Abstract
Objective
Fructose consumption has risen alongside obesity and diabetes. Gut hormones involved in hunger and satiety (ghrelin and PYY) may respond differently to fructose compared to glucose ingestion. We evaluated the effects of glucose and fructose ingestion on ghrelin and PYY in lean and obese adolescents with differing insulin sensitivity.
Methods
Adolescents were divided into lean (n=14), obese insulin sensitive (n=12) (OIS), and obese insulin resistant (n=15) (OIR). In a double-blind, cross-over design, subjects drank 75g of glucose or fructose in random order, serum was obtained every 10 minutes for 60 minutes.
Results
Baseline acyl-ghrelin was highest in lean and lowest in OIR (p=0.02). After glucose ingestion acyl-ghrelin decreased similarly in lean and OIS, but appeared lower in OIR (vs lean p=0.03). Suppression differences were more pronounced after fructose (lean vs. OIS p=0.008, lean vs. OIR p<0.001). OIS became significantly hungrier after fructose (p=0.015). PYY was not significantly different at baseline, varied minimally after glucose, and rose after fructose.
Conclusion
Compared to lean, OIS adolescents have impaired acyl-ghrelin responses to fructose but not glucose, whereas OIR adolescents have blunted responses to both. Diminished suppression of acyl-ghrelin in childhood obesity, particularly if accompanied by insulin resistance, may promote hunger and overeating.
Keywords: Glucose, fructose, acyl-ghrelin, PYY, insulin resistant, adolescents
Introduction
The worldwide increased consumption of sugar-sweetened beverages (SSBs) among both adults and children is considered a potential contributor to the obesity pandemic (1). A common component of added sugars is fructose, a glucose isomer, whose metabolism is distinct from that of glucose (2). Rodent studies indicate that fructose consumed orally or injected centrally increases food intake more than glucose (2). A recent study of lean adults by Page et al. (3) suggests that ingestion of glucose, but not fructose, decreases activation in the hypothalamus, insula, and striatum, brain regions known to be involved in regulating appetite, motivation, and reward processing. The distinct response to fructose may be due at least in part to the differential effects of fructose, compared to glucose, on gut-derived hunger and satiety hormones, such as ghrelin and peptide YY (PYY). Greater ghrelin suppression has been described after glucose compared to fructose ingestion alone (4) and in response to meals accompanied by glucose rather than fructose containing beverages (5) in lean men or women. However, Page et al. failed to detect significant differences in circulating levels of total ghrelin following glucose and fructose ingestion in lean adults (3), and Bowen et.al found no differences in obese men (6). Comparisons of the effect of glucose and fructose on ghrelin have not been studied in obese and lean children. Furthermore, although it has been suggested that the glucose-mediated insulin response may play a role in ghrelin suppression (7) the effects of adiposity and insulin resistance on ghrelin responses have not been clarified in children (8–12).
Ghrelin increases prior to mealtime, even in the absence of circadian and meal cues, and is suppressed by caloric intake (13). Obese, as compared to lean adults, have lower fasting ghrelin levels and limited postprandial ghrelin suppression (14). Given that the ghrelin levels inversely relate to insulin resistance in adults (15,16) and the responses of total and acyl-ghrelin correlate with insulin sensitivity in kids (17), to better evaluate the relationship of acyl-ghrelin with insulin resistance we divided the obese group into insulin sensitive and insulin resistant, removing the effect of body weight. Data is conflicting on whether obesity itself affects fasting ghrelin levels in children (9–12,18–21). However in all but one of these studies (12) total circulating ghrelin was measured rather than both the acylated (acyl) and unacylated forms of this hormone (22), whereas ghrelin’s orexigenic effect is dependent on its acylation at the serine 3 residue by the enzyme Ghrelin-O-Acyl-Transferase (GOAT) (23). Thus, studies of how specific macronutrients influence the action of ghrelin require measurement of its main biologically active form, acyl-ghrelin (24). In contrast to ghrelin, PYY is low during fasting and increases postprandially (25). Data is conflicting regarding PYY levels in lean and obese individuals (10,12,18,26).
In the United States adolescents consume 12.1% of their daily calories as fructose (27), yet the effects of fructose as compared to glucose ingestion on acyl-ghrelin and PYY, the two major gut-derived hormones implicated in the regulation of hunger, are not known. Therefore we studied how ingestion of glucose and fructose drinks affect the acute dynamic changes of acyl-ghrelin and PYY, and related these changes to subjective appetite ratings of hunger and fullness in adolescent children. Given that both ghrelin and PYY responses may be affected by adiposity and modulated by insulin sensitivity, the obese adolescents were matched for body weight and divided into insulin sensitive and insulin resistant groups. We hypothesized that the gut hormonal responses to ingested glucose and fructose would be altered to a greater extent in obese insulin resistant (OIR) adolescents as compared to the obese insulin sensitive (OIS) and lean adolescents and that these hormonal changes would be associated with greater hunger and less fullness.
Methods and Procedures
Lean and obese adolescents with similar distributions of age, gender, pubertal development, and ethnicity were recruited from the Yale Pediatric Obesity Clinic and the community. Based on the whole body insulin sensitivity index (WBISI), the obese adolescents were further divided into OIS and OIR groups. Each participant had a physical exam and detailed medical history. Eligible subjects were healthy and taking no chronic medications, or medications that effect glucose metabolism. Subjects were excluded for the following; diabetes, prediabetes (impaired fasting glucose or impaired glucose tolerance), abnormal renal function, pregnancy, endocrinopathies, chronic illness, psychiatric disorders, substance abuse, or use of anorexic agents.
Prior to enrollment in the study all subjects completed an oral glucose tolerance test (OGTT) as previously described (28) to assess glucose tolerance and WBISI, a dual-energy X-ray absorptiometry (Hologic Scanner, Boston, MA, USA) to evaluate body composition, and underwent MRI to evaluate hepatic fat fraction (HFF) (procedure described in supplemental methods). WBISI was calculated as reported by Matsuda (29). Anthropometric measures included height (measured by stadiometer), weight, and BMI both measured by body fat analyzer (TBF 300, Tanita, Arlington Heights, IL). BMI percentile determinations were made based on Center for Disease Control (CDC) growth charts. Subjects were divided into lean (BMI >25th to <75th percentile) and obese, (BMI >95th percentile), and the obese were further divided into OIS or OIR based on the median of the WBISI (3 l2/mg × µU) of our large multiethnic obese cohort. The Yale University School of Medicine Human Investigation Committee approved the study. Written consent and assent were obtained prior to the study.
