Abstract
Many anti-cancer drugs, such doxorubicin (DXR), intercalate into nuclear DNA of cancer cells thereby inhibiting their growth. However, it is not well understood how such drugs interact with mitochondrial DNA (mtDNA). Using cell and molecular studies of cultured cells we show that DXR and other DNA intercalators such as ethidium bromide, can rapidly intercalate into mtDNA within living cells, causing aggregation of mtDNA nucleoids and altering the distribution of nucleoid proteins. Remodelled nucleoids excluded DXR and maintained mtDNA synthesis, whereas non-remodelled nucleoids became heavily intercalated with DXR, which inhibited their replication leading to mtDNA depletion. Remodelling was accompanied by extensive mitochondrial elongation or interconnection, and was suppressed in cells lacking MFN 1 and OPA1, key proteins for mitochondrial dynamics. In contrast, remodelling was significantly increased by p53 or ATM inhibition, indicating a link between nucleoid dynamics and the genomic DNA damage response.
Collectively, our results show that DNA intercalators can trigger a common mitochondrial response, which likely contributes to the marked clinical toxicity associated with these drugs.
Keywords: Anthracyclines, mtDNA, mitochondria, nucleoids, doxorubicin, ethidium, Mitofusin, OPA1, TFAM, DRP1
Introduction
DNA intercalating drugs form the cornerstone of many anti-cancer regimes. Prominent amongst these are the anthracyclines, a group of compounds including DXR and daunorubicin (Hande, 1998). DNA intercalators function by inserting between the base pairs of the DNA double helix, thereby altering its molecular topology. Consequently, DNA polymerases and other DNA related proteins are inhibited and DNA replication is reduced, causing the death of rapidly dividing cancer cells. Unfortunately anthracyclines can cause severe cardiotoxicity (Singal and Iliskovic, 1998), often manifesting months or even years after treatment (Steinherz and Steinherz, 1991). The reason for this delayed toxicity remains unknown, but one possibility is that anthracyclines induce a lesion capable of damaging the cell over a long period. A potential candidate for such a lesion is the mitochondrion, as cardiac specific mitochondrial dysfunction is an early and specific feature of anthracycline cardiotoxicity (Conklin, 2005; Jung and Reszka, 2001; Tokarska-Schlattner et al., 2006; Wallace, 2003), which can persist even after drug cessation (Zhou et al., 2001). Mitochondria contain a 16.5kb multi-copy genome known as mitochondrial DNA (mtDNA), and extensive mtDNA damage in the form of depletion and deletions has been found in cardiac tissue of DXR treated patients (Lebrecht et al., 2005), as well as rodent and cell culture models (Cullinane et al., 2000; Lebrecht et al., 2004; Suliman et al., 2007). Using the fluorescent DNA dye PicoGreen, that we have previously shown labels specifically nuclear and mtDNA within living cells (Ashley et al., 2005; Ashley et al., 2008), we recently demonstrated that anthracyclines are capable of rapidly penetrating into mitochondria to directly intercalate into mtDNA (Ashley and Poulton, 2009).
In vivo, mtDNA is arranged into punctate nucleoid structures (Garrido et al., 2003; Legros et al., 2004), which consist of several copies of mtDNA bound to ‘nucleoid’ proteins such mtSSB, TFAM and POLG. Although the functional significance of nucleoids remains obscure, yeast nucleoids can undergo structural remodelling in response to metabolic stress in order to protect mtDNA from damage (Chen et al., 2005b; Kucej et al., 2008). Loss of the TFAM homologue and nucleoid protein Abf2p sensitises yeast mtDNA to damage by the DNA intercalator ethidium bromide (Chen et al., 2005b). Within human cells, the tumour suppressor p53 can translocate to mitochondria and interact with POLG in order to protect mtDNA from damage by ethidium bromide (Achanta et al., 2005).
Mitochondria are dynamic organelles which can undergo rapid transient fission and fusion, mediated by pro-fusion proteins such as OPA1 and Mitofusins 1/2, and pro-fission proteins such as DRP1 (Chen et al., 2005a). Recently the anticancer DNA intercalator actinomycin D has been shown to trigger OPA1 and MFN1 mediated stress induced mitochondrial hyperfusion (SIMH), which has a protective anti-apoptotic effect within cells (Tondera et al., 2009). Collectively this evidence suggests that mitochondria and mtDNA may undergo a coordinated stress response in the presence of DNA intercalating drugs.
In this study we sought to study the effects of anti-cancer DNA intercalating drugs on mtDNA and mitochondria. We show that anthracyclines can directly interact with mtDNA at clinically relevant concentrations, producing major alterations of nucleoids and mitochondrial morphology and ultimately resulting in the depletion of mtDNA.
Experimental Procedures
Materials
All chemicals were from Sigma-Aldrich, Axxora or Invitrogen. Antibodies to β-actin, cytochrome c, p53, POLG and TID1 were from Thermo-Fisher, anti-TFAM was raised in our own lab. Anti-DNA (IgM1) was from ProGen Biotechnick, or a kind gift of Peter Cook (IgM2). Anti-BrdU was from Roche. Anti-mtSSB, Anti-ATAD3 and Ditercalinium were generous gifts from Prof. Zeviani, Dr. I. Holt and Prof. B.P. Roques, respectively. MFN1/2 (Chen et al., 2003) and POLG exonuclease null (Trifunovic et al., 2004) mouse embryonic fibroblasts (MEFs) were the generous gift of Dr. D. Chan and Dr. A. Trifunovic, respectively.
Cell culture and drug treatment
Mycoplasma free normal human fibroblasts, H9C2 rat cardiomyocytes, RH30 and A549 cells were cultured as previously (Ashley et al., 2005). For drug treatments cells were seeded at ~2 × 106/cm2 in dishes and allowed to attach overnight before exposure to 1 ml of drug/vehicle medium per cm2 of vessel surface area.
Cytochemical labelling
Immuno-cytochemical labelling, histochemical staining of cytochrome c oxidase (COX) activity, bromodeoxyuridine (brdU) labelling of newly synthesised DNA, and PicoGreen labelling were performed as described previously (Ashley et al., 2005). TMRM co-staining was done using 30 nM for 20 minutes. Cells were visualised using a Leica DMI50 microscope fitted with a Hamamatsu ORCA-II camera.
Propidium Iodide/ calcein-AM determination of viability
Cells were incubated with 1 μM calcein-AM and 0.5 μg/ml propidium iodide for 10 minutes and analysed by microscopy.
TUNEL labelling
Detection of methanol fixed apoptotic cells was done using a kit from Takara Bio Inc. (Shigo, Japan).
Digital image analysis
SimplePCI 6 was used for image capture. Image analysis was performed on unprocessed, 8-bit digital images using ImageJ. Fluorescent intensities (pixel grey values) of defined regions of interest (nuclei or nucleoids), corrected for cellular background, were determined as described (Abramoff, 2004). Cell counts where done by manually counting cells from randomly acquired images.
Real-time quantitative PCR
Determination of relative mtDNA to nDNA was performed as described previously (Ashley et al., 2005), except the nuclear probe was labelled with Vic at the 5’ end and amplifications were done simultaneously using a GeneAmp 7700 sequence detection system.
Oxygen consumption
Cells were harvested, counted with a haemocytometer and re-suspended in DMEM without serum or glucose, at 5 × 106 cells/ml. Oxygen consumption was performed on whole cells at 37°C, using a Hansatech Clarke type oxygen electrode.
ATP measurements
Cells were harvested and counted using a haemocytometer and ATP measured using a Promega CellTiter-Glo assay as per manufacturer instructions, using a Turner Biosystems luminometer.
Radio-labelling of mtDNA synthesis
α32P-dCTP labelling of newly synthesised mtDNA in permeabilised cells was performed as described previously (Ashley et al., 2007; Emmerson et al., 2001).
siRNA transfections
For OPA1/p53/DRP1 knock-downs we used the previously validated siRNA sequences, 5′-GTTATCAGTCTGAGCCAGGdTdT-3′ for OPA1(Olichon et al., 2003), 5’-GCAUGAACCGGAGGCCCAUdTdT-3’ for p53 (Martinez et al., 2002), and 5’-GCAGAAGAATGGGGTAAATdTdT -3’ for Drp-1 (Griparic et al., 2007). Qiagen AllStars siRNA was used as a negative control. 100 nM siRNA was tranfected using Dharmacon Dharmafect, as per manufacturer’s protocols.
