Abstract
The endocannabinoid system consists of the cannabinoid (CB) receptors, CB1 and CB2, the endogenous ligands anandamide (AEA, arachidonoylethanolamide) and 2-arachidonoylglycerol (2-AG), and their synthetic and metabolic machinery. The use of cannabis has been described in classical and recent literature for the treatment of pain, but the potential for psychotropic effects as a result of the activation of central CB1 receptors places a limitation upon its use. There are, however, a number of modern approaches being undertaken to circumvent this problem, and this review represents a concise summary of these approaches, with a particular emphasis upon CB2 receptor agonists. Selective CB2 agonists and peripherally restricted CB1 or CB1/CB2 dual agonists are being developed for the treatment of inflammatory and neuropathic pain, as they demonstrate efficacy in a range of pain models. CB2 receptors were originally described as being restricted to cells of immune origin, but there is evidence for their expression in human primary sensory neurons, and increased levels of CB2 receptors reported in human peripheral nerves have been seen after injury, particularly in painful neuromas. CB2 receptor agonists produce antinociceptive effects in models of inflammatory and nociceptive pain, and in some cases these effects involve activation of the opioid system. In addition, CB receptor agonists enhance the effect of μ-opioid receptor agonists in a variety of models of analgesia, and combinations of cannabinoids and opioids may produce synergistic effects. Antinociceptive effects of compounds blocking the metabolism of anandamide have been reported, particularly in models of inflammatory pain. There is also evidence that such compounds increase the analgesic effect of non-steroidal anti-inflammatory drugs (NSAIDs), raising the possibility that a combination of suitable agents could, by reducing the NSAID dose needed, provide an efficacious treatment strategy, while minimizing the potential for NSAID-induced gastrointestinal and cardiovascular disturbances. Other potential “partners” for endocannabinoid modulatory agents include α2-adrenoceptor modulators, peroxisome proliferator-activated receptor α agonists and TRPV1 antagonists. An extension of the polypharmacological approach is to combine the desired pharmacological properties of the treatment within a single molecule. Hopefully, these approaches will yield novel analgesics that do not produce the psychotropic effects that limit the medicinal use of cannabis.
Keywords: Cannabinoid, Anandamide, CB2 receptor, Fatty acid amide hydrolase, Neuropathic pain, Inflammatory pain
1. Introduction
In 1993 it was reported in Nature that a carbonized material recovered from the abdomen of a young girl who had died in childbirth in the fourth century AD contained traces of a stable constituent of cannabis (Zias et al., 1993). Although this represents a documented early use of cannabis for the presumed treatment of pain, the use of cannabis extracts for this and other medical indications, as well as for recreational uses, has been described in both the ancient world and in more modern times (review, see Mechoulam, 1986). Currently, Sativex™, a buccal formulation of cannabis extract with defined ratios of Δ9-tetrahydroxannabinol (THC, the main psychoactive ingredient of cannabis) and cannabidiol, is licensed in Canada for the treatment of pain in multiple sclerosis patients (Perez and Ribera, 2008). A major issue, however, for all treatments based on cannabis is the potential for psychotropic effects and concerns about the long-term use of such medications. These concerns place a limitation upon the dosages that can be given and hence the potential level of pain relief. There are, however, a number of approaches that can be taken to circumvent this problem.
2. The endocannabinoid system
The endocannabinoid system consists of the G-protein coupled cannabinoid (CB) receptors, CB1 and CB2, the endogenous ligands anandamide (AEA, arachidonoylethanolamide) and 2-arachidonoylglycerol (2-AG), and their synthetic and metabolic machinery. The role of these endocannabinoids and other putative endocannabinoids in pain modulation and pathways for their synthesis and degradation has recently been reviewed (Hohmann and Suplita 2006). CB1 receptors are primarily neuronal (although they have been found in non-neuronal tissue) and mediate the psychotropic actions of cannabis (see Monory et al. (2007) for a genetic dissection of the neuronal populations involved in the different behavior al and autonomic effects of THC).
