Abstract
Flagella propel bacteria during both swimming and swarming, dispersing them widely. However, while swimming bacteria use chemotaxis to find nutrients and avoid toxic environments, swarming bacteria appear to suppress chemotaxis and to use the dynamics of their collective motion to continuously expand and acquire new territory, barrel through lethal chemicals in their path, carry along bacterial and fungal cargo that assists in exploration of new niches, and engage in group warfare for niche dominance. Here we focus on two aspects of swarming, which if understood, hold the promise of revealing new insights into microbial signaling and behavior, with ramifications beyond bacterial swarming. These are: how bacteria sense they are on a surface and turn on programs that promote movement, and how as dense packs they override scarcity and adversity.
Keywords: Flagellar motor, surface sensing, group migration, antibiotic resistance, biofilms, bacterial warfare
Introduction
Swarming motility was first recorded for Proteus species [1, 2], and subsequently observed to be widespread among flagellated bacteria [3, 4]. Swarming is studied on agar surfaces in the laboratory, and to our knowledge is not confirmed to occur (i.e. by observing motility under the microscope) in natural habitats. However, the wide variety of bacteria that swarm in the laboratory provide a compelling argument that they must also swarm in nature [3-6]. Swarming tactics are as diverse as the bacteria that utilize them, and have been recently reviewed [5-8]. This perspective highlights two areas of swarming which we believe have the greatest potential for revealing new biological mechanisms both at the individual and communal level, the general principles being applicable to other bacterial communities and even beyond bacteria to other organisms which migrate collectively. We will first review novel properties that emerge during group migration, and then tackle unsolved problems in surface sensing. But first, a brief primer on the flagellum and on chemotaxis.
The flagellum and chemotaxis
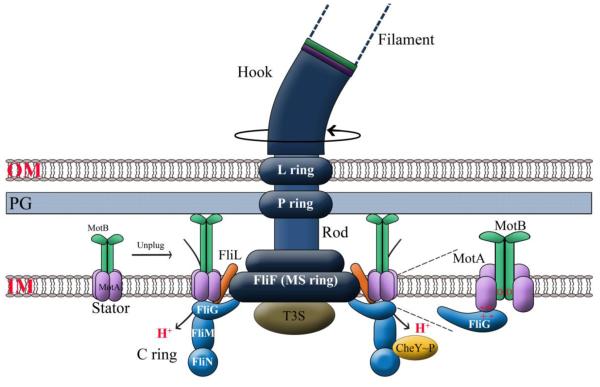
A typical ion-driven bacterial flagellum is shown in Fig. 1. The basic structural arrangement shown is common to all the bacteria discussed in this article, whether Gram-positive or Gram-negative, whether positioned at polar or at lateral locations. The term ‘motor’ refers to two functional entities: the rotor (rotating basal structure: C ring, MS ring, rod) and the stator (stationary ion-conducting complexes: MotAB proteins) (Fig.1) [9]. In E. coli, there are a maximum of 11-13 stator units located around the MS ring within the inner membrane. These units turn over, transiently engaging and disengaging with the rotor; they conduct protons only when engaged [10]. Protonation of a specific Asp32 residue in MotB is thought to cause conformational changes in the cytoplasmic region of MotA that sets up charge-charge interactions with residues in FliG in the C ring (Fig. 1, expanded inset). This causes FliG to move and generate torque for flagellar rotation [11, 12]. Torque is transmitted via the MS ring and rod to the external hook and filament. In marine Vibrios, homologous units conduct Na+ ions that drive rotation of the polar flagellum [13].
Fig. 1.
A typical bacterial flagellum. The bidirectional rotating unit consists of the cytoplasmic C ring composed of three proteins (FliG, FliM, FliN), inner membrane MS ring, periplasmic rod, external hook and a long helical filament. Rod-hook-filament proteins are secreted via a Type IIII secretion system (T3S) at the base of the MS ring. Rotation is indicated by a curved arrow. MotAB stators insert randomly and drift in the membrane till they encounter the basal MS - C rings, upon which their plugged ion channel opens to initiate ion flow, which powers rotation. FliL interacts with both the stators and the rotor to modulate motor speed and directional bias [107]. The L and P rings serve as bushings and are stationary. Expanded Inset: During ion flow, protonation of the critical Asp (D) residue in MotB is thought to induce a conformational change in the cytoplasmic region of MotA which sets up charge-charge interactions with FliG. This interaction generates torque, causing FliG to move. CheY~P is a response regulator generated by the chemotaxis system. It interacts with FliN and FliM in the C ring to cause changes in FliG on top of the ring, which switches rotational direction from the default CCW state to CW; the switching bias is important for chemotaxis. See text. OM, outer membrane; PG, peptidoglycan; IM, inner membrane.
In E. coli and Salmonella, a CCW rotor direction is the default state and causes the bacterium to swim forward or run. Bacteria tumble if the rotation is switched to a CW direction. Switching is controlled by binding of the chemotaxis response regulator phospho-CheY (CheY~P) to the bottom of the C ring, causing a conformational change in FliG at the top of the ring, which results in a change in rotational direction from CCW to CW (Fig. 1) [14]. The swimming movement of E. coli and Salmonella has been described as a ‘random walk’, in which periods of smooth swimming (or runs) are interrupted by short re-orientations (or tumbles) [15]. The chemotaxis system encodes a short-term memory that enables the bacteria to remember temporal changes in chemoeffector concentrations and to bias their random walk towards higher concentrations of attractants and avoid higher concentrations of repellents [16]. Abundant quantitative data on the chemotaxis pathway have led to several mathematical models of chemotaxis [17, 18].
Moving in a group
Due to ease of viewing, microfluidic devices are becoming popular for monitoring the behavior of dense groups of bacteria, with the presumption of gaining insight into swarming [19, 20]. These closed devices with no-slip boundaries are very useful for observing short-range interactions within the group, but the boundaries suppress long-range hydrodynamic interactions [19]. In these devices, swimmer cells are concentrated and packed into high densities, or made longer by antibiotic treatment to mimic the phenotype of some swarming bacteria, or smooth swimming mutants are used. A cautionary note regarding use of these devices is that, as discussed under ‘Sensing’ below, concentrated swimmers are not equivalent to swarmers, temperate swarmers (bacteria that require softer agar to swarm; [8]) do not elongate substantially [21, 22], and the ability to switch motor direction is important for swarming [23, 24]. Also, placing bacteria between two no-slip boundaries creates an environment that is different from a natural swarm whose upper surface is not stationary [25]. The best platform on which to observe the swarm would be the natural open one, where the bacteria have themselves initiated movement [26-28].
Collective migration in a swarm
The most fascinating feature common to all swarms is the ceaseless streaming and swirling motion of millions of bacteria, packed side to side, continuously pushing the swarm outward, acquiring more nutrients and colonizing more and more surface territory (see movies in [8]). One might expect that the bacteria would use chemotaxis to search for food in their outward journey. Yet, the temperate swarmers E. coli, Salmonella and Serratia marcescens largely suppress chemotaxis during swarming as determined by the prolonged smooth swimming behavior displayed by these bacteria when picked from the swarm and suspended in a drop of liquid (reported under ‘Migration’ in [4]). This was also noted for E. coli swarms by videotaping cells at the edge of a moving colony [29]. How and why bacteria suppress chemotaxis during swarming is an interesting problem for future studies of this collective motion, which is being increasingly analyzed as a model to derive insights into the swarming behavior of large animals such as schooling fish or flocking birds [5, 8, 30].
