Abstract
Objective
Angiopoietin-2 (Ang-2) blocking agents are currently undergoing clinical trials for use in cancer treatment. Ang-2 has also been associated with rupture-prone atherosclerotic plaques in humans, suggesting a role for Ang-2 in plaque stability. Despite the availability of Ang-2 blocking agents, their clinical use is still lacking. Our aim was to establish if Ang-2 has a role in atheroma development and in the transition of subclinical to clinically relevant atherosclerosis. We investigated the effect of antibody-mediated Ang-2 blockage on atherogenesis after in a mouse model of atherosclerosis.
Methods
Hypercholesterolemic (low-density lipoprotein receptor−/− apolipoprotein B100/100) mice were subjected to high-cholesterol diet for eight weeks, one group with and one group without Ang-2 blocking antibody treatment during weeks 4–8.To enhance plaque development, a peri-adventitial collar was placed around the carotid arteries at the start of antibody treatment. Aortic root, carotid arteries and brachiocephalic arteries were analyzed to evaluate the effect of Ang-2 blockage on atherosclerotic plaque size and stable plaque characteristics.
Results
Anti-Ang-2 treatment reduced the size of fatty streaks in the brachiocephalic artery (−72%, p < 0.05). In addition, antibody-mediated Ang-2 blockage reduced plasma triglycerides (−27%, p < 0.05). In contrast, Ang-2 blockage did not have any effect on the size or composition (collagen content, macrophage percentage, adventitial microvessel density) of pre-existing plaques in the aortic root or collar-induced plaques in the carotid artery.
Conclusions
Ang-2 blockage was beneficial as it decreased fatty streak formation and plasma triglyceride levels, but had no adverse effect on pre-existing atherosclerosis in hypercholesterolemic mice.
Keywords: Angiogenesis, Microvascular leakage, Plaque stability, Angiopoietin-2, Atherosclerosis
Abbreviations: Ang-1, angiopoietin-1; Ang-2, angiopoietin-2; ApoB, apolipoprotein B; LDLr, low density lipoprotein receptor; MMP, matrix metalloproteinase; VEGF-A, vascular endothelial growth factor-A
Highlights
-
•
Antibody-mediated Ang-2 blockage delays fatty streak formation in mice.
-
•
Ang-2 blockage lowers plasma triglyceride levels.
-
•
Ang-2 blockage has no negative effects on preexisting atherosclerosis.
1. Introduction
Increased plasma levels of the vascular growth factor angiopoietin-2 (Ang-2) occur in several diseases, including cancer [1], systemic infections [2], and in cardiovascular diseases such as acute myocardial infarction [3]. Ang-2 is also increased in highly vascularized, rupture-prone human atherosclerotic plaques [4,5]. Despite the detrimental role of Ang-2 in atherosclerosis suggested by these results, Ang-2 overexpression decreased plaque size in a mouse model of atherosclerosis [6]. However, we considered that systemic Ang-2 overexpression fails to mimic the function of endogenous Ang-2 in atherogenesis.
Despite increased Ang-2 levels in clinically relevant atherosclerosis and the availability of Ang-2 blocking agents, there have been no attempts to study Ang-2 blockage in cardiovascular diseases. Therefore, we considered it important to study the role of Ang-2 blocking as a therapeutic strategy in atherosclerosis. Because the blocking of vascular endothelial growth factor in cancer therapy increased the risk of unpredicted cardiovascular side effects, such as arterial thrombosis, heart failure or cardiomyopathy [7–10], and because anti-Ang-2 therapy is currently used for cancer treatment as well, we aimed to evaluate the potential cardiovascular risk of anti-Ang-2 treatment in an experimental setting of pre-existing atherosclerosis. In order to investigate the role of endogenous Ang-2, a blocking strategy was adopted. Currently, a wide variety of Ang-2 blocking agents are being tested in clinical trials (some of them in Phase III) against different types of cancer (reviewed in [11]). We used an antibody that inhibits tumor growth in mice by blocking Ang-2 binding to its receptor Tie-2 [12].
The therapeutic potential of Ang-2 blockage is based on the adverse effects of Ang-2 on processes leading to plaque instability: microvessel growth (angiogenesis), decreased pericyte coating of microvasculature and increased vascular permeability [2,13,14], that promotes leukocyte recruitment [13,15,16]. In addition, Tie-2 expressing macrophages respond to Ang-2 by secreting TNFα [17], which can contribute to the inflammation in the vessel wall.
Microvessel growth and permeability, and inflammation are also involved in the transition of a clinically silent, stable atherosclerotic plaque into a vulnerable plaque at risk of fibrous cap rupture and ensuing clinical events [18]. The abundance and compromised structural integrity of plaque microvessels are detrimental to plaque stability, increasing the likelihood of plaque rupture [18–20]. Structurally abnormal microvessels involve the risk of plaque hemorrhage and lead to increased lipoprotein deposition and influx of leukocytes, triggering a pro-inflammatory cycle that results in plaque weakening.
In this study, we investigated the effects of the Ang-2 blocking antibody on fatty streak formation and atherosclerotic plaque progression in aortic root, brachiocephalic arteries and in collar-induced carotid atherosclerosis in LDLr−/− ApoB100/100 mice.