Immunoblotting
Was performed as described previously (Poulton et al., 1994).
Statistical analysis
Statistical analysis was calculated using Microsoft Excel or SPSS. Significance was calculated using Student t-test.
Results
Doxorubicin and ethidium bromide alter nucleoid and mitochondrial morphology
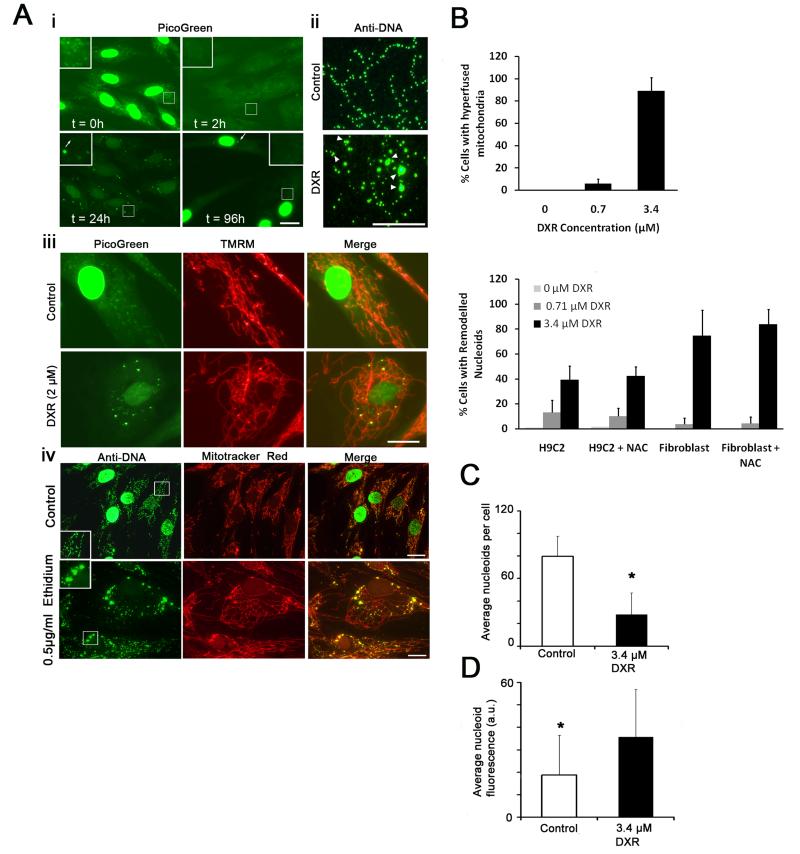
To determine the long term effect of DXR on mtDNA we utilised PicoGreen fluorescence quenching to monitor DXR/DNA interactions within normal primary human fibroblasts, as described previously (Ashley and Poulton, 2009). In untreated cells nuclear and mtDNA were brightly labelled by PicoGreen (Fig. 1A i), and both signals were substantially quenched by addition of DXR, indicating intercalation of DXR into nuclear and mitochondrial DNA. Following 24 hours of DXR exposure PicoGreen fluorescence partially recovered in both the nucleus and mitochondria, the latter containing a small number of brightly labelled and grossly enlarged nucleoids. The average nucleoid diameter of these enlarged nucleoids was 1-3 μm, compared to ~ 0.8 μm of normal sized nucleoids. Although normally sized nucleoids remained within the DXR treated cell, they were often heavily quenched by DXR (t = 24h arrowed in inset). Hereafter, we term these DXR induced nucleoid alterations ‘nucleoid remodelling’. Following DXR exposure, surviving cells allowed to recover in drug free medium for 72 hours displayed marked reduction in nucleoid PicoGreen labelling, despite recovery of the nuclear signal, suggestive of mtDNA depletion, which was confirmed using QPCR (Fig. 3D). Some remodelled nucleoids were still visible (arrowed)
Figure 1.
DXR and ethidium alter mtDNA nucleoids and mitochondrial morphology. (Ai) Time-course of PicoGreen labelling of live primary human fibroblasts incubated with DXR (t = hours of exposure). (Aii) Anti-DNA (IgM1) labelling of mtDNA within DXR (3.4 μM 24 hours) and vehicle treated fibroblasts. (Aiii) PicoGreen/TMRM co-labelling of DXR/vehicle treated fibroblasts (2 μM 24 hours). (Aiv) Anti-DNA (IgM1)/Mitotracker red labelling of mtDNA/mitochondria within fibroblasts treated with vehicle or ethidium bromide (0.5μg/ml 24 hours). (B upper) Percentage of DXR/vehicle treated fibroblasts exhibiting mitochondrial interlinking after 24 hours (n = 200). (B lower) Percentage of DXR/vehicle treated human fibroblasts and H9C2 rat cardiomyocytes exhibiting nucleoid remodelling after 24 hours, in the presence/absence of 100 μg/mL N-acetylcysteine (n = 200). (C) Mean nucleoids per cell within fibroblasts treated with DXR (3.4 μM 24 hours) or vehicle (n = 10). (D) Average nucleoid fluorescence (a.u. = arbitrary units) within fibroblasts treated with DXR/vehicle (3.4 μM 24 hours) (n = 100). * P = <0.05 versus control. Error bars + S.D. Results are representative of three independent experiments. Size bars: 20μm except Aii (10μm).
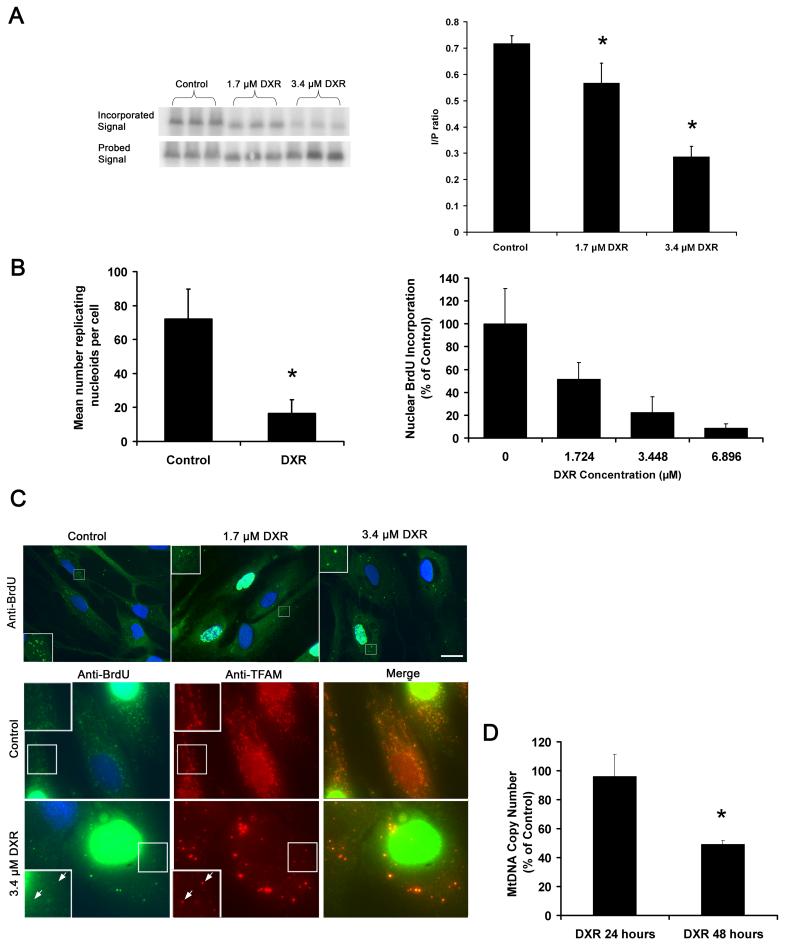
Figure 3.
