Abstract
Although circulating tumor cells (CTCs) in blood have been widely investigated as a potential biomarker for diagnosis and prognosis of metastatic cancer, their inherent rarity and heterogeneity bring tremendous challenges to develop a CTC detection method with clinically significant specificity and sensitivity. With advances in nanotechnology, a series of new methods that are highly promising have emerged to enable or enhance detection and separation of CTCs from blood. In this review, we systematically categorize nanomaterials, such as gold nanoparticles, magnetic nanoparticles, quantum dots, graphenes/graphene oxides, and dendrimers and stimuli-responsive polymers, used in the newly developed CTC detection methods. This will provide a comprehensive overview of recent advances in the CTC detection achieved through application of nanotechnology as well as the challenges that these existing technologies must overcome to be directly impactful on human health.
Introduction
Circulating tumor cell (CTC) detection from blood, often as referred to “liquid biopsy”, has attracted a great deal of scientific and clinical interests, particularly because this method can be potentially used to diagnose metastatic cancer and to monitor the disease progress without invasive tissue biopsy.1-4 After escaping from the primary tumor site, CTCs travel through the bloodstream, extravasating and initiating secondary tumor colonies, or dying in bloodstream. First observed in the blood of a metastatic cancer patient by Dr. Ashworth in 1869,5 CTCs have received a great amount of attention since the mid 1990’s for their clinical value as a biomarker that is closely correlated to cancer metastasis.6 To isolate the CTCs, a number of technologies have been developed to differentiate CTCs based on their unique biological and/or physical properties that are distinct from hematological cells.7 Among those, CellSearch™, ISET™, and CTC-chip are three CTC detection methods that are in advanced stages of clinical translation. CellSearch™ (Janssen Diagnostics), the first and currently only FDA-approved system for the automated CTC detection for breast, prostate, and colorectal metastatic cancer, relies on the immunomagnetic separation of CTCs using an antibody against a CTC marker, epithelial cell adhesion molecule (EpCAM).1 Using size-based separation, ISET™ discriminates CTCs from hematological cells by filtration through an 8 μm pore filter due to the larger size of CTCs relative to hematologic cells. CTC-chip is an immunoaffinity-based microfluidic device functionalized with silane chemicals, neutravidin, and biotinylated anti-EpCAM. The 1st and 2nd Generations of CTC-chip8, 9 were based on microfluidic devices with microposts and herringbone mixers, respectively, to increase hydrodynamic efficiency of the flow and thereby capture efficiency. However, due to the rarity (as few as one in the background of 106-109 hematologic cells) among blood cells10 and heterogeneity of CTCs,11 clinically significant detection of CTCs still remains a tremendous technical challenge. In the process of achieving sensitive CTC detection, a variety of new detection methods have been extensively investigated, which is reflected in that the number of publications on the subject has exponentially increased since the 1990’s (Figure 1).
Figure 1. Trend in CTC capture research.
Number of publications regarding CTC capture from 1950 to the present (Based on a search result for “separation or isolation or enrichment or detection or capture or recovery” and “circulating tumor cells” as keywords from ISI-Web of Science).
In many of the emerging CTC detection techniques, nanomaterials, such as gold nanoparticles, magnetic nanoparticles, quantum dots, graphenes/graphene oxides, and dendrimers/stimuli-responsive polymers, have played a central role in the enhancement of immunoaffinity-based detection of CTCs. Although many reviews have summarized the recent advances in CTC detection,7,12-14 the critical role played by nanomaterials in the field has not been thoroughly reviewed. In this review, we therefore focus on emerging nanomaterials that have been utilized in the enhancement of immunoaffinity-based detection of CTCs. Chemical modification methods, detection mechanisms, and advantages of the frequently used nanomaterials are summarized in Table 1. By categorizing based on the nanomaterials, we discuss the advantages and disadvantages of each nanomaterial, along with recent advances in the related technologies, providing a comprehensive overview regarding the use of nanoparticles to enhance CTC detection and to overcome the challenges of the existing technologies.
Table 1.
Summary of the nanomaterials used in emerging CTC detection platforms.
| Type | Chemical modification |
Detection mechanism |
Advantages | Ref. |
|---|---|---|---|---|
| Gold nanoparticles |
Polymeric coating or linkers combined with CTC targeting ligands |
Photoacoustic imaging after in vivo injection |
|
17, 18, 23, 24 |
|
| ||||
| Raman reporter molecule or silver coating combined with CTC targeting ligands |
Ultrasensitive surface-enhanced Raman scattering (SERS) |
|
27-31 | |
|
| ||||
| Magnetic nanoparticles (MNPs) |
Polymeric coating or linkers combined with CTC targeting ligands |
Isolation of CTCs from the drawn blood specimens |
|
1, 35, 38-43 |
|
| ||||
| Quantum dots (QDs) |
Polymeric coating or linkers combined with QDs and CTC targeting ligands |
Fluorescence-based identification |
|
45, 46 |
|
| ||||
| Graphene/ graphene oxides (GO) |
Adsorption of dye- labelled biomolecules via pi-pi stacking |
|
|
49-51 |
|
| ||||
| Dendrimers/ Stimuli- responsive polymers |
Covalent conjugation or modification with CTC specific antibodies/ligands |
Immunostaining confirmation after CTC capture on the platform |
|
54, 58, 59, 61-66 |
1. Nanotechnology used in emerging CTC detection platforms
1.1. Gold nanoparticles
Enhanced light absorption and scattering properties of gold nanoparticles have been employed in detecting CTCs as the binding between gold nanoparticles and CTCs can be quantitatively measured via photoacoustic signals or surface plasmon resonance (SPR) shifts. A variety of gold nanoparticles, such as gold nanospheres, nanorods, and nanoshells, can be prepared and integrated with targeting ligands, imaging labels, therapeutic drugs, and other functionalities.15 Gold nanoparticles can be used in in vivo imaging and ex vivo diagnostic sensors given its capacity to provide enormous sensitivity, throughput, and flexibility. Depending on the particle size and shape, the surface plasmon resonance (SPR) of gold nanoparticles is varied: the narrow ranges of nanospheres (~520-550 nm); splitting into two modes of nanorods (one longitudinal mode parallel to the long axis of the rod and a transverse mode perpendicular to the long axis, 520-550 nm and 720-750 nm); and NIR-closing ranges of nanoshells (850-900 nm).16 In particular, due to the unique SPR splitting, gold nanorods have been frequently utilized for CTC detection using techniques such as photoacoustic imaging.17, 18
1.1.1. Gold nanoparticles for CTC targeting in vivo
CTCs in blood stream can be targeted in vivo by injecting nanomaterials targeting CTCs, enabling in situ monitoring of the number of CTCs. The real-time CTC monitoring in vivo eliminates the necessity of blood sampling, sample preparation, or CTC isolation, and induces the phagocytic clearance of CTCs upon binding. However, for the CTC-targeted nanoparticles to be effective, the nanoparticles should overcome high shear stress of blood circulation, induce no immune responses, and prevent undesired accumulation in organs.19 This method can also result in potential false positive signals due to expression of the target antigens/markers on normal cells, non-specific binding of the nanoparticles, and background noise (the signal-to-background ratio).19 PEGylation of the nanoparticles has been frequently used to address some of the issues by enhancing their circulation time while reducing their non-specific binding.20, 21
To detect CTCs in the bloodstream in a xenograft mouse model, PEGylated gold nanorods conjugated with CTC targeting ligands have been employed for photoacoustic/photothermal flow cytometry. CTCs in the blood stream of a human breast cancer xenograft model were bound to urokinase plasminogen activator fragment or anti-CD44 conjugated to 30 nm spherical magnetic nanoparticles (MNP, cylindrical neodymium-iron-boron MNP). Following the accumulation of CTCs bound with magnetic nanorods through the application of a magnetic field on the ear of the xenograft mouse, the enriched CTCs were then targeted by PEGylated gold rods with a carbon nanotube (GNT) core and folate (FA) or anti-CD44 coating for use as a photoacoustic contrast agent.17, 18 Photoacoustic tomography (PAT) is a hybrid imaging modality that uses light to rapidly heat elements within the tissue, which results in photoacoustic waves that can be detected with an ultrasonic transducer.22 With photoacoustic imaging using photoacoustic/photothermal flow cytometry, this novel MNP/GNT cocktail achieved a targeting efficiency reaching around 95% at flow rates from 5 mm/s up to 5 cm/s in the vasculature of mouse ear (Figure 2). Furthermore, photoacoustic flow cytometry was conducted using gold nanorods conjugated with FA (GNR670-FA) and anti-EpCAM (GNR670-EpCAM) or anti-CD45 (GNR820-CD45). The peak optical intensities at different wavelengths (either 670 nm or 820 nm) were used to simultaneously discriminate the different cells.23 When the multilayer nanomaterials of gold nanorods (core)-silica coating (middle layer)-iron oxide nanocrystal (outer layer) were developed to combine multiple detection methods and reduce the nanoparticle injection frequency, efficient CTC detection was achieved at efficacies above 90% with high specificity.24 By the simultaneous use of targeting, magnetic enrichment, signal amplification, and multicolor recognition, sensitive CTC capture at 1 cell/mL concentrations was attained using photoacoustic/photothermal flow cytometry.24, 25
Figure 2. Magnetic enrichment and gold nanoparticle-based photoacoustic detection of CTCs in vivo.18.