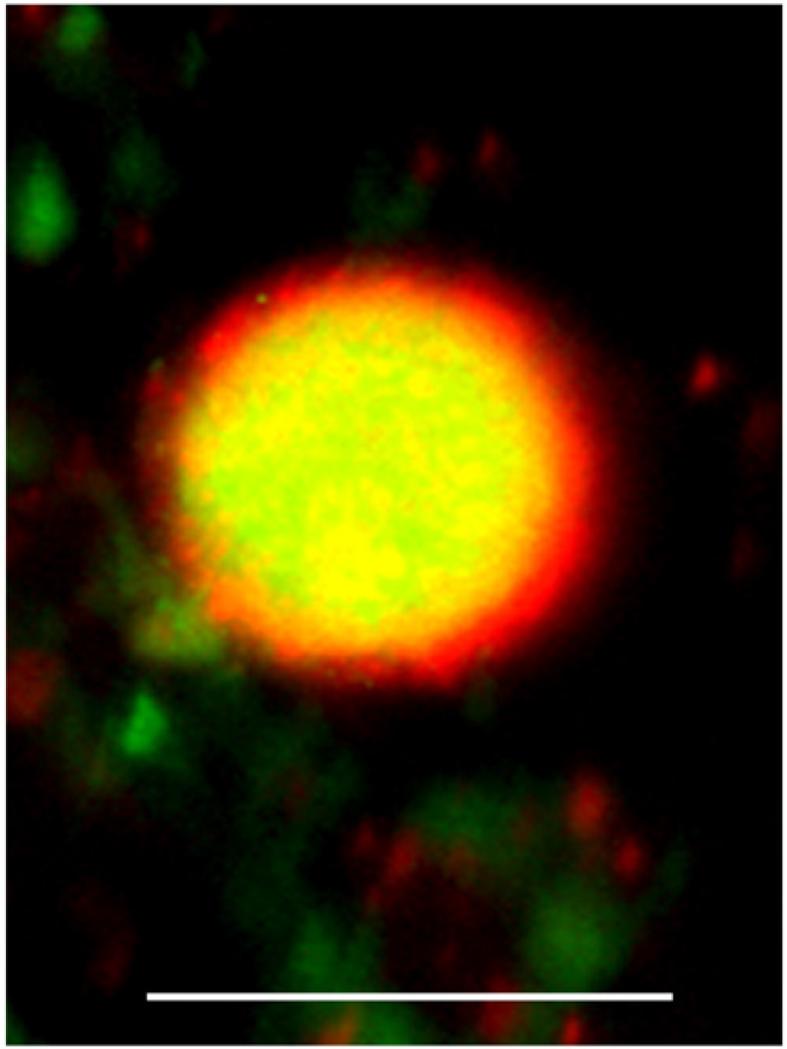
CB1 receptors are expressed in neurons of the CNS (Matsuda et al. 1990), and in DRG neurons (Hohmann and Herkenham 1999, Price et al. 2003), but there is also evidence for the expression of CB1 receptors in non-neural tissue (see e.g. Tokanovic et al., 2007). Similarly, while the CB2 receptor was originally considered to be expressed primarily in lymphoid tissues in the periphery (Munro et al. 1993, Di Marzo et al. 2004), recent evidence has pointed to a neuronal localization in some regions of the rodent brain (Onaivi et al. 2006), and CB2 immunoreactivity has been shown in activated microglia in affected regions of multiple sclerosis and amyotrophic lateral sclerosis post mortem human spinal cord (Yiangou et al. 2006). A recent study has also presented evidence for the localization of CB2 receptor-like immunoreactivity in human DRG sensory neurons in vitro (see Fig. 1), in injured nerves including neuromas, and in nerve fibers in human synovium and digit skin (Anand et al. 2008).
Fig. 1.
Membrane bound CB2 receptor (red) and cytoplasmic Gap43 (green) immunostaining in a human DRG small neuron in vitro. Bar=50 μm.
