SUMMARY
Mutations in the Pax6 gene cause ocular defects in both vertebrate and invertebrate animal species, and the disease aniridia in humans. Despite extensive experimentation on this gene in multiple species, including humans, we still do not understand the earliest effects on development mediated by this gene. This prompted us to develop pax6 mutant lines in Xenopus tropicalis taking advantage of the utility of the Xenopus system for examining early development and in addition to establish a model for studying the human disease aniridia in an accessible lower vertebrate. We have generated mutants in pax6 by using Transcription Activator-Like Effector Nuclease (TALEN) constructs for gene editing in X. tropicalis. Embryos with putative null mutations show severe eye abnormalities and changes in brain development, as assessed by changes in morphology and gene expression. One gene that we found is downregulated very early in development in these pax6 mutants is myc, a gene involved in pluripotency and progenitor cell maintenance and likely a mediator of some key pax6 functions in the embryo. Changes in gene expression in the developing brain and pancreas reflect other important functions of pax6 during development. In mutations with partial loss of pax6 function eye development is initially relatively normal but froglets show an underdeveloped iris, similar to the classic phenotype (aniridia) seen in human patients with PAX6 mutations. Other eye abnormalities observed in these froglets, including cataracts and corneal defects, are also common in human aniridia. The frog model thus allows us to examine the earliest deficits in eye formation as a result of pax6 lesions, and provides a useful model for understanding the developmental basis for the aniridia phenotype seen in humans.
Keywords: Xenopus tropicalis, Transcription Activator-Like Effector Nuclease (TALEN), lens, retina, neural patterning
INTRODUCTION
The transcription factor Pax6 plays an essential role in a highly conserved network of genes controlling eye development in both invertebrates and vertebrates (Callaerts et al., 1997; Gehring and Ikeo, 1999). Pax6 also plays critical roles outside of the eye, in brain and spinal cord development (Manuel and Price, 2005; Stoykova et al., 1996) as well as in development of the pancreas and intestinal enteroendocrine cells (Hill et al., 1999; St-Onge et al., 1997) and is active throughout development in different phases of organogenesis and into adult life (Osumi et al., 2008). Homozgyous mutations in animals ranging from Drosophila (Sturtevant, 1951) to mammals (Hogan et al., 1986; Roberts, 1967) result in eyelessness and phenotypes in other sites of activity as well. In humans heterozygotes develop the syndrome aniridia, so named because patients have no iris (or a poorly formed iris), though the defect is far more pervasive in the eye, leading to defects in the cornea, lens and retina that can result in severe visual deficits that typically increase with age (Hingorani et al., 2012). In this study we use gene-editing tools to generate mutations in the amphibian Xenopus tropicalis to study the role of pax6 both in development and disease.
Study of the function of pax6 in the amphibian X. tropicalis offers the prospect of clarifying its activity at key early stages. In addition, as a diploid Xenopus species it is well suited to genetic studies to examine function, and also provides the many overall advantages of the Xenopus system (e.g. ready access to large numbers of embryos, suitability for surgical manipulations) for examining early developmental phenomena (Harland and Grainger, 2011).
Mutations in Pax6 have been discovered or induced in a number of model organisms, providing insight into the function of this gene from a number of perspectives. The Small eye (Sey) mutant in the mouse is semi-dominant, resulting in heterozygotes with eye deficits (Hogan et al., 1988; Hogan et al., 1986), including an aniridia-like phenotype similar to that seen in humans, though in the mouse the phenotype includes a small eye, which is not reported in human patients (Kokotas and Petersen, 2010). Rat Small-eye mutations (rSey and rSey2) cause a similar phenotype (Matsuo et al., 1993; Osumi et al., 1997). Both mouse and rat Small eye mutations result in premature stop codons. Other missense mutations in the mouse are hypomorphs with varying degrees of different eye abnormalities (Favor et al., 2008; Favor et al., 2001). Homozgyous null mutations are lethal in mice, with severe brain and olfactory deficits (Hogan et al., 1986), and in the rare cases of compound heterozygotes in humans most individuals die perinatally (Glaser et al., 1994).
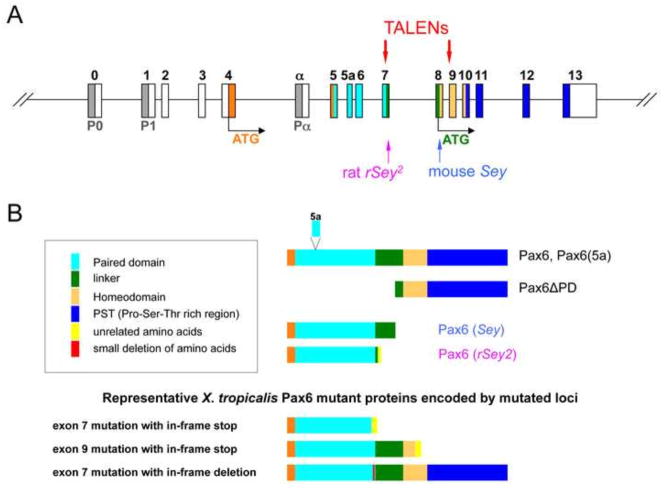
The Pax6 gene has a complex structure and regulation (Shaham et al., 2012) that reflect the many functions of this gene and which are relevant to the mutations discussed in this study. The gene has 16 exons, and three promoters drive its expression yielding several transcripts. Transcription is further controlled by a large number of regulatory elements that reside both within the gene and at significant distances 5′ and 3′ of the coding region. Details relevant to this paper are described in Fig. 1 and discussed in the Results section. This transcription factor has the characteristic structural features of many genes in the Pax family, including the bi-partite DNA-binding paired domain toward the 5′ end of the gene, found in all members of this gene family. The paired domain is connected to a glycine-rich linker bridging it to a paired-like homeodomain. At the C-terminal end of the protein is a proline-serine-threonine (PST) domain that is essential for the transactivation properties of Pax6. The mouse Pax6Sey mutation described above is due to a nonsense mutation upstream of the 5′ end of the homeodomain and is presumed to be a null mutation based on the fact that it is allelic to Dickie’s Small eye (SeyDey), which deletes the Pax6 gene (Hogan et al., 1988) and generates an identical phenotype to SeyDey in homozygotes. Similarly it is presumed that in human aniridia the PAX6 gene is likewise generally a null since it presents with the same aniridia phenotype in WAGR (Wilms tumor, aniridia, genitourinary abnormalities, mental retardation) syndrome that occurs because of a chromosomal deletion that includes the PAX6 gene (Prosser and van Heyningen, 1998).
Fig. 1.
Strategy for targeted mutagenesis of X. tropicalis pax6 locus. (A) Structure of pax6 gene is shown schematically (not to scale). P0, P1, Pα are promoters (shaded in grey). Exons (boxes) are numbered or named on top (0 to 13 or α) and color coded: white, untranslated regions (UTRs); orange, N-terminal region before the paired domain; other colors correspond to color codes for protein domains shown in (B). Two translation start sites are shown (ATG): orange, for full-length isoforms (Pax6 with or without 5a); green, for paired-less isoform (Pax6ΔPD). Positions of mouse Sey and rat rSey2 mutations are indicated by arrows on the bottom. Two pairs of TALEN targets in exon 7 and exon 9 are shown on top. (B) Structures of Pax6 wild-type and representative mutant proteins are shown schematically (not to scale). Color codes of protein domains are shown in the box except the first three amino acids, which are shown in orange. Note that the paired domain is located between amino acids 4–131 and the homeodomain between amino acids 212–272 in X. tropicalis due to insertion of two amino acids between the two domains. The amino acid sequence of each domain is as shown in Walther and Gruss (1991). Most but not all mutations have some non-Pax6 amino acids at the C-terminus. Note that X. tropicalis mutations in exon 7 could theoretically encode Pax6ΔPD if mutations were not simultaneously introduced in exon 9 (not shown in the drawing).
Some embryonic gene targets of Pax6 that may result in the numerous phenotypes described above have been studied but significant questions remain, particularly about the earliest changes in targets associated with Pax6 mutations, but also those associated with later events, including brain development and later eye defects. In Xenopus, pax6 is activated at the late gastrula stage in the presumptive anterior neural plate and is continuously expressed thereafter (Hirsch and Harris, 1997), a pattern seen in mammals as well. In the mouse, following neurulation, a member of the Necab gene family (N-terminal EF-hand calcium binding proteins) is downregulated in the Sey mutant but a number of key regulatory genes important in retina and forebrain formation that have been examined at this stage of development (e.g. the homeobox genes Six3, Otx2, Rax and Lhx2) were not found to be immediate targets of Pax6 (Bernier et al., 2001). Nuclear protein gene Mab21l1 (Yamada et al., 2003) and the secreted frizzled-related protein gene Sfrp2 (Wawersik et al., 1999) are also downregulated in the Sey mutant. In a zebrafish study of pax6 targets (Coutinho et al., 2011) a number of genes are downregulated upon morpholino antisense oligonucleotide treatment of embryos and studies on specific differentiated tissues in the mouse, e.g. the lens, provide a more global analysis of later Pax6 targets (Wolf et al., 2009). However there remains very little information about the changes in gene expression, and their effects on development, that accompany the period just after Pax6 activation at the end of gastrulation and during early neurula stages when important elements in patterning of the retina and neural plate are being established (Saha and Grainger, 1992).
The rapid progress in gene editing with TALEN and CRISPR constructs in the past few years (Peng et al., 2014) has allowed rapid genetic manipulations in Xenopus as in many other organisms. Both TALENs (Ishibashi et al., 2012; Lei et al., 2012; Nakajima et al., 2013; Nakajima and Yaoita, 2013) and CRISPRs (Blitz et al., 2013; Guo et al., 2014; Nakayama et al., 2013) have generated mutant phenotypes in a number of genes and genetically modified lines in the case of production of albino animals mutated in the tyrosinase gene. The study described here, in which we target the pax6 gene with TALEN constructs, is the first in depth study in which F1 and/or F2 non-mosaic animals carrying putative mutations in a key developmentally regulated gene have been examined using targeted gene editing in Xenopus. A previous study described the F0 mosaic phenotype in Xenopus laevis embryos where the pax6 gene was targeted (Suzuki et al., 2013).
In this study we find that gene targeting of pax6 results in a highly consistent, putative null phenotype in F1 and F2 homozygote and/or compound heterozygote embryos: a severely reduced eye which fails to form a lens. The consistent mutant platform permits us to begin to examine gene expression changes that accompany the initial phases of retina and brain formation and provides a powerful starting point for elaborating the initial functions of the pax6 gene during development. An allele was also identified that appears to reduce but not inactivate pax6 activity, revealing a hypomorphic phenotype in which early development appears normal but froglets exhibit eye abnormalities very similar to those seen in aniridia patients, providing a highly accessible model system for studying the ontogeny leading to the aniridia syndrome and for potentially mitigating the disease phenotype.
MATERIALS AND METHODS
Construction of TALENs to target the pax6 gene
TALEN scaffolds with the obligate heterodimeric FokI (ELD/KKR) were obtained from Christopher H. K. Cheng (pCS2+TALEN-ELD/KKR; (Lei et al., 2012). For an experimental control, we used pCS+TALEN-ELD/KKR-Tyr I (to target the tyrosinase gene), which was described previously (Nakajima and Yaoita, 2013), hereafter referred to as pCS-ELD/KKR-Tyr I for simplicity. To target exon 7 and exon 9 of the pax6 gene, we chose 5′-GTCATCAATAAACCGAGTGCTGCGCAACCTGGCGAGCGAAAAGCA-3′ for exon 7 and 5′-TTGAACGAACACATTACCCCGACGTGTTTGCCAGGGAAAGATTAGCTGCC-3′ for exon 9, where underlined sequences are left targets and double-underlined sequences are right targets (in antisense-strands). The DNA binding domains to recognize these targets were assembled as previously described (Cermak et al., 2011) with minor modifications as reported (Nakajima et al., 2013). TALENs recognizing left targets were constructed in pCS+TALEN-ELD to yield pCS+TALEN-ELD-pax6 E7 (for exon 7) or E9 (for exon 9), and pCS+TALEN-KKR-pax6 E7 or E9 were similarly constructed for right targets. Hereafter these are referred to as pCS-ELD/KKR-pax6 E7/E9 for simplicity. The EMBOSS program fuzznuc (Rice et al., 2000) was used to search for possible off-target sites of these TALENs based on the X. tropicalis genome sequence Assembly v4.1 (Hellsten et al., 2010). Since the repeat variable di-residue NN for recognizing the nucleotide G could also bind to the nucleotide A, R (i.e., G or A) was used in the off-target search of left targets and Y (i.e., C or T) in the right targets search. Thus the off-target sites of pCS-ELD/KKR-pax6 E7 used both the sense ‘RTCATCAATAAACCRN(10,30)GGYGAGYGAAAAGYA’ and the antisense sequence ‘TRCTTTTCRCTCRCCN(10,30)YGGTTTATTGATGAY’, ELD/KKR-pax6 E9 used ‘TTRAACRAACACATTN(10,30)YYAGGGAAAGATTAGYTGYY’ and ‘RRCARCTAATCTTTCCCTRRN(10,30)AATGTGTTYGTTYAA’, and pCS-ELD/KKR-Tyr I used ‘TTCTTCTTCCTCCATRTN(10,30)YYYAAGGGYATGTAGYA’ and ‘TRCTACATRCCCTTRRRN(10,30)AYATGGAGGAAGAAGAA’. Since TALENs from pCS-ELD-pax6 E7 and pCS-KKR-pax6 E9 or pCS-ELD-pax6 E9 and pCS-KKR-pax6 E7 could make a heterodimer when 4 TALEN mRNAs are co-injected, we also tested combinations of the exon 7 left target and the exon 9 right target, ‘RTCATCAATAAACCRN(10,30)YYAGGGAAAGATTAGYTGYY’ and ‘RRCARCTAATCTTTCCCTRRN(10,30)YGGTTTATTGATGAY’, as well as the exon 9 left target and the exon 7 right target, ‘TTRAACRAACACATTN(10,30)GGYGAGYGAAAAGYA’ and ‘TRCTTTTCRCTCRCCN(10,30)AATGTGTTYGTTYAA’. We saw a few theoretically possible off-target sites with 10 to 30 spacer nucleotides having three or more mismatched nucleotides, but no complete matches to off-target sites were found in any of the combinations examined.
