Abstract
Infection with respiratory syncytial virus (RSV) generally presents as a mild, upper airway disease in human patients but may cause severe lower airway disease in the very young and very old. Progress toward understanding the mechanisms of RSV pathogenesis has been hampered by a lack of relevant rodent models. Mice, the species most commonly used in RSV research, are resistant to upper respiratory infection and do not recapitulate the pattern of virus spread in the human host. To address the need for better rodent models of RSV infection, we have characterized the acute and chronic pathology of RSV infection of a relatively permissive host, cotton rats (Sigmodon hispidus). We demonstrate that virus delivered to the upper airway results in widespread RSV replication in the ciliated respiratory epithelial cells of the nasal cavity and, to a lesser extent, of the lung. Although acute inflammation is relatively mild and rapidly eliminated after viral clearance, chronic, eosinophilic lung pathology persists. These data support the use of cotton rats as a robust rodent model of human RSV disease, including the association between RSV pneumonia and subsequent development of allergic asthma.
Abbreviations: BAL, bronchoalveolar lavage; RSV, human respiratory syncytial virus
Human respiratory syncytial virus (RSV) is the primary cause of lower airway disease in infants and children worldwide.39 Although generally limited to the upper airway, RSV infection can also manifest as bronchiolitis and pneumonia in young children and the elderly and has been implicated as a major cause of middle ear infections (otitis media).3,55 In addition to the problems associated with acute illness, children who experience severe RSV disease in infancy are at increased risk for development of asthma and recurrent wheezing later in childhood.35,53,56
RSV is ubiquitous worldwide, with most children infected in infancy, and essentially all children are infected by 3 y of age.20 Although reinfection throughout life has been documented, the true incidence of upper airway infection is difficult to quantify, given that treatment for such infections typically is not sought. In comparison, lower airway infection is more likely to come to medical attention, and it is currently estimated that at least 33.8 million episodes of RSV-induced acute pulmonary infection in children younger than 5 y occur yearly and that as many as 200,000 of those episodes are fatal.40 Although supportive treatment for severe RSV disease is highly effective in infants, access to such treatment is generally only available in industrialized countries. More than 90% of fatal RSV cases occur in the developing world.40
Mice have been used extensively to study RSV infection, yet there are key limitations of the mouse model for the study of human RSV disease and immunity. The most important of these is the poor permissiveness of the mouse for human RSV. RSV replicates to a limited extent in the mouse lung, and large viral loads delivered in a relatively large volume are generally used with this model. Even with intranasal inoculation, primarily lower, and not upper, airway infection is achieved in WT mice. This scenario is unlike the human disease, in which the upper airway is the primary target of RSV replication.24,25 Mice are essentially resistant to upper airway infection, with little to no virus detected in nasal washes evaluated by plaque assay, and only rare RSV-antigen–positive cells are detected by immunohistochemistry.13,18,21 In addition to this altered route of entry, the pattern of lung infection is markedly divergent between humans and mice. In humans, RSV primarily infects ciliated bronchiolar epithelial cells and, to a much lesser extent, alveolar cells.32 In contrast, bronchiolar epithelial cells are infected only rarely in the mouse lung; instead RSV targets the pneumocytes.37
Unlike mice, cotton rats are susceptible to RSV infection of the upper airway, and the pattern of lower airway infection mirrors that seen in human patients. The cotton rat was established as a model of RSV infection more than 3 decades ago and has since emerged as the preferred rodent model in which to evaluate RSV therapeutics and vaccine candidates.19,23,33,41,49,57,60 In addition, as the availability of species-specific reagents has improved, cotton rats have become an increasingly useful model in which to study RSV pathogenesis.6 However, whereas the susceptibility of cotton rats to RSV infection has been established firmly, the pathology of RSV infection in this species has not yet been extensively characterized.
Asthma clearly is a multifactorial disease, dependent on both genetic and environmental factors (see references 28 and 30 for recent reviews), and many studies have pointed to a role for respiratory virus infection in the induction of asthma. In one study,17 the combination of atopy and the presence of virus in nasal secretions synergistically increased the odds ratio for wheezing in children 25-fold, and another study29 showed that repeated rhinovirus infections in the first 3 y of life increased by 26-fold the risk of developing asthma by the age of 6 y. This relationship is obviously a complex one, influenced both by the nature and the timing of the viral infection. Nonetheless, as many as 80% of acute asthma exacerbations in children and approximately 50% in adults are associated with viral infection.33,42 The majority of these infections are attributed to rhinoviruses, but other respiratory viruses, including influenza virus, RSV, and coronaviruses, can provoke these attacks.33,42 Beyond the exacerbation of established asthma, evidence that severe RSV disease in infancy is correlated with development of asthma and recurrent wheezing in later childhood is mounting. An association between lower airway RSV infection and subsequent development of recurrent wheezing and asthma was demonstrated more than 30 y ago,16 and several recent prospective studies have strengthened this correlation.27,53
In the current study, we expand upon existing knowledge of the cotton rat model of RSV infection by characterizing the spread of virus after droplet inhalation and the early and late inflammatory response to viral challenge. We confirm the observation that cotton rats are relatively permissive in regard to RSV infection as compared with mice, and we demonstrate that the infection of cotton rats, like that in the human host, is primarily an upper airway phenomenon. In addition, we show that primary RSV infection of the cotton rat lung, even at the low levels in this study, induces chronic changes consistent with those in cases of human allergic asthma. This finding is of interest because of the substantial literature associating early, severe respiratory infection with the development of asthma later in life. The studies of RSV infection in cotton rats that we describe here suggest that this species may be predisposed to atopy and that, long after virus clearance, changes associated with allergic inflammation persist in human hosts. Therefore, in addition to the usefulness of cotton rats for testing RSV treatments and vaccine candidates, we suggest that this species may serve as a model system for determining the involvement of virus infection in the development of allergic asthma.
Materials and Methods
Cotton rats.
Female cotton rats (Sigmodon hispidus; age, 6 to 10 wk; Sigmovir Biosystems, Bethesda, MD) were used in this study. Cotton rats were housed in groups of 4 in a barrier facility and received a commercial pelleted rat chow and water free choice. On the basis of physical examination by a licensed veterinarian, all cotton rats were considered healthy on arrival at our facility. Testing for specific pathogens was not performed, because naturally occurring infectious diseases of cotton rats are not well characterized. The cotton rats were acclimated for 7 to 10 d prior to viral challenge. All procedures used here were conducted humanely. Animal housing and experimental procedures were approved by the IACUC at the New York University School of Medicine and Rutgers New Jersey Medical School. Cohorts of 4 to 5 animals were used for each experimental point.
Virus and viral infection.
Human RSV strain A2, originally obtained from R Chanock at the NIH, was passaged on murine STAT1−/− fibroblast monolayers. For preparation of virus stock, STAT1−/− fibroblasts were infected at a multiplicity of infection of approximately 0.02 and incubated in DMEM with 5% fetal calf serum for 36 to 48 h until nearly all cells were involved in syncytia. Medium was collected and centrifuged at 1000 × g for 5 min, then pelleted cells were resuspended in E3E (3% ethanol, 0.025 M glucose, 0.15 M NaCl, 0.00533 M KCl, 0.001 M Na2EDTA, 0.00916 M Na2HPO4, 0.025 M HEPES pH 7.6, 0.0015% phenol red), 1 mL per 60 cm2 cells. This preparation was centrifuged at 3000 × g for 5 min. The supernatant was then centrifuged at 20,000 × g for 2 h and the resulting pellet resuspended in 0.1 M NaCl, 0.02M HEPES pH7.6, 0.005M MgCl2. After an additional centrifugation at 3000 × g for 5 min, the resulting supernatant was used as virus stock. In experiments using UV-inactivated virus, the virus was inactivated by exposure to a 30-W germicidal UV bulb for 2 min at a distance of 60 cm from the source.
