Abstract
Adipose tissue-derived stem cells (ADSCs) are adult stem cells that can be easily harvested from subcutaneous adipose tissue. Many studies have demonstrated that ADSCs differentiate into vascular endothelial cells (VECs), vascular smooth muscle cells (VSMCs), and cardiomyocytes in vitro and in vivo. However, ADSCs may fuse with tissue-resident cells and obtain the corresponding characteristics of those cells. If fusion occurs, ADSCs may express markers of VECs, VSMCs, and cardiomyocytes without direct differentiation into these cell types. ADSCs also produce a variety of paracrine factors such as vascular endothelial growth factor, hepatocyte growth factor, and insulin-like growth factor-1 that have proangiogenic and/or antiapoptotic activities. Thus, ADSCs have the potential to regenerate the cardiovascular system via direct differentiation into VECs, VSMCs, and cardiomyocytes, fusion with tissue-resident cells, and the production of paracrine factors. Numerous animal studies have demonstrated the efficacy of ADSC implantation in the treatment of acute myocardial infarction (AMI), ischemic cardiomyopathy (ICM), dilated cardiomyopathy, hindlimb ischemia, and stroke. Clinical studies regarding the use of autologous ADSCs for treating patients with AMI and ICM have recently been initiated. ADSC implantation has been reported as safe and effective so far. Therefore, ADSCs appear to be useful for the treatment of cardiovascular disease. However, the tumorigenic potential of ADSCs requires careful evaluation before their safe clinical application.
Keywords: Adipose tissue-derived stem cells, Cardiovascular disease, Acute myocardial infarction, Ischemic cardiomyopathy, Hindlimb ischemia, Stroke
Core tip: Adipose tissue-derived stem cells (ADSCs) have been used for the treatment of cardiovascular disease with the efficacy of ADSC implantation demonstrated in animal models. However, the mechanisms underlying the capacity of ADSCs for regenerating the cardiovascular system remain controversial. ADSCs may differentiate into blood vessels and cardiomyocytes, fuse with other cell types, obtaining the characteristics of those cells, and secrete paracrine factors that have proangiogenic and/or antiapoptotic activities. This review also discusses recently initiated clinical trials using autologous ADSCs.
INTRODUCTION
Cardiovascular disease (CVD) is a leading cause of morbidity and mortality worldwide. Despite advances in the treatment of acute myocardial infarction (AMI) using percutaneous coronary intervention, the treatment of heart failure (HF), which occurs as a result of the death of myocardial tissues and subsequent tissue remodeling, is still a challenging problem. As cardiomyocytes are terminally differentiated cells with minimal regenerative capacity, heart transplantation is currently the only treatment option for end-stage ischemic heart disease. The development of new therapies for AMI and HF is required to meet this substantial clinical requirement. Thus, stem cell therapy for CVD has recently gained substantial attention.
Stem cells are defined as cells capable of self-renewal and differentiation into a variety of phenotypes[1]. Stem cells comprise embryonic stem cells (ESCs) and adult stem cells (ASCs). ESCs were originally isolated from the inner cell mass of blastocysts[2], and are pluripotent stem cells capable of giving rise to all three germ layers. However, several issues, including ethical concerns and teratoma formation, limit the clinical use of ESCs. Induced pluripotent stem (iPS) cells are also pluripotent stem cells that have very similar characteristics to ESCs[3,4]. As ethical problems can be avoided, iPS cells represent a potentially promising option for stem cell therapy. However, cancer formation is a major issue that needs to be overcome before widespread acceptance of the use of iPS cells in clinical settings. ASCs are multipotent stem cells that reside in various adult tissues. Among ASCs, bone marrow-derived mesenchymal stem cells (BMMSCs) and adipose tissue-derived stem cells (ADSCs) are the most extensively studied. BMMSCs are reported to have the potential to differentiate into various cell types including bone, cartilage, cardiac muscle, skeletal muscle, vascular endothelial cells (VECs), and vascular smooth muscle cells (VSMCs)[5,6]. BMMSCs have been used to treat CVD in clinical settings, with promising results reported in a number of studies[7-14], although other studies failed to demonstrate positive outcomes[15,16]. ADSCs have gained substantial attention recently as subcutaneous adipose tissues are abundant and can be easily harvested using liposuction, a procedure that is less invasive than bone marrow aspiration, with minimal donor discomfort. Adipose tissue contains a significantly greater proportion of stem cells than the bone marrow (5% vs 0.01%) and is therefore a convenient source of stem cells[17]. Furthermore, ADSCs reportedly do not express class II major histocompatibility complexes[18,19], suggesting that ADSCs may be suitable for allogenic transplantation in addition to autologous transplantation. In this review, we discuss the characteristics of ADSCs and their potential use in the treatment of CVD.
CLASSIFICATION OF ADSCS
ADSCs can be obtained from subcutaneous adipose tissues with the use of collagenase digestion. Freshly isolated ADSCs (fADSCs) are known to be heterogeneous and contain hematopoietic cells (CD45+ and/or CD34+) and VECs (CD34+/CD31+) in addition to stem cells (CD44+ and CD105+)[20]. fADSCs can be cultured on plastic dishes in the presence of fetal bovine serum (FBS). Non-adherent cells, those that do not attach to plastic dishes, can be removed to obtain cultured ADSCs (cADSCs), a relatively homogeneous population that expresses stem cell markers, such as CD44 and CD105, but not hematopoietic lineage markers, including CD11b, CD45, and CD34 or the VEC marker CD31[21,22]. Artificially-modified ADSCs (mADSCs) are a type of ADSCs produced through the introduction of specific genes[23,24] or pre-treatment with drugs[25] before administration. The purpose of artificial modification is to improve the function of ADSCs, such as proangiogenic and antiapoptotic activities. cADSCs have been the most widely used type, particularly in animal studies. However, fADSCs may be more suitable for clinical applications for several reasons. First, fADSCs can be rapidly prepared compared with cADSCs as cell culture is not required while preparing fADSCs. The rapid preparation and administration of stem cells may be required to achieve sufficient recovery from tissue ischemia when treating AMI or critical hindlimb ischemia. Second, the preparation of fADSCs is technically less challenging compared with that of cADSCs as it does not require the use of foreign materials such as FBS. ADSCs used in clinical settings must not contain any foreign materials derived from animals or humans other than the individual patient receiving the stem cell therapy. Therefore, it is desirable to avoid culturing in the preparation of ADSCs for clinical applications.