2. Materials and methods
2.1. Experimental animals
Ten to twelve week old male LDLR−/− ApoB100/100 mice (n = 26) were placed on a high cholesterol diet (42% of calories from fat and 0.15% from cholesterol, no sodium cholate; TD 88173 Harlan Teklad, Boxmeer, NL) for 8 weeks. After 3 weeks of diet, mice underwent collar placement surgery as previously described [21]. In brief, the mice were anaesthetized with isoflurane (induction: 4.5% isoflurane, 450 ml air, maintenance: 2.0% isoflurane, 200 ml air; Baxter International, Helsinki, Finland) and injected with 0.1 mg Rimadyl s.c. (Pfizer, Helsinki, FI). Anesthesia was controlled regularly by visual inspection and toe pinch reflex. Carotid arteries were accessed via a sagittal anterior neck incision and dissected from the surrounding tissue without damaging the vagus nerve or the carotid arteries. A 2 mm silastic collar was placed bilaterally, right under the bifurcation and fixed with three circumferential surgical silk knots.
One day after collar placement surgery, the mice were distributed randomly and injected intraperitoneally with either a monoclonal Ang-2 blocking antibody [12] (n = 14) or a control immunoglobulin (n = 12; IgG) at a dose of 10 mg/kg, as previously described [22]. The injections were repeated three times weekly over a total time of five weeks.
Food and water were provided ad libitum during the entire study. All animal experiments were approved by National Experimental Animal Board of Finland and carried out in accordance with guidelines of the Finnish Act on Animal Experimentation.
2.2. Plasma Ang-2 levels
Plasma Ang-2 concentrations were measured using enzyme linked immunosorbent assay for murine Ang-2 (R&D systems, Abingdon, UK).
2.3. Echocardiography and carotid artery ultrasound
Echocardiographic measurements were performed before the collar operations and at 5 weeks after the operation/antibody treatment using the Vevo® 2100 Ultrasound System (VisualSonics®, Amsterdam, NL). The animals were anesthetized with isoflurane. The Ejection fraction (EF), fractional shortening (FS), left ventricle (LV) mass, LV diastolic and systolic volume were analyzed from parasternal short axis M-mode measurements. EF was calculated by Vevo2100 software using the Teicholz formula [23]. Carotid strain was measured as percent change in the arterial diameter: (SD – DD)/DD, where SD was the systolic and DD the diastolic CCA diameter. Peak wall shear stress at 1 mm proximal to the collar was calculated by the Poiseuille equation: τ (dyn/cm2) = 4 × V × η/ID, where V is the peak systolic flow velocity (cm/s), η is the blood viscosity (taken as 0.035 P), and ID is the maximal lumen diameter of the targeted carotid artery (cm) [24,25].
2.4. Total serum cholesterol and triglycerides
Plasma was separated by centrifugation and stored at −80 °C until further use. Cholesterol was determined using standard enzymatic assays (CHOD-PAP method - Cholesterol FS Ecoline product no. 1 1300 99 90 314, DiaSys, Holzheim, GE), as were triglyceride levels (GPO method - Triglycerides FS Ecoline REF 1 5760 99 90 314 both DiaSys).
2.5. Atherosclerotic plaque quantification and immunohistochemistry
Atherosclerotic plaque development in the murine arterial tree starts in the aortic root, extending to the aortic arch and brachiocephalic trunk and, after an extended time period, developing into the carotid bifurcation [26]. In this study, the mice were fed a high cholesterol diet for 8 weeks and subjected to antibody treatment during the last 5 weeks. The plaques in the brachiocephalic artery consisted mainly of foamy macrophages without an overlying cap or extracellular cholesterol crystals, thus termed fatty streaks. The plaques in the aortic root and carotid arteries were classified as advanced plaques composed of immune cells, smooth muscle cells and a necrotic core, all covered by a fibrous cap. The antibody effect on fatty streak formation could thus be studied in the brachiocephalic artery, while the effect on progression of pre-existing, advanced plaques was studied in the aortic root and carotid arteries with a collar placement, respectively.
Mice were euthanized by CO2 asphyxiation and blood was collected via the right ventricle for ELISA, total cholesterol- and triglyceride analysis. Remaining blood was cleared by perfusion with 20 ml PBS via the left ventricle. Brachiocephalic arteries, aortic root and right common carotid arteries were excised and immersion fixed in 1% paraformaldehyde overnight.
Tissue samples were paraffin embedded, serially sectioned (4 μm) and stained with hematoxylin and eosin (HE, Sigma, Zwijndrecht, NL) for quantification of the plaque areas using computerized morphometry (Leica QWin V3, Cambridge, UK). Total plaque area and necrotic core content were obtained by averaging measurements of five representative sections of the brachiocephalic artery, aortic root and right common carotid artery. The necrotic core was defined by cholesterol clefts, lipid droplets and acellular regions. Collagen content was determined from representative sections stained with Sirius Red (Sirius Red + area/plaque area; Sigma). Plaque macrophages and the intra-plaque and adventitial microvessels were quantified using immunohistochemistry for Mac-3 (Mac-3+ area/plaque area; BD Pharmingen, Breda, NL) and PCAM-1 (BD Pharmingen, Breda, NL) respectively.
2.6. Statistical analysis
All data are presented as mean ± SEM. Following Shapiro–Wilk test for normal distribution, the groups were compared with student's t-test or Mann–Whitney rank-sum test. (GraphPad Prism4, La Jolla, CA, USA). A p-value of p < 0.05 was considered significant.
