Abstract
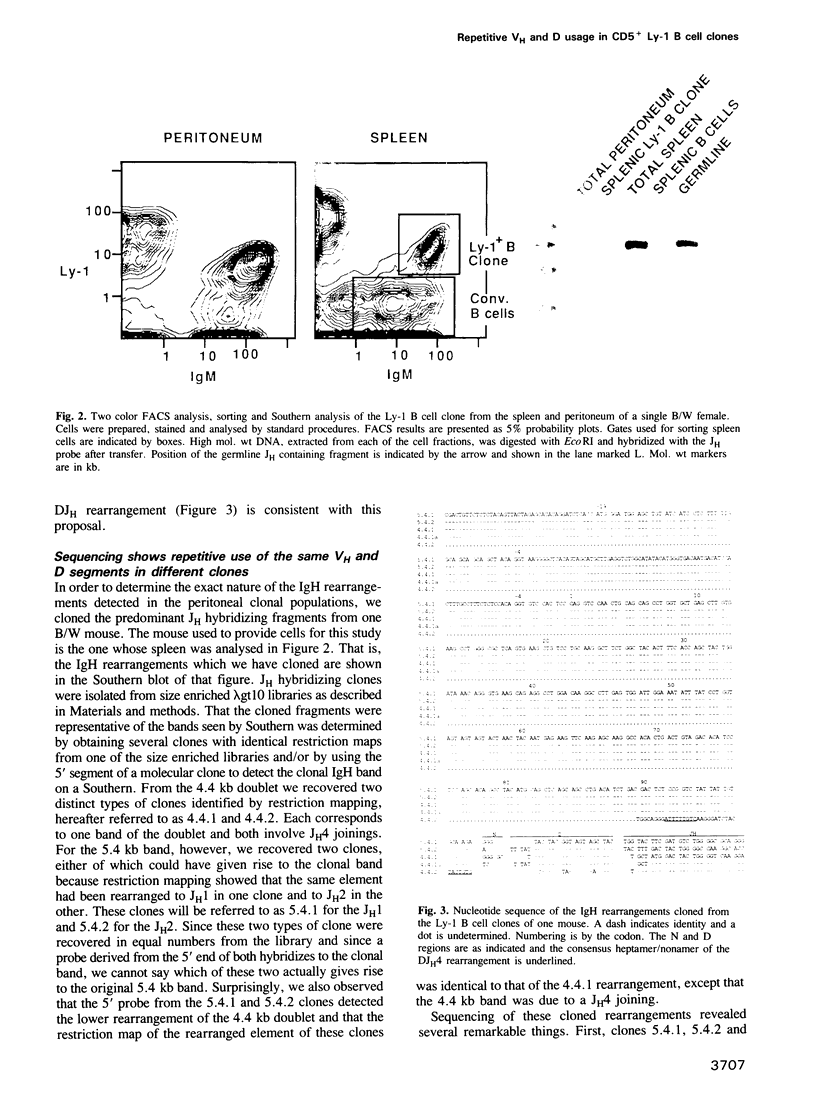
The usually small Ly-1 B cell population is markedly increased in older mice by expansion of certain clones. This results in a cellular picture very similar to human B chronic lymphocytic leukemia. Here we report a molecular analysis of the immunoglobulin gene rearrangements of the Ly-1 B cell populations in (NZB x NZW)F1 females. We find that (i) the number of clones found in the peritoneum (a major tissue source of Ly-1 B cells) decreases with age till mono- or biclonality is common by approximately 6 months, (ii) many clones from different mice show the same size rearrangements at both the Ig heavy and light chain loci and (iii) the IgH rearrangements found in a clone isolated from the spleen of one mouse are a subset of those found in the peritoneum of the same mouse, implying migration occurs from the peritoneum to the spleen. Molecular cloning and sequencing of the IgH rearrangements from the peritoneal clones of one B/W mouse revealed that all productive rearrangements used the identical unmutated VH and D elements joined to different JHS. Indeed, two VDJH4 rearrangements were recovered which were identical but for six junctional (N region) nucleotides. The conservation of VH and D segment usage in the rearrangements of these Ly-1 B cell clones could indicate some strong selective pressure for clonal expansion (for example antigen selection) operates via the immunoglobulin molecules of these cells. Southern analyses of other (NZB x NZW)F1 mice with this cloned VH and the usage of the same or similar VH genes among a number of Ly-1 B origin tumors in other mouse strains indicate the generality of this repetitive VH gene usage in individual mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Galili N., Trela M., Levy R., Sklar J. Single cell origin of bigenotypic and biphenotypic B cell proliferations in human follicular lymphomas. J Exp Med. 1988 Feb 1;167(2):582–597. doi: 10.1084/jem.167.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dildrop R., Krawinkel U., Winter E., Rajewsky K. VH-gene expression in murine lipopolysaccharide blasts distributes over the nine known VH-gene groups and may be random. Eur J Immunol. 1985 Nov;15(11):1154–1156. doi: 10.1002/eji.1830151117. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Förster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987 Apr;17(4):521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Bishop G. A., Mercolino T. J. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986 Oct;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Stall A. M., Herzenberg L. A., Herzenberg L. A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986 Oct;16(10):1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T., Huang S. Y., Gefter M. L. Influence of clonal selection on the expression of immunoglobulin variable region genes. Science. 1984 Dec 14;226(4680):1283–1288. doi: 10.1126/science.6334361. [DOI] [PubMed] [Google Scholar]
- Osmond D. G. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986 Oct;93:103–124. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Hardy R. R., Herzenberg L. A. Three-color immunofluorescence analysis of mouse B-lymphocyte subpopulations. Cytometry. 1984 Mar;5(2):159–168. doi: 10.1002/cyto.990050210. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Reininger L., Ollier P., Poncet P., Kaushik A., Jaton J. C. Novel V genes encode virtually identical variable regions of six murine monoclonal anti-bromelain-treated red blood cell autoantibodies. J Immunol. 1987 Jan 1;138(1):316–323. [PubMed] [Google Scholar]
- Reth M., Gehrmann P., Petrac E., Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. 1986 Aug 28-Sep 3Nature. 322(6082):840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Hillson J. L., Perlmutter R. M. Early restriction of the human antibody repertoire. Science. 1987 Nov 6;238(4828):791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Fariñas M. C., Tarlinton D. M., Lalor P. A., Herzenberg L. A., Strober S., Herzenberg L. A. Ly-1 B-cell clones similar to human chronic lymphocytic leukemias routinely develop in older normal mice and young autoimmune (New Zealand Black-related) animals. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7312–7316. doi: 10.1073/pnas.85.19.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Chiang N. Y. Proliferation of Ly-1 B cells in autoimmune NZB and (NZB x NZW)F1 mice. Eur J Immunol. 1987 Jun;17(6):809–814. doi: 10.1002/eji.1830170612. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]