Both AEA and 2-AG are synthesized upon demand rather than being pre-stored, and have relatively short durations of action, due to effective metabolic pathways. In the case of AEA, this is brought about by a process of cellular accumulation followed primarily by hydrolysis to arachidonic acid, catalyzed by the enzyme fatty acid amide hydrolase (FAAH) (although it can also act as a substrate for both cyclooxygenase-2 and lipoxygenases). In the case of 2-AG, a similar pattern is seen, although in the brain the enzyme monoacylglycerol lipase is more important. At the outset, it should be pointed out that the exact process whereby cells accumulate AEA is not known and is a current area of controversy; mechanisms ranging from FAAH-gated diffusion to as yet unidentified designated transporter proteins having been postulated (see Fowler, 2008). In addition to the formal components of the endocannabinoid system summarized above, there are additional candidate cannabinoid receptors and endogenous ligands that have been described in the literature (reviews see Bradshaw and Walker, 2005; Brown, 2007). Therefore, endocannabinoid system can and should be regarded as a “work in progress” rather than an absolutely defined entity. The recent report that mice lacking GPR55 receptors (a putative CB receptor) do not show mechanical hyperalgesia following either complete Freund’s adjuvant treatment or partial nerve ligation (Staton et al., 2008) underlines the fact that the detailed characterization of the endocannabinoid system is an important research priority. Notwithstanding, a number of compounds are available that target different components of the currently accepted endocannabinoid system, compounds that have been found to have therapeutic potential in models of inflammatory and in some cases neuropathic pain (see Table 1 for a description of the main compounds forming the core of this review).
Table 1. The pharmacology of the endocannabinoid system–selected compounds.
| Compound | Mechanism of action | Effect in pain |
|---|---|---|
THC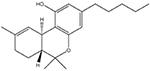
|
Primarily activation of CB receptors, although has off-target actions (e.g. Barann et al., 2002) |
Inflammatory: + Neuropathic: + |
AM1241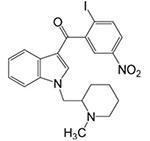
|
CB2-receptor selective ligand; acts as a “protean” agonist in vitro (Yao et al., 2006) and CB2 agonist in vivo |
Inflammatory: + Neuropathic: + |
GW405833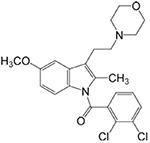
|
CB2-receptor selective ligand (efficacy dependent upon assay used, see Yao et al., 2008) |
Inflammatory: + Neuropathic: + |
LY2318912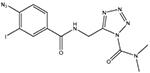
|
Blocks the accumulation and metabolism of AEA (Moore et al., 2005). Acts primarily as a potent FAAH inhibitor, but with many off-target actions (Alexander and Cravatt, 2006) |
Inflammatory: + |
URB597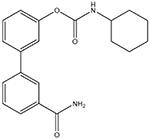
|
Selective FAAH inhibitor (Kathuria et al., 2003). Some off-target actions have been reported, but their importance is unclear |
Inflammatory: + Visceral: + Neuropathic: +/− |
ibu-am5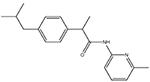
|
Dual COX- and FAAH-inhibitory compound (Holt et al., 2007). |
Visceral: + |
| N-arachidonoylserotonin |
Dual TRPV1 antagonist/FAAH-inhibitory compound (Maione et al., 2007). |
Inflammatory: + Neuropathic: + |
Pravadoline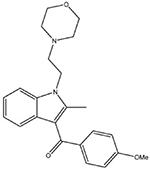
|
Dual CB agonist/COX-inhibitory compound (D’Ambra et al., 1992). |
Inflammatory: + Visceral: + |
The compounds shown in this table have been selected since they are those principally discussed in this review.“+” indicates efficacy in a preclinical model of this pain type (for details, see text). For a more complete list, see Fowler (2008).