3. Results
3.1. Ang-2 blocking decreases plasma triglycerides but not plasma cholesterol levels
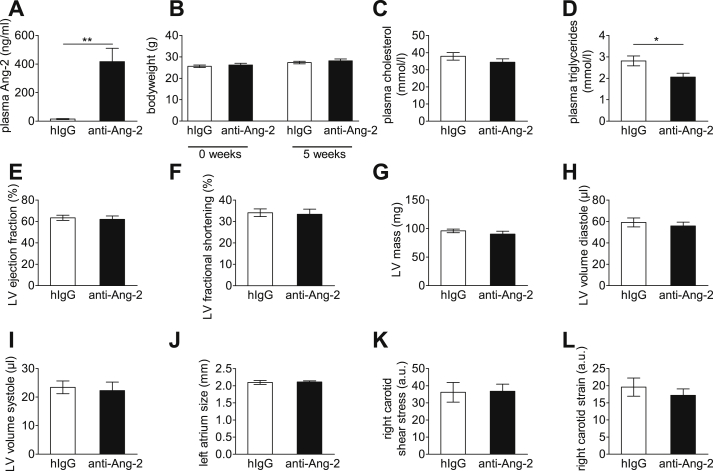
The Ang2 antibody used in the study blocks the Ang-2 fibrinogen binding domain that is responsible for receptor binding [12], and this results in increased Ang-2 levels in the circulation [27]. The antibody injections resulted in an almost 30-fold increased plasma Ang-2 concentration after five weeks of treatment (Fig. 1A). An increase in plasma Ang-2 has also been described in humans treated with the anti-Ang-2 antibody [27]. During five weeks of administration, the antibody treatment did not affect the general health status, including body weight (Fig. 1B), cardiac function (Fig. 1E–J) or shear stress in carotid artery after collar implantation (Fig. 1K and L). However, the treatment led to a 27% decrease in plasma triglycerides (Fig. 1D) without any effects on plasma cholesterol (Fig. 1C).
Fig. 1.
Anti Ang-2 antibody treatment increases plasma Ang-2 levels and decreases plasma triglycerides. Plasma Ang-2 levels in anti-Ang and IgG treated mice (A). During the five weeks of administration, the antibody treatment did not affect the general health status, body weight (B), cardiac function (E–J) or shear stress in carotid artery after collar implantation (K + L). However, the treatment led to a decrease in plasma triglycerides (D) without effects on plasma cholesterol (C). IgG n = 12; anti-Ang-2 n = 14; *p < 0.05; **p < 0.01.
3.2. Ang-2 blocking decreases fatty streak formation
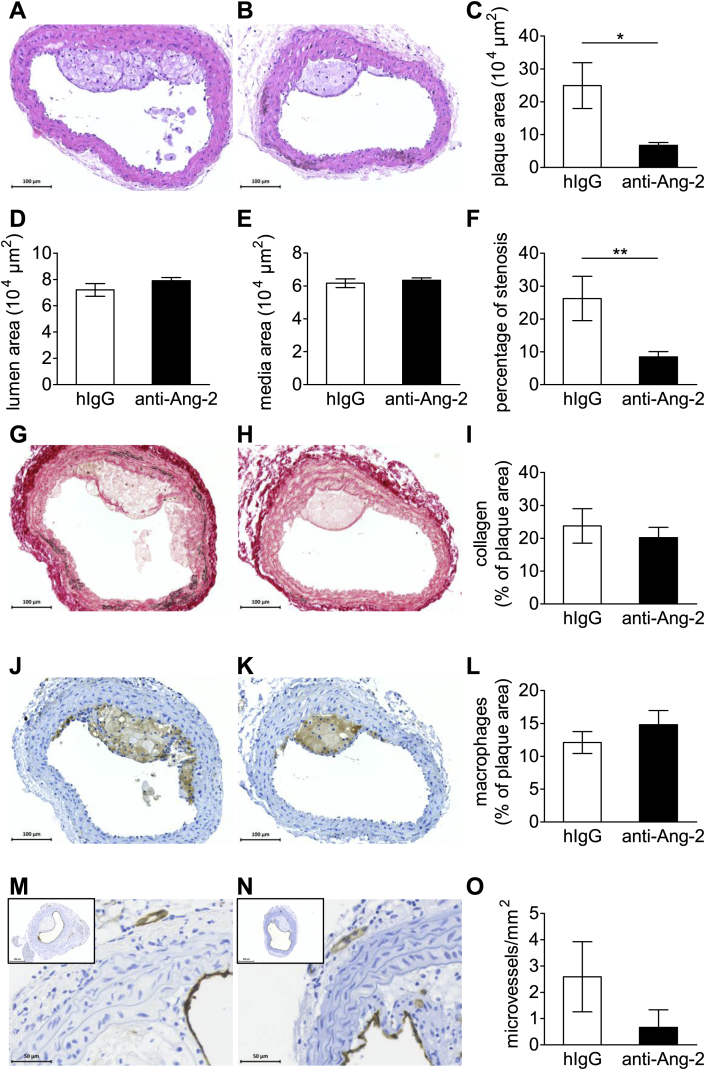
In order to investigate if Ang-2 blockage affects fatty streak formation, the brachiocephalic artery [28] was analyzed after eight weeks of high-cholesterol diet. Interestingly, fatty streak formation in the brachiocephalic artery was significantly less in mice treated with the anti-Ang-2 antibody than control IgG (Fig. 2A–C). While anti-Ang-2 therapy reduced the percentage of stenosis, the lumen and media size remained unchanged (Fig. 2D–F). Furthermore, Ang-2 blockage did not alter the fatty streak composition: the collagen content and the percentage of Mac-3 positive macrophages in the plaques (Fig. 2G–I and J–L, respectively) remained unaltered. No microvessels were found in the fatty streaks and adventitial microvessel density was not altered by the antibody treatment (Fig. 2M–O and data not shown).