Xenopus tropicalis, synthesis of TALEN mRNAs and microinjection
We used in house breeding stocks of Xenopus tropicalis, which are not highly inbred but originated from the same ancestors of the Nigerian inbred line that was used for genome sequencing (Hellsten et al., 2010). X. tropicalis eggs were fertilized in vitro, dejellied, and injections made into one cell stage embryos within 40 min after fertilization as in earlier studies (Ogino et al., 2006). Embryos were raised to adults as described previously (Hirsch et al., 2002).
The capped mRNAs encoding each TALEN that were injected into embryos were synthesized from pCS-ELD/KKR-Tyr I and pCS-ELD/KKR-pax6 E7/E9 using the mMESSAGE mMACHINE® SP6 Kit (Invitrogen). A pair of each 100 pg or 200 pg of TALEN mRNAs for left and right targets was mixed for injection. As for dual targeting exon 7 and exon 9 simultaneously, two pairs of TALENs (each 100 or 200 pg) were mixed and injected.
Genotyping of pax6 and rax mutant and wild-type embryos
Since we can easily distinguish between pax6 “null” mutant and wild-type phenotypes at tadpole stages due to different morphologies of the eye region, we do not necessarily perform genotyping on embryos older than st.35 [all stages according to Nieuwkoop and Faber (1994)]. In this case, the genotype of embryos with a wild-type appearance is designated as (+/?). All embryos shown at earlier stages were genotyped. Embryos were lysed as described (Fish et al., 2012), followed by genomic PCR of the pax6 region (PCR primers are listed in Fig. 3). The PCR amplicon was purified and sequenced, or as needed, re-cloned and sequenced. In most experiments, phenotypes are indistinguishable between +/+ and +/−, and thus one representative embryo of either genotype has been chosen as a “wild-type” exemplar to image and labeled as (+/?) unless otherwise described. Genotyping of rax mutant and wild-type embryos was performed as described (Fish et al., 2014).
Fig. 3.
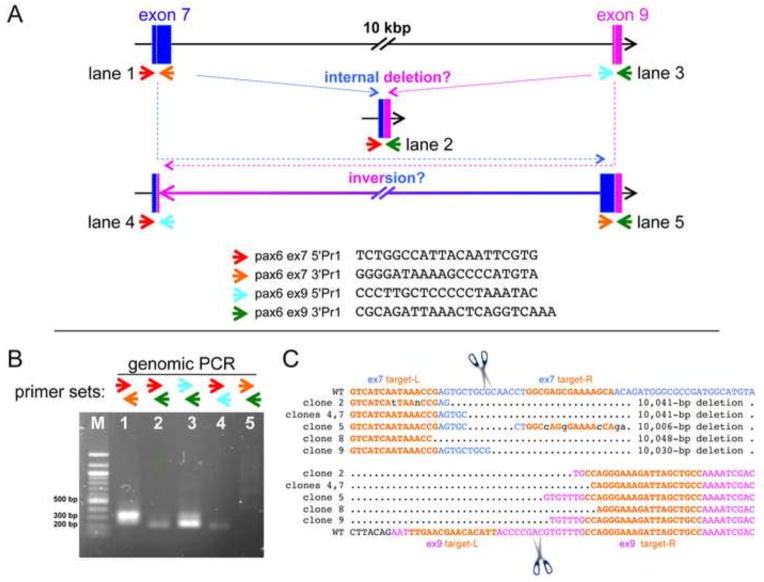
Strategy for screening mutations in the pax6 locus edited by two pairs of TALENs targeting exon 7 and exon 9 simultaneously. (A) Schematic drawing of targeting regions, only showing exon 7 (blue) and exon 9 (magenta) for simplicity (not to scale). Small colored arrows indicate different primers for genomic PCR of exon 7 and exon 9 (each sequence is shown on the bottom, from 5′ to 3′). Different combinations of primers can amplify other possible junction regions created by deletion or inversion between exons 7 and 9 as schematically shown. (B) A representative gel pattern of genomic-PCR bands from an embryo injected with both pairs of TALENs mRNAs. In this specific case, one of the possible junctions representing a 3′ inversion site (where a 3′ piece of exon 7 would have been fused inverted to 3′ piece of exon 9, lane 5) was not detected, but in other cases, we have seen such a band. (C) The genomic-PCR band corresponding to what is shown in lane 2 in (B) from 3 pooled embryos was gel-purified, cloned, and sequenced. The results of 6 clones are aligned. Only regions between two target sites (target-Ls, left target; target-Rs, right target in orange) are shown. Corresponding wild-type sequences are shown on top for exon 7 and on the bottom for exon 9. Multiple 10-kb deletion events were confirmed, and junctions where 5′ parts of exon 7 (blue) fused to 3′ parts of exon 9 (magenta) were seen in the expected cleavage regions (in between left and right targets, indicated by scissors) in both exons 7 and 9, suggesting that 10-kbp deletions were due to cleavages by TALENs and thus both TALENs were functional.
Tissue Processing and Histology
For X. tropicalis studies, to analyze mutant phenotypes, embryos were fixed overnight in Bouin’s fixative and embedded either in JB-4 Plus® resin (Polysciences) or in Paraplast® Plus. Plastic embedded tissue was sectioned at 3 μm and stained with toluidine blue. Paraffin embedded tissue was sectioned at 10 μm and stained with hematoxylin. For assessing expression domains of certain transcription factors following in situ hybridization, some embryos were supported in a clay dish and sliced with a small scalpel.
For preparation of human corneal samples, tissues were placed in 4% paraformaldehyde and stored at 4°C. For mouse samples, immediately after euthanization (CO2 and cervical dislocation), both eyes from mice were enucleated and immersed in Davidson’s fixative (Poly Scientific R & D Corp. Bay Shore, NY) at room temperature for 24 hr. The eyes were then rinsed briefly in water and then transferred into 10% neutral buffered formalin for storage. Mouse globes were measured (Castroviejo calipers) at the horizontal equator using 2.5 × magnification. Both mouse globes and human corneal tissues were hand-processed and embedded in paraffin wax according to the following protocol: 70% ethanol for 30 min, 3 × 100% ethanol for 30 min, 2 × xylene for 30 min, 3 × 100% wax (Surgipath Paraplast, Leica) for 30 min at 62°C. For mouse eyes, 7 μm sagittal sections one quarter of the way into the globe based on prior measurements were made using a rotary microtome; sections from human corneas were cut at 10 μm. Sections were captured on SuperFrost Ultra Plus slides. For routine microscopy, sections were then deparaffinized in two changes of xylene, rehydrated in graded ethanol (100%, 100%, 95%, 95%, and 70%), rinsed in water, stained with 10 minutes each of Gill II Hematoxylin (Surgipath™, Richmond, Il.) and Eosin (Surgipath™, Richmond, Il.), followed by a dehydration in graded ethanol (70%, 95%, 95%, 100%, 100%) and then two changes of xylene. Upon completion of staining, a coverslip was applied with Cytoseal 60 (Richard-Allan Scientific).
In situ hybridization
Whole-mount in situ hybridization was carried out essentially according to the procedure in (Sive et al., 2000), though modified when genotyping was required, omitting acetic anhydride and post-color fixation steps as described (Fish et al., 2014). A minimum of 3 (up to 5) mutant embryos were examined by in situ hybridization for alteration of expression of downstream genes, showing highly consistent results. All antisense probes were labeled with digoxigenin (Roche) and detected by BCIP/NBT (Roche) or BM Purple (Roche). Probes for myc and pax6 were prepared as described previously (Fish et al., 2014). mab21l1 cDNA in pCS107 vector was obtained from The Wellcome Trust Sanger Institute and digested with EcoRI and used as template for T7 in vitro transcription to make the probe. Probes for ins, gsx2, and neurog2 were made from templates created by genomic PCR as described (Fish et al., 2014). Primers used for these probes are as follows (5′ to 3′ direction, underlined nucleotides indicate T7 sequences):
ins 5′ primer, CCAGGACAACGAGTTGGATG
ins 3′ primer, TAATACGACTCACTATAGGGAGAAGCTCTTCAGCTTACATTTATTTGC
gsx2 5′ primer, CTGCGGCAATGTCTAGGTCT
gsx2 3′ primer, TAATACGACTCACTATAGGGAGATCATGCAATGGAATCTCCTG
neurog2 5′ primer, ACTGCCATCATTACCCGAAG
neurog2 3′ primer, TAATACGACTCACTATAGGGAGAGCACAGAGAAAGGCAACACA
Frogs
The frogs used for this study were from our colony maintained at the University of Virginia. All procedures involving frogs were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory animals and approved by the University of Virginia Animal Care and Use Committee.
Mice
The mice used for this study were maintained as a Pax6Sey-Neu/+ colony on a majority CD1 genetic background with enough C57BL6/J to provide iris pigmentation. Wild-type (Pax6+/+) littermates were used as controls. The genotype of each animal was determined by PCR as previously described (Kim and Lauderdale, 2006). All experiments involving mice were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were performed with approval and oversight of the University of Georgia Institutional Animal Care and Use Committee.
Human tissues
Cadaveric PAX6-normal human corneoscleral tissues derived from consented donors according to Institutional Review Board (IRB)-approved protocols were obtained from the Georgia Eye Bank. Aniridia tissue derived from consented donors according to Institutional Review Board (IRB)-approved protocols were obtained from Dr. John M. Freeman (Memphis Eye and Cataract Associates, Memphis, TN); tissue was obtained as byproduct of scheduled procedures.
RESULTS
Strategy for targeted mutagenesis of Xenopus tropicalis pax6 gene and resultant mutations
As discussed earlier, the pax6 gene locus has a complex structure (Fig. 1A), i.e., there are three promoters (P0, P1 and Pα) and alternative splicing events to create different mRNAs, some of which encode the same protein(s), and others which can create different protein(s). Translation is initiated at the ATG in exon 4, to yield a full-length protein, or at the ATG in exon 8 to yield a paired-less protein (Kim and Lauderdale, 2008). Although to date only one cDNA for X. tropicalis is reported (i.e., NM_001006762) which lacks the exon 5a-coding amino acids (used in one alternative spliced form; illustrated in Fig. 1B) and encodes a 424 amino-acid protein, there are EST clones containing exon 5a (e.g., CR438572). In a previous study we showed evidence that promoter α appears to be functional in X. tropicalis (Fish et al., 2012), suggesting that in X. tropicalis there must also be multiple versions of Pax6 mRNAs and proteins being expressed. Mouse and rat mutations are thought to be null mutations. The mouse Pax6 mutant Sey has a substitution in exon 8 that creates a premature stop codon (Hill et al., 1991), and rat rSey2 has an insertion in exon 7 (Osumi et al., 1997) to create (downstream) an in-frame stop codon (Fig. 1B). In order to create similar null mutations in X. tropicalis and knock out the function of proteins resulting from both start sites the following strategy was undertaken: to block or inactivate proteins from the first ATG (Fig. 1A), we designed a target in exon 7 using TALENs as shown schematically in Fig. 1, and to block all protein forms, including the paired-less protein (Pax6ΔPD) derived from the second ATG, we also targeted exon 9.