The power of swarms
1. Elevated resistance to antibiotics
Many temperate swarmers show increased resistance to a variety of structurally and mechanistically different antibiotics albeit only when swarming [31-37] (Fig. 2a). The resistance dissipates when the bacteria are transferred to liquid, and is therefore non-genetic. Various mechanisms have been proposed to explain this resistance, which is different from that of slow-growing ‘persisters’ that are also non-genetically resistant to a variety of antibiotics [38]. The proposed mechanisms include altered outer membrane composition [32], decreased membrane permeability [39, 40], induced oxidative stress response [41], lower metabolic activity ([39, 42]) and altruism [36]. (P. mirabilis is reported to use anaerobic respiration during swarming [43, 44]). These explanations must reconcile the observation that when cells swarming on an antibiotic surface are lifted and transferred directly to a fresh swarm plate, they are killed [36] (Fig. 2a, right panel). If cellular changes such as decreased antibiotic permeability, slower metabolism or increased defense against reactive oxygen species (thought to be the ultimate killing mechanism of many antibiotics [45]) were responsible for the elevated resistance of swarmers, why should this property dissipate when cells are transferred to a fresh swarm surface?
Fig. 2.

Survival in a swarm. (a) Elevated antibiotic resistance of bacterial swarms. Top left: Border-crossing assay in Salmonella. On a divided Petri dish containing LB media solidified with 0.3% agar (w/v; swimming condition) or 0.6% agar (swarming condition), cells inoculated in the left no-antibiotic chamber were allowed to migrate to the right antibiotic-containing chamber. Numbers refer to μg/mL of the antibiotic Kanamycin (Kan). At both Kan concentrations that stopped the swimmers at the border (20 and 50), swarmers crossed the border and continued to colonize the antibiotic chamber. Top right: Antibiotic sensitivity of swarmer cells that crossed the border. The horizontal labels indicate plate composition, either no-antibiotic control (Ctrl) solidified with hard agar (1.5%; non-swarming) or Kan (K50 Swrm) solidified with 0.6% agar. The vertical labels indicate the source of the cells, taken either from the no-antibiotic side (Ctrl Swrm) or from the antibiotic side (K50 Swrm), transferred to the indicated plates by the flat end of a cylindrical toothpick. Bottom: Bacteria endure cell death as they continue to swarm. Swarmer cells from the no-antibiotic side (control) and from the antibiotic side of K50 plates were stained with the live/dead stain. The control plate had 6% dead (red) cells, the K50 plate had 38% dead cells. Data taken from [36]. (b) Mutual co-operation between two bacterial species. Top: A mixture of ampicillin (Amp)-sensitive but swarming-proficient P. vortex, and Amp-resistant but non-motile E. coli was inoculated in the center of a swarm plate and imaged after incubation for 72 h on a plate with 200 μg/ml Amp, stained with Coomassie blue to enhance contrast. Bottom: An identical experiment, but using hexidium iodide to identify P. vortex (red) and GFP expression for E. coli (green), the two colonies imaged by fluorescence microscopy. Data from [51]. (c) Warfare between different swarming Proteus strains manifested as Dienes lines. Strains A, B and C were propagated in pairs on swarm media. Dienes lines form between different but not identical strains. Data from [108]. All reproduced with permission. See text.
One property that obviously changed during the transfer of cells between swarm plates was the cell density. Bacterial inoculum size has been noted to influence the MIC (minimal inhibitory concentration) of an antibiotic, lowering it with decreasing density [46]. However, this effect is generally observed for β-lactam antibiotics, and less often with quinolones and aminoglycosides [47], the MICs for all of which increase in swarmers. Even if one assumes that the high density of cells might reduce the effective concentration of the free antibiotic in the medium say by binding dead as well as viable bacteria, the total number of antibiotic molecules per bacterium remains enormous in the swarm plate. Perhaps a combination of mechanisms operate. For example, according to the altruism hypothesis, cells directly in contact with antibiotic get killed (Fig. 2a, bottom panel) and provide a protective barrier for those on top [36]. The transfer may have lifted only cells at the top of the colony, leaving their protectors behind. Whether the protective barrier is physical, or whether ‘signals’ from the dead subpopulation are continuously intercepted by the living as an alarm to induce protective mechanisms including an oxidative response, and whether such mechanisms also operate in biofilms, would be interesting to test.
2. Carrying beneficial cargo
Motile microorganisms can move objects such as microscopic beads and gears [48-50], so it is no surprise that Paenibacillus vortex swarms have been reported to move fungal spores and other bacteria (dubbed ‘cargo’) over long distances, provided the swarm was multilayered [37, 51]. Such transport can be mutually beneficial, for example, when the cargo was non-motile β-lactamase-producing E. coli, it allowed ampicillin-sensitive P. vortex to swarm and colonize an ampicillin containing plate, dispersing both bacteria (Fig. 2b). One would imagine that the swirling bacterial mass would trap the cargo passively, but there appeared to be some specificity to this phenomenon in that cargo transport was not observed with other Paenibacillus spp., nor with swarming P. mirabilis, nor were all species of spores or bacteria carried along by P. vortex. Related observations have been reported for P. vortex is assisting the dispersal of Xanthomonas perforans [52]. Examples of such mutual cooperation abound in nature, including between bacteria and eukaryotic organisms [53].
A common theme that appears to be emerging in bacterial communes, whether in swarms, during flagella-independent migration, or within biofilms, is that bi-stability of certain traits can set up a phenotypic heterogeneity that leads not only to bet-hedging as has been previously proposed, but also to division of labor [54, 55]. For example, swarms of P. vortex appear to have two phenotypic variants, one more motile than the other; the hypermotile population spearheads colony expansion, has lower ATP levels, higher tolerance to antibiotics, and cargo-carrying capacity [42, 51]. Another study observed that swarming cells of B. subtilis propagated on sub-lethal kanamycin concentrations (0.1μg/ml) generated immotile cell clusters that segregated from the motile cells, forming stationary barriers around which the motile cells navigated [56]. The barriers changed the surrounding local fluid flow [56]. Whether and how these barriers and the altered fluid flow influence antibiotic susceptibility would be interesting to investigate, but the differential response to the antibiotic by a subset of bacteria again reveals the phenotypic heterogeneity of a swarming population. Even a colony of non-motile B. subtilis can promote expansion by differentiating into two kinds of cells, one producing extracellular matrix (ECM) and the other producing a surfactant [57]. The ECM promotes adherence of bacteria to each other as well as to the surface, while the surfactant weakens surface tension. The signals that set up this heterogeneity are known [54]. Matrix producers form bundles that are hypothesized to drive outward expansion of the colony, which is facilitated by the surfactant producers.
The phenotypic heterogeneity observed in both motile and non-motile bacterial colonies are changing our notion of bacterial communes, where a deceptively uniform colony is in reality quite non-uniform [58]. A bacterial swarm, not unlike a swarm of bees, is composed of genetically identical but phenotypically different individuals that respond appropriately to their environment to perform different jobs, occupy different spaces, establish mutually beneficial associations with other bacteria or even fungi, and make sacrifices that allow the group to find shelter and survive in a hostile environment. These studies suggest that specific bacterial heterogeneities could be exploited to direct bacterial groups to perform desired tasks.