Fig. 2.
Antibody mediated Ang-2 blockage delays fatty streak formation in the brachiocephalic artery. Representative images of HE-stained brachiocephalic arteries (A + B) show a decrease in fatty streak size in animals treated with anti Ang-2 antibody (C). There is no difference in lumen area or medial thickness (D + E) but a reduction in stenosis after anti-Ang-2 treatment (F). Plaque collagen content (percentage of Sirius Red positive area/total plaque area, G–I), percentage of macrophage area (Mac3+ area/total plaque area, J–L) and adventitial microvessel density (microvessels/mm2) (MVD) (M–O). Representative images: left panels IgG, right panels anti-Ang-2 antibody. IgG n = 12; anti-Ang-2 n = 14; *p < 0.05.
3.3. Existing and advanced plaques are not affected by antibody-mediated Ang-2 blockage
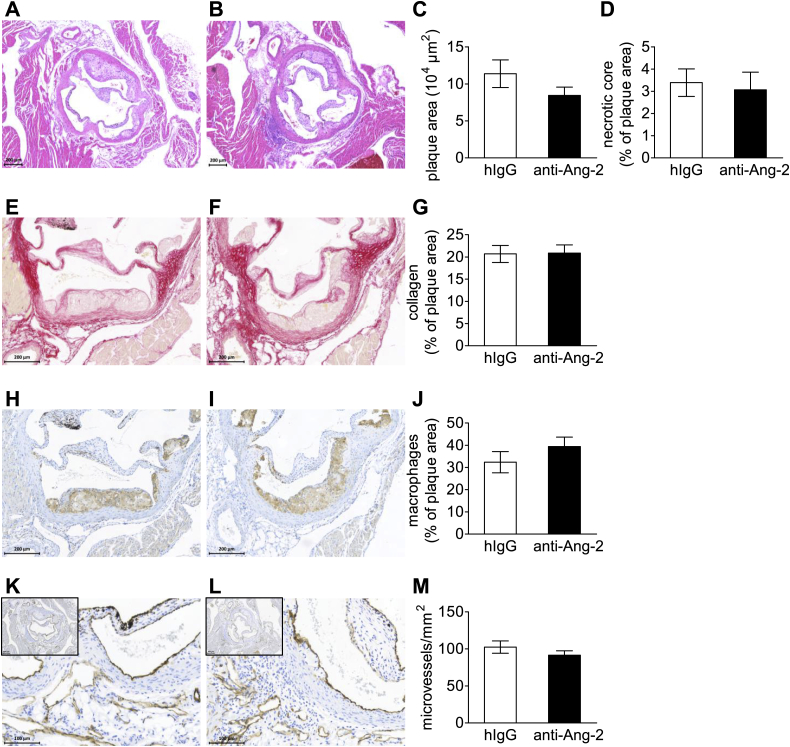
Mice were fed a high-cholesterol diet for three weeks followed by anti-Ang-2 antibody treatment while the diet was continued. This allowed us to analyze the pre-existing plaques in the aortic root in addition to fatty streaks in the brachiocephalic artery. Plaque sizes in aortic roots of mice treated with anti-Ang-2 did not differ from control IgG injected mice after eight weeks of high-cholesterol diet and five weeks of antibody administration (Fig. 3A–C). In addition, the treatment did not alter the amount of necrotic core (Fig. 3A, B and D), collagen content (Fig. 3E–G) or the macrophages in the plaques (Fig. 3H–J). No changes in intra-plaque vessels or adventitial microvessel density were observed (Fig. 3K–M and data not shown). Hence, Ang-2 blockage did not interfere with the progression or stability of pre-existing plaques.
Fig. 3.
Anti Ang-2 antibody treatment does not alter pre-existing atherosclerosis in the aortic root. Representative images of HE-stained plaques in the aortic root (A + B) show no difference in plaque size (C) or necrotic core (D) in pre-existing plaques of the aortic root after antibody treatment. Also, collagen (percentage of Sirius Red positive area/total plaque, E–G), macrophage content (Mac3+ area/total plaque area, H–J) and adventitial microvessel density (microvessels/mm2, K–M) were not altered by the anti-Ang-2 antibody treatment. Representative pictures: left panels control IgG, right panels anti-Ang-2. IgG n = 12; anti-Ang-2 n = 14.
