduced CO derivative. The observed changes at 559 nm but not at 551 nm in the truncated enzyme compared to the wild type suggest CO binding to the truncated enzyme at the heme b3 active site but not to the remaining heme c. The difference between these spectra is accounted for by realizing that in the wild type cbb3 from P. stutzeri, CO has been previously shown to bind to both heme b3 and to one of the heme c components of CcoP (27, 28). Since the truncated enzyme lacks the heme c components of CcoP, it is not surprising that the spectroscopic features of CO binding to heme c are absent in the CO-bound minus reduced difference spectrum of the truncated enzyme.
The hemes were further characterized by resonance Raman spectroscopy. The oxidized truncated enzyme is substantially photoreduced when exposed to the laser beam during resonance Raman measurements to a much greater extent than the oxidized wild type enzyme. This is shown by the presence of a large contribution at 1363 cm−1 in the spectrum from the truncated enzyme (Spectrum a in Figure 7B) compared to the weaker contribution in the wild type enzyme (Spectrum b in Figure 7B). The 1362–1363 cm−1 band shown in spectra e and f in Figure 7B originates from the fraction of the enzyme that has been reduced. For both the wild type and truncated enzymes, subtraction of the appropriate amount of the spectrum of the reduced enzyme from that of the oxidized enzyme so as to eliminate the band at 1363 cm−1 reveals nearly identical spectra corresponding to that of the fully oxidized enzymes (Spectra c and d in Figure 7B).
Figure 7. Resonance Raman spectra of the oxidized (a), reduced (e) and reduced CO-bound (g) truncated enzyme, CcoNOQPX, compared to those of the wild type cytochrome cbb3 (Spectra b, f and h) in the low frequency region (A) and the high frequency region (B).
Spectra c and d are the difference spectra with respect to the reduced forms of the truncated enzyme (e) and the wild type (f), respectively. (C) Resonance Raman difference spectrum (Spectrum a) of the CO-bound wild type enzyme minus its reduced form, compared to the difference spectrum (Spectrum c) of the CO-bound truncated enzyme minus its reduced form. The reduced five-coordinate heme b32+(5C) is absent from the difference spectra because it is not enhanced with the 413.1 nm excitation and the reduced hemes c and the low spin heme b cancel out in the a and c difference spectra leaving only the spectra of the CO-bound species, which are assigned as the heme b3-CO adduct in Spectrum c but in Spectrum a in addition to the heme b3-CO another species is present. When the heme b3-CO spectrum (Spectrum c) is subtracted from Spectrum a, Spectrum b is formed, which is assigned as the spectrum of a heme c-CO species. The laser power at the samples was ∼ 7 mW for the oxidized and reduced forms and ∼1.5 mW for the CO adducts.
Spectra e and f in Figure 7A show the low frequency spectra of the reduced enzymes. The 676 cm−1 line is assigned to heme b in the reduced and oxidized spectra and the lines at 686 and 694 cm−1 are assigned to heme c in the reduced and oxidized spectra, respectively. The band at 686 cm−1 is stronger in the spectrum of the reduced wild type enzyme (Spectrum f versus Spectrum e) due to the presence of the two additional heme c units. The relatively large contribution from the line at 694 cm−1 in the spectrum of the oxidized truncated enzyme (Spectrum a) suggests that the photoreduction occurs primarily in the heme c moieties rather than in the heme b. The greater photoreduction in the truncated enzyme may indicate a higher redox potential of the remaining heme c in CcoO, consistent with the slower oxidation of heme c observed in the single-turnover experiments with the truncated enzyme (see Figure 9A and 9C).
Figure 9. Absorbance changes monitoring the reaction of O2 with the fully reduced wild type cytochromecbb3 and the CcoNOQPX variant.
