Abstract
Previous studies have employed different experimental approaches to enhance visual function in adults with amblyopia including perceptual learning, videogame play, and dichoptic training. Here, we evaluated the efficacy of a novel dichoptic action videogame combining all three approaches. This experimental intervention was compared to a conventional, yet unstudied method of supervised occlusion while watching movies.
Adults with unilateral amblyopia were assigned to either playing the dichoptic action game (n = 23; ‘game’ group), or to watching movies monocularly while the fellow eye was patched (n = 15; ‘movies’ group) for a total of 40 h.
Following training, visual acuity (VA) improved on average by ≈0.14 logMAR (≈27%) in the game group, with improvements noted in both anisometropic and strabismic patients. This improvement is similar to that described after perceptual learning, video game play or dichoptic training. Surprisingly, patients with anisometropic amblyopia in the movies group showed similar improvement, revealing a greater impact of supervised occlusion in adults than typically thought. Stereoacuity, reading speed, and contrast sensitivity improved more for game group participants compared with movies group participants. Most improvements were largely retained following a 2-month no-contact period.
This novel video game, which combines action gaming, perceptual learning and dichoptic presentation, results in VA improvements equivalent to those previously documented with each of these techniques alone. Interestingly, however, our game intervention led to greater improvement than control training in a variety of visual functions, thus suggesting that this approach has promise for the treatment of adult amblyopia.
Keywords: Amblyopia, Videogames, Perceptual learning, Suppression, Stereopsis, Visual acuity
1. Introduction
Amblyopia is a developmental disorder which results from physiological alterations in the visual cortex early in life (Ciuffreda, Levi, & Selenow, 1991). It is considered the most frequent cause of vision loss in infants and young children aside from refractive error, affecting roughly 1–4% of the population worldwide (Birch, 2013; Drover et al., 2008; Friedman et al., 2009; McKean-Cowdin et al., 2013; Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) Group, 2009; Williams et al., 2008). In addition to the reduced visual acuity, amblyopic individuals experience a broad range of lowand high-level visual deficits. These include reduced contrast sensitivity (Bradley & Freeman, 1981; Hess & Holliday, 1992; Levi & Harwerth, 1977), high levels of spatial uncertainty (Hess & Holliday, 1992; Levi & Klein, 1982, 1985) spatial distortion (Bedell & Flom, 1981, 1983), and impaired reading abilities (Levi, Song, & Pelli, 2007; see reviews in Kiorpes, 2006; and in Levi, 2006), among others.
Traditionally, it was thought that the visual deficits in amblyopia (and particularly visual acuity) could only be reversed if amblyopia treatment was implemented before the end of the critical period for visual development, by the age of 6–8 years (Von Noorden, 1981). The standard treatment for childhood amblyopia is occlusion therapy (patching of the good eye), with 120 h of occlusion resulting in, on average, a one-line (0.1 logMAR) improvement in visual acuity at 6 years of age (Stewart et al., 2007). No data on the efficacy of patching is available for adults. Interestingly, however, the notion that the adult visual system is beyond the critical period for plasticity has been challenged with several studies providing compelling evidence for improved vision in amblyopic adults following training. These studies have mostly employed three different kinds of intervention: monocular perceptual learning (PL), monocular videogame play (VGP) and dichoptic PL/VGP.
The initial studies employing PL did so under monocular viewing with the participants being required to perform fine discriminations of basic stimulus features over thousands of trials with their amblyopic eye only (Astle, Webb, & McGraw, 2011; Chung, Li, & Levi, 2006, 2008; see recent reviews in Levi & Li, 2009; Levi & Polat, 1996; Levi, Polat, & Hu, 1997; Li, Klein, & Levi, 2008; Li & Levi, 2004; Li et al., 2005; Polat, 2008; Polat et al., 2004; Zhang et al., 2013, 2014; Zhou et al., 2006). Improvements, although sometimes taskand stimulus-specific (see Zhang et al., 2014), often show some transfer to visual acuity (Levi & Li, 2009; Levi & Polat, 1996; Levi, Polat, & Hu, 1997; Li & Levi, 2004) and even stereovision (Zhang et al., 2014).
One serious limitation of this approach is that PL is typically repetitious and boring. Thus, several recent studies have investigated retraining the amblyopic eye through video game play. Video games have been shown to enhance vision and visual attention in normally sighted adults (see Achtman, Green, & Bavelier, 2008; Bavelier et al., 2012; Green & Bavelier, 2012; Green, Li, & Bavelier, 2010). For example, playing an off-the-shelf action video game (Medal of Honor) monocularly for 40 h results in improvements in visual acuity and other visual functions (Li et al., 2011) and reduces the “attentional blink” (Li, Ngo, & Levi, 2015). Recently, Hussain et al. (2014) have developed a contrast-based videogame for treating both adults and children with amblyopia.
While these monocular training methods are directed toward improving the visual performance of the amblyopic eye, an alternative approach is to consider amblyopia as a binocular problem, involving among other abnormalities, suppression of the amblyopic eye by the dominant eye (Baker, Meese, & Hess, 2008; Bi et al., 2011; Ding, Klein, & Levi, 2013; Ding & Levi, 2014; Harrad & Hess, 1992; Harrad, Sengpiel, & Blakemore, 1996; Hess, Thompson, & Baker, 2014; Levi, Harwerth, & Smith, 1979; Maehara et al., 2011; Mansouri, Thompson, & Hess, 2008; Sengpiel & Blakemore, 1996; Worth & Chevasse, 1950). Viewed from this perspective, an alternative approach is to treat amblyopia by reducing the suppression by training dichoptically. Hess and colleagues have applied dichoptic PL and dichoptic videogame play to retrain adults with amblyopia and documented significant improvements in visual acuity and in stereopsis (Hess, Mansouri, & Thompson, 2010a, 2010b, 2011; Hess et al., 2012, 2014; Li et al., 2013; To et al., 2011).
Interestingly, despite the very different methodologies employed (PL or videogame play; monocular or dichoptic presentation, a few hours of training to several months), most studies report, on average, improvement in visual acuity of between 1 and 2 lines on a LogMAR chart (for recent reviews see Hussain et al., 2014; Levi, 2012; Levi & Li, 2009), and variable improvement in stereopsis (Levi, Knill, & Bavelier, 2015 – this issue).
In the present study, we evaluate the potential benefits of combining PL, video game play and dichoptic presentation, by asking adults with amblyopia to play a dichoptic, custom-made action videogame with an embedded, monocular PL task (see Bayliss et al., 2012, 2013) for 40 h. The dichoptic action game was designed to incorporate the benefits of action video game play, including an immersive and engaging game environment, with those of binocular dichoptic treatment, by using a split screen view that allows independent control of image luminance and contrast in each window. The PL task required participants to discriminate the orientation of a Gabor patch that was presented to the amblyopic eye only (see Method section below).
A control group underwent ‘active patching’ for the same amount of time, having subjects watch movies with their amblyopic eye. This control allowed us to estimate the potential benefits of supervised patching while actively stimulating the amblyopic eye in this population. We hypothesized that the benefits from the combined game treatment would exceed the benefits from the ‘movies plus patching’ treatment.
Finally, we were interested to learn whether there are differences in responsiveness to treatment between the two main types of amblyopia: anisometropic amblyopia (different refractive errors in the two eyes) and strabismic amblyopia (misalignment of the two eyes with or without refractive errors). Although both conditions result in reduced visual acuity in the amblyopic eye despite appropriate optical correction, the causes and the consequences may be different (McKee, Levi, & Movshon, 2003). Surprisingly, this question has seldom been addressed in previous studies, potentially due to the relatively small number of participants.
2. Methods
2.1. Study participants and ethics statement
The Research Subjects Review Boards at the University of Rochester and the University of California, Berkeley approved the study protocol, and did not ask for the study to be registered as a clinical trial. The study was conducted according to the tenets of the Declaration of Helsinki and informed consent was obtained from each participant. Thirty-eight (n = 38) adults (mean age: 39.7 ± 15.4, range 19–66 years) with unilateral amblyopia completed the study (see Fig. 1 for numbers of participants screened, qualified and dropped). Participants were recruited through referrals from local eye doctors, through the eye clinic at UC Berkeley and through print advertisements. Two experienced optometrists provided complete eye exams for all participants prior to enrolling. The inclusion criteria included: (1) age 18 years or older; (2) anisometropic amblyopia, strabismic amblyopia, or mixed (i.e., anisometropic and strabismic); (3) interocular visual acuity difference of at least 0.2 logMAR; and (4) no history of eye surgery except those to correct strabismus. Exclusion criteria included: (1) non-comitant or large angle constant strabismus (>30 prism diopters); and (2) any ocular pathological conditions (e.g., macular abnormalities) and nystagmus. All of our participants had 20/12–20/20−3 vision in the non-amblyopic eye. The retinal health of all participants was assessed as normal, and they all had clear ocular media (as assessed by ophthalmoscopy). Cover tests were used to assess ocular alignment at both distance and near. Clinical data of all study participants is summarized in Table 1. The study took place at two research laboratories, at University of Rochester and at University of California, Berkeley.
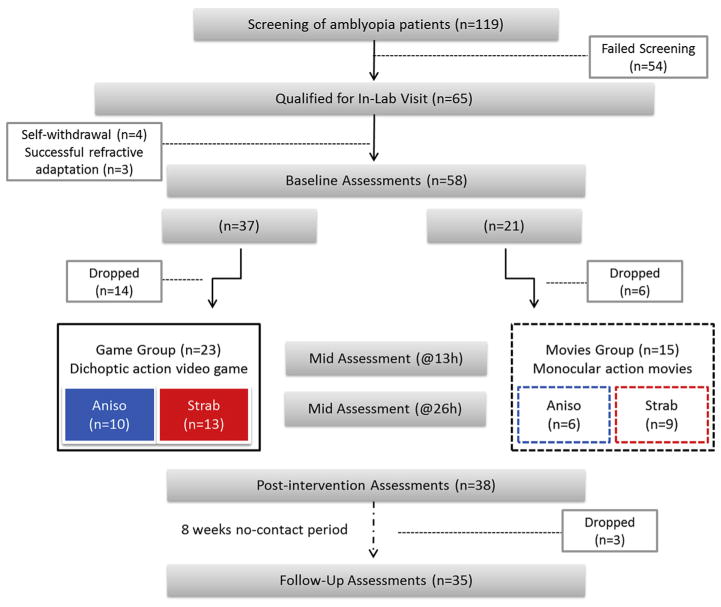
Fig. 1.
General Study Design. 119 potential participants were screened for participation in the study. 54 failed screening for various reasons (e.g. resolved amblyopia, other pathologies present). The 65 participants that qualified for in-lab visit following screening (55% of screened) were scheduled to complete the study baseline assessment battery. Seven participants were subsequently excluded from the study: four could not make the required time commitment, while the other three no longer qualified after being given a refractive adaptation period (see text). Fifty-eight (n = 58) participants completed the baseline assessments, and were allocated into one of two intervention groups: game group (n = 37) or movies group (n = 21). 23 participants from the game group and 15 from the movies group completed a total of 40 h of intervention. During the intervention, visual acuity and stereoacuity only were assessed after 13 and 26 h of intervention (‘mid assessment’). At the completion of 40 h, participants repeated the complete assessment battery (‘post-intervention’). Following an 8-week period of no-contact, participants (n = 35) repeated the complete assessment battery a third time (‘follow up’). Abbreviations: Aniso: subjects with anisometropic amblyopia (no strabismus); Strab: subjects with strabismic amblyopia (both strabismic and mixed aetiologies are included).
Table 1.