DXR inhibits mtDNA synthesis and causes mtDNA depletion. (A) Phosphor-screen image of 32P-dCTP incorporation of PVUII digested mtDNA, representing newly synthesised mtDNA, isolated from DXR/vehicle treated A549 (3.4μM DXR 24 hours), and the corresponding signal of the same mtDNA fragments hybridised to a total human mtDNA probe, representing the total mtDNA mass per lane. Graph shows quantification of the relative mtDNA fragment intensities (mtDNA replication) based on the mean of incorporated (I) vs probed (P) signal ratios (n = 3). (B left) Quantification of the average number of brdU labelled (replicating) nucleoids of DXR/vehicle treated fibroblasts (3.4μM DXR 24 hours) (n = 100), following labelling with brdU (10 μM 6 hours), and immuno-detection by anti-brdU. (B right) Average nuclear brdU incorporation of S-phase cells from the same experiment (n = 100). (C upper) Image of brdU incorporation into cellular DNA of fibroblasts used to generate data for (B). DAPI was used to stain nuclei so S-phase nuclei appear white. (C lower) Anti-TFAM/brdU co-labelling of DXR/vehicle treated fibroblasts (3.4μM DXR 24 hours). (D) MtDNA to nuclear DNA ratios of fibroblasts treated with vehicle/DXR (1.7 μM) for up to 48 hrs, as determined by real-time quantitative PCR (n = 3). Note that the drug was washed out after 24 hours to reduce cellular toxicity. This had no effect on nucleoid remodelling or PicoGreen quenching (not shown). * P = <0.05. Error bars +S.D. Size bars: 20μm. Results are representative of three independent experiments.
Immuno-labelling of DXR treated fibroblasts with anti-DNA IgM (IgM1) capable of detecting nucleoids (Legros et al., 2004) confirmed that DXR caused gross enlargement of nucleoids (Fig. 1A ii). The enlarged remodelled nucleoids appeared to be composed of aggregates of smaller nucleoids and there was noticeable clustering of nucleoids (arrowed), which was not generally seen in untreated cells. Co-labelling DXR treated fibroblasts with PicoGreen and the mitochondrial probe TMRM revealed mitochondria within DXR treated cells had undergone marked interlinking, with highly interconnected mitochondria (Fig. 1A iii and supplementary Figure 1B). Remodelled nucleoids frequently co-localised with small mitochondrial swellings not commonly observed in controls. Similar results were observed in DXR treated A549 cancer cells and H9C2 cardiomyocytes (supplementary Figure 1A and C), although H9C2s did not demonstrate mitochondrial interlinking (supplementary Figure 1D).
Anti-DNA and Mitotracker Red (which detects mitochondria in fixed cells) showed that ethidium bromide also caused marked nucleoid remodelling accompanied by mitochondrial interlinking (Figure 1A iv).
Quantification of a similar experiment to 1A showed that DXR could induce mitochondrial interlinking and remodelling at doses below 1 μM. H9C2 rat cardiomyocytes were more sensitive at low concentrations of DXR than human fibroblasts, but the level of remodelling was unaffected by incubating cells with the well known ROS scavenger N-acetylcysteine (Fig. 1B). DXR significantly reduced nucleoid numbers within individual fibroblasts (Fig. 1C), and significantly increased the average nucleoid fluorescence (Fig. 1D).
Remodelled nucleoids are enriched with TFAM, mtSSB and POLG
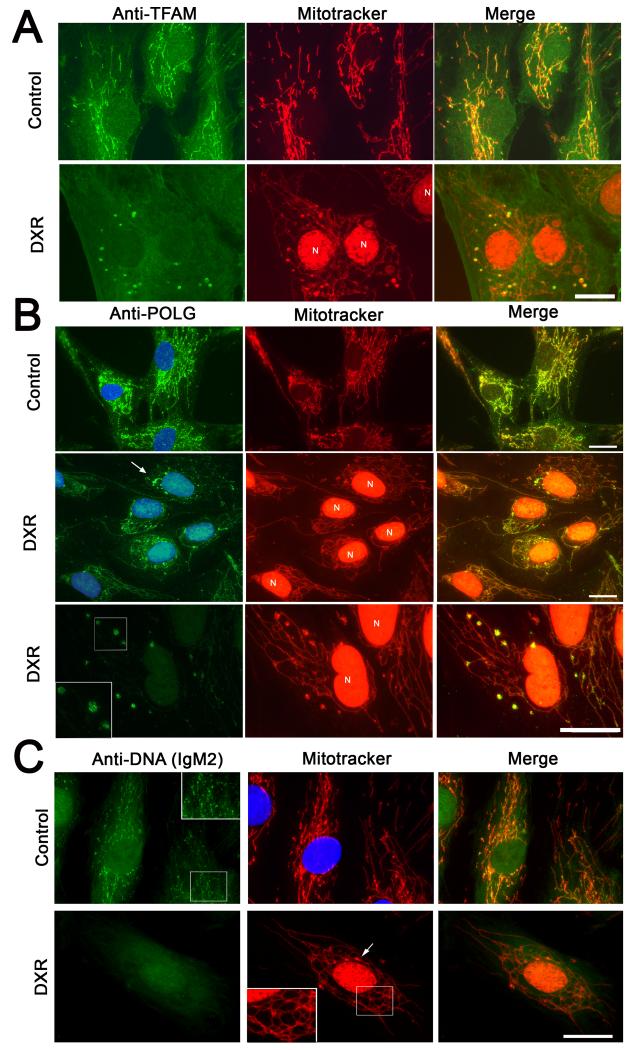
Within nucleoids, mtDNA is complexed with ‘core’ nucleoid proteins including mtSSB, POLG and TFAM, and associated, perhaps indirectly, with TID1 and ATAD3 (Bogenhagen et al., 2008; He et al., 2007). To determine if DXR affected these proteins, we immuno-labelled fibroblasts treated with DXR or vehicle. In untreated cells, TFAM was concentrated into small dots (nucleoids) distributed evenly along mitochondria, but was heavily concentrated into large mitochondrial blobs after DXR exposure (Fig. 2A). Large swathes of the mitochondria thus became depleted of detectable TFAM. The focal accumulations of TFAM co-localised with mitochondrial swellings, and with remodelled nucleoids (detected with anti-DNA IgM1 - not shown). Similar results were obtained using ethidium (not shown). Note that the strong red nuclear signal in DXR treated cells is due to auto-fluorescence of DXR intercalated into nuclear DNA, and was not due to Mitotracker. Mitochondrial DXR was not visible. Western blot of TFAM showed the total amount of TFAM remained un-affected by DXR treatment (Supplementary Fig. 2D).
Figure 2.
DXR alters the distribution of TFAM, mtSSB and POLG and reduces mtDNA binding by Anti-DNA IgM2. (A) Anti-TFAM/Mitotracker labelling of fibroblasts incubated with vehicle/DXR (3.4 μM 24 hours). Retained DXR fluorescence within the nuclei is indicated by ‘N’. (B) Anti-POLG/Mitotracker labelling of fibroblasts incubated with vehicle or DXR for 24 hours (3.4 μM 24 hours). DAPI was used to stain nuclei blue. DXR induced POLG accumulations are arrowed. The bottom panels show a higher magnification of a POLG labelling cell acquired using reduced camera exposure. (C) Anti-DNA IgM2/Mitotracker labelling of fibroblasts incubated with vehicle/DXR (3.4 μM 24 hours). Arrow shows a mitochondrial swelling indicating a remodelled nucleoid. Size bars: 20μm. Results are representative of at least three independent experiments.
Immuno-labelling of POLG1 (Fig. 2B) and mtSSB (supplementary Fig. 2C) also showed marked re-organisation into remodelled nucleoids in response to DXR treatment, although rarely as marked as TFAM. Lower camera exposure revealed that POLG enriched nucleoids consisted of small ‘dot like’ structures (Fig. 2B, bottom panels). Tid1 and ATAD3 showed re-localisation to a much lesser degree in response to DXR, but generally remained evenly distributed though-out mitochondria (supplementary figure 2A and B). Surprisingly, DXR treatment substantially reduced the mtDNA binding affinity of a second anti-DNA antibody (IgM2) (Fig. 2C), suggesting a structural alteration to the mtDNA.
In conclusion, remodelled nucleoids were enriched with the core nucleoid proteins TFAM, mtSSB and POLG, and had reduced affinity for certain anti-DNA antibodies.