(a) Gold nanoparticle-based CTC targeting from 70 mm veins in mouse ear. (b and c) After enrichment using urokinase plasminogen activator-conjugated MNPs (b) under the external magnet, CTCs in blood vasculature are quantitatively detected using FA-coated GNP (c) and two-color photoacoustic detection. (Reproduced by permission of Nature Publishing Group)
These efforts of using gold nanoparticles as an in vivo CTC capture platform promoted their potential use as agents for microsurgical removal or the laser ablation of CTCs. Additionally, the fast clearance rates of these particles (~10 min) provide a solution to the potential toxicity caused by unwanted accumulation of gold nanoparticles used in animals.18 It is further argued that gold nanorods offer the greatest absorption efficiency per unit volume in photoacoustic imaging methods.24 The gold nanorods, combined with various magnetic and other biocompatible nanoparticles, hold promise in the application of these methods in clinical setting. However, the complicated preparation of gold nanoparticles and intrinsic issues of in vivo nanoparticle injection need to be overcome before translating this approach to clinics.
1.1.2. Gold nanoparticles for CTC capture ex vivo
Nanoparticles functionalized with targeting ligands for CTCs can be used ex vivo either to directly bind to and separate the CTCs in blood samples or to functionalize a surface to capture CTCs from blood. The CTC detection ex vivo has advantages including the potentially enabled post-capture analysis after cell culture and zero risk of potential toxicity of the CTC-capturing nanoparticles to the patients. However, an efficient CTC-capturing nanoparticles must be able to capture extremely rare and heterogeneous CTCs, while maintaining viability of the capture CTCs, in order to prevent potential false negative signals and enable CTC culture, which is of technical challenges.
Gold nanoparticles can be functionalized on a nanostructured chip surface enabling binding with CTCs and enumeration of CTCs without labeling.26, 27 For instance, a gold nanostructured chip was fabricated from the SiO2 surface of a chip using the wet-lithographic method and functionalized to capture CTCs via coating and activation of a self-assembled monolayer of 11-mercaptoundecanoic acid, followed by antibody immobilization.26 The amount of antibodies bound to the gold nanostructured surface and biomolecular interaction processes on the sensor chip surface could be quantified in real time by measuring signal changes in phase shift and amplitude of the electroacoustic resonance. Using a relatively low concentration of antibodies, the gold nanostructured chip successfully detected CD4-positive human lymphoblastic leukemia cell JEG-3 and human placental choriocarcinoma cell MOLT-17, whereas a reference surface covered with human immunoglobulins did not.26
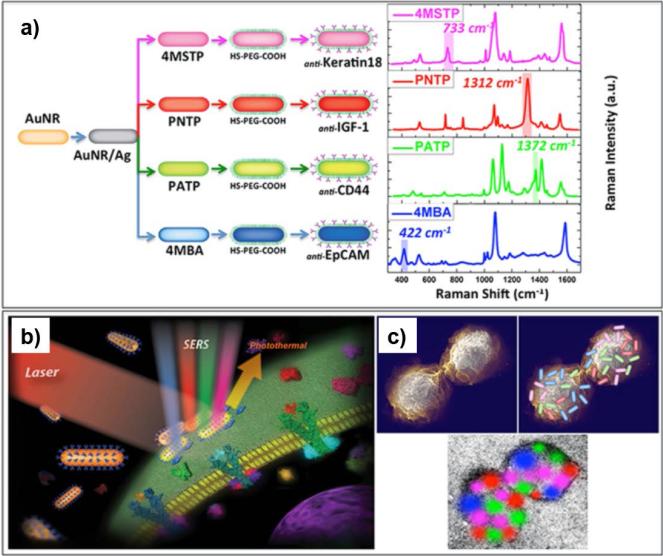
Gold nanoparticles coated with Raman reporter molecules or silver can also be used as ultrasensitive surface-enhanced Raman scattering (SERS) probes to capture and confirm CTCs without labeling.28-30 For example, after capturing on an antibody-functionalized nitrocellulose membrane substrate, Zhang et al. counted tumor cells on the membrane using gold nanoparticles and large-scale surface-enhanced Raman scattering (SERS) imaging technology.28 In this study, the colloid gold nanoparticles (60 nm) were prepared with Raman reporter molecules (pMBA) for SERS imaging and functionalized with anti-EpCAM for specific binding to CTCs.28 Of the 100 of non-small cell lung cancer (NSCLC) NCI-H1650 cells in 1 mL of whole human blood, 34 cells were captured on the nitrocellulose membrane and counted successfully according to the SERS imaging.28 For SERS-based biosensing, Nima et al. functionalized silver-coated gold nanorods with four pairs of organic Raman-active molecules and antibodies specific to breast cancer markers: 4-mercaptobenzoic acid (4MSTP)-anti-Keratin 18; p-nitrothiophenol (PNTP)-anti-Insulin-like growth factor antigen (IGF-1); p-aminothiophenol (PATP)-anti-CD44; and 4-(methylsulfanyl)thiophenol (4MBA)-anti-EpCAM.31 Compared to conventional pMBA–coated gold nanorods, a 129-fold SERS signal enhancement of these hybrid nanoparticles allowed for shorter detection time while maintaining highly specific detection of individual breast cancer MCF-7 cells in whole human blood.31 The specificity of detection (1 CTC per 90,000 fibroblast cells) and the distinguishability between various targets (IGF1, EpCAM, CD-44, and Keratin18) further attested to the potential application of the silver-coated gold nanoparticles as CTC imaging platforms via SERS (Figure 3).
Figure 3. Silver-Gold nanoparticles for CTC detection through surface-enhanced Raman Scattering.31.
(a) Four families of SERS nanoparticles (Blue: AuNR/Ag/4MBA/anti-EpCAM, Red: AuNR/Ag/PNTP/anti-IGF-1 Receptor β, Green: AuNR/Ag/PATP/anti-CD44, Magenta: AuNR/Ag/4MSTP/anti-Keratin18) were used for CTC detection using 2D multi-color SERS/PT detection technique (b and c). (Reproduced by permission of Nature Publishing Group)
Such gold nanoparticles for ex vivo CTC capture could be also used as agents for CTC confirmation and quantitative measurement. The increased sensitivity of CTC capture as well as improved photoacoustic imaging can be attributed to the unique characteristics of gold nanoparticles. Although additional studies using whole blood samples are required, the gold-coated CTC capture chips and silver/gold nanorod hybrids show great potential to achieve clinically significant detection and quantification of CTCs.
1.2. Magnetic nanoparticles (MNPs)
One of the commonly used strategies to isolate CTCs is to utilize MNP complexes that bind to the cells for in vivo and in vitro separation under a magnetic field. MNPs are composed of magnetic elements, commonly iron, nickel, cobalt and their oxides such as magnetite (Fe3O4), maghemite (Υ-Fe2O3), cobalt ferrite (Fe2CoO4), and chromium dioxide (CrO2). Among iron oxide MNPs that are chemically stable, biocompatible MNPs such as magnetite (Fe3O4) have been most commonly used in biological applications. Superparamagnetic MNPs display the features with a fast response to applied magnetic fields with negligible remanence (residual magnetism), which is attractive for a broad range of biomedical applications to prevent agglomeration at room temperature. However, naked metallic nanoparticles are highly reactive and are easily oxidized in air, which often results in loss of magnetism and dispersibility (aggregates). Therefore, for many applications it is crucial to develop protection strategies to chemically stabilize the naked magnetic nanoparticles by the grafting of or coating with surfactants, polymers (e.g. PEG, dextran, polyvinylpyrrolidone (PVP), Polyvinyl alcohol (PVA), chitosan, and polypeptides), or inorganic materials (e.g. silica, carbon, and gold).32 The protecting shells not only stabilize MNPs, but can also be used for further functionalization with ligands for applications in biolabeling, bioseparation, and multimodal imaging. Some examples of in vivo injectable applications of MNPs, based on gold and magnetic nanoparticle complexes, were already discussed in Section 1.1.1. In this section, the ex vivo applications of MNPs for CTC capture will be primarily discussed.