While under isofluorane anesthesia, cotton rats were intranasally challenged with 1 × 106 pfu RSV (or were mock challenged with sterile saline) in a 50-µL total volume divided equally between nares. The viral inoculum was delivered slowly, over approximately 15 s, in a small volume to restrict initial virus infection to the upper airway; 8 cotton rats were used for each time point. Saline-challenged controls were examined at a single time point only, that is, 1 d after saline instillation. Infected cotton rats were examined on days 2, 4, 5, 6, 7, 10 and 28 after inoculation. At each time point, nasal wash and bronchoalveolar lavage (BAL) fluids were collected from 4 cotton rats; lung (right lung for plaque assay and left lung for histology and immunohistochemistry) and nasal cavities (for histology and immunohistochemistry) were collected from the remaining 4 cotton rats in the group. Thus, each sample or tissue was used for only one purpose (for example, lung histology was not performed on lungs that had been lavaged with saline). After inoculation with live or UV-inactivated virus, cohorts of 5 cotton rats were euthanized, with collection of BAL fluids and lung tissue, at day 5 after treatment.
Viral plaque assay.
Cotton rats were euthanized by CO2 asphyxiation at multiple time points until 28 d after virus challenge. Nasal wash fluids and lungs were collected immediately after euthanasia and stored at –80 °C until use. Viral plaque assay was performed on murine STAT1−/− fibroblast monolayers as previously described.18
BAL analysis.
Immediately after CO2 asphyxiation of cotton rats, BAL fluids were collected by washing the lung with 2 mL of sterile saline. Cell differentials were determined on Wright–Geimsa-stained preparations (CytoSpin, Thermo Scientific, Waltham, MA).
Histology and immunohistochemistry.
Tissue specimens were collected immediately after CO2 asphyxiation. Nasal cavities were fixed in neutral buffered formalin then decalcified with 0.35 M EDTA in 0.1 M Tris (pH 6.95). The right lung and decalcified nasal cavities were processed routinely, paraffin-embedded and sectioned at 5 µm. Tissues sections were stained with hematoxylin and eosin or were left unstained for immunohistochemistry. For immunohistochemistry, tissue sections were incubated with goat polyclonal RSV antiserum (Biodesign, Saco, ME) diluted 1:500 followed by incubation with biotinylated rabbit antigoat antibody (ScyTek, Logan, UT), streptavidin linked to horseradish peroxidase, and 3-amino-9-ethylcarbazole chromagen (Scytek). Labeled tissue sections were counterstained with hematoxylin. Parental consent was obtained for the use of autopsy material for research.
Statistical analysis.
SigmaPlot 12.0 (Systat Software, San Jose, CA) was used to perform Student t tests where appropriate. A P value less than 0.05 was considered statistically significant.
Results
Intranasal RSV inoculation causes diffuse upper airway and focal lower airway infection in cotton rats.
Although the cotton rat is considered the ‘gold standard’ for preclinical evaluation of RSV therapeutics and vaccine candidates, the pathology of RSV infection in this species has not been extensively characterized. To address this gap, we performed intranasal RSV infections of cotton rats by using a small-volume viral inoculum, delivered slowly, to restrict initial infection to the nasopharynx. We then tracked viral replication and associated pathology during the acute infection and at a late time point (28 d after infection), when active virus infection had resolved. Virus replication was assayed in the nasal cavity and lung tissues via immunohistochemistry and plaque assay. As expected, plaque assay results were consistent with those published previously,46 with peak lung titers on the order of 104 to 105 pfu per gram of tissue and nasal wash titers of 103–104 pfu/mL. Immunohistochemisty for RSV antigens showed diffuse viral replication in the nasal cavity, which peaked on day 5 (Figure 1), but only scattered foci of viral infection were seen in the lung (Figure 2). By day 4 after infection, the peak of viral replication in the lung, RSV antigen was visualized in the lungs of 75% of rats, dropping to 25% at day 6, when extensive upper airway infection was still present in 100% of rats. RSV antigen was not detected in the lung beyond day 6 (data not shown). No clinical signs of illness were apparent at any time point. These findings are in agreement with previous work.46
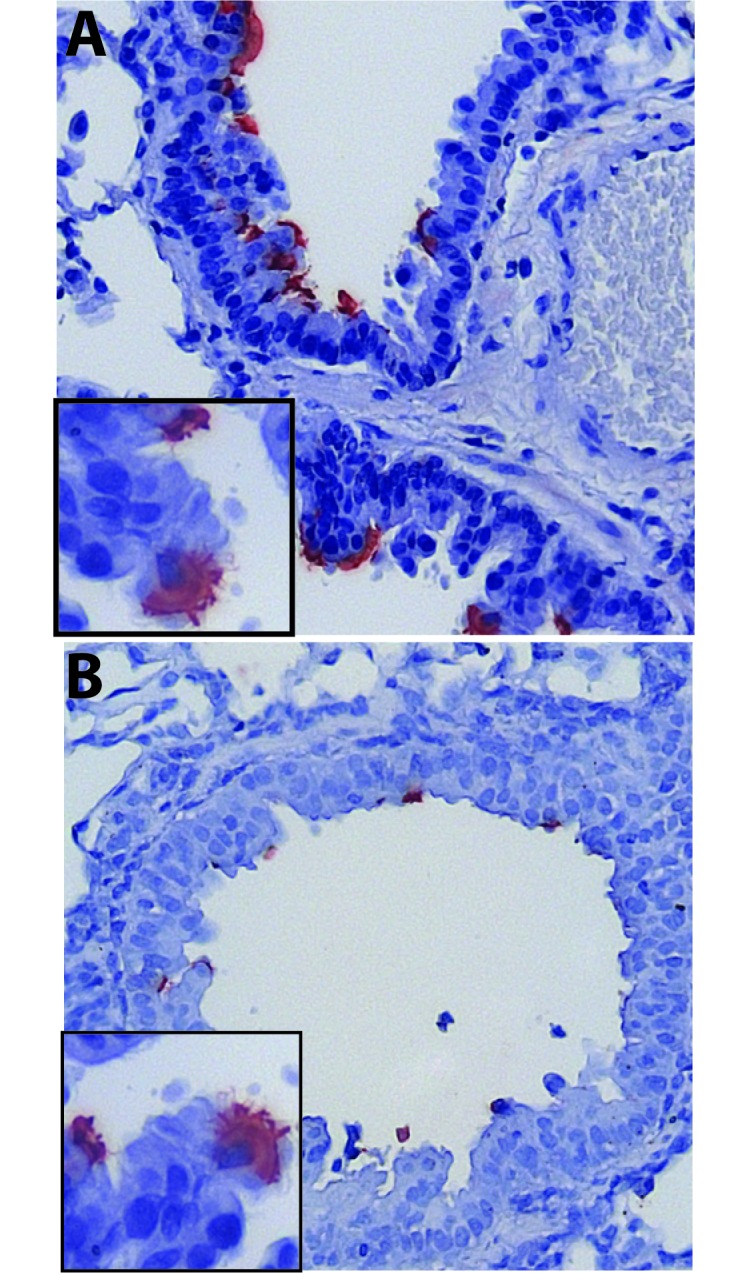
Figure 1.