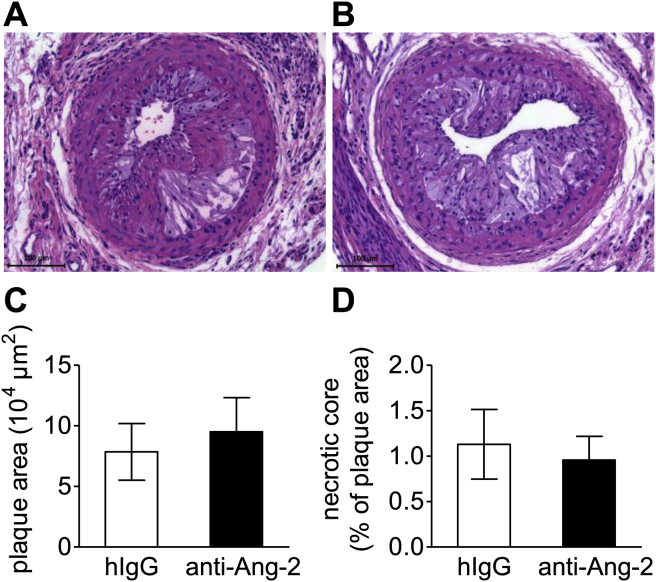
In addition to the pre-existing plaques in the aortic root, we were interested in the effects of the antibody on collar-induced fast-growing advanced plaques. The plaque size and necrotic core content in these rapidly progressing collar-induced advanced plaques did not differ between the anti-Ang-2 and control IgG-treated mice (Fig. 4A–D). The effect of anti-Ang-2 on fatty streak formation and atherosclerotic plaque development has been summarized in Table 1.
Fig. 4.
Advanced lesions in the carotid arteries are not affected by Ang-2 blockage. Representative images of HE-stained collar induced advanced plaques (A + B) in the right common carotid artery. Plaque size (C) necrotic core (D). Representative images and quantification of (E–G) collagen, (H–J) macrophages, (K–M) microvessels: left control IgG, right anti-Ang-2. IgG n = 12; anti-Ang-2 n = 14.
Table 1.
Summarizing table of the described data.
| Parameter | Tissue | Alteration in anti-Ang-2 mice | Statistics | |
|---|---|---|---|---|
| Plaque burden | Plaque size | Brachiocephalic artery | ↓ | * |
| Collar model | = | n.s. | ||
| Aortic root | = | n.s. | ||
| Degree of stenosis | Brachiocephalic artery | ↓ | ** | |
| Plaque phenotype | Necrotic core | Brachiocephalic artery | Absent in fatty streak | – |
| Collar model | = | n.s. | ||
| Aortic root | = | n.s. | ||
| Plaque collagen | Brachiocephalic artery | = | n.s. | |
| Aortic root | = | n.s. | ||
| Plaque macrophages | Brachiocephalic artery | = | n.s. | |
| Aortic root | = | n.s. | ||
| Microvessel density | Brachiocephalic artery | = | n.s. | |
| Aortic root | = | n.s. | ||
| Systemic effects | Angiopoetin-2 | Plasma | ↑ | ** |
| Cholesterol | Plasma | = | n.s. | |
| Triglycerides | Plasma | ↓ | * | |
| Cardiac function | US of the heart | = | n.s. | |
| Carotid strain + shear stress | US of carotid artery | = | n.s. | |
| Body weight | Before sacrifice | = | n.s. |
US = ultra sound; n.s. = not significant.
*p < 0.05, **p < 0.01.
4. Discussion
Our study is the first one to investigate Ang-2 depletion in experimental atherosclerosis and the results demonstrate that antibody-mediated Ang-2 depletion reduces fatty streak formation in the brachiocephalic arteries. In addition, anti-Ang-2 treatment reduces plasma triglycerides while plasma cholesterol levels remain unchanged. The anti-Ang-2 antibody did not interfere with pre-existing plaques of the aortic root or with collar-induced plaques in the carotid artery. Moreover, Ang-2 depletion had no effect on the stability of the plaque phenotype as microvessel density, macrophage and collagen content and the necrotic cores remained unchanged (Table 1).
4.1. Ang-2 loss-of-function model contradictory to gain-of-function model in atherosclerosis
To date, inhibition or knock-out of endogenous Ang-2 in human or murine atherosclerosis have not been reported. However, Ang-2 overexpression was shown to decrease plaque formation in the aortic root of ApoE knock-out mice via inhibition of LDL oxidation [6]. However, the impact on plaque stability was not studied in great detail. The authors only observed an absolute decrease in plaque macrophages after Ang-2 overexpression, albeit that data were not corrected for the reduced overall plaque size [6]. Therefore, the reduction in macrophage area probably only reflects the plaque stage. Concordant with this notion, in our study, we did not observe any difference in relative macrophage content (macrophage area normalized to the plaque area) after Ang-2 blockage. Enhancing Ang-2 may seem an attractive therapy at first sight. However, transgenic Ang-2 overexpression does not mimic the endogenous role of Ang-2 in atherogenesis, which is crucial for the translation to the human situation.
4.2. No role for Ang-2 blockage in plaque stabilization
Considering the stimulatory role of Ang-2 in various processes associated with vulnerable plaques [18], such as increased inflammation [13], plaque vascularity [5], microvascular leakage [15] and MMP expression [5], we expected profound effects of Ang-2 antagonism on the stable plaque phenotype. However, Ang-2 deletion was only able to prevent fatty streak formation; it did not prevent plaque progression or changes in plaque stability.