Clinical profile of study participants.
| Subj. code | Age/sex | AE | Refractive error
|
Visual acuity
|
Ocular alignment (prism diopters)
|
SeA (arc sec) | AE fixation | Treatment history | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | L | R | L | Distance | Near | ||||||
| Game group | |||||||||||
| A1 | 21/F | R | +3.50/−2.75 × 170 | +0.50 | 0.18 | −0.08 | Ortho | Ortho | 70 | Central | Patched at age 10 |
| A2 | 20/F | L | +0.50/−0.75 × 130 | +1.50/−0.75 × 20 | −0.06 | 0.16 | Ortho | 5 XP | 25 | Central | No treatment |
| A3 | 23/F | L | −4.00/−1.25 × 178 | −2.00/−4.75 × 9 | −0.12 | 0.28 | 2XP | 4 XP | 50 | Central | No treatment |
| A4 | 35/M | R | +3.50/−7.50 × 5 | +0.5/−0.25 × 5 | 0.22 | −0.24 | Ortho | 3XP | 200 | Central | Patched at ages 3–6 |
| A5 | 59/F | L | +0.75/−0.25 × 134 | +5.00/−0.50 × 074 | −0.10 | 0.66 | 2XP | 4XP | F | Central | Patched at ages 6–10/11 |
| A6 | 48/M | L | Plano/−0.50 × 95 | +4.00/−1.50 × 80 | 0.04 | 0.44 | 6XP | 2XP | F | Central | Patched at ages 8–10/11 |
| A7 | 20/M | L | +0.75/−0.75 × 180 | +2.00/−1.00 × 130 | 0.00 | 0.26 | Ortho | Ortho | 70 | Central | No treatment |
| A8 | 19/M | R | −13.50 | −4.00 | 1.22 | −0.14 | Ortho | Ortho | 200 | Central | No treatment |
| A9 | 62/F | L | +1.75/−0.50 × 10 | +5.75/−0.75 × 165 | 0.02 | 0.86 | Ortho | Ortho | F | Central | No treatment |
| A10 | 35/F | L | −1.75/−0.50 × 172 | +2.00/−1.00 × 168 | 0.00 | 0.80 | Ortho | Ortho | F | Central | Patched at age 5 |
| S1 | 42/F | L | Plano/−0.25 × 111 | −0.25/−0.75 × 18 | −0.20 | 0.54 | Ortho | 4 IXT | 100 | Central | Patched at ages 9–11 |
| S2* | 52/M | L | +7.00/−1.00 × 109 | +6.75/−0.75 × 93 | 0.10 | 0.88 | 10 LET | 15 LET | F | Central | No treatment |
| S3 | 59/M | L | −0.75/−0.75 × 100 | Plano/−0.75 × 100 | 0.04 | 0.36 | 12 L HypoT | 12 L HypoT | F | Central unsteady | No treatment |
| S4 | 60/M | L | Plano | +1.00/−2.25 × 90 | 0.04 | 0.62 | 25 LXT, 3 LHyperT | 28 LXT, 3 LHyperT | F | N/A | Strabismus Sx at age 7 |
| S5 | 52/F | L | +6.50/−1.00 × 160 | +7.00/−1.00 × 20 | 0.06 | 0.78 | 12 LXT | 8–10 LXT | F | Central | Patched at 18 months old |
| S6* | 57/F | L | +3.25/−1.50 × 123 | +3.25/−2.00 × 47 | −0.06 | 0.22 | 5 LET, 12 L HypoT | 5 LET, 12 LHypoT | 400 | Central | Strabismus Sx at age 5 |
| S7 | 21/F | L | +4.00 | +5.00/−1.00 × 10 | −0.12 | 0.44 | 6 LET | 5 LET | F | Central | Patching, atropine at age 6 |
| S8 | 22/M | R | Plano | −1.25/−0.25 × 130 | 0.88 | 0.04 | 8 RET | 1 RET | F | Central | N/A |
| S9 | 35/F | L | +0.50/−0.50 × 180 | +3.50/−5.25 × 172 | −0.10 | 0.28 | 4XP | 12–14 LXT | 100 | Central | Patched at preschool age for ~2 years |
| S10 | 50/F | L | +1.75/−0.50 × 129 | +4.50/−1.25 × 054 | −0.10 | 0.74 | 8 LET, 2 LHyperT | 8 LET, 2 LHyperT | F | Central | Patched at ages 4–5 |
| S11 | 24/F | L | −4.75/−1.00 × 30 | −1.75 | 0.02 | 0.62 | 16 LXT 6 LHypoT | 20 LXT, 6 LHypoT | F | Central, unsteady | Patched at preschool, non-compliant |
| S12 | 40/M | R | +0.25/−1.00 × 143 | −4.75/−1.00 × 6 | 0.92 | 0.04 | 6 RET | 8 RET | F | Central | Three Sx: at infancy and in 2000 & 2006 |
| S13 | 54/F | R | +5.50 | +1.50/−0.50 × 155 | 0.92 | −0.02 | 6 RXT, 3LHypo T | 6 RXT, 3 LHypoT | F | 2L ± 30′ TEF | Patched for a year at age 6 |
| Movies group | |||||||||||
| A11 | 31/M | L | +1.75 | +8.5/−2.25 × 60 | −0.14 | 0.66 | Ortho | 2XP | F | Central | Patched at age 8 for 1.5 years |
| A12 | 30/F | R | −6.00/−0.75 × 180 | −4.5 | 0.64 | −0.20 | Ortho | 3EP | 70 | Central | Patched for a year at age 15 |
| A13 | 29/F | L | −0.25 | +2.5/−0.25 × 07 | −0.16 | 0.50 | Ortho | Ortho | 140 | Central | Patched, age8–9 |
| A14 | 23/F | L | −0.25/−0.75 × 180 | +4.50/−5.25 × 180 | 0.00 | 0.48 | Ortho | Ortho | 200 | Central | Patched at age 17–18 and vision therapy |
| A15 | 46/M | R | −12.75/−0.75 × 067 | −8.75/−0.75 × 045 | 0.86 | 0.08 | 8XP | 5XP | F | Central | Patched at age 6–7 |
| A16 | 45/M | R | +5.50/−2.25 × 160 | Plano | 0.48 | −0.10 | Ortho | Ortho | F | Central | Patched at age 5 |
| S14 | 66/M | R | +5.50/−2.5 × 10 | +5.00/−2.50 × 180 | 0.46 | 0.04 | 15 RXT, 6 L HypoT | 12 RXT, 5 L Hypo T | F | 1.5L TEF | Patched at age 1 for a few weeks |
| S15* | 56/M | R | −1.50/−0.25 × 90 | −1.50/−0.25 × 140 | 0.32 | 0.12 | 12–14 RET | 15–16 RET | F | Central | No treatment |
| S16 | 59/F | L | −3.75 | −2.5 | 0.02 | 0.30 | 4R HT | 3EP, 4 R HT | 70 | Central | Patching at age 18– 30 months |
| S17 | 23/F | L | +7.25/−0.25 × 120 | +9/−0.5 × 155 | −0.02 | 0.24 | 3XP | 3LXT | 30 | Central | Patched at age 4 |
| S18 | 63/F | R | +5.00/−1.50 × 90 | +0.75 | 0.22 | −0.10 | 2 RET | 2 RET | 140 | Central | No treatment |
| S19 | 41/F | L | +3.00 | +5.25 | −0.14 | 0.72 | 10 LET | 5 LET | F | Central | Patched at age 4–5 |
| S20 | 41/F | L | −5.00/−0.25 × 026 | +1.00/−3.50 × 158 | 0.00 | 0.28 | 2 LXT | 2 LET | F | Central | No treatment |
| S21 | 25/M | R | −9.25 | −2.00/−1.00 × 005 | 0.82 | −0.20 | 6 RXT | 6RXT | 140 | Not Central★ | Patched at ages 7–8 |
| S22 | 23/M | L | −7.00 | −3.25/−1.00 × 160 | 0.02 | 0.30 | L6HT,8XT | L4HT, 8XT | F | N/A | Patched at ages 11–12 |
Abbreviations: (1) Amblyopic etiology; A, anisometropic; S/, strabismic; S, both strabismus and anisometropia; (2) AE, amblyopic eye (R – right; L – left); (3) Ocular Alignment. Ortho, Orthophoria; XP, exophoria; EP, esophoria; XT, exotropia; ET, esotropia; IXT, Intermittent exotropia; ALT ET, Alternating esotropia; HyperT, hypertropia; HypoT, hypotropia; (4) SeA, stereoacuity. F, failed (>400 arcsec); (5) Fixation. TEF, temporal eccentric fixation;
, magnitude not measured; (6) N/A, missing data; (7) Sx, surgery. Note that treatment history includes any treatment beyond glass prescription. Age appropriate near correction was used for the various test distances. Units: visual acuity is given in logMAR units.
2.1.1. Subject classification
Study participants were classified as either anisometropic (‘Aniso’) or strabismic (‘Strab’) amblyopes. Anisometropia was defined as ≥0.50D difference in spherical equivalent refraction or ≥1.5D difference in astigmatism in any meridian, between the two eyes (Wallace et al., 2011). Amblyopic subjects with anisometropia and an absence of manifest ocular deviation were classified as anisometropic amblyopes. Those with an ocular deviation (strabismus), as indicated by the cover test, were classified as strabismic amblyopes, irrespective of their refractive state, meaning that participants with both strabismus and anisometropia were classified as ‘strabismic’.
2.2. Study design overview
The complete experimental design is detailed in Fig. 1. Following consent and screening, participants were assigned into one of two intervention groups: (1) Game Group (n = 23): playing the custom-made dichoptic videogame using a mirror stereoscope (see description below); (2) Movies group (n = 15): watching movies monocularly with the fellow (non-amblyopic, NAE) eye occluded with a black eye patch.
Because we anticipated a higher dropout rate for the game group, participants were allocated with a 2:1 ratio to the game and movies groups respectively. This resulted in 37/58 (≈64%) being allocated to the game group and 21/58 (≈36%) to the movies group. The dropout rate was higher for the game (38%) than for the movies group (28%; See Fig. 1), mainly because of the substantial time commitment required for training in the lab, resulting in 23 of the game and 15 of the movies participants completing the study. We note that the two groups were similar in age (39.6 ± 16 and 40.1 ± 15 years in game and movies groups, respectively), and in distribution of amblyopia type (≈60% strabismic and 40% anisometropic in each group), but differed slightly, although not significantly, in their baseline visual acuity (0.58 ± 0.06 vs. 0.49 ± 0.06 logMAR in game and movies groups, respectively; t-test: p = 0.32). Subject allocation was not based on the clinical characteristics of participants.
Importantly, at the time of enrollment, participants were told that the study compared the efficiency of two active interventions, and that they would be assigned to one of the two groups without them being able to choose. Before starting the 40-h intervention, participants completed a test battery to assess vision and related functions (‘baseline assessments’). Participants repeated the battery at the completion of the 40 h (‘post-intervention’) and following a 2-month no-contact period (‘follow-up’). A subset of the assessments was also conducted following 13 and 26 h of intervention (‘mid assessment’). Because both interventions are experimental, patients assigned to the movies group were offered the possibility to undergo the game training regimen upon completion of their study, and vice versa. This cross-over will however not be discussed any further in this paper.
2.3. Study interventions
Participants from both groups were required to complete a total of 40 h of intervention, in sessions lasting 1.5–2 h, for at least 2 and up to 5 times/week. Participants were given full optical correction for the viewing distance (68 cm). Five participants, who needed new prescriptions at the time of enrollment, were given 6–8 weeks of refractive adaptation prior to starting the study. Among these, three actually achieved near-normal VA in their amblyopic eye after the period of refractive adaptation and were therefore excluded from the study (see Fig. 1).