1.2.1. MNPs for CTC capture ex vivo
MNPs containing CTC-targeting molecules have been used for CTC detection upon mixing with blood specimens drawn from patients. CellSearchTM is based on iron oxide (Fe3O4, Ferrofluid™) MNPs coated with antibodies (anti-EpCAM) against CTC surface markers (EpCAM) through polymeric linkers. Upon binding to CTCs in blood specimens, the MNPs bound to CTCs can be isolated by magnetic active cell sorting (MACS) upon applying an external magnetic field.1 This CellSearchTM approach has been used as a diagnostic and prognostic test, monitoring CTCs in blood from patients with various types of cancers, such as breast, prostate, colorectal, pancreatic, gastrointestinal, and small lung cancer. However, the CTC detection sensitivity of this assay is highly dependent on the binding capacity of the MNPs and sensitivity/selectivity of anti-EpCAM.33, 34 In particular, the size of the currently used Fe3O4 MNPs is approximately 100 nm in diameter, which has a relatively low surface-to-volume ratio causing lower binding capacity and less stability in whole blood (aggregation or precipitation).35 In order to enhance the detection properties of the inorganic particles, Xu et al. reported that MNPs with smaller diameter (30 nm) had enhanced diffusivity in viscous samples and binding affinity.35 The iron oxide MNPs were coated with an amphiphilic triblock polymer consisting of polybutylacrylate (PBA, hydrophobic), polyethylacrylate (PEA, hydrophobic), and polymethacrylic acid (PMA, hydrophilic), along with a hydrophobic hydrocarbon side chain, which enhanced physical stability and allowed further surface functionalization of the nanoparticle surfaces.
1.2.2. MNPs combined with other strategies for ex vivo CTC capture
The fluorescent-magnetic bifunctional nanoparticles composed of MNPs and optical components, such as fluorescence dyes, quantum dots (QDs), or X-ray contrast agents, are of particular interest in multimodal imaging.36-38 By means of encapsulation, direct reaction, and inorganic synthesis, magnetic-optical bifunctional nanoparticles with different structures have been prepared and successfully applied for multimodal imaging.38 Fluorescent-MNPs can be used for the simultaneous detection and isolation of multiple types of tumor cells. For instance, anti-CD3- and anti-PSMA-conjugated MNPs were labeled with red and yellow fluorescence dyes to target leukemia Jurkat T cells and prostate LNCaP cancer cells, respectively. Under a magnet and a fluorescence microscope, Jurkat cells and LNCaP cells in mixture were distinguishable and separated based on fluorescence.39 Guo et al. also showed that ferricacetylacetonate-based MNPs and trioctylphosphine oxide-capped CdSe/ZnS QDs were spontaneously encapsulated by amphiphilic (2-hydroxyl-3-dodecanoxyl) propylcarboxymethylchitosans (HDP-CMCHSs) via a facile ultrasonication.38 The fluorescent-MNPs were modified with streptavidin, facilitating bioconjugation with biotin-labeled human epidermal growth factor (hEGF) for specific recognition and detection of rare EGFR-positive HeLa cells from whole blood using a magnet and a fluorescence microscope.38 In addition, superparamagnetic iron oxide and X-ray absorbing bismuth nanoparticles were separately conjugated with FA via a reaction between 3-aminopropyl-triethoxysilane on nanoparticles and activated carboxylic group of FA.40 After adding both nanoparticles in cell suspension, nanoparticles could bind to the surface of FA receptor-overexpressing tumor cells, which were localized in a small area using a micro-magnet, excited using an incoming X-ray beam, and detected by X-ray based detection.40 Using this nanoparticle mixture of iron oxide nanoparticles-FA and X-ray absorbing bismuth nanoparticles-FA, X-ray based CTC detection and CTC killing occurred simultaneously by increasing X-ray intensity to damage DNA and locally induce cell death of CTCs.40
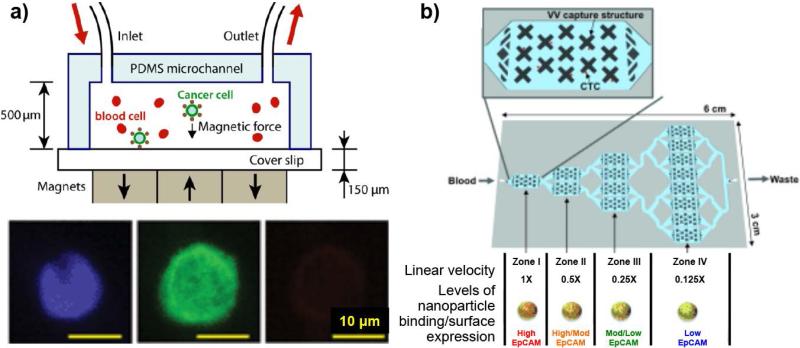
Besides the magnetic-optical bifunctional nanoparticles mentioned above, microfluidic systems made of poly(dimethylsiloxane) (PDMS) have been combined with MNPs to magnetically capture and identify CTCs on a microfluidic chip. After conjugating with anti-EpCAM, Fe3O4 MNPs used for CellSearch™ system were added along with the CellSearch™ capture enhancement reagent to the cancer cell-spiked blood samples.41, 42 A typical PDMS microfluidic chip was combined with a defined magnetic field gradient in the vicinity of arrayed magnets with alternating polarities, as illustrated in Figure 4a, which led to an effective capture of MNP-labeled cancer cells. This microchip-based immunomagnetic detection demonstrated great cancer cell capture rates of 90% and 86% for an EpCAMlow colon cancer cell line, COLO205, and an EpCAMhigh breast cancer cell line, SKBR3 cells, respectively.41 This microchip-based immunomagnetic detection required fewer (by 25%) magnetic particles to achieve a comparable capture rate than the CellSearch™ system, while maintaining the screening speed (at an optimal blood flow rate of 10 mL/hr) more than five times faster than those of other microchip-based assays.41 In another example, a cancer cell mixture with different levels of EpCAM expression was tagged with MNPs functionalized with an antibody against the surface marker EpCAM.43 The labeled CTCs were magnetically captured and sorted in the local velocity valleys (VVs) generated by a multizone velocity valley device that features four different regions with different linear velocities (Figure 4b): EpCAMhigh cells trapped in zone I (1 × speed); EpCAMmedium cells trapped in zone II – III (0.5 × and 0.25 × speed); and EpCAMlow cells trapped in zone IV (0.125 × speed). The surface-marker-guided sorting and profiling of CTCs with different phenotypes within this unique microchip performed well for cancer cell lines with varying surface expression as well as prostate cancer patient samples.43
Figure 4. Immunomagnetic-based microfluidic devices for CTC detection.
(a) A scheme of the device for CTCs labeled by anti-EpCAM-MNP being captured in a magnetic field while undergoing flow. The captured COLO205 cells were identified via immunostaining (DAPI (blue), cytokeratin (green), and CD45 (red).41 (Reproduced by permission of Royal Society of Chemistry) (b) EpCAM-expression-level-dependent CTC sorting. Anti-EpCAM-MNP-labeled CTCs were sorted in a device with multiple velocity valley zones with different linear velocities: EpCAMHigh cells trapped in zone I and EpCAMLow cells trapped in zone IV.43 (Reproduced by permission of WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim)
As seen in the CellSearch™ system that has already been clinically implemented, these MNP-based systems as an ex vivo CTC capture platform clearly have potential to be used for CTC enrichment upon magnet exposure. Additionally, the combination with other materials can expand the application of MNPs from CTC capture and sorting to more complicated CTC identification and differentiation using multimodal imaging.