Changes in fatty streak formation could not be attributed to effects on plaque inflammation or angiogenesis. Rather, Ang-2 blockage may retard fatty streak formation by a reduction in plasma triglycerides, prevention of endothelial dysfunction or reduction of monocyte chemotaxis. We observed lower plasma triglycerides after Ang-2 antibody treatment, which might explain the decrease in fatty streak formation. Until recently, hypertriglyceridemia was regarded as a risk factor of cardiovascular disease based on epidemiological association only, with much controversy on possible causality. However, recent Mendelian randomization studies increasingly support the causative role for increased plasma triglycerides in cardiovascular disease and atherosclerosis [29,30]. Cellular effects are mediated by remnant lipoproteins, rich in triglycerides, rather than chylomicrons (reviewed in [31]). Mechanistically, direct uptake of these remnant lipoproteins by macrophages stimulates cholesterol ester accumulation and macrophage foam cell formation, an important step in fatty streak formation [32]. Neutral and oxidized free fatty acids generated by lipolysis of these triglyceride-rich lipoproteins can also stimulate endothelial dysfunction, subsequently enhancing vascular inflammation and lipoprotein retention [33], and hence fatty streak formation [31]. Thus, lower triglycerides may prevent endothelial dysfunction and foam cell formation, explaining the reduction in fatty streak formation. Although high triglyceride levels have also been associated with clinically relevant atherosclerosis [34], reduced levels were without effect on pre-existing atherosclerosis in our model.
Ang-2 blockage could decrease fatty streak formation also via processes that are regulated by the angiopoietin receptor Tie2, which is highly expressed in endothelial cells and monocytes, both involved in the early development of atherosclerosis. Ang-2 expressed by endothelial cells has been shown to stimulate monocyte chemotaxis [35], hence Ang-2 blockage may reduce monocyte recruitment to developing fatty streaks. However, as we did not observe alterations in macrophage content of fatty streaks, this seems unlikely. More likely, is the involvement of endothelial cell dysfunction and the subsequent permeability for lipoproteins. This is thought to be the initial step at the onset of atherosclerosis. Ang-2 is known to induce endothelial destabilization and vascular permeability [36], thus suggesting a beneficial effect of Ang-2 blockage.
4.3. Used Ang-2 antibody proven to be effective in other disease models
The Ang-2 blocking capacity of anti-Ang2 antibodies in vitro [12] and in vivo [12,22,27,37] has been firmly established and was confirmed in our study by using Ang-2 ELISA. It has been reported that treatment of cardiac allografts with anti-Ang2 protects from transplant inflammation and rejection [37]. This is in line with atherosclerosis inhibition by overexpression of the Tie2 agonist ligand, Ang1 in cardiac allografts [38]. Antibody-mediated Ang-2 blockage has been proven to reduce tumor growth [12] and lung metastasis formation [22] in murine cancer models by increasing tumor vessel stability. Moreover, Ang-2 is released by endothelial cells [39], thus anti-Ang-2 antibody has perfect access to the target through the adventitial microvasculature and the main arteries. It is thus unlikely that Ang-2 depletion or impaired tissue availability of the antibody could explain the lack of effect on plaque progression and stable plaque phenotype.
4.4. Inhibiting Ang-2 alone might not be sufficient
Alternatively, inhibiting Ang-2 only might not suffice to affect pre-existing atherosclerosis. Additional factors, such as vascular endothelial growth factor A (VEGF-A) should be targeted as well. For example, one could employ a bifunctional anti-VEGF-A/anti-Ang-2 antibody, which combines the inhibition of both growth factors; this has already provided a stronger anti-tumor effect than targeting the two growth-factors separately [40].
4.5. Ang-2 cause or consequence of atherosclerosis
The lack of effect on plaque growth or the stable plaque phenotype is in contrast to previously reported associations between Ang-2 and cardiovascular disease. Increased Ang-2 plasma levels have been correlated with cardiovascular disease progression, but have also been suggested to provide a biomarker for future cardiovascular events such as myocardial infarction or stroke [3,41–47]. Furthermore, Ang-2 was detected in advanced vascularized human plaques [48] and it was associated with a high microvessel content and destabilizing MMP-2 expression [5]. The outcomes of these studies support our findings that Ang-2 depletion helps to inhibit atherosclerosis development. Nevertheless, not all studies could show a correlation of Ang-2 with plaques at risk of rupture [49,50]. Perhaps the clinical correlation of Ang-2 expression and atherosclerosis reflects a compensatory expression in response to plaque vulnerability. Overall, Ang-2 may be correlated with cardiovascular diseases, but the results described in the present work do not support a causal role for it in atherosclerosis progression or plaque vulnerability. Importantly, our study gives no reason to think that Ang-2 blockage could have adverse effects on pre-existing atherosclerosis of treated cancer patients.
5. Conclusions
This is the first time Ang-2 blockage has been tested in an experimental model of atherosclerosis and found to have significant protective effects on fatty streak formation. Ang-2 blockage reduces plasma triglycerides and decreases early plaque formation, suggesting a beneficial effect of Ang-2 depletion on the early phase of atherosclerosis. In addition, no effects on pre-existing atherosclerosis or cardiac function were found, suggesting that anti-Ang-2 therapy is safe in various clinical settings.