DIFFERENTIATION POTENTIAL OF ADSCS IN VITRO
ADSCs have the potential to differentiate into cartilage, bone, tendon, and fat when cultured under lineage-specific conditions[26-29]. Furthermore, ADSCs have the potential to differentiate into VECs, VSMCs, and cardiomyocytes in vitro (Table 1), the main components of the cardiovascular system. Miranville et al[30] isolated and examined the characteristics of human fADSCs. Human fADSCs were found to express CD34 (27.6%-63.4%) with CD34 positive cells shown to be composed of two populations: CD34+/CD31+ cells (probably VECs) and CD34+/CD31- cells. The authors demonstrated CD34+/CD31- cells expressed CD31 and von Willebrand factor (vWF) when cultured in a medium containing vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF). Planat-Benard et al[31] used relatively fresh human cADSCs cultured on plastic dishes for 3 d without passaging. Approximately 90% of these cells were found to express CD34, and they expressed VEC markers, including CD31 and vWF, when cultured in a semisolid medium. Rodríguez et al[32] studied human cADSCs cultured in MCDB 131 medium supplemented with 1% FBS. The authors found these cells expressed VSMC markers, including α-smooth muscle actin (SMA), calponin, caldesmon, myosin heavy chain, and smooth muscle protein 22-α (SM22α). Furthermore, differentiated cells contracted in response to carbachol demonstrated contractile capacity. Jeon et al[33] demonstrated the use of sphingosylphosphorylcholine (SPC) to induce the differentiation of human cADSCs into VSMCs, as determined by SMA, calponin, and SM22α expression. They also found that SPC-induced differentiation of ADSCs into VSMCs depended on transforming growth factor-β (TGF-β), shown to be secreted by ADSCs in an autocrine manner. Rangappa et al[34] incubated rabbit cADSCs with 5-azacytidine. The authors demonstrated that these cells differentiated into spontaneously beating cardiomyocytes with expression of myosin heavy chain, sarcomeric α-actinin, and troponin I. Gaustad et al[35] incubated human cADSCs with rat cardiomyocyte extracts and demonstrated ADSC expression of cardiomyocyte markers, including sarcomeric α-actinin, desmin, and cardiac troponin I. Differentiated cells were also shown to beat autonomously. Planat-Bénard et al[36] cultured murine fADSCs in a semisolid methylcellulose medium without 5-azacytidine and found that ADSCs expressed cardiac-specific markers, such as transcription factors, GATA-4, and Nkx2.5. These cells demonstrated spontaneous beating with acceleration in response to isoproterenol, a β-agonist, and deceleration in response to carbamylcholine, an acetylcholine agonist.
Table 1.
Differentiation potential of adipose tissue-derived stem cells in vitro
| Cell type | Expression of VEC markers | Expression of VSMC markers | Expression of cardiomyocyte markers | Production of paracrine factors | Ref. |
| Human fADSCs | CD31, vWF | NE | NE | NE | Miranville et al[30] |
| Human cADSCs | CD31, vWF | NE | NE | NE | Planat-Benard et al[31] |
| Human cADSCs | NE | SMA, calponin, caldesmon, myosin heavy chain, SM22α | NE | NE | Rodríguez et al[32] |
| Human cADSCs | NE | SMA, calponin, SM22α | NE | NE | Jeon et al[33] |
| Rabbit cADSCs | NE | NE | Myosin heavy chain, sarcomeric α-actinin, troponin I | NE | Rangappa et al[34] |
| Human cADSCs | NE | NE | Sarcomeric α-actinin, desmin, cardiac troponin | NE | Gaustad et al[35] |
| Murine fADSCs | NE | NE | GATA-4, Nkx2.5 | NE | Planat-Bénard et al[36] |
| Human cADSCs | NE | NE | NE | VEGF, HGF, TGF-β | Rehman et al[44] |
| Murine cADSCs | ND | ND | NE | VEGF, HGF | Nakagami et al[45] |
| Human cADSCs | NE | NE | NE | VEGF, IGF-1 | Sadat et al[46] |
NE: Not examined; ND: Not detected; ADSCs: Adipose tissue-derived stem cells; VEC: Vascular endothelial cell; TGF: Transforming growth factor; VSMC: Vascular smooth muscle cell; HGF: Hepatocyte growth factor; IGF-1: Insulin-like growth factor-1; vWF: von Willebrand factor.
DIFFERENTIATION POTENTIAL OF ADSCS IN VIVO
It has also been suggested that ADSCs express VEC, VSMC, and cardiomyocyte markers in vivo (Table 2). For example, cADSCs administered in a hindlimb ischemia model[31] and AMI model[37] were reportedly incorporated into tissues and were found to express VEC markers, such as CD31 and vWF. ADSC implantation has been shown to improve blood flow in a murine hindlimb ischemia model[31]. Jack et al[38] injected human cADSCs into the bladder and urethra and demonstrated the expression of SMA, a marker for VSMCs, by engrafted cells. Valina et al[37] injected porcine cADSCs into the coronary artery following the induction of AMI and found that a proportion of engrafted cells expressed SMA. The authors also found that left ventricular function recovered following administration of ADSCs. Strem et al[39] prepared fADSCs from Rosa 26 mice ubiquitously expressing β-galactosidase and injected these cells into the intraventricular chamber following myocardial cryoinjury. The authors demonstrated co-expression of β-galactosidase with myosin heavy chain, Nkx2.5, and troponin I. Yamada et al[40] transplanted the CD29 positive fraction of murine cADSCs into the infarct border zone of an AMI model and demonstrated the expression of cardiomyocyte markers, such as sarcomeric actin and GATA-4. Furthermore, improved left ventricular function was observed in this study.
Table 2.
Differentiation potential of adipose tissue-derived stem cells in vivo
| Cell type | Animal model | Expression of VEC markers | Expression of VSMC markers | Expression of cardiomyocyte markers | Functional recovery | Ref. |
| Human cADSCs | Murine hindlimb ischemia | CD31 | NE | NE | Yes | Planat-Benard et al[31] |
| Porcine cADSCs | Porcine AMI | vWF | SMA | NE | Yes | Valina et al[37] |
| Human cADSCs | Bladders and urethras of athymic rats and SCID mice | NE | SMA | NE | NE | Jack et al[38] |
| Murine fADSCs | Murine AMI | NE | NE | Myosin heavy chain, Nkx2.5, troponin I | Yes | Strem et al[39] |
| Murine fADSCs | Rat AMI | NE | NE | Sarcomeric actin, GATA-4 | Yes | Yamada et al[40] |
| Conditioned medium from human cADSCs | Murine hindlimb ischemia | NE | NE | NE | Yes | Bhang et al[48] |
NE: Not examined; VSMC: Vascular smooth muscle cell; ADSCs: Adipose tissue-derived stem cells; vWF: von Willebrand factor; VEC: Vascular endothelial cell; SMA: Smooth muscle actin; AMI: Acute myocardial infarction.