Distribution and extent of RSV infection of the upper airways of cotton rats. Nasal cavity tissues were collected from cotton rats at multiple early time points after RSV infection and examined for RSV antigens by immunohistochemistry. Widespread infection of ciliated respiratory epithelial cells was detected in 100% of cotton rats (A) 4 d, (C) 5 d, and (E) 6 d after RSV infection, with peak infection observed at the 5-d time point. Infection was not limited to ciliated respiratory epithelial cells but also was detected in neuronal cells of the olfactory epithelium (B, 4 d after RSV infection). On days 5 and 6, mucus with sloughed, RSV-antigen–positive, epithelial cells was present in upper airway lumina (D and F). Images were captured at magnifications of 100× (A, C, E), 200× (B, D, F), and 400× (insets).
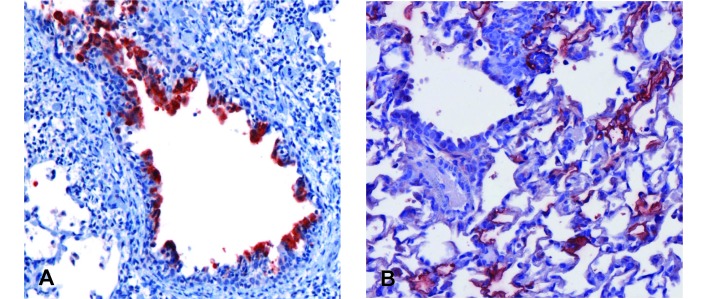
Figure 2.
Distribution and extent of acute RSV infection of the cotton rat lower airway. Lung tissue was collected from cotton rats at multiple time points early after RSV infection and examined for RSV antigens by using immunohistochemistry. Sporadic infection of ciliated respiratory epithelial cells lining bronchioles was detected in the lungs of 100%, 75%, and 25% of rats at 2 d, (A) 4 d, and (B) 6 d after RSV infection, respectively. Lung involvement, always focal, decreased markedly by 6 after infection with detection of only rare, weakly antigen-positive cells, with antigen restricted to the cilia. RSV antigen was not detected in lung collected after the 6-d time point (not shown). Images were captured at a magnification of 200× or 400× (insets).
Like in human patients but unlike in mice, the ciliated respiratory epithelial cells, which line the nasal cavity, were the primary target for RSV infection in cotton rats.26,32 However, in addition to these cells, we noted RSV infection of the olfactory epithelium of the caudal nasal cavity (Figure 1 B). The olfactory epithelium, which lines the rostral portion of the nasal cavity, comprises bipolar olfactory neurons surrounded by supporting sustentacular cells and the excretory ducts from submucosal mucus glands. Viral antigen was present in the ductal epithelium and neuronal cell bodies. We previously noted RSV infection of the olfactory epithelium in both the mouse and chinchilla models;18 this pattern has not been observed in human patients, but examination of this tissue is not standard medical practice. As acute infection progressed through day 6, the upper airway contained abundant mucus with sloughed epithelial cells, many of which were positive for RSV antigens (Figure 1 D and F). Copious mucus production is a feature of RSV infection in human hosts.9
RSV infection persists in the upper airway of cotton rats.
One of the remaining uncertainties in RSV epidemiology is the basis for seasonal outbreaks. RSV infection occurs only in the winter months in temperate climates, yet no animal reservoir, which could sustain the virus between human outbreaks, has been identified. Although not demonstrated convincingly, low-levels of virus are suspected to be harbored in the upper respiratory tract of some subjects throughout the year. During seasonal outbreaks, for reasons unknown, virus replication resumes, virus particles are shed, and infection of the human population is maintained.52,54 In support of this hypothesis, we previously demonstrated that viral antigen can be detected in the upper airway of chinchillas as long as 14 d after RSV infection, when productive infection is no longer detectable by plaque assay.22 We sought to determine whether the upper airway of cotton rats is similarly permissive to persistent RSV infection. To this end, we collected nasal cavities 28 d after RSV infection and performed immunohistochemistry for RSV antigen. Low levels of RSV antigen were present in the upper airways of 100% of rats at this late time point (Figure 3). This result was in contrast to that for the lower airway, which was negative for virus by 6 d after infection (data not shown).
Figure 3.
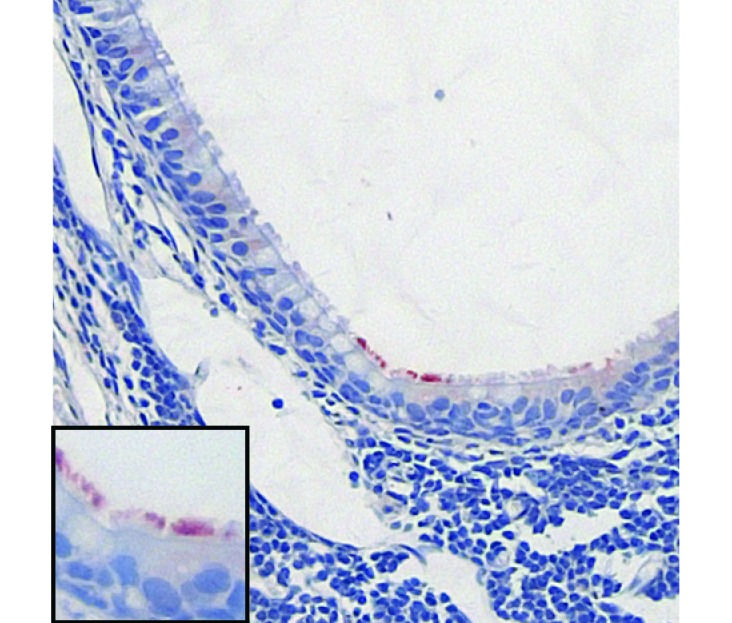
Persistence of RSV infection in the upper airway of cotton rats. Nasal cavity tissues were collected from cotton rats 28 d after RSV infection and examined for RSV antigens by using immunohistochemistry. Rare antigen-positive ciliated respiratory epithelial cells were detected. Epithelium shown here overlies the nasal associated lymphoid tissue (NALT). Images were captured at a magnification of 200× or 400× (inset).
RSV infection of the upper airway of cotton rats triggers a mixed inflammatory infiltrate followed by rapid resolution.