1.3. Quantum dots (QDs) and other fluorescent nanomaterials
QDs, based on their strong fluorescence intensity, present a unique opportunity to isolate CTCs in a quantitative manner. Colloidal QDs made of ZnS, CdS, ZnSe, CdTe and PbSe, emit a wide spectrum of fluorescence ranging from ultraviolet (UV) to infrared (IR). Compared to other fluorescence dyes, QD properties of interest include high quantum yield, high molar extinction coefficients (around 10–100 folds higher), broad absorption with narrow, symmetric photoluminescence spectra, the ability to size-tune the photoluminescence emission, high resistance to photo bleaching, and exceptional resistance to photo- and chemical degradation. However, intermittency (blinking) under continuous excitation and aqueous insolubility of QDs need to be addressed for CTC detection. The aqueous solubility issue of QDs can be solved by surface functionalization with hydrophilic ligands, either through cap exchange or by coating. After functionalizing QDs to target to specific biomolecules, QDs have been used in deep-tissue imaging, fluorescence resonance energy transfer (FRET)-based cellular labeling, and CTC detection.44 Among those applications, ex vivo CTC capture using QDs holds the greatest clinical significance, given strong, stable fluorescence and yet with known toxicity concerns of QDs when injected into the body.
1.3.1. QDs for CTC capture ex vivo
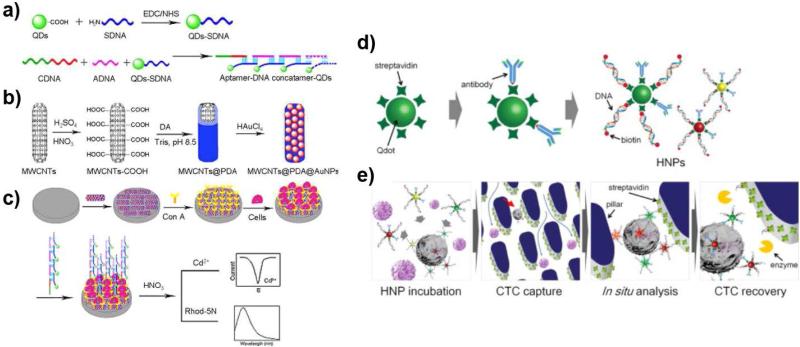
The strong and stable fluorescence emission of QDs has led to the development of QD-based fluorescence probes for CTC identification and confirmation. For example, aptamer-DNA concatemer (a DNA complex containing multiple copies of the same DNA sequences)-QDs hybrids were prepared by hybridization of multiple signal DNA aptamer (sDNA)-conjugated QDs, capture DNA (cDNA) targeting CTCs, and auxiliary DNA (aDNA), as depicted in Figure 5a. As a substrate, a conventional electrode was modified to capture CTCs using a hybrid nanomaterial of multiwall carbon nanotubes (MWCNT, core)-polydopamine (PD, linker)-gold nanoparticles (Figure 5b), and then functionalized with a tumor cell-binding lectin, concanavalin A (Con A), as shown in Figure 5b and 5c. The binding of CCRF-CEM and Ramos cells was successfully confirmed via dual electrochemical and fluorescence measurements using the Con A-coated electrode and the QD signal probe, respectively. Furthermore, the stable and high fluorescence signal intensity of QDs was further amplified in the presence of Cd2+ by QD coating with a Cd2+-sensitized Rhod-5N dye. A linear relation of the peak current (R2 = 0.9960) and fluorescence intensity (not provided) with the number of loaded CCRF-CEM cells were found in the range of 102 and 106 cells/mL.45 Although lower than those of EIS sensor (6,000 cells/mL) and a quartz crystal microbalance sensor (8,000 cells/mL), their detection limit of 50 cells/mL of this QD probe-based system at 3σ is still high and needs to be significantly improved (lowered) for its clinical translation.
Figure 5. QD-based CTC detection.
(a-c) QDs were conjugated with aptamer-DNA concatemer that has binding affinity to CTCs (QD probes, a). Gold nanoparticles were deposited on polydopamine-coated multiwall carbon nanotube (Material B, b). The detection of CTCs on the Material B-conjugated platform was monitored using QD probes (c).45 (Reproduced by permission of American Chemical Society) (d-e) The biotinylated antibody and DNA linker on QDs (d) were used to capture CTCs on streptavidin-coated pillars of the device.46 (Reproduced by permission of Elsevier)
The various fluorescence colors of QDs have been used for surface marker-dependent CTC capturing and sorting.46, 47 Lee et al. showed that size-tuned QDs with different emission wavelengths upon the same excitation wavelength could be used for surface marker-dependent capturing and sorting of heterogeneous CTCs.46 Three different types (emission wavelengths of 525 nm, 565 nm, and 625 nm) of streptavidin-modified QDs were functionalized with biotinylated antibodies for EpCAM, epidermal growth factor receptor (EGFR), and human epidermal growth factor receptor (HER-2), respectively. The QDs with specific antibodies were then hybridized with biotinylated complementary DNA to block the unbound streptavidin and to decorate biotins on the QDs (Figure 5d). Based on the fluorescence emission wavelengths of the QDs with three different antibodies, in situ expression of heterogeneous surface markers on three breast cancer cell lines (MCF-7, MDA-MB-231, and SK-BR-3) were identified and sorted. The biotin ends on the QDs were used to capture the QD-bound tumor cells on a streptavidin-coated chip, as depicted in Figure 5e. The average capture efficiency and surface marker-dependent sorting accuracy of the breast cancer cells were 87.5% and 92.4%, respectively. Additionally, the DNA linker was cleaved using restriction enzymes to recover CTCs from the chip without affecting cellular viability for post-capture analysis.46 The releasing efficiency of the captured cells from the streptavidin-coated chip was 86.1%. However, the cytotoxicity of QDs could potentially affect the viability of the cells recovered from the chip, which should be addressed for further investigations.
QDs have been demonstrated to provide advantages in ex vivo CTC capture due to their wide spectrum of fluorescence, strong intensities, and various emission wavelengths. Additionally, the surface functionalization with other materials has shown that the application of QDs for CTC capturing and sorting can be expanded to CTC identification and differentiation using fluorescence imaging. However, for clinical translations, further investigations of QDs are required to improve the intrinsic properties of QDs, such as intermittency (blinking) under continuous excitation, aqueous insolubility, aggregation, and toxicity.
1.3.2. Fluorescence-labeled nanoparticles for CTC capture in vivo
Because QDs have not been widely used in vivo, we will discuss about another quantification assay of CTCs using other fluorescence nanoparticles in this section. The Low group injected FA-FITC and FA-DiD into mice, and FA fluorescence overlap (yellow) of FITC (green) and DiD (red) were monitored for in vivo quantification of FAR-overexpressed CTCs. Using multiphoton intravital microscopy after injecting non-toxic, FA-fluorescence nanoparticles, circulating L1210A cells were detected in the vasculature of the mouse ear, which shows its potential to be used for non-invasive in vivo labeling and quantitation of CTCs. These nanoparticles were also used for ex vivo flow cytometry to detect CTCs in peripheral blood samples from ovarian cancer patients.19 However, this method has a few potential problems, such as low tissue penetration of fluorescence light in multiphoton intravital microscopy, missing of FAR-negative tumors, and competitive binding with serum FA for binding to CTCs. Furthermore, the fast photobleaching of FITC and relatively strong background fluorescence in the mice hindered the highly sensitive detection of CTCs. Nonetheless, when optimized with other tumor-specific fluorescent ligands (e.g. 2-[3-(1,3-dicarboxylpropyl)reido] pentanedioic acid (DUPA) for prostate cancer)19 and with stronger, longer-wavelength fluorescence probes, this approach has potential to be developed as a simple in situ CTC detection method.19
1.4. Graphenes and graphene oxides
Graphene is an atomically thick, two-dimensional (2-D) sheet of sp2 hybridized carbon arranged in a honeycomb structure. Graphene is the basic building block for graphitic materials of all other dimensionalities: graphite (3-D carbon allotrope of graphene sheets stacked on top of each other and separated by 3.37 A°) and carbon nanotubes (CNT, 1-D carbon allotropes). Graphene has numerous extraordinary physicochemical properties, such as high theoretical specific surface area (2,630 m2g−1), high intrinsic mobility (200,000 cm2 v−1 s−1), strong mechanical strength (high Young’s modulus, ~1.0 TPa), and excellent thermal conductivity (~5,000 Wm−1K−1), along with its optical transmittance (~97.7% opacity) and electrical conductivity.48 As charge transfer between the adsorbed molecules and graphene is responsible for the chemical response, graphene has shown excellent performance in electrochemical detection of small biomolecules. As a result, graphenes and its oxidized form graphene oxides (GO) dispersed on materials or devices have been investigated as a platform for electrical detection of CTCs.49
1.4.1. Graphene/GO for CTC capture ex vivo
The fluorescence quenching properties of GO have increasingly been used in optical biosensing applications and applied for the CTC capture. Ionic hydroxyl and carboxyl groups of GO allow for formation of molecular complexes and electrostatic interactions with charged molecules, while its aromatic, sp2 domains facilitate pi-pi stacking and fluorescence quenching. The quenched fluorescence gets recovered upon interaction of a targeting biomolecule with a target protein on a cell membrane, which is the basis of several detection and imaging applications using GO. In this regard, dye-labeled biomolecules (ssDNA and proteins) were adsorbed and functionalized on GO for use as optical sensors. Cy2-conjugated S6, Cy5-conjugated A9, and Alexa Fluor 488-conjugated YJ-1 aptamers were attached to the surface of a microporous (20–40 μm) GO membrane. Specific binding of these aptamers to tumor biomarkers, such as HER-2, prostate specific membrane antigen (PSMA), and carcinoembryonic antigen (CEA), were capable of capturing multiple types of tumor cells for the separate and simultaneous label-free detection.50 By filtering citrated whole rabbit blood spiked with 10 CTCs/mL mixture of SKBR3 (breast), LNCaP (prostate), and SW-948 (colon) cancer cells in addition to 105 cells/mL of peripheral blood mononuclear cells (PBMCs) and HaCaT (negative control skin cells), the platform achieved 95% capture efficiency with 97% cell viability. Using multi-color fluorescence imaging, labeled aptamers bound to their respective cancer cell lines recovered fluorescence and the cells were visualized and differentiated in the GO membrane.