However, cell fusion should be considered carefully before concluding that ADSCs have the potential to differentiate into VECs, VSMCs, or cardiomyocytes in vivo. The in vivo fusion of administered ADSCs with tissue-resident VECs, VSMCs, and/or cardiomyocytes may lead to ADSCs acquiring the phenotypes of the corresponding fused cell types, making it appear as if ADSCs are directly differentiating into these cell types. In fact, cell fusion has been shown to occur with the in vivo administration of BMMSCs. Alvarez-Dolado et al[41] used R26R mice that contain a lacZ reporter gene downstream of a stop codon flanked by loxP sites (floxed). The lacZ reporter gene was therefore only expressed when the loxP-flanked stop codon was excised by Cre recombinase (Figure 1). The authors lethally irradiated these mice and transplanted BMMSCs from mice that ubiquitously express Cre recombinase and green fluorescent protein (GFP). If cells from the donor and recipient fused, the Cre enzyme would excise the Lox P-flanked stop codon, thereby allowing the expression of the lacZ gene. The results of this study revealed β-gal+ (fused) and GFP+ cells in the brain, heart, and liver of recipients, at 2 and 4 mo post-transplantation. Thus, BMMSCs potentially fuse with other cell types in vivo. There have been no reports so far clearly demonstrating the fusion of ADSCs with other cell types in vivo. Bai et al[42] injected both human fADSCs and cADSCs into murine hearts and examined the occurrence of cell fusion using fluorescence in situ hybridization to detect human X chromosomes and murine Y chromosomes. The authors did not detect co-localization of human X chromosomes with murine Y chromosomes in individual cells, excluding the possibility of cell fusion events. However, similar techniques used to detect cell fusion by BMMSCs (e.g., the transplantation of ADSCs derived from transgenic mice expressing Cre recombinase into recipients expressing a lacZ reporter gene downstream of a floxed stop codon) should be used to conclusively determine whether ADSCs fuse with other tissue-resident cell types. Interestingly, Metzele et al[43] artificially fused human cADSCs with neonatal rat cardiomyocytes using hemagglutinating virus of Japan. The authors demonstrated spontaneous beating of fused ADSCs and the expression of human troponin I, suggesting fused ADSCs produced cardiomyogenic proteins. Furthermore, fused ADSCs were positive for the cell proliferation marker Ki67, suggesting proliferating capacity in marked contrast to terminally differentiated cardiomyocytes that are unable to proliferate. Therefore, ADSCs may stimulate the regeneration of heart muscles through in vivo fusion with cardiomyocytes.
Figure 1.
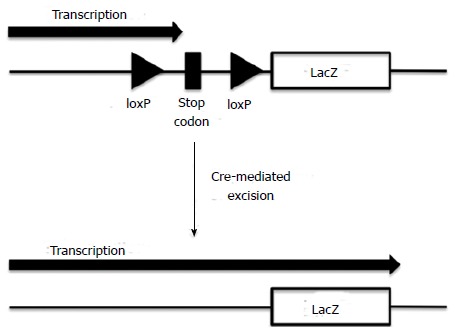
Schematic representation of LacZ expression following the excision of a floxed stop codon by Cre recombinase.
PRODUCTION OF PARACRINE FACTORS BY ADSCS
ADSCs have been shown to produce a variety of proangiogenic and antiapoptotic factors. Rehman et al[44] examined the production of paracrine factors by human cADSCs. The authors showed that ADSCs produced VEGF, hepatocyte growth factor (HGF), and TGF-β. VEGF production increased five-fold when ADSCs were cultured under hypoxic conditions. Conditioned medium (CM) obtained from hypoxic ADSCs significantly increased the proliferation and survival of VECs. Furthermore, the administration of these ADSCs significantly improved perfusion in a hindlimb ischemia model. Nakagami et al[45] reported murine cADSCs produce VEGF and HGF. The authors also administered ADSCs in a mouse hindlimb ischemia model and found transplanted ADSCs improved blood flow. However, transplanted ADSCs did not express VEC or VSMC markers, suggesting that ADSCs did not differentiate into vascular components in this study. Sadat et al[46] demonstrated human cADSCs produce VEGF and IGF-I and that these cytokines contribute to the antiapoptotic effects of ADSCs on cardiomyocytes. The authors implicated the secretion of VEGF by ADSCs in the ADSC-induced stimulation of tube formation by VECs. Yeghiazarians et al[47] administered BMMSCs and their lysates into the heart in a murine AMI model. The authors revealed that both BMMSCs and their lysates improved cardiac function and histology to similar extents, suggesting cytokines produced by BMMSCs, but not cells per se, are required for the recovery of cardiac function. Bhang et al[48] used a three-dimensional spheroid culture of human ADSCs to prepared CM. The authors injected CM into ischemic regions in a murine hindlimb ischemia model. They detected restoration of blood perfusion in this model. Albersen et al[49] injected rat cADSCs and their lysates into the penis in a rat model of cavernous nerve injury. The authors found that both ADSCs and their lysates restored erectile function to similar extents. These results suggest substances secreted by ADSCs, rather than cells per se, are critical for their regenerative function. Collectively, these results suggest that paracrine factors produced by ADSCs play a major, if not all, role in the regeneration of the cardiovascular system, although the differentiation and cell fusion of ADSCs may also be involved. The possible mechanisms underlying the regenerative effects of ADSCs on the cardiovascular system are summarized in Figure 2.
Figure 2.
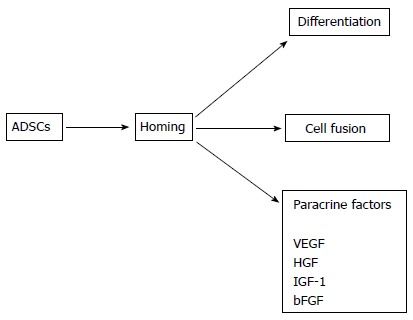
Possible mechanisms underlying the effect of adipose tissue-derived stem cells on regeneration of the cardiovascular system. ADSCs: Adipose tissue-derived stem cells; VEGF: Vascular endothelial growth factor; HGF: Hepatocyte growth factor; IGF-1: Insulin-like growth factor-1; bFGF: Basic fibroblast growth factor.
SURVIVAL OF ADSCS IN VIVO
The survival and engraftment of ADSCs in vivo have been examined within 30 d of ADSC implantation in the majority of studies[37,50-53]. Yin et al[54] injected swine cADSCs into the coronary artery following induction of AMI and examined the fate of ADSCs 8 wk after injection. The authors found that many ADSCs expressed troponin T and α-sarcomeric actin, indicating the ability of ADSCs to survive for 8 wk. Bai et al[42] introduced a luciferase reporter gene into human cADSCs and transplanted these cells into the murine heart muscle using an AMI model. The authors detected luciferase-positive ADSCs by bioluminescence imaging. Bioluminescence was observed 16 wk after ADSC transplantation, indicating that some ADSCs survived for 16 wk. However, murine cADSCs transplanted in a hindlimb ischemia model were found to barely remain in ischemic tissues 28 d after transplantation[45]. Therefore, the survival and engraftment of ADSCs in recipient tissues appear to vary according to the animal species and experimental models used.