The absorbance changes were monitored at (A) 420 nm, (B) 430 nm, (C) 550 nm and (D) 560 nm. The absorbance decrease at 420 nm (A) and 550 nm (C) is associated with the oxidation of heme c, while the absorbance decrease at 430 nm (B) and 560 nm (D) is mainly due to the oxidation of low spin heme b. The inset in C shows the first 3 milliseconds of the changes at 550 nm with the amplitudes arbitrarily normalized in order to emphasize the difference in rate constant. The solutions contained 1 to 2 μM of cytochrome cbb3, 50 mM Hepes, 50 mM KCl, 0.03% DDM and 1 mM O2 at pH 7.4 and 298K. Results with the wild type and the CcoNOQPX variant are shown in black and in red, respectively. A laser artifact at t=0 has been truncated for clarity.
In addition to vibrational modes of the porphyrin macrocycles in the resonance Raman bands in the 200–800 cm−1 range, modes associated with Fe-ligand motions along the axis normal to the heme are also present. The CO adducts of heme proteins exhibit Fe-C-O vibrations in the 400–600 cm−1 region. Spectra g and h show the CO-bound forms of the dithionite-reduced enzymes in Figure 7A and 7B, for the low- and high-frequency regions, respectively. In order to determine the spectra of the isolated components we have subtracted the spectra of the dithionite-reduced enzyme from those of the CO-bound, dithionite-reduced forms (Figure 7C). Note that in the truncated enzyme, if CO only coordinates to the heme b3 moiety, this should represent c2+b2+b3-CO minus c2+b2+b32+ (5C), where the b32+ (5C) designates the five-coordinate reduced heme b3. The contributions from the reduced heme c2+ and heme b2+ cancel out of the difference spectra leaving a spectrum of b3-CO minus b32+(5C) but the b32+(5C) makes no contribution to the difference spectrum as it is not enhanced with the 413.1 nm excitation. This results in spectrum c in Figure 7C which we assign as that of pure b3-CO. The difference spectra of the wild type enzyme (Spectrum a in Figure 7C), which should be represented as c2+c2+c2+b2+b3-CO minus c2+c2+c2+b2+b32+ (5C), are quite different from the spectra of the truncated enzyme. This is due to CO binding to the heme c moieties of CcoP, consistent with the optical absorption data (Figure 2D) and the data reported previously (27, 28). By subtracting the Spectrum c, the b3-CO spectrum, from Spectrum a, we obtained Spectrum b, which we assign as the pure spectrum of heme c2+-CO. This fully accounts for the observed spectroscopic differences between spectra a and c in Figure 7C.
CO recombination kinetics
One test of the integrity of the heme b3/CuB active site of the truncated enzyme is to determine the kinetics of recombination of CO following photolysis of CO bound to the active site of the fully reduced enzyme. Monitoring the photolysis/rebinding process at 430 nm includes CO binding to heme b3 component of the enzyme. Figure 8 shows that the wild type and truncated variations of the dithionite-reduced enzyme rebind to CO to a similar extent and with similar kinetics. The rate of CO recombination to heme b3 (seen as the phase with positive amplitude on the millisecond time-scale in the figure) is the same for the wild type and the truncated variants; ∼450 s−1 (Figure 8A) suggesting that the truncation does not greatly perturb the rate of CO rebinding to the active site after being photolytically expelled into solution. The results indicate no substantial conformational change around the active site due to the truncation.
