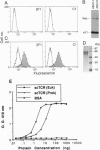
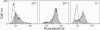Abstract
T-cell receptors (TCRs) are membrane anchored heterodimers structurally related to antibody molecules. Single-chain antibodies can be engineered by linking the two variable domains, which fold properly by themselves. However, proper assembly of the variable domains of a human TCR (V alpha and V beta) that recognize the HLA-DR2b/myelin basic protein-(85-99) peptide complex was critically dependent on the addition of a third domain, the constant region of the TCR beta chain (C beta), to the single-chain construct. Single-chain molecules with the three-domain design, but not those with the two-domain design, expressed in a eukaryotic cell as chimeric molecules linked either to glycosyl phosphatidylinositol or to the transmembrane/cytoplasmic domains of the CD3 zeta chain were recognized by a conformation-sensitive monoclonal antibody. The chimeric three-domain single-chain TCR linked to CD3 zeta chain signaled in response to both the specific HLA-DR/peptide and the HLA-DR/superantigen staphylococcal enterotoxin B complexes. Thus, by using this three-domain design, functional single-chain TCR molecules were expressed with high efficiency. The lipid-linked single-chain TCR was solubilized by enzymatic cleavage and purified by affinity chromatography. The apparent requirement of the constant domain for cooperative folding of the two TCR variable domains may reflect significant structural differences between TCR and antibody molecules.
Full text
PDF

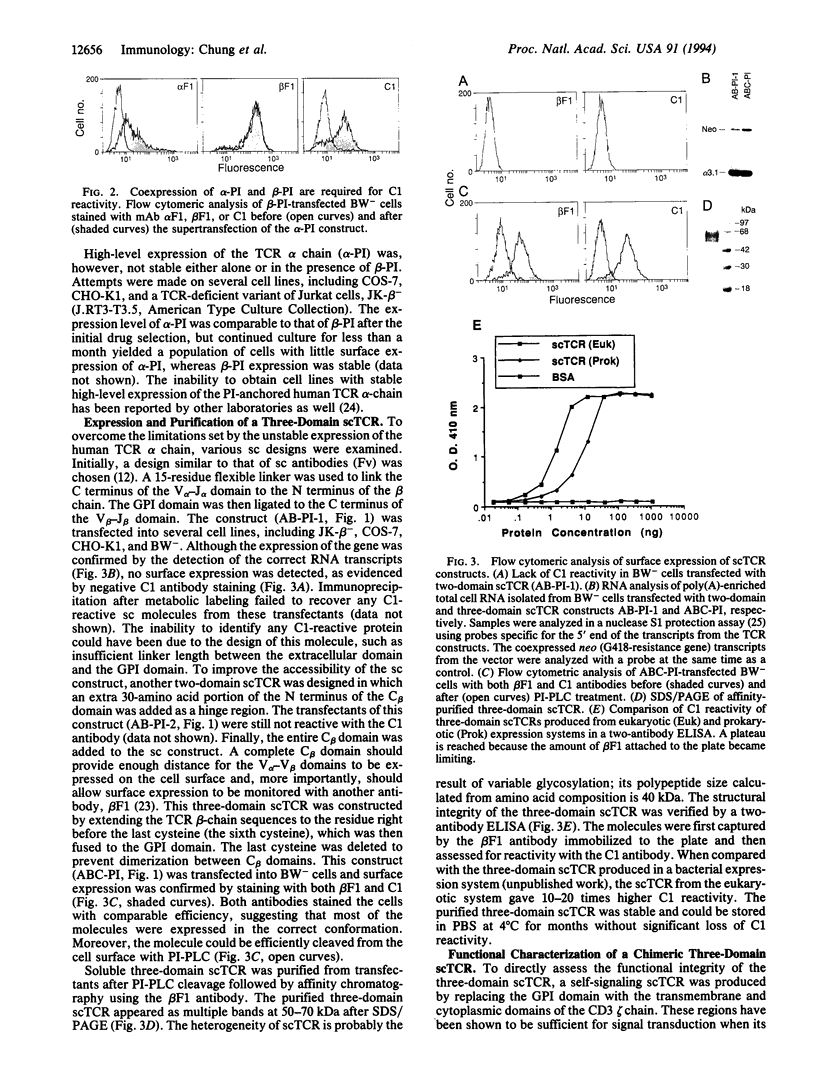
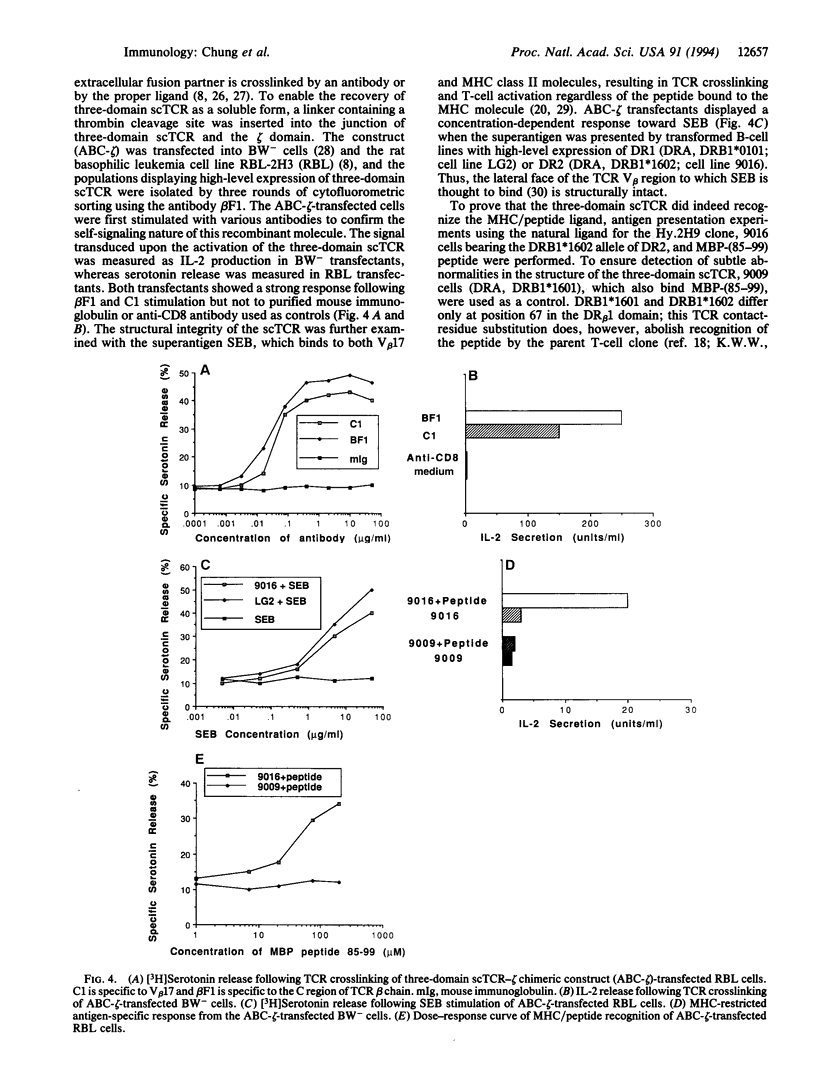

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., Klusner R. D. Genetic and mutational analysis of the T-cell antigen receptor. Annu Rev Immunol. 1990;8:139–167. doi: 10.1146/annurev.iy.08.040190.001035. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Pattern P., Chien Y., Yokota T., Eshhar Z., Giedlin M., Gascoigne N. R., Goodnow C., Wolf R., Arai K. Variability and repertoire size of T-cell receptor V alpha gene segments. Nature. 1985 Oct 3;317(6036):430–434. doi: 10.1038/317430a0. [DOI] [PubMed] [Google Scholar]
- Behlke M. A., Spinella D. G., Chou H. S., Sha W., Hartl D. L., Loh D. Y. T-cell receptor beta-chain expression: dependence on relatively few variable region genes. Science. 1985 Aug 9;229(4713):566–570. doi: 10.1126/science.3875151. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Warnke R. A., Jones N., Strominger J. L. Characterization and expression of the human alpha beta T cell receptor by using a framework monoclonal antibody. J Immunol. 1987 Mar 1;138(5):1502–1509. [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Perry R. P. The importance of downstream delta-factor binding elements for the activity of the rpL32 promoter. Nucleic Acids Res. 1993 Jul 11;21(14):3301–3308. doi: 10.1093/nar/21.14.