STUDY PROCEDURES
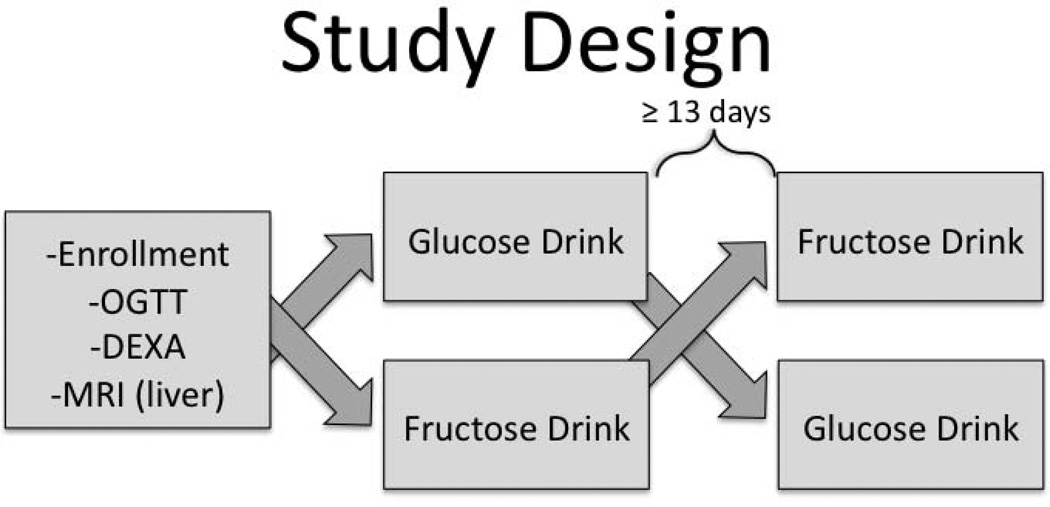
Subjects arrived at 0700 h to the Yale MRRC (Magnetic Resonance Research Center) after a 12 hour overnight fast. An IV was placed in an antecubital vein and two baseline (−20 and 0 minute) serum samples were obtained. Then in a double-blind, cross-over manner, subjects drank in random order either a 75g glucose drink or a 75g fructose drink, dissolved in 300 ml cherry flavored water (Figure 1). Of note 75g of glucose was an appropriate dose for all subjects if considering standard OGTT dosing. The testing dates from the glucose and fructose drinks were at minimum 13 days apart. Samples were analyzed for glucose, insulin, PYY, total ghrelin, and acyl-ghrelin at 10 minute intervals for 60 minutes. Additionally, baseline samples were collected for leptin and adiponectin. Samples were also obtained for measurement of plasma fructose levels at baseline as well as at 0, 20, 40, and 60 minutes following glucose and fructose ingestion.
Figure 1.
Study Design
OGTT: oral glucose tolerance test
DEXA: dual-energy X-ray absorptiometry
Behavioral ratings
Before drink consumption and after study completion, a visual analog scale (VAS) (30) was used to assess appetite ratings with higher scores indicating greater feelings of hunger, satiety, and fullness.
ANALYTICAL METHODS
Plasma glucose was determined using a glucose analyzer (YSI 2700 STAT Analyzer, Yellow Springs Instruments, Yellow Springs, OH) and plasma fructose levels were measured using gas chromatography–tandem mass spectrometry. Radioimmunoassays from Millipore were used for measurements of leptin, total ghrelin, acyl-ghrelin, PYY, and adiponectin. Blood samples obtained for acyl-ghrelin were prepared in EDTA tubes immediately after collection at the bedside. Specifically, the whole blood was promptly treated with Pefabloc SC (purchased from Sigma-aldrich, St. Louis, MO) to a final concentration of 1mg/ml, and then centrifuged for 10 minutes at 4° degrees Celsius 4000 rpm. The plasma obtained was acidified by adding HCL to a final concentration of 0.05 normal and stored at −80° Celsius until they were analyzed using the Millipore kit. The PYY assay measured both the hormone PYY3–36 and the full-length hormone PYY1–36. Intra assay variation was as follows: PYY 8.5–9.7%, total ghrelin 4.9–8.4%, and acyl- ghrelin 7.1–12.4%. All hormones were determined in duplicate and both studies for each individual subject were analyzed in the same assay to eliminate potential effects of interassay variation.
STATISTICAL ANALYSIS
Based on preliminary data in OIS and OIR adolescents, a two-sided 0.05 significance level and sample size of 20 per group would provide greater than 95% power to detect a 20% relative difference in the area under the curve of acyl-ghrelin percent change. Additionally to identify effects of obesity in addition to insulin resistance, a lean group was recruited, and goal recruitment was 25 per group, accommodating for 20% loss to follow-up.
Data are represented as the mean and standard deviation for continuous variables and frequencies for categorical variables. Baseline levels of hormones are summarized as the mean of the −20 and 0 minute time intervals from the glucose day and compared using analysis of variance. To evaluate the effect of the glucose and fructose drinks, the changes in plasma hormone levels from baseline were compared using a linear mixed model repeated measures analysis with SAS PROC MIXED (SAS 9.2, Cary, NC). Fixed effects in the model included drink type (Glucose or Fructose ingestion), time (10, 20, 30, 40, 50, 60 minutes after drinking), group (Lean, OIS, OIR), and their interactions. The effect of the order of drink was examined and not significant. To compare the changes after different drinks, the baseline value was also included as a covariate. Based on the type III tests for main effects and interaction effects, linear contrasts were used to further compare the differential responses to the glucose or fructose drink averaged across 60 minutes within each group, and to compare these differences between groups. The Bonferroni correction was used to adjust the multiple pair-wise group comparisons. Therefore p< 0.017 for those group comparisons was considered significant. Otherwise the significance level was p < 0.05. Changes in VAS within a group were compared using a paired T test. Spearman correlations were used to evaluate the relationships of adiposity, insulin resistance, insulin, and HFF with hormones.