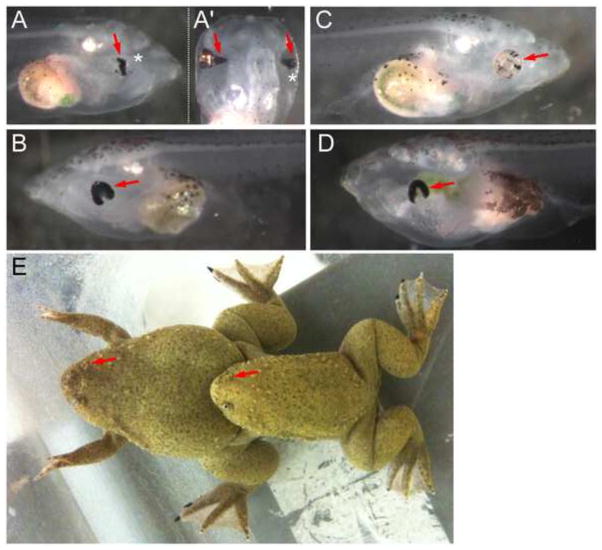
We injected mRNAs for a pair of TALENs to target exon 7 and a pair of TALENs to target exon 9 individually or simultaneously. We also tested a pair of TALENs to target tyrosinase (tyr) (Nakajima and Yaoita, 2013) as a positive control for the experimental procedure as well as a negative control for morphological phenotypes that may be caused by the injection of any TALENs. Since our ultimate purpose was to establish mutant lines, we did not necessarily score F0 embryos systematically. However, at both doses we tested (100 pg or 200 pg of each TALEN mRNA), we observed morphologically abnormal eyes in pax6 TALENs-injected embryos (Fig. 2). The most frequent eye phenotype seen at tadpole stage is a horseshoe-shaped retina as seen from a lateral view, i.e., the ventral part of the eye is not formed (Fig. 2B, D). Although F0 animals sometimes had more severely degenerated eyes (Fig. 2A, lateral view), this degree of abnormality was not seen in F1 non-mosaic mutant animals (F1 mutants will be described in more detail later). Another interesting feature of mutants apparent when viewing animals from the dorsal aspect was a variable disturbance of the transformation of the initially broad connection between the brain and optic vesicle/cup, i.e. the optic stalk, into the optic nerve. In some, but not all, phenotypic F0 animals the optic stalk would persist in the form of a broad, cone-shaped extension of the retinal tissue spanning part (Fig. 2A′, left eye), or all (see example in Fig. 5 for F1 animals), of the distance between the eye and the brain. By contrast, tyr TALENs-injected embryos had eyes with variably reduced pigmentation which were otherwise morphologically normal as expected (Fig. 2C).
Fig. 2.
Representative phenotypes of F0 embryos injected with TALENs. Lateral (A, B) and dorsal (A′, the same embryo shown in A) views of two embryos injected with a pair of TALENs targeting pax6 exon 9 (200 pg mRNA each). Asterisks in (A) and (A′) indicate the same abnormal eye. The most frequently seen eye phenotype is shown in (B) (arrow). (C) Lateral view of an embryo injected with a pair of TALENs targeting tyr (200 pg mRNA each). The eye is morphologically normal but less pigmented (arrow). (D) Lateral view of an embryo injected with two pair of TALENs targeting pax6 exons 7 and 9 (200 pg mRNA each). The abnormality here (arrow) is similar to what is shown in (B). (E) Mating of mature frogs raised from embryos injected with two pairs of pax6 TALENs. Eyes are missing in one side of each animal (arrows).
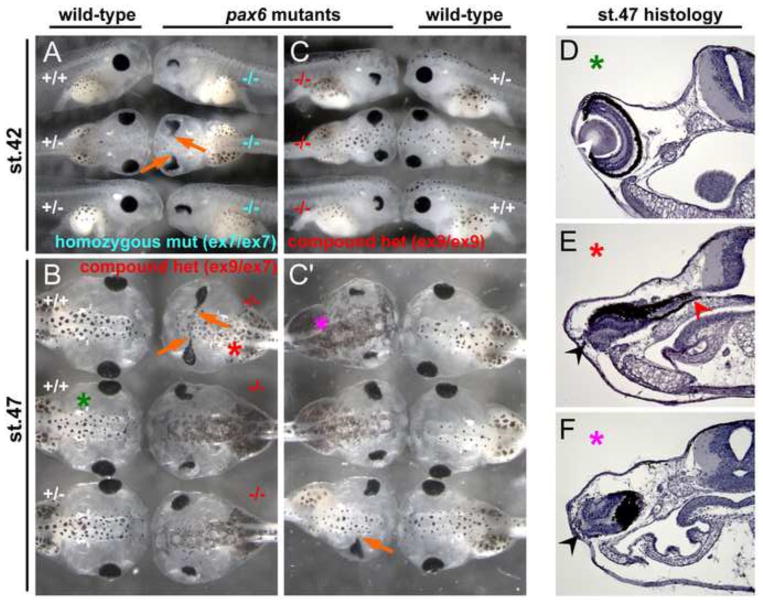
Fig. 5.
Phenotype of Xenopus pax6 mutants at tadpole stages. (A) Phenotype of F2 embryos at stage 42 from an intercross of F1 heterozygotes carrying a 13-bp deletion in exon 7. −/−, homozygous mutant; +/−, heterozygous; +/+, wild-type. These are siblings of embryos shown in Fig. 4B. (B) Phenotype of F2 embryos at stage 47 from cross between an F1 female carrying a 1-bp deletion (#2) in exon 9 and an F1 male carrying a 13-bp deletion in exon 7. −/−, compound heterozygous mutant (genotype is ex9 Δ1-bp#2/ex7 Δ13-bp); +/−, heterozygous (genotype, +/ex7 Δ13-bp); +/+, wild-type. These are siblings of embryos shown in Fig. 4D. (C, C′) Phenotype of F2 embryos at stage 42 (C) and stage 47 (C′) from mating of an F1 female carrying a 1-bp deletion (#2) in exon 9 and F1 male carrying a 1-bp deletion (#3) in exon 9. Embryos shown in C (mixture of lateral and dorsal views) are the same embryos as shown in C′ (all dorsal views) at the same axial position, the only difference being their ages. −/−, compound heterozygous mutant (genotype is ex9 Δ1-bp#2/ex9 Δ1-bp#3); +/−, heterozygous wild-type (genotype, ex9 Δ1-bp#2/+ for top embryo, +/ex9 Δ1-bp#3 for second embryo); +/+, homozygous wild-type. These are siblings of embryos shown in Fig. 4C. Orange arrows show the eye fused to the brain, which is often, but not always, seen in pax6 mutants regardless of genotype. (D–F) Cross sections of the eye region of embryos shown in (B) and (C′). Corresponding embryo and section are marked by the same colored asterisks. Wild-type embryos (D) have well organized retina and lens (white arrowhead), whereas mutants (E, F) do not have lens and the retina is disorganized (black arrowheads). By this stage, mutant eyes were separated from the brain in some embryos (F) or still connected to the brain in other embryos (E, red arrowhead).
It is important to note that the dual targeting of exons 7 and 9 simultaneously can, in addition to creating deletions within exon7 or exon 9, create 10-kbp inversions or deletions between exons 7 and 9. This was demonstrated by a genomic PCR assay using different combinations of primer sets to amplify each exon, making it possible to detect deletions spanning exon 7 and 9 and inversions of the region between exon 7 and 9 (see Fig. 3A, B). We further focused on examining the 10-kbp deletion event by cloning and subsequent sequencing of genomic-PCR fragments to verify the existence of this large deletion, demonstrating that both pairs of TALENs had indeed cut simultaneously resulting in the large deletion (Fig. 3C). Although we did not sequence other PCR fragments individually from other F0 embryos, this result suggested that small indels in exon 7 and/or exon 9, and 10-kb inversions were likely occurring in many mosaic F0 embryos injected with both pairs of TALENs, which we confirmed in F1 animals (see below). Embryos (injected with both exons 7/9 TALENs together and exon 9 TALENs alone) that had abnormal eyes but which were otherwise normal in appearance were sorted and raised to adults (Fig. 2E) to establish F0 populations. We hypothesized that animals with an abnormal or missing eye would likely be mosaics with a high level of mutations in part of the animal but which were viable because other regions were either wild-type or had non-lethal (heterozygous) mutations. The stock 716 population (st.716) is comprised of F0 frogs targeting both exons 7 and 9, and the st.718 population targets exon 9 alone.
Germline transmission of mutations and establishment of lines carrying pax6 gene mutations
Germline transmission of mutations in mosaic F0 frogs was evaluated by generating F1 animals from matings of F0 adults (as illustrated in Fig. 2E). In crosses of these F0 frogs we observed mutant phenotypes in the resulting compound heterozygote F1 animals, as described in more detail below, similar to what we saw in F0 animals, verifying germline transmission of the mutations. We then systematically outcrossed F0 frogs with wild-type frogs to assay for germline transmission frequencies and allelic variation. All six of the st.716 frogs tested had germline transmission at various frequencies (notably, male frog #3 had 100% germline transmission of mutations), whereas only three out of seven st.718 frogs tested had germline transmission. The mutation profiles and their frequencies are summarized in Supplementary data Table S1. To date, we have not yet confirmed germline transmission of 10-kbp deletions between exons 7 and 9, but we have confirmed at least one 10-kbp inversion between exons 7 and 9. Others are small (up to 17-bp deletion) indels created in exon 7 or exon 9 alone, or simultaneously in both exons, which in many cases cause frame-shifts, resulting in premature stop codons. The predicted protein products are schematically shown in Fig. 1B (bottom). As a consequence, such mutations in exon 7 encode proteins truncated after amino acid positions between 125–128 depending on the individual mutation, thus lacking a few amino acids at the end of the paired domain (that is located between amino acid residues 4–131) and the remainder of the coding sequence. Exon 7 mutants could still theoretically encode the Pax6ΔPD isoform if mutations were not simultaneously introduced in exon 9. Out-of-frame mutations in exon 9 encode proteins truncated after amino acid positions between 236–239 depending on the individual mutation, thus lacking amino acids after the middle of the homeodomain (located between amino acid residues 212–272). As we describe below, these mutations seem to behave as null mutations and the proteins, if they are expressed, do not seem to be functional.
In some cases, small indels created in-frame deletions of [3 × n] bp to result in 1–5 amino-acid deletions without changing the remainder of the protein. In case of exon 7, 1 to 5 amino acid deletions are found in the region between 125 and 129 of the paired domain, whereas in exon 9, one case shows a deletion of amino acid 238 in the homeodomain. We do not know at this time if one amino acid deletion due to the in-frame exon 9 mutation has any impact on the mutated protein function. However, we do see an impact of small in-frame deletions in exon 7 on the resultant protein, discussed in detail below.
Several F1outcross animals were raised to adults, establishing F1 heterozygous frogs that have small indels in either exon 7 or 9, or in both, and one F1 heterozygous frog that has a 10-kb inversion between exons 7 and 9. Both F0 and F1 mutant carriers are used in this study. Several F2 lines are now being raised for future study.
Consistent phenotypes are seen in mutants with premature stop codons in exon7, or combinations of mutations in exon 7 and exon 9
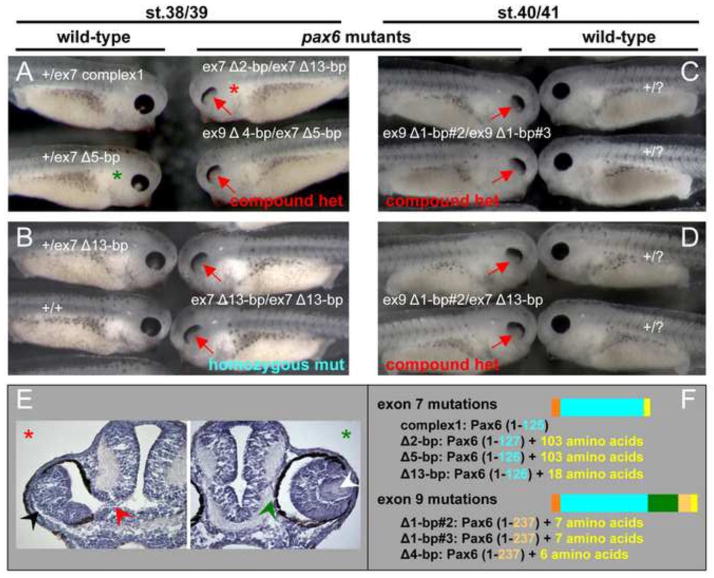
The various induced pax6 mutations leading to frame shifts and resulting in premature stop codons are expected to abrogate gene activity, particularly the exon 7 target site. As expected we find that there is a consistent phenotype in compound heterozygous or homozygous frame shift mutations in exon 7. But in addition we see an identical phenotype in all combinations examined of compound heterozygotes involving exon 7 and exon 9 out-of-frame mutants. Thus we hypothesize that both exon 7 and exon 9 mutations (and exon 7/9 deletions and inversions) yield inactive Pax6 protein. Fig. 4 (stage 38–41) and Fig. 5 (stage 42 and 47) summarize pax6 mutant phenotypes of tadpoles from different matings of F0 and F1 mutant carrier frogs. The features of mutant embryos are consistent in any pax6 mutant regardless of whether they are homozygous mutants that have the 13-bp deletion in exon 7 or compound heterozygous mutants, and regardless of whether mutations are in exon 7 or exon 9.
Fig. 4.