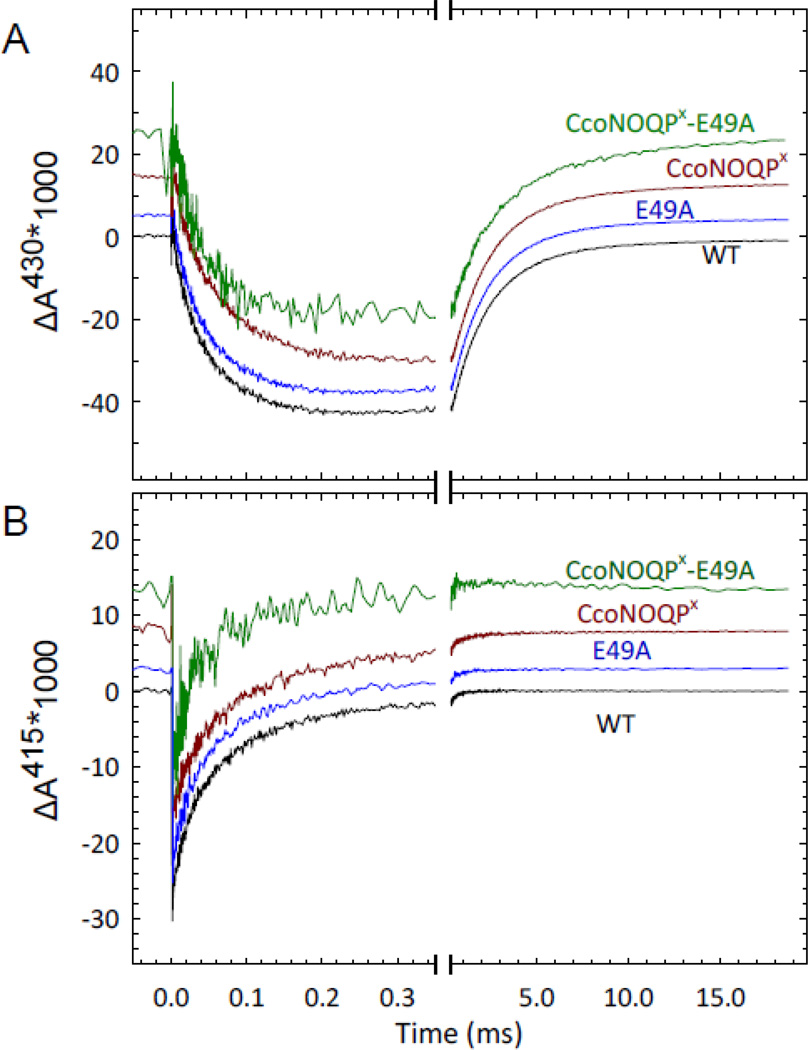
Figure 8. CO-recombination kinetics following flash photolysis of the fully reduced wild type cytochromecbb3, the E49IIIA, CcoNOQPX and CcoNOQPX- E49IIIA mutants, monitored at 430 nm (A) and at 415 nm (B).
(A) CO rebinding to hemes c and b3 is monitored at 430 nm where both processes contribute. The rapid decrease and subsequent slow increase in absorbance correspond to CO recombination to the heme c and to the heme b components of the enzyme, respectively. (B) CO rebinding monitored at 415 nm mostly shows CO binding to a heme c component. Experimental conditions: 5 μM enzyme, 50 mM Hepes, 50 mM KCl, 0.03% DDM, 3 μM PMS, 3 mM ascorbate, ∼100 μM dithionite and 1 mM CO at pH 7.4 and 298K. The traces have been normalized to the same absorbance change at 0.3 ms and then spaced for better comparison. A laser artifact at t=0 has been truncated for clarity.
The CO recombination kinetics data were complicated by the presence of a second component (the rapid phase with negative amplitude in Figure 8A). This has been previously observed with the wild type enzymes from both P. stutzeri and R. sphaeroides and shown to be due to CO rebinding to one of the heme c components of CcoP (27, 28). The surprising presence of the second kinetic component of CO recombination in the truncated enzyme (Figure 8A and B) cannot be due to flash-induced dissociation of CO from the remaining heme c in CcoO, since the resonance Raman spectroscopy (Figure 7C) and the UV-visible spectroscopy (Figure 2D) show no evidence of CO binding to heme c. This kinetic feature is instead likely due to photodissociation of a labile axial ligand from heme c in CcoO, which is followed by either rebinding of the endogenous ligand or CO. Flash-induced dissociation of intrinsic ligands and competition with extrinsic ligand (CO, O2) binding has been reported for other heme proteins (29–31). The details of the origin of the rapid CO-binding phase in both wild type and truncated cbb3 will be investigated further separately as it is out of the scope of this study.