Results
Baseline
As shown in Table 1, the three groups (lean n=14, OIS n=12, OIR n=15) were similar in terms of age (p=0.19), gender (p=0.30) and ethnicity (p=0.17). The mean Tanner stage of the cohort was 3.9±1.2. BMI varied amongst the three groups as expected based on the study design (p=<0.0001), however BMI in the two obese groups was indistinguishable (OIS 33.9±4.9, OIR 34.3±5.1, p=0.84). Furthermore, there were no significant differences in percent body fat (OIS 38.7±7.6, OIR 42.1±5.4, p=0.18) and lean body mass (OIS 59.1±12.2, OIR 54.7±7.2, p=0.26) among the obese groups. Despite having similar levels of adiposity, the OIR group had significantly higher fasting glucose and insulin levels than the OIS and lean groups. By study design, their level of insulin sensitivity was significantly different from both the OIS and lean groups as reflected by WBISI. Notably, WBISI (lean 3.7±1.6, OIS 3.2±0.9, p=0.40) and adiponectin levels (lean 11.0±3.7, OIS 12.1±8.4, p=0.66) were similar in both the lean and OIS groups, despite markedly different levels of adiposity. Baseline PYY was similar among the three groups and did not correlate with BMI (r= −0.039, p=0.825) or WBISI (r= −0.202, p=0.246). In contrast, baseline total and acyl-ghrelin levels were lowest in the OIR group and highest in the lean group (Table 1), and these differences were significant across the groups. Fasting acyl-ghrelin correlated positively with WBISI (r=0.406, p=0.013) and inversely with HFF (r= −0.603, p<0.001) (Table 2).
Table 1.
Main anthropometric and biochemical values at baseline
| Baseline Characteristics |
Lean N=14 |
Obese Insulin Sensitive N = 12 |
Obese Insulin Resistant N = 15 |
P-value |
|---|---|---|---|---|
| Female/Male | 4/10 | 7/5 | 7/8 | 0.30‡ |
| AA/C/H | 4/5/5 | 6/5/1 | 3/4/8 | 0.17‡ |
| Age (years) | 15.9±1.6 | 16.0±2.1 | 14.9±1.6 | 0.188 |
| BMI (kg/m2) | 21.4±2.92,3 | 33.9±4.91 | 34.3±5.11 | <.0001 |
| Metabolic Profile | ||||
| Fasting Glucose (mg/dl) | 86.9±5.33 | 87.9±5.53 | 93.7±6.11,2 | 0.005 |
| Fasting Insulin (µU/ml) | 14.5±62,3 | 21.2±7.41,3 | 41.6±14.21,2 | <.0001 |
| 2 Hour Glucose (mg/dl) | 97.5±15.13 | 101.6±13.13 | 123.9±20.51,2 | 0.0003 |
| 2 Hour Insulin (µU/ml) | 62.9±42.63 | 73.1±30.33 | 309.3±169.31,2 | 0.012 |
| Leptin (ng/ml) | 8.3±9.42,3 | 37.8±21.51 | 37.8±15.81 | <0.0001 |
| Adiponectin (µg/ml) | 11.0±3.73 | 12.1±8.4 | 7.0±2.41 | 0.039 |
| WBISI* (l2/mg × µU) | 3.7±1.63 | 3.2±0.93 | 1.1±0.51,2 | <.0001 |
| % Body Fat | 22.2±8.22,3 | 38.7±7.61 | 42.1±5.41 | <.0001 |
| Lean Body Mass (kg) | 49.3±9.72 | 59.1±12.21 | 54.7±7.2 | 0.049 |
| Hepatic Fat Fraction (%) | 0.22±0.8 | 3.7±10.9 | 7.5±10.8 | 0.107 |
| Total Cholesterol (mg/dl) | 133.3±20.4 | 151±10.7 | 148±27.2 | 0.079 |
| LDL (mg/dl) | 68.2±17.62 | 91.8±11.51 | 86±23.4 | 0.006 |
| HDL (mg/dl) | 51.4±12.53 | 46.3±9.2 | 38±10.41 | 0.007 |
| Triglycerides (mg/dl) | 68.5±28.8 | 65.1±19.7 | 128.7±129.3 | 0.075 |
| Gut Derived Hormones | ||||
| Total Ghrelin (pg/ml) | 817.6±258.63 | 595.1±293.0 | 575.7±174.81 | 0.024 |
| Acyl-ghrelin (pg/ml) | 126.5±41.83 | 108.8±45.5 | 84.4±29.61 | 0.022 |
| PYY (pg/ml) | 83.7±20.1 | 73.0±20.7 | 89.1±17.3 | 0.159 |
Data are reported as frequencies for gender and ethnicity and mean value ± standard deviation for all other characteristics.
1, 2 and 3 indicate significant post-hoc pair-wise difference between Lean1, OIS2, and OIR3 after Bonferroni correction for multiple comparisons.
AA: African Americans, C: Caucasians, H: Hispanics
Chi-Square
Whole Body Insulin Sensitivity Index
Table 2.
Baseline acyl-ghrelin and PYY correlations
| Baseline Acyl-Ghrelin (pg/ml) | Baseline PYY (pg/ml) | |||
|---|---|---|---|---|
| r | p | r | p | |
| WBISI (l2/mg × µU) | 0.4057 | 0.0127 | −0.2016 | 0.2456 |
| % Body Fat | −0.1822 | 0.31 | −0.0627 | 0.7419 |
| Hepatic Fat Fraction (%) | −0.6025 | 0.0001 | 0.1304 | 0.4695 |
Data indicate results of the Spearman correlation.