3. Waging war
The type VI secretion system (T6SS) found in Gram-negative bacteria delivers toxins into other bacteria upon contact, puncturing and killing these bacteria [59]. This system was recently observed to be employed by P. mirabilis during swarming [60]. Strains of Proteus have long been observed to form boundaries called ‘Dienes lines’ at the intersection of swarms from different isolates [61] (Fig. 2c). Such boundaries do not form between swarming colonies of a single strain, and can be used as a practical method to type isolates. The boundaries suggest a mechanism for discriminating self from non-self. One study found that the discrimination resided in a six-gene locus termed ids (identification of self), which mediated self-recognition by a diffusible signal using a mechanism that did not involve killing [62]. Another study showed that the boundaries are killing zones that depend on the T6SS [60]. Interestingly, the T6S apparatus is assembled only upon initiation of swarming, as if in preparation for ground warfare, and deployed apparently indiscriminately when one swarm meets another [60]. The dominant strain continues to fire and penetrate into the opposing swarm. Understanding the rules of this battle will shed light on conflict and cooperation between bacterial swarms and the evolution of optimal multicellular interactions controlling migration and niche dominance.
Sensing the surface to initiate swarming
Unlike swimming, bacteria must prepare for swarming as judged by the fact that when swimmers are transferred to the surface, they exhibit a lag before moving, must reach higher cell densities, and generally require energy-rich media for swarming to begin. In many bacteria, mutations that overcome these requirements map to two-component signaling systems or to regulators that increase flagella synthesis [22, 63-66]. Thus, there must be surface-sensing mechanisms that, during the initiation period, transduce the surface-contact signal to trigger new transcriptional as well as post-transcriptional pathways in preparation for swarming (reviewed in [8]). These mechanisms are sure to be varied. Robust swarmers that swarm on "hard" agar surfaces (Proteus and Vibrio spp.) have a dramatically different morphology, becoming hyperflagellated and hyperelongated when propagated on a surface, while temperate swarmers that require a "softer" agar surface (E. coli, Bacillus, Pseudomonas, Salmonella, Serratia, spp.) do not exhibit such a dramatic morphology [8]. Here we will consider two sensors implicated in sensing surface contact in more than one bacterial species - the flagellum and the cell envelope. Despite nearly a quarter century of observations, there have been no breakthroughs in understanding how the surface-contact signal is transduced. We will briefly review and distill the most clear-cut of these cases, unify disparate observations, and propose new ideas, with the hope of provoking new experimentation challenging these ideas, so that the field can move forward.
The flagellum as a sensor
1. Vibro parahaemolyticus
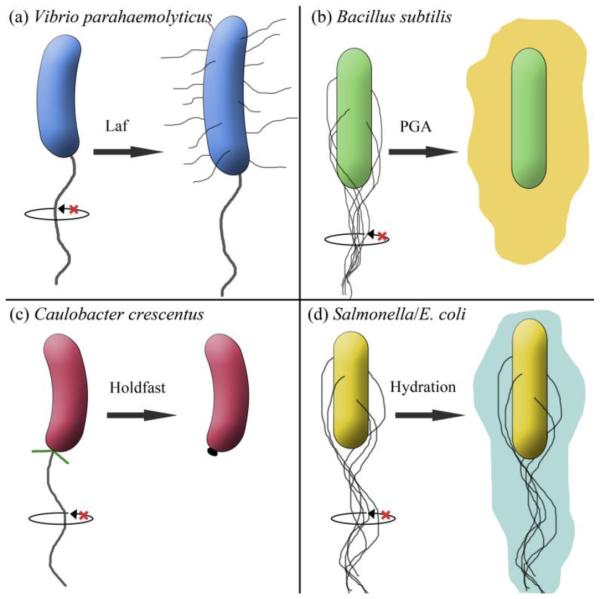
Surface contact is expected to slow the flagellum due to increased viscous drag on the surface. The best evidence for the flagellum as a sensor comes from the marine bacterium Vibrio parahaemolyticus, where slowing rotation of the polar flagellum using two different experimental regimes – either increasing external load on the filament (and hence the motor) via increased viscosity or antibody tethering [67, 68], or blocking Na+ ion flow through the stators by adding the Na+ channel blocker phenamil [69] – leads to synthesis of hundreds of lateral flagella (reviewed in [70]) (Fig. 3a). That both load on the motor (viscosity, antibody) and no load (blocking ion flow) lead to the same output, suggests that the perceived signal may not be load itself but a consequence of high load such as slowing or stalling the motor, and diminishing ion flux. Speed and ion flux are intimately linked and not separable [71]. The key question is whether there is a reasonable mechanism that the cell could use as a read-out for decreased speed/ion flux. We would like to propose that the read-out might be conformational changes at the rotor-stator interface, which monitor ion flux. We use the following observations to support this suggestion. Stators can attach (engage) to the motor only when they are conducting ions and are able to generate torque [72]. In the marine bacterium Vibrio alginolyticus, which is closely related to V. parahaemolyticus, blocking ion flow with phenamil, inhibited the stators from engaging with the rotor [73]. Therefore, the induction of lateral flagella synthesis in V. parahaemolyticus upon phenamil treatment is likely a consequence of stator disengagement [69]. What might be the state of stators under high viscosity/antibody tethering conditions, which stall the motor by placing a high load on the filament [67, 68]? In E. coli, motors subjected to a sudden load increase, recruit additional stators to increase speed [74]. Stators are also fully engaged when external magnetic fields are applied to generate a torque so high that the motors stall [75]. Thus, at high load, with stators fully engaged, motors can either be running or stalled. However, even with fully engaged stators, the rotor-stator interface is likely to have a different conformation between a running motor (ion-conducting) and a stalled one (non-conducting). On a surface, when the load on the V. parahaemolyticus polar flagellum is expected to be high due to viscous drag, the motor could be stalled and non-conducting. Thus, we reconcile the two sets of experiments in V. parahaemolyticus that stop the motor by suggesting that the common denominator in these manipulations is absence of the normal rotor-stator configuration found in a running motor, and that it is the loss of this configuration that is the input signal. Consistent with this proposal, deletions of genes encoding the stators also induce lateral flagella synthesis [68].
Fig. 3.
The Flagellum as a Surface Sensor. (a-d) Surface contact initially restricts flagellar rotation (indicated by x), signaling varied downstream events in the different bacteria, which promote either motility or sessility, as described in the text.
The insight we derive above from the Vibrio experiments is useful because it unifies the role of the flagellum as a sensor in bringing about two completely opposite outcomes for life on a surface i.e. swarming vs biofilm formation. In the soil bacterium Bacillus subtilis, inhibition of flagellar rotation results in increased transcription and secretion of poly-γ-glutamate (PGA), a polymer that forms an external slime layer and promotes adhesion to surfaces [76-78] (Fig. 3b). Some of the experimental methods to inhibit rotation used antibodies to tether flagella. Others involved complete deletion of the genes encoding both stator proteins (MotA and MotB) or introducing a point mutation in a critical protonated Asp residue of MotB (see Fig. 1). In E. coli, an equivalent mutation in MotB, expected to prevent proton flux, disrupts stable binding of the stators to the rotor [75]. Thus, PGA production requires stators to be absent or to not be in their proton-conducting state. Transcription of the operon responsible for PGA synthesis is additionally dependent on the two-component signaling system DegS/DegU, which is activated by the reported flagellar disruptions [76, 77].