Xenopus pax6 mutants have abnormal eyes without lenses. (A–D) Offspring from crosses between F0 mutation carriers (A) or F1 carriers (B–D) with two mutant alleles, showed a consistent phenotype in the eye (red arrows), regardless of the genotype of mutant alleles. F0 crosses (st.716 female #2 × male #3) created a variety of combinations of genotypes in mutant offspring (A), whereas offspring from F1 crosses (i.e., parents are not mosaic) are about 25% homozygous mutant (B–D). Note that mutants homozygous for a 13-bp deletion in exon 7 (B) are indistinguishable from other compound heterozygous mutants, regardless of the combination of exon 7 mutations (A, top right embryo), combination of exon 7 and exon 9 mutations (A, bottom right embryo and D), or combination of exon 9 mutations (C). Each embryo shown here was genotyped except for phenotypic wild-type embryos in C and D, thus shown as +/?. Judging from the mutation profiles (see Table S1), we could infer the origins of mutated alleles of offspring from F0 crosses, or in the case of F1 crosses, the genotypes of parents are known in advance, thus we show offspring genotypes in order of parental female locus/male locus except for the top wild-type embryo in (B), where the parental origin of alleles is undeterminable. Actual sequences of mutations can be found in Table S1. (E) Cross sections of embryos marked by red (mutant) and green (wild-type) asterisks in (A) to show morphology of the eyes. The wild-type embryo (green asterisk) has a lens (white arrowhead) and the retina is surrounded by retinal pigment epithelial (RPE) and separated from the brain (green arrowhead), whereas the mutant embryo (red asterisk) has no lens and the retina is disorganized (black arrowhead) and still connected to the brain (red arrowhead). (F) Schematic presentation of proteins encoded by the mutations shown here. Also refer to Fig. 1 for color codes. Exon 7 mutations cause truncation at the C-terminus of the paired domain (located between amino acid residues 4–131), whereas exon 9 mutations cause truncation in the middle of the homeodomain (located between amino acid residues 212–272). Truncations in many cases but not all are followed by unrelated amino acids due to the frame-shift. Note that mutant embryos shown in (C) make almost identical mutant proteins with only one amino acid change at position 238, thus this is very close to being a homozygous mutation.
The first sign of a morphological phenotype in the mutant can be detected between late stage 20’s to early stage 30’s, but in some cases at such stages the phenotype is hard to distinguish from normal morphological variation in wild-type embryos (data not shown). The mutant phenotype becomes more obvious when pigmentation of the retinal pigment epithelium (RPE) is completed, i.e., at late stage 30’s to early 40’s. Surprisingly, pax6 mutants in X. tropicalis, unlike Pax6 mutants in mouse Sey (Hill et al., 1991) or rat rSey2 (Osumi et al., 1997) do not lose the eye completely, i.e., there is a persistent eye-like structure with abnormal retina and no lens. As of stage 38–41 (Fig. 4), the mutant eye has a characteristic appearance showing the RPE only in the region corresponding to the anterior-dorsal side of the wild-type eye, and has a highly disorganized retina and no lens as seen in histological sections (Fig. 4E). As development proceeds (Fig. 5), the RPE acquires a flattened or arc shape because morphogenesis of the ventral optic cup fails (Fig. 5A, C, lateral views of mutants and Fig. 5E, F, transverse sections), a feature which was also observed in F0 mosaic embryos (Fig. 2B, D). In some cases, the RPE extends along the persistent optic stalk (Fig. 5A, B, C′, orange arrows) that contains differentiated retina tissue (Fig. 5E, red arrowhead).
Given that the rodent Pax6 mutations that are similarly positioned to our frog mutations result in no or non-functional proteins (Engelkamp et al., 1999; Numayama-Tsuruta et al., 2007), it seems unlikely that the truncated protein(s) in the X. tropicalis mutants, if expressed at all, would be sufficiently functional to account for the incomplete loss of eyes that we see. Rather, there is another possibility that a second pax6-related gene, pax6.2, may have redundant function in Xenopus embryos and may partially rescue pax6 mutations. It was recently discovered that non-mammalian vertebrates all appear to have this second pax6-like gene and that the gene has been lost in birds and mammals (Ravi et al., 2013). X. tropicalis Pax6.2 (XM_004912020) consists of 281 amino acids and has 85% amino-acid identity with the region of amino acids 137–424 of X. tropicalis Pax6. It consists of the homeodomain and PST but no paired domain, as is characteristic of all pax6.2 genes. To assess the possible overlapping role of pax6.2 with pax6, we examined its expression by in situ hybridization (see Fig. S1). At early stages the expression is not detectable by in situ hybridization, but by stage 25 a low level of expression is detected in the dorsal half of the retina, and becomes more obvious at later stages. Expression at later stages is also seen in the notochord (pax6 is not expressed in this tissue), and no expression is detected in the brain (again, unlike pax6). The pax6.2 expression domain in the retina is coincident with the dorsal location where the partial retina is formed in pax6 mutants, consistent with the possibility of involvement of pax6.2 in ameliorating the pax6 mutant phenotype in frog compared to mammals. Although evaluation of function of pax6.2 in pax6 mutants remains to be studied in the future, since all homozygous and compound heterozygous out-of-frame mutants involving exon 7 and 9 deletions show consistent phenotypes, at present we consider those phenotypes to result from in-frame, premature stop codons caused by indel-generated frameshifts (see Table S1) leading to protein products with no activity. These “null” mutant embryos at later stages have some axial defects (data not shown) and eventually die some time after stage 46. In the following analyses of the “null” phenotypes, we do not distinguish different mutated genotypes but simply regard embryos as falling into two classes: 1) frame-shift mutations causing a premature stop codon (and a clear, consistent, embryonic phenotype); or 2) “wild-type” embryos with a normal appearance (but which may carry cryptic mutations).
Expression of genes downstream of pax6 at early and late stages during tadpole development
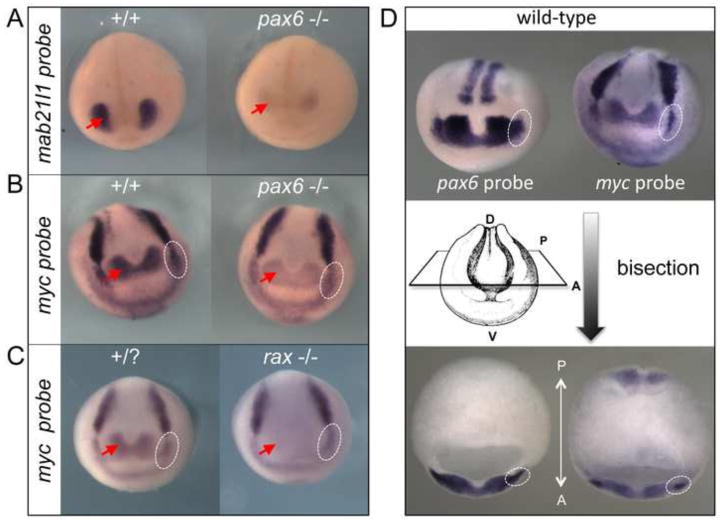
We have examined expression levels of several marker genes that are expressed in the same tissues and organs as pax6 by in situ hybridization. Although more extensive studies will be performed in the future using homozygous mutant lines, in this initial study we focus on only a few genes. Mab21l1 is a known downstream gene of Pax6 and has important roles in eye development (Yamada et al., 2003). As expected and consistent with the report in mouse Pax6 mutant [i.e., Sey/Sey, (Yamada et al., 2003)], we also observed a reduction of expression of mab21l1 in “null” pax6 mutant frog embryos (Fig. 6A). We also tested myc expression that is known to be downstream of the key eye gene rax in the retina region (Fish et al., 2014) as shown in Fig. 6C, but has not been identified as a downstream target of pax6 to our knowledge. We found that myc expression in pax6 “null” mutant is indeed downregulated specifically in the regions where its expression overlaps with pax6 (Fig. 6B), i.e., the retina and brain (red arrows) and presumptive lens ectoderm (PLE) at stage 15 (dotted white circles, Fig. 6D shows both expression of pax6 and myc in the PLE). This suggests that myc is downstream of both rax and pax6, and potentially plays a role in eye and brain development, and also that myc may be involved in lens development under the control of pax6.
Fig. 6.
mab21l1 and myc are downstream of pax6. (A–B) In situ hybridization of wild-type (+/+) and pax6 mutant embryos (−/−, compound heterozygote of complex1 and 10-kbp inversion mutations, see Table S1 for sequence) at st, 21 (A) or st. 15 (B) with mab21l1 (A) or myc (B) probe. Red arrows indicate expression of mab21l1 (A) and myc (B) in the eye region. Expression of both genes is reduced in mutants. (C) In situ hybridization of wild-type (+/?) and rax mutant (−/−, see Fish et al., 2014) embryos with myc probe. Note that myc expression in the presumptive retina region (red arrow) is almost completely lost in the rax mutant as reported previously (Fish et al., 2014), whereas in the pax6 mutant myc expression is consistently reduced, but to a lesser extent as shown in (B). White dotted circles show the presumptive lens ectoderm (PLE) region, in which expression of myc is indistinguishable between wild-type and rax mutant embryos. By contrast, expression of myc in the PLE of pax6 mutant is reduced compared to the wild-type embryo (B, white dotted circles). (D) In situ hybridization of wild-type embryos with pax6 (top left) and myc (top right) probes at stage 15. The same embryos are bisected at the level shown schematically, clearly showing the expression of pax6 and myc in the PLE (bottom, white dotted circles).
While disruption of eye development is the most obvious effect of pax6 mutation in frog embryos/larvae, examination of expression of a few genes involved with pax6 in regulating regional forebrain development show that process to be compromised as well.
Although pax6 function seems to be completely knocked out in our mutants, in situ hybridization reveals that the mRNA continues to be expressed throughout most of the normal expression domains. In the forebrain of late embryonic to larval stages of Xenopus, pax6 is expressed in the pretectum (prosomere 1), dorsal thalamus (prosomere2), prethalamus (prosomere 3) and in the telencephalon in the lateral and ventral pallium as well as in the lateral ganglionic eminence (lge) region of the subpallium and in the olfactory bulbs (Moreno et al., 2008). Interestingly, by stage 38 there is a clear loss of pax6 mRNA from the pretectum and dorsal thalamus of mutants (Fig. 7A) also seen in mouse mutants (Mastick et al., 1997), suggesting that Pax6 protein must be required for continued expression in these regions.
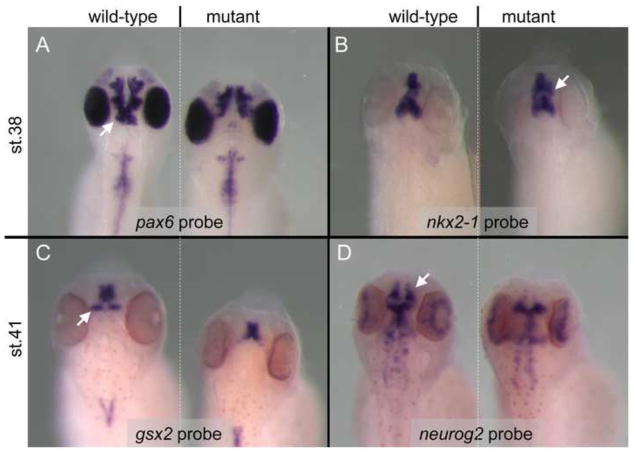
Fig. 7.
pax6 mutants display altered forebrain patterning. In situ hybridization comparison of expression patterns of several genes show altered brain patterns in mutant versus wild-type embryos. pax6 mRNA expression is lost from the pretectum (white arrow) and ventral thalamus of mutants, but appears to be maintained normally in more anterior parts of the forebrain including retina at stage 38 (A). By contrast, as seen in panel (B) the expression domain of nkx2-1 appears to be expanded in the telecephalon (white arrow) of pax6 mutants compared to wild-type. gsx2 expression is lost from the prethalamus (C, white arrow) of the mutant, and neurog2 is greatly reduced in the telencephalon (D, white arrow) of mutant embryos.
To compare the effects of pax6 mutations in our frog model with those described in Pax6 mutants in other organisms, we looked at expression of a small number of genes either known to be Pax6 targets or whose expression domain overlaps with that of Pax6. Nkx2-1 is a homeobox gene that is essential for specification of the basal forebrain structure the medial ganglionic eminence (mge) (Sussel et al., 1999). In Pax6 homozygous mutant mice, Nkx2-1 expression expands laterally into the lge (Stoykova et al., 2000). Similarly, in frog nkx2-1 is expressed in mge as well as the preoptic area and the hypothalamus (Gonzalez et al., 2002), and the mge domain appears to be expanded in our mutants (Fig. 7B).
Gsx2 (previously Gsh2) is another homeobox gene expressed in the developing vertebrate forebrain where, along with Pax6 and Nkx2-1, it is known to play a key role in regional specification. In mice its primarily subpallial expression domain in the telencephalon is largely complementary to the mainly pallial domain of Pax6, but there is some overlap across the pallial/subpallial boundary (Yun et al., 2001). That these two genes act together to establish pallial vs subpallial territories is suggested by the observations that in Pax6 mutant mice part of the ventral pallium is respecified into a structure with characteristics of lge, while in Gsx2 mutant mice part of the lge is respecified into tissue with ventral pallium characteristics (Carney et al., 2009; Yun et al., 2001). Similarly, in Xenopus gsx2 is expressed in the lge and in the prethalamus (Illes et al., 2009). Interestingly, when we looked at stage 41 the telencephalic expression of gsx2 appeared similar in mutant and wild-type, however the prethalamic expression domain was missing in the mutants (Fig. 7C).
Lastly, we looked at expression of neurog2, another gene expressed during forebrain development and shown to be a direct target of pax6 regulation (Holm et al., 2007; Scardigli et al., 2003). It is co-expressed in developing cerebral cortex (pallium) in mice, and expression is reduced in Pax6 homozygous mutant mice (Toresson et al., 2000; Yun et al., 2001). In the frog, the telencephalic (pallial) domain of neurog2 expression appears to be reduced/missing in the pax6 mutant (Fig 7D), consistent with what has been observed in mouse Pax6 mutants.