3. Targeting the endocannabinoid system for the treatment of pain
3.1. Peripherally-restricted cannabinoid agonists
It has been well established that the ability of cannabinoids to affect pain perception has supra-spinal, spinal and peripheral components (for review, see Hohmann, 2002; Walker and Hohmann, 2005). With respect to the peripheral component, local administration of both synthetic cannabinoids and exogenous anandamide and 2-AG produce antinociceptive effects in the formalin model of inflammatory pain (see e.g. Calignano et al., 1998; Guindon et al., 2007). Recently, Agarwal et al. (2007) reported the effects in pain models of the conditional deletion of CB1 receptors in nociceptive neurons of the dorsal root ganglia of the mouse. These mice were more sensitive to the effects of intraplantar administration of capsaicin and formalin, whereas their motor performance on a rotorod test was not affected. The hyperalgesic response to administration of complete Freund’s adjuvant was also greater than for wild-type mice, and the ability of a synthetic cannabinoid to alleviate this response was also greatly reduced (Agarwal et al., 2007). These data would suggest that peripherally restricted cannabinoids may have utility in inflammatory pain. In this respect, Fride et al. (2004) have identified analog s of the (+)-enantiomer of cannabidiol that are active towards CB receptors and reduce the pain response in the formalin model of inflammatory pain, without producing overt signs of central CB1 receptor activation. Other peripherally restricted CB1 or CB1/CB2 receptor agonists are currently being investigated as potential approaches to the treatment of pain. A good example of this is the study of Dziadulewicz et al. (2007). These authors reported that naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone bound as an agonist to human CB1 and CB2 receptors with IC50 values of 15 and 98 nM, respectively, and produced a good separation of effects upon neuropathic pain and catalepsy, consistent with a limited penetration of the compound into the brain.
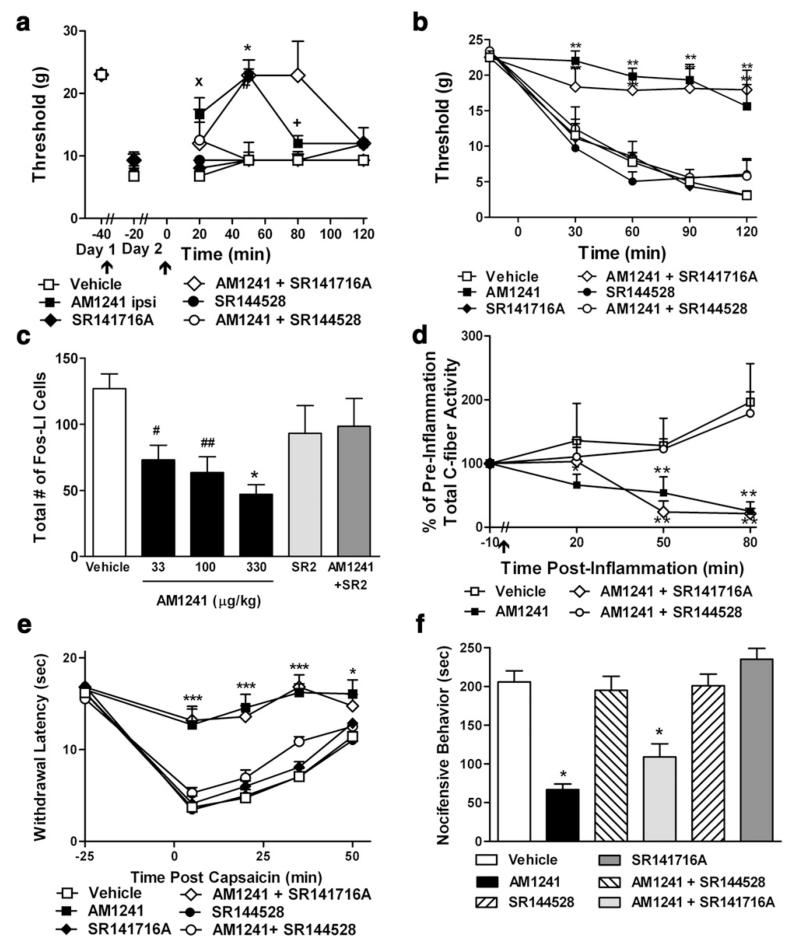
3.2. CB2 receptor-selective agonists (see Fig. 2)
Fig. 2.