Single-turnover reaction of the fully reduced enzyme with O2
To further evaluate the properties of the truncated cytochrome cbb3, the kinetics of the reaction of the fully reduced enzyme with O2 was studied using the flow-flash technique. Note that the fully reduced wild type cbb3 contains six available electrons, whereas the truncation contains four, so we expect different end products of the reaction. The end product in the wild type cbb3 is not fully understood (see ref 37), but is presumed to be a partly re-reduced intermediate. In this experiment, the CO-adduct of the fully reduced enzyme is rapidly mixed with O2 saturated buffer and then, prior to thermal dissociation of CO, the CO is expelled from the active site by photolysis. This allows O2 to bind to the active site, where it is reduced and the hemes are oxidized. Heme b is monitored at 430 nm and 560 nm whereas heme c is monitored at 420 nm and 550 nm. The results are shown in Figure 9.
For the wild type enzyme, the results show that both hemes b and hemes c are oxidized in a single phase with time constant τ ≈ 0.3 ms (k ≈ 3300 s−1)(25). This is shown for both hemes b (Figure 9B and D) and hemes c (Figure 9A and C). The rate of oxidation of the hemes b in the truncated enzyme is similar to that of the wild type, τ ≈ 0.3 ms (25) (Figure 9D), but the heme c component of the truncated enzyme is more slowly oxidized, with a time constant of τ ≈ 1 ms (k ≈ 1000 s−1) (Figure 9C+ inset). The amplitude of the τ ≈ 1 ms phase is significantly smaller for the truncated enzyme at 420 nm and 550 nm than for the τ ≈ 0.3 ms in wild type, consistent with only one instead of two or three hemes c oxidizing.
Discussion
The current work definitively shows that the final assembly of cytochrome cbb3, which is the addition of the CcoP subunit to the pre-assembled CcoNO “core” (15, 18), does not require the hydrophilic diheme domain of CcoP. The first 88 amino acids of CcoP from the V. cholerae enzyme are sufficient for the final assembly. These data complement the previous observation that the transmembrane portion of the assembly factor CcoH is required for the interaction with CcoQP and plays a critical part in the postulated stepwise assembly of the active enzyme (22, 23). The stepwise model of assembly proposes that 1) the pre-assembled CcoNO and CcoQP subcomplexes each bind to and are stabilized by the CcoH assembly protein, forming CcoNOH and CcoQPH, and then 2) the two sub-complexes are brought together initially by interactions between the CcoH subunits in each sub-complex, forming CcoNO(H)2QP. Loss of CcoH, possibly during purification, yields the final CcoNOQP active enzyme. The current results are also consistent with data from the Daldal laboratory (32) showing that the CcoN subunit could be assembled even in the absence of a fully functional cytochrome c maturation process and is independent of the c-type cytochromes.
Remarkably, the truncated CcoNOQPX cytochrome cbb3 is expressed in amounts similar to the wild type enzyme, is as stable as the wild type after purification, and retains about 9% of the steady state oxygen reductase activity of the wild type enzyme using TMPD as the electron donor (Table 1, Figure 4). The absence of the hydrophilic domain of CcoP eliminates the docking site for the physiological electron donor, reduced cytochrome c4, which therefore does not act as a reductant for the truncated enzyme (Figure 6). Furthermore, the one remaining cytochrome c in the CcoO subunit of the truncated enzyme, is not readily accessible from solution (4) and is not rapidly reduced by the artificial electron donor TMPD. The slow steady state activity of the enzyme (about 18 s−1) is likely mostly due to the slow reaction of TMPD with the truncated enzyme. This is confirmed by the rapid reaction of the fully reduced truncated enzyme with O2, indicating that steps during the catalytic cycle other than those observed during this single-turnover experiment must be rate limiting during steady state reaction of the truncated enzyme.
Other than the slow kinetics of reduction of the enzyme during steady state catalysis due to the absence of the heme c components of CcoP, the truncation has only very subtle functional consequences. The remaining heme c associated with CcoO is more readily photoreduced (Figure 7), and one of the axial ligands appears to be more labile upon flash photolysis (Figure 8). It appears that the midpoint potential of this heme c is increased by removal of the hydrophilic domain of CcoP.