Although we have looked at only a few gene markers so far, we find alterations in gene expression patterns in the developing brains of pax6 mutant frogs that are generally consistent with those predicted for downstream targets of pax6 function and those reported in Pax6 mutations in other organisms.
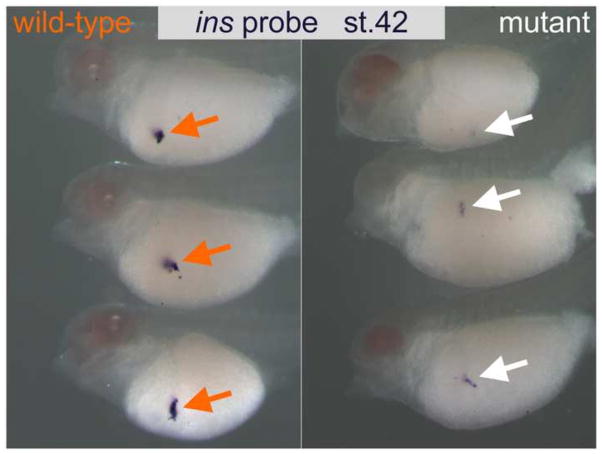
Finally, to expand our initial survey of sites of tissues where pax6 is known to be essential, we looked for evidence of pax6 malfunction in the developing pancreas where we examined insulin (ins) expression as an indicator of pancreas development, and as expected its expression was reduced in mutant embryos (Fig. 8), as has been reported in mouse Pax6 mutants (Sander et al., 1997; St-Onge et al., 1997).
Fig. 8.
insulin (ins) expression is downregulated in pax6 mutants. Wild-type (left) and pax6 mutant (right) embryos at stage 42 were subjected to in situ hybridization with an ins probe. Expression of ins gene in this lateral view of mutant embryos (white arrows) is reduced compared to the level seen in wild-type embryos (orange arrows).
Hypomorphic alleles in combination with “null” alleles cause an eye phenotype similar to human aniridia
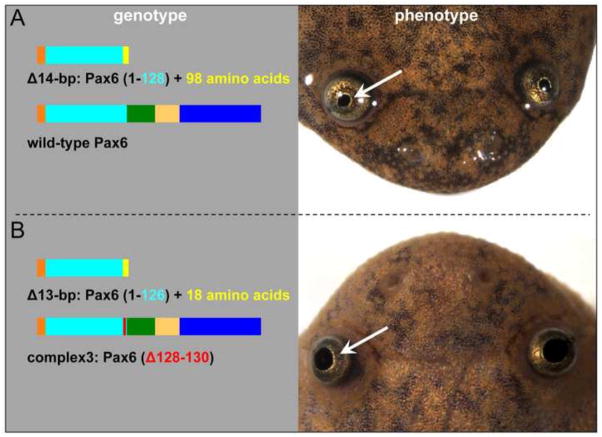
While establishing F1 lines, in one mating between F0 st.716 female #2 and male #3 we found a number of the offspring that developed into froglets which had a common abnormality, namely enlarged pupil size. These (total 12) froglets developed from the apparently wild-type 96 survivors of this mating, i.e. after those embryos showing the characteristic “null” phenotype (Fig. 4A) died during tadpole stages. That is, the froglets with the enlarged pupil phenotype appear to have had no distinctive embryonic phenotype. Fig. 9 shows typical gross morphological appearance of the mutant froglets. Genotyping of all 12 frogs (see Table S1) revealed that a specific combination of mutations was associated with what we postulate to be a hypomorphic genotype. All frogs have one allele with frame-shifted mutations that encode truncated proteins lacking a few amino acids of the end of the paired domain and the rest of C-terminal portion of the Pax6 protein (as seen in “null” mutants). The other allele has an in-frame small deletion that deletes a few amino acids that in almost all cases includes arginine at amino acid 128 (R128), though in one case only amino acid residue 127 is deleted (see Fig. 9B left panel and Table 1) with the rest of the protein remaining intact. We hypothesize that this results in production of a Pax6 protein with reduced activity. We have recently confirmed that these froglets can survive, and some have reached sexual maturity. Thus. the features of their eye abnormality and the non-lethality of this condition are similar to what is seen in human aniridia patients. Because this mutation results in a phenotype that is significantly less severe than animals with two out-of-frame deletions (which are presumed to be null), we refer to the compound heterozygotes with an aniridia-like appearance as having a “hypomorphic phenotype.”
Fig. 9.
Combination of truncated Pax6 protein and Pax6 protein with small in-frame deletion causes an aniridia-like phenotype in X. tropicalis froglets. (A) An example of a heterozygous genotype (left) of phenotypically wild-type froglet (right). (B) An example of the compound heterozygous genotype (left) of an aniridia-like froglet (right). All other genotypes of aniridia-like froglets are listed in Table S1.
Table 1.
Pax6 proteins encoded by mutations found in aniridia-like frogs
| Combination of proteins | number of cases (n=12) |
|---|---|
| Pax6 (1–126) + additional 103 amino acids Pax6 (Δ128–130) |
2 |
| Pax6 (1–127) + additional 103 amino acids Pax6 (Δ128–129) |
1 |
| Pax6 (1–126) + additional 18 amino acids Pax6 (Δ127) |
1 |
| Pax6 (1–127) + additional 101 amino acids Pax6 (Δ128–130) |
2 |
| Pax6 (1–126) + additional 18 amino acids Pax6 (Δ128–130) |
5* |
| Pax6 (1–125) Pax6 (Δ128–130) |
1 |
Their genotypes for in-frame deletions are in two patterns (9-bp deletion and complex3, see Table S1), but encode the same protein [Pax6 (Δ128–130)].
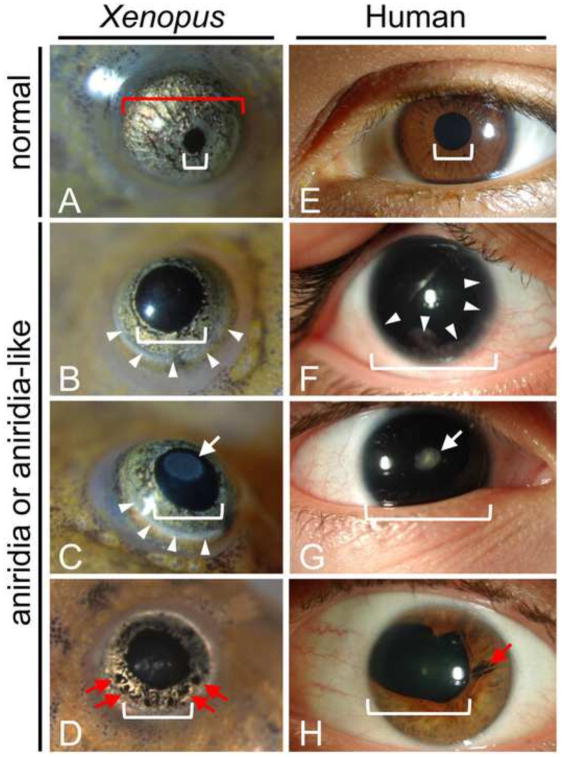
Unlike human aniridia patients who commonly have no visible iris, all of the hypomorphic mutants have clearly visible, but substantially reduced iris tissue (Fig. 10). As in human patients, the eye phenotypes are somewhat variable among individuals and can even vary between the two eyes of one animal. Examination of hypomorphic and normal frog eyes revealed that the frog hypomorphic mutants show similar abnormalities as seen in humans including: most commonly, clear lenses (Fig. 10B and F), occasional focal cataracts (lens opacities) (Fig. 10C and G, white arrows), frequent peripheral clouding of the cornea (Fig. 10B, C and F, white arrowheads) and occasional focal losses of remaining iris tissue (Fig. D and H, red arrows). One hypomorphic mutant frog appeared to be missing one eye entirely, however upon dissection a very small rudimentary eye with retinal tissue was found beneath the skin (not shown).
Fig. 10.
Comparison of aniridia-like frog eyes with human aniridic eyes. For purposes of comparison the images of froglet eyes shown here are approximately half the size of the human eyes. Actual froglet eye diameter is approximately one-tenth that of human. Normal frog (A) and human (E) eyes have small pupil (indicated by white brackets) and relatively large, pigmented iris. Aniridia-like mutant froglets (B–D) and human aniridia patients (F–H) show variable eye phenotypes including reduced (B–D, H) or absent (F, G) iris tissue. Remaining iris tissue may show focal losses (red arrows D, H). The lenses may be clear (B, F) or have a focal cataract (white arrows in C, G). Also, both frog and human eyes may show peripheral clouding of the cornea due to keratopathy (white arrowheads in B, C, F).
Since the frog mutants generally retain some iris tissue, we made measurements of pupil diameter (Fig 10A, white bracket) and corneal diameter (Fig. 10A, red bracket) in our 12 hypomorphic mutants and 12 age-matched, wild-type froglets to better assess the degree of iris tissue reduction. We found that pupil diameter was about twice as large in mutant as compared to wild-type (0.63 ±0.03mm vs 0.34 ±0.02mm, p=<0.0001), while the overall eye size as represented by measurement of the corneal diameters was about 20% less in mutants compared to wild-type (1.22 ±0.03mm vs 1.46 ±0.02mm, p< 0.0001) (Mean ± SEM, n=24 wild-type eyes, n=23 mutant eyes).
Histologic examination of three hypomorphic mutant frog eyes reveal several key features of the aniridia-like phenotype (Fig. 11) and permit further comparison with human aniridia and that seen in heterozygous Pax6 mutant mice (Figs. 12 and 13). In overview, frog hypomorphic mutant eyes are somewhat smaller than normal, as indicated by the measurements described above, have lenses that are also slightly reduced in size (Fig. 11) and show abnormalities in both fiber cells and lens epithelium (Fig. 12). The corneas show abnormalities in layering (Fig. 13), and the irises are consistently reduced compared with normal (Fig. 11). In general, the hypomorphic eyes have well developed retinas although they may be folded, and the ganglion cell layer was sparse and irregular in the cases examined (Fig. 11), features also seen in some SeyDey mice (Theiler et al., 1978). In one case the retina was partially missing, but the remainder appeared normally developed (Fig. 11C).
Fig. 11.
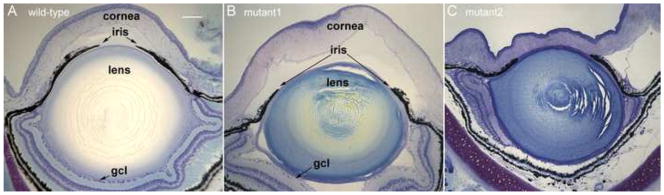
General histologic features of aniridia-like mutant froglet eyes. Compared with wild-type eyes (A), these mutants show variable defects in cornea, iris, lens and retina. Most notably, the pupil diameter is much wider in the mutants (B, C). Lenses show variable reduction in size and differentiation consistent with the variable presence of cataracts visible upon gross inspection of eyes of intact animals. While the retina is usually present and grossly normal, it may contain folds and the ganglion cell layer (gcl) is somewhat sparse and irregular. The retina may be partially absent as shown in C. Scale bar in panel A = 100μm.
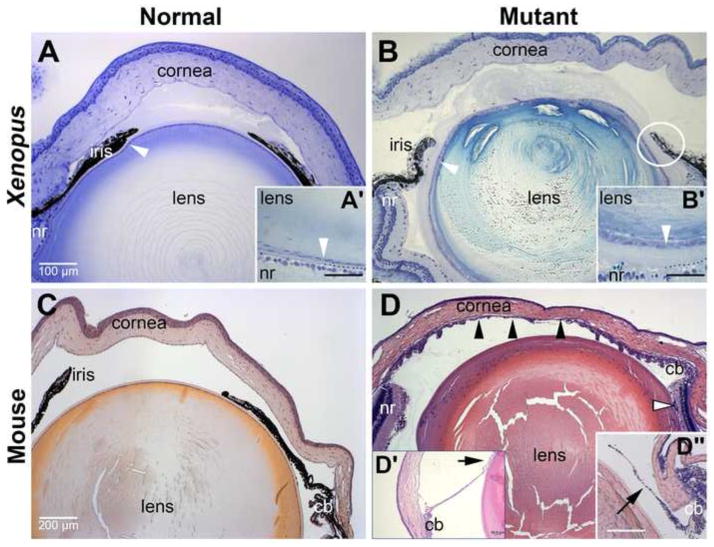
Fig. 12.
Xenopus mutants for pax6 exhibit anterior eye defects comparable to those observed in Pax6 mutant mice. Histological sections cut through eyes from either wild-type or Pax6 mutant X. tropicalis and mice showing anterior structures. (A) Wild-type froglet eye. White arrowhead denotes lens epithelial cells. (A′) High magnification view of lens epithelial cells in region of lens adjacent to retina. Dotted line denotes boundary between lens and retina. (B) pax6 hypomorphic froglet eye. In addition to the eye being smaller, defects in the cornea, iris, and lens are evident. Circle indicates region with likely iridolenticular adhesion. (B′) High magnification view of lens epithelial cells in region of lens adjacent to retina comparable to A′. Dotted line denotes boundary between lens and retina. Note expansion of acellular material between lens and retina compared to wild-type. (C) 8-week-old adult wild-type mouse (D) 8-week-old adult Pax6Sey-Neu/+ mouse. Black arrowheads denote iridokeratotic adhesion. Open arrowhead denotes iris or ciliary tissues. (D′) Arrow shows iridolenticular adhesion in section cut from different Pax6Sey-Neu/+ mouse. (D″) Arrow shows iris hypoplasia typically observed in these Pax6Sey-Neu/+ mice; this section was cut from a third Pax6Sey-Neu/+ mouse at a plane comparable to that of the wild-type mouse in C. cb, ciliary body; nr, neuroretina. Scale bar in panel A applies to panel B; scale bar in C applies to panel D, including D″. Scale bar in A′, B′ and D′ denote 50 micrometers.