Hormonal changes in response to the Glucose drink and Fructose drink
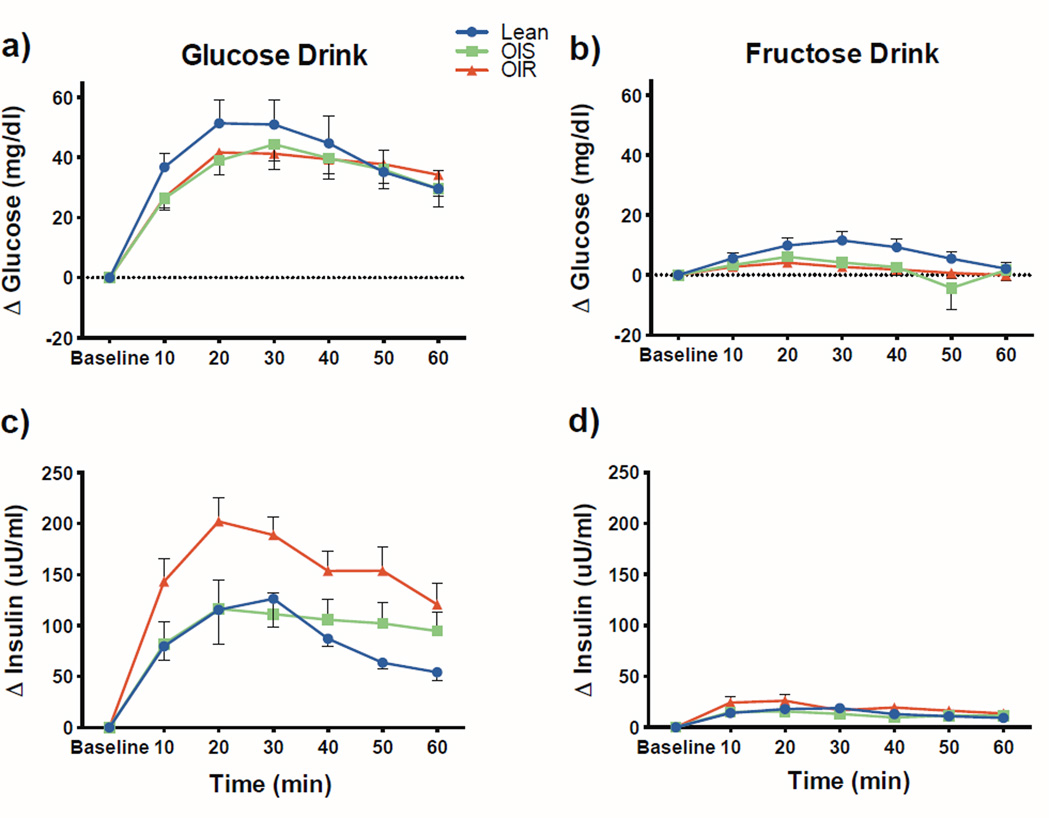
As expected the overall mean change in plasma glucose from baseline was markedly lower for the fructose drink compared to the glucose drink, as indicated by the main effect of drink type (3.6 ± 0.7 vs. 37.2 ± 2.8, p<.0001) and this was true for each individual group (p<0.0001) (Figure 2). Moreover, the group by drink type interaction was not significant (p=0.92), indicating that the difference between the two drinks was similar in all groups. In keeping with these findings, the glucose drink induced a markedly greater insulin response as compared to the fructose drink in all three groups (p<0.0001). However, the effect of drink type differed across groups (interaction effect p=0.006). Specifically, insulin stimulation was significantly greater in the OIR as compared to the lean group (difference 67.6 ± 19.9, p=0.0008) and tended to be lower in the OIS group (difference 52.4 ± 23.5, p=0.027).
Figure 2.
Glucose (a and b) and insulin (c and d) changes from baseline after the glucose and fructose drinks in lean (blue) obese insulin sensitive (OIS, green) and obese insulin resistant (OIR, red) adolescents. Plotted points represent mean and error bars reflect the standard error.
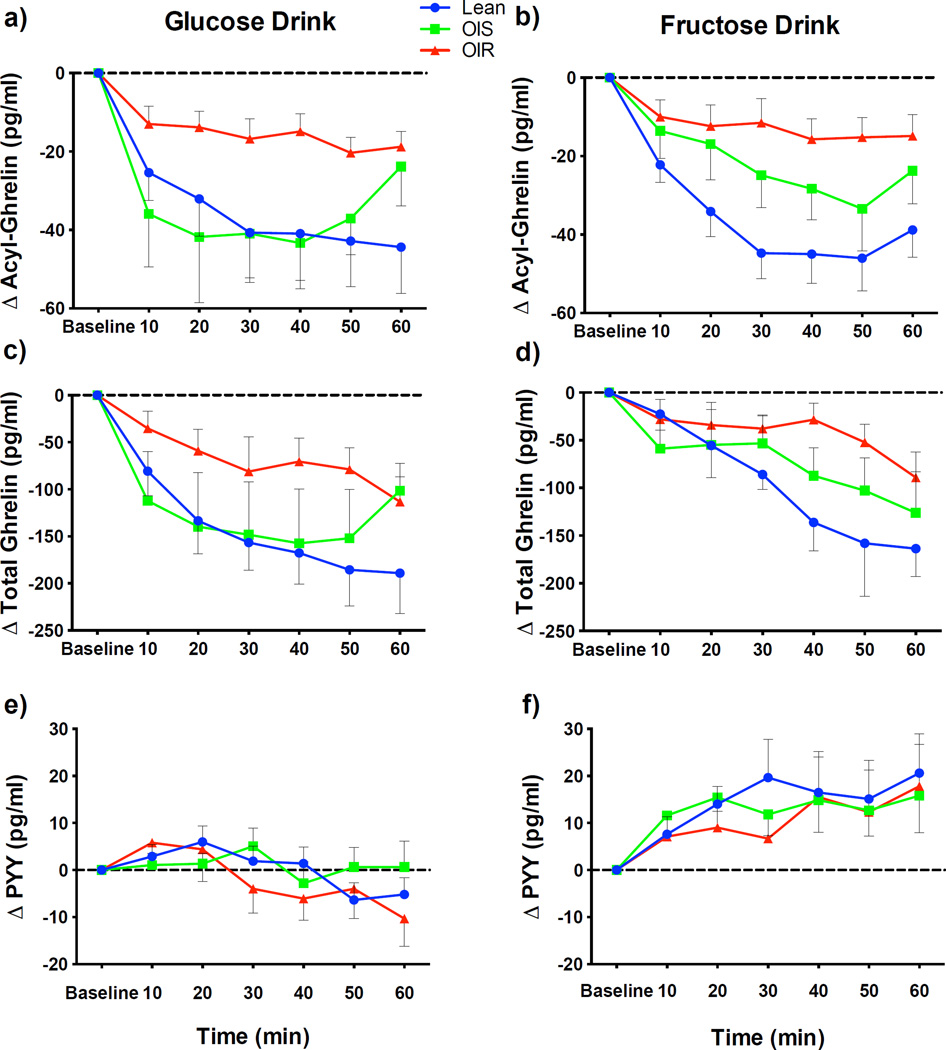
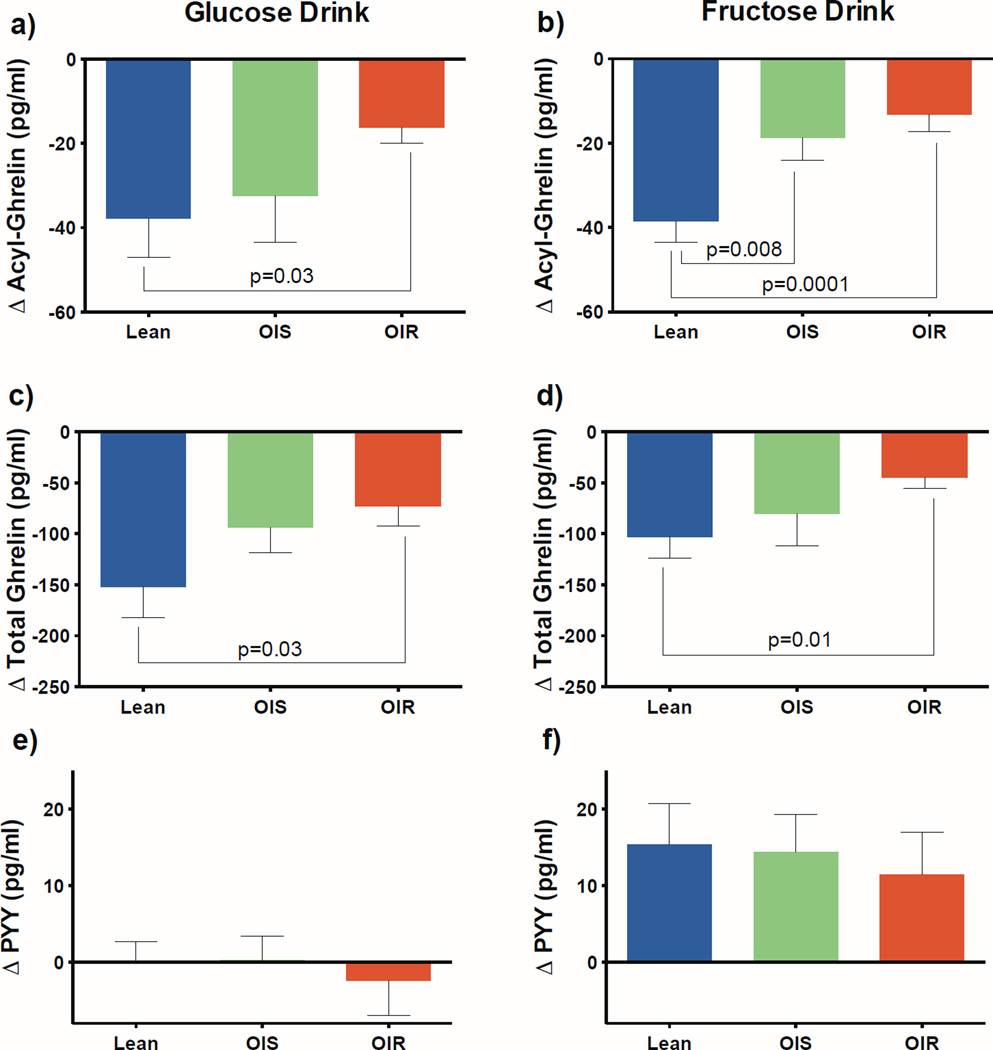
Acyl-ghrelin suppression during the 60 minutes differed by group (main effect of group for glucose p=0.01 and fructose p=0.001). In lean subjects, both the glucose and fructose drinks promptly suppressed circulating total ghrelin as well as acyl-ghrelin (Figure 3). The glucose drink was accompanied by a similar mean suppression in acyl-ghrelin between the OIS and lean (p=0.72), whereas the fructose drink caused a blunted suppression of acyl-ghrelin in the OIS compared to the lean group (Figure 4, −18.7 ± 5.4 vs. −38.5 ± 5.0, p=0.008 OIS vs. lean control). Suppression of total and