Activation of cannabinoid CB2 receptors suppresses the development and maintenance of inflammatory nociception in behavioral, electrophysiological and neurochemical studies. (a) AM1241 (33 μg/kg i.p.), administered locally in the inflamed paw, suppresses established carrageenan-evoked mechanical allodynia. Effects were blocked by the CB2 antagonist SR144528 (33 μg/kg i.p.), but not by SR141716A (33 μg/kg i.p.). (b) AM1241 (330 μg/kg i.p.) induces a CB2-mediated suppression of the development of carrageenan-evoked (b) mechanical allodynia and (c) spinal Fos protein expression. (d) AM1241 (330 μg/kg, i.v.) suppressed total C-fiber-mediated neuronal excitability in spinal wide dynamic range neurons through a CB2-specific mechanism. AM1241 (330 μg/kg i.p.) also suppresses capsaicin-evoked (e) thermal hyperalgesia and (f) nocifensive behavior. (b–f) Effects were completely blocked by SR144528 (1 mg/kg), but not by SR141716A (1 mg/kg). Data (Mean+SEM). Sources: (a) Gutierrez et al. (2007); (b–c) Nackley et al. (2003a), (d) Nackley et al. (2004); (e–f) Hohmann et al. (2004).
Expression of CB2 receptors in the peripheral and central nervous systems is modulated in a number of rodent pain models. No increase in CB2 receptor mRNA was apparent in the Freund’s Complete Adjuvant (FCA) model of inflammatory pain, while upregulation of mRNA and protein were seen in the ipsilateral dorsal horn of the lumbar spinal cord using two models of neuropathic pain, the chronic constriction injury (CCI) (Bennett and Xie, 1988) and spinal nerve ligation (SNL) (Kim and Chung, 1992) models (Zhang et al., 2003). The authors suggest that the increased expression was in microglia as it colocalized with OX-42, a marker of microglia. The upregulation of CB2 receptor mRNA in spinal cord was confirmed in a subsequent study using the SNL model (Beltramo et al., 2006). Here, the cell type was ambiguous, though in a separate set of experiments the authors did provide evidence for upregulation of CB2 receptor mRNA in cultured rat spinal microglia. Expression of CB2 receptors on microglia is supported by additional studies showing interferon γ (IFNγ) induces upregulation (Carlisle et al., 2002) with a distinct subcellular localization at the surface of activated microglia in culture (Walter and Stella, 2004). Upregulation of CB2 receptors in spinal cord was also demonstrated immunohistochemically following either SNL or axotomy (Wotherspoon et al., 2005). However, in contrast to the earlier studies, colocalization with GAP-43 and galanin in the superficial lamina suggested expression on primary afferent (C fiber) terminals (Wotherspoon et al., 2005). This upregulation was absent in CB2 receptor knockout mice. Supporting neuronal localization, increased CB2 receptor immunoreactivity was also seen in nerve sections proximal, but not distal, to the site of ligation (Wotherspoon et al., 2005) (Fig. 2).