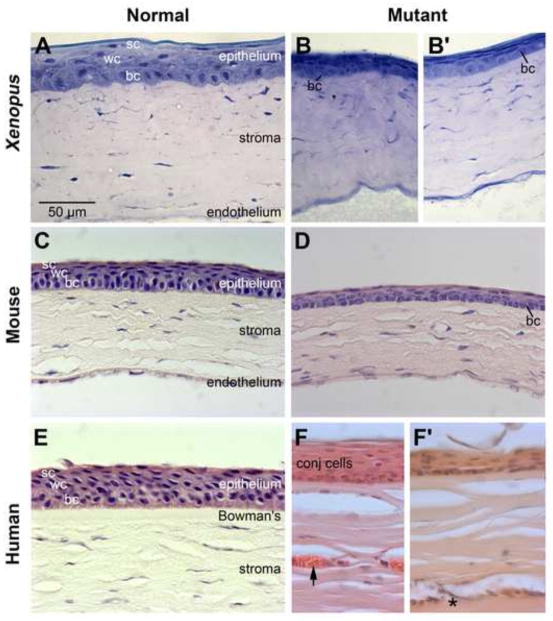
Fig. 13.
Xenopus pax6 mutant exhibits corneal defects similar to those observed in Pax6 mutant mice and human aniridia. Histology of the cornea of wild-type and Pax6 mutant frog, mice, and human. (A) wild-type froglet. (B) pax6 hypomorphic froglet. (C) 8-week-old adult wild-type mouse (D) 8-week-old adult Pax6Sey-Neu/+ mouse. (E) 70-year-old human. The cornea did not show clinical signs of keratopathy. (F–F′) 49-year-old individual with classical aniridia. The patient was diagnosed with stage III keratopathy and exhibited full conjunctivalization of the cornea. Arrow denotes blood vessel. Asterisk denotes area of cellular infiltration in the superficial corneal stroma. The samples here were of peripheral cornea. Note: the endothelial layer was not captured in these images of human cornea. bc, basal cells; conj, conjunctival cells, sc, superficial cells; wc, wing cells. Scale bar in panel A applies to panels (B–F).
To examine the phenotype in more detail, histological sections of the eyes from these animals were compared to wild-type frogs, 8-week-old wild-type adult mice and siblings heterozygous mutant for Pax6 (Figs. 12 and 13). Because corneal defects are progressive in both individuals with aniridia and mice mutant for Pax6, young adult mice were chosen for these comparisons. The Pax6Sey-Neu/+ mice chosen for these experiments were on a majority CD1 genetic background to more closely model the genetics of human aniridia and selected for more normal eye sizes, again to more closely model human aniridia.
Like mice heterozygous mutant for Pax6, Xenopus harboring the hypomorphic pax6 genotype had defects in the anterior segment, which included the cornea, lens and iris. The iris in mutant animals, while bilayered, was generally reduced in length and dysmorphic compared to wild-type animals (3 out of 3 hypomorphic mutants examined so far). In the most severely affected mutant examined, iridolenticular and keratolenticular adhesions were apparent (circle in Fig. 12B). For comparison, iris hypoplasia was observed in 90% of the mice examined (Fig. 12D″), and iridokeratotic, iridolenticular and keratolenticular adhesions were observed in about 30% of the same animals (n=40). An example of an iridolenticular adhesion is shown in Fig. 12D′ and iridokeratotic adhesions are denoted with black arrowheads in Fig. 12D. No iris defects were observed in wild-type littermates (Fig. 12C, n = 40). Lens defects were comparable between pax6 mutant frogs and mutant mice, and included cataracts in 16% of frogs (n=12) and approximately 67% of the mice (n = 40). In the hypomorphic Xenopus mutants, the lens epithelium was typically more cuboidal than the highly flattened epithelium of wild-type frogs (arrowheads in Fig. 12A′ and B′). Larger epithelial cells are occasionally observed in the lenses of more severely affected mutant mice (data not shown).
Most if not all individuals with aniridia will develop a keratopathy known as aniridia-related keratopathy (ARK), which is caused by a breakdown of the corneal epithelium and invasion of conjunctival cells (Holland et al., 2003; Mayer et al., 2003; Netland et al., 2011). To assess the effect of pax6 mutations in frogs compared to mammals, histological sections of cornea were compared to those from Pax6Sey-Neu/+ mice and the cornea of an individual with aniridia (Fig. 13). The patient was diagnosed with stage III keratopathy and had essentially full conjunctivalization of the cornea (i.e., corneal epithelial cells were largely replaced by cells from the conjunctiva). The sample here was taken from the limbal area as a result of a surgical procedure to repair the cornea.
The cornea of the wild-type frog is structurally similar to that of mouse and human (Fig. 13A, C and E, respectively) and consists of an outer epithelium, stroma and endothelial layer (Fig. 13A). The epithelium, which accounts for approximately 10% of the corneal thickness, consists of a basal layer composed of a single row of cuboidal to short columnar basal cells, followed by 1–2 layers of intermediate (wing) cells and one to two layers of flattened superficial cells. The epithelium is separated from the stroma by an acellular (Bowman’s) layer, which can be difficult to see by light microscopy. The corneal stroma, which accounts for approximately 90% of the corneal thickness, exhibits a generally well organized, horizontally arranged laminar appearance with flattened spindle-shaped keratocytes, and is avascular. A corneal endothelium, which consists of a single layer of cells, is present on the posterior surface of the cornea.
Histological analysis of the corneas from pax6 mutant frogs revealed that, similar to mice and humans heterozygous null for Pax6 (Fig. 13D and F′, respectively), the corneal epithelium of pax6 hypomorphic frogs appeared to have fewer cell layers and was generally lacking the more differentiated superficial cells compared to the cornea of wild-type frogs (compare Fig. 13B, B′ to A). At the histological level, whereas basal cells in the wild-type animals contained round to oval nuclei that stained darkly with toluidine blue, basal cell nuclei in the mutant corneas tended to be lightly stained with a punctate appearance. This staining pattern was also observed in the corneas from Pax6 mutant mice using hematoxylin (compare Fig. 13D). Both touidine blue and hematoxylin stain chromatin. A similar comparison could not be performed on the aniridia cornea because the normal basal cells were largely absent from the samples provided (Fig. 13F, data not shown). Rather the peripheral epithelial layer was composed primarily of cells with a conjunctival like morphology (Fig. 13F); more centrally (Fig. 13F′), the aniridic cornea exhibited a thinned and roughened appearance, consistent with a loss of corneal epithelial cells.
In both frogs and mice mutant for Pax6, the anterior stroma typically contained densely packed and disorganized collagenous lamella, which had a wavy appearance, and this was similar to the stroma observed in the cornea from the individual with aniridia (Fig. 13B, B′, D, F and F′). In contrast with the corneas from mutant frogs, goblet cells were present in the epithelium of approximately 80% of Pax6Sey-Neu/+ mice (n = 39) and in the human aniridia sample. Additionally, blood vessels were observed in the peripheral to mid anterior stroma in all corneal samples from Pax6Sey-Neu/+ mice (n = 39) and in the human aniridia sample (arrow in Fig. 13F). Areas of cellular infiltration, indicative of inflammatory response, were also observed in the superficial aniridic corneal stroma (asterisk in Fig. 13F′); similar infiltrations were present in the corneal stroma of the more severely affected Pax6Sey-Neu/+ mice (data not shown). Both neovascularization of the cornea and cellular infiltration in the stroma are progressive changes associated with breakdown of corneal epithelium and become more pronounced with age. Children with aniridia and juvenile Small eye mice generally have clear corneas without evidence of inappropriate vascularization or evidence of inflammation. Therefore, it is likely that the apparent lack of neovascularization in the corneas of the froglets is due to their young age rather than a fundamental difference in the progression of keratopathy between frogs, mice, and humans.
DISCUSSION
By utilizing gene editing TALENs technology we have generated an allelic series of mutant lines in the pax6 gene in Xenopus tropicalis that provide a powerful context for studying pax6 function in development and disease, augmenting in significant new ways what can be accomplished with other model organisms. These lines have already begun to provide valuable new insights about the role of pax6 gene regulation during development and will continue to be an important source of clearer understanding about pax6 function during development.
Generation and phenotypic analysis of out-of-frame exon 7 and 9 pax6 mutations
The mutations that we have generated, while targeted to a particular locus, are heterogeneous within this locus because the TALEN technology used here generates different size deletions and inserts within single embryos at different times and in different cell lineages. While this approach creates some complexity in genotyping F0 and F1 animals it also has presented opportunities to identify a variety of alleles that yielded the different phenotype outcomes we discuss here. In the future this methodology should be very valuable in generating other alleles that would permit novel investigations of Pax6 protein activity, dosage and phenotypic effects. We consider here first the group of exon 7 and 9 out-of-frame mutations that lead to a consistent strong embryonic phenotype and are likely null mutations.
These mutants highlight the utility of the Xenopus system for capitalizing on this gene editing technology but generating them requires careful titration of injected constructs. Targeting genes essential for development, like pax6, likely implies that one will generate lethal phenotypes if the mutation rate is high. While one can reduce the mutation frequency by reducing TALEN concentrations, at a low hit rate, germline transmission is inefficient and screening of F1 offspring becomes problematic. We find that injecting at relatively high doses, in conjunction with the mosaic nature of the process, provides the advantage that some animals have high mutation rates in only some cells, giving rise to individual animals with a very high germline transmission rate (up to 100% in a few animals we have generated). As described in the Results section, with the pax6 gene we can often identify animals of this kind because one eye will be severely impacted but not the other. Having animals (particularly males, which can be mated weekly) carrying such a high level of mutations is clearly advantageous in generating a high frequency of F1 mutant embryos for detailed study. This is an especially valuable feature when one considers the long reproductive lifespan of Xenopus (we have animals which are 10 years old and still fertile).
The diversity of mutations produced also allowed us to compare phenotypes generated in F1 animals by various mutations. In this paper we show that putative null mutations toward the end of the paired domain in exon 7, predicted to be null based on the target site similarity to mutations in mouse and rat that are thought to be null (discussed earlier), have a characteristic eye phenotype (loss of ventral retina and no lens). Mutations in exon 9 (in the pax6 homeodomain), and deletions that include the region between exon 7 and 9 all have this characteristic phenotype, suggesting that all of these are likely to be null mutations. In addition these results imply that the unique transcript generated from the α promoter, the paired-less form of pax6, Pax6ΔPD, which would be truncated by the exon 9 deletion within the homeodomain but remains intact in exon 7 mutations, does not contribute in a discernable way to early eye development.
As noted in the Introduction, the rationale for proposing that the mouse Sey mutation is a null and that typical aniridia mutations in humans are as well (and our mutations in X. tropicalis pax6) come from the observation that the phenotypes of the mammalian mutants are indistinguishable from those resulting from deletions spanning the Pax6 gene. It has also been argued that nonsense mediated decay (NMD), which is known to cause mRNA degradation in response to premature stop codon mutations by a well characterized mechanism (Chang et al., 2007), is responsible for the null phenotype in such PAX6 mutations (e.g. Vincent et al., 2003). Although it has been shown that NMD machinery exists in Xenopus, at least in oocytes (Whitfield et al., 1994), we found that pax6 transcripts persist in our mutants based on in situ hybridization in the tadpole brain (Fig. 7) and at earlier stages as well (data not shown), even though there is a stop codon shortly following the mutation site in the 7th exon responsible for the mutant shown in Fig. 7 as in all exon 7 mutations of this type. Similarly, mutant Pax6 transcripts are present in mid-gestation mouse embryos homozygous mutant for the SeyNeu allele (Pax6Sey-Neu/Sey-Neu) at levels comparable to wildtype Pax6 [Mastick et al., (1997) and J. Lauderdale, unpublished]. In addition, another study on a premature stop codon mutation in Xenopus, in this case the eye regulatory gene rax (Fish et al., 2014) also shows embryonic expression of the mutant RNA. Within the nervous system there is also evidence that a microRNA, miR-128, inhibits NMD in embryos of several vertebrates, including Xenopus (Bruno et al., 2011). We have preliminary evidence (Qiu, Nakayama and Grainger, unpublished) that this microRNA is expressed in the embryonic eye, brain and neural tube. All of this data together with recent reports suggesting that NMD is not a universal phenomenon in stop codon mutations [e.g. Neu-Yilik et al., (2011)] argue that NMD is not a primary mechanism for regulating expression of premature stop codon mutants in pax6 and possibly more broadly in embryogenesis.