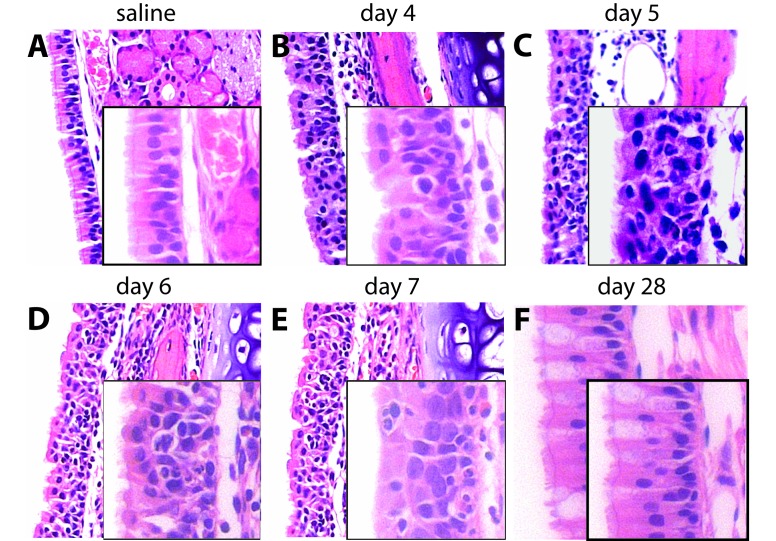
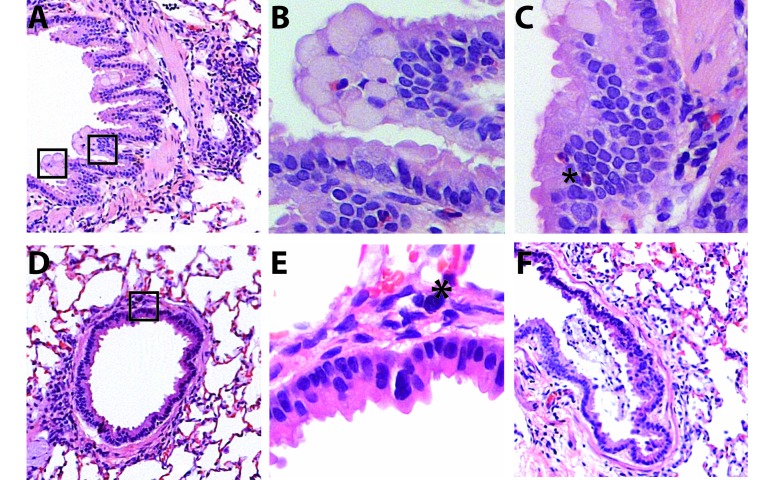
The upper airway of cotton rats, as compared with mice, has long been recognized as permissive for RSV replication. Nonetheless, most studies using this model have focused on lung, rather than nasal, pathology.8,14,34,59 Because RSV is primarily an upper airway infection in humans, we wanted to characterize disease that followed intranasal delivery of a small volume of inoculum rather than a volume sufficient to infect the nose and lung simultaneously, as had been done previously.46 Using the small-volume approach, we observed that the diffuse virus spread was accompanied by a mixed inflammatory response that peaked at day 5 after infection but was essentially resolved by the 28-d time point despite evidence of viral persistence. The infiltrate was composed of neutrophils, eosinophils, macrophages, and lymphocytes and involved the epithelium and underlying submucosa (Figure 4 A through C). Mucosal injury was evident by day 5, with regenerative changes, marked by basal cell proliferation, observed as early as 6 d after RSV infection (Figure 4 D). Resolution of nasal mucosal inflammation was essentially complete by 28 d after RSV infection, in that only rare inflammatory cells remained, but goblet cell metaplasia persisted (Figure 4 F).
Figure 4.
Histopathology of acute and chronic RSV infection of the upper airway in cotton rats. Histology was performed on tissues from the nasal cavity collected until 28 d after viral challenge. The surfaces of the nasal septum and turbinates in the anterior portion of the nasal cavity are covered primarily by columnar or pseudocolumnar respiratory epithelium, and sections from that area are shown here. (A) Naïve upper respiratory mucosa is characterized by an orderly arrangement of ciliated respiratory epithelial cells with small numbers of more granular-appearing, mucus-producing, goblet cells. RSV infection resulted in acute inflammation most severe at the 5-d time point, with resolution beginning by day 6. (B) A mixed inflammatory infiltrate in the epithelial layer and the submucosa was present in nasal mucosa collected 4 d after infection. (C) Increasingly severe inflammation was present 5 d after RSV infection. (D) By 6 d after RSV infection, inflammation persisted, but early evidence of epithelial regeneration emerged, characterized by basal cell proliferation. (E) Lingering histopathologic evidence of inflammation, including intraepithelial abscesses and loss of cilia, persisted at the 7-d time point. (F) Finally, regeneration of nasal cavity mucosa and resolution of inflammation was essentially complete by 28 d after RSV infection, with only goblet cell metaplasia remaining. Images were captured at a magnification of 200× or 400× (insets).
RSV infection of the lung of cotton rats is characterized by rapidly resolving bronchiolitis and persistent eosinophilia.
Given the clinical significance of RSV pneumonia, most rodent studies have focused on the effects of RSV infection in lung. Nonetheless, because of our interest in vaccine design and the necessity of determining whether any aspect of pathology after immunization challenge is enhanced relative to infection alone, we sought a more complete picture of the development and resolution of lower airway infection and inflammation in the lung of RSV-infected cotton rats.
We noted moderate, multifocal, peribronchiolar inflammation, with occasional involvement of the alveolar septae, which was maximal 5 d after RSV inoculation (Figure 5 C and D). Inflammation was characterized by a primarily lymphocytic infiltrate that also contained macrophages and eosinophils. Alveolar septae adjacent to foci of bronchiolitis were expanded by this infiltrate, and a small number of inflammatory cells were present within alveolar spaces. Eosinophils were present within the epithelial layer.
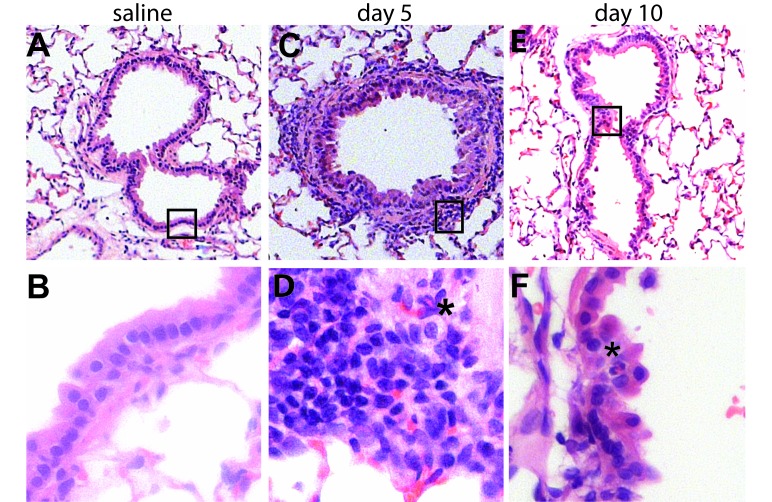
Figure 5.
Histopathology of acute RSV infection of the lower airway in cotton rats. Lungs collected at 5 and 10 d after virus challenge were processed after fixation; and hematoxylin- and eosin-stained sections were examined. (A–C) Naïve lung is characterized by thin alveolar septate and bronchioles lined by an orderly arrangement of ciliated respiratory epithelial cells surrounded by a thin layer of smooth muscle. (D–F) By 5 d after infection, there were multiple foci of bronchiolitis, with mucosal and peribronchial infiltrates consisting primarily of lymphocytes and histiocytes, with rare eosinophils (*). Some areas showed involvement of adjacent alveolar septae. Mucosal changes included epithelial disorganization and hyperplasia. (F) By 10 d after infection, inflammation had largely resolved, with only rare eosinophils present within the bronchiolar epithelium (*). Mucosal repair was ongoing at the 10-d time point, characterized by epithelial hypertrophy and squamous metaplasia. Images were captured at a magnification of 40× (top panels), 100× (middle panels), or 400× (bottom panels). Areas shown at higher magnification in the lower panels are highlighted by boxes in upper-panel images.