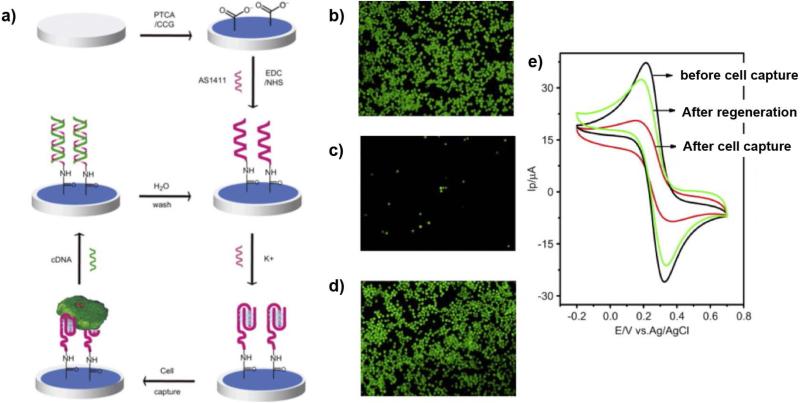
The excellent electrochemical properties, particularly its high conductivity and electron mobility in the pi system, of graphene have also been utilized in various biosensors for label-free detection of CTCs. Feng et al. modified a glass carbon electrode with 3,4,9,10-perylene tetracarboxylic acid (PTCA)-adsorbed graphene and an AS1411 aptamer with specificity for cancer cell surface marker, nucleolin.51 Necessary for binding to nucleolin, the aptamer conjugated with carboxylic groups of PTCA was then folded into its quadraplex structure, by treatment of potassium ion solution. Changes in electrical current through the electrode upon interaction with cells were observed using electrochemical impedance spectroscopy and cyclic voltammetry. A significant increase in impedance was observed upon binding with nucleolin-positive MDA-MB-231 and K562 cells, while changes in impedance was negligible upon binding with neculolin-negative NIH3T3 serving as a negative control, with a detection range of 103 to 106 cells/mL (Figure 6). Additionally, via the treatment of AS1411 with its complementary DNA (cDNA), whose competitive interaction prevents interaction with nucleolin, viable tumor cells and the electrode were able to be released from the electrode and be recycled.
Figure 6. Graphene-modified aptasensors.51.
(a) Electrochemical sensor with aptamer, AS1411, and graphene were developed for selective label-free detection of CTCs. Green labeled HeLa cells were captured on aptasensor (b) and released after 5 μM cDNA treatment (c). The regenerated aptasensor after releasing the cells were able to capture HeLa cells again (d). (e) The cell capture on aptasensor can be monitored by cyclic voltammograms of [Fe(CN)6]3−/4−. (Reproduced by permission of Elsevier)
Although the CTC detection platforms using graphene and GO are promising, the development of such graphene-based CTC detection is still in its infancy. Potential issues, such as the lack of methods for the scale-up and well-controlled, high quality synthesis/processing of graphene, need to be addressed by intensive research for clinical translation.
1.5. Dendrimers and Stimuli-responsive polymers
Recent advances in polymeric nanomaterials have enabled to design biomedical devices with significantly improved functions. For example, multivalent binding that occurs in a variety of physiological processes has been exploited to significantly increase the sensitivity and selectivity of detection assays.52 Dendrimers offer a unique opportunity to precisely control the multivalent binding effect with their unique properties obtained from their well-defined chemical structure and a high density of peripheral functional groups.53-56 Another promising approach is to use stimuli-responsive polymers for CTC capture and release. Stimuli-responsive polymers have been used to release the captured CTCs upon exposure to various stimuli, such as light, temperature, pH, and physical stress. In this section, these polymer-based approaches in CTC detection will be discussed.
1.5.1. Dendrimers for CTC capture ex vivo
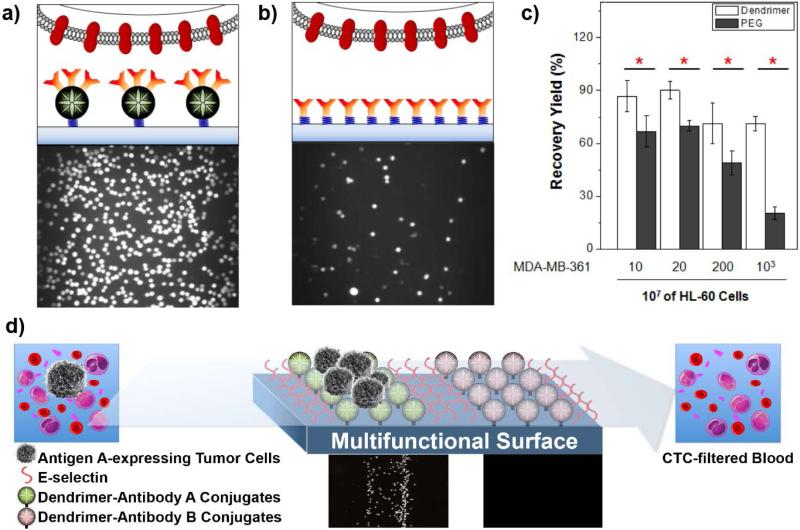
The binding strength between CTCs and a capture surface can be enhanced through dendrimer-mediated multivalent binding effect, which can significantly improve the sensitivity and selectivity of the surfaces for CTC detection.54 Dendritic nanomaterials, such as poly(amidoamine) (PAMAM) dendrimers, have been demonstrated to effectively mediate multivalent binding effect due to their capability to preorganize/orient ligands, polymer backbone topology, and easy deformability.53 In our recent studies, we have developed a CTC detection platform for efficient surface capture of tumor cells through the PAMAM dendrimer-mediated multivalent binding effect.54 Briefly, a glass slide surface with epoxy groups was functionalized with NH2-PEG-COOH that was subsequently conjugated with partially carboxylated generation 7 (G7) PAMAM dendrimers. The dendrimer-functionalized surface was then incubated with cancer cell-specific antibodies, resulting in a CTC detection surface.57-59 To confirm the multivalent binding, we first measured the dissociation constant of the dendrimers conjugated with anti-EpCAM using surface plasmon resonance, which was observed to be over one million-fold lower than that of free anti-EpCAM. Importantly, the surfaces functionalized with the anti-EpCAM-dendrimer conjugates exhibited dramatically enhanced cell adhesion and binding stability of three breast cancer cell lines (MDA-MB-361, MCF-7, and MDA-MB-231). As shown in Figure 7, the surface capture of tumor cells on the dendrimer-coated surface was markedly improved, compared to that on the linear polymer PEG-coated surface. Furthermore, a significantly higher number of bound cancer cells, particularly MDA-MB-231 cells which have lower EpCAM expression, remained on the dendrimer-coated surface after strong agitation (up to 15.2 fold), further confirming the multivalent cell capture.54 The surface sensitivity and specificity towards tumor cells were further improved when another biomimetic approach was introduced to the surface. Additional immobilization of E-selectin that induces cell rolling has been shown to enhance the surface capture of tumor cells (up to 10 fold compared to the PEG-coated surface).57-60 The significant enhancement of the dendrimer-coated surfaces was recently expanded to various antibodies and proven effective in capturing tumor cells from clinically relevant blood samples as well.59
Figure 7. Dendrimer-mediated multivalent binding for enhanced detection of CTCs.