Another clear-cut case linking flagellar rotation to a cellular response is seen in the fresh water bacterium Caulobacter crescentus, where arrest of the polar flagellum generates an immediate or ‘just-in-time’ secretion of an adhesive stalk [79] (Fig. 3c). This normally happens when the bacterium attaches to a surface using Type IV pili located next to the flagellum (green whiskers in 3c). When the pili retract, they jam the bacterial cell body on the surface, pinning the flagellum and arresting its rotation. Holdfast synthesis is independent of new protein synthesis and is also stimulated by viscous agents that slow the flagellum. While the state of the stators under these conditions is not yet known, arrested flagellar rotation is clearly implicated in the secretion response.
Based on the selected systems discussed above we propose the following model for sensing through the flagellum. We suggest that a normal running motor sequesters and inactivates a regulator of the downstream response (Fig. 4a, blue capsule). When the motor conformation deviates from normal in having non-conducting stators (or no stators), the regulator is released for action. The observed graded response to viscosity or ion-channel blockers in V. parahaemolyticus can be explained by graded inactivation of the stators [69]. Alternatively, the regulator could be bound and activated by a stalled motor, but this model requires that the rotor be present for the response to occur.
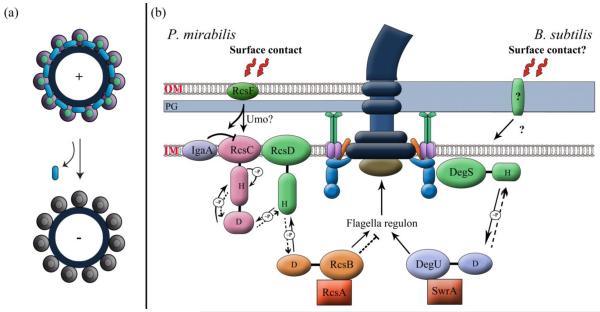
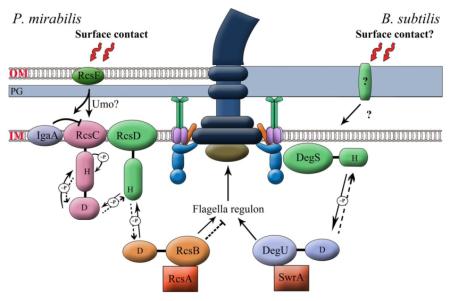
Fig. 4.
Models for surface sensing. (a) The flagellar motor as a sensor. The rotor is represented by a large black circle. Ion conducting stators are purple-and-green colored circles and non-conducting ones are grey. A running motor is (+), and a stalled motor is (−). We propose that a running motor sequesters and inactivates a regulator (blue), which is released for action when the motor stalls. The (−) state may also be achieved in the absence of the stators or of the rotor (not shown). The regulator could alternatively be bound and activated by a stalled motor, but this model would require that the rotor be present for the response. (b) The cell envelope as a sensor. Contact with a solid surface is detected by an outer membrane / cell wall protein. In P. mirabilis (left), RcsF is implicated in the sensory pathway. We propose that upon surface contact, RcsF is released into the periplasm [95], where it directly or indirectly, via Umo or IgaA proteins, activates the phosphatase activity of RcsC kinase, reversing phosphate (~P) flow to dephosphorylate and inactivate RscB~P, the repressor of flagella biosynthesis (Dotted lines indicate that the normal phosphorylation reaction is suppressed). RcsA is an auxiliary transcriptional regulator in Gram-negative bacteria, which complexes with RscB~P (see text). The flagellum is also implicated in sensing in P. mirabilis, but the mechanism is unclear. In B. subtilis (right), the soluble DegS/DegU signaling system senses events at the rotor-stator interface [76, 77, 109]. In response to surface contact, this signaling system could play a role equivalent to that depicted for the Rcs system in P. mirabilis, except that the surface signal activates the kinase activity of DegS. We propose that SwrA is an auxiliary regulator of DegU~P [22] (see text). The DegS/DegU system could be sequestered by a running motor as in (a), receiving surface signals either via the flagellum or by an independent route.
2. E. coli/Salmonella
These enteric bacteria swarm, but unlike V. parahaemolyticus, do not induce a swarming-specific transcriptional program [80]. The cells double in size, so they have twice the number of flagella, but flagella synthesis is not upregulated [21, 80, 81]. The critical swarming requirement for these bacteria is adequate hydration of the surface colony (Fig. 3d). Hydration is promoted by the ability of flagella to periodically switch their direction of rotation as deduced by the following curious interplay between the flagellum and the chemotaxis system in enabling swarming. Chemotaxis is not required for outward migration of the swarm [23, 24, 81, 82], and is actually suppressed during swarming as discussed above. Nevertheless, a functional chemotaxis system is required for colony hydration because null mutants in the chemotaxis pathway (Che), which are locked in the rotational direction of their motors (either CW or CCW) do not swarm [83]. Non-chemotactic suppressors that restored swarming to a CCW-biased mutant mapped to a component of the flagellar switching apparatus (FliM in the C ring; Fig. 1); these suppressors concomitantly restored switching and colony hydration [21, 24]. This finding confirms that the chemotaxis system is required because it generates the CheY~P response regulator that controls motor switching [16]. Thus, it is not chemotaxis per se, but the ability to switch motor direction that controls colony hydration. Indeed, the Che mutants can be rescued for swarming by hydrating the swarm colony [81].
How changes in the direction flagellar rotation controls colony hydration is still not clear. It has been speculated that flagellar filaments normally stick to swarm agar and thus get tethered or restricted; motor switching unsticks or liberates them [81]. Once free, rotating filaments could aid colony hydration either by secreting water through the flagellum, by creating turbulence on the surface that draws up the water by capillary action from the agar beneath [21], or by stripping LPS off the bacterial outer membrane, the free LPS penetrating the agar to act as an osmotic agent [81, 84]. LPS defects in Salmonella can be bypassed by use of a highly wettable surface [84], and MotA/B mutants that cannot rotate their filaments also have a dry colony morphology [21]. Support for the LPS-release hypothesis was provided in a study of osmolarity in different regions of an advancing E. coli swarm utilizing fluorescent liposomes as reporters, which concluded that high osmotic pressure at the leading edge of the swarm extracts water from the underlying agar and promotes motility, and that the osmolyte is of high molecular weight and is likely LPS [85]. However, in B. subtilis, there is no outer membrane or LPS, and a non-flagellate mutant apparently lacks hydration [25]. We note that in E. coli, mutations that increase flagella numbers can bypass LPS defects [86]. Thus, flagellar filaments appear to be important for colony hydration by more than one mechanism.
Borrowing elements from the model proposed in Fig. 4a, the following two alternate scenarios can be envisaged to explain how E. coli/Salmonella exploit the flagellum to hydrate their swarm colonies. In the first scenario, the surface initially restricts flagellar rotation, which is the sensory input or signal like in the other examples discussed above. The resulting stalled motor configuration transduces this signal to activate just-in-time osmolyte secretion (Fig. 3d). With the arrival of water, it follows that the ‘dry’ signal will dissipate and the flagella will now be free to rotate. The ability to switch rotor directions is important at this stage to unstick from the surface. Hydration may be promoted by the rotating flagella bundles by any one of the mechanisms considered above. In this model, flagella play two active roles: they initially restrict motor rotation to generate the signal that primes hydration, and they subsequently allow rotation to facilitate continued hydration. In an alternate scenario, temperate swarmers may not sense the surface by this mechanism at all (see ‘Bacillus subtilis’ below), but the ability to switch may be important to unstick the flagella so that they can continue rotating to enable colony hydration. In either case, the flagella clearly play a role in addition to motility to promote swarming [21].