Coincident with inflammatory influx were bronchial and bronchiolar epithelial changes. Unlike the extensive epithelial necrosis seen in influenza or adenoviral infections,26 dropout of individual epithelial cells occurred after RSV infection. The mucosal layer remained intact, but showed acute changes in cell morphology, for example, loss of cilia and a cuboidal rather than columnar appearance. This pattern was followed by regenerative changes, with hypertrophy of basal cells. The acute inflammatory response resolved quickly, and by day 10 after infection, only rare eosinophils remained (Figure 5 E and F).
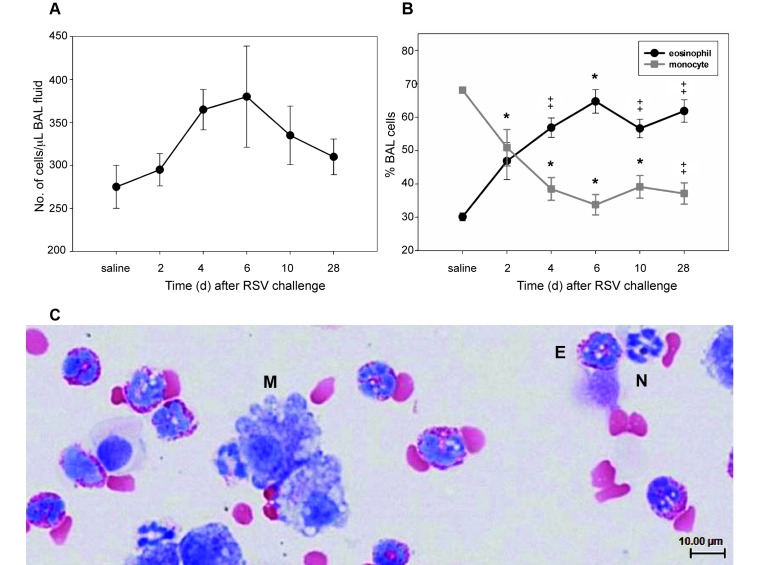
For a more quantitative assessment of the inflammatory process. BAL fluids were examined at 2, 4, 6, 10, and 28 d after inoculation. We noted a mild increase in BAL fluid cellularity (Figure 6 A) that peaked on day 6 and returned to baseline by the 28-d time point. However, despite only modest changes in overall cell numbers, BAL fluid cytology revealed a marked shift in cellular composition in response to RSV infection (Figure 6 B). At baseline, after saline challenge, BAL fluid was composed predominantly of macrophages. This finding was not unexpected, given that the BAL fluid from healthy animals of many species, including humans, is composed predominantly of this cell type. What was unexpected—and previously unreported—was the presence of eosinophils, averaging 30% of the total cell number, in unchallenged cotton rats. After RSV infection, the number of eosinophils doubled, becoming the predominant cell type present in the airways throughout the 28-d course of the study.
Figure 6.
Cytology of RSV infection of the lower airway in cotton rats. BAL fluid was collected from cotton rats until 28 d after RSV infection, and cytospin preparations were examined after fixation. (A) RSV infection induced a mild, not statistically significant, increase in total number of BAL cells that was most pronounced at the 6 d time point. (B) Differential cell counts revealed a marked increase in the percentage of eosinophils as early as 2 d after RSV infection. The increase (P < 0.001) in eosinophil percentage was accompanied by a corresponding decrease in monocyte percentage and persisted to 28 d. The increase in eosinophil percentage is supported by (C) BAL cytology (magnification, 400×), where eosinophils (E) were differentiated from neutrophils (N) by the presence of abundant brightly eosinophilic cytoplasmic granules. Monocytes (M), the second-most predominant cell type observed, are large cells characterized by abundant basophilic granular to vacuolated cytoplasm and frequently ruffled cell borders. *, P < 0.05; ‡ P < 0.001; data points represent the mean (error bars, SEM).
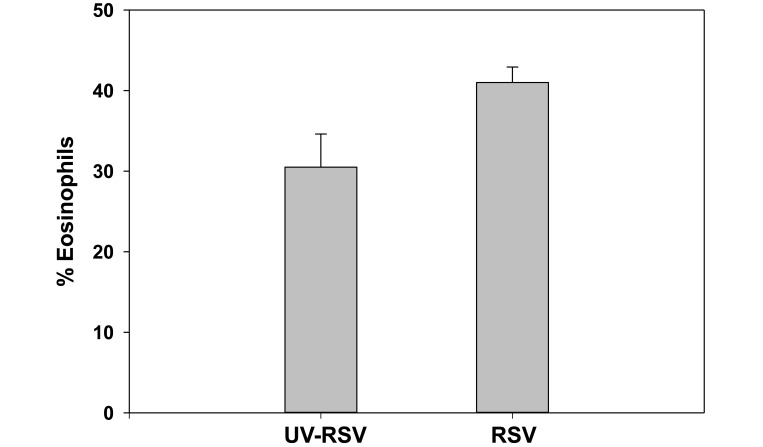
To eliminate the possibility that some aspect of virus preparation was eliciting the influx of eosinophils, we repeated this study, inoculating cotton rats (n = 5) with 106 pfu of virus, either live or UV-irradiated. At day 5 after infection, BAL fluids were analyzed for cell composition. As in saline-treated cotton rats, eosinophils made up 30% of the lavage specimens from cotton rats treated with UV-inactivated virus (Figure 7), with significantly (P < 0.05) higher levels in those receiving live RSV. No virus was detected by plaque assay in lung homogenates from cotton rats that received UV-inactivated RSV; the mean titer in cotton rats given live virus was 8.4 ± 5.6 × 104 pfu/g lung tissue.
Figure 7.
BAL cytology in cotton rats treated with UV-inactivated RSV. BAL fluid was collected from cotton rats that received either 106 pfu RSV,or an equivalent volume of UV-inactivated virus from the same preparation. Samples were harvested at 5 d after inoculation. Differential counts revealed an average of 30% eosinophils in cotton rats given the inactivated virus preparation, consistent with prior values after saline instillation, and an increase (P = 0.042) in lung eosinophils in cotton rats that received live RSV. Bars represent the mean value (error bars, SEM).
To ensure that no other aspect of our treatment was causing an eosinophilic response, we performed BAL on 5 untreated cotton rats. These BAL samples contained eosinophils that comprised a mean of 21.4% ± 5.2% of the total cells present; according to a t test, there was no significant difference (P = 0.08) in the percentage of eosinophils between untreated cotton rats and those inoculated with UV-inactivated RSV. Lungs harvested from the untreated cohort were harvested and examined for any microscopic evidence of pathology. No pathology was evident in any of the 5 cotton rats (Figure 8), but rare eosinophils could be identified in the alveolar spaces.
Figure 8.