(a-c) The dendrimer-coated surface exhibited greatly enhanced tumor cell detection. Compared to the surface with anti-EpCAM-conjugated linear polymers, polyethylene glycol (PEG, b), the dendritic nanoparticle-immobilized platform captured significantly more tumor cells (a) and improved detection of tumor cells from a mixture with 107 HL-60 cells (c).54 (Reproduced by permission of WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim) (d) A combination of dendrimers and E-selectin (a cell rolling inducing agent), along with multiple antibodies achieved highly sensitive differential detection of tumor cells.59 (Reproduced by permission of American Chemical Society)
1.5.2. Nanomaterials for effective release of captured CTCs
CTCs captured from patient blood provide opportunities to perform post-capture analysis to identify signaling pathways and investigate molecular profiling of the individual CTCs. A number of approaches to efficiently release the captured CTCs have been explored.61-63 Proteolytic enzymes and/or stimuli-responsive polymers have been commonly used to engineer the CTC capture surface to release the cells as a result of surface degradation or in response to external stimuli such as light, temperature, and mechanical forces, respectively.
Alginate hydrogels and self-assembled DNA nanostructures have been incorporated onto the surface to increase CTC capture efficiency by altering the surface topography and efficiently release the isolated CTCs from the surface upon simple stimulation. Alginate in solution containing CaCl2 was injected into the 1st generation CTC-chip for in situ hydrogel formation on the chip surface, which was further functionalized with a mixture of PEG, EDC, sulfo-NHS and anti-CD34. This ionically crosslinked hydrogel was used to capture and release CD34-expressing endothelial progenitor cells in heparin-treated blood specimens without the need for enzymatic digestion,61 with the principle of calcium chelation driving the substrate degradation. When the ionically crosslinked alginates on the chip surface were covalently crosslinked using a photoinitiator irgacure 2959 and functionalized with anti-EpCAM, EpCAM-expressing CTCs were captured and released via a treatment with alginate lyase.64 In another study, self-assembled DNA nanostructures were incorporated into the avidin-coated, 2nd generation CTC-chip via rolling circle amplification at 37°C using a biotinylated primer-circular template complex, nucleotide triphosphate containing deoxyribose (dNTP), and DNA polymerase.65 Long multiple aptamers in the matrix of DNA nanostructures on the chip had highly specific binding affinity with lymphoblast CCRF-CEM cells over monovalent aptamers and anti-EpCAM.65 The degradation of DNA matrix under exposure to DNase/endonucleases induced to release the captured cells from the chip.65 However, these approaches have potential issues. The calcium-sensitive approach is limited to the use of chelating agents as blood anticoagulants. The use of enzymes (alginate lyase and DNase) has resulted in poor efficiency (<10%) in release and limited viability of the cells.66 To release the captured CTCs without the need for enzymatic digestion, various types of polymers with stimuli-responsive properties have been employed.
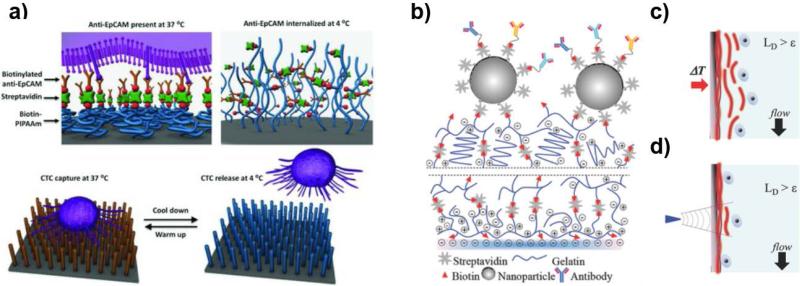
Thermally responsive polymers have been used to release the isolated CTCs from the surface upon external temperature changes. For instance, thermally responsive poly(N-isopropylacrylamide) (PNIPAAm) polymers have been grafted onto a silicon nanowire substrates (SiNWS)-based CTC detection surface.66 The self-assembled monolayer (SAM) of aminosiloxane on the SiNWS was reacted with a mixture of isopropylacrylamide and methyl aminoethylmethacrylate, resulting in PNIPAAm via the atom transfer radical polymerization (ATRP). The amino groups on the PNIPAAm-grafted SiNWS were then conjugated with biotin-NHS and streptavidin for further functionalization with biotinylated anti-EpCAM. As shown in Figure 8a, this thermally responsive platform demonstrated the effective capture of tumor cells in the presence of human white blood cells at 37°C and release of the captured cells with retained viability and functionality at 4°C. Recently, a thermally responsive gelatin-based nanostructured coating by a layer-by-layer (LbL) deposition of biotinylated gelatin and streptavidin was also developed for temperature-responsive release (for bulk-population recovery) of the captured CTCs (Figure 8b and 8c).62 Raising the device temperature to 37 °C degraded the nanocoating from the whole surface within minutes for a bulk-population release of CTCs. In addition, local regions of the gelatin nanocoating were sensitive to mechanical stresses from a frequency-controlled microtip, which was used for mechanosensitive single cell release of CTCs (Figure 8b and 8d).62 This dual release strategy from the gelatin-coated chip has successfully driven to characterize the PIK3CA and EGFR oncogene mutations in the released CTCs.
Figure 8. Stimulus-responsive release of captured CTCs.
(a) Biotin-functionalized PNIPAAm polymer brushes were used as temperature-sensitive linkers between anti-EpCAM and the surface of silicon nanowire substrate, which resulted in the releasing of captured CTCs from the substrate upon cooling down from 37°C to lower than 4°C.66 (Reproduced by permission of WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim) (b-c) The surface of a microfluidic device was coated with temperature- and mechano-sensitive gelatin: A bulk release mechanism of captured cells upon a temperature change (c) and a cell-specific release by applying vibration force of a microtip (d).62 (Reproduced by permission of WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim)
While promising, these nanomaterials that are responsive to enzymes or external stimuli may face challenges to be clinically implemented due to the requirement to run the samples at certain temperature (for thermally responsive nanomaterials) and conditions (DNase/endonuclease-free conditions for DNA/aptamer-based materials). Additionally, the exposure of individual cells to a certain stimulus (e.g. enzyme, light, chemical, temperature, or mechanical stress) during the release process may affect the cell viability.
2. Challenges in Clinical Translation
Although biomedical nanotechnologies have made significant progress in the detection of rare CTCs in cancer patient blood over the past two decades, most of the CTC detection methods have yet to be clinically translated. To successfully translate these recently developed CTC detection methods to a clinical setting, several limitations must be overcome. First, the synthetic procedures of typical nanomaterials are complicated and require multiple steps to achieve well-defined architecture, which makes the scalability and reproducibility of the promising nanomaterials difficult. In particular, the methods using hard-to-fabricate materials, including graphene, multilayer magnetic-silica-gold nanoparticles, and quantum dots, will have issues of inconsistency in quality control and device fabrication. Novel methods or nanomaterials that involve simple preparation steps with a high level of controllability and processability would be required for facile translation of the highly promising technologies described in this review. Second, the development of CTC detection methods often faces poor or unpredictable correlation between the results of in vitro cancer cell lines and clinical samples. This is because cancer cell lines do not fully represent the heterogeneous phenotypes and epithelial mesenchymal transition (EMT)-induced phenotypic changes observed in clinical CTCs. Post-capture analysis and culture expansion of the captured CTCs from blood specimens would be helpful to find representative CTC biomarkers and establish representative cell models for CTCs. Third, the high frequency of non-specific binding of normal hematological cells and low viability of captured CTCs are unavoidable challenges due to intrinsic characteristics of CTCs in blood. More effort and study for individual platforms are obviously needed to overcome these inevitable challenges for CTC detection and to transform promising preliminary results into clinical products.
Conclusion
Given that accurate enumeration of CTCs in blood can provide valuable clinical insight into the progress of metastatic cancers, technologies that achieve highly sensitive and reliable CTC detection are urgently required. As summarized in this review, several types of nanoparticles, such as gold nanoparticles, MNPs, QDs, graphenes, and polymers, have been used in recently developed CTC detection systems that hold great promise to be clinically translated. Those nanomaterials have been demonstrated to enhance the sensitivity and specificity of the CTC devices and/or to accommodate additional functions such as label-free detection and stimuli-responsive release of CTCs. Although the extensive clinical validation of the emerging CTC detection methods is still required, the development of the promising nanomaterials for CTC detection is under progress at an exponential rate because of their profound potential impact to cancer diagnosis and prognosis. Clinically significant detection of CTCs using nanotechnology will likely allow accurately monitoring CTC changes in individual patients and subsequently molecular biological analysis of the captured CTCs, ultimately leading to the development of personalized medicine against debilitating metastatic cancers.