6. Significance in the context of atherosclerosis
The present study is the first to investigate the effect of blocking Ang-2 on experimental atherosclerosis. In addition, the cardiovascular safety of the Ang-2 blocking was studied. It was shown that anti-Ang-2 therapy delayed fatty streak formation and decreased plasma triglyceride levels. Pre-existing atherosclerosis and cardiac function were unaltered. The results suggest a favorable safety profile for the clinical use of antibody mediated Ang-2 blockage.
Sources of funding
Research was supported by Finnish Academy (141069), ERC (ERC-2009-AdG-250050, FutureGenes) grant, Sigrid Juselius Foundation, Finnish Foundation for Cardiovascular Research (all to S.Y.); the European Research Council (ERC-2010-AdG-268804, VESSEL network), Leducq Transatlantic Network of Excellence on Lymph Vessels in Obesity and Cardiovascular Disease (11CVD03) (all to K.A.), a VENI fellowship of the Netherlands Organization of Scientific research (to J.C.S. 016.116.017); a PhD-student fellowship from the Cardiovascular Research Institute Maastricht (to T.L.T.).
Disclosures
None.
Acknowledgements
The authors would like to thank Dr. ChingChing Leow and MedImmune for the anti-Ang-2 antibodies, and the staff of the Experimental animal facility at the University of Eastern Finland, Kuopio.
References
- 1.Llovet J.M. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2012;18:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler T. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J. Clin. Invest. 2013;123(8):3436–3445. doi: 10.1172/JCI66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iribarren C. Circulating angiopoietins-1 and -2, angiopoietin receptor tie-2 and vascular endothelial growth factor-a as biomarkers of acute myocardial infarction: a prospective nested case-control study. BMC Cardiovasc. Disord. 2011;11:31. doi: 10.1186/1471-2261-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Dall J. Immaturity of microvessels in haemorrhagic plaques is associated with proteolytic degradation of angiogenic factors. Cardiovasc. Res. 2010;85:184–193. doi: 10.1093/cvr/cvp253. [DOI] [PubMed] [Google Scholar]
- 5.Post S. Balance between angiopoietin-1 and angiopoietin-2 is in favor of angiopoietin-2 in atherosclerotic plaques with high microvessel density. J. Vasc. Res. 2008;45:244–250. doi: 10.1159/000112939. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed A. Angiopoietin-2 confers atheroprotection in apoe-/- mice by inhibiting ldl oxidation via nitric oxide. Circ. Res. 2009;104:1333–1336. doi: 10.1161/CIRCRESAHA.109.196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowles E.J. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J. Natl. Cancer Inst. 2012;104:1293–1305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J. Am. Coll. Cardiol. 2012;60:2504–2512. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 9.Choueiri T.K. Congestive heart failure risk in patients with breast cancer treated with bevacizumab. J. Clin. Oncol. 2011;29:632–638. doi: 10.1200/JCO.2010.31.9129. [DOI] [PubMed] [Google Scholar]
- 10.Scappaticci F.A. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J. Natl. Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 11.Saharinen P. Vegf and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med. 2011;17:347–362. doi: 10.1016/j.molmed.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Leow C.C. Medi3617, a human anti-angiopoietin 2 monoclonal antibody, inhibits angiogenesis and tumor growth in human tumor xenograft models. Int. J. Oncol. 2012;40:1321–1330. doi: 10.3892/ijo.2012.1366. [DOI] [PubMed] [Google Scholar]
- 13.Fiedler U. Angiopoietin-2 sensitizes endothelial cells to tnf-alpha and has a crucial role in the induction of inflammation. Nat. Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 14.Lobov I.B. Angiopoietin-2 displays vegf-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benest A.V. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS One. 2013;8:e70459. doi: 10.1371/journal.pone.0070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhandari V. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat. Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murdoch C. Expression of tie-2 by human monocytes and their responses to angiopoietin-2. J. Immunol. 2007;178:7405–7411. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 18.Yla-Herttuala S. Stabilization of atherosclerotic plaques: an update. Eur. Heart J. 2013;34:3251–3258. doi: 10.1093/eurheartj/eht301. [DOI] [PubMed] [Google Scholar]
- 19.Moreno P.R. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 20.Sluimer J.C. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J. Am. Coll. Cardiol. 2009;53:1517–1527. doi: 10.1016/j.jacc.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von der Thusen J.H. Induction of rapid atherogenesis by perivascular carotid collar placement in apolipoprotein e-deficient and low-density lipoprotein receptor-deficient mice. Circulation. 2001;103:1164–1170. doi: 10.1161/01.cir.103.8.1164. [DOI] [PubMed] [Google Scholar]
- 22.Holopainen T. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J. Natl. Cancer Inst. 2012;104:461–475. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huusko J. Aav9-mediated vegf-b gene transfer improves systolic function in progressive left ventricular hypertrophy. Mol. Ther. 2012;20:2212–2221. doi: 10.1038/mt.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feintuch A. Hemodynamics in the mouse aortic arch as assessed by mri, ultrasound, and numerical modeling. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H884–H892. doi: 10.1152/ajpheart.00796.2006. [DOI] [PubMed] [Google Scholar]