The cell envelope as a sensor
1. Proteus mirabilis
Like in V. parahaemolyticus, swarming is accompanied by increased flagellar synthesis in this soil bacterium [87-89]. The flagella are thought to be involved in surface sensing, but the data are not unequivocal, as discussed elsewhere [90]. In P. mirabilis, Umo (upregulator of the master flagellar operon flhDC) proteins associated with the cell envelope are implicated in sensing the surface; one of these proteins is likely located in the inner membrane (IM) and one in the periplasm [91, 92]. Genetic experiments suggest that these proteins signal through a pathway that somehow intersects with the Rcs signaling pathway [93], and inactivates the RscB response regulator that normally inhibits flhDC [92, 94] (Fig. 4b). In E. coli, the Rcs pathway is known to be activated by outer membrane (OM) stress. Here, RcsF, an OM protein, is continuously funneled to the OM, separating it from the IM protein IgaA, which negatively regulates the RcsC kinase [95]. During envelope stress, RcsF is no longer funneled to the OM but remains in the periplasm, interacting with IgaA to relieve its inhibition of the RcsC kinase. RcsC now phosphorylates RscB, which represses flhDC [95]. Inspired by these findings, we propose that in P. mirabilis, surface contact leads to a similar series of events through RcsF and via Umo proteins, but instead of activating the kinase activity of RcsC, these events activate its phosphatase activity, reversing phosphate flow through the system and relieving inhibition of the flagellar operon (Fig. 4b, left panel). Whether this pathway also communicates with the flagellum or whether restriction of rotation is independently sensed, remains to be determined [89].
2. Bacillus subtilis
This bacterium doubles its flagella numbers during swarming, a response essential for swarming to occur [22]. There is no evidence as yet that restricting flagella rotation is responsible for this increase. On the contrary, tethering flagella with antibody leads to PGA synthesis as discussed above. Surface contact in B. subtilis causes a rise in the levels of SwrA, the master regulator of flagella biosynthesis, by a mechanism that slows its degradation by the Lon protease [22]. Mutation of Lon causes hyperflagellated cells in liquid, and abolishes the swarming lag. In addition to SwrA, the two-component regulatory system DegS/DegU is also required to increase flagella synthesis in B. subtilis [78, 94] (Fig. 4b). Overexpression of SwrA cannot compensate for a loss of the response regulator DegU in restoring swarming motility [96]. Mutation of Lon homologs in V. parahaemolyticus and P. mirabilis also produce hyperflagellated cells [63, 97]. In E. coli and Salmonella, the Lon protease degrades RcsA [93], an auxiliary transcriptional regulator that complexes with RcsB~P [98-100] (Fig. 4b). Activation of the Rcs system could provide a mechanism for sequestering RcsA away from Lon by binding to RcsB~P. We propose that in B. subtilis, activation of the DegS kinase by surface contact leads to generation of DegU~P, which forms a complex with SwrA, sequestering SwrA away from Lon and leading to upregulation of flagella synthesis (Fig. 4b, right panel). The DegS/DegU system also senses stator disruption (see above), and could conform to the sequestered regulator modeled in Fig. 4a [78]. This signaling system may intercept the surface-contact signal at the rotor-stator interface, or independently of it. If the former, then other pathways monitoring the environment might intercede, channeling events at this interface to either favor PGA accumulation or motility.
We note that just as sensing through the flagellum can signal two opposing lifestyles - swarming or biofilm formation – sensing through the cell envelope can do the same. For example, growth of Pseudomonas aeruginosa on a surface can trigger an increase in c-di-GMP levels through two chemoreceptor-like complexes, one of which clusters in the IM [101], and the other collaborates with type IV pili (TFP) to secrete an OM-associated protein required for generating c-di-GMP, both complexes ultimately controlling the production of biofilms [102]. C-di-GMP is an important signaling molecule in the transition between motile and sessile stages of bacterial life, where high levels of c-di-GMP inhibit motility and promote biofilm formation, and low levels favor motile behaviors [103]. In V. parahaemolyticus, contact with a surface decreases c-di-GMP levels and favors swarming [104] by a pathway independent of sensing through the flagellum [105]. Closing the circle, c-di-GMP signaling in P. aeruginosa also controls the choice of stators incorporated into the flagellum, which affects the decision of P. aeruginosa to swarm [106].
Concluding remarks
The molecular principles of sensing and surviving derived from the study of swarming are applicable to bacterial groups that move without flagella, or those that don’t move at all but form biofilms. Individuals within a swarm cooperate to favor survival and dispersal when faced with a hostile environment, underscoring the power vested in the collective. This power could be potentially harnessed for beneficial tasks. The study of collective motion within a swarm is expected to lead to new models for this migration pattern, with insights beyond bacteria into migration patterns in the animal world. As this perspective highlights, much remains to be discovered about conflict, cooperation, and signaling within a swarm. The future looks exciting.
Highlights.
A bacterial swarm has unique survival and dispersal mechanisms
For swarming to ensue, bacteria sense and respond to surface contact
New models for surface-sensing are proposed
Acknowledgements
Motility work in our laboratory is supported by National Institutes of Health Grants GM112507 and AI116514 and in part by the Robert Welch Foundation Grant F-1811 to R.M.H..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972;36:478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Williams FD, Schwarzhoff RH. Nature of the swarming phenomenon in Proteus. Annu Rev Microbiol. 1978;32:101–22. doi: 10.1146/annurev.mi.32.100178.000533. [DOI] [PubMed] [Google Scholar]
- [3].Harshey RM. Bees aren't the only ones: swarming in gram-negative bacteria. Mol Microbiol. 1994;13:389–94. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- [4].Harshey RM. Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol. 2003;57:249–73. doi: 10.1146/annurev.micro.57.030502.091014. [DOI] [PubMed] [Google Scholar]
- [5].Copeland MF, Weibel DB. Bacterial Swarming: A Model System for Studying Dynamic Self-assembly. Soft matter. 2009;5:1174–87. doi: 10.1039/B812146J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–44. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Armbruster CE, Mobley HL. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol. 2012;10:743–54. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Partridge JD, Harshey RM. Swarming: flexible roaming plans. J Bacteriol. 2013;195:909–18. doi: 10.1128/JB.02063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Minamino T, Imada K, Namba K. Molecular motors of the bacterial flagella. Curr Opin Struct Biol. 2008;18:693–701. doi: 10.1016/j.sbi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [10].Leake MC, Chandler JH, Wadhams GH, Bai F, Berry RM, Armitage JP. Stoichiometry and turnover in single, functioning membrane protein complexes. Nature. 2006;443:355–8. doi: 10.1038/nature05135. [DOI] [PubMed] [Google Scholar]