acyl-ghrelin levels were significantly impaired after the fructose drink in the OIR group as compared to the response seen in the lean adolescents, and were approaching significance in the glucose drink after the correction for multiple comparisons (Figure 4, fructose drink total ghrelin −44.9 ± 10.5 vs. −103.0 ± 20.7, p=0.01, acyl-ghrelin −13.3 ± 4.0 vs. −38.5 ± 5.0, p=0.0001, glucose drink total ghrelin −73.0 ± 19.4 vs. −152.1 ± 30.0, p=0.03, acyl-ghrelin −16.3 ± 3.6 vs. −37.7 ± 9.4, p=0.03). Hence, while in the lean group glucose and fructose ingestion suppressed acyl-ghrelin to a similar extent (lean glucose vs. fructose difference 3.4 ± 5.7 SE, p=0.55), the presence of obesity and more importantly insulin resistance impacted the magnitude of acyl-ghrelin suppression, which was further diminished in response to the fructose drink. Furthermore, lower sensitivity (WBISI) correlated with a smaller mean decrement in acyl-ghrelin after the glucose drink (r= −0.325, p=0.049) and was stronger after the fructose drink (r= −0.470, p =0.004). The mean change in plasma insulin did not correlate significantly with the change in acyl-ghrelin for either drink.
Figure 3.
Hormonal changes from baseline after the glucose and fructose drinks in lean (blue) obese insulin sensitive (OIS, green) and obese insulin resistant (OIR, red) adolescents.
Acyl-Ghrelin (a,b), Total Ghrelin (c,d), PYY (e,f). Plotted points represent mean and error bars reflect the standard error.
Figure 4.
Least squared means of the hormonal change and standard error during the 60 minutes after either the glucose or fructose drink in lean (blue) obese insulin sensitive (OIS, green) and obese insulin resistant (OIR, red) adolescents. Acyl-Ghrelin (a,b), Total Ghrelin (c,d), PYY (e,f).
Mean hormonal change represents the mean of the 10, 20, 30, 40, 50, and 60 minute changes from baseline. P values <0.017 are significant. No significant differences were noted between groups for PYY.