In summary, the current work shows that the hydrophobic domain of CcoP is not only essential for rapid proton uptake through the KC-channel during catalysis, but is required for both the assembly and stability of cytochrome cbb3. The CcoH protein requires the hydrophobic domain of CcoP for the final assembly of the enzyme. The hydrophilic domain of CcoP, in contrast, though it is required for electron transfer from the physiological electron donor, is not required either for the assembly or stability of cytochrome cbb3.
Highlights.
Hydrophobic domain of CcoP is sufficient for assembly of cytochrome cbb3
Assembly of cytochrome cbb3 does not require pre-assembly of the heme c’s in CcoP
Cytochrome cbb3 without the hydrophilic domain of CcoP retains 10% activity
Role of CcoP includes both proton entry and electron entry to cytochrome cbb3
ACKNOWLEDGMENTS
We thank Dr. Ashtamurthy Pawate for help and guidance during the DLS measurements. This work was supported by grants from the National Institutes of Health grants GM098799 to D.L.R., GM086482 to S.-R.Y. and HL16101 to R.B.G, and by grants from the Swedish Research council and the Faculty of Science at Stockholm University to PÄ
This work was supported by grants from the National Institutes of Health grants GM098799 to D.L.R., GM086482 to S.-R.Y. and HL16101 to R.B.G, and by grants from the Swedish Research council and the Faculty of Science at Stockholm University to PÄ
Abbreviations
- TMPD
N,N,N’,N’-tetramethyl-p-phenylenediamine
- PMS
phenazine methosulphate
- HCO
heme-copper oxygen reductase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sousa FL, et al. The superfamily of heme-copper oxygen reductases: Types and evolutionary considerations. Biochimica et Biophysica Acta (BBA) -Bioenergetics. 2012;1817(4):629–637. doi: 10.1016/j.bbabio.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Rauhamäki V, Bloch DA, Wikström M. Mechanistic stoichiometry of proton translocation by cytochrome cbb3. Proceedings of the National Academy of Sciences. 2012;109(19):7286–7291. doi: 10.1073/pnas.1202151109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han H, et al. Adaptation of aerobic respiration to low O2 environments. Proc Natl Acad Sci U S A. 2011;108(34):14109–14114. doi: 10.1073/pnas.1018958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buschmann S, et al. The structure of cbb3 cytochrome oxidase provides insights into proton pumping. Science. 2010;329(5989):327–330. doi: 10.1126/science.1187303. [DOI] [PubMed] [Google Scholar]
- 5.Xie H, Buschmann S, Langer JD, Ludwig B, Michel H. Biochemical and biophysical characterization of the two isoforms of cbb3-type cytochrome c oxidase from Pseudomonas stutzeri. J Bacteriol. 2014;196(2):472–482. doi: 10.1128/JB.01072-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toledo-Cuevas M, Barquera B, Gennis RB, Wikström M, García-Horsman JA. The cbb3-type Cytochrome c Oxidase from Rhodobacter sphaeroides, a Proton-pumping Heme-copper Oxidase. Biochim. Biophys. Acta. 1998;1365:421–434. doi: 10.1016/s0005-2728(98)00095-4. [DOI] [PubMed] [Google Scholar]
- 7.Koch H-G, Hwang O, Daldal F. Isolation and Characterization of Rhodobacter capsulatus Mutants Affected in Cytochrome cbb3 Oxidase Activity. J. of Bacteriology. 1998;180(4):969–978. doi: 10.1128/jb.180.4.969-978.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raitio M, Wikström M. An Alternative Cytochrome Oxidase of Paracoccus Denitrificans Functions as a Proton Pump. Biochim. Biophys. Acta. 1994;1186:100–106. [Google Scholar]