- [11].Zhou J, Lloyd SA, Blair DF. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc Natl Acad Sci U S A. 1998;95:6436–41. doi: 10.1073/pnas.95.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kojima S, Blair DF. Conformational change in the stator of the bacterial flagellar motor. Biochemistry. 2001;40:13041–50. doi: 10.1021/bi011263o. [DOI] [PubMed] [Google Scholar]
- [13].Li N, Kojima S, Homma M. Sodium-driven motor of the polar flagellum in marine bacteria Vibrio. Genes Cells. 2011;16:985–99. doi: 10.1111/j.1365-2443.2011.01545.x. [DOI] [PubMed] [Google Scholar]
- [14].Paul K, Brunstetter D, Titen S, Blair DF. A molecular mechanism of direction switching in the flagellar motor of Escherichia coli. Proc Natl Acad Sci U S A. 2011;108:17171–6. doi: 10.1073/pnas.1110111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Berg HC. Bacterial flagellar motor. Curr Biol. 2008;18:R689–91. doi: 10.1016/j.cub.2008.07.015. [DOI] [PubMed] [Google Scholar]
- [16].Hazelbauer GL, Falke JJ, Parkinson JS. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bray D, Levin MD, Lipkow K. The chemotactic behavior of computer-based surrogate bacteria. Curr Biol. 2007;17:12–9. doi: 10.1016/j.cub.2006.11.027. [DOI] [PubMed] [Google Scholar]
- [18].Tu Y. Quantitative modeling of bacterial chemotaxis: signal amplification and accurate adaptation. Ann Rev Biophys. 2013;42:337–59. doi: 10.1146/annurev-biophys-083012-130358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wensink HH, Dunkel J, Heidenreich S, Drescher K, Goldstein RE, Lowen H, et al. Meso-scale turbulence in living fluids. Proc Natl Acad Sci U S A. 2012;109:14308–13. doi: 10.1073/pnas.1202032109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Swiecicki JM, Sliusarenko O, Weibel DB. From swimming to swarming: Escherichia coli cell motility in two-dimensions. Integr Biol (Camb) 2013;5:1490–4. doi: 10.1039/c3ib40130h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Partridge JD, Harshey RM. More than motility: Salmonella flagella contribute to overriding friction and facilitating colony hydration during swarming. J Bacteriol. 2013;195:919–29. doi: 10.1128/JB.02064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mukherjee S, Bree AC, Liu J, Patrick JE, Chien P, Kearns DB. Adaptor-mediated Lon proteolysis restricts Bacillus subtilis hyperflagellation. Proc Natl Acad Sci U S A. 2015;112:250–5. doi: 10.1073/pnas.1417419112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burkart M, Toguchi A, Harshey RM. The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proc Natl Acad Sci U S A. 1998;95:2568–73. doi: 10.1073/pnas.95.5.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mariconda S, Wang Q, Harshey RM. A mechanical role for the chemotaxis system in swarming motility. Mol Microbiol. 2006;60:1590–602. doi: 10.1111/j.1365-2958.2006.05208.x. [DOI] [PubMed] [Google Scholar]
- [25].Be'er A, Harshey RM. Collective motion of surfactant-producing bacteria imparts superdiffusivity to their upper surface. Biophys J. 2011;101:1017–24. doi: 10.1016/j.bpj.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang HP, Be'er A, Florin EL, Swinney HL. Collective motion and density fluctuations in bacterial colonies. Proc Natl Acad Sci U S A. 2010;107:13626–30. doi: 10.1073/pnas.1001651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Du H, Xu Z, Anyan M, Kim O, Leevy WM, Shrout JD, et al. High density waves of the bacterium Pseudomonas aeruginosa in propagating swarms result in efficient colonization of surfaces. Biophys J. 2012;103:601–9. doi: 10.1016/j.bpj.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lin SN, Lo WC, Lo CJ. Dynamics of self-organized rotating spiral-coils in bacterial swarms. Soft matter. 2014;10:760–6. doi: 10.1039/c3sm52120f. [DOI] [PubMed] [Google Scholar]
- [29].Darnton NC, Turner L, Rojevsky S, Berg HC. Dynamics of bacterial swarming. Biophys J. 2010;98:2082–90. doi: 10.1016/j.bpj.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Elgeti J, Winkler RG, Gompper G. Physics of microswimmers-single particle motion and collective behavior: a review. Reports on progress in physics Physical Society. 2015;78:056601. doi: 10.1088/0034-4885/78/5/056601. [DOI] [PubMed] [Google Scholar]
- [31].Kim W, Killam T, Sood V, Surette MG. Swarm-cell differentiation in Salmonella enterica serovar Typhimurium results in elevated resistance to multiple antibiotics. J Bacteriol. 2003;185:3111–7. doi: 10.1128/JB.185.10.3111-3117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim W, Surette MG. Swarming populations of Salmonella represent a unique physiological state coupled to multiple mechanisms of antibiotic resistance. Biol Proced Online. 2003;5:189–96. doi: 10.1251/bpo61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Overhage J, Bains M, Brazas MD, Hancock RE. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J Bacteriol. 2008;190:2671–9. doi: 10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lai S, Tremblay J, Deziel E. Swarming motility: a multicellular behaviour conferring antimicrobial resistance. Environ Microbiol. 2009;11:126–36. doi: 10.1111/j.1462-2920.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- [35].Tambalo DD, Yost CK, Hynes MF. Characterization of swarming motility in Rhizobium leguminosarum bv. viciae. FEMS Microbiol Lett. 2010;307:165–74. doi: 10.1111/j.1574-6968.2010.01982.x. [DOI] [PubMed] [Google Scholar]
- [36].Butler MT, Wang Q, Harshey RM. Cell density and mobility protect swarming bacteria against antibiotics. Proc Natl Acad Sci U S A. 2010;107:3776–81. doi: 10.1073/pnas.0910934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ingham CJ, Kalisman O, Finkelshtein A, Ben-Jacob E. Mutually facilitated dispersal between the nonmotile fungus Aspergillus fumigatus and the swarming bacterium Paenibacillus vortex. Proc Natl Acad Sci U S A. 2011;108:19731–6. doi: 10.1073/pnas.1102097108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kester JC, Fortune SM. Persisters and beyond: mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Crit Rev Biochem Mol Biol. 2014;49:91–101. doi: 10.3109/10409238.2013.869543. [DOI] [PubMed] [Google Scholar]
- [39].Kim W, Surette MG. Metabolic differentiation in actively swarming Salmonella. Mol Microbiol. 2004;54:702–14. doi: 10.1111/j.1365-2958.2004.04295.x. [DOI] [PubMed] [Google Scholar]
- [40].Wang L, Zhang C, Gong F, Li H, Xie X, Xia C, et al. Influence of Pseudomonas aeruginosa pvdQ gene on altering antibiotic susceptibility under swarming conditions. Curr Microbiol. 2011;63:377–86. doi: 10.1007/s00284-011-9979-0. [DOI] [PubMed] [Google Scholar]
- [41].Turnbull AL, Surette MG. Cysteine biosynthesis, oxidative stress and antibiotic resistance in Salmonella typhimurium. Res Microbiol. 2010;161:643–50. doi: 10.1016/j.resmic.2010.06.004. [DOI] [PubMed] [Google Scholar]
- [42].Roth D, Finkelshtein A, Ingham C, Helman Y, Sirota-Madi A, Brodsky L, et al. Identification and characterization of a highly motile and antibiotic refractory subpopulation involved in the expansion of swarming colonies of Paenibacillus vortex. Environ Microbiol. 2013;15:2532–44. doi: 10.1111/1462-2920.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Armitage JP. Changes in metabolic activity of Proteus mirabilis during swarming. J Gen Microbiol. 1981;125:445–50. doi: 10.1099/00221287-125-2-445. [DOI] [PubMed] [Google Scholar]