Circulating plasma PYY levels increased in response to the fructose drink in all three groups compared to the response seen after glucose (lean 15.1 ± 5.8, p=0.01, OIS 13.6 ± 6.0, p=0.02, OIR 13.9 ± 7.3, p=0.06). The stimulation of PYY did not differ by group (group by drink interaction (p=0.98) although the overall main effect of drink type was significant (p<0.005). Mean change in PYY was positively correlated with WBISI, more strongly during the fructose than the glucose drink (glucose r= −0.325, p=0.049, fructose r= −0.470, p=0.004), and was not correlated with insulin levels.
Behavioral Ratings
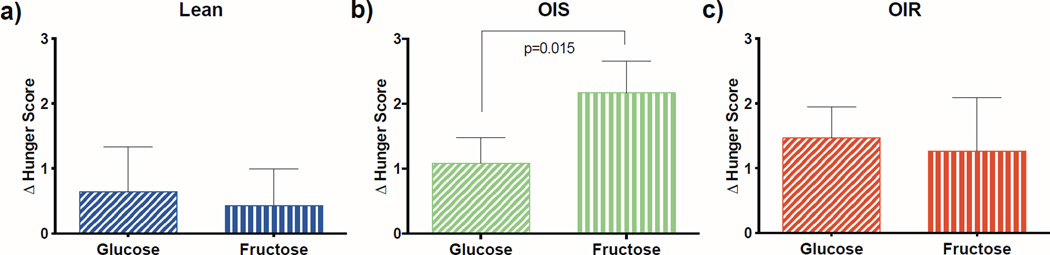
There were no significant differences in baseline hunger, fullness, or satiety on the glucose versus fructose days. While the changes in hunger scores were similar following ingestion of both glucose and fructose in lean and OIR adolescents, the OIS adolescents had a significantly greater change in hunger score following the fructose as compared to the glucose drink (p=0.015), indicating that they become hungrier after consumption of fructose (Figure 5).
Figure 5.
Change in hunger score by group a) lean, b) OIS, and c) OIR, for the glucose (slashed lines) and fructose (vertical lines) drinks. P value <0.05 is indicated.
Discussion
This study describes the differential effects of glucose and fructose drinks on the acute dynamic changes in two major gut-derived satiety hormones, acyl-ghrelin and PYY, in adolescents with different levels of adiposity and insulin sensitivity. The following key findings emerged: 1) in lean adolescents acyl-ghrelin levels were suppressed by 30% in response to either glucose or fructose drinks; 2) in OIS adolescents the glucose drink suppressed acyl-ghrelin to a similar extent as it did in the lean, whereas the fructose drink failed to diminish acyl-ghrelin levels to the same extent as that seen in lean adolescents; and 3) in the OIR adolescents suppression of acyl-ghrelin was blunted following both the glucose and fructose drinks. Suppression in acyl-ghrelin was correlated with insulin sensitivity, particularly during the fructose drink. Additionally hunger in the OIS group increased significantly during the fructose drink while acyl-ghrelin suppression was blunted, consistent with a role for acyl-ghrelin in satiety signaling. PYY on the other hand was fairly stable after the glucose drink, but rose after the fructose drink in all groups. Unlike ghrelin, the PYY response appeared to be independent of adiposity and insulin resistance, but rather influenced by the type of sugar ingested.
Insulin resistance impacted the capacity of glucose and fructose ingestion to diminish acyl-ghrelin, the active form of the hormone. Whereas glucose ingestion suppressed acyl-ghrelin identically in lean and OIS adolescents, this response approached significant impairment in OIR adolescents (Figures 3 and 4). In contrast, the acyl-ghrelin suppression seen in lean individuals following fructose ingestion was diminished in both obese groups; but this effect of obesity was more pronounced in OIR than OIS adolescents. Thus it would appear that in addition to obesity in adolescents, the presence of insulin resistance further limits the capacity of fructose to suppress this key orexigenic hormone and may continue to promote hunger and overconsumption of fructose (or other calories), particularly in obese adolescents who are insulin resistant. Fructose consumption in diet promotes hepatic de novo lipogenesis and the accumulation of fatty acids into the liver. The accumulation of fatty acids in the hepatocytes leads to the increased production of diacylglycerol, whose accumulation impairs insulin signaling (31).
Clamp studies have shown that inducing a hyperinsulinemic state rapidly reduces total ghrelin levels in healthy individuals, but the reduction is blunted in adults with type 2 diabetes (32). In obese and overweight postmenopausal women without diabetes, the euglycemic hyperinsulinemic clamp was found to significantly decrease acyl-ghrelin in the overweight or OIS women, but not OIR women (32). Our findings in response to the glucose drink are in agreement with these studies, indicating that physiologic changes present in the insulin resistant state affect ghrelin signaling. The fructose drink, however has a minimal effect on insulin levels, yet a clear gradation is seen in the acyl-ghrelin responses of the three groups, with the OIS response between that of the robust lean and limited OIR response. One can speculate that simple obesity, without insulin resistance, may improve the glucose induced ghrelin response, making the impairment in acyl-ghrelin apparent when insulin is removed from the equation.
Ghrelin administration has been shown to increase appetite and food intake in lean and obese adults (33–35). Acyl-ghrelin acts by binding to its receptor, the GHS-R1a (22) that in turn promotes hunger through the release of neuropeptide Y and agouti-related protein from neurons in the arcuate nucleus of the hypothalamus (36). It is intriguing to speculate that the persistent elevation in acyl-ghrelin after drinking or eating fructose may help explain the reduced satiety feelings often reported in obese children. This was apparent in the OIS group, where the change in hunger was significantly greater after the fructose compared to the glucose drink.
PYY3–36 also acts at the arcuate nucleus, but in contrast to ghrelin promotes satiety via Y2 receptors (25). However, unlike acyl-ghrelin, we observed that fasting levels of PYY were not altered by obesity (as previously reported (10,12)) or insulin resistance. Surprisingly, the satiety hormone PYY was stimulated by fructose ingestion in all groups, while there was negligible change in PYY after the glucose drink. Prior studies found that while PYY increased after meals in the lean, this response was inconsistent in obese children (10,12). Additional studies will be needed to clarify the effects of macronutrients and obesity on PYY responses.