- 9.Preisig O, Zufferey R, Thony-Meyer L, Appleby CA, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178(6):1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira MM, Carita JN, Anglin R, Saraste M, Teixeira M. Heme Centers of Rhodothermus marinus Respiratory Chain. Characterization of Its cbb3 Oxidase. J. Bioenerg. Biomemb. 2000;32(2):143–152. doi: 10.1023/a:1005555829301. [DOI] [PubMed] [Google Scholar]
- 11.Hemp J, Christian C, Barquera B, Gennis RB, Martinez TJ. Helix Switching of a Key Active-Site Residue in the Cytochrome cbb 3 Oxidases. Biochemistry. 2005;44:10766–10775. doi: 10.1021/bi050464f. [DOI] [PubMed] [Google Scholar]
- 12.Peters A, Kulajta C, Pawlik G, Daldal F, Koch H-G. Stability of the cbb3-Type Cytochrome Oxidase Requires Specific CcoQ-CcoP Interactions. J. Bacteriol. 2008;190(16):5576–5586. doi: 10.1128/JB.00534-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh JI, Kaplan S. Oxygen adaptation. The role of the CcoQ subunit of the cbb3 cytochrome c oxidase of Rhodobacter sphaeroides 2.4.1. J Biol Chem. 2002;277(18):16220–16228. doi: 10.1074/jbc.M200198200. [DOI] [PubMed] [Google Scholar]
- 14.Oh J-I, Kaplan S. The cbb3 Terminal Oxidase of Rhodobacter sphaeroides 2.4.1: Structural and Functional Implciatioins for the Regulation of Spectral Complex Formation. Biochemistry. 1999;38:2688–2696. doi: 10.1021/bi9825100. [DOI] [PubMed] [Google Scholar]
- 15.Zufferey R, Presig O, Hennecke H, Thöny-Meyer L. Assembly and Function of the Cytochrome cbb 3 Oxidase Subunits in Bradyrhizobium japonicum . J. Biol. Chem. 1996;271(15):9114–9119. doi: 10.1074/jbc.271.15.9114. [DOI] [PubMed] [Google Scholar]
- 16.Ducluzeau A-L, Ouchane S, Nitschke W. The cbb3 Oxidases Are an Ancient Innovation of the Domain Bacteria. Molecular Biology and Evolution. 2008;25(6):1158–1166. doi: 10.1093/molbev/msn062. [DOI] [PubMed] [Google Scholar]
- 17.de Gier J-WL, et al. Structural and Functional Analysis of aa 3-type and cbb 3-type Cytochrome c Oxidases of Paracoccus denitrificans Reveals Significant Differences in Proton-pump Design. Mole Microbiol. 1996;20(6):1247–1260. doi: 10.1111/j.1365-2958.1996.tb02644.x. [DOI] [PubMed] [Google Scholar]
- 18.Kulajta C, Thumfart JO, Haid S, Daldal F, Koch HG. Multi-step Assembly Pathway of the cbb(3)-type Cytochrome c Oxidase Complex. J Mol Biol. 2006;355(5):989–1004. doi: 10.1016/j.jmb.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Oh JI, Kaplan S. Redox signaling: globalization of gene expression. The EMBO journal. 2000;19(16):4237–4247. doi: 10.1093/emboj/19.16.4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch HG, Winterstein C, Saribas AS, Alben JO, Daldal F. Roles of the ccoGHIS Gene Products in the Biogenesis of the ccb(3)-Type Cytochrome c Oxidase. J. Mol. Biol. 2000;297(1):49–65. doi: 10.1006/jmbi.2000.3555. [DOI] [PubMed] [Google Scholar]
- 21.Preisig O, Zufferey R, Hennecke H. The Bradyrhizobium japonicum fixGHIS Genes are Required for the Formation of the High-affinity cbb 3-type Cytochrome Oxidase. Arch. Microbiol. 1996;165:297–305. doi: 10.1007/s002030050330. [DOI] [PubMed] [Google Scholar]