DXR inhibits mtDNA replication but remodelled nucleoids remain replicatively active
As DXR inhibits polymerases (Ellis et al., 1987; Hixon et al., 1981) we investigated the effect of DXR on DNA replication using α 32P-dNTP labelling of permeabilised A549 cells (Emmerson et al., 2001), which undergo remodelling and mitochondrial interlinking in response to DXR (supplementary Fig. 1A). DXR substantially reduced the amount of mtDNA α 32P-dCTP incorporation relative to total mtDNA mass (determined with a total human mtDNA probe), indicating that DXR inhibited mtDNA replication (Fig. 3A left). Quantification of the bands showed a dose response effect, with a greater than 50% reduction of mtDNA synthesis after exposure to 3.4 μM DXR (Fig. 3A right). We also examined DNA synthesis in DXR treated fibroblasts using bromodeoxyuridine (brdU) labelling (Magnusson et al., 2003). DXR and vehicle treated fibroblasts were incubated with brdU, and brdU labelled nuclear/mtDNA visualised with anti-brdU antibody. Measurements of the brdU intensity of S-phase nuclei and the number of brdU labelled nucleoids showed that DXR strongly inhibited nuclear synthesis, and sharply reduced the number of replicating nucleoids (Figure 3B). Most replicating nucleoids in DXR treated cells were enlarged i.e. re-modelled (Fig. 3C, upper panels). Since previous anti-DNA labelling had shown that numerous non-remodelled nucleoids remained within DXR treated cells (Fig. 1Aii), we determined if DXR had preferentially inhibited their replication, by double labelling DXR treated cells with brdU and anti-TFAM (Fig. 3C, lower panels). Within untreated cells, most TFAM positive nucleoids showed brdU labelling but within DXR treated cell only the largest remodelled nucleoids showed brdU incorporation comparable or greater than that of untreated nucleoids. A large number of non or partially remodelled nucleoids labelled by TFAM showed little or no brdU label (examples arrowed in insets).
To determine if DXR caused mtDNA depletion, we measured the total mtDNA copy number using quantitative real-time PCR (Fig. 3D). MtDNA levels were unaffected following a 24hour drug exposure, but showed a significant depletion of mtDNA after 48 hours, compared to controls. DXR was removed after 24 hours to reduce cellular toxicity but this did not alter the level of PicoGreen quenching (not shown), indicating DXR remained intercalated.
Thus, DXR preferentially inhibits mtDNA replication of non-remodelled nucleoids causing mtDNA depletion.
Nucleoid remodelling is not secondary to apoptosis and does not alter mitochondrial function
We next determined if remodelling preceded cell death or compromised mitochondrial dysfunction. Using TUNEL labelling we determined the cellular apoptotic index of DXR/vehicle treated (supplementary figure. 3A i). Following a 24-48 hour DXR exposure (3.4 μM), less than 10% of cells were apoptotic, well below the number of cells exhibiting remodelled nucleoids or increased mitochondrial interconnection. Calcein/propidium labelling showed less than 5% of the attached cell population were necrotic (supplementary Fig. 3A ii). DAPI/cytochrome c immuno-labelling showed no differences between vehicle and DXR treated cells, unlike cells treated with hydrogen peroxide, which showed nuclear located cytochrome c and chromosomal condensation (very bright DAPI signal), hallmarks of apoptosis (supplementary Fig. 3A iii). Remodelling did not occur during staurosporine-induced apoptosis (supplementary Fig. 3A iv). DXR did not significantly compromise mitochondrial function of fibroblasts or H9C2 cells, as determined by ATP generation, cellular oxygen consumption, cytochrome c oxidase histochemisty, and JC-1 fluorescence (supplementary Fig. 3B).
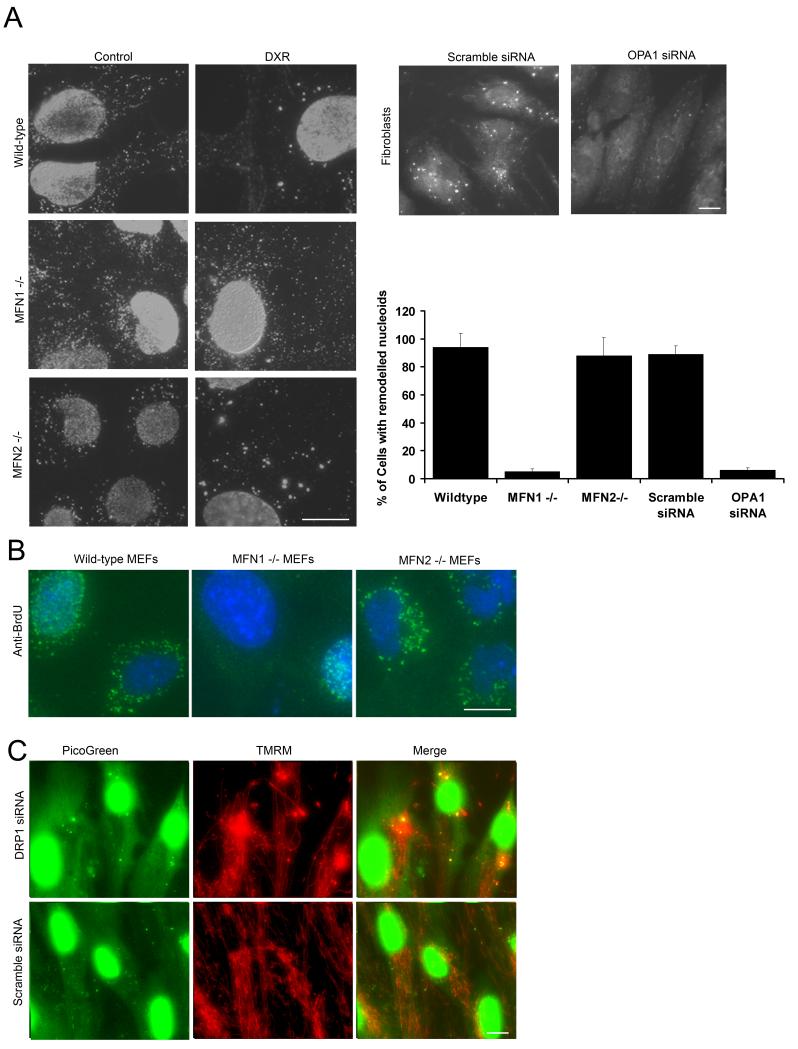
DXR induced nucleoid remodelling is mediated by OPA1 and mitofusin1 but not mitofusin 2
We next determined the influence of mitochondrial fusion on DXR induced remodelling by comparing wild-type mouse embryonic fibroblasts (MEFs) with mitochondrial fusion deficient MEFs harbouring null mutations in either MFN1 or MFN2 (Chen et al., 2003). These MFN mutants contained fragmented mitochondria (supplementary Fig. 4A). Anti-DNA (IgM1) labelling indicated that whilst DXR induced substantial nucleoid remodelling within wild-type and MFN2 null MEFs, remodelling was almost totally suppressed within MFN1 null MEFs (Fig. 4A). Next we determined if OPA1 was also involved in nucleoid remodelling by ablating OPA1 levels in human fibroblasts using siRNA, confirmed by Western blot (supplementary Fig. 4B). DXR nucleoid remodelling, as detected by PicoGreen, was inhibited within the OPA1 siRNA transfected fibroblasts, but not in scramble siRNA transfected cells. Quantification showed that remodelling was almost totally suppressed by loss of OPA1 and MFN1, but not by loss of MFN2 (Fig. 4A, graph).
Figure 4.
Influence of MFN1/2,OPA1 and DRP1 on DXR induced nucleoid remodelling. (A left) Anti-DNA IgM1 labelling of DXR/vehicle treated wild-type, MFN1 null and MFN2 null MEFs (7.2 μM DXR 24 hrs). (A right) PicoGreen labelling of fibroblasts transfected with either OPA1 targeting siRNA (48 hours) or a scramble siRNA control, after treatment with DXR (7.2 μM 24 hours). (A graph) Quantification of % cells with DXR induced remodelled nucleoids (n = 200). (B) Representative brdU labelling (20μM 24 hours) of wild-type, MFN1 and MFN2 null cells, treated with vehicle/DXR (3.4 μM 24 hours). (C) PicoGreen/TMRM labelling of DRP1/Scramble siRNA treated cells (6 days). Bars 20 μm. Results + S.D. Results are representative of three independent experiments.