- [44].Alteri CJ, Himpsl SD, Engstrom MD, Mobley HL. Anaerobic respiration using a complete oxidative TCA cycle drives multicellular swarming in Proteus mirabilis. mBio. 2012:3. doi: 10.1128/mBio.00365-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dwyer DJ, Kohanski MA, Collins JJ. Role of reactive oxygen species in antibiotic action and resistance. Curr Opin Microbiol. 2009;12:482–9. doi: 10.1016/j.mib.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Udekwu KI, Parrish N, Ankomah P, Baquero F, Levin BR. Functional relationship between bacterial cell density and the efficacy of antibiotics. J Antimicrob Chemother. 2009;63:745–57. doi: 10.1093/jac/dkn554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Brook I. Inoculum effect. Rev Infec Dis. 1989;11:361–8. doi: 10.1093/clinids/11.3.361. [DOI] [PubMed] [Google Scholar]
- [48].Darnton N, Turner L, Breuer K, Berg HC. Moving fluid with bacterial carpets. Biophys J. 2004;86:1863–70. doi: 10.1016/S0006-3495(04)74253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Weibel DB, Garstecki P, Ryan D, DiLuzio WR, Mayer M, Seto JE, et al. Microoxen: microorganisms to move microscale loads. Proc Natl Acad Sci U S A. 2005;102:11963–7. doi: 10.1073/pnas.0505481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sokolov A, Apodaca MM, Grzybowski BA, Aranson IS. Swimming bacteria power microscopic gears. Proc Natl Acad Sci U S A. 2010;107:969–74. doi: 10.1073/pnas.0913015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Finkelshtein A, Roth D, Ben Jacob E, Ingham CJ. Bacterial swarms recruit cargo bacteria to pave the way in toxic environments. mBio. 2015:6. doi: 10.1128/mBio.00074-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hagai E, Dvora R, Havkin-Blank T, Zelinger E, Porat Z, Schulz S, et al. Surface-motility induction, attraction and hitchhiking between bacterial species promote dispersal on solid surfaces. The ISME journal. 2014;8:1147–51. doi: 10.1038/ismej.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Brock DA, Douglas TE, Queller DC, Strassmann JE. Primitive agriculture in a social amoeba. Nature. 2011;469:393–6. doi: 10.1038/nature09668. [DOI] [PubMed] [Google Scholar]
- [54].Lopez D, Vlamakis H, Losick R, Kolter R. Paracrine signaling in a bacterium. Genes Dev. 2009;23:1631–8. doi: 10.1101/gad.1813709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Veening JW, Smits WK, Kuipers OP. Bistability, epigenetics, and bet-hedging in bacteria. Annu Rev Microbiol. 2008;62:193–210. doi: 10.1146/annurev.micro.62.081307.163002. [DOI] [PubMed] [Google Scholar]
- [56].Benisty S, Ben-Jacob E, Ariel G, Be'er A. Antibiotic-induced anomalous statistics of collective bacterial swarming. Phy Rev Lett. 2015;114:018105. doi: 10.1103/PhysRevLett.114.018105. [DOI] [PubMed] [Google Scholar]
- [57].van Gestel J, Vlamakis H, Kolter R. From cell differentiation to cell collectives: Bacillus subtilis uses division of labor to migrate. PLoS biology. 2015;13:e1002141. doi: 10.1371/journal.pbio.1002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shapiro JA. Thinking about bacterial populations as multicellular organisms. Annu Rev Microbiol. 1998;52:81–104. doi: 10.1146/annurev.micro.52.1.81. [DOI] [PubMed] [Google Scholar]
- [59].Costa TR, Felisberto-Rodrigues C, Meir A, Prevost MS, Redzej A, Trokter M, et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol. 2015;13:343–59. doi: 10.1038/nrmicro3456. [DOI] [PubMed] [Google Scholar]
- [60].Alteri CJ, Himpsl SD, Pickens SR, Lindner JR, Zora JS, Miller JE, et al. Multicellular bacteria deploy the type VI secretion system to preemptively strike neighboring cells. PLoS Pathog. 2013;9:e1003608. doi: 10.1371/journal.ppat.1003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dienes L. Reproductive processes in Proteus cultures. J Bacteriol. 1946;51:585. [PubMed] [Google Scholar]
- [62].Gibbs KA, Urbanowski ML, Greenberg EP. Genetic determinants of self identity and social recognition in bacteria. Science. 2008;321:256–9. doi: 10.1126/science.1160033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Stewart BJ, Enos-Berlage JL, McCarter LL. The lonS gene regulates swarmer cell differentiation of Vibrio parahaemolyticus. J Bacteriol. 1997;179:107–14. doi: 10.1128/jb.179.1.107-114.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Belas R, Schneider R, Melch M. Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior. J Bacteriol. 1998;180:6126–39. doi: 10.1128/jb.180.23.6126-6139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lai HC, Soo PC, Wei JR, Yi WC, Liaw SJ, Horng YT, et al. The RssAB two-component signal transduction system in Serratia marcescens regulates swarming motility and cell envelope architecture in response to exogenous saturated fatty acids. J Bacteriol. 2005;187:3407–14. doi: 10.1128/JB.187.10.3407-3414.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Soo PC, Horng YT, Wei JR, Shu JC, Lu CC, Lai HC. Regulation of swarming motility and flhDC(Sm) expression by RssAB signaling in Serratia marcescens. J Bacteriol. 2008;190:2496–504. doi: 10.1128/JB.01670-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Belas R, Simon M, Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986;167:210–8. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–51. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- [69].Kawagishi I, Imagawa M, Imae Y, McCarter L, Homma M. The sodium-driven polar flagellar motor of marine Vibrio as the mechanosensor that regulates lateral flagellar expression. Mol Microbiol. 1996;20:693–9. doi: 10.1111/j.1365-2958.1996.tb02509.x. [DOI] [PubMed] [Google Scholar]
- [70].McCarter LL. Dual flagellar systems enable motility under different circumstances. J Mol Microbiol Biotechnol. 2004;7:18–29. doi: 10.1159/000077866. [DOI] [PubMed] [Google Scholar]
- [71].Meister M, Lowe G, Berg HC. The proton flux through the bacterial flagellar motor. Cell. 1987;49:643–50. doi: 10.1016/0092-8674(87)90540-x. [DOI] [PubMed] [Google Scholar]
- [72].Tipping MJ, Steel BC, Delalez NJ, Berry RM, Armitage JP. Quantification of flagellar motor stator dynamics through in vivo proton-motive force control. Mol Microbiol. 2013;87:338–47. doi: 10.1111/mmi.12098. [DOI] [PubMed] [Google Scholar]
- [73].Fukuoka H, Wada T, Kojima S, Ishijima A, Homma M. Sodium-dependent dynamic assembly of membrane complexes in sodium-driven flagellar motors. Mol Microbiol. 2009;71:825–35. doi: 10.1111/j.1365-2958.2008.06569.x. [DOI] [PubMed] [Google Scholar]