APPLICATION OF ADSCS TO TREAT CVD
AMI and ischemic cardiomyopathy
Many studies have demonstrated the efficacy of ADSC administration in recovering cardiac function in AMI models. cADSCs have been predominantly used in animal models[37,39,40,51-57], although fADSCs[58] and mADSCs[23-25,59] have also been used. ADSCs have been transplanted via the coronary artery[37,53,54,57] and directly into cardiac muscles[23-25,39,40,51,55,56,58,59] in previous studies. Although ADSC implantation into the heart showed efficacy in recovering cardiac function in most studies, the underlying mechanisms remain controversial. Transplanted ADSCs expressed VEC, VSMC, or cardiomyocyte markers in numerous studies[37,39,40,52-55,57,59]; however, the “differentiation” of ADSCs was either not detected or examined in other studies[23-25,51,56,58]. Bai et al[42] transplanted both fADSCs and cADSCs in a murine AMI model and found both cell types recovered cardiac function to a similar extent. A proportion of transplanted fADSCs and cADSCs were found to express cardiomyocyte markers, including troponin I and connexin 43. These results are encouraging as fADSCs may be more suitable for clinical applications than cADSCs for reasons outlined above. ADSCs differentiated into specific cell types have been used to treat chronic MI. Okura et al[60] induced the differentiation of human cADSCs into cardiomyoblast-like cells (CLCs) in vitro and transplanted these cells into the swine coronary arteries 4 wk following the induction of MI. Cardiac function was recovered by CLC implantation. Furthermore, implanted CLCs expressed human α-cardiac actin, Nkx2.5, and GATA-4. Several studies have used a monolayer sheet to transplant ADSCs into chronic MI models. Miyahara et al[61] cultured rat ADSCs on a temperature-responsive polymer to prepare a monolayer of ADSCs. The authors transplanted these cells onto scarred myocardium at 4 wk following coronary ligation. Transplanted cells grew in situ to form a thick stratum containing newly-formed blood vessels. The transplantation of monolayered cells prevented ventricular wall scarring and improved cardiac function. Okura et al[62] induced the differentiation of human cADSCs into CLCs in vitro and prepared monolayer sheets of human CLCs and ADSCs using a temperature-responsive polymer. The authors then transplanted these cells onto the infarcted areas of rats 4 wk following the induction of MI. The authors demonstrated that the implantation of CLCs, but not ADSCs, resulted in a long-term recovery of cardiac function and improved survival. Furthermore, CLCs, but not ADSCs, were found to express human troponin I.
Clinical trials of ADSCs in the treatment of AMI have recently been initiated. The AdiPOse-derived stem ceLLs in the treatment of patients with ST-elevation myOcardial infarction (APOLLO) trial is a double-blind, placebo-controlled, phase I/IIa trial[63]. Autologous fADSCs were transplanted into the coronary artery of AMI patients with ST-segment elevation following successful revascularization. During the 6-mo follow-up period, improvements in the left ventricular ejection fraction and myocardial perfusion and reductions in the infarct size were demonstrated. The subsequent phase II/III trial, called ADVANCE, is currently ongoing. In this trial, AMI patients with ST elevation are treated with intracoronary implantation of autologous fADSCs. The primary endpoint is reduction in the infarct size as measured by magnetic resonance imaging. The adiPose-deRived stEm Cells In the treatment of patients with non revaScularizable ischEmic myocardium (PRECISE) trial enrolled patients who had chronic ischemic cardiomyopathy (ICM) not amenable to any revascularization procedures[64]. Autologous fADSCs were transplanted into cardiac muscles from endocardial sites. Maximal oxygen consumption and total left ventricular mass were significantly improved by ADSCs implantation. The ATHENA trial is an ongoing clinical trial intending to treat patients who have chronic ICM with HF symptoms using autologous fADSCs. The endpoints of this trial include peak oxygen consumption, perfusion defects, HF symptoms, left ventricle end-systolic and diastolic volume, and ejection fraction.
Dilated cardiomyopathy
Several studies have demonstrated the efficacy of ADSC implantation in the recovery of cardiac function using dilated cardiomyopathy (DCM) models. Lin et al[65] used a rat DCM model induced by the injection of porcine myosin and implanted cADSCs into cardiac muscle. The effect of combination therapy with ADSCs and sildenafil, a phosphodiesterase type-5 inhibitor, was also evaluated. This study found that either ADSCs implantation alone or sildenafil treatment alone was effective for the recovery of cardiac function, with combination therapy being the most effective. Hamdi et al[66] transplanted a monolayer sheet of murine cADSCs onto the heart surface in a murine DCM model, in which a floxed serum response factor gene is conditionally deleted using the expression of Cre recombinase. The authors found many blood vessels in transplanted sheets and some transplanted ADSCs in the cardiac muscle, a proportion of which expressed CD31. The authors further demonstrated the recovery of cardiac function and significant reduction of cardiac fibrosis following ADSC transplantation. Pınarlı et al[67] transplanted cADSCs into a doxorubicin-induced HF model. They further examined combination therapy of ADSC transplantation with resveratrol, a polyphenolic compound found in red grapes with an antioxidant activity. This study found either ADSC implantation alone, or resveratrol administration alone, was effective in recovering cardiac function, although combination therapy was found to be most effective.
Hindlimb ischemia
A number of studies have demonstrated that ADSC implantation improves blood flow in animal hindlimb ischemia models. fADSCs[30,68], cADSCs[31,45,48,69-76], and mADSCs[77] have all been used in these studies. Although the efficacy of ADSC administration in the recovery of blood flow appears conclusive, the mechanisms underlying the ability of ADSCs to recover blood flow remain controversial. ADSCs have been shown to engraft and express VEC and/or VSMC markers in some studies[30,31,69,70,72,75,77]. However, other studies have shown that engraftment was either not observed or examined[68,76] or paracrine factors secreted by ADSCs appeared to predominantly mediate the recovery of blood flow[45,48,70,71,73,74,77]. Lee et al[76] performed the transplantation of autologous cADSCs in 15 patients with critical limb ischemia. Although this was a pilot study, ADSC implantation caused no complications during the follow-up period and clinical improvement was observed in 66.7% patients. Larger-scale clinical studies are required to conclusively evaluate the efficacy and safety of ADSC transplantation in the treatment of limb ischemia.