To determine if the suppression of nucleoid remodelling within MFN1 null cells led to increased sensitivity of mtDNA to DXR, we pulse-labelled wild-type, MFN1 and MFN2 MEFs with BrdU following a 24 hour treatment with DXR (1.7 μM). As shown in Figure 4D, mtDNA synthesis was markedly suppressed within MFN1 null cells, yet was well maintained by MFN2 and wild-type cells.
To determine if suppression of mitochondrial fission could alter nucleoids, we ablated DRP1 using siRNA. PicoGreen/TMRM co-staining showed that prolonged knock-down of DRP1 in fibroblasts lead to the formation of hyper-fused mitochondria, and large mitochondrial swellings which co-localised with apparently re-modelled nucleoids (Fig. 4C).
Collectively, the data above suggest that the nucleoid remodelling induced by DXR is mediated by MFN1 and OPA1, but not MFN2, and that suppression of remodelling renders mtDNA replication more susceptible to DXR inhibition. Furthermore nucleoid remodelling can be induced in the absence of DXR by inhibition of mitochondrial fission.
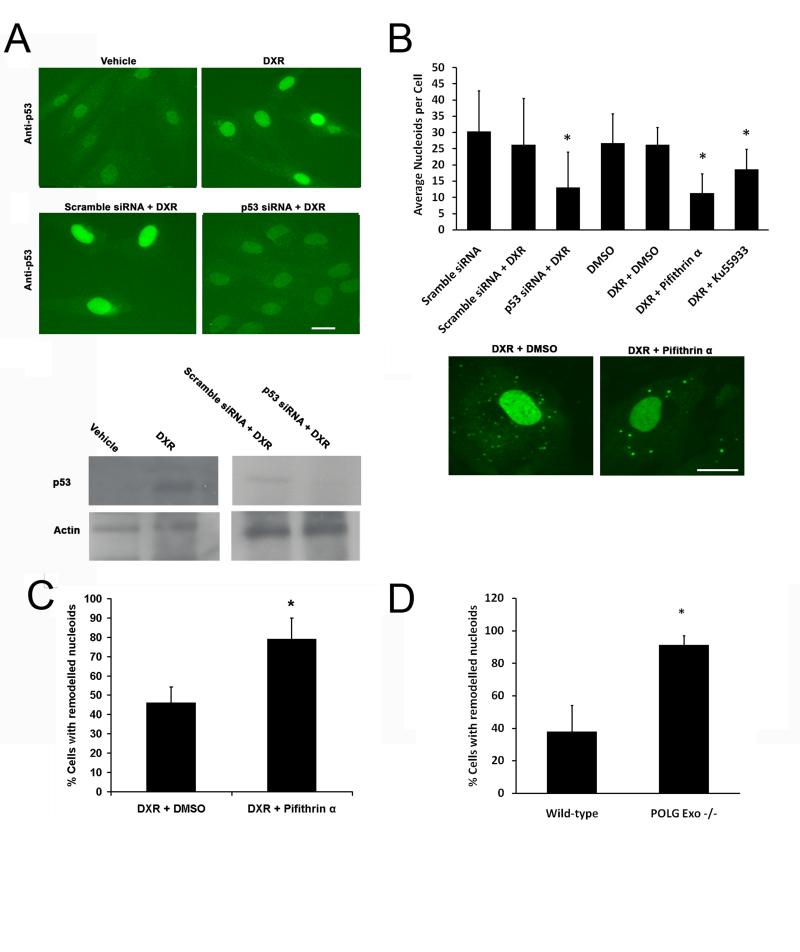
p53, ATM and POLG suppresses DXR induced remodelling
As DNA damage induced by DXR can trigger up regulation of p53 and its translocation to mitochondria where it is involved in mtDNA protection (Nithipongvanitch et al., 2007a), we determined if altering p53 activity altered nucleoid remodelling. In DXR treated fibroblasts, p53 was up-regulated and this could be inhibited by p53 siRNA (Fig. 5A). Using PicoGreen we found that DXR remodelling was substantially increased by p53 knockdown (Fig. 5B). Chemical inhibition of p53 by pifithrin-α also increased remodelling confirming the specificity of the result. Remodelled nucleoids were larger and brighter in the p53 inhibited cells than in scramble or vehicle treated controls (Fig. 5B lower). As p53 activation by DXR is mediated mainly by ATM (Kurz et al., 2004), we compared DXR remodelling in cells treated with either vehicle or the ATM inhibitor KU55933. ATM inhibition substantially increased the level of DXR remodelling (Fig. 5B).
Figure 5.
p53, ATM and POLG suppress DXR induced nucleoid remodelling. (A upper panel) Anti-p53 immuno-labelling of fibroblasts treated with vehicle or DXR (1.7 μM for 24 hours) or transfected with p53/control, following exposure to 3.4 μM DXR for 24 hours. (A lower) Immuno-blot of similar experiment to A. (B upper panels) Quantification of nucleoid remodelling of DXR treated (3.4 μM 24 hours) fibroblasts in which p53 was inhibited by siRNA or pifithrin-α (10 μg/ml), or ATM inhibited by KU55933 (10 μM) (n = 20). (B lower panels) Typical PicoGreen staining of cells treated with DXR in the presence of vehicle or pifithrin-α. (C) Effect of vehicle or pifithrin-α (10 μg/ml) on DXR (3.4 μM 24 hours) induced nucleoid remodelling in RH30 cells expressing a p53 transcription mutant (n = 20). (D) Nucleoid remodelling exhibited by wild-type or POLG exonuclease null MEFs treated with DXR (3.4 μM 24 hours) (n =100). Results +S.D. *P <0.05. Bars 20μm. Results are representative of three independent experiments.
To determine if the transcriptional activity of p53 was necessary for its influence on remodelling, we treated RH30 cells with DXR in the presence or absence of pifithrin-α, and counted the proportion of cells exhibiting strong nucleoid remodelling. RH30 cells express a mutant p53 protein unable to initiate transcription of p53 responsive genes (Felix et al., 1992; McKenzie et al., 2002). The amount of remodelling was increased by pifithrin-α treatment within RH30, suggesting p53 transcriptional activity was not required for the suppression of nucleoid remodelling (Fig. 5C).
To determine the influence of POLG exonuclease (proofreading) activity on DXR nucleoid remodelling we compared POLG wild-type and POLG exonuclease −/− MEFs derived from a trans-genetic knock-in mouse (Trifunovic et al., 2004). POLG Exo −/− MEFs exhibited significantly more DXR induced nucelodi remodelling than identically treated wild-type controls (Fig. 5D).
In summary the data above indicates p53/ATM and POLG exonuclease activity suppresses DXR nucleoid remodelling.
DNA intercalators induce nucleoid remodelling
To determine the forms of DNA damage capable of triggering nucleoid remodelling we tested several drugs with known DNA damaging effects for their ability to induce remodelling, detected by either anti-DNA or PicoGreen labelling (Table 1). Certain anti-cancer drugs were found to induce varying levels of remodelling, including several anthracyclines, actinomycin D and ditercalinium. All compounds that induced remodelling were known DNA intercalators. Remodelling was not observed with specific inhibitors of topoisomerases, ROS generators, gamma radiation, DNA alkylating agents or nucleoside analogues, indicating DNA intercalation and not other forms of DNA damage triggered nucleoid remodelling.
Table 1.