If we accept the hypothesis that pax6 mutations in exon 7 and exon 9 are null, we would invoke the proposal discussed earlier that pax6.2 plays a role in mitigating the degree of the retinal phenotype in our mutants. We have already used CRISPR-mediated gene targeting to mutate the pax6.2 and are currently raising animals to evaluate the contribution of this gene to eye development. It has been shown that in zebrafish, an antisense morpholino oligonucleotide directed against pax6.2 does reduce eye size in wild-type embryos (Ravi et al., 2013). Our analysis of expression of pax6.2 is consistent with a role in retinal phenotype in the pax6 mutant because, as noted earlier, expression is restricted to the dorsal retina (which persists in the mutant), but also we note that pax6.2 is not expressed in the lens, and that the lens is completely absent in the pax6 mutant. In addition pax6.2 is not expressed early in development, nor in the brain and the alterations we see in expression of genes like myc and mab21l1 in the early retina (Fig. 6) and brain later (Fig. 7) are consistent with a null phenotype in these domains of pax6 activity. The significant effect of pax6.2 on retina formation is somewhat surprising given its late onset of expression.
pax6 gene targets in putative null mutants
We report here some preliminary findings about expression of putative gene targets of pax6 that both highlight new observations and the gaps in our understanding of pax6 regulation of patterning. As noted in the Introduction, there are few studies of the earliest pax6 targets, likely to be a group of genes that would be highly informative about the primary roles of this gene both in development and disease. In our work we have shown, as in mammals, that the nuclear protein gene mab21l1 is highly reduced in the eye region in our putative pax6 null embryos. Gene targeting of mab21l1 in mice reveals that this gene is essential for lens formation (Yamada et al., 2003) and a future area of interest will be to ascertain the extent to which the failure of the lens to form in the pax6 mutant is related to its effect on mab21l1 expression.
Because the myc gene is expressed in a large part of the early pax6-expressing domain and is so important in pluripotency and maintaining progenitor cells in a number of developmental contexts (Prasad et al., 2012; Smith and Dalton, 2010), roles which fit within the framework of pax6 function in pluripotency regulation and neurogenesis (e.g. Sansom et al., 2009), we decided to examine the expression of myc at the neural plate stage, shortly after pax6 activation. Previously, we found myc to be downregulated in another key eye gene required for retina specification, rax (Fish et al., 2014). As shown in Fig. 6 myc is indeed downregulated in the retina, and in the lens ectoderm as well, implying a potentially important role in both of these components of the eye. In other studies, targeted downregulation of the myc gene by morpholino antisense oligonucleotide injection into the blastomeres giving rise to the head region in Xenopus laevis embryos is shown to interfere with eye development (Bellmeyer et al., 2003). A recent study also highlights the role of Myc in cell proliferation during mouse lens development (Cavalheiro et al., 2014). Further studies on the function of myc as a potential mediator of pax6 activity will be an important goal for the future. A more thorough study of putative gene targets by RNA-Seq analysis, as was performed in our rax mutant study in X. tropicalis (Fish et al., 2014), will be instrumental in elucidating the core genes involved in the eye and neural gene regulatory network involved in the specification events that occur by the neural plate stage (Saha and Grainger, 1992).
Preliminary examinations of expression of putative pax6 targets in the brain and pancreas are also informative. In the work presented here we see expression of several key brain regulators that have been shown to be pax6 targets in previous studies and find that they modulate gene expression in ways similar to those seen in mammals, illustrating the requirement for positive feedback to maintain Pax6 expression in some regions of the diencephalon (Mastick et al., 1997) and substantiating its role in dorsal/ventral patterning in the forebrain (Georgala et al., 2011). However, as discussed above, the role of pax6 in initial patterning events remains largely unexamined, though Xenopus appears to preserve many of the brain functions of Pax6 in mammals and again should serve as a valuable model for examining these early events. Turning to the activity of pax6 in the pancreas, again our data on insulin in the pax6 mutant Xenopus indicates the conservation of function in this organ as well, as reported elsewhere (Pearl et al., 2009).
Hypomorphic mutations in pax6 lead to an aniridia-like phenotype
The genetic lesions that do not lead to obvious embryonic phenotypes, but elicit an aniridia-like phenotype in froglets, reveal further important features of the TALEN-mediated mutations in X. tropicalis. As background information, we note that animals carrying heterozygous mutations that we believe are null (e.g. frameshift mutations in exon 7) have no obvious eye phenotype, whereas in mammals mutations of this kind would yield an aniridia phenotype. This may suggest a compensatory role of the pax6.2 gene. In addition it is also possible that the activity of pax6 itself, or the genetic context in Xenopus differs slightly from that in mammals. It is known that in mice eye development is adversely affected by modest changes (either reductions or increases) in the level of Pax6 (Schedl et al., 1996). While raising F1 froglets with complex genotypes we noticed some which developed aniridia and it was at this point that we ascertained that animals carrying one putative null allele and one allele with a small in-frame deletion might be hypomorphs, which are likely to have less than one-half of the wild-type level of Pax6 activity. As noted in the Results section, all of the animals with the hypomorphic phenotype include deletions at or adjacent to amino acid 128, a conserved paired domain residue that causes aniridia in a number of patients as a result of missense mutations and which contacts the major groove of DNA (van Heyningen and Williamson, 2002). These results highlighted to us a previously unappreciated potential value of small in-frame deletions in helping to pinpoint key regions associated with a particular gene function. In the future we intend to identify this kind of lesion in targeted regions of the pax6 gene outside of the paired domain (where most aniridia-causing mutations are found). As pointed out by others (van Heyningen and Williamson, 2002), while most of the human mutations in PAX6 that have been identified affect the eye, this finding may be a product of observational bias, as generally only patients presenting with eye-related diseases would be likely to have their PAX6 locus sequenced. Further identification of vertebrate model phenotypes in other tissues where pax6 is expressed, and which are caused by pax6 mutant alleles with lesions in other key regions of the protein, may reveal novel connections to human disease.
Importantly, we show for the first time that mutations in the Pax6 gene cause similar anterior eye defects in both amphibians and mammals. The structures of the anterior segment of the vertebrate eye, which include the cornea, iris, ciliary body, and lens, are required to focus the light that enters the eye and to regulate intraocular pressure. Developmental defects of these structures can lead to several debilitating eye diseases, including cataracts, corneal dystrophies, and glaucoma. Despite much work in rodent models over the past several decades, a detailed understanding of the molecular mechanisms and genetic hierarchies involved in anterior segment formation is still lacking. Our results demonstrate that, at least in the case of Pax6, the mechanisms controlling these processes have been conserved between humans, rodents, and Xenopus tropicalis, which last shared a common ancestor approximately 350 million years ago (Hedges and Kumar, 2002), and demonstrate Xenopus as an important new vertebrate model for investigations addressing eye problems of significant interest to human health.
Supplementary Material
Highlights.
X. tropicalis pax6 mutants were isolated by TALEN gene editing.
Gene expression studies in pax6 mutant reveal its role in eye and neural patterning.
Homozygous pax6 mutants are lethal and have malformed retina and no lens.
Hypomorphic pax6 mutant results in human aniridia-like phenotype.
Acknowledgments
Funding
This work was funded by grants from the National Institutes of Health [NIH R01 EY017400, EY018000 and EY022954] to R.M.G. and research awards from the Sharon Stewart Aniridia Trust to R.M.G and J.D.L. and also by the Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, [Grant Number 25430089] to K.N. and [Grant Number 26440057] to Y.Y.
We thank past and current Grainger lab members for valuable discussions. We also thank Christopher H. K. Cheng and Hui Zhao for sharing pCS2+TALEN-ELD/KKR plasmids, and Daniel F. Voytas for the Golden Gate TALEN and the TAL effector kit (Addgene, #1000000016). We appreciate consultations with Veronica van Heyningen and Noriko Osumi regarding information about mouse and rat Pax6 mutations, and Ira Blitz for pointing out us the possibility of the presence of second pax6 gene (pax6.2) locus found while annotating the Xenopus tropicalis genome. We also gratefully acknowledge Sharon Levasseur for her participation in the clinical evaluation of the aniridic phenotype in X. tropicalis mutants. In addition we would like to thank John Freeman for providing human tissue used in these studies. Finally, the authors gratefully acknowledge support of the National Xenopus Resource and its staff, from which pax6 mutant frogs described here will be available.
Footnotes
Author contributions
T.N., M.F., A.O.O., K.B.Z., M.B.F., J.L.C., and J.D.L. performed experiments. K.N. and Y.Y. designed and constructed TALEN plasmids, and performed preliminary experiments to test activity of TALENs to choose the best target sequences to use. T.N., M.F., A.O.O., J.L.C., J.D.L., P.A.N. and R.M.G analyzed data. T.N. and R.M.G. conceived the study, and wrote the manuscript with M.F. and J.D.L.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellmeyer A, Krase J, Lindgren J, LaBonne C. The protooncogene c-myc is an essential regulator of neural crest formation in Xenopus. Developmental Cell. 2003;4:827–839. doi: 10.1016/s1534-5807(03)00160-6. [DOI] [PubMed] [Google Scholar]
- Bernier G, Vukovich W, Neidhardt L, Herrmann BG, Gruss P. Isolation and characterization of a downstream target of Pax6 in the mammalian retinal primordium. Development. 2001;128:3987–3994. doi: 10.1242/dev.128.20.3987. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Biesinger J, Xie X, Cho KW. Biallelic genome modification in F0 Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis. 2013;51:827–834. doi: 10.1002/dvg.22719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno IG, Karam R, Huang L, Bhardwaj A, Lou CH, Shum EY, Song HW, Corbett MA, Gifford WD, Gecz J, Pfaff SL, Wilkinson MF. Identification of a microRNA that activates gene expression by repressing nonsense-mediated RNA decay. Molecular Cell. 2011;42:500–510. doi: 10.1016/j.molcel.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaerts P, Halder G, Gehring WJ. PAX-6 in development and evolution. Annual Review of Neuroscience. 1997;20:483–532. doi: 10.1146/annurev.neuro.20.1.483. [DOI] [PubMed] [Google Scholar]
- Carney RS, Cocas LA, Hirata T, Mansfield K, Corbin JG. Differential regulation of telencephalic pallial-subpallial boundary patterning by Pax6 and Gsh2. Cerebral Cortex. 2009;19:745–759. doi: 10.1093/cercor/bhn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro GR, Matos-Rodrigues GE, Gomes AL, Rodrigues PM, Martins RA. c-myc regulates cell proliferation during lens development. PloS One. 2014;9:e87182. doi: 10.1371/journal.pone.0087182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Research. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YF, Imam JS, Wilkinson MF. The nonsense-mediated decay RNA surveillance pathway. Annual Review of Biochemistry. 2007;76:51–74. doi: 10.1146/annurev.biochem.76.050106.093909. [DOI] [PubMed] [Google Scholar]
- Coutinho P, Pavlou S, Bhatia S, Chalmers KJ, Kleinjan DA, van Heyningen V. Discovery and assessment of conserved Pax6 target genes and enhancers. Genome Research. 2011;21:1349–1359. doi: 10.1101/gr.124115.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelkamp D, Rashbass P, Seawright A, van Heyningen V. Role of Pax6 in development of the cerebellar system. Development. 1999;126:3585–3596. doi: 10.1242/dev.126.16.3585. [DOI] [PubMed] [Google Scholar]
- Favor J, Gloeckner CJ, Neuhauser-Klaus A, Pretsch W, Sandulache R, Saule S, Zaus I. Relationship of Pax6 activity levels to the extent of eye development in the mouse, Mus musculus. Genetics. 2008;179:1345–1355. doi: 10.1534/genetics.108.088591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favor J, Peters H, Hermann T, Schmahl W, Chatterjee B, Neuhauser-Klaus A, Sandulache R. Molecular characterization of Pax62Neu through Pax610Neu: an extension of the Pax6 allelic series and the identification of two possible hypomorph alleles in the mouse Mus musculus. Genetics. 2001;159:1689–1700. doi: 10.1093/genetics/159.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish MB, Nakayama T, Fisher M, Hirsch N, Cox A, Reeder R, Carruthers S, Hall A, Stemple DL, Grainger RM. Xenopus mutant reveals necessity of rax for specifying the eye field which otherwise forms tissue with telencephalic and diencephalic character. Developmental Biology. 2014;395:317–330. doi: 10.1016/j.ydbio.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish MB, Nakayama T, Grainger RM. Simple, fast, tissue-specific bacterial artificial chromosome transgenesis in Xenopus. Genesis. 2012;50:307–315. doi: 10.1002/dvg.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends in Genetics. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Georgala PA, Carr CB, Price DJ. The role of Pax6 in forebrain development. Developmental Neurobiology. 2011;71:690–709. doi: 10.1002/dneu.20895. [DOI] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nature Genetics. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Lopez JM, Marin O. Expression pattern of the homeobox protein NKX2-1 in the developing Xenopus forebrain. Brain research. Gene Expression Patterns. 2002;1:181–185. doi: 10.1016/s1567-133x(02)00015-7. [DOI] [PubMed] [Google Scholar]