Stroke
Several studies have demonstrated ADSC implantation induces functional recovery following brain ischemia in animal models of cerebral infarction (CI)[78-81]. Kang et al[78] occluded the middle cerebral artery (MCA) to induce CI and transplanted human cADSCs into the lateral ventricle. Transplanted ADSCs migrated to the border zone of the injured area and intact brain tissue and into injured areas. A proportion of ADSCs were found to express microtubule-associated protein 2 (MAP2), a neuron marker, and glial fibrillary acid protein (GFAP), an astrocyte marker. Furthermore, ADSC implantation improved motor and somatosensory behavior following CI, although no reduction in the area of CI was observed following ADSC implantation. Gutiérrez-Fernández et al[79] injected cADSCs intravenously following MCA occlusion in rats and found a significant recovery of motor function, although no reduction in the infarct size or ADSC engraftment into damaged tissues was observed. Furthermore, the expression of VEGF, synaptophysin, a neuron marker, and neurofilament was significantly increased following ADSC injection, although it was not examined whether ADSCs per se produced these molecules. Liu et al[80] transplanted human cADSCs into the right corpus striatum and cerebral cortex of rats following MCA occlusion. Neurological deficits were significantly attenuated by ADSC administration. Significantly increased expression of brain-derived neurotrophic factor (BDNF), nerve growth factor, and basic fibroblast growth factor mRNA and increased protein levels of BDNF and Bcl-2 were observed following ADSC transplantation, although it was not examined whether ADSCs produced these molecules.
Coronary artery restenosis
Balloon injury of the carotid artery and wire injury of the femoral artery have been widely used as models of coronary artery restenosis. We implanted rat cADSCs around the femoral artery from the adventitial side following wire injury of the femoral artery and found that ADSC implantation significantly inhibited neointimal formation and stimulated re-endothelialization[82]. We also demonstrated that ADSCs produced angiopoietin-1 (Ang-1) and that the effect of ADSC administration diminished when expression of Ang-1 was suppressed using small interfering RNA (siRNA) against Ang-1[83] (Figure 3), indicating that Ang-1 produced by ADSCs plays a critical role in the inhibition of neointimal formation. Although drug-eluting stents (DES) are widely used and they potently inhibit restenosis, the use of DES does not always improve patient outcomes, most likely due to increased risk of late thrombosis[84,85]. Because DES inhibit the proliferation of VECs as well as VSMCs by secreting antiproliferative drugs, DES may delay re-endothelialization, resulting in thrombus formation. Therefore, agents that stimulate re-endothelialization, such as Ang-1, may be more suitable for the suppression of neointimal formation than currently used inhibitors of cell proliferation. Systematic analysis of ADSC cytokine production is required to identify molecules that inhibit neointimal formation and stimulate re-endothelialization.
Figure 3.
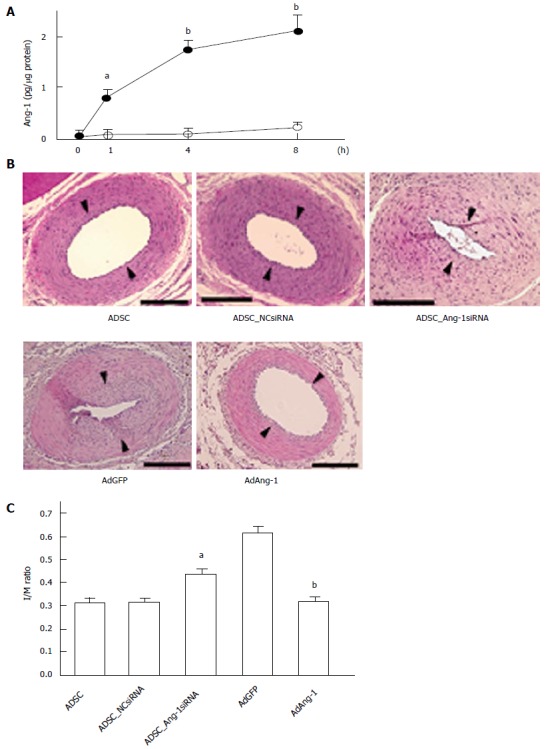
Ang-1 is implicated in Adipose tissue-derived stem cell-induced suppression of neointimal formation. A: ADSCs produce Ang-1, particularly when cultured in medium containing growth factors for VECs. Rat ADSCs were plated in 24-well plates and cultured in control medium (open circles) or medium containing growth factors for VECs (EGM: closed circles) for 1 wk. After washing with PBS, the medium was replaced with serum-free Dulbecco’s modified Eagle medium and incubated for the indicated periods. Ang-1 accumulation was measured with an enzyme-linked immunosorbent assay kit. aP < 0.05, bP < 0.01 vs 0 h (n = 6 per group); B: Effect of knockdown of endogenous Ang-1 in ADSCs and forced expression of Ang-1 on neointimal formation. ADSCs were infected with lentivirus expressing negative control siRNA (NCsiRNA), which does not suppress the expression of mammalian mRNA, or lentivirus expressing Ang-1 siRNA (Ang-1siRNA). ADSCs not infected with lentivirus were used as positive controls (ADSC). ADSCs were cultured in EGM for 1 wk. ADSCs (106 cells) were seeded from the adventitial side immediately after wire injury of the rat femoral artery. Adenoviruses expressing green fluorescent protein (AdGFP) or Ang-1 (AdAng-1) were also injected into the femoral artery from the adventitial side following wire injury. Femoral arteries were harvested 14 d after injury for histological analyses. Arrowheads indicate the position of internal elastic lamina. Bars represent 100 μm; C: I/M ratios were compared among the groups (n = 8 per group). aP < 0.05 vs NCsiRNA infection and bP < 0.01 vs AdGFP infection. PBS: Phosphate-buffered saline; ADSCs: Adipose tissue-derived stem cells; VECs: Vascular endothelial cells.
FUTURE DIRECTIONS
Careful examination of the following points is required before the safe and effective clinical application of ADSCs.
Tumorigenesis
Although ADSCs may be less prone to forming tumors than ESCs, it has been reported that BMMSCs form tumors in vivo[86]. Furthermore, several reports have suggested ADSCs promote the proliferation of cancer cells both in vitro and in vivo[87-89]. Therefore, ADSCs may stimulate the growth of pre-existing tumors even if ADSCs per se do not form tumors.
Effects of age and comorbid diseases on the function of ADSCs
Patients suffering from CVD are often older and have comorbid diseases, such as hypertension and diabetes. When considering the autologous transplantation of ADSCs in these patients, it is necessary to examine whether age and comorbid diseases affect the function of ADSCs. Several studies have demonstrated that ADSCs collected from aged patients have less capacity for proliferation and differentiation compared to those collected from young donors[90-92]. Furthermore, several reports have shown that ADSCs collected from diabetic mice, hemodialysis patients, and HF patients have less capacity for proliferation, differentiation, or proangiogenic cytokine production[93-95]. Therefore, patients requiring ADSC transplantation for the treatment of CVD may not have access to high-quality autologous ADSCs. Allogenic transplantation of ADSCs may be required in these patients.