In addition to studies demonstrating the presence of CB2 receptor in components of the pain pathway and alterations in expression level in pain models, agonists both selective and non-selective have been used to investigate the role of CB2 in nociception. The non-selective endogenous agonists, AEA and PEA, have been combined with the CB1 receptor-selective antagonists, AM251, AM281 and SR141716A and the CB2 receptor-selective antagonists, AM630 and SR144528. Administration of AEA locally into the rat hindpaw reverses pain due to both intraplantar carrageenan and formalin (Sokal et al., 2003; Guindon et al., 2006a). While the AEA-induce analgesia following formalin was blocked by the CB1 receptor antagonist AM251 (Guindon et al., 2006a), both the CB2 and CB1 receptor-selective antagonists, SR144528 and SR141716A, respectively, blocked the effect of AEA on carrageenan-induced hypersensitivity of spinal neurons (Sokal et al., 2003). These results imply a contribution of both peripherally expressed CB1 and CB2 receptors to the antinociceptive response. Importantly in the study by Sokal et al. (2003), local administration had no effect in non-inflamed tissue. Likewise, systemic administration has been shown to reverse CFA-induced mechanical hyperalgesia in a non-CB1 receptor sensitive manner (Smith et al., 1998).
The non-selective synthetic cannabinoid agonists HU-210, nabilone, CP55,940 and WIN55,212-2, have also been combined with CB1 and CB2 receptor-selective antagonists to confirm the role of CB2 receptors in pain transmission. HU-210 reversed carrageenan-induced edema through the CB2 receptor, as the CB2 receptor-selective antagonist SR144528 blocked this effect (Clayton et al., 2002). A similar result was found when nabilone was combined with SR144528 (Conti et al., 2002). Studies have also been conducted with CP55,940 and WIN55,212-2. Both compounds are analgesic in assays of acute pain; the effect of CP55,940 is only partially blocked by CB1 receptor-selective antagonists (Scott et al., 2004) while that of WIN55,212-2 is reduced in CB2 receptor knockout animals compared to wild type, but remains in CB1 receptor knockout animals (Ibrahim et al., 2006). CP55,940 administered either systemically or intrathecally has also proven efficacious against neuropathic pain in rats. Interestingly, only the antihyperalgesic effect of systemically administered CP55,940 could be partially blocked by SR144528 (Scott et al., 2004). This result could be due to insufficient CNS penetraton of the antagonist, or may imply that the effects in the spinal cord are due to CB1 receptor activation alone. In the same study, the dose-dependent catalepsy that was observed was not blocked by a CB2 receptor antagonist, reinforcing the notion that side-effects track with activation of CB1 receptors. Finally, WIN55,212-2 has been shown to inhibit carrageenan-induced allodynia and mechanical hyperalgesia and burn-induced thermal and mechanical hyperalgesia after local administration, as well as bone cancer pain and inflammatory muscle pain after systemic administration (Nackley et al., 2003b; Kehl et al., 2003; Johanek and Simone, 2004). All effects, except for muscle pain, were shown to be at least partially blocked by CB2 receptor-selective antagonists.
Concerning studies investigating selective CB2 agonists, a relatively large number of the pharmacological reports that have been published on the role of CB2 receptors in pain have been generated using a limited number of selective agonists, namely HU-308, AM1241, GW405833, JWH 015 and JWH 133. HU-308 is a THC derivative that is reported to have an in vitro Ki value of ~23 nM at CB2 receptors (versus a Ki value that is greater than 10 mM for the CB1 receptor) (Hanuš et al., 1999). When administered intraperitoneally, it reduced both the inflammation associated with arachidonic acid-induced ear swelling and the late phase of formalin-induced pain behavior in mice, in an SR144528-sensitive manner (Hanuš et al., 1999). In addition, in the plantar incision model of post-surgical pain, it has been shown to reduce allodynia. This effect was blocked by the CB2 receptor-selective antagonist SR144528 (LaBuda et al., 2005). HU-308 appears to have minimal off target effects as it did not affect acute nociception as measured on a 55 °C hot plate, did not cause catalepsy and did not inhibit ambulation or rearing in the open field (Hanuš et al., 1999).