2.3.1. Game group: a dichoptic custom-made unreal tournament video game
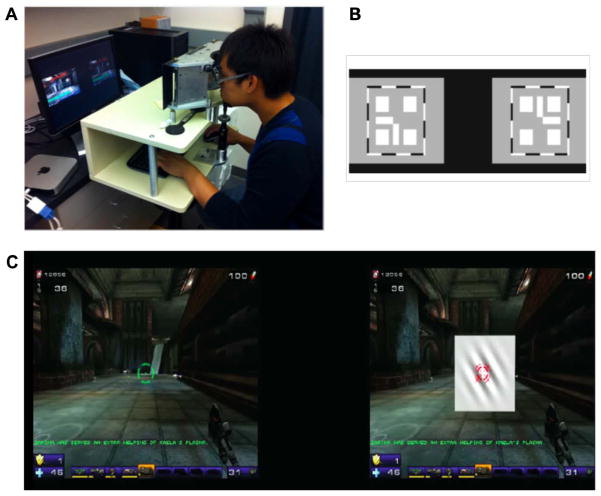
We developed a dichoptic version of a commercial first-person-shooter action video game, Unreal Tournament 2004 (Epic Games, 2004). The dichoptic videogame combines both the highly motivating aspects of commercial action video games as well as several adaptations custom made for amblyopic patients. Specifically, the game is played under dichoptic viewing conditions in order to reduce suppression and promote fusion, while challenging the amblyopic eye with an embedded psychophysical resolution task. This custom-made game has five main innovative features (Fig. 2; see Bayliss et al., 2012, 2013 for full details):
Fig. 2.
The dichoptic custom-made Unreal Tournament video game. (A) Game group participants used a mirror stereoscope to achieve alignment and play the dichoptic game. (B) Nonius lines appearing at the beginning of a training session, to allow for alignment and fusion of the two eyes. Participants viewed this through a stereoscope, when each eye receives half a cross. Participants were asked to align the two images until they perceived a complete cross in the center. (C) A screen shot of the actual game while being played by an amblyopic participant. At the start of each training session, participants adjust the alpha level of the image seen by the non-amblyopic eye (NAE) in order to overcome suppression and to achieve fusion. The set alpha level is then used to play the game, so that the amblyopic eye (AE) image is usually brighter than the NAE image. Green and red targets (see cross hairs) are also aligned prior to game play. In addition, an adaptive Gabor discrimination task is embedded in the scene viewed by the AE (gray square in center of left image). Participants were instructed to play the action game, by shooting enemies or bots as quickly as possible. A demo of the game can be seen at: http://www.youtube.com/watch?v_71RML96XxCI.
The game presents a split screen view, allowing independent control of the images presented to the right and left eyes (which are viewed in a mirror stereoscope), and in particular their respective alpha level.
Alpha blending was used to balance the perceived image strength of the non-amblyopic eye (NAE) with that of the AE eye at the start of each play session, in an effort to reduce suppression and facilitate fusion.
The game includes several easier tutorial levels, allowing individuals with little or no video game experience to gradually master the skills required to become a video game player.
An orientation discrimination perceptual learning task is seamlessly embedded within the game. It consists of a Gabor patch embedded in a gray square and presented to the AE only. The user is required to decide whether the Gabor patch is tilted left or right, with one orientation requiring responding by shooting the patch, and the other to just ignore the patch until it goes away. An incorrect response transforms the Gabor into a particularly powerful game enemy. The spatial frequency of the Gabor patch is adapted to maintain participant’s performance at 79% correct (Levitt, 1971). The Gabor patch task enables us to monitor the AE’s resolution limit under dichoptic conditions, while simultaneously serving as a suppression check, ensuring that the AE is actively engaged during game play.
Additional suppression checks (see below) were interleaved with the videogame play to ensure the use of AE during dichoptic gameplay.
The videogame was displayed on a gamma corrected monitor (Mitsubishi Diamond Pro 2070 SB), with resolution 1024 × 768 pixels and refresh rate 60 Hz. The split screen images of the game were viewed in a custom designed stereoscope at a distance of 68 cm. All participants were given trial frames with their refractive correction if needed. Details on the establishment of alignment and fusion during gameplay, progression of game difficulty during gameplay, suppression checks and the embedded PL task can be found in the Supplemental Methods.
We note that there are important differences between our method of dichoptic presentation and that used by others. Our action video game presented the same image to each eye (except for Gabor patches and suppression checks) with reduced luminance/contrast in the fellow eye, in an attempt to promote binocular fusion, whereas other dichoptic video game studies have presented different game elements to each eye so that binocular combination is required to play the game (see Hess, Thompson, & Baker, 2014 for a review). Both approaches have been shown to reduce binocular suppression as well as to improve visual acuity and stereopsis (Vedamurthy et al., 2015).
2.3.2. Movies group: monocular watching of action TV series
Participants in the movies group were asked to watch pre-selected TV series on a computer monitor, for a total of 40 h. Participants were instructed to watch those monocularly, wearing a black eye patch on their non-amblyopic eye (NAE). The TV series were self-chosen by the users from a compiled list (e.g. Heroes, season 1; Firefly: The complete series; Terminator: The Sarah Connor Chronicles). Titles on that list were selected by experimenters to include movies that are rich in action content (adventure, action movies, road races, etc.) and to provide enough variety for patients to comply. Thus, while we tried to include action components in the movies training, it remains unclear to what extent the action content of the movies group was matched to that of the game group.
This ‘movies’ intervention provides an active control for our ‘game’ intervention. Patching is considered the “gold standard” treatment for amblyopia in children, but interestingly the type of supervised patching (plus movies watching for 40 h) we present here has never been tested in adults.
At the inception of the study, our intention was that all training (game and movies) would be carried out in the lab. However, for many subjects, the time commitment of in-lab training was too burdensome, a difficulty reflected in the high drop-out rates (Fig. 1). While we continued to assign subjects to one or the other group regardless of their availability for in-lab training, once assigned, participants in the movies group were given the choice of either completing their intervention in the lab or at their home while being monitored through Skype. The latter ensured that participants training from home complied with the paradigm. This option was not available to the game group since their training required specialized equipment. Thus, all 23 video game trainees completed their training in the lab, while 6 of the movies group participants completed their training in the lab and 9 completed it at home. All participants were required to come to the lab to complete their assessments, regardless of whether they trained at home or in the lab.
2.4. Visual function assessments
Participants were required to wear their best optical correction (if any) given each test distance for all visual assessments. Our assessments included two primary measures, VA and stereoacuity, and three secondary measures (contrast sensitivity, reading speed and the Amblyopia Strabismus Questionnaire Evaluation) mostly aimed at documenting the impact of training on every day functioning.
2.4.1. Visual acuity (VA)
Clinical visual acuity (VA) at distance was measured using either Bailey–Lovie logMAR letter charts (UCB site), or the high-contrast ETDRS format chart with Sloan optotypes (catalog No. 2104; Precision Vision, La Salle, Illinois; U of R site). Monocular and binocular acuity were measured. In addition to the standard assessment times (baseline, post-intervention and follow-up), VA was also assessed following 13 and 26 h of training (‘mid assessments’).
2.4.2. Stereoacuity
Stereopsis was measured using the Randot Stereotest (Stereo Optical Co., Inc.; See description in Simons, 1981). In addition to the standard assessment times (baseline, post-intervention and follow-up), stereoacuity was also assessed following 13 and 26 h of training (‘mid assessments’). Analyses were performed on the logarithm (base 10) of the stereoacuity values, with those patients having non-measurable thresholds being assigned a value of 600 arcsec (similar to Wallace et al., 2011). To ensure that this arbitrary selection did not affect the results, we repeated all analyses with nil stereo assigned the value of 6000 arcsec, and got similar results. Results are reported as improvement in log arcsec (log stereoacuity pre – log stereoacuity post), and as the corresponding percent improvement.
2.4.3. Contrast sensitivity function
We used the quick Contrast Sensitivity Function (qCSF; Lesmes et al., 2010), a Bayesian adaptive procedure, to measure the contrast sensitivity function. A detailed description of this measure can be found in Lesmes et al. (2010). Stimuli were displayed on Mitsubishi Diamond Pro 2070 SB CRT monitor. Gamma nonlinearity correction was applied prior to conducting the experiments. A special circuit was used to obtain high (>14 bit) grayscale resolution (Li et al., 2003). The mean luminance of the display was 30.9 cd/m2. Screen resolution was set to 1920 × 1440 at 90 Hz.
Here we report area under the log CSF curve (AULCSF) as a summary measure for contrast sensitivity (Lesmes et al., 2010). Measurements were made for each eye separately using 250 trials per eye.
2.4.4. Reading speed
Reading speed for reading out-loud was evaluated using the standardized MN Read Acuity Chart (Legge et al., 1989). The test was run for each eye separately and then binocularly. Basic reading speeds were calculated in words per minute (wpm) after accounting for reading errors. We then derived, for each participant, a difference reading speed score: this was derived by first calculating the reading speed difference (post minus pre or follow-up minus pre) for each print size value, summing all reading speeds and dividing by the number of print sizes used. This difference measure was used for data analysis.
2.4.5. Self-report of amblyopia state (ASQE)
We used the Amblyopia Strabismus Questionnaire Evaluation (ASQE; Felius et al., 2007), a self-administered questionnaire includes 26 items and contains five scales: fear of losing the better eye, distance estimation, visual disorientation, double vision, and social contact and appearance. ASQE has good psychometric properties (internal consistency reliability of 0.8–0.92), and has shown strong correlations with clinical characterization of patients. This questionnaire was administered at the standard assessment times (baseline, post-intervention and follow-up).
2.5. Data analysis
Our primary hypothesis concerns the efficiency of the ‘game’ versus ‘movies’ intervention on VA which is best documented by focusing on pre versus post-intervention differences in VA. For all measures, we conducted repeated-measures Analysis of Variance (ANOVA) with within-subjects factor of time (2 levels: pre- and post-intervention) and between-subjects factors of treatment group (2 levels: game and movies) and amblyopia type (2 levels: anisometropic and strabismic; note that ‘strabismic’ definition included both individuals with purely strabismic amblyopia and those with both strabismus and anisometropia) on the five main dependent variables. We also report similar repeated-measures ANOVAs but with preand follow-up data as time factors. These latter analyses are indicative of the long lasting effects of the interventions. Finally, to best capture changes in each group separately when needed, we conducted a separate 2 × 2 ANOVA for each group (with within-subjects factor of time and between-subjects factor of amblyopia type), and corrected for multiple comparisons using Bonferroni correction.
2.5.1. Missing data
Three participants dropped out following post-training and before the follow-up assessments, hence their data is missing from follow-up. In addition, post-training qCSF data is missing for one subject, and MN Read data for 2 subjects, in all cases due to data not being recorded correctly. In cases of missing data, these participants were omitted from the analysis.
3. Results
3.1. Changes in clinical visual acuity (VA)
3.1.1. Omnibus ANOVA
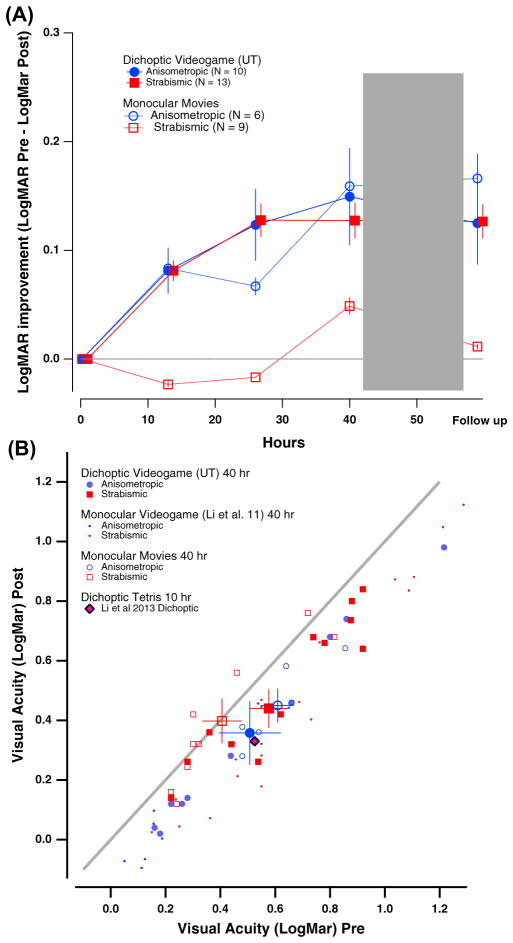
VA results are summarized in Fig. 3. Following 40 h of intervention, VA (logarithm of the minimum angle of resolution – logMAR) improved significantly (effect of time: F(1,34) = 75.8; p < 0.00001). The improvement was statistically different between the game group (0.14 ± 0.01 logMAR, on average, equivalent to 28 ± 2.% improvement) and the movies group (0.07 ± 0.03 logMAR, 15 ± 6.4%; time X treatment group: F(1,34) = 4.5, p < 0.05; no effect of treatment group: F(1,34) = 0.15, p = 0.7).