Improved ADSC survival and function
The use of mADSCs may improve the survival and/or function of ADSCs. The incubation of ADSCs with chemical compounds, culture in hypoxic conditions, or the introduction of ectopic genes are all potential methods for the pre-implantation modification of ADSCs. It is noteworthy that ADSCs cultured under hypoxic conditions have demonstrated increased capacity for proliferation, proangiogenic cytokine production, and maintenance of stemness[96-98]. The incubation of fADSCs under hypoxic conditions prior to implantation into patients may be a feasible strategy for improving the results of ADSC implantation.
Identification of paracrine factors
ADSCs produce a variety of paracrine factors, as aforementioned, and these factors appear to play a major role in the regeneration of the cardiovascular system. Elucidation of cytokine combinations with the greatest efficacy in the regeneration of the cardiovascular system may remove the need for ADSC implantation in the future.
CONCLUSION
Evidence accumulated from animal studies has indicated that ADSCs show efficacy in the treatment of CVD including AMI, ICM, and critical limb ischemia. Clinical trials have reported the safety and efficacy of ADSC implantation in the treatment of CVD. ADSCs may regenerate tissues through a number of mechanisms including direct differentiation into VECs, VSMCs, and cardiomyocytes, fusion with tissue-resident cells, and secretion of proangiogenic and antiapoptotic cytokines. The malignant potential of ADSCs should be carefully examined in the future.
Footnotes
Conflict-of-interest statement: There exist no conflicts of interest in this study.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 11, 2015
First decision: June 3, 2015
Article in press: June 19, 2015
P- Reviewer: Kato M, Sabate M, Skobel E S- Editor: Song XX L- Editor: A E- Editor: Wu HL
References
- 1.Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 6.Grove JE, Bruscia E, Krause DS. Plasticity of bone marrow-derived stem cells. Stem Cells. 2004;22:487–500. doi: 10.1634/stemcells.22-4-487. [DOI] [PubMed] [Google Scholar]
- 7.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 9.Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Tillmanns H, Yu J, Corti R, Mathey DG, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 10.Leistner DM, Fischer-Rasokat U, Honold J, Seeger FH, Schächinger V, Lehmann R, Martin H, Burck I, Urbich C, Dimmeler S, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clin Res Cardiol. 2011;100:925–934. doi: 10.1007/s00392-011-0327-y. [DOI] [PubMed] [Google Scholar]
- 11.Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schlüter M, Tonn T, Seeger F, Dimmeler S, Lindhoff-Last E, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 12.Williams AR, Trachtenberg B, Velazquez DL, McNiece I, Altman P, Rouy D, Mendizabal AM, Pattany PM, Lopera GA, Fishman J, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiavetta A, Maione C, Botti C, Marino G, Lillo S, Garrone A, Lanza L, Pagliari S, Silvestroni A, Signoriello G, et al. A phase II trial of autologous transplantation of bone marrow stem cells for critical limb ischemia: results of the Naples and Pietra Ligure Evaluation of Stem Cells study. Stem Cells Transl Med. 2012;1:572–578. doi: 10.5966/sctm.2012-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 16.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Niemeyer P, Kornacker M, Mehlhorn A, Seckinger A, Vohrer J, Schmal H, Kasten P, Eckstein V, Südkamp NP, Krause U. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng. 2007;13:111–121. doi: 10.1089/ten.2006.0114. [DOI] [PubMed] [Google Scholar]
- 19.Alipour F, Parham A, Kazemi Mehrjerdi H, Dehghani H. Equine adipose-derived mesenchymal stem cells: phenotype and growth characteristics, gene expression profile and differentiation potentials. Cell J. 2015;16:456–465. doi: 10.22074/cellj.2015.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Planells Roig MV, Pallas Regueria JA, Carbonell Tatay F, Sancho Fornos S. Immune thrombocytopenia and HIV-1 infection. Response to splenectomy. Rev Esp Enferm Dig. 1990;77:225–226. [PubMed] [Google Scholar]
- 21.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 22.Bai X, Alt E. Myocardial regeneration potential of adipose tissue-derived stem cells. Biochem Biophys Res Commun. 2010;401:321–326. doi: 10.1016/j.bbrc.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Paul A, Nayan M, Khan AA, Shum-Tim D, Prakash S. Angiopoietin-1-expressing adipose stem cells genetically modified with baculovirus nanocomplex: investigation in rat heart with acute infarction. Int J Nanomedicine. 2012;7:663–682. doi: 10.2147/IJN.S26882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi CZ, Zhang XP, Lv ZW, Zhang HL, Xu JZ, Yin ZF, Yan YQ, Wang CQ. Adipose tissue-derived stem cells embedded with eNOS restore cardiac function in acute myocardial infarction model. Int J Cardiol. 2012;154:2–8. doi: 10.1016/j.ijcard.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 25.Berardi GR, Rebelatto CK, Tavares HF, Ingberman M, Shigunov P, Barchiki F, Aguiar AM, Miyague NI, Francisco JC, Correa A, et al. Transplantation of SNAP-treated adipose tissue-derived stem cells improves cardiac function and induces neovascularization after myocardium infarct in rats. Exp Mol Pathol. 2011;90:149–156. doi: 10.1016/j.yexmp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 27.Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, Bae YC, Jung JS. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 28.Dicker A, Le Blanc K, Aström G, van Harmelen V, Götherström C, Blomqvist L, Arner P, Rydén M. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res. 2005;308:283–290. doi: 10.1016/j.yexcr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 31.Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 32.Rodríguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci USA. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Bae YC, Jung JS, Kim JH. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J Cell Sci. 2006;119:4994–5005. doi: 10.1242/jcs.03281. [DOI] [PubMed] [Google Scholar]
- 34.Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75:775–779. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 35.Gaustad KG, Boquest AC, Anderson BE, Gerdes AM, Collas P. Differentiation of human adipose tissue stem cells using extracts of rat cardiomyocytes. Biochem Biophys Res Commun. 2004;314:420–427. doi: 10.1016/j.bbrc.2003.12.109. [DOI] [PubMed] [Google Scholar]
- 36.Planat-Bénard V, Menard C, André M, Puceat M, Perez A, Garcia-Verdugo JM, Pénicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 37.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 38.Jack GS, Almeida FG, Zhang R, Alfonso ZC, Zuk PA, Rodríguez LV. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. 2005;174:2041–2045. doi: 10.1097/01.ju.0000176489.96993.84. [DOI] [PubMed] [Google Scholar]