The small molecule CB2 receptor-selective compound that has most substantially penetrated the scientific literature is AM1241, that is reported to have an affinity for CB2 receptors of 2 nM with a high degree of selectivity over CB1 receptors (95-340 fold) (Malan et al., 2001a). This high degree of selectivity is maintained in membranes prepared from mouse spleen and brain, for CB2 and CB1 receptors, respectively (Ibrahim et al., 2003). AM1241 is a racemic mix; considering the individual enantiomers R,S-AM1241 act as an agonist at human CB2, but an inverse agonist at rat and mouse CB2 receptors. R-AM1241 binds with more than 40-fold higher affinity than S-AM1241, to human rat and mouse CB2 receptors and displays a functional profile similar to that of the racemate. In contrast, S-AM1241 is an agonist at human, rat and mouse CB2 receptors. AM1241 has been described as a protean agonist implying the state of constitutive receptor activity can determine the functional effect of a ligand-receptor interaction (Yao et al., 2006). Behaviorally, AM1241 did not elicit deficits in the rotarod or catalepsy assays (Malan et al., 2001a). AM1241 did however show antinociceptive effects towards an acute thermal stimulus (Hargreaves apparatus) when administered either systemically or locally; this effect could be reversed with the CB2 receptor-selective antagonist, AM630 (Malan et al., 2001a). AM1241 has also been shown to be anti-inflammatory and efficacious against inflammatory, neuropathic and post-surgical pain, in addition to chemical-(capsaicin and formalin) induced pain and substance P-induced plasma extravasation, when administered either locally or systemically (Malan et al., 2001b; Nackley et al., 2003a; Quartilho et al., 2003; Hohmann et al., 2004; LaBuda et al., 2005). The effects of AM1241 are reversible with selective CB2 receptor antagonists (AM630 and SR144528), were not reversible with selective CB1 receptor antagonists (AM251 and SR141716A), and remained in animals that were null mutants for the CB1 receptor (Ibrahim et al., 2003).
GW405833 (L-768,242) is a CB2 receptor agonist with a reported in vitro Ki value of 4–12 nM at recombinant human CB2 receptors (Gallant et al., 1996; Green et al., 1999; Valenzano et al., 2005). At recombinant human CB1 receptors, GW405833 has reported Ki values ranging from 1900 to 4800 nM, leading to a 160–1200-fold selectivity (Gallant et al., 1996; Valenzano et al., 2005). GW405833 has shown agonist properties in both direct cAMP accumulation assays (Valenzano et al., 2005) and a cAMP reporter system. Against native rat CB2 receptors the Ki value is comparable to human, but the compound is more potent at CB1 receptors compared to the human ortholog leading to reduced selectivity (80-fold) (Valenzano et al., 2005). In vivo, GW405833 reduces edema and inhibits hypersensitivity associated with intraplantar injection of carrageenan (Clayton et al., 2002). These effects were inhibited by SR144528, providing evidence that the effect of GW405833 is mediated by CB2 receptors (Clayton et al., 2002). The compound (up to 30 mg/kg) elicits potent and efficacious antihyperalgesic effects in the rat SNL and PSN (Seltzer et al., 1990) models of neuropathic pain, as well as incisional, chemical-induced (formalin) and inflammatory (CFA) pain (Valenzano et al., 2005; LaBuda et al., 2005; Beltramo et al., 2006). Analgesia, sedation and catalepsy were not seen in this dose range, but were apparent at 100 mg/kg (Valenzano et al., 2005). Comparable effects were seen in mouse models of inflammatory and neuropathic pain but were absent in CB2 receptor knockout mice. The side effects and frank analgesia remained in these knockout animals suggesting a CB1 receptor-related mechanism of action at these high doses (Whiteside et al., 2005).