Compounds tested for ability to induce nucleoid remodelling in Fibroblasts. Cells were incubated with medium containing compounds at the specified concentrations for 24 hours, and nucleoid remodelling detected with PicoGreen.
| Drug/Process | Tested Concentration | Physiological Effect | Nucleoid remodelling inducer |
|---|---|---|---|
| Doxorubin (Anthracycline) | 0.7 – 7.5 μM | DNA intercalator, topoisomerase II inhibitor+ | Yes |
| Daunorubicin (Anthracycline) | 0.5 – 3.4 μM | DNA intercalator, topoisomerase II inhibitor+ | Yes |
| Epirubicin (Anthracycline) | 0.5 – 3.4 μM | DNA intercalator, topoisomerase II inhibitor+ | Yes |
| Idarubicin (Anthracycline) | 0.5 – 3.4 μM | DNA intercalator, topoisomerase II inhibitor+ | Yes |
| Aclarubicin (Anthracycline) | 1.7 – 3.4 μM | DNA intercalator, topoisomerase II inhibitor$ | Yes |
| Ethidium bromide | 0.5 – 1μg/ml | DNA intercalator | Yes |
| Actinomycin D | 5 – 10μM | DNA intercalator, topoisomerase inhibitor+ | Yes |
| Ditercalinium | 0.5 – 1 μg/ml | DNA intercalator, topoisomerase inhibitor$ | Yes |
| Etoposide | 10 - 20 μM | Topoisomerase II inhibitor$ | No |
| Ciprofloxacin | 10 – 50 μM | Topoisomerase II inhibitor+ | No |
| Sobzuzoxane | 50 – 100 μM | Topoisomerase II inhibitor$ | No |
| Campthofecin | 10 -20 μM | Topoisomerase I inhibitor | No |
| ICRF-193 | 50 - 100 μM | Topoisomerase II inhibitor$ | No |
| Cisplatin | 10 – 50 μM | DNA cross-linker | No |
| Bleomycin | 50 μg/ml | DNA damager | No |
| Hydroxyurea | 0.5 - 1 mM | Ribonucleotide reductase inhibitor | No |
| Menadione | 5 - 10 μM | Mitochondrial ROS generator | No |
| Bromodeoxyuridine | 10-20 μM | Nucleoside analogue | No |
| Dideoxycytidine | 5 - 15 μM | Nucleoside analogue | No |
| Gamma irradiation | 3000 Rad | DNA damager | No |
| Chloramphenicol | 25-50 μg/ml | Mitochondrial translation inhibitor | No |
| Xanthine/Xanthine oxidase | 50 U | ROS generator | No |
| H2O2 | 200-500 mM | ROS generator | No |
Key:
Catalytic inhibitor
Poison
Discussion
This study addresses the hypothesis that anti-cancer DNA intercalating drugs can directly affect mtDNA. We have previously shown that such drugs can rapidly intercalate into mtDNA of living cells (Ashley and Poulton, 2009). Here we show how these drugs progressively alter the structure and arrangement of nucleoids and mitochondria, by causing nucleoid aggregation and mitochondrial interlinking. These effects were influenced by mitochondrial dynamics and ATM/ p53 activation, thereby showing mitochondrial nucleoids are linked to the genomic DNA damage response.
Within fibroblasts exposed to DXR, initial strong fluorescent quenching of PicoGreen by DXR showed that substantial intercalation occurred rapidly within nuclear and mtDNA (Fig. 1A), consistent with previous findings (Ashley and Poulton, 2009). However, after several hours, mtDNA fluorescence started to recover and was accompanied by the formation of giant, abnormally bright nucleoids, a process we describe as ‘nucleoid remodelling’. Although many non-remodelled nucleoids remained most failed to recover their fluorescence, indicating they lacked the ability to exclude DXR unlike their remodelled counterparts. Similar results were found within DXR treated H9C2 cardiomyocytes and A549 cancer cells. Anti-DNA labelling confirmed DXR exposed nucleoids were abnormal and showed ethidium also induced nucleoid alterations. Because these changes largely preceded DXR induced cell death, were not induced by staurosporine, and were induced by non-toxic doses of ethidium, we conclude that remodelling was not secondary to apoptosis.
TFAM became strongly enriched into remodelled nucleoids, and to a lesser extent mtSSB and POLG also. In contrast nucleoid proteins not believed to be directly bound to mtDNA such as ATAD3 and Tid1 showed much less alteration in their mitochondrial distribution. MtDNA binding by another anti-DNA IgM (IgM2) capable of detecting mtDNA was markedly reduced by DXR, suggesting nucleoid structure was altered so as to reduce its binding affinity. As IgM2 and IgM1 were raised against differing cellular epitopes, remodelling may alter the access of the antibodies to one but not the other epitope. α32P-dCTP radiolabel and brdU incorporation showed mtDNA synthesis was significantly reduced by DXR (Fig. 3A) and DXR exposed cells consequently became depleted of mtDNA (Fig. 3D).
Remodelling was closely linked to mitochondrial dynamics as remodelling was accompanied by marked mitochondrial interlinking in certain cell types. Furthermore remodelling required the mitochondrial pro-fusion proteins MFN1 and OPA1 (Fig. 4), suggesting remodelling requires the aggregation of nucleoids from separate mitochondria. Remodelling did not require MFN2, which has a lower pro-fusion activity than that of MFN1 and is not essential for fusion (Chen et al., 2005a; Cipolat et al., 2004; Tondera et al., 2009). DXR mediated mitochondrial interlinking may represent stress induced mitochondrial hyperfusion (SIMH), which has recently been shown to be a pro-survival response of cells treated with toxic agents (Tondera et al., 2009). Notably we found that actinomycin D, which can induce SIMH, also triggers nucleoid remodelling (supplementary Fig. 2C), suggesting both phenomena are linked. Strikingly both SIMH and nucleoid remodelling similarly require OPA1 and MFN1, but not MFN2 activity. Mitochondrial fission may also play a role in nucleoid remodelling, as prolonged inhibition of fission via knock-down of DRP 1 caused the formation of highly interlinked mitochondria and enlarged nucleoids which co-localised with mitochondrial swellings (Figure 4C). However, immunoblotting failed to detect any DXR induced alterations of the expression of OPA1, DRP1, MFN2 or MFN2 (unpublished observations).
Nucleoid remodelling was specific to DNA intercalators, and not other forms of damage such as reactive oxygen species (table 1). DNA damage induced by DXR triggered the accumulation of p53 within the nuclei of treated cells, and inhibition of p53, or its upstream activator ATM, increased DXR nucleoid remodelling (Fig. 5B), indicating p53 repressed remodelling. Because this was apparently not due to p53s role as a transcription factor (Fig. 5C), it might well be due to its direct involvement in mtDNA protection. p53 can interact with POLG (Achanta et al., 2005), and is important for protecting mtDNA from damage by ethidium bromide (Achanta et al., 2005) and DXR (Nithipongvanitch et al., 2007b). POLG exonuclease null MEFs exhibited markedly increased remodelling compared to wild-type counterparts (Fig. 5D) indicating the proofreading exonuclease activity of POLG also suppresses nucleoid remodelling. Since p53 enhances POLGs exonuclease activity (Bakhanashvili et al., 2009; Bakhanashvili et al., 2008) p53 may exert its influence on nucleoid remodelling via its enhancement of POLG exonuclease activity.
In yeast, nucleoids can undergo structural alterations to protect mtDNA from damage during metabolic stress, possibly by allowing mtDNA to adopt a compact state (Chen et al., 2005b; Kucej et al., 2008). It is therefore conceivable that the ‘nucleoid remodelling’ we have characterised within mammalian cells helps to protect mtDNA from damaging intercalating agents. Several lines of evidence support this theory. Firstly, the absence of PicoGreen quenching by DXR within remodelled nucleoids shows they were more efficient at excluding DXR than non-remodelled nucleoids. Secondly, brdU/TFAM co-labelling demonstrated mtDNA synthesis was better maintained by large remodelled nucleoids than by smaller or non-remodelled nucleoids (Fig. 3C). Thirdly, mtDNA synthesis in the presence of DXR was markedly reduced within MFN1 null cells in which remodelling was suppressed, when compared to that of MFN2 null or wild type cells (Fig. 4A). This suggests that reducing nucleoid remodelling renders mtDNA more susceptible to DXR by, for example, increasing DXR intercalation. Finally, mitochondrial function was well maintained within DXR treated cells (supplementary Fig. 3) indicating remodelling did not compromise short term mitochondrial function as might be expected were the process pathogenic.
The present study and previous work (Ashley and Poulton, 2009), shows that mtDNA is a direct target for several clinically useful anti-cancer DNA drugs. This is consistent with the extensive mtDNA damage associated with human DXR pathology (Lebrecht et al., 2005). Ethidium bromide, which had a similar affect on mtDNA as DXR, can trigger long-term mitochondrial dysfunction by damaging mtDNA (von Wurmb-Schwark et al., 2006). Thus, in vivo, DXR intercalation into cardiac mtDNA and subsequent damage may contribute to the longer-term tissue dysfunction characteristic of DXR pathology.