- 39.Strem BM, Zhu M, Alfonso Z, Daniels EJ, Schreiber R, Beygui R, MacLellan WR, Hedrick MH, Fraser JK. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7:282–291. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 40.Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342:662–670. doi: 10.1016/j.bbrc.2006.01.181. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 42.Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, Bankson JA, Vykoukal D, Alt E. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 43.Metzele R, Alt C, Bai X, Yan Y, Zhang Z, Pan Z, Coleman M, Vykoukal J, Song YH, Alt E. Human adipose tissue-derived stem cells exhibit proliferation potential and spontaneous rhythmic contraction after fusion with neonatal rat cardiomyocytes. FASEB J. 2011;25:830–839. doi: 10.1096/fj.09-153221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 45.Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, et al. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 46.Sadat S, Gehmert S, Song YH, Yen Y, Bai X, Gaiser S, Klein H, Alt E. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007;363:674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 47.Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, Takagawa J, Sievers RE, Wong ML, Kapasi NK, Mirsky R, et al. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–1256. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhang SH, Lee S, Shin JY, Lee TJ, Jang HK, Kim BS. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol Ther. 2014;22:862–872. doi: 10.1038/mt.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, Lue TF. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazo M, Planat-Bénard V, Abizanda G, Pelacho B, Léobon B, Gavira JJ, Peñuelas I, Cemborain A, Pénicaud L, Laharrague P, et al. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bagó JR, Soler-Botija C, Casaní L, Aguilar E, Alieva M, Rubio N, Bayes-Genis A, Blanco J. Bioluminescence imaging of cardiomyogenic and vascular differentiation of cardiac and subcutaneous adipose tissue-derived progenitor cells in fibrin patches in a myocardium infarct model. Int J Cardiol. 2013;169:288–295. doi: 10.1016/j.ijcard.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Rigol M, Solanes N, Roura S, Roqué M, Novensà L, Dantas AP, Martorell J, Sitges M, Ramírez J, Bayés-Genís A, et al. Allogeneic adipose stem cell therapy in acute myocardial infarction. Eur J Clin Invest. 2014;44:83–92. doi: 10.1111/eci.12195. [DOI] [PubMed] [Google Scholar]
- 54.Yin Q, Pei Z, Wang H, Zhao Y. Cyclosporine A-nanoparticles enhance the therapeutic benefit of adipose tissue-derived stem cell transplantation in a swine myocardial infarction model. Int J Nanomedicine. 2014;9:17–26. doi: 10.2147/IJN.S52005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayes-Genis A, Soler-Botija C, Farré J, Sepúlveda P, Raya A, Roura S, Prat-Vidal C, Gálvez-Montón C, Montero JA, Büscher D, et al. Human progenitor cells derived from cardiac adipose tissue ameliorate myocardial infarction in rodents. J Mol Cell Cardiol. 2010;49:771–780. doi: 10.1016/j.yjmcc.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Danoviz ME, Nakamuta JS, Marques FL, dos Santos L, Alvarenga EC, dos Santos AA, Antonio EL, Schettert IT, Tucci PJ, Krieger JE. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS One. 2010;5:e12077. doi: 10.1371/journal.pone.0012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rigol M, Solanes N, Farré J, Roura S, Roqué M, Berruezo A, Bellera N, Novensà L, Tamborero D, Prat-Vidal C, et al. Effects of adipose tissue-derived stem cell therapy after myocardial infarction: impact of the route of administration. J Card Fail. 2010;16:357–366. doi: 10.1016/j.cardfail.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Schenke-Layland K, Strem BM, Jordan MC, Deemedio MT, Hedrick MH, Roos KP, Fraser JK, Maclellan WR. Adipose tissue-derived cells improve cardiac function following myocardial infarction. J Surg Res. 2009;153:217–223. doi: 10.1016/j.jss.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SW, Lee DW, Yu LH, Zhang HZ, Kim CE, Kim JM, Park TH, Cha KS, Seo SY, Roh MS, et al. Mesenchymal stem cells overexpressing GCP-2 improve heart function through enhanced angiogenic properties in a myocardial infarction model. Cardiovasc Res. 2012;95:495–506. doi: 10.1093/cvr/cvs224. [DOI] [PubMed] [Google Scholar]
- 60.Okura H, Saga A, Soeda M, Miyagawa S, Sawa Y, Daimon T, Ichinose A, Matsuyama A. Intracoronary artery transplantation of cardiomyoblast-like cells from human adipose tissue-derived multi-lineage progenitor cells improve left ventricular dysfunction and survival in a swine model of chronic myocardial infarction. Biochem Biophys Res Commun. 2012;425:859–865. doi: 10.1016/j.bbrc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 62.Okura H, Matsuyama A, Lee CM, Saga A, Kakuta-Yamamoto A, Nagao A, Sougawa N, Sekiya N, Takekita K, Shudo Y, et al. Cardiomyoblast-like cells differentiated from human adipose tissue-derived mesenchymal stem cells improve left ventricular dysfunction and survival in a rat myocardial infarction model. Tissue Eng Part C Methods. 2010;16:417–425. doi: 10.1089/ten.TEC.2009.0362. [DOI] [PubMed] [Google Scholar]
- 63.Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, Geleijnse ML, Fernandez-Aviles F, Zijlsta F, Serruys PW, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59:539–540. doi: 10.1016/j.jacc.2011.09.065. [DOI] [PubMed] [Google Scholar]
- 64.Perin EC, Sanz-Ruiz R, Sánchez PL, Lasso J, Pérez-Cano R, Alonso-Farto JC, Pérez-David E, Fernández-Santos ME, Serruys PW, Duckers HJ, et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am Heart J. 2014;168:88–95.e2. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 65.Lin YC, Leu S, Sun CK, Yen CH, Kao YH, Chang LT, Tsai TH, Chua S, Fu M, Ko SF, et al. Early combined treatment with sildenafil and adipose-derived mesenchymal stem cells preserves heart function in rat dilated cardiomyopathy. J Transl Med. 2010;8:88. doi: 10.1186/1479-5876-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamdi H, Boitard SE, Planat-Benard V, Pouly J, Neamatalla H, Joanne P, Perier MC, Bellamy V, Casteilla L, Li Z, et al. Efficacy of epicardially delivered adipose stroma cell sheets in dilated cardiomyopathy. Cardiovasc Res. 2013;99:640–647. doi: 10.1093/cvr/cvt149. [DOI] [PubMed] [Google Scholar]
- 67.Pınarlı FA, Turan NN, Pınarlı FG, Okur A, Sönmez D, Ulus T, Oğuz A, Karadeniz C, Delibaşı T. Resveratrol and adipose-derived mesenchymal stem cells are effective in the prevention and treatment of doxorubicin cardiotoxicity in rats. Pediatr Hematol Oncol. 2013;30:226–238. doi: 10.3109/08880018.2012.762962. [DOI] [PubMed] [Google Scholar]
- 68.Harada Y, Yamamoto Y, Tsujimoto S, Matsugami H, Yoshida A, Hisatome I. Transplantation of freshly isolated adipose tissue-derived regenerative cells enhances angiogenesis in a murine model of hind limb ischemia. Biomed Res. 2013;34:23–29. doi: 10.2220/biomedres.34.23. [DOI] [PubMed] [Google Scholar]