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clackson T., Hoogenboom H. R., Griffiths A. D., Winter G. Making antibody fragments using phage display libraries. Nature. 1991 Aug 15;352(6336):624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Devaux B., Bjorkman P. J., Stevenson C., Greif W., Elliott J. F., Sagerström C., Clayberger C., Krensky A. M., Davis M. M. Generation of monoclonal antibodies against soluble human T cell receptor polypeptides. Eur J Immunol. 1991 Sep;21(9):2111–2119. doi: 10.1002/eji.1830210920. [DOI] [PubMed] [Google Scholar]
- Engel I., Ottenhoff T. H., Klausner R. D. High-efficiency expression and solubilization of functional T cell antigen receptor heterodimers. Science. 1992 May 29;256(5061):1318–1321. doi: 10.1126/science.1598575. [DOI] [PubMed] [Google Scholar]
- Friedman S. M., Crow M. K., Tumang J. R., Tumang M., Xu Y. Q., Hodtsev A. S., Cole B. C., Posnett D. N. Characterization of human T cells reactive with the Mycoplasma arthritidis-derived superantigen (MAM): generation of a monoclonal antibody against V beta 17, the T cell receptor gene product expressed by a large fraction of MAM-reactive human T cells. J Exp Med. 1991 Oct 1;174(4):891–900. doi: 10.1084/jem.174.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoire C., Rebaï N., Schweisguth F., Necker A., Mazza G., Auphan N., Millward A., Schmitt-Verhulst A. M., Malissen B. Engineered secreted T-cell receptor alpha beta heterodimers. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8077–8081. doi: 10.1073/pnas.88.18.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L., Tian W. T., Rittershaus C., Ko J. L., Marsh H. C., Jr, Ip S. H. Two distinct immunogenic epitopes on the alpha chain of human T cell antigen receptor. Hybridoma. 1989 Dec;8(6):577–588. doi: 10.1089/hyb.1989.8.577. [DOI] [PubMed] [Google Scholar]
- Hilyard K. L., Reyburn H., Chung S., Bell J. I., Strominger J. L. Binding of soluble natural ligands to a soluble human T-cell receptor fragment produced in Escherichia coli. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9057–9061. doi: 10.1073/pnas.91.19.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoo W. F., Lacy M. J., Denzin L. K., Voss E. W., Jr, Hardman K. D., Kranz D. M. Characterization of a single-chain T-cell receptor expressed in Escherichia coli. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4759–4763. doi: 10.1073/pnas.89.10.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J. S., McCartney J., Tai M. S., Mottola-Hartshorn C., Jin D., Warren F., Keck P., Oppermann H. Medical applications of single-chain antibodies. Int Rev Immunol. 