It is important to note that glucose and fructose are rarely consumed in isolation, and when consumed together their effects on gut hormones may be different; however, to clearly understand the unique physiologic response to each monosaccharide we chose to study them separately in this initial study. Future studies should investigate the effects of these sugars in combination, as commonly consumed in high fructose corn syrup. A limitation of this study was use of a PYY assay which measured both PYY1–36 and PYY3–36, which have been described to have different effects on appetite (25). Studying obestatin, an appetite regulating hormone encoded by the same gene as ghrelin and known to be altered in obese youth (37,38), would also have been valuable and should be included in future studies. Baseline leptin values were obtained as a secondary measure of adiposity, however leptin values at additional time points could have provided more insight. Additionally subjects fasted overnight prior to the studies, however a standardized meal and activity plan for the day prior to each visit would have strengthened this study. Although a possible confounder in our study is the potential different timing of insulin resistance in males and females during puberty, recently a study by Jeffery et al. has shown that timing of the insulin resistance peak does not differ much between boys and girls (39). Finally, it proved difficult to recruit subjects and match them by ethnicity, thus future studies should pay particular attention to subject number and ensure a similar distribution of ethnicity in each group.
Estimates of obesity in the US by the year 2030 are alarming, forecasting that 50–51% of adult men and 45–52% of women will be obese, which can be expected to increase prevalence rates of diabetes, stroke, heart disease, and cancer in these individuals (40). Obese children are likely to remain obese as adults, thus the hormonal responses to fructose in this population may have far reaching effects and deserve serious consideration. Glucose and fructose drinks suppressed acyl-ghrelin similarly in lean adolescents. In contrast, compared to lean adolescents, suppression of acyl-ghrelin is impaired to a greater extent following fructose than following glucose ingestion in obese insulin sensitive adolescents, and to both drinks in obese insulin resistant adolescents. We speculate that the impaired orexigenic hormonal responses to fructose seen in the obese adolescents may contribute to altered satiety and overeating.
Supplementary Material
What is already known on this subject
A rise in fructose consumption has accompanied the obesity epidemic.
In adults glucose and fructose ingestion have different effects on brain areas involved in appetite regulation.
What this study adds
An understanding of how glucose as compared to fructose affects gut hormones involved in appetite regulation.
An understanding of how adiposity and insulin resistance affect gut hormone responses to glucose and fructose in adolescents.
Acknowledgements
We wish to thank the staff and nurses of the Yale Hospital Research Unit of the Yale Center for Clinical Investigation (YCCI) funded by the National Center for Advancing Translational Sciences (UL1-RR-024139) for their assistance with collecting serum samples, and the YCCI Core Lab for conducting the hormone assays. This work was also supported in part by the Yale Diabetes Research Center (DK-045735) as well as by NIH grants 1R01DK085577-01 (PI: Caprio, Co-PI: Sherwin, Co-I: Sinha), R01-HD40787 (PI: Caprio), R01-HD-28016 (PI: Caprio), DRC P30DK045735, K12DK094714-02 (PI: Tamborlane), and the Pediatric Endocrine Society (Van Name).
Footnotes
Conflicts of Interest: The authors declare that they have no conflicts of interest associated with this manuscript. Bob Sherwin consulted for or served on Data Safety Monitoring Boards within the last year for BMS, AstraZeneca, Lilly, Novo Nordisk, MannKind, Merck, Novartis. Ania Jastreboff provided assistance to Atrium Staffing for the New Haven Pfizer Clinical Research Unit. All other authors have nothing to disclose.
Author Contributions: SC, RS, RSS, and CG were responsible for the study design and funding. SC, RS, CG, JK, SM, MS, RK, GC and ED were responsible for the data collection; MV, AMJ, SC, FL, and JD conducted data analysis; MV, NS, CG, AMJ, RS, RSS, and SC contributed to the interpretation of the data; MV, SC, AMJ, RS, and RSS wrote the manuscript.
Clinical Trial Registration Number: # NCT01808846
References
- 1.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics. 2008 Jun;121(6):e1604–e1614. doi: 10.1542/peds.2007-2834. [DOI] [PubMed] [Google Scholar]
- 2.Cha SH, Wolfgang M, Tokutake Y, Chohnan S, Lane MD. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proceedings of the National Academy of Sciences of the United States of America. 2008 Nov 4;105(44):16871–16875. doi: 10.1073/pnas.0809255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Page KA, Chan O, Arora J, Belfort-Deaguiar R, Dzuira J, Roehmholdt B, et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA : the journal of the American Medical Association. 2013 Jan 2;309(1):63–70. doi: 10.1001/jama.2012.116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prodam F, Me E, Riganti F, Gramaglia E, Bellone S, Baldelli R, et al. The nutritional control of ghrelin secretion in humans: the effects of enteral vs. parenteral nutrition. European journal of nutrition. 2006 Oct;45(7):399–405. doi: 10.1007/s00394-006-0613-z. [DOI] [PubMed] [Google Scholar]
- 5.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, et al. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. The Journal of clinical endocrinology and metabolism. 2004 Jun;89(6):2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 6.Bowen J, Noakes M, Clifton PM. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int J Obes (Lond) 2007 Nov;31(11):1696–1703. doi: 10.1038/sj.ijo.0803665. [DOI] [PubMed] [Google Scholar]
- 7.Griffen SC, Oostema K, Stanhope KL, Graham J, Styne DM, Glaser N, et al. Administration of Lispro insulin with meals improves glycemic control, increases circulating leptin, and suppresses ghrelin, compared with regular/NPH insulin in female patients with type 1 diabetes. The Journal of clinical endocrinology and metabolism. 2006 Feb;91(2):485–491. doi: 10.1210/jc.2005-1338. [DOI] [PubMed] [Google Scholar]
- 8.Soriano-Guillen L, Barrios V, Martos G, Chowen JA, Campos-Barros A, Argente J. Effect of oral glucose administration on ghrelin levels in obese children. European journal of endocrinology / European Federation of Endocrine Societies. 2004 Jul;151(1):119–121. doi: 10.1530/eje.0.1510119. [DOI] [PubMed] [Google Scholar]
- 9.Baldelli R, Bellone S, Castellino N, Petri A, Rapa A, Vivenza D, et al. Oral glucose load inhibits circulating ghrelin levels to the same extent in normal and obese children. Clinical endocrinology. 2006 Mar;64(3):255–259. doi: 10.1111/j.1365-2265.2006.02441.x. [DOI] [PubMed] [Google Scholar]