Acknowledgement
This work was partially supported by National Science Foundation (NSF) under grant # DMR1409161, National Institutes of Health (NIH) Grant # R01CA182528, and the Technological Innovation R&D Program (grant # S2083505) funded by the Small and Medium Business Administration of Korea.
Contributor Information
Ja Hye Myung, Department of Biopharmaceutical Sciences, College of Pharmacy, University of Illinois, Chicago, IL 60612.
Kevin A. Tam, Department of Biopharmaceutical Sciences, College of Pharmacy, University of Illinois, Chicago, IL 60612
Sin-jung Park, Department of Biopharmaceutical Sciences, College of Pharmacy, University of Illinois, Chicago, IL 60612.
Ashley Cha, Department of Biopharmaceutical Sciences, College of Pharmacy, University of Illinois, Chicago, IL 60612.
Seungpyo Hong, Department of Biopharmaceutical Sciences, College of Pharmacy, University of Illinois, Chicago, IL 60612; Integrated Science and Engineering Division, Underwood International College, Yonsei University, Incheon 406-840, KOREA.
References
- 1.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Janicke F, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8:98–101. 1889. [PubMed] [Google Scholar]
- 3.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 4.van de Stolpe A, Pantel K, Sleijfer S, Terstappen LW, den Toonder JM. Circulating tumor cell isolation and diagnostics: toward routine clinical use. Cancer Res. 2011;71:5955–5960. doi: 10.1158/0008-5472.CAN-11-1254. [DOI] [PubMed] [Google Scholar]
- 5.Ashworth TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aus Med J. 1869;15:146–149. [Google Scholar]
- 6.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LWMM, Uhr JW. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A. 1998;95:4589–4594. doi: 10.1073/pnas.95.8.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myung JH, Gajjar KA, Han YE, Hong SP. The role of polymers in detection and isolation of circulating tumor cells. Polym Chem-UK. 2012;3:2336–2341. [Google Scholar]
- 8.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, Herold CI, Marcom PK, George DJ, Garcia-Blanco MA. Circulating Tumor Cells from Patients with Advanced Prostate and Breast Cancer Display Both Epithelial and Mesenchymal Markers. Molecular Cancer Research. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253:180–204. doi: 10.1016/j.canlet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. J Cell Biol. 2011;192:373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes AD, King MR. Nanobiotechnology for the capture and manipulation of circulating tumor cells. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:291–309. doi: 10.1002/wnan.168. [DOI] [PubMed] [Google Scholar]
- 15.Cai W, Gao T, Hong H, Sun J. Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl. 2008;1:17–32. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Chen X. Gold nanoparticles for photoacoustic imaging. Nanomedicine (Lond) 2015;10:299–320. doi: 10.2217/nnm.14.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galanzha EI, Kim JW, Zharov VP. Nanotechnology-based molecular photoacoustic and photothermal flow cytometry platform for in-vivo detection and killing of circulating cancer stem cells. J Biophotonics. 2009;2:725–735. doi: 10.1002/jbio.200910078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galanzha EI, Shashkov EV, Kelly T, Kim JW, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat Nanotechnol. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci U S A. 2007;104:11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Kohler N, Zhang M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials. 2002;23:1553–1561. doi: 10.1016/s0142-9612(01)00267-8. [DOI] [PubMed] [Google Scholar]
- 22.Zharov VP, Galanzha EI, Shashkov EV, Khlebtsov NG, Tuchin VV. In vivo photoacoustic flow cytometry for monitoring of circulating single cancer cells and contrast agents. Opt Lett. 2006;31:3623–3625. doi: 10.1364/ol.31.003623. [DOI] [PubMed] [Google Scholar]
- 23.Nedosekin DA, Juratli MA, Sarimollaoglu M, Moore CL, Rusch NJ, Smeltzer MS, Zharov VP, Galanzha EI. Photoacoustic and photothermal detection of circulating tumor cells, bacteria and nanoparticles in cerebrospinal fluid in vivo and ex vivo. J Biophotonics. 2013;6:523–533. doi: 10.1002/jbio.201200242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu XG, Wei CW, Xia JJ, Pelivanov I, O'Donnell M, Gao XH. Trapping and Photoacoustic Detection of CTCs at the Single Cell per Milliliter Level with Magneto-Optical Coupled Nanoparticles. Small. 2013;9:2046–2052. doi: 10.1002/smll.201202085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galanzha EI, Shashkov E, Sarimollaoglu M, Beenken KE, Basnakian AG, Shirtliff ME, Kim JW, Smeltzer MS, Zharov VP. In Vivo Magnetic Enrichment, Photoacoustic Diagnosis, and Photothermal Purging of Infected Blood Using Multifunctional Gold and Magnetic Nanoparticles. Plos One. 2012;7 doi: 10.1371/journal.pone.0045557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broker P, Lucke K, Perpeet M, Gronewold TMA. A nanostructured SAW chip-based biosensor detecting cancer cells. Sensor Actuat B-Chem. 2012;165:1–6. [Google Scholar]
- 27.Safaei TS, Mohamadi RM, Sargent EH, Kelley SO. In Situ Electrochemical ELISA for Specific Identification of Captured Cancer Cells. ACS Appl Mater Interfaces. 2015 doi: 10.1021/acsami.5b02404. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Zhang R, Gao MX, Zhang XM. Novel Nitrocellulose Membrane Substrate for Efficient Analysis of Circulating Tumor Cells Coupled with Surface-Enhanced Raman Scattering Imaging. Acs Appl Mater Inter. 2014;6:370–376. doi: 10.1021/am404406c. [DOI] [PubMed] [Google Scholar]
- 29.Wu X, Luo L, Yang S, Ma X, Li Y, Dong C, Tian Y, Zhang L, Shen Z, Wu A. Improved SERS Nanoparticles for Direct Detection of Circulating Tumor Cells in the Blood. ACS Appl Mater Interfaces. 2015;7:9965–9971. doi: 10.1021/acsami.5b02276. [DOI] [PubMed] [Google Scholar]
- 30.Pallaoro A, Hoonejani MR, Braun GB, Meinhart CD, Moskovits M. Rapid Identification by Surface-Enhanced Raman Spectroscopy of Cancer Cells at Low Concentrations Flowing in a Microfluidic Channel. Acs Nano. 2015;9:4328–4336. doi: 10.1021/acsnano.5b00750. [DOI] [PubMed] [Google Scholar]
- 31.Nima ZA, Mahmood M, Xu Y, Mustafa T, Watanabe F, Nedosekin DA, Juratli MA, Fahmi T, Galanzha EI, Nolan JP, et al. Circulating tumor cell identification by functionalized silver-gold nanorods with multicolor, super-enhanced SERS and photothermal resonances. Sci Rep-UK. 2014;4 doi: 10.1038/srep04752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew Chem Int Edit. 2007;46:1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 33.Hejazian M, Li W, Nguyen NT. Lab on a chip for continuous-flow magnetic cell separation. Lab Chip. 2015;15:959–970. doi: 10.1039/c4lc01422g. [DOI] [PubMed] [Google Scholar]
- 34.Gorges TM, Tinhofer I, Drosch M, Rose L, Zollner TM, Krahn T, von Ahsen O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12 doi: 10.1186/1471-2407-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Aguilar ZP, Yang L, Kuang M, Duan H, Xiong Y, Wei H, Wang A. Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials. 2011;32:9758–9765. doi: 10.1016/j.biomaterials.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CH, Cook J, Emelianov S, Sokolov K. Multimodal Magneto-Plasmonic Nanoclusters for Biomedical Applications. Adv Funct Mater. 2014;24:6862–6871. [Google Scholar]
- 37.Fan Z, Senapati D, Singh AK, Ray PC. Theranostic magnetic core-plasmonic shell star shape nanoparticle for the isolation of targeted rare tumor cells from whole blood, fluorescence imaging, and photothermal destruction of cancer. Mol Pharm. 2013;10:857–866. doi: 10.1021/mp300468q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo S, Chen YQ, Lu NN, Wang XY, Xie M, Sui WP. Ultrasonication-assisted one-step self-assembly preparation of biocompatible fluorescent-magnetic nanobeads for rare cancer cell detection. Nanotechnology. 2014;25:505603. doi: 10.1088/0957-4484/25/50/505603. [DOI] [PubMed] [Google Scholar]