- 25.Castier Y. P47phox-dependent nadph oxidase regulates flow-induced vascular remodeling. Circ. Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
- 26.Lutgens E. Atherosclerosis in apoe*3-leiden transgenic mice: from proliferative to atheromatous stage. Circulation. 1999;99:276–283. doi: 10.1161/01.cir.99.2.276. [DOI] [PubMed] [Google Scholar]
- 27.Natale R.B. Safety, pharmacokinetics, and antitumor activity of medi3617, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J. Clin. Oncol. 2012;30 doi: 10.1200/JCO.2008.19.6683. (suppl; abstr TPS2621) [DOI] [PubMed] [Google Scholar]
- 28.Lutgens E. Requirement for cd154 in the progression of atherosclerosis. Nat. Med. 1999;5:1313–1316. doi: 10.1038/15271. [DOI] [PubMed] [Google Scholar]
- 29.Johansen C.T. Using mendelian randomization to determine causative factors in cardiovascular disease. J. Intern Med. 2013;273:44–47. doi: 10.1111/j.1365-2796.2012.02586.x. [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen A.B. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 2013;34:1826–1833. doi: 10.1093/eurheartj/ehs431. [DOI] [PubMed] [Google Scholar]
- 31.Watts G.F. Demystifying the management of hypertriglyceridaemia. Nat. Rev. Cardiol. 2013;10:648–661. doi: 10.1038/nrcardio.2013.140. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein J.L. Cholesteryl ester accumulation in macrophages resulting from receptor-mediated uptake and degradation of hypercholesterolemic canine beta-very low density lipoproteins. J. Biol. Chem. 1980;255:1839–1848. [PubMed] [Google Scholar]
- 33.Wang L. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized ffas that induce endothelial cell inflammation. J. Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triglyceride Coronary Disease Genetics C Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholz A. Angiopoietin-2 promotes myeloid cell infiltration in a beta(2)-integrin-dependent manner. Blood. 2011;118:5050–5059. doi: 10.1182/blood-2011-03-343293. [DOI] [PubMed] [Google Scholar]
- 36.Hakanpaa L. Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat. Commun. 2015;6:5962. doi: 10.1038/ncomms6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syrjala S.O. Angiopoietin-2 inhibition prevents transplant ischemia-reperfusion injury and chronic rejection in rat cardiac allografts. Am. J. Transpl. 2014;14:1096–1108. doi: 10.1111/ajt.12672. [DOI] [PubMed] [Google Scholar]
- 38.Nykanen A.I. Angiopoietin-1 protects against the development of cardiac allograft arteriosclerosis. Circulation. 2003;107:1308–1314. doi: 10.1161/01.cir.0000054623.35669.3f. [DOI] [PubMed] [Google Scholar]
- 39.Augustin H.G. Control of vascular morphogenesis and homeostasis through the angiopoietin-tie system. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 40.Kienast Y. Ang-2-vegf-a crossmab, a novel bispecific human igg1 antibody blocking vegf-a and ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin. Cancer Res. 2013;19:6730–6740. doi: 10.1158/1078-0432.CCR-13-0081. [DOI] [PubMed] [Google Scholar]
- 41.Chung N.A. Measurement of the soluble angiopoietin receptor tie-2 in patients with coronary artery disease: development and application of an immunoassay. Eur. J. Clin. Invest. 2003;33:529–535. doi: 10.1046/j.1365-2362.2003.01173.x. [DOI] [PubMed] [Google Scholar]
- 42.David S. Angiopoietin 2 and cardiovascular disease in dialysis and kidney transplantation. Am. J. Kidney Dis. 2009;53:770–778. doi: 10.1053/j.ajkd.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 43.David S. Circulating angiopoietin-2 in essential hypertension: relation to atherosclerosis, vascular inflammation, and treatment with olmesartan/pravastatin. J. Hypertens. 2009;27:1641–1647. doi: 10.1097/HJH.0b013e32832be575. [DOI] [PubMed] [Google Scholar]
- 44.Golledge J. Increased serum angiopoietin-2 is associated with abdominal aortic aneurysm prevalence and cardiovascular mortality in older men. Int. J. Cardiol. 2013;167:1159–1163. doi: 10.1016/j.ijcard.2012.03.120. [DOI] [PubMed] [Google Scholar]
- 45.Lee K.W. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation. 2004;110:2355–2360. doi: 10.1161/01.CIR.0000138112.90641.7F. [DOI] [PubMed] [Google Scholar]
- 46.Patel J.V. Angiopoietin-2 levels as a biomarker of cardiovascular risk in patients with hypertension. Ann. Med. 2008;40:215–222. doi: 10.1080/07853890701779586. [DOI] [PubMed] [Google Scholar]
- 47.Wang X. Changes and significance of serum angiopoietin-2 levels in patients with coronary heart disease. Biomarkers. 2012;17:745–749. doi: 10.3109/1354750X.2012.727028. [DOI] [PubMed] [Google Scholar]
- 48.Calvi C. Angiopoietin 2 induces cell cycle arrest in endothelial cells: a possible mechanism involved in advanced plaque neovascularization. Arterioscler. Thromb. Vasc. Biol. 2004;24:511–518. doi: 10.1161/01.ATV.0000116864.86607.35. [DOI] [PubMed] [Google Scholar]
- 49.Chen F. Apoptosis and angiogenesis are induced in the unstable coronary atherosclerotic plaque. Coron. Artery Dis. 2005;16:191–197. doi: 10.1097/00019501-200505000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Pelisek J. Neovascularization and angiogenic factors in advanced human carotid artery stenosis. Circ. J. 2012;76:1274–1282. doi: 10.1253/circj.cj-11-0768. [DOI] [PubMed] [Google Scholar]