- 10.Lomenick JP, Clasey JL, Anderson JW. Meal-related changes in ghrelin, peptide YY, appetite in normal weight and overweight children. Obesity (Silver Spring) 2008 Mar;16(3):547–552. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 11.Gil-Campos M, Aguilera CM, Ramirez-Tortosa MC, Canete R, Gil A. Fasting and postprandial relationships among plasma leptin, ghrelin, and insulin in prepubertal obese children. Clin Nutr. 2010 Feb;29(1):54–59. doi: 10.1016/j.clnu.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Mittelman SD, Klier K, Braun S, Azen C, Geffner ME, Buchanan TA. Obese adolescents show impaired meal responses of the appetite-regulating hormones ghrelin and PYY. Obesity (Silver Spring) 2010 May;18(5):918–925. doi: 10.1038/oby.2009.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings DE, Frayo RS, Marmonier C, Aubert R, Chapelot D. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. American journal of physiology. Endocrinology and metabolism. 2004 Aug;287(2):E297–E304. doi: 10.1152/ajpendo.00582.2003. [DOI] [PubMed] [Google Scholar]
- 14.le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. The Journal of clinical endocrinology and metabolism. 2005 Feb;90(2):1068–1071. doi: 10.1210/jc.2004-1216. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin TAF, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. The Journal of clinical endocrinology and metabolism. 2004;89(4):1630–1635. doi: 10.1210/jc.2003-031572. [DOI] [PubMed] [Google Scholar]
- 16.Delhanty PJ, van der Lely AJ. Ghrelin and glucose homeostasis. Peptides. 2011 Nov;32(11):2309–2318. doi: 10.1016/j.peptides.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Prodam F, Monzani A, Ricotti R, Marolda A, Bellone S, Aimaretti G, et al. Systematic review of ghrelin response to food intake in pediatric age, from neonates to adolescents. The Journal of clinical endocrinology and metabolism. 2014 May;99(5):1556–1568. doi: 10.1210/jc.2013-4010. [DOI] [PubMed] [Google Scholar]
- 18.Stock S, Leichner P, Wong AC, Ghatei MA, Kieffer TJ, Bloom SR, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. The Journal of clinical endocrinology and metabolism. 2005 Apr;90(4):2161–2168. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 19.Maffeis C, Bonadonna RC, Consolaro A, Vettor R, Banzato C, Silvagni D, et al. Ghrelin, insulin sensitivity and postprandial glucose disposal in overweight and obese children. European journal of endocrinology / European Federation of Endocrine Societies. 2006 Jan;154(1):61–68. doi: 10.1530/eje.1.02055. [DOI] [PubMed] [Google Scholar]
- 20.Zou CC, Liang L, Wang CL, Fu JF, Zhao ZY. The change in ghrelin and obestatin levels in obese children after weight reduction. Acta Paediatr. 2009 Jan;98(1):159–165. doi: 10.1111/j.1651-2227.2008.00997.x. [DOI] [PubMed] [Google Scholar]
- 21.Bascietto C, Giannini C, D'Adamo E, de Giorgis T, Chiarelli F, Mohn A. Implications of gastrointestinal hormones in the pathogenesis of obesity in prepubertal children. Journal of pediatric endocrinology & metabolism : JPEM. 2012;25(3–4):255–260. doi: 10.1515/jpem-2011-0478. [DOI] [PubMed] [Google Scholar]
- 22.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999 Dec 9;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008 Feb 8;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Davis JF, Perello M, Choi DL, Magrisso IJ, Kirchner H, Pfluger PT, et al. GOAT induced ghrelin acylation regulates hedonic feeding. Hormones and behavior. 2012 Nov;62(5):598–604. doi: 10.1016/j.yhbeh.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballantyne GH. Peptide YY(1–36) and peptide YY(31–36): Part I. Distribution, release and actions. Obesity surgery. 2006 May;16(5):651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 26.Haltia LT, Savontaus E, Vahlberg T, Rinne JO, Kaasinen V. Acute hormonal changes following intravenous glucose challenge in lean and obese human subjects. Scandinavian journal of clinical and laboratory investigation. 2010 Jul;70(4):275–280. doi: 10.3109/00365511003792975. [DOI] [PubMed] [Google Scholar]
- 27.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape journal of medicine. 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 28.Yeckel CW, Weiss R, Dziura J, Taksali SE, Dufour S, Burgert TS, et al. Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. The Journal of clinical endocrinology and metabolism. 2004 Mar;89(3):1096–1101. doi: 10.1210/jc.2003-031503. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes care. 1999 Sep;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 30.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2000 Jan;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 31.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. The New England journal of medicine. 2014 Sep 18;371(12):1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 32.Chabot F, Caron A, Laplante M, St-Pierre DH. Interrelationships between ghrelin, insulin and glucose homeostasis: Physiological relevance. World journal of diabetes. 2014 Jun 15;5(3):328–341. doi: 10.4239/wjd.v5.i3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, et al. Ghrelin enhances appetite and increases food intake in humans. The Journal of clinical endocrinology and metabolism. 2001 Dec;86(12):5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 34.Druce MR, Wren AM, Park AJ, Milton JE, Patterson M, Frost G, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 2005 Sep;29(9):1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 35.Druce MR, Neary NM, Small CJ, Milton J, Monteiro M, Patterson M, et al. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. Int J Obes (Lond) 2006 Feb;30(2):293–296. doi: 10.1038/sj.ijo.0803158. [DOI] [PubMed] [Google Scholar]
- 36.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001 Jan 11;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 37.Balagopal PB, Gidding SS, Buckloh LM, Yarandi HN, Sylvester JE, George DE, et al. Changes in circulating satiety hormones in obese children: a randomized controlled physical activity-based intervention study. Obesity (Silver Spring) 2010 Sep;18(9):1747–1753. doi: 10.1038/oby.2009.498. [DOI] [PubMed] [Google Scholar]
- 38.Reinehr T, de Sousa G, Roth CL. Obestatin and ghrelin levels in obese children and adolescents before and after reduction of overweight. Clinical endocrinology. 2008 Feb;68(2):304–310. doi: 10.1111/j.1365-2265.2007.03042.x. [DOI] [PubMed] [Google Scholar]
- 39.Jeffery AN, Metcalf BS, Hosking J, Streeter AJ, Voss LD, Wilkin TJ. Age before stage: insulin resistance rises before the onset of puberty: a 9-year longitudinal study (EarlyBird 26) Diabetes care. 2012 Mar;35(3):536–541. doi: 10.2337/dc11-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011 Aug 27;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.