- 39.Song EQ, Hu J, Wen CY, Tian ZQ, Yu X, Zhang ZL, Shi YB, Pang DW. Fluorescent-Magnetic-Biotargeting Multifunctional Nanobioprobes for Detecting and Isolating Multiple Types of Tumor Cells. Acs Nano. 2011;5:761–770. doi: 10.1021/nn1011336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hossain M, Luo Y, Sun ZY, Wang CM, Zhang MH, Fu HY, Qiao Y, Su M. X-ray enabled detection and eradication of circulating tumor cells with nanoparticles. Biosens Bioelectron. 2012;38:348–354. doi: 10.1016/j.bios.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Hoshino K, Huang YY, Lane N, Huebschman M, Uhr JW, Frenkel EP, Zhang X. Microchip-based immunomagnetic detection of circulating tumor cells. Lab Chip. 2011;11:3449–3457. doi: 10.1039/c1lc20270g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Besant JD, Mohamadi RM, Aldridge PM, Li Y, Sargent EH, Kelley SO. Velocity valleys enable efficient capture and spatial sorting of nanoparticle-bound cancer cells. Nanoscale. 2015;7:6278–6285. doi: 10.1039/c5nr00797f. [DOI] [PubMed] [Google Scholar]
- 43.Mohamadi RM, Besant JD, Mepham A, Green B, Mahmoudian L, Gibbs T, Ivanov I, Malvea A, Stojcic J, Allan AL, et al. Nanoparticle-Mediated Binning and Profiling of Heterogeneous Circulating Tumor Cell Subpopulations. Angew Chem Int Edit. 2015;54:139–143. doi: 10.1002/anie.201409376. [DOI] [PubMed] [Google Scholar]
- 44.Medintz IL, Uyeda HT, Goldman ER, Mattoussi H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater. 2005;4:435–446. doi: 10.1038/nmat1390. [DOI] [PubMed] [Google Scholar]
- 45.Liu HY, Xu SM, He ZM, Deng AP, Zhu JJ. Supersandwich Cytosensor for Selective and Ultrasensitive Detection of Cancer Cells Using Aptamer-DNA Concatamer-Quantum Dots Probes. Anal Chem. 2013;85:3385–3392. doi: 10.1021/ac303789x. [DOI] [PubMed] [Google Scholar]
- 46.Lee HJ, Cho HY, Oh JH, Namkoong K, Lee JG, Park JM, Lee SS, Huh N, Choi JW. Simultaneous capture and in situ analysis of circulating tumor cells using multiple hybrid nanoparticles. Biosens Bioelectron. 2013;47:508–514. doi: 10.1016/j.bios.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 47.Liu Q, Yeh YC, Rana S, Jiang Y, Guo L, Rotello VM. Differentiation of cancer cell type and phenotype using quantum dot-gold nanoparticle sensor arrays. Cancer Letters. 2013;334:196–201. doi: 10.1016/j.canlet.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim H, Abdala AA, Macosko CW. Graphene/Polymer Nanocomposites. Macromolecules. 2010;43:6515–6530. [Google Scholar]
- 49.Yoon HJ, Kim TH, Zhang Z, Azizi E, Pham TM, Paoletti C, Lin J, Ramnath N, Wicha MS, Hayes DF, et al. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat Nanotechnol. 2013;8:735–741. doi: 10.1038/nnano.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nellore BPV, Kanchanapally R, Pramanik A, Sinha SS, Chavva SR, Hamme A, Ray PC. Aptamer-Conjugated Graphene Oxide Membranes for Highly Efficient Capture and Accurate Identification of Multiple Types of Circulating Tumor Cells. Bioconjugate Chem. 2015;26:235–242. doi: 10.1021/bc500503e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng L, Chen Y, Ren J, Qu X. A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials. 2011;32:2930–2937. doi: 10.1016/j.biomaterials.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Edit. 1998;37:2755–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 53.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Jr., Banaszak Holl MM. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chem Biol. 2007;14:107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 54.Myung JH, Gajjar KA, Saric J, Eddington DT, Hong S. Dendrimer-mediated multivalent binding for the enhanced capture of tumor cells. Angew Chem Int Edit. 2011;50:11769–11772. doi: 10.1002/anie.201105508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearson RM, Sunoqrot S, Hsu H, Bae JW, Hong S. Dendritic nanoparticles: the next generation of nanocarriers? Ther Deliv. 2012;3:941–959. doi: 10.4155/tde.12.76. [DOI] [PubMed] [Google Scholar]
- 56.Bugno J, Hsu H, Hong S. Recent advances in targeted drug delivery approaches using dendritic polymers. Biomater Sci. 2015 doi: 10.1039/C4BM00351A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myung JH, Gajjar KA, Pearson RM, Launiere CA, Eddington DT, Hong S. Direct measurements on CD24-mediated rolling of human breast cancer MCF-7 cells on E-selectin. Anal Chem. 2011;83:1078–1083. doi: 10.1021/ac102901e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Launiere C, Gaskill M, Czaplewski G, Myung JH, Hong S, Eddington DT. Channel Surface Patterning of Alternating Biomimetic Protein Combinations for Enhanced Microfluidic Tumor Cell Isolation. Anal Chem. 2012;84:4022–4028. doi: 10.1021/ac2033408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myung JH, Gajjar KA, Chen JH, Molokie RE, Hong S. Differential Detection of Tumor Cells Using a Combination of Cell Rolling, Multivalent Binding, and Multiple Antibodies. Anal Chem. 2014;86:6088–6094. doi: 10.1021/ac501243a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myung JH, Launiere CA, Eddington DT, Hong S. Enhanced tumor cell isolation by a biomimetic combination of E-selectin and anti-EpCAM: implications for the effective separation of circulating tumor cells (CTCs) Langmuir. 2010;26:8589–8596. doi: 10.1021/la904678p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatch A, Hansmann G, Murthy SK. Engineered Alginate Hydrogels for Effective Microfluidic Capture and Release of Endothelial Progenitor Cells from Whole Blood. Langmuir. 2011;27:4257–4264. doi: 10.1021/la105016a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reategui E, Aceto N, Lim EJ, Sullivan JP, Jensen AE, Zeinali M, Martel JM, Aranyosi AJ, Li W, Castleberry S, et al. Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells. Adv Mater. 2015;27:1593–1599. doi: 10.1002/adma.201404677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gaddes ER, Gydush G, Li S, Chen N, Dong C, Wang Y. Aptamer-based polyvalent ligands for regulated cell attachment on the hydrogel surface. Biomacromolecules. 2015;16:1382–1389. doi: 10.1021/acs.biomac.5b00165. [DOI] [PubMed] [Google Scholar]
- 64.Shah AM, Yu M, Nakamura Z, Ciciliano J, Ulman M, Kotz K, Stott SL, Maheswaran S, Haber DA, Toner M. Biopolymer System for Cell Recovery from Microfluidic Cell Capture Devices. Anal Chem. 2012 doi: 10.1021/ac300190j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao WA, Cui CH, Bose S, Guo DG, Shen C, Wong WP, Halvorsen K, Farokhzad OC, Teo GSL, Phillips JA, et al. Bioinspired multivalent DNA network for capture and release of cells. Proc Natl Acad Sci U S A. 2012;109:19626–19631. doi: 10.1073/pnas.1211234109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou S, Zhao HC, Zhao LB, Shen QL, Wei KS, Suh DY, Nakao A, Garcia MA, Song M, Lee T, et al. Capture and Stimulated Release of Circulating Tumor Cells on Polymer-Grafted Silicon Nanostructures. Adv Mater. 2013;25:1547–1551. doi: 10.1002/adma.201203185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading/Resources
- 1.Alix-Panabières Catherine, Pantel Klaus. Challenges in circulating tumour cell research. Nature Reviews Cancer. 2014;14:623–631. doi: 10.1038/nrc3820. DOI:10.1038/nrc3820. [DOI] [PubMed] [Google Scholar]
- 2.Pantel Klaus, Brakenhoff Ruud H., Brandt Burkhard. Detection, clinical relevance and specific biological properties of disseminating tumor cells. Nature Reviews Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. DOI: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 3.Myung Ja Hye, Gajjar Khyati A., Han Ye Eon, Hong Seungpyo. The role of polymers in detection and isolation of circulating tumor cells. Polymer Chemistry. 2012;3:2336–2341. DOI: 10.1039/C2PY20420G. [Google Scholar]