Supplementary Material
Supplementary Figure 1 Nucleoid remodelling within A549 and H9C2 cardiomyocytes. (A) PicoGreen/TMRM labelling of DXR/vehicle treated A549 cells (3.4 μM 24 hours). (B) Mitotracker red labelling of mitochondria within DXR/vehicle treated fibroblasts. Note that DXR intercalated into the nucleus is visible in the red channel after fixation. (C) PicoGreen labelling of H9C2 cardiomyocytes treated with DXR/vehicle. (D) Mitotracker labelling of vehicle/ DXR treated H9C2 cells. Bars: 20μm except B (10μm).
Supplementary Figure 2 Effects of DXR on TFAM, mtSSB, Tid1 and ATAD3. (A) Anti-Tid1/Mitotracker labelling of fibroblasts treated with vehicle/DXR (3.4 μM 24 hours). (B) Anti-ATAD/Mitotracker labelling of fibroblasts treated with vehicle/DXR. (C) Anti-DNA (IgM1)/anti-mtSSB labelling of DXR labelling of fibroblasts treated with vehicle/DXR. (D) Immunoblot of beta-actin and TFAM in fibroblasts treated with vehicle/DXR (3.4 μM 24 hours). Bars: 20μm. (DXR 3.4 μM 24 hours).
Supplementary Figure 3 DXR induced mitochondrial alterations are not secondary to cell death and does not compromise mitochondrial function. (Ai) Percentage apoptotic (TUNEL +ve) of vehicle/DXR treated fibroblasts (3.4μM) (n=200) * P = <0.05, *** P = <0.005 vs control. (Aii) Percentage necrotic (calcein −ve propidium + ve) of vehicle/DXR treated fibroblasts (3.4μM) (n=200). (Aiii) Cytochrome c/DAPI co-labelling of DXR (3.4 μM 24 hours), vehicle or H2O2 (80 mM 24 hours) treated fibroblasts. (Aiv) PicoGreen labelling of apoptotic fibroblasts following treatment with staurosporine (1 μM 24 hours). (Bi) Histochemical staining of cytochrome c oxidase activity of H9C2 or fibroblasts cells treated with DXR/ vehicle (3.4 μM 24 hours). (Bii) JC-1 labelling of fibroblasts treated with vehicle/DXR (3.4 μM 24 hours) - red ‘J-aggregates’ are present when mitochondria are polarised. (Biii) Polargraphic oxygen consumption of H9C2s treated with DXR/vehicle (3.4 μM 24 hours) or sodium azide (5mM 20 minutes) ($ p = 0.053, **P = <0.005 vs control, n = 3). (Biv) ATP levels measured by luciferase assay of DXR/vehicle (3.4 μM 24 hours) or rotenone (10 μM, 20 minutes) treated fibroblasts. Error bars +S.D. Size bars 20μm. Results are representative of three independent experiments. ‘a.u.’ = arbitrary units.
Supplementary Figure 4 Influence of MFN1/2 and OPA1 on mitochondrial morphology. (A) TMRM labelling of wild-type, MFN1 null and MFN2 null MEFs. (B) TMRM labelling of OPA1 or scramble siRNA treated cells. Immunoblotting blot of siRNA treated fibroblasts showing OPA1 and beta-actin protein levels.
Acknowledgements
We thank I. Sargent for use of equipment, E. Brampton, M. Zeviani, I. Holt, B. Roques, A.Trifunovic, P. Cook and D. Chan for materials, and K. Morten, and F. Brook for technical support. This work was funded by the MRC/Wellcome Trust.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Achanta G, Sasaki R, Feng L, Carew JS, Lu W, Pelicano H, et al. Novel role of p53 in maintaining mitochondrial genetic stability through interaction with DNA Pol gamma. EMBO J. 2005;24:3482–92. doi: 10.1038/sj.emboj.7600819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley N, Adams S, Slama A, Zeviani M, Suomalainen A, Andreu AL, et al. Defects in maintenance of mitochondrial DNA are associated with intramitochondrial nucleotide imbalances. Hum Mol Genet. 2007;16:1400–11. doi: 10.1093/hmg/ddm090. [DOI] [PubMed] [Google Scholar]
- Ashley N, Harris D, Poulton J. Detection of mitochondrial DNA depletion in living human cells using PicoGreen staining. Exp Cell Res. 2005;303:432–46. doi: 10.1016/j.yexcr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Ashley N, O’Rourke A, Smith C, Adams S, Gowda V, Zeviani M, et al. Depletion of mitochondrial DNA in fibroblast cultures from patients with POLG1 mutations is a consequence of catalytic mutations. Hum Mol Genet. 2008;17:2496–506. doi: 10.1093/hmg/ddn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley N, Poulton J. Mitochondrial DNA is a direct target of anti-cancer anthracycline drugs. Biochem Biophys Res Commun. 2009;378:450–5. doi: 10.1016/j.bbrc.2008.11.059. [DOI] [PubMed] [Google Scholar]
- Bakhanashvili M, Grinberg S, Bonda E, Rahav G. Excision of nucleoside analogs in mitochondria by p53 protein. AIDS. 2009;27:779–88. doi: 10.1097/QAD.0b013e328329c74e. [DOI] [PubMed] [Google Scholar]
- Bakhanashvili M, Grinberg S, Bonda E, Simon AJ, Moshitch-Moshkovitz S, Rahav G. p53 in mitochondria enhances the accuracy of DNA synthesis. Cell Death Differ. 2008;15:1865–74. doi: 10.1038/cdd.2008.122. [DOI] [PubMed] [Google Scholar]
- Bogenhagen DF, Rousseau D, Burke S. The layered structure of human mitochondrial DNA nucleoids. J Biol Chem. 2008;283:3665–75. doi: 10.1074/jbc.M708444200. [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005a;280:26185–92. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XJ, Wang X, Kaufman BA, Butow RA. Aconitase couples metabolic regulation to mitochondrial DNA maintenance. Science. 2005b;307:714–7. doi: 10.1126/science.1106391. [DOI] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–32. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin KA. Coenzyme q10 for prevention of anthracycline-induced cardiotoxicity. Integr Cancer Ther. 2005;4:110–30. doi: 10.1177/1534735405276191. [DOI] [PubMed] [Google Scholar]
- Cullinane C, Cutts SM, Panousis C, Phillips DR. Interstrand cross-linking by adriamycin in nuclear and mitochondrial DNA of MCF-7 cells. Nucleic Acids Res. 2000;28:1019–25. doi: 10.1093/nar/28.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CN, Ellis MB, Blakemore WS. Effect of adriamycin on heart mitochondrial DNA. Biochem J. 1987;245:309–12. doi: 10.1042/bj2450309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerson CF, Brown GK, Poulton J. Synthesis of mitochondrial DNA in permeabilised human cultured cells. Nucleic Acids Res. 2001;29:E1. doi: 10.1093/nar/29.2.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix CA, Kappel CC, Mitsudomi T, Nau MM, Tsokos M, Crouch GD, et al. Frequency and diversity of p53 mutations in childhood rhabdomyosarcoma. Cancer Res. 1992;52:2243–7. [PubMed] [Google Scholar]
- Garrido N, Griparic L, Jokitalo E, Wartiovaara J, van der Bliek AM, Spelbrink JN. Composition and dynamics of human mitochondrial nucleoids. Mol Biol Cell. 2003;14:1583–96. doi: 10.1091/mbc.E02-07-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–64. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hande KR. Clinical applications of anticancer drugs targeted to topoisomerase II. Biochim Biophys Acta. 1998;1400:173–84. doi: 10.1016/s0167-4781(98)00134-1. [DOI] [PubMed] [Google Scholar]
- He J, Mao CC, Reyes A, Sembongi H, Di Re M, Granycome C, et al. The AAA+ protein ATAD3 has displacement loop binding properties and is involved in mitochondrial nucleoid organization. J Cell Biol. 2007;176:141–6. doi: 10.1083/jcb.200609158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixon SC, Ellis CN, Daugherty JP. Heart mitochondrial DNA synthesis: preferential inhibition by adriamycin. J Mol Cell Cardiol. 1981;13:855–60. doi: 10.1016/0022-2828(81)90242-x. [DOI] [PubMed] [Google Scholar]