- 69.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 70.Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279–290. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 71.Cai L, Johnstone BH, Cook TG, Liang Z, Traktuev D, Cornetta K, Ingram DA, Rosen ED, March KL. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells. 2007;25:3234–3243. doi: 10.1634/stemcells.2007-0388. [DOI] [PubMed] [Google Scholar]
- 72.Sumi M, Sata M, Toya N, Yanaga K, Ohki T, Nagai R. Transplantation of adipose stromal cells, but not mature adipocytes, augments ischemia-induced angiogenesis. Life Sci. 2007;80:559–565. doi: 10.1016/j.lfs.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 73.Cho HH, Kim YJ, Kim JT, Song JS, Shin KK, Bae YC, Jung JS. The role of chemokines in proangiogenic action induced by human adipose tissue-derived mesenchymal stem cells in the murine model of hindlimb ischemia. Cell Physiol Biochem. 2009;24:511–518. doi: 10.1159/000257495. [DOI] [PubMed] [Google Scholar]
- 74.Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 75.Kang Y, Park C, Kim D, Seong CM, Kwon K, Choi C. Unsorted human adipose tissue-derived stem cells promote angiogenesis and myogenesis in murine ischemic hindlimb model. Microvasc Res. 2010;80:310–316. doi: 10.1016/j.mvr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 76.Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, Park JH, Lee SY, Kim SP, Kim YD, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76:1750–1760. doi: 10.1253/circj.cj-11-1135. [DOI] [PubMed] [Google Scholar]
- 77.Madonna R, Taylor DA, Geng YJ, De Caterina R, Shelat H, Perin EC, Willerson JT. Transplantation of mesenchymal cells rejuvenated by the overexpression of telomerase and myocardin promotes revascularization and tissue repair in a murine model of hindlimb ischemia. Circ Res. 2013;113:902–914. doi: 10.1161/CIRCRESAHA.113.301690. [DOI] [PubMed] [Google Scholar]
- 78.Kang SK, Lee DH, Bae YC, Kim HK, Baik SY, Jung JS. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183:355–366. doi: 10.1016/s0014-4886(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 79.Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdán S, Díez-Tejedor E. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther. 2013;4:11. doi: 10.1186/scrt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu XL, Zhang W, Tang SJ. Intracranial transplantation of human adipose-derived stem cells promotes the expression of neurotrophic factors and nerve repair in rats of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol. 2014;7:174–183. [PMC free article] [PubMed] [Google Scholar]
- 81.Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, Otero-Ortega L, Fuentes B, Vallejo-Cremades MT, Sanz-Cuesta BE, Díez-Tejedor E. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: proof of concept in rats. J Transl Med. 2015;13:46. doi: 10.1186/s12967-015-0406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi M, Suzuki E, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y. Adipose tissue-derived stem cells inhibit neointimal formation in a paracrine fashion in rat femoral artery. Am J Physiol Heart Circ Physiol. 2010;298:H415–H423. doi: 10.1152/ajpheart.00391.2009. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi M, Suzuki E, Kumano S, Oba S, Sato T, Nishimatsu H, Kimura K, Nagano T, Hirata Y. Angiopoietin-1 mediates adipose tissue-derived stem cell-induced inhibition of neointimal formation in rat femoral artery. Circ J. 2013;77:1574–1584. doi: 10.1253/circj.cj-12-0930. [DOI] [PubMed] [Google Scholar]
- 84.Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med. 2006;119:1056–1061. doi: 10.1016/j.amjmed.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 85.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 86.Jeong JO, Han JW, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–1347. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, Devarajan E, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 88.Heo SC, Lee KO, Shin SH, Kwon YW, Kim YM, Lee CH, Kim YD, Lee MK, Yoon MS, Kim JH. Periostin mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth in a xenograft lung adenocarcinoma model. Biochim Biophys Acta. 2011;1813:2061–2070. doi: 10.1016/j.bbamcr.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Ji SQ, Cao J, Zhang QY, Li YY, Yan YQ, Yu FX. Adipose tissue-derived stem cells promote pancreatic cancer cell proliferation and invasion. Braz J Med Biol Res. 2013;46:758–764. doi: 10.1590/1414-431X20132907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madonna R, Renna FV, Cellini C, Cotellese R, Picardi N, Francomano F, Innocenti P, De Caterina R. Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. Eur J Clin Invest. 2011;41:126–133. doi: 10.1111/j.1365-2362.2010.02384.x. [DOI] [PubMed] [Google Scholar]
- 91.Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laschke MW, Grässer C, Kleer S, Scheuer C, Eglin D, Alini M, Menger MD. Adipose tissue-derived microvascular fragments from aged donors exhibit an impaired vascularisation capacity. Eur Cell Mater. 2014;28:287–298. doi: 10.22203/ecm.v028a20. [DOI] [PubMed] [Google Scholar]
- 93.Cianfarani F, Toietta G, Di Rocco G, Cesareo E, Zambruno G, Odorisio T Diabetes impairs adipose tissue-derived stem cell function and efficiency in promoting wound healing. Wound Repair Regen ; 21: 545-553. doi: 10.1111/wrr.12051. [DOI] [PubMed] [Google Scholar]
- 94.Fortini C, Cesselli D, Beltrami AP, Bergamin N, Caragnano A, Moretti L, Cecaro F, Aquila G, Rizzo P, Riberti C, et al. Alteration of Notch signaling and functionality of adipose tissue derived mesenchymal stem cells in heart failure. Int J Cardiol. 2014;174:119–126. doi: 10.1016/j.ijcard.2014.03.173. [DOI] [PubMed] [Google Scholar]
- 95.Yamanaka S, Yokote S, Yamada A, Katsuoka Y, Izuhara L, Shimada Y, Omura N, Okano HJ, Ohki T, Yokoo T. Adipose tissue-derived mesenchymal stem cells in long-term dialysis patients display downregulation of PCAF expression and poor angiogenesis activation. PLoS One. 2014;9:e102311. doi: 10.1371/journal.pone.0102311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rasmussen JG, Frøbert O, Pilgaard L, Kastrup J, Simonsen U, Zachar V, Fink T. Prolonged hypoxic culture and trypsinization increase the pro-angiogenic potential of human adipose tissue-derived stem cells. Cytotherapy. 2011;13:318–328. doi: 10.3109/14653249.2010.506505. [DOI] [PubMed] [Google Scholar]
- 97.Yamamoto Y, Fujita M, Tanaka Y, Kojima I, Kanatani Y, Ishihara M, Tachibana S. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. Biores Open Access. 2013;2:199–205. doi: 10.1089/biores.2013.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chung DJ, Wong A, Hayashi K, Yellowley CE. Effect of hypoxia on generation of neurospheres from adipose tissue-derived canine mesenchymal stromal cells. Vet J. 2014;199:123–130. doi: 10.1016/j.tvjl.2013.10.020. [DOI] [PubMed] [Google Scholar]