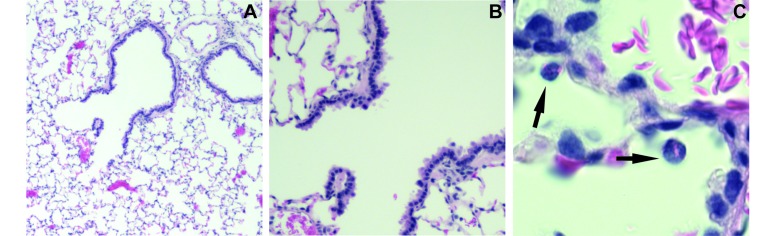
Eosinophils in the airway of untreated cotton rats with no microscopic evidence of disease. Lungs from 5 untreated rats showed no evidence of infection or inflammation. A representative animal is shown here, with images taken at a magnification of (A) 40×, (B) 100×, and (C) 600×. Rare eosinophils (C, →) were present in alveolar spaces. The eosinophil is in an alveolar space adjacent to a small vessel filled with RBCs.
Asthma-like changes persist in the lungs following clearance of RSV infection.
Severe RSV disease in infancy is increasingly recognized as a major predisposing factor for development of asthma and recurrent wheezing.53,56 To determine whether RSV infection in cotton rats is associated with the chronic changes found in human patients with allergic asthma, tissues collected 28 d after viral challenge were examined. The histology of the upper airway had returned to normal at this time point, therefore no images from the nasal cavity are shown. In contrast, inflammatory changes persisted in the lower airways (Figure 9).
Figure 9.
Chronic changes in the lung after RSV infection. Lungs were collected 28 d after viral challenge and examined microscopically. (A–F) Chronic changes included persistent peribronchiolar inflammatory infiltrates, (B, C) mucosal and goblet cell hyperplasia, (C) the continuing presence of eosinophils (*) in the bronchiolar mucosa and submucosa, and (E) the presence of mast cells (*) in the smooth muscle layer. (F) Mucus was present in terminal airways. Images were taken at a magnification of 100× (A, D, E) or 400× (B, C, F)). Panels B and C show high-power images of fields outlined in panel A. Panel E is a high-power image of the field outlined in panel D.
Chronic changes included mucosal and goblet cell hyperplasia, with persistence of eosinophils in the mucosa and submucosa, and mast cells in the smooth muscle surrounding the airways. In addition, the smooth muscle layer surrounding bronchioles appeared hypertrophied when compared with bronchioles of similar diameter in uninfected rats (Figure 5 A and B), and mucus was present in airways even at the late (day 28) time point. Taken together, these findings are consistent with the chronic changes seen in human patients with asthma and are consistent with the observed association between RSV-induced lower airway disease and later onset of asthma and recurrent wheezing in children.
Discussion
Our understanding of RSV pathogenesis, and the role played by this ubiquitous virus in the development of allergic responses in the lung, has been limited by the lack of a small animal model that faithfully recapitulates human infection. Mice are the most common animal used to study RSV, but studies using other rodent models and, much less frequently, NHP11 have been reported. Studies of other pneumoviruses in their primary hosts, including bovine RSV infection of calves and pneumonia virus of mice infection of mice, have been used to gain a better understanding of RSV biology.38,50,51 The relative merits of these models have been reviewed recently.4 Each of these systems has unique advantages and disadvantages, but because of the permissivity of cotton rats in regard to RSV replication, this species is increasingly used to evaluate potential RSV vaccines and therapeutics.
Cotton rats are susceptible to a wide range of human pathogens. Although there are, as yet, no genetically modified strains, inbred and microbiologically defined cotton rats are commercially available, as are a growing number of cotton-rat–specific reagents.7,43,44 Experimental RSV infection of cotton rats was first reported in 1971,12 and the pathology of RSV-infected cotton rats was first described in 1978.46 Most subsequent studies of RSV infection in cotton rats have focused on the effect of a specified preventative or treatment on virus replication and clearance, with little attention paid to the pathology.8,14,33,34,45,47 The source of animals used in one study12 is not specified, but the colony established in the United States by other authors46 has been used subsequently by many researchers and is the source of the animals we have studied here. This landmark paper46 showed that, after intranasal inoculation, RSV replicated primarily in the nasal epithelium and in the respiratory epithelium lining the bronchi and bronchioles. Using the strain of cotton rat developed by that group,46 we have replicated those findings here. This pattern of spread is similar to that described in human patients32,61 and unlike that in mice, where the viral antigen is largely absent from the nasal cavity and airways but is present in pneumocytes lining the alveolar spaces rather than the airway-lining cells18 (Figure 10). The inflammatory response to infection described in the earlier study46 was limited, consisting of intraepithelial neutrophils and a sparse peribronchial and perivascular inflammatory infiltrate made up of lymphocytes and neutrophils. Although the black-and-white photography in the earlier publication46 make direct comparison difficult, the pathology we have observed is markedly different. These differences may be due to a number of factors, including the use of the A2 rather than the Long strain of RSV, an increased virus dose, the relatively small volume used for inoculation, and the difference in morphology between human and rodent eosinophils. Although eosinophils from both species have brightly eosinophilic cytoplasmic granules, human eosinophils contain a distinctive bilobed nucleus, a feature key to their histologic identification. In mice and cotton rats, eosinophil nuclei are less uniform, often with a donut-shaped nucleus58 (Figure 6), but remain distinct from the multilobed nuclei of neutrophils.
Figure 10.
Differing patterns of RSV infection in the lungs of mice and humans. (A) Photomicrograph (magnification, 100×) of a lung lesion in an infant found to have RSV infection at autopsy.31 (B) RSV replication in the lung of a BALB/c mouse 4 d after intranasal inoculation with 107 pfu RSV A2 (magnification, 200×).
Here we have described in detail the acute and chronic lung pathology after intranasal RSV A2 infection of cotton rats. After the delivery of low-volume viral inoculum to the nasal cavity, we observed diffuse infection of the uppermost airway by RSV, with more focal involvement of the lungs. This conclusion relies primarily on our immunohistochemistry studies. Virus was detected by plaque assay in both nasal wash and lung homogenate specimens, but direct comparison of those samples tells us relatively little about the spread of infection and the nature of the disease.
In our hands, RSV infection of the nose of cotton rats was accompanied by marked acute inflammation accompanied by degenerative epithelial changes. Inflammation persisted at day 7, but by 28 d after infection, an increase in goblet cell number was the only evidence of prior injury (Figure 4) despite immunohistochemical evidence of prolonged upper airway infection (Figure 3). Acute inflammatory lesions in the lung, limited to the bronchioles and adjacent parenchyma, were more focal and less severe and accompanied by epithelial damage and regenerative changes. However, in contrast to that in the nose, lung eosinophilia persisted. Interestingly, even BAL fluid collected from naïve cotton rats that were untreated or challenged only with saline contained a high baseline level of eosinophils, 25% on average. The number of lung eosinophils increased in response to RSV infection and remained elevated until the 28-d point. Chronic changes in lung tissue that remained at 1 mo after infection also included the persistence of eosinophils within the epithelial layer, goblet cell metaplasia, and a thickened bronchiolar smooth muscle layer with infiltrating mast cells. This chronic, RSV-induced, lung pathology recapitulates the chronic changes seen in human patients with asthma.1,15
The predominance of eosinophils in BAL fluid and the persistent eosinophilic infiltrates in response to primary RSV challenge of cotton rats were unexpected findings and are contrary to what has been reported in human patients, in whom BAL fluids contain predominately lymphocytes and neutrophils.32 Despite the prominent eosinophilia in lungs from naïve cotton rats, the number of circulating eosinophils in the blood was not elevated above that in other laboratory species2,48 or human patients36 nor is it increased in response to RSV infection (data not shown). The lung eosinophilia we noted here may be a feature of paramyxoviral infection in this species, given that high levels of eosinophils in BAL fluid also occur in cotton rats infected with measles virus.10 Pulmonary eosinophilia, however, is not known to be a general reaction to virus infection in cotton rats, given that the inflammatory response to the hantavirus Black Creek Canal virus, a natural pathogen of cotton rats, is mediated by mononuclear cells.5 Whether other pathogens induce an eosinophilic response in this species is unknown, and in the absence of specific pathogen testing for cotton rats, the possibility of intercurrent infection cannot be eliminated.