Fig. 3.
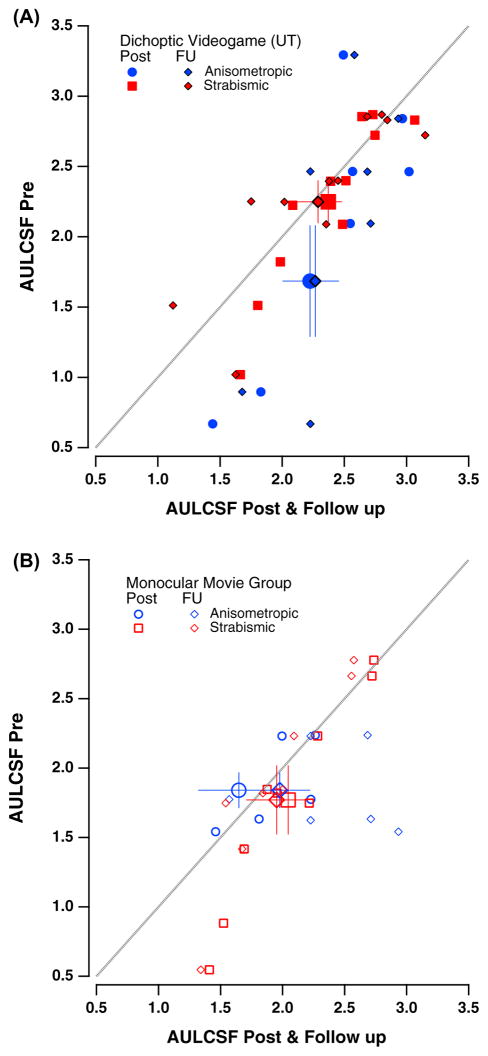
Changes in visual acuity (VA) as a function of hours of either video game play or movies watching. Color coding is used throughout the figures to represent the type of amblyopia. Red squares, strabismic (either pure strabismics or mixed etiology); blue circles, anisometropic. Solid symbols: game group; Open symbols: movies group. (A) Average VA (in logMAR units) as a function of hours of training for game (solid symbols) and movies (open symbols) groups. Error bars: one SEM (here and in all subsequent figures). (B) Post-intervention VA (y-axis) as a function of baseline VA (x-axis) for individual participants. Values below the diagonal represent improved VA at post-intervention relative to baseline. Larger colored symbols show averaged VA data for anisometropic (blue) and strabismic (red) individuals. Data from previous studies using either monocular videogame play (Li et al., 2011) or dichoptic tetris (Li et al., 2013) are shown for comparison.
Our analysis also included amblyopia type, and there, an interesting difference also emerged. While subjects with anisometropic amblyopia showed similar improvements following either game play (by 0.15 ± 0.01 logMAR; 29 ± 2%) or movies (by 0.16 ± 0.03 logMAR, 31 ± 5.8%), subjects with strabismic amblyopia improved only following game play (by 0.13 ± 0.02 logMAR; 26 ± 4%). No improvement was seen in subjects with strabismic amblyopia after watching movies monocularly (0.008 ± 0.03 logMAR; 2 ± 6.8%). Accordingly, a significant interaction of time X treatment group X amblyopia type was present (F(1,34) = 6.6, p < 0.02; also time X amblyopia type: F(1,34) = 11.3; p < 0.005).
Given our interest in the efficacy of different interventions, the impact of the game and movies interventions was considered separately, using 2 × 2 ANOVAs with time and amblyopia type as factors and Bonferroni correction for multiple comparisons. For the game group, overall improvement was statistically significant (effect of time: F(1,21) = 82.2, p < 0.00001), but not the effect of amblyopia type (p = 0.56) or their interaction (p > 0.9). For the movies group, the time factor was also significant (F(1,13) = 15.6, p < 0.01), as well as the time X amblyopia type interaction (F(1,13) = 12.7, p < 0.005). The amblyopia type was not significant (F(1,13) = 1.6, p = 0.44).
VA improvements were retained at follow-up (effect of time: F(1,31) = 32.8; p < 0.0001) with retention being numerically larger for the game group (by 0.12 ± 0.02 logMAR; 24 ± 4%), compared with the movies group (by 0.05 ± 0.03 logMAR; 11 ± 6.5%). Yet, the time X treatment group interaction effect was not significant (p = 0.19), perhaps due to large inter-individual variations (no effect for treatment group: p = 0.7). Compared with their respective baseline assessments, and in line with post-intervention data, subjects with anisometropic amblyopia demonstrated better VA with both game (by 0.13 ± 0.02 logMAR; 26 ± 4%) and movies (by 0.16 ± 0.05 logMAR; 31 ± 9.6%) interventions at follow-up. Subjects with strabismic amblyopia demonstrated better VA only following the game (by 0.11 ± 0.04 logMAR; 22 ± 8.1%) but not the movies intervention (by × −0.008 ± 0.03 logMAR; −2 ± 7%). This pattern was supported by a significant interaction between time and amblyopia type (F(1,31) = 6.7; p < 0.02) and, importantly, a significant 3-way interaction of time X treatment group X type (F(1,31) = 5.2, p < 0.03).
3.1.2. Controlling for baseline VA differences
Finally, we tested whether improvements in VA depended on the baseline VA, by performing an Analysis of Covariance (ANCOVA) with baseline VA as covariate and VA difference (post–pre) as the dependent variable. The covariate effect was marginally significant (baseline VA: F(1,33) = 2.89; p = .098). The effects of treatment group, amblyopia type and their interaction, albeit slightly weaker, did not depart from those in the original analyses (treatment group: F(1,33) = 3.9, p = .054; amblyopia type: F(1,33) = 11.1, p < .005; interaction: F(1,33) = 3.8, p = .058).
3.1.3. Controlling for different drop-out rates between the two groups
Since the two treatment groups had different drop-out rates during intervention (38% and 28% for game and movies groups, respectively), we conducted a secondary analysis, to test whether these drop-out rates biased the results of our main analysis. This analysis took into account the data of the participants who dropped-out at various stages of the study, ‘carrying forward’ the data from their last data point. The 2 × 2 × 2 ANOVA, with within-subjects factor of time and between-subjects factors of treatment groups and amblyopia type, was therefore run with additional data from the 18 participants who dropped out (n = 12 from the game group, and n = 6 from the movies group; data from 2 additional game group participants was lost for analysis).
The results of this additional analysis were similar to those of the main analysis: we found significant effects of time (F(1,52) = 44.4, p < .00001), as well as significant interaction between time and amblyopia type (F(1,52) = 5.08, p < .03), and a significant 3-way time X treatment group X amblyopia type interaction (F(1,52) = 6.08, p < .02). The time X treatment group interaction was, however, not significant (F(1,52) = .57, p = .45).
We further examined differences in baseline VA between the various groups, and found that baseline VA did not differ significantly between participants who completed the 40-h intervention (n = 38; VA: 0.54 ± 0.04) and those who dropped out (n = 18; VA: 0.54 ± 0.07 logMAR; t(54) = .03, p = .97). Moreover, baseline VA was similar for game group drop-outs and game group completers (t(33) = .49, p = .62), for movies group drop-outs and movies group completers (t(19) = .93, p = .36), and for game group drop-outs and movies group drop-outs (t(16) = .37, p = .71).
Together, these results confirm that there is likely no bias in our primary analyses due to higher drop-out rates in the game compared with the movies group.
3.1.4. Dynamics of VA change
To gain a better understanding of how differences between amblyopia type and treatment group emerged over the timecourse of training, we now turn to the VA assessment performed at mid1 (after 13 h) and at mid2 (after 26 h) (See Fig. 3 top panel). After 13 h of intervention, VA improved, on average, by 0.08 ± 0.02 logMAR (15.7 ± 3.1%) for the game group, and only by 0.02 ± 0.02 logMAR (3.7 ± 3.7%) for movies group. While in the game group, this improvement was similar for both subjects with anisometropic (by 0.08 ± 0.02 logMAR; 15.7 ± 5.1%) and strabismic (by 0.08 ± 0.02 logMAR; 15.8 ± 4.1%) amblyopia, in the movies group, subjects with anisometropic amblyopia did improve (by 0.08 ± 0.03 logMAR; by 16.6 ± 5.5%) while strabismics did not (by −0.02 ± 0.01 logMAR; −4.8 ± 2%).
This pattern became stronger after 26 h (‘mid2’) of training. VA improved significantly for game group (by 0.13 ± 0.01 logMAR relative to baseline, 24.3 ± 2.4%) and only slightly for movies group (by 0.02 ± 0.02 logMAR; 2.7 ± 3.7%). In the game group, improvements were again comparable for subjects with anisometropic (by 0.12 ± 0.02 logMAR; 24 ± 3.3%) and strabismic (by 0.13 ± 0.02 logMAR; 24.5 ± 3.5%) amblyopia, whereas in the movies group, subjects with anisometropic amblyopia improved by 0.07 ± 0.02 logMAR (13.8 ± 3.9%), while those with strabismic amblyopia did not improve (by −0.02 ± 0.02 logMAR; −4.7 ± 4.1%).
We ran a further analysis to examine whether performance differences exist between 26 and 40 h of training, as it is important for practical consideration to consider the length of intervention. An omnibus 2 × 2 × 2 ANOVA with mid2 and post-training as time points confirmed a significant effect of time (F(1,34) = 8.7, p < 0.01), which showed that VA kept improving with additional training. The effects of time from mid2 to post-training remained only marginally significant between treatment groups (time X group: F(1,34) = 3.5, p = 0.06), and between amlyopia types (time X type: F(1,34) = 3.6, p = 0.06). The time X group X type was not significant (F(1,34) = 0.68, p = 0.41).
To summarize, improvements were noted already after 13 h of training, however participants continued to improve after 26 h as well. The numerical differences between amblyopia types was evident already at 13 h of training.
3.2. Changes in stereoacuity
Thirteen of the 23 participants in the game group (56%) and 8 of the 15 movies group participants (53%) failed the Randot stereo (circles) test at the baseline visit (we label them as ‘stereo blind’). Thus our two groups were quite balanced in terms of stereo vision.
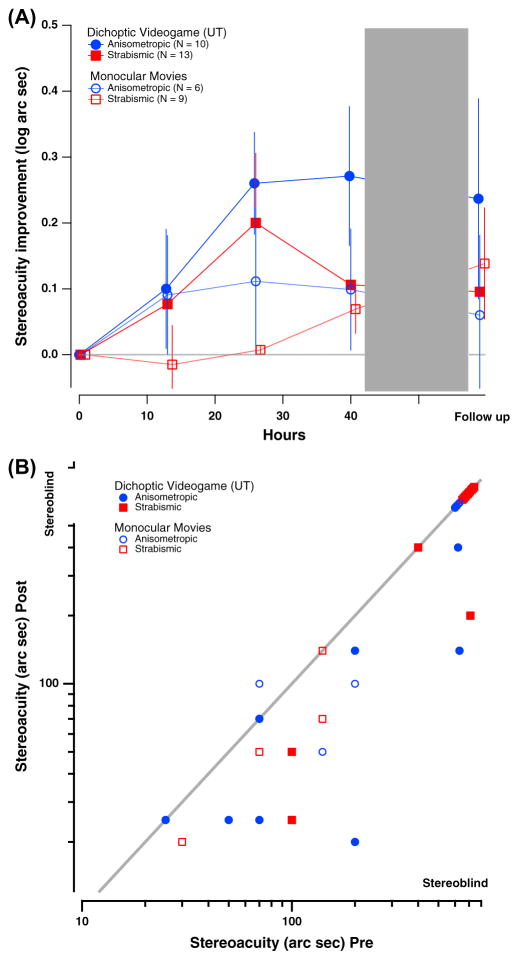
Following 40 h of intervention, stereoacuity improved significantly overall, by on average of 0.18 ± 0.05 log arcsec (34 ± 9.4%) and of 0.08 ± 0.04 log arcsec (17 ± 8.4%) for the game and movies groups respectively (Fig. 4, top; effect of time: F(1,34) = 11.7; p < 0.005; no effect of group: p = 0.92; no effect for time X group: p = 0.2).