- Guo X, Zhang T, Hu Z, Zhang Y, Shi Z, Wang Q, Cui Y, Wang F, Zhao H, Chen Y. Efficient RNA/Cas9-mediated genome editing in Xenopus tropicalis. Development. 2014;141:707–714. doi: 10.1242/dev.099853. [DOI] [PubMed] [Google Scholar]
- Harland RM, Grainger RM. Xenopus research: metamorphosed by genetics and genomics. Trends in Genetics. 2011;27:507–515. doi: 10.1016/j.tig.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges SB, Kumar S. Genomics. Vertebrate genomes compared. Science. 2002;297:1283–1285. doi: 10.1126/science.1076231. [DOI] [PubMed] [Google Scholar]
- Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, Ovcharenko I, Putnam NH, Shu S, Taher L, Blitz IL, Blumberg B, Dichmann DS, Dubchak I, Amaya E, Detter JC, Fletcher R, Gerhard DS, Goodstein D, Graves T, Grigoriev IV, Grimwood J, Kawashima T, Lindquist E, Lucas SM, Mead PE, Mitros T, Ogino H, Ohta Y, Poliakov AV, Pollet N, Robert J, Salamov A, Sater AK, Schmutz J, Terry A, Vize PD, Warren WC, Wells D, Wills A, Wilson RK, Zimmerman LB, Zorn AM, Grainger R, Grammer T, Khokha MK, Richardson PM, Rokhsar DS. The genome of the Western clawed frog Xenopus tropicalis. Science. 2010;328:633–636. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill ME, Asa SL, Drucker DJ. Essential requirement for Pax6 in control of enteroendocrine proglucagon gene transcription. Molecular Endocrinology. 1999;13:1474–1486. doi: 10.1210/mend.13.9.0340. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hingorani M, Hanson I, van Heyningen V. Aniridia. European Journal of Human Genetics. 2012;20:1011–1017. doi: 10.1038/ejhg.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch N, Harris WA. Xenopus Pax-6 and retinal development. Journal of Neurobiology. 1997;32:45–61. [PubMed] [Google Scholar]
- Hirsch N, Zimmerman LB, Gray J, Chae J, Curran KL, Fisher M, Ogino H, Grainger RM. Xenopus tropicalis transgenic lines and their use in the study of embryonic induction. Developmental Dynamics. 2002;225:522–535. doi: 10.1002/dvdy.10188. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Hirst EM, Horsburgh G, Hetherington CM. Small eye (Sey): a mouse model for the genetic analysis of craniofacial abnormalities. Development. 1988;103(Suppl):115–119. doi: 10.1242/dev.103.Supplement.115. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. Journal of Embryology and Experimental Morphology. 1986;97:95–110. [PubMed] [Google Scholar]
- Holland EJ, Djalilian AR, Schwartz GS. Management of aniridic keratopathy with keratolimbal allograft: a limbal stem cell transplantation technique. Ophthalmology. 2003;110:125–130. doi: 10.1016/s0161-6420(02)01451-3. [DOI] [PubMed] [Google Scholar]
- Holm PC, Mader MT, Haubst N, Wizenmann A, Sigvardsson M, Gotz M. Loss- and gain-of-function analyses reveal targets of Pax6 in the developing mouse telencephalon. Molecular and Cellular Neurosciences. 2007;34:99–119. doi: 10.1016/j.mcn.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Illes JC, Winterbottom E, Isaacs HV. Cloning and expression analysis of the anterior parahox genes, Gsh1 and Gsh2 from Xenopus tropicalis. Developmental Dynamics. 2009;238:194–203. doi: 10.1002/dvdy.21816. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Cliffe R, Amaya E. Highly efficient bi-allelic mutation rates using TALENs in Xenopus tropicalis. Biology Open. 2012;1:1273–1276. doi: 10.1242/bio.20123228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lauderdale JD. Analysis of Pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of Pax6 in the eye and olfactory bulb. Developmental Biology. 2006;292:486–505. doi: 10.1016/j.ydbio.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Kim J, Lauderdale JD. Overexpression of pairedless Pax6 in the retina disrupts corneal development and affects lens cell survival. Developmental Biology. 2008;313:434–454. doi: 10.1016/j.ydbio.2007.10.043. [DOI] [PubMed] [Google Scholar]
- Kokotas H, Petersen MB. Clinical and molecular aspects of aniridia. Clinical Genetics. 2010;77:409–420. doi: 10.1111/j.1399-0004.2010.01372.x. [DOI] [PubMed] [Google Scholar]
- Lei Y, Guo X, Liu Y, Cao Y, Deng Y, Chen X, Cheng CH, Dawid IB, Chen Y, Zhao H. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs) Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17484–17489. doi: 10.1073/pnas.1215421109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Price DJ. Role of Pax6 in forebrain regionalization. Brain Research Bulletin. 2005;66:387–393. doi: 10.1016/j.brainresbull.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Mastick GS, Davis NM, Andrew GL, Easter SS., Jr Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development. 1997;124:1985–1997. doi: 10.1242/dev.124.10.1985. [DOI] [PubMed] [Google Scholar]
- Matsuo T, Osumi-Yamashita N, Noji S, Ohuchi H, Koyama E, Myokai F, Matsuo N, Taniguchi S, Doi H, Iseki S, et al. A mutation in the Pax-6 gene in rat small eye is associated with impaired migration of midbrain crest cells. Nature Genetics. 1993;3:299–304. doi: 10.1038/ng0493-299. [DOI] [PubMed] [Google Scholar]
- Mayer KL, Nordlund ML, Schwartz GS, Holland EJ. Keratopathy in congenital aniridia. The Ocular Surface. 2003;1:74–79. doi: 10.1016/s1542-0124(12)70130-1. [DOI] [PubMed] [Google Scholar]
- Moreno N, Retaux S, Gonzalez A. Spatio-temporal expression of Pax6 in Xenopus forebrain. Brain Research. 2008;1239:92–99. doi: 10.1016/j.brainres.2008.08.052. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Nakai Y, Okada M, Yaoita Y. Targeted gene disruption in the Xenopus tropicalis genome using designed TALE nucleases. Zoological Science. 2013;30:455–460. doi: 10.2108/zsj.30.455. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Yaoita Y. Comparison of TALEN scaffolds in Xenopus tropicalis. Biology Open. 2013;2:1364–1370. doi: 10.1242/bio.20136676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Fish MB, Fisher M, Oomen-Hajagos J, Thomsen GH, Grainger RM. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis. 2013;51:835–843. doi: 10.1002/dvg.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland PA, Scott ML, Boyle JW, IV, Lauderdale JD. Ocular and systemic findings in a survey of aniridia subjects. J AAPOS. 2011;15:562–566. doi: 10.1016/j.jaapos.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Neu-Yilik G, Amthor B, Gehring NH, Bahri S, Paidassi H, Hentze MW, Kulozik AE. Mechanism of escape from nonsense-mediated mRNA decay of human beta-globin transcripts with nonsense mutations in the first exon. RNA. 2011;17:843–854. doi: 10.1261/rna.2401811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieukoop PD, Faber J. Normal Table of Xenopus laevis. Garland Publishing Inc; New York: 1994. [Google Scholar]
- Numayama-Tsuruta K, Arai Y, Osumi N. The rat Small eye homozygote (rSey2/rSey2) can be regarded as a Pax6 null mutant. In: Esashi M, Ishii K, Ohuchi N, Osumi N, Sato M, Yamaguchi T, editors. Future Medical Engineering Based on Bionanotechnology: Proceedings of the Final Symposium of the Tohoku University 21st Century Center for Excellence Program. Imperial College Press; 2007. pp. 151–161. [Google Scholar]
- Ogino H, McConnell WB, Grainger RM. High-throughput transgenesis in Xenopus using I-SceI meganuclease. Nature Protocols. 2006;1:1703–1710. doi: 10.1038/nprot.2006.208. [DOI] [PubMed] [Google Scholar]
- Osumi N, Hirota A, Ohuchi H, Nakafuku M, Iimura T, Kuratani S, Fujiwara M, Noji S, Eto K. Pax-6 is involved in the specification of hindbrain motor neuron subtype. Development. 1997;124:2961–2972. doi: 10.1242/dev.124.15.2961. [DOI] [PubMed] [Google Scholar]
- Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M. Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells. 2008;26:1663–1672. doi: 10.1634/stemcells.2007-0884. [DOI] [PubMed] [Google Scholar]
- Pearl EJ, Bilogan CK, Mukhi S, Brown DD, Horb ME. Xenopus pancreas development. Developmental dynamics. 2009;238:1271–1286. doi: 10.1002/dvdy.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Clark KJ, Campbell JM, Panetta MR, Guo Y, Ekker SC. Making designer mutants in model organisms. Development. 2014;141:4042–4054. doi: 10.1242/dev.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad MS, Sauka-Spengler T, LaBonne C. Induction of the neural crest state: control of stem cell attributes by gene regulatory, post-transcriptional and epigenetic interactions. Developmental Biology. 2012;366:10–21. doi: 10.1016/j.ydbio.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J, van Heyningen V. PAX6 mutations reviewed. Human Mutation. 1998;11:93–108. doi: 10.1002/(SICI)1098-1004(1998)11:2<93::AID-HUMU1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ravi V, Bhatia S, Gautier P, Loosli F, Tay BH, Tay A, Murdoch E, Coutinho P, van Heyningen V, Brenner S, Venkatesh B, Kleinjan DA. Sequencing of Pax6 loci from the elephant shark reveals a family of Pax6 genes in vertebrate genomes, forged by ancient duplications and divergences. PLoS Genetics. 2013;9:e1003177. doi: 10.1371/journal.pgen.1003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends in genetics. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Roberts RC. Small eyes - a new dominant mutation in the mouse. Genetic Res Camb. 1967;9:121–122. [Google Scholar]
- Saha MS, Grainger RM. A labile period in the determination of the anterior-posterior axis during early neural development in Xenopus. Neuron. 1992;8:1003–1014. doi: 10.1016/0896-6273(92)90123-u. [DOI] [PubMed] [Google Scholar]
- Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes & Development. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, van Heyningen V, Rubenstein JL, Livesey FJ. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genetics. 2009;5:e1000511. doi: 10.1371/journal.pgen.1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scardigli R, Baumer N, Gruss P, Guillemot F, Le Roux I. Direct and concentration-dependent regulation of the proneural gene Neurogenin2 by Pax6. Development. 2003;130:3269–3281. doi: 10.1242/dev.00539. [DOI] [PubMed] [Google Scholar]
- Schedl A, Ross A, Lee M, Engelkamp D, Rashbass P, van Heyningen V, Hastie ND. Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell. 1996;86:71–82. doi: 10.1016/s0092-8674(00)80078-1. [DOI] [PubMed] [Google Scholar]
- Shaham O, Menuchin Y, Farhy C, Ashery-Padan R. Pax6: a multi-level regulator of ocular development. Progress in Retinal and Eye Research. 2012;31:351–376. doi: 10.1016/j.preteyeres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger R, Harland R. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 2000. [Google Scholar]
- Smith K, Dalton S. Myc transcription factors: key regulators behind establishment and maintenance of pluripotency. Regenerative Medicine. 2010;5:947–959. doi: 10.2217/rme.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Fritsch R, Walther C, Gruss P. Forebrain patterning defects in Small eye mutant mice. Development. 1996;122:3453–3465. doi: 10.1242/dev.122.11.3453. [DOI] [PubMed] [Google Scholar]
- Sturtevant AH. A map of the fourth chromosome of Drosophila melanogaster, based on crossing over in triploid females. Proceedings of the National Academy of Sciences of the United States of America. 1951;37:405–407. doi: 10.1073/pnas.37.7.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Suzuki KT, Isoyama Y, Kashiwagi K, Sakuma T, Ochiai H, Sakamoto N, Furuno N, Kashiwagi A, Yamamoto T. High efficiency TALENs enable F0 functional analysis by targeted gene disruption in Xenopus laevis embryos. Biology Open. 2013;2:448–452. doi: 10.1242/bio.20133855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler K, Varnum DS, Stevens LC. Development of Dickie’s Small eye, a mutation in the house mouse. Anatomy and Embryology. 1978;155:81–86. doi: 10.1007/BF00315732. [DOI] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- van Heyningen V, Williamson KA. PAX6 in sensory development. Human Molecular Genetics. 2002;11:1161–1167. doi: 10.1093/hmg/11.10.1161. [DOI] [PubMed] [Google Scholar]
- Vincent MC, Pujo AL, Olivier D, Calvas P. Screening for PAX6 gene mutations is consistent with haploinsufficiency as the main mechanism leading to various ocular defects. European Journal of Human Genetics. 2003;11:163–169. doi: 10.1038/sj.ejhg.5200940. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wawersik S, Purcell P, Rauchman M, Dudley AT, Robertson EJ, Maas R. BMP7 acts in murine lens placode development. Developmental Biology. 1999;207:176–188. doi: 10.1006/dbio.1998.9153. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Sharpe CR, Wylie CC. Nonsense-mediated mRNA decay in Xenopus oocytes and embryos. Developmental Biology. 1994;165:731–734. doi: 10.1006/dbio.1994.1289. [DOI] [PubMed] [Google Scholar]
- Wolf LV, Yang Y, Wang J, Xie Q, Braunger B, Tamm ER, Zavadil J, Cvekl A. Identification of pax6-dependent gene regulatory networks in the mouse lens. PloS One. 2009;4:e4159. doi: 10.1371/journal.pone.0004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Hasegawa T, Osumi N, Koseki H, Takahashi N. Cell-autonomous involvement of Mab21l1 is essential for lens placode development. Development. 2003;130:1759–1770. doi: 10.1242/dev.00399. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.