- Jung K, Reszka R. Mitochondria as subcellular targets for clinically useful anthracyclines. Adv Drug Deliv Rev. 2001;49:87–105. doi: 10.1016/s0169-409x(01)00128-4. [DOI] [PubMed] [Google Scholar]
- Kucej M, Kucejova B, Subramanian R, Chen XJ, Butow RA. Mitochondrial nucleoids undergo remodeling in response to metabolic cues. J Cell Sci. 2008;121:1861–8. doi: 10.1242/jcs.028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz EU, Douglas P, Lees-Miller SP. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J Biol Chem. 2004;279:53272–81. doi: 10.1074/jbc.M406879200. [DOI] [PubMed] [Google Scholar]
- Lebrecht D, Kokkori A, Ketelsen UP, Setzer B, Walker UA. Tissue-specific mtDNA lesions and radical-associated mitochondrial dysfunction in human hearts exposed to doxorubicin. J Pathol. 2005;207:436–44. doi: 10.1002/path.1863. [DOI] [PubMed] [Google Scholar]
- Lebrecht D, Setzer B, Rohrbach R, Walker UA. Mitochondrial DNA and its respiratory chain products are defective in doxorubicin nephrosis. Nephrol Dial Transplant. 2004;19:329–36. doi: 10.1093/ndt/gfg564. [DOI] [PubMed] [Google Scholar]
- Legros F, Malka F, Frachon P, Lombes A, Rojo M. Organization and dynamics of human mitochondrial DNA. J Cell Sci. 2004;117:2653–62. doi: 10.1242/jcs.01134. [DOI] [PubMed] [Google Scholar]
- Magnusson J, Orth M, Lestienne P, Taanman JW. Replication of mitochondrial DNA occurs throughout the mitochondria of cultured human cells. Exp Cell Res. 2003;289:133–42. doi: 10.1016/s0014-4827(03)00249-0. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Naguibneva I, Lehrmann H, Vervisch A, Tchenio T, Lozano G, et al. Synthetic small inhibiting RNAs: efficient tools to inactivate oncogenic mutations and restore p53 pathways. Proc Natl Acad Sci U S A. 2002;99:14849–54. doi: 10.1073/pnas.222406899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie PP, McPake CR, Ashford AA, Vanin EF, Harris LC. MDM2 does not influence p53-mediated sensitivity to DNA-damaging drugs. Mol Cancer Ther. 2002;1:1097–104. [PubMed] [Google Scholar]
- Nithipongvanitch R, Ittarat W, Velez JM, Zhao R, St Clair DK, Oberley TD. Evidence for p53 as Guardian of the Cardiomyocyte Mitochondrial Genome Following Acute Adriamycin Treatment. J Histochem Cytochem. 2007a doi: 10.1369/jhc.6A7146.2007. [DOI] [PubMed] [Google Scholar]
- Nithipongvanitch R, Ittarat W, Velez JM, Zhao R, St Clair DK, Oberley TD. Evidence for p53 as guardian of the cardiomyocyte mitochondrial genome following acute adriamycin treatment. J Histochem Cytochem. 2007b;55:629–39. doi: 10.1369/jhc.6A7146.2007. [DOI] [PubMed] [Google Scholar]
- Olichon A, Baricault L, Gas N, Guillou E, Valette A, Belenguer P, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278:7743–6. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- Poulton J, Morten K, Freeman-Emmerson C, Potter C, Sewry C, Dubowitz V, et al. Deficiency of the human mitochondrial transcription factor h-mtTFA in infantile mitochondrial myopathy is associated with mtDNA depletion. Hum Mol Genet. 1994;3:1763–9. doi: 10.1093/hmg/3.10.1763. [DOI] [PubMed] [Google Scholar]
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–5. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- Steinherz L, Steinherz P. Delayed cardiac toxicity from anthracycline therapy. Pediatrician. 1991;18:49–52. [PubMed] [Google Scholar]
- Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–41. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarska-Schlattner M, Wallimann T, Schlattner U. Alterations in myocardial energy metabolism induced by the anti-cancer drug doxorubicin. C R Biol. 2006;329:657–68. doi: 10.1016/j.crvi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- von Wurmb-Schwark N, Cavelier L, Cortopassi GA. A low dose of ethidium bromide leads to an increase of total mitochondrial DNA while higher concentrations induce the mtDNA 4997 deletion in a human neuronal cell line. Mutat Res. 2006;596:57–63. doi: 10.1016/j.mrfmmm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Wallace KB. Doxorubicin-induced cardiac mitochondrionopathy. Pharmacol Toxicol. 2003;93:105–15. doi: 10.1034/j.1600-0773.2003.930301.x. [DOI] [PubMed] [Google Scholar]
- Zhou S, Palmeira CM, Wallace KB. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett. 2001;121:151–7. doi: 10.1016/s0378-4274(01)00329-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Nucleoid remodelling within A549 and H9C2 cardiomyocytes. (A) PicoGreen/TMRM labelling of DXR/vehicle treated A549 cells (3.4 μM 24 hours). (B) Mitotracker red labelling of mitochondria within DXR/vehicle treated fibroblasts. Note that DXR intercalated into the nucleus is visible in the red channel after fixation. (C) PicoGreen labelling of H9C2 cardiomyocytes treated with DXR/vehicle. (D) Mitotracker labelling of vehicle/ DXR treated H9C2 cells. Bars: 20μm except B (10μm).
Supplementary Figure 2 Effects of DXR on TFAM, mtSSB, Tid1 and ATAD3. (A) Anti-Tid1/Mitotracker labelling of fibroblasts treated with vehicle/DXR (3.4 μM 24 hours). (B) Anti-ATAD/Mitotracker labelling of fibroblasts treated with vehicle/DXR. (C) Anti-DNA (IgM1)/anti-mtSSB labelling of DXR labelling of fibroblasts treated with vehicle/DXR. (D) Immunoblot of beta-actin and TFAM in fibroblasts treated with vehicle/DXR (3.4 μM 24 hours). Bars: 20μm. (DXR 3.4 μM 24 hours).
Supplementary Figure 3 DXR induced mitochondrial alterations are not secondary to cell death and does not compromise mitochondrial function. (Ai) Percentage apoptotic (TUNEL +ve) of vehicle/DXR treated fibroblasts (3.4μM) (n=200) * P = <0.05, *** P = <0.005 vs control. (Aii) Percentage necrotic (calcein −ve propidium + ve) of vehicle/DXR treated fibroblasts (3.4μM) (n=200). (Aiii) Cytochrome c/DAPI co-labelling of DXR (3.4 μM 24 hours), vehicle or H2O2 (80 mM 24 hours) treated fibroblasts. (Aiv) PicoGreen labelling of apoptotic fibroblasts following treatment with staurosporine (1 μM 24 hours). (Bi) Histochemical staining of cytochrome c oxidase activity of H9C2 or fibroblasts cells treated with DXR/ vehicle (3.4 μM 24 hours). (Bii) JC-1 labelling of fibroblasts treated with vehicle/DXR (3.4 μM 24 hours) - red ‘J-aggregates’ are present when mitochondria are polarised. (Biii) Polargraphic oxygen consumption of H9C2s treated with DXR/vehicle (3.4 μM 24 hours) or sodium azide (5mM 20 minutes) ($ p = 0.053, **P = <0.005 vs control, n = 3). (Biv) ATP levels measured by luciferase assay of DXR/vehicle (3.4 μM 24 hours) or rotenone (10 μM, 20 minutes) treated fibroblasts. Error bars +S.D. Size bars 20μm. Results are representative of three independent experiments. ‘a.u.’ = arbitrary units.
Supplementary Figure 4 Influence of MFN1/2 and OPA1 on mitochondrial morphology. (A) TMRM labelling of wild-type, MFN1 null and MFN2 null MEFs. (B) TMRM labelling of OPA1 or scramble siRNA treated cells. Immunoblotting blot of siRNA treated fibroblasts showing OPA1 and beta-actin protein levels.