- [74].Lele PP, Hosu BG, Berg HC. Dynamics of mechanosensing in the bacterial flagellar motor. Proc Natl Acad Sci U S A. 2013;110:11839–44. doi: 10.1073/pnas.1305885110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tipping MJ, Delalez NJ, Lim R, Berry RM, Armitage JP. Load-dependent assembly of the bacterial flagellar motor. mBio. 2013:4. doi: 10.1128/mBio.00551-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cairns LS, Marlow VL, Bissett E, Ostrowski A, Stanley-Wall NR. A mechanical signal transmitted by the flagellum controls signalling in Bacillus subtilis. Mol Microbiol. 2013 doi: 10.1111/mmi.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chan JM, Guttenplan SB, Kearns DB. Defects in the flagellar motor increase synthesis of poly-gamma-glutamate in Bacillus subtilis. J Bacteriol. 2014;196:740–53. doi: 10.1128/JB.01217-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mukherjee S, Kearns DB. The structure and regulation of flagella in Bacillus subtilis. Annu Rev Genet. 2014 doi: 10.1146/annurev-genet-120213-092406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Li G, Brown PJ, Tang JX, Xu J, Quardokus EM, Fuqua C, et al. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol Microbiol. 2012;83:41–51. doi: 10.1111/j.1365-2958.2011.07909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wang Q, Frye JG, McClelland M, Harshey RM. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol Microbiol. 2004;52:169–87. doi: 10.1111/j.1365-2958.2003.03977.x. [DOI] [PubMed] [Google Scholar]
- [81].Wang Q, Suzuki A, Mariconda S, Porwollik S, Harshey RM. Sensing wetness: a new role for the bacterial flagellum. Embo J. 2005;24:2034–42. doi: 10.1038/sj.emboj.7600668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kearns DB, Losick R. Swarming motility in undomesticated Bacillus subtilis. Mol Microbiol. 2003;49:581–90. doi: 10.1046/j.1365-2958.2003.03584.x. [DOI] [PubMed] [Google Scholar]
- [83].Harshey RM, Matsuyama T. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc Natl Acad Sci U S A. 1994;91:8631–5. doi: 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Toguchi A, Siano M, Burkart M, Harshey RM. Genetics of swarming motility in Salmonella enterica serovar Typhimurium: critical role for lipopolysaccharide. J Bacteriol. 2000;182:6308–21. doi: 10.1128/jb.182.22.6308-6321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ping L, Wu Y, Hosu BG, Tang JX, Berg HC. Osmotic pressure in a bacterial swarm. Biophys J. 2014;107:871–8. doi: 10.1016/j.bpj.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Girgis HS, Liu Y, Ryu WS, Tavazoie S. A comprehensive genetic characterization of bacterial motility. PLoS Genet. 2007;3:1644–60. doi: 10.1371/journal.pgen.0030154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rather PN. Swarmer cell differentiation in Proteus mirabilis. Environ Microbiol. 2005;7:1065–73. doi: 10.1111/j.1462-2920.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- [88].Pearson MM, Rasko DA, Smith SN, Mobley HL. Transcriptome of swarming Proteus mirabilis. Infect Immun. 2010;78:2834–45. doi: 10.1128/IAI.01222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Morgenstein RM, Szostek B, Rather PN. Regulation of gene expression during swarmer cell differentiation in Proteus mirabilis. FEMS Microbiol Rev. 2010;34:753–63. doi: 10.1111/j.1574-6976.2010.00229.x. [DOI] [PubMed] [Google Scholar]
- [90].Harshey RM. The flagellum as a sensor. In: de Bruijn FJ, editor. Stress and Environmental control of Gene Expression in Bacteria. Wiley Blackwell; Hoboken, USA: 2016. In Press. [Google Scholar]
- [91].Dufour A, Furness RB, Hughes C. Novel genes that upregulate the Proteus mirabilis flhDC master operon controlling flagellar biogenesis and swarming. Mol Microbiol. 1998;29:741–51. doi: 10.1046/j.1365-2958.1998.00967.x. [DOI] [PubMed] [Google Scholar]
- [92].Morgenstein RM, Rather PN. Role of the Umo proteins and the Rcs phosphorelay in the swarming motility of the wild type and an O-antigen (waaL) mutant of Proteus mirabilis. J Bacteriol. 2012;194:669–76. doi: 10.1128/JB.06047-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Majdalani N, Gottesman S. The Rcs phosphorelay: a complex signal transduction system. Annu Rev Microbiol. 2005;59:379–405. doi: 10.1146/annurev.micro.59.050405.101230. [DOI] [PubMed] [Google Scholar]
- [94].Patrick JE, Kearns DB. Swarming motility and the control of master regulators of flagellar biosynthesis. Mol Microbiol. 2012;83:14–23. doi: 10.1111/j.1365-2958.2011.07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cho SH, Szewczyk J, Pesavento C, Zietek M, Banzhaf M, Roszczenko P, et al. Detecting envelope stress by monitoring beta-barrel assembly. Cell. 2014;159:1652–64. doi: 10.1016/j.cell.2014.11.045. [DOI] [PubMed] [Google Scholar]
- [96].Calvio C, Osera C, Amati G, Galizzi A. Autoregulation of swrAA and motility in Bacillus subtilis. J Bacteriol. 2008;190:5720–8. doi: 10.1128/JB.00455-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Clemmer KM, Rather PN. The Lon protease regulates swarming motility and virulence gene expression in Proteus mirabilis. J Med Microbiol. 2008;57:931–7. doi: 10.1099/jmm.0.47778-0. [DOI] [PubMed] [Google Scholar]
- [98].Torres-Cabassa AS, Gottesman S. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J Bacteriol. 1987;169:981–9. doi: 10.1128/jb.169.3.981-989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kelm O, Kiecker C, Geider K, Bernhard F. Interaction of the regulator proteins RcsA and RcsB with the promoter of the operon for amylovoran biosynthesis in Erwinia amylovora. Mol Gen Genet. 1997;256:72–83. doi: 10.1007/s004380050547. [DOI] [PubMed] [Google Scholar]
- [100].Wang Q, Zhao Y, McClelland M, Harshey RM. The RcsCDB signaling system and swarming motility in Salmonella enterica serovar typhimurium: dual regulation of flagellar and SPI-2 virulence genes. J Bacteriol. 2007;189:8447–57. doi: 10.1128/JB.01198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Guvener ZT, Harwood CS. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Mol Microbiol. 2007;66:1459–73. doi: 10.1111/j.1365-2958.2007.06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Luo Y, Zhao K, Baker AE, Kuchma SL, Coggan KA, Wolfgang MC, et al. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. mBio. 2015:6. doi: 10.1128/mBio.02456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ferreira RB, Antunes LC, Greenberg EP, McCarter LL. Vibrio parahaemolyticus ScrC modulates cyclic dimeric GMP regulation of gene expression relevant to growth on surfaces. J Bacteriol. 2008;190:851–60. doi: 10.1128/JB.01462-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Boles BR, McCarter LL. Vibrio parahaemolyticus scrABC, a novel operon affecting swarming and capsular polysaccharide regulation. J Bacteriol. 2002;184:5946–54. doi: 10.1128/JB.184.21.5946-5954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Kuchma SL, Delalez NJ, Filkins LM, Snavely EA, Armitage JP, O'Toole GA. Cyclic di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa PA14 requires the MotAB stator. J Bacteriol. 2015;197:420–30. doi: 10.1128/JB.02130-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Partridge JD, Nieto V, Harshey RM. A new player at the flagellar motor: FliL controls both motor output and bias. mBio. 2015:6, e02367. doi: 10.1128/mBio.02367-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Pfaller MA, Mujeeb I, Hollis RJ, Jones RN, Doern GV. Evaluation of the discriminatory powers of the Dienes test and ribotyping as typing methods for Proteus mirabilis. J Clin Microbiol. 2000;38:1077–80. doi: 10.1128/jcm.38.3.1077-1080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hsueh YH, Cozy LM, Sham LT, Calvo RA, Gutu AD, Winkler ME, et al. DegU-phosphate activates expression of the anti-sigma factor FlgM in Bacillus subtilis. Mol Microbiol. 2011;81:1092–108. doi: 10.1111/j.1365-2958.2011.07755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]