Fig. 4.
Changes in stereopsis as a function of hours of either video game play or movies watching. (A) Log stereoacuity improvement (Log stereoacuity Pre – Log stereoacuity Post) as a function of time in intervention (hours) for both game (solid symbols) and movies (open symbols) groups. The dotted gray line indicates no improvement. (B) Individual stereoacuity data at post-intervention as a function of baseline stereoacuity for game (solid symbols) and movies (open symbols) groups, plotted in log–log coordinates. Stereoacuity of 20–40 arcsec is within the normal stereo vision range; stereoacuity larger than 400 arcsec on the Randot circles test is considered stereo-blindness and was assigned a value of 600 arcsec. Color coding is similar to previous figures. Values below the diagonal represent improved stereoacuity. Note that not all individual data points are visible due to observations with overlapping values.
We further examined the amblyopia type effects. Subjects with anisometropic amblyopia in the game group showed the largest improvements (0.27 ± 0.1 log arcsec; 46 ± 17%) compared with all other subjects: strabismic amblyopia in the game group (0.1 ± 0.06 log arcsec; 21 ± 12.3%), anisometropic amblyopia in the movies group (0.1 ± 0.09 log arcsec; 21 ± 18.5% and strabismic amblyopia in the movies group (0.07 ± 0.04 log arcsec; 15 ± 8.5%). However, the effect of group X time X type was not significant (all ps > .1).
Given our interest in the efficacy of different interventions, we analyzed the data from the two treatment groups, game and movies, separately, i.e. two 2 × 2 ANOVAs of time and amblyopia type, with Bonferroni correction. For the game group, overall improvement was statistically significant (effect of time: F(1,21) = 10.9, p < 0.005, Bonferroni Corrected). An effect of amblyopia type (F(1,21) = 6.3, p = 0.02, Bonferroni Corrected) revealed better stereoacuity for patients with anisometropic amblyopia, and there was no interaction between time X amblyopia type (p = 0.16, Bonferroni Corrected). For the movies group, the effect of time failed to reach statistical significance (time: F(1,13) = 3.7, p = 0.07, Bonferroni Corrected) and no other effect was seen (ps > 0.7 for both amblyopia type and time X type interaction).
The pattern of results was largely retained at follow-up, with retention being numerically larger for game group participants (0.16 ± 0.08 log arcsec; improvement relative to the baseline assessment (31 ± 15.4%) compared with the movies group (0.11 ± 0.07 log arcsec; 22 ± 14.2%). Although the effect of time was significant (F(1,31) = 5.4; p < 0.03), the effect of time X group was not: (p = 0.56; no effect for group: p = 0.72). As with the post-training data, effects were numerically largest for subjects with anisometropic amblyopia in the game group (0.24 ± 0.1 log arcsec change; 42 ± 17.7%) compared with all other groups. Statistically, however, none of the effects were significant (all ps > 0.1).
In summary, improvements were numerically larger for the game group, especially for the anisometropic patients. However, since over half of participants were stereo-blind, the data remain noisy, despite our relatively large sample of patients.
3.3. Contrast sensitivity (qCSF)
We use the area under the log CSF curve (AULCSF) as a summary measure for contrast sensitivity. As can be seen in Fig. 5, the AULCSF increased for both treatment groups, with game group participants showing an average increase of 0.3 log units2 (from 2 ± 0.19 to 2.3 ± 0.13; Fig. 5A), and movies group participants of only 0.1 (from 1.8 ± 0.15 to 1.9 ± 0.16; Fig. 5B). The 2 × 2 × 2 omnibus ANOVA indicated a main effect of time (F(1,33) = 5.1, p < 0.04), with the treatment group X time failing to reach significance (F(1,33) = 3.08, p = 0.09).
Fig. 5.
Contrast Sensitivity Data. Area under the contrast sensitivity curve (AULCSF) for amblyopic eye (AE) at pre-training (y-axis) as a function of post-training and follow-up AULCSF (x-axis). (A) Game group data (solid symbols). (B) Movies group data (open symbols). Blue symbols denote data for subjects with anisometropic amblyopia at post-training (blue circles) and follow up (blue diamonds); Red symbols denote data for subjects with strabismic amblyopia at post-training (red squares) and follow-up (red diamonds). Larger symbols denote averages (±SEM). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The same analysis revealed amblyopia type effects with the greatest gains made by the game group anisometropes (from 1.68 ± 0.4 to 2.2 ± 0.2), while changes in the other groups were smaller (movies group anisometropes: from 1.8 ± 0.13 to 1.6 ± 0.32; game group strabismics: from 2.2 ± 0.15 to 2.4 ± 0.11; movies group strabismic: from 1.77 ± 0.2 to 2.04 ± 0.16). This greater improvement for individuals with anisometropic amblyopia in the game group was confirmed by a significant three-way interaction of time X treatment group X amblyopia type (F(1,33) = 7.2, p < 0.02).
Looking at each group separately (Bonferroni correction), we find that for the game group, overall improvement was statistically significant (effect of time: F(1,20) = 10.2, p < 0.005, Bonferroni corrected), with no effect of either amblyopia type (p = 0.24) or their interaction (p = 0.057, Bonferroni corrected). In contrast, for the movies group, none of the effects were statistically significant (time and amblyopia type: p > 0.5; interaction: F(1,13) = 3.3, p = 0.09, Bonferroni corrected).
Effects at follow-up were similar to post-intervention outcomes, albeit quite weaker: AULCSF changed from 2 ± 0.2 (at pre) to 2.25 ± 0.13 (at follow-up) for game group participants (n = 20 with follow-up data), and from 1.9 ± 0.17 to 1.96 ± 0.12 for movies group participants (n = 12). The overall change in AULCSF at follow-up was not statistically significant (effect of time: F(1,30) = 3.7, p = 0.062; effect of group: p = 0.28; group X time: p = 0.23).
3.4. Changes in reading speed
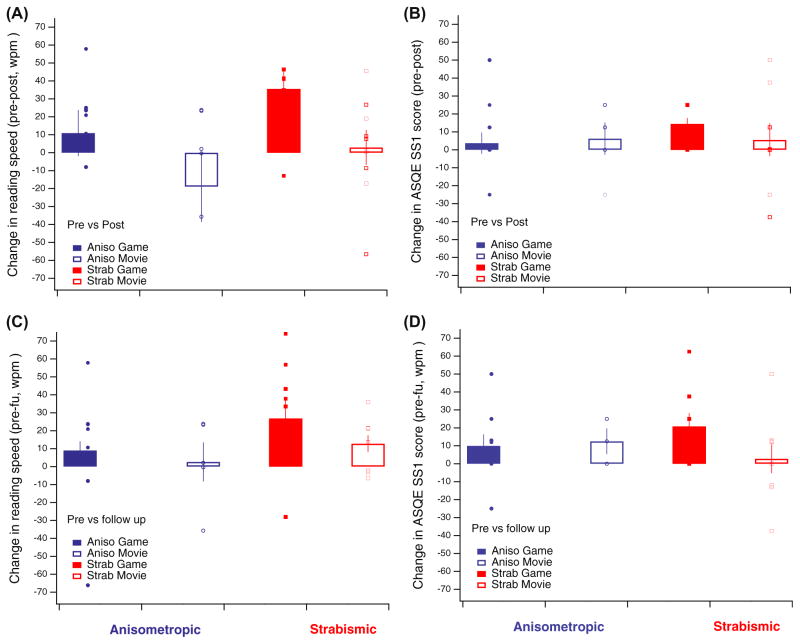
We examined changes in reading speed as a function of intervention using the MN Read chart-based test. Since we used the difference scores from pre- to post- or follow-up for analysis (see Methods section above), the omnibus ANOVA included a 2 × 2 analysis with treatment group and amblyopia type.
The Omnibus 2 × 2 ANOVA on reading speed difference scores indicated a main effect of treatment group (F(1,32) = 5.7, p < 0.03). Thus, the improvement in reading speed post-intervention was larger for the game group (in 26.2 ± 8.5 words-per-minute, wpm), as compared to the movies group (−5.8 ± 9.7 wpm) group. Amblyopia type did not reach statistical significance (F(1,32) = 3.1, p = 0.08, see Fig. 6A, top), and there was no significant interaction between treatment group and amblyopia type (p = 0.9). The follow-up data showed a similar trend, where game group patients improved on average by 18.4 ± 6.2 wpm, whereas movies group patients improved less (9.2 ± 4.8 wpm). However, the difference was not statistically significant (effect of group: p = 0.22; effect of type: p = 0.09; see Fig. 6A, bottom).
Fig. 6.
Changes in reading speed and in subjective fear of losing the good eye following training. (A) Difference reading speed from preto post-intervention (top) and from baseline to follow-up (bottom). Changes are denoted as difference in averaged reading speed, averaged across all attempted sizes. (B) Changes in sub-scale 1 (SS1) of the Amblyopia and Strabismus questionnaire (ASQE), ‘fear of losing the good eye’ at post training (top) and at follow-up (bottom). Note that larger values denote less fear of losing the good eye. On all panels, boxes denote first and third quartile data (±SEM as vertical bars), and small circles and rectangles denote individual participant data. Data is shown separately for subjects with anisometropic (blue symbols) and strabismic (red symbols) amblyopia, as well as for game (filled symbols) and movies (open symbols) groups. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Self-report measures of improvement: the ASQE questionnaire
Study participants were asked to complete the Amblyopia and Strabismus Questionnaire (ASQE) pre and post-intervention. ASQE has 5 different sub-scales, and provides a self-report measure for the deficits associated with amblyopia. Four of the 5 subscales did not show any numerical difference following intervention. However, the ‘fear of losing the good eye’ subscale (SS1) did show a suggestive pattern. The omnibus ANOVA with treatment group, amblyopia type and time indicated a significant effect of time (F(1,34) = 5.35, p < 0.03), with the game group showing a decrease in the fear of loosing the good eye from 53.3 ± 5.7 to 63 ± 4.9 (higher values mean less fear of losing good eye), and the movies group from 48.3 ± 8.5 to 54 ± 7.7. No other effect was significant, indicating that improvement was similar for both groups and for both amblyopia types.
As in the other analyses we followed up with separate analyses for game and movies group, Bonferroni corrected. For the game group, there was a significant effect of time (F(1,21) = 9.03, p < 0.01, Bonferroni corrected), as well as a significant effect of amblyopia type (F(1,21) = 8.98, p < 0.01, Bonferroni corrected), while interaction between time and type did not reach statistical significance (p = 0.09, Bonferroni corrected; Fig. 6B, top). For the movies group, none of the effect were significant (all p values > 0.35).
The same pattern of results as at post-intervention was observed at follow-up with the main effect of time remaining significant (F(1,29) = 6.1, p < 0.02), and no other effects being significant (Fig. 6B, bottom).
We conclude that intervention, whether games or movies, tended to reduce the fear of losing the good eye, with the game intervention appearing, at least numerically, to be the most promising.