Human studies of RSV infection are limited by safety issues and the fact that, despite its ubiquity, RSV infection does not usually result in severe disease. Therefore, relatively little clinical or autopsy material is available for study and none from patients with the typical, mild course. Published studies focus on differences between more and less severely ill, hospitalized patients, thus yielding relatively little insight into the mechanisms that, although insufficiently effective to prevent infection, can limit spread. RSV infections in the majority of in human patients are limited to the upper airway,9 with only 40% to 50% of primary infections in infants resulting in clinically significant lower airway disease.55 Therefore animal models of RSV pathogenesis that, unlike mice, are susceptible to infection of the uppermost airway are urgently needed. We note that RSV infection of cotton rats shows a pattern of virus growth and spread similar that found in human patients, although no clinical signs of illness were apparent in cotton rats. In addition, the virus-mediated development of asthma-like pathology in cotton rats, even in the absence of severe disease, may represent a new opportunity to study the connection between RSV infection and the development of allergic asthma.
Acknowledgments
This study was funded by the National Institute of Allergy and Infectious Disease (grant R01 AI088770-01 to JED). JLG was supported by grant 5T32 RR007073-08, the NIH NIDCD Pediatric Loan Repayment Plan, and the Presidential Fellowship from the Ohio State University. We wish to thank Michael Oglesbee, Mark Peeples, Prosper Boyaka, and Stefan Niewiesk for thoughtful discussions; Basil Kahwash and Heidi Risman for technical assistance; and the NYU and New Jersey Medical School Histology Cores for slide preparation.
References
- 1.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. 1992. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 101:916–921. [DOI] [PubMed] [Google Scholar]
- 2.Aleman CL, Noa M, Mas R, Rodeiro I, Mesa R, Menendez R, Gamez R, Hernandez C. 2000. Reference data for the proncipal physiological indicators in 3 species of laboratory animals. Lab Anim 34:379–385. [DOI] [PubMed] [Google Scholar]
- 3.Alper CM, Winther B, Mandel EM, Hendley JO, Doyle WJ. 2009. Rate of concurrent otitis media in upper respiratory tract infections with specific viruses. Arch Otolaryngol Head Neck Surg 135:17–21. [DOI] [PubMed] [Google Scholar]
- 4.Bem RA, Domachowske JB, Rosenberg HF. 2011. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol 301:L148–L156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billings AN, Rollin PE, Milazzo ML, Molina CP, Eyzaguirre EJ, Livingstone W, Ksiazek TG, Fulhorst CF. 2010. Pathology of Black Creek Canal virus infection in juvenile hispid cotton rats (Sigmodon hispidus). Vector Borne Zoonotic Dis 10:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boukhvalova MS, Prince GA, Blanco JC. 2009. The cotton rat model of respiratory viral infections. Biologicals 37:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC. 2006. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine 24:5027–5035. [DOI] [PubMed] [Google Scholar]
- 8.Brideau RJ, Walters RR, Stier MA, Wathen MW. 1989. Protection of cotton rats against human respiratory syncytial virus by vaccination with a novel chimeric FG glycoprotein. J Gen Virol 70:2637–2644. [DOI] [PubMed] [Google Scholar]
- 9.Buchman CA, Doyle WJ, Pilcher O, Gentile DA, Skoner DP. 2002. Nasal and otologic effects of experimental respiratory syncytial virus infection in adults. Am J Otolaryngol 23:70–75. [DOI] [PubMed] [Google Scholar]
- 10.Carsillo M, Klapproth K, Niewiesk S. 2009. Cytokine imbalance after measles virus infection has no correlation with immune suppression. J Virol 83:7244–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cormier SA, You D, Honnegowda S. 2010. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert Rev Anti Infect Ther 8:1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dreizin RS, Vyshnevetskaia LO, Bagdamian EE, Iankevich OD, Tarasova LB. 1971. [Experimental RS virus infection of cotton rats. A viral and immunofluorescent study] Vopr Virusol 16:670–676.[Article in Russian]. [PubMed] [Google Scholar]
- 13.Durbin JE, Johnson TR, Durbin RK, Mertz SE, Morotti RA, Peebles RS, Graham BS. 2002. The role of IFN in respiratory syncytial virus pathogenesis. J Immunol 168:2944–2952. [DOI] [PubMed] [Google Scholar]
- 14.Faverio LA, Piazza FM, Johnson SA, Darnell ME, Ottolini MG, Hemming VG, Prince GA. 1997. Immunoprophylaxis of group B respiratory syncytial virus infection in cotton rats. J Infect Dis 175:932–934. [DOI] [PubMed] [Google Scholar]
- 15.Fixman ED, Stewart A, Martin JG. 2007. Basic mechanisms of development of airway structural changes in asthma. Eur Respir J. 29:379–389. [DOI] [PubMed] [Google Scholar]
- 16.Frick OL, German DF, Mills J. 1979. Development of allergy in children. I. Association with virus infections. J Allergy Clin Immunol 63:228–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gern JE, Busse WW. 2002. Relationship of viral infections to wheezing illnesses and asthma. Nat Rev Immunol 2:132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gitiban N, Jurcisek JA, Harris RH, Mertz SE, Durbin RK, Bakaletz LO, Durbin JE. 2005. Chinchilla and murine models of upper respiratory tract infections with respiratory syncytial virus. J Virol 79:6035–6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glenn GM, Smith G, Fries L, Raghunandan R, Lu H, Zhou B, Thomas DN, Hickman SP, Kpamegan E, Boddapati S, Piedra PA. 2013. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine 31:524–532. [DOI] [PubMed] [Google Scholar]
- 20.Glezen WP, Taber LH, Frank AL, Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 140:543–546. [DOI] [PubMed] [Google Scholar]
- 21.Graham BS, Perkins MD, Wright PF, Karzon DT. 1988. Primary respiratory syncytial virus infection in mice. J Med Virol 26:153–162. [DOI] [PubMed] [Google Scholar]
- 22.Grieves JL, Jurcisek JA, Quist B, Durbin RK, Peeples ME, Durbin JE, Bakaletz LO. 2010. Mapping the anatomy of respiratory syncytial virus infection of the upper airways in chinchillas (Chinchilla lanigera). Comp Med 60:225–232. [PMC free article] [PubMed] [Google Scholar]
- 23.Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC, Tristram DA, Siber GR, Prince GA, Van Raden M, Hemming VG, Respiratory Syncytial Virus Immune Globulin Study Group 1993. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med 329:1524–1530. [DOI] [PubMed] [Google Scholar]
- 24.Hall C, Walsh E. 1998. Respiratory syncytial virus. In: Feigin R, Cherry J, Kaplan S, Demmler-Harrison G, Textbook of pediatric infectious disease, 4th ed Philadelphia (PA): Saunders–Elsevier. [Google Scholar]
- 25.Hall CB, Geiman JM, Biggar R, Kotok DI, Hogan PM, Douglas GR., Jr 1976. Respiratory syncytial virus infections within families. N Engl J Med 294:414–419. [DOI] [PubMed] [Google Scholar]