1993;10(2-3):195–217. doi: 10.3109/08830189309061696. [DOI] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Irwin M. J., Hudson K. R., Ames K. T., Fraser J. D., Gascoigne N. R. T-cell receptor beta-chain binding to enterotoxin superantigens. Immunol Rev. 1993 Feb;131:61–78. doi: 10.1111/j.1600-065x.1993.tb01530.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. L., Reay P. A., Ehrich E. W., Davis M. M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Lippincott-Schwartz J., Bonifacino J. S. The T cell antigen receptor: insights into organelle biology. Annu Rev Cell Biol. 1990;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- Kurucz I., Jost C. R., George A. J., Andrew S. M., Segal D. M. A bacterially expressed single-chain Fv construct from the 2B4 T-cell receptor. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3830–3834. doi: 10.1073/pnas.90.9.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. Y., Devaux B., Green A., Sagerström C., Elliott J. F., Davis M. M. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science. 1990 Aug 10;249(4969):677–679. doi: 10.1126/science.1696397. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Novotny J., Ganju R. K., Smiley S. T., Hussey R. E., Luther M. A., Recny M. A., Siliciano R. F., Reinherz E. L. A soluble, single-chain T-cell receptor fragment endowed with antigen-combining properties. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8646–8650. doi: 10.1073/pnas.88.19.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991 Mar 8;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Ward E. S. Expression and secretion of T-cell receptor V alpha and V beta domains using Escherichia coli as a host. Scand J Immunol. 1991 Aug;34(2):215–220. doi: 10.1111/j.1365-3083.1991.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Ward E. S. Secretion of T cell receptor fragments from recombinant Escherichia coli cells. J Mol Biol. 1992 Apr 20;224(4):885–890. doi: 10.1016/0022-2836(92)90455-s. [DOI] [PubMed] [Google Scholar]
- Weber S., Traunecker A., Oliveri F., Gerhard W., Karjalainen K. Specific low-affinity recognition of major histocompatibility complex plus peptide by soluble T-cell receptor. Nature. 1992 Apr 30;356(6372):793–796. doi: 10.1038/356793a0. [DOI] [PubMed] [Google Scholar]
- White J., Blackman M., Bill J., Kappler J., Marrack P., Gold D. P., Born W. Two better cell lines for making hybridomas expressing specific T cell receptors. J Immunol. 1989 Sep 15;143(6):1822–1825. [PubMed] [Google Scholar]
- Wucherpfennig K. W., Zhang J., Witek C., Matsui M., Modabber Y., Ota K., Hafler D. A. Clonal expansion and persistence of human T cells specific for an immunodominant myelin basic protein peptide. J Immunol. 1994 Jun 1;152(11):5581–5592. [PubMed] [Google Scholar]