4. Discussion
4.1. Summary of results
In the current study, our aim was to test the benefits of a novel treatment for adult amblyopia, that combined dichoptic viewing, videogame play and monocular PL. We compared this novel treatment to an active control treatment of supervised occlusion therapy. Interestingly, while occlusion therapy is considered the gold-standard treatment in children with amblyopia, occlusion has not been systematically assessed in amblyopic adults.
Following our dichoptic/PL game play, significant improvements were noted in VA and stereopsis, as well as in contrast sensitivity, reading speed and reduced fear of loosing the good eye. These improvements were weaker but still visible following a 2-months no-contact period. In contrast, following supervised patching with movies viewing, participants showed no significant changes in any of these functions, except VA. In the movies group, VA improvement was restricted to anisometropic amblyopes with strabismic amblyopes showing no changes. Our two interventions varied along several different dimensions. In particular while the game treatment took extra-care to balance the inputs between the two eyes, the movies group was trained exclusively monocularly (with the amblyopic eye). We recognize that other confounds also exist, especially the fact that while all game group participants were trained in the laboratory, a significant portion of the movies group was allowed to train at home. While there are several successful at home training studies, the respective impact of at-home versus in-lab training in amblyopia remains largely unknown.
Overall, the game intervention led to significantly greater benefits than the movies intervention. Amblyopia type also qualified these effects, especially for VA and contrast sensitivity. Interestingly, the present study suggests that active supervised patching in adults with anisometropic (but not strabismic) amblyopia may be more potent than what any of the existing literature may suggest.
We review below results for each intervention and measure in turn, before turning to possible implications and caveats for the treatment of adult amblyopia.
4.2. The benefits of playing a dichoptic videogame with a PL task
4.2.1. Improvements in visual acuity
The game we have developed and tested in the current study was designed to incorporate the benefits of three different approaches, each of which has been shown to positively affect vision in adult amblyopia. By using an intervention that combines all three methods, we could have expected to see an additive effect, leading to larger improvements in VA than each of the method on its own. This was not the case. In our study, the magnitude of improvement was ≈1.4 lines on a logMAR chart.
It is striking that, despite a wide range of stimuli, tasks, methods, durations of training and subject ages, studies in the adult amblyopia literature typically report VA improvements in the magnitude of 1–2 lines on a logMAR chart. This is the case for multihour monocular PL training applied to the amblyopic eye (Astle, Webb, & McGraw, 2010, 2011; Chung, Li, & Levi, 2008, 2012; Hussain et al., 2012; Levi, 2012; see recent reviews in Levi & Li, 2009; Levi & Polat, 1996; Levi, Polat, & Hu, 1997; Zhang et al., 2014), and for video game training, either off-the shelf action games (Jeon, Maurer, & Lewis, 2012; e.g. Li et al., 2011) or customized games (Hussain et al., 2014).
Indeed, even studies that applied dichoptic training methods, aimed at reducing suppression of the amblyopic eye by the dominant eye, also report similar magnitude of improvements in VA (Hess, Mansouri, & Thompson, 2010a, 2010b, 2011). Hess and colleagues have used a dichoptic version of Tetris, in which stimulus elements are presented separately to each eye, and image strength is controlled separately to each eye during training, to facilitate fusion (Hess et al., 2012, 2014; Li et al., 2013; To et al., 2011). Using this paradigm, the magnitude of improvement in VA was again of about 1.6 lines, but with as little as 10 h of training, rather than 40 h or the kilo trials used in PL.
The fairly stable magnitude of a 1 to 2-line improvement in VA noted in adults with amblyopia may correspond to a ceiling on the amount of VA improvement that can be achieved in adult amblyopes, at least with the methods used so far. A similar conclusion was reached by Hussain et al. (2014) in a study that tested the benefits of a monocular videogame with a PL task. The dichoptic game we tested here, resulted in a similar level of improvement in VA (Fig. 3B replots the data from several monocular and dichoptic studies along with our data). Thus it appears that dichoptic presentation with balanced image strength in the two eyes, and an integrated PL task, does not result in any greater improvement in VA than does monocular training. However, as noted below, it may have advantages in promoting fusion and stereopsis. The relative advantage of each of these methods separately remains the target of further investigations, as does the application of other methods, such as transcranial direct current stimulation (Spiegel et al., 2013).
4.2.2. Improvement in stereo vision and suppression following dichoptic training
In the current study, we found an average improvement of 23% in stereopsis, with about half of videogame group participants showing some improvement in stereopsis. Subjects with anisometropic amblyopia made numerically greater improvements in stereopsis than those with strabismic amblyopia. Specifically, 6/10 (60%) of the anisometropic amblyopes improved, compared with only 3/13 strabismic amblyopes (23%). The relatively high proportion of anisometropic amblyopes showing improvement in stereopsis is in line with previous reports in the monocular training literature (see Levi et al., 2015; Li et al., 2011; Zhang et al., 2014). However, we note that the proportion of strabismic amblyopes showing improved stereoacuity is much higher than the roughly 5% reported in previous studies of monocular PL and videogame play (Levi et al., submitted). For example, Li et al. (2011) found no improvement in stereopsis in their strabismic subjects following 40 h of monocular videogame play. This pattern of results suggests that dichoptic training may not be necessary for improving stereopsis in anisometropic amblyopes, but may be advantageous in strabismic amblyopes. Indeed, in their recent review, Hess, Thompson, and Baker (2014) report that ≈37% of strabismic subjects showed improved stereopsis following dichoptic training (for a review see also Levi et al., 2015 – this issue).
4.2.3. Improvements in contrast sensitivity, reading speed and quality of life
Contrast sensitivity improved as a result of videogame training, as reflected by the increase in the area under the CSF. Several recent studies have shown that extensive PL can result in improved contrast sensitivity for adults with amblyopia (Huang, Zhou, & Lu, 2008; Polat et al., 2004; Zhou et al., 2006). It is interesting to note that most of these studies have focused on anisometropic amblyopia, the sub-population that showed significantly most improvement in AULCSF following our training. To the best of our knowledge, contrast sensitivity changes have not been tested in the several recent dichoptic PL or videogame studies (Hess et al., 2014; Li et al., 2013), hence direct comparison with the effectiveness of these methods cannot be derived.
Two other secondary outcomes of our study are the effects on reading speed and quality of life. Surprisingly, despite the relatively large pool of studies documenting benefits in PL, dichoptic training or video game play for amblyopic adults, there are no reports, to the best of our knowledge, of generalization of training to everyday life activities. Here we used reading speed and quality of life, two aspects of behavior that seem especially important in case such treatment ever becomes the clinical standard-of-care for adults with amblyopia.
We found that reading speed significantly improved following game, but not movies intervention, regardless of amblyopia etiology. Previous studies have reported improvement in letter contrast following monocular PL training (Chung, Li, & Levi, 2006, 2008), but have not addressed the question of reading speed. We hypothesize that the improved reading speed is a direct result of the fast-paced nature of first-person-shooter action video games, which require fast actions and eye movements to identify game bots. Indeed, fast-paced games have been found to speed up reaction times in individuals with normal vision (Dye, Green, & Bavelier, 2009). This conclusion is further supported by the fact that these improvements were not limited to anisometropic amblyopia, but were evident, and strikingly pronounced, in strabismic amblyopia as well, hence may be the result of a more generalized effect induced by the nature of the action game play.
Significant changes in quality of life following game play were only found for a single domain, the fear of losing the good eye. For this specific subscale, game group participants showed less fear of losing the good eye following intervention. Although in need of further confirmation, the effect was numerically larger for strabismic than for anisometropic amblyopes. This may be, however, driven by the initial lower scores for game group strabismics, as this sub-group had more fear of losing their good eye initially.
Overall, our findings on reading speed and fear of loosing the good eye suggest that the changes brought about by game play can be of significance to the everyday life of the participants.
4.2.4. Retention of effects
Intervention benefits were retained, albeit weakened, for at least 2 months following the completion of training. Indeed, for all our measures a main effect of baseline-to follow-up assessment remained; however, there was little evidence for greater improvements in the game group as compared to the movies group at follow-up. The only exception was VA, for which all groups except for strabismic patients in the movies group retained some improvement at follow-up.
This sustained improvement in VA is in line with several previous reports in the literature, showing that VA improvements are retained for at least a year (Chen et al., 2008; e.g. Li & Levi, 2004; Polat et al., 2004) or even 18 months (Zhou et al., 2006) following training (see Levi & Li, 2009 for review). However, while these previous studies only tested the retention of VA improvements, our study extends these PL training results by showing that following dichoptic/PL videogame training, effects are also still visible for other measures, including stereopsis, contrast sensitivity, reading speed and fear of losing the good eye.
Interestingly, all previous studies testing the retention of effects over time have used PL paradigms, which involve repetition of the same stimuli for a very large number of trials. The improvements are maintained for long periods of time for normal-sighted people as well (Sagi, 2011). For videogame play in amblyopic patients, we are aware of only one study that tested retention of effects following training and brain transcranial stimulation (Spiegel et al., 2013). Retention effects were tested three months post training on a small subset of study patients, and VA and stereo effects were found to sustain 3 months after treatment. We are not aware of any ‘videogame only’ training study, monocular or dichoptic, which has tested retention of effects following training in adult amblyopia. Our study is the first to show that gains made following 40 h of gameplay are still visible 2 months following completion of training, without the need for an additional ‘training boost’. Both the gains after training and the loss at follow-up appear larger in amblyopic subjects than in those with normal vision. Overall, however, our results are in line with reports in the videogame training literature in normal subjects, showing training benefits are still visible months to years after the end of training (Li et al., 2009, 2010). Future studies are needed to test retention beyond 2 months, and optimize the amount and schedule of training to generate durable improvements in visual function (dose–response trials).
4.3. The surprisingly positive effects of supervised patching on anisometropic amblyopia
An unexpected outcome of our study is that our supervised patching paradigm resulted in improved VA, an effect entirely driven by patients with anisometropic amblyopia. Previous studies, in which adults were given 20 h of unsupervised patching, found no improvement in VA (Li, Ngo, & Levi, 2015; Li et al., 2011). Although our result was unexpected, successful treatment of adults with anisometropic amblyopia has been previously reported (e.g. Wick et al., 1992). It is noteworthy that the magnitude of VA improvement in this ‘movies anisometropic’ control group was again within 1–2 lines on a logMAR chart and comparable to that seen in the anisometropic patients playing the dichoptic videogame. We note that VA is the only measure for which the active supervised patching training was found to have some efficacy. Clearly, patching, although beneficial for a subset of patients, is less effective than dichoptic/PL videogame play.
Patching has been considered the gold-standard treatment in young children and even adolescents (Chen et al., 2008; Erdem et al., 2011; Holmes et al., 2005; Scheiman et al., 2005; Sen, 1982), but not in adults with amblyopia (Wu & Hunter, 2006). Moreover, the few experimental studies that did employ patching as the control intervention reported no benefits to patching in adults (Li et al., 2011; Polat et al., 2004). To the best of our knowledge, our study is the first to employ a multi-hour supervised patching in a large study involving only adults with amblyopia (but see Chen et al., 2008 for a study involving children and adults), and the first to report positive effects of supervised patching in adult amblyopia, in our case, anisometropia. We recognize, however, that our sample size in this cell is small, being limited to only 6 patients, and thus calling for caution as to the replicability of this effect.
For strabismic individuals, improvements in the movies group were overall minor. This null effect of patching in strabismic amblyopia is in line with the Li et al. (2011) study. They too found no improvement following 20 h of patching, and their patching group (in their case, non-supervised) included only subjects with strabismic amblyopia.