- 26.Hammond S, Chenever E, Durbin JE. 2007. Respiratory virus infection in infants and children. Pediatr Dev Pathol 10:172–180. [DOI] [PubMed] [Google Scholar]
- 27.Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. 2005. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy, and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 16:386–392. [DOI] [PubMed] [Google Scholar]
- 28.Holgate ST. 2010. A brief history of asthma and its mechanisms to modern concepts of disease pathogenesis. Allergy Asthma Immunol Res 2:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr 2008. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 178:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson DJ, Lemanske RF., Jr 2010. The role of respiratory virus infections in childhood asthma inception. Immunol Allergy Clin North Am 30:513–522 [vi.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS, Jr, Bakaletz LO, Durbin RK, Flano E, Durbin JE. 2007. Differential type I interferon induction by respiratory syncytial virus and influenza A virus in vivo. J Virol 81:9790–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol. 20:108–119. [DOI] [PubMed] [Google Scholar]
- 33.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O'Grady J, Koenig S, Tamura JK, Woods R, Bansal G, Couchenour D, Tsao E, Hall WC, Young JF. 1997. Development of a humanized monoclonal antibody (MEDI493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis 176:1215–1224. [DOI] [PubMed] [Google Scholar]
- 34.Jones B, Zhan X, Mishin V, Slobod KS, Surman S, Russell CJ, Portner A, Hurwitz JL. 2009. Human PIV2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with 2 additional rSeVs to protect against hPIV1, hPIV2, hPIV3, and RSV. Vaccine 27:1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korppi M, Piippo-Savolainen E, Korhonen K, Remes S. 2004. Respiratory morbidity 20 years after RSV infection in infancy. Pediatr Pulmonol 38:155–160. [DOI] [PubMed] [Google Scholar]
- 36.Kratz A, Ferraro M, Sluss PM, Lewandrowski KB. 2004. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med 351:1548–1563. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Sobrido L, Gitiban N, Fernandez-Sesma A, Cros J, Mertz SE, Jewell NA, Hammond S, Flano E, Durbin RK, Garcia-Sastre A, Durbin JE. 2006. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol 80:1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer G, Deplanche M, Schelcher F. 2008. Human and bovine respiratory syncytial virus vaccine research and development. Comp Immunol Microbiol Infect Dis 31:191–225. [DOI] [PubMed] [Google Scholar]
- 39.Murata Y, Falsey AR. 2007. Respiratory syncytial virus infection in adults. Antivir Ther 12:659–670. [PubMed] [Google Scholar]
- 40.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen TN, Power UF, Robert A, Haeuw JF, Helffer K, Perez A, Asin MA, Corvaia N, Libon C. 2012. The respiratory syncytial virus G protein conserved domain induces a persistent and protective antibody response in rodents. PLoS One 7:e34331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholson KG, Kent J, Ireland DC. 1993. Respiratory viruses and exacerbations of asthma in adults. Br Med J 307:982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niewiesk S. 2009. Current animal models: cotton rat animal model. Curr Top Microbiol Immunol 330:89–110. [DOI] [PubMed] [Google Scholar]
- 44.Niewiesk S, Prince G. 2002. Diversifying animal models: the use of hispid cotton rats (Sigmodon hispidus) in infectious diseases. Lab Anim 36:357–372. [DOI] [PubMed] [Google Scholar]
- 45.Piazza FM, Johnson SA, Darnell ME, Porter DD, Hemming VG, Prince GA. 1993. Bovine respiratory syncytial virus protects cotton rats against human respiratory syncytial virus infection. J Virol 67:1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prince GA, Jenson AB, Horswood RL, Camargo E, Chanock RM. 1978. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol 93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 47.Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. 2003. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol 77:13156–13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robel GL, Lochmiller RL, McMurry ST, Qualls CW., Jr 1996. Environmental, age, and sex effects on cotton rat (Sigmodon hispidus) hematology. J Wildl Dis 32:390–394. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez WJ, Gruber WC, Groothuis JR, Simoes EA, Rosas AJ, Lepow M, Kramer A, Hemming V. 1997. Respiratory syncytial virus immune globulin treatment of RSV lower respiratory tract infection in previously healthy children. Pediatrics 100:937–942. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg HF, Bonville CA, Easton AJ, Domachowske JB. 2005. The pneumonia virus of mice infection model for severe respiratory syncytial virus infection: identifying novel targets for therapeutic intervention. Pharmacol Ther 105:1–6. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg HF, Domachowske JB. 2008. Pneumonia virus of mice: severe respiratory infection in a natural host. Immunol Lett 118:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarze J, O'Donnell DR, Rohwedder A, Openshaw PJ. 2004. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med 169:801–805. [DOI] [PubMed] [Google Scholar]
- 53.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 171:137–141. [DOI] [PubMed] [Google Scholar]
- 54.Sikkel MB, Quint JK, Mallia P, Wedzicha JA, Johnston SL. 2008. Respiratory syncytial virus persistence in chronic obstructive pulmonary disease. Pediatr Infect Dis J 27:S63–S70. [DOI] [PubMed] [Google Scholar]
- 55.Simoes EA, Carbonell-Estrany X. 2003. Impact of severe disease caused by respiratory syncytial virus in children living in developed countries. Pediatr Infect Dis J 22:S13–20. [DOI] [PubMed] [Google Scholar]
- 56.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. 1999. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 354:541–545. [DOI] [PubMed] [Google Scholar]
- 57.Swanson KA, Settembre EC, Shaw CA, Dey AK, Rappuoli R, Mandl CW, Dormitzer PR, Carfi A. 2011. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc Natl Acad Sci USA 108:9619–9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Treuting PM, Dintzis SM. 2012. Comparative anatomy and histology: a mouse and human atlas. London (UK): Academic Press. [Google Scholar]
- 59.Wyde PR, Laquerre S, Chetty SN, Gilbert BE, Nitz TJ, Pevear DC. 2005. Antiviral efficacy of VP14637 against respiratory syncytial virus in vitro and in cotton rats following delivery by small-droplet aerosol. Antiviral Res 68:18–26. [DOI] [PubMed] [Google Scholar]
- 60.Zeitlin L, Bohorov O, Bohorova N, Hiatt A, Kim do H, Pauly MH, Velasco J, Whaley KJ, Barnard DL, Bates JT, Crowe JE, Jr, Piedra PA, Gilbert BE. 2013. Prophylactic and therapeutic testing of Nicotiana-derived RSV-neutralizing human monoclonal antibodies in the cotton rat model. MAbs 5:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 76:5654–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]