- 22.Ekici S, Pawlik G, Lohmeyer E, Koch HG, Daldal F. Biogenesis of cbb(3)-type cytochrome c oxidase in Rhodobacter capsulatus. Biochim Biophys Acta. 2012;1817(6):898–910. doi: 10.1016/j.bbabio.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pawlik G, et al. The putative assembly factor CcoH is stably associated with the cbb3-type cytochrome oxidase. J Bacteriol. 2010;192(24):6378–6389. doi: 10.1128/JB.00988-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HJ, Gennis RB, Ädelroth P. Entrance of the proton pathway in cbb3-type heme-copper oxidases. Proceedings of the National Academy of Sciences. 2011;108(43):17661–17666. doi: 10.1073/pnas.1107543108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahn YO, et al. Conformational coupling between the active site and residues within the KC-channel of the Vibrio cholerae cbb3-type (C-family) oxygen reductase. Proc Natl Acad Sci U S A. 2014;111(42):E4419–4428. doi: 10.1073/pnas.1411676111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang HY, et al. The diheme cytochrome c(4) from Vibrio cholerae is a natural electron donor to the respiratory cbb(3) oxygen reductase. Biochemistry. 2010;49(35):7494–7503. doi: 10.1021/bi1004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Reimann J, Singh LM, Adelroth P. Substrate binding and the catalytic reactions in cbb3-type oxidases: the lipid membrane modulates ligand binding. Biochim Biophys Acta. 2010;1797(6–7):724–731. doi: 10.1016/j.bbabio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Pitcher RS, Brittain T, Watmough NJ. Complex interactions of carbon monoxide with reduced cytochrome cbb3 oxidase from Pseudomonas stutzeri. Biochemistry. 2003;42(38):11263–11271. doi: 10.1021/bi0343469. [DOI] [PubMed] [Google Scholar]
- 29.Liebl U, et al. Ligand binding dynamics to the heme domain of the oxygen sensor Dos from Escherichia coli. Biochemistry. 2003;42(21):6527–6535. doi: 10.1021/bi027359f. [DOI] [PubMed] [Google Scholar]
- 30.Hargrove MS. A flash photolysis method to characterize hexacoordinate hemoglobin kinetics. Biophys J. 2000;79(5):2733–2738. doi: 10.1016/S0006-3495(00)76512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cianetti S, Negrerie M, Vos MH, Martin JL, Kruglik SG. Photodissociation of heme distal methionine in ferrous cytochrome C revealed by subpicosecond time-resolved resonance Raman spectroscopy. J Am Chem Soc. 2004;126(43):13932–13933. doi: 10.1021/ja046442i. [DOI] [PubMed] [Google Scholar]
- 32.Ekici S, et al. Intracytoplasmic copper homeostasis controls cytochrome c oxidase production. MBio. 2014;5(1):e01055–01013. doi: 10.1128/mBio.01055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemp J, et al. Comparative genomics and site-directed mutagenesis support the existence of only one input channel for protons in the C-family (cbb3 oxidase) of heme-copper oxygen reductases. Biochemistry. 2007;46(35):9963–9972. doi: 10.1021/bi700659y. [DOI] [PubMed] [Google Scholar]
- 34.Jasaitis A, Verkhovsky MI, Morgan JE, Verkhovskaya ML, Wikström M. Assignment and Charge Translocation Stoichiometries of the Major Electrogenic Phases in the Reaction of Cytochrome c Oxidase with Dioxygen . Biochemistry. 1999;38:2697–2706. doi: 10.1021/bi982275l. [DOI] [PubMed] [Google Scholar]
- 35.Egawa T, Yeh SR. Structural and functional properties of hemoglobins from unicellular organisms as revealed by resonance Raman spectroscopy. J Inorg Biochem. 2005;99(1):72–96. doi: 10.1016/j.jinorgbio.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Brändén M, et al. On the Role of the K-proton Transfer Pathway in Cytochrome c Oxidase. PNAS. 2001;98(9):5013–5018. doi: 10.1073/pnas.081088398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y, Reimann J, Lepp H, Drici N, Adelroth P. Vectorial proton transfer coupled to reduction of O2 and NO by a heme-copper oxidase. Proc Natl Acad Sci U S A. 2008;105(51):20257–20262. doi: 10.1073/pnas.0805429106. [DOI] [PMC free article] [PubMed] [Google Scholar]