Although our results are in need of confirmation, they highlight the importance of including active control groups when evaluating the efficacy of a training regimen. Most previous studies have included no control group (Chung, Li, & Levi, 2008; Hess, Thompson, & Baker, 2014; Hess, Mansouri, & Thompson, 2010a, 2010b; Hess et al., 2012; Hussain et al., 2012, 2014; Li, Klein, & Levi, 2008) or a no-contact control (Zhou et al., 2006), while others have included a control condition that either had a very small number of participants (Li et al., 2011), had a very small number of strabismic participants (Li et al., 2013), or a control that did not match the duration of the active intervention (Chen et al., 2008). As we demonstrate here, the visual system of amblyopic patients may be more plastic than once thought. How the present result relates to the status quo on patching will require future studies. Our patching paradigm was quite unique in being supervised and requiring participants to watch engaging movies with preferably fast-paced, action-packed sequences while patched. We cannot at this point separate the relative contribution of the type of attention-grabbing content we were seeking from the use of supervised patching on the magnitude of improvement reported. Future studies would need to include larger number of subjects and more testing to determine whether the differential effects as a function of amblyopia etiology are replicable, and which factors in the training may drive these changes.
4.4. Caveats
We note several caveats to our study. First, our dichoptic videogame training utilized custom-built stereoscopes to enable us to present separately controlled stimuli to the two eyes, and enable subjects to fuse them. Although constructed from a highly popular game platform, the dichoptic game intervention required subjects to perform the extensive training (40 h) in the lab. While this provided us with excellent control and monitoring of the training, it was a hardship for participants, resulting in a large dropout rate (38%). We note that our secondary analysis shows that the differential dropout rate between the two groups did not bias the outcome. We suspect that home training would substantially improve compliance.
Unfortunately, most previous perceptual learning/videogame studies do not report dropout rate, hence it is difficult to compare ours with other forms of active treatment. Although not directly comparable, it is well documented that in children with amblyopia, compliance with prescribed at-home patching is poor. On average, amblyopic children patch for less than half the prescribed dose (Stewart et al., 2004). Future studies should aim to better document attrition rate, as well as test ways to motivate participants to continue in training.
Finally, as noted above, it is not clear that dichoptic videogames/perceptual learning result in greater improvement in visual acuity than monocular videogames/perceptual learning. On the other hand, a key goal of dichoptic training is to foster binocular cooperation and stereopsis. While the jury is still out on which approach is best, a review of the extant studies suggests that stereopsis can be improved in a substantial proportion of individuals with anisometropic amblyopia through either monocular or dichoptic training; however, individuals with strabismic amblyopia fare better with dichoptic training than with monocular training and better yet with direct training of stereopsis (Levi et al., 2015).
5. Conclusion
Our novel dichoptic/PL video game, which combines action gaming with perceptual learning, suppression checks and image strength matching across eyes, results in a broad range of improvements in adults with amblyopia, and provides some pointers toward principles for improving treatment for amblyopia. Our game training was more effective in recovering visual acuity than “supervised patching”. Our study also highlights a surprising positive impact of supervised patching for anisometropic, but not strabismic amblyopia.
Supplementary Material
Acknowledgments
We thank our research assistants and study coordinators at UCB and Uof R sites: Rachel Albert, Aleksandra Fazlipour, Jennifer Luu, Charlie Ngo, Sheena Song, Olga Pikul, Rachel Spencer, Gary Volkell, Eunice Yang. We also thank the creators of the Unreal Tournament 2004 mod for amblyopia treatment: Joshua Alway, Ari Check, Yunien Chen, Justin Coburn, Michael Culek, Elora Krzanich, Bradley Lubahn, Stuart Monske, Joel Ogden, Rachel Orosz, Bill Phillips, Stephen Smith, and Rob Yates. We are indebted to Jian Ding who kindly provided the software for the nonius alignment task.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.visres.2015.04.008.
Footnotes
Authors DB and DL have filed a patent for a dichoptic treatment method, however none of the authors have a commercial conflict of interest.
Author contributions
IV and MN share co-first authorship. Study design and conceptualization: DB & DL; Video Game Development and Design: primarily JB with contributions from DB, IV and DL; Video Game play test during game development: IV and SH with contributions from DB & DL & MN; Piloting and fine-tuning of vision experiments: primarily IV with contributions of MN, FZ and SH; Running the Study: IV, MN, FZ and SH; Data analysis: MN &IV; Writing: primarily MN with all authors contributing. Finally, this work could not have happened without the support of Drs. Gearinger and DePaolis who referred patients to our study. We thank Grants from the National Eye Institute #R01s EY020976 to DL and DB, Grant EY016880 to DB and P30EY001319 to the Center for Visual Science at Rochester.
References
- Achtman RL, Green CS, Bavelier D. Video games as a tool to train visual skills. Restorative Neurology and Neuroscience. 2008;26:435–446. [PMC free article] [PubMed] [Google Scholar]
- Astle AT, Webb BS, McGraw PV. Spatial frequency discrimination learning in normal and developmentally impaired human vision. Vision Research. 2010;50:2445–2454. doi: 10.1016/j.visres.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle AT, Webb BS, McGraw PV. Can perceptual learning be used to treat amblyopia beyond the critical period of visual development? Ophthalmic Physiology Optics, Journal of British College of Ophthalmic Opticians. 2011;31:564–573. doi: 10.1111/j.1475-1313.2011.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DH, Meese TS, Hess RF. Contrast masking in strabismic amblyopia: Attenuation, noise, interocular suppression and binocular summation. Vision Research. 2008;48:1625–1640. doi: 10.1016/j.visres.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Green CS, Pouget A, Schrater P. Brain plasticity through the life span: Learning to learn and action video games. Annual Review of Neuroscience. 2012;35:391–416. doi: 10.1146/annurev-neuro-060909-152832. [DOI] [PubMed] [Google Scholar]
- Bayliss J, Vedamurthy I, Bavelier D, Nahum M, Levi DM. Lazy eye shooter: A novel game therapy for visual recovery in adult amblyopia. 4th International IEEE Consumer Electronic Society – Games Innovation Conference.2012. [Google Scholar]
- Bayliss J, Vedamurthy I, Nahum M, Levi DM, Bavelier D. HCI International. 2013. Lazy eye shooter: making a game therapy for visual recovery in adult amblyopia usable. [Google Scholar]
- Bedell HD, Flom MC. Monocular spatial distortion in strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1981;20:263–268. [PubMed] [Google Scholar]
- Bedell HE, Flom MC. Normal and abnormal space perception. American Journal of Optometry and Physiological Optics. 1983;60:426–435. doi: 10.1097/00006324-198306000-00003. [DOI] [PubMed] [Google Scholar]
- Bi H, Zhang B, Tao X, Harwerth RS, Smith EL, Chino YM. Neuronal responses in visual area V2 (V2) of macaque monkeys with strabismic amblyopia. Cerebral Cortex (New York, NY) 2011;1991(21):2033–2045. doi: 10.1093/cercor/bhq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch EE. Amblyopia and binocular vision. Progress in Retinal and Eye Research. 2013;33:67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Investigative Ophthalmology & Visual Science. 1981;21:467–476. [PubMed] [Google Scholar]
- Chen PL, Chen JT, Fu JJ, Chien KH, Lu DW. A pilot study of anisometropic amblyopia improved in adults and children by perceptual learning: An alternative treatment to patching. Ophthalmic Physiology Optics, Journal of British College of Ophthalmic Opticians. 2008;28:422–428. doi: 10.1111/j.1475-1313.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- Chung STL, Li RW, Levi DM. Identification of contrast-defined letters benefits from perceptual learning in adults with amblyopia. Vision Research. 2006;46:3853–3861. doi: 10.1016/j.visres.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Li RW, Levi DM. Learning to identify near-threshold luminance-defined and contrast-defined letters in observers with amblyopia. Vision Research. 2008;48:2739–2750. doi: 10.1016/j.visres.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung STL, Li RW, Levi DM. Learning to identify near-acuity letters, either with or without flankers, results in improved letter size and spacing limits in adults with amblyopia. PLoS ONE. 2012;7:e35829. doi: 10.1371/journal.pone.0035829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda K, Levi DM, Selenow A. Amblyopia: Basic and clinical aspects. Boston, MA: Butterworth-Heinemann; 1991. [Google Scholar]
- Ding J, Klein SA, Levi DM. Binocular combination in abnormal binocular vision. Journal of Vision. 2013;13:14. doi: 10.1167/13.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Levi DM. Rebalancing binocular vision in amblyopia. Ophthalmic Physiology Optics, Journal of British College of Ophthalmic Opticians. 2014;34:199–213. doi: 10.1111/opo.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drover JR, Kean PG, Courage ML, Adams RJ. Prevalence of amblyopia and other vision disorders in young Newfoundland and Labrador children. Canadian Journal of Ophthalmology Journal Canadien D’ophtalmologie. 2008;43:89–94. doi: 10.3129/i07-187. [DOI] [PubMed] [Google Scholar]
- Dye MWG, Green CS, Bavelier D. Increasing speed of processing with action video games. Current Directions in Psychological Science. 2009;18:321–326. doi: 10.1111/j.1467-8721.2009.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem E, Cınar GY, Somer D, Demir N, Burcu A, Ornek F. Eye patching as a treatment for amblyopia in children aged 10–16 years. Japanese Journal of Ophthalmology. 2011;55:389–395. doi: 10.1007/s10384-011-0029-z. [DOI] [PubMed] [Google Scholar]
- Felius J, Beauchamp GR, Stager DR, Van De Graaf ES, Simonsz HJ. The amblyopia and strabismus questionnaire: English translation, validation, and subscales. American Journal of Ophthalmology. 2007;143:305–310. doi: 10.1016/j.ajo.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Friedman DS, Repka MX, Katz J, Giordano L, Ibironke J, Hawse P, et al. Prevalence of amblyopia and strabismus in white and African American children aged 6 through 71 months the Baltimore pediatric eye disease study. Ophthalmology. 2009;116(2128–2134):e1–e2. doi: 10.1016/j.ophtha.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epic Games. [accessed 02.11.04];Unreal tournament, 2004. 2004 < http://www.unrealtournament.com/utgoty/>.
- Green CS, Bavelier D. Learning, attentional control, and action video games. Current Biology CB. 2012;22:R197–R206. doi: 10.1016/j.cub.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CS, Li R, Bavelier D. Perceptual learning during action video game playing. Topics in Cognitive Science. 2010;2:202–216. doi: 10.1111/j.1756-8765.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- Harrad RA, Hess RF. Binocular integration of contrast information in amblyopia. Vision Research. 1992;32:2135–2150. doi: 10.1016/0042-6989(92)90075-t. [DOI] [PubMed] [Google Scholar]
- Harrad R, Sengpiel F, Blakemore C. Physiology of suppression in strabismic amblyopia. British Journal of Ophthalmology. 1996;80:373–377. doi: 10.1136/bjo.80.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Babu RJ, Clavagnier S, Black J, Bobier W, Thompson B. The iPod binocular home-based treatment for amblyopia in adults: Efficacy and compliance. Clinical & Experimental Optometry: Journal Australia Optometrists Association. 2014;97:389–398. doi: 10.1111/cxo.12192. [DOI] [PubMed] [Google Scholar]
- Hess RF, Holliday IE. The spatial localization deficit in amblyopia. Vision Research. 1992;32:1319–1339. doi: 10.1016/0042-6989(92)90225-8. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. A binocular approach to treating amblyopia: Antisuppression therapy. Optometry and Vision Science: Official Publication of the American Academy of Optometry. 2010a;87:697–704. doi: 10.1097/OPX.0b013e3181ea18e9. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. A new binocular approach to the treatment of amblyopia in adults well beyond the critical period of visual development. Restorative Neurology and Neuroscience. 2010b;28:793–802. doi: 10.3233/RNN-2010-0550. [DOI] [PubMed] [Google Scholar]
- Hess RF, Mansouri B, Thompson B. Restoration of binocular vision in amblyopia. Strabismus. 2011;19:110–118. doi: 10.3109/09273972.2011.600418. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: Structure, suppression and plasticity. Ophthalmic Physiology Optics, Journal of British College of Ophthalmic Opticians. 2014;34:146–162. doi: 10.1111/opo.12123. [DOI] [PubMed] [Google Scholar]
- Hess RF, Thompson B, Black JM, Machara G, Zhang P, Bobier WR, et al. An iPod treatment of amblyopia: An updated binocular approach. Optometrists in Saint Louis, MO. 2012;83:87–94. [PubMed] [Google Scholar]
- Holmes JM, Edwards AR, Beck RW, Arnold RW, Johnson DA, Klimek DL, et al. A randomized pilot study of near activities versus non-near activities during patching therapy for amblyopia. Journal of AAPOS: the Official Publication of the American Association for Pediatric Ophthalmology and Strabismus/American Association for Pediatric Ophthalmology and Strabismus. 2005;9:129–136. doi: 10.1016/j.jaapos.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Huang CB, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proceedings of the National academy of Sciences of the United States of America. 2008;105:4068–4073. doi: 10.1073/pnas.0800824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z, Astle AT, Webb BS, McGraw PV. The challenges of developing a contrast-based video game for treatment of amblyopia. Frontiers in Psychology. 2014;5:1210. doi: 10.3389/fpsyg.2014.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z, Webb BS, Astle AT, McGraw PV. Perceptual learning reduces crowding in amblyopia and in the normal periphery. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32:474–480. doi: 10.1523/JNEUROSCI.3845-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon ST, Maurer D, Lewis TL. The effect of video game training on the vision of adults with bilateral deprivation amblyopia. Seeing Perceiving. 2012;25:493–520. doi: 10.1163/18784763-00002391. [DOI] [PubMed] [Google Scholar]
- Kiorpes L. Visual processing in amblyopia: Animal studies. Strabismus. 2006;14:3–10. doi: 10.1080/09273970500536193. [DOI] [PubMed] [Google Scholar]
- Legge GE, Ross JA, Luebker A, LaMay JM. Psychophysics of reading. VIII. The minnesota low-vision reading test. Optometry and Vision Science: Official Publication of the American Academy of Optometry. 1989;66:843–853. doi: 10.1097/00006324-198912000-00008. [DOI] [PubMed] [Google Scholar]
- Lesmes LA, Lu ZL, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: The quick CSF method. Journal of Vision. 2010;10(17):1–21. doi: 10.1167/10.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi D. Visual processing in amblyopia: Human studies. Strabismus. 2006;14:11–19. doi: 10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- Levi D. Prentice award lecture 2011: Removing the brakes on plasticity in the amblyopic brain. Optometry and Vision Science: Official Publication of the American Academy of Optometry. 2012;89:827–838. doi: 10.1097/OPX.0b013e318257a187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Investigative Ophthalmology & Visual Science. 1977;16:90–95. [PubMed] [Google Scholar]
- Levi D, Harwerth RS, Smith EL. Humans deprived of normal binocular vision have binocular interactions tuned to size and orientation. Science. 1979;206:852–854. doi: 10.1126/science.493988. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982;298:268–270. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein SA. Vernier acuity, crowding and amblyopia. Vision Research. 1985;25:979–991. doi: 10.1016/0042-6989(85)90208-1. [DOI] [PubMed] [Google Scholar]
- Levi D, Knill D, Bavelier D. Stereopsis and amblyopia: A mini-review. Vision Research. 2015 doi: 10.1016/j.visres.2015.01.002. http://dx.doi.org/10.1016/j.visres.2015.01.002, pii:S0042-6989(15)00008-5. [DOI] [PMC free article] [PubMed]
- Levi D, Li RW. Perceptual learning as a potential treatment for amblyopia: A mini-review. Vision Research. 2009;49:2535–2549. doi: 10.1016/j.visres.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proceedings of the National academy of Sciences of the United States of America. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U, Hu YS. Improvement in Vernier acuity in adults with amblyopia. Practice makes better. Investigative Ophthalmology & Visual Science. 1997;38:1493–1510. [PubMed] [Google Scholar]
- Levi D, Song S, Pelli DG. Amblyopic reading is crowded. Journal of Vision. 2007;7(21):1–17. doi: 10.1167/7.2.21. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. Journal of the Acoustical Society of America. 1971;49(Suppl 2):467. [PubMed] [Google Scholar]
- Li RW, Klein SA, Levi DM. Prolonged perceptual learning of positional acuity in adult amblyopia: Perceptual template retuning dynamics. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28:14223–14229. doi: 10.1523/JNEUROSCI.4271-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. Journal of Vision. 2004;4:476–487. doi: 10.1167/4.6.7. [DOI] [PubMed] [Google Scholar]
- Li X, Lu ZL, Xu P, Jin J, Zhou Y. Generating high gray-level resolution monochrome displays with conventional computer graphics cards and color monitors. Journal of Neuroscience Methods. 2003;130:9–18. doi: 10.1016/s0165-0270(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Li RW, Ngo C, Levi DM. Relieving the attentional blink in the amblyopic brain with video games. Science Report. 2015;5:8483. doi: 10.1038/srep08483. http://dx.doi.org/10.1038/srep08483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biology. 2011;9:e1001135. doi: 10.1371/journal.pbio.1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Polat U, Makous W, Bavelier D. Enhancing the contrast sensitivity function through action video game training. Nature Neuroscience. 2009;12:549–551. doi: 10.1038/nn.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Polat U, Scalzo F, Bavelier D. Reducing backward masking through action game training. Journal of Vision. 2010:10. doi: 10.1167/10.14.33. [DOI] [PubMed] [Google Scholar]
- Li J, Thompson B, Deng D, Chan LYL, Yu M, Hess RF. Dichoptic training enables the adult amblyopic brain to learn. Current Biology CB. 2013;23:R308–R309. doi: 10.1016/j.cub.2013.01.059. [DOI] [PubMed] [Google Scholar]
- Li RW, Young KG, Hoenig P, Levi DM. Perceptual learning improves visual performance in juvenile amblyopia. Investigative Ophthalmology & Visual Science. 2005;46:3161–3168. doi: 10.1167/iovs.05-0286. [DOI] [PubMed] [Google Scholar]
- Maehara G, Thompson B, Mansouri B, Farivar R, Hess RF. The perceptual consequences of interocular suppression in amblyopia. Investigative Ophthalmology & Visual Science. 2011;52:9011–9017. doi: 10.1167/iovs.11-7748. [DOI] [PubMed] [Google Scholar]
- Mansouri B, Thompson B, Hess RF. Measurement of suprathreshold binocular interactions in amblyopia. Vision Research. 2008;48:2775–2784. doi: 10.1016/j.visres.2008.09.002. [DOI] [PubMed] [Google Scholar]
- McKean-Cowdin R, Cotter SA, Tarczy-Hornoch K, Wen G, Kim J, Borchert M, Varma R Multi-Ethnic Pediatric Eye Disease Study Group. Prevalence of amblyopia or strabismus in asian and non-Hispanic white preschool children: Multi-ethnic pediatric eye disease study. Ophthalmology. 2013;120:2117–2124. doi: 10.1016/j.ophtha.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. Journal of Vision. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- Multi-Ethnic Pediatric Eye Disease Study (MEPEDS) Group. Prevalence and causes of visual impairment in African–American and Hispanic preschool children: The multi-ethnic pediatric eye disease study. Ophthalmology. 2009;116(1990–2000):e1. doi: 10.1016/j.ophtha.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U. Restoration of underdeveloped cortical functions: Evidence from treatment of adult amblyopia. Restorative Neurology and Neuroscience. 2008;26:413–424. [PubMed] [Google Scholar]
- Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proceedings of the National academy of Sciences of the United States of America. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi D. Perceptual learning in vision research. Vision Research. 2011;51:1552–1566. doi: 10.1016/j.visres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Scheiman MM, Hertle RW, Beck RW, Edwards AR, Birch E, Cotter SA, et al. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Archives of Ophthalmology. 2005;123:437–447. doi: 10.1001/archopht.123.4.437. [DOI] [PubMed] [Google Scholar]
- Sen DK. Results of treatment of anisohypermetropic amblyopia without strabismus. British Journal of Ophthalmology. 1982;66:680–684. doi: 10.1136/bjo.66.10.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengpiel F, Blakemore C. The neural basis of suppression and amblyopia in strabismus. Eye (London, England) 1996;10(Pt 2):250–258. doi: 10.1038/eye.1996.54. [DOI] [PubMed] [Google Scholar]
- Simons K. A comparison of the Frisby, Random-Dot E, TNO, and Randot circles stereotests in screening and office use. Archives of Ophthalmology. 1981;99:446–452. doi: 10.1001/archopht.1981.03930010448011. [DOI] [PubMed] [Google Scholar]
- Spiegel DP, Li J, Hess RF, Byblow WD, Deng D, Yu M, et al. Transcranial direct current stimulation enhances recovery of stereopsis in adults with amblyopia. Neurotherapy Journal of the American Society for Experimental Neurotherapeutics. 2013;10:831–839. doi: 10.1007/s13311-013-0200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Stephens DA, Fielder AR, Moseley MJ, Cooperative MOTAS. Modeling dose–response in amblyopia: Toward a child-specific treatment plan. Investigative Ophthalmology & Visual Science. 2007;48:2589–2594. doi: 10.1167/iovs.05-1243. [DOI] [PubMed] [Google Scholar]
- To L, Thompson B, Blum JR, Maehara G, Hess RF, Cooperstock JR. A game platform for treatment of amblyopia. IEEE Transactions on Neural Systems and Rehabilitation Engineering: A Publication of the IEEE Engineering in Medicine and Biology Society. 2011;19:280–289. doi: 10.1109/TNSRE.2011.2115255. [DOI] [PubMed] [Google Scholar]
- Vedamurthy I, Nahum M, Bavelier D, Levi DM. Mechanisms of recovery of visual function in adult amblyopia through a tailored action video game. Science Reports. 2015;5:8482. doi: 10.1038/srep08482. http://dx.doi.org/10.1038/srep08482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Noorden GK. New clinical aspects of stimulus deprivation amblyopia. American Journal of Ophthalmology. 1981;92:416–421. doi: 10.1016/0002-9394(81)90534-1. [DOI] [PubMed] [Google Scholar]
- Wallace DK, Lazar EL, Melia M, Birch EE, Holmes JM, Hopkins KB, et al. Stereoacuity in children with anisometropic amblyopia. Journal of AAPOS: the Official Publication of the American Association for Pediatric Ophthalmology and Strabismus/American Association for Pediatric Ophthalmology and Strabismus. 2011;15:455–461. doi: 10.1016/j.jaapos.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick B, Wingard M, Cotter S, Scheiman M. Anisometropic amblyopia: Is the patient ever too old to treat? Optometry and Vision Science. 1992;69(11):866–878. doi: 10.1097/00006324-199211000-00006. [DOI] [PubMed] [Google Scholar]
- Williams C, Northstone K, Howard M, Harvey I, Harrad RA, Sparrow JM. Prevalence and risk factors for common vision problems in children: Data from the ALSPAC study. British Journal of Ophthalmology. 2008;92:959–964. doi: 10.1136/bjo.2007.134700. [DOI] [PubMed] [Google Scholar]
- Worth A, Chevasse FB. Squint: Its causes, pathology and treatment. Philadelphia, PA: Blakiston; 1950. [Google Scholar]
- Wu C, Hunter DG. Amblyopia: Diagnostic and therapeutic options. American Journal of Ophthalmology. 2006;141:175–184. doi: 10.1016/j.ajo.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Cong LJ, Klein SA, Levi DM, Yu C. Perceptual learning improves adult amblyopic vision through rule-based cognitive compensation. Investigative Ophthalmology & Visual Science. 2014;55:2020–2030. doi: 10.1167/iovs.13-13739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yang X, Liao M, Zhang N, Liu L. Internet-based perceptual learning in treating amblyopia. European Journal of Ophthalmology. 2013;23:539–545. doi: 10.5301/ejo.5000269. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang C, Xu P, Tao L, Qiu Z, Li X, et al. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Research. 2006;46:739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.