JWH 015 is a synthetic cannabinoid agonist with literature Ki values of 14–54 nM at recombinant human CB2 receptors and 380 nM at recombinant human CB1 receptors (Showalter et al., 1996; Aung et al., 2000; Mukherjee et al., 2004). At recombinant rat CB2 receptors, the compound is reported to be less potent, with a Ki value of 150 nM (Mukherjee et al., 2004). Additionally, JWH 015 decreases FSK-mediated cAMP accumulation in vitro (Mukherjee et al., 2004). It is perhaps due to this limited selectivity for CB2 over CB1 receptors that this pharmacological tool has not been as extensively profiled in vivo compared to those compounds discussed earlier. Importantly however, when administered systemically it can reduce microglial activation following infection with TMEV (Theiler’s murine encephalomyelitis virus) in mice (Arévalo-Martin et al., 2003), an effect that may indicate the mechanism of action of CB2 receptor agonists in neuropathic pain. Lastly, JWH 133 is a published CB2 receptor-selective compound with a reported Ki value against recombinant human CB2 receptors of ~3 nM and against native rat CB1 receptors of ~670 nM (Huffman et al., 1999). No in vitro functional data have been published for JWH 133. When administered systemically, JWH 133 can inhibit citric acid-induced cough in conscious guinea pigs, possibly indicating a neuronal mechanism of action (Patel et al., 2003). In support of this conclusion JWH 133 has been shown to decrease capsaicin-induced depolarizations of guinea pig and human vagus nerve (Patel et al., 2003). In addition, it has been shown to reverse hyperalgesia due to intraplantar injection of carrageenan with a concurrent reduction of edema (Elmes et al., 2005). Importantly, JWH 133 has been shown to reduce the responses of wide dynamic range dorsal horn neurons to both innocuous and noxious intensities of mechanical stimuli. This effect was seen in models of inflammatory and neuropathic pain, in addition to sham operated animals and was partially blocked by the CB2 receptor antagonist SR144528 (Elmes et al., 2004). A comparable effect was observed following direct spinal application of JWH 133 (Sagar et al., 2005).
Functional studies in cultured human sensory neurons demonstrated CB2 agonists, GW842166 and GW833972, produced inhibition of capsaicin-induced Ca2+ influx. Further mechanistic studies showed CB2 agonist inhibition of capsaicin responses was reversed in the presence of the CB2 antagonist, GW818646, and exogenous 8-bromo cAMP, but was unaffected in the presence of the opioid blocker, naloxone, or the CB1 receptor antagonist SR141716A. Studies demonstrating the inhibition of sensory nerve activity by CB2 receptor activation in guinea pigs (Patel et al. 2003), and rat models of acute and chronic pain (Ross et al. 2001, Nackley et al. 2004, Elmes et al. 2004, Sagar et al. 2005) also suggest direct action on sensory neurons. In addition, there is evidence for the expression of CB2 receptors in keratinocytes, which in the presence of CB2 agonists, release endogenous β-endorphins to activate opioid receptors in the peripheral nerve terminals of sensory neurons (Ibrahim et al. 2005).
Taken together these studies summarized above imply both a peripheral and spinal site of actions at different cell types. Regarding clinical trials, GlaxoSmithKline has undertaken a CB2 agonist Phase II trial for inflammatory pain. Pharmos is still considering clinical trials for their CB2-selective agonist PRS-639058 (structure not disclosed) as is Glenmark with their most advanced candidate GRC 10693 (structure not disclosed).
3.3. Inhibitors of endocannabinoid metabolism
The finding that formalin administration to the hindpaw of rats produced a release of AEA into the periaqueductal gray (Walker et al., 1999) led those authors to suggest that compounds preventing the metabolism of this endocannabinoid may be useful agents for the treatment of pain. This has subsequently been shown to be the case, in particular for inflammatory pain. Thus, selective inhibitors of fatty-acid amide hydrolase such as URB597 (structure see Table 1) and OL-135 produce beneficial effects in a variety of models of inflammatory pain, whereas their effects upon neuropathic pain are less clear (Lichtman et al., 2004; Jayamanne et al., 2006; Chang et al., 2006; Russo et al., 2007a,b). Inhibitors of FAAH and MGL also enhance endocannabinoid-mediated analgesia that is induced by exposure to environmental stressors and increase the bioavailability of anandamide and/or 2-AG (Hohmann et al. 2005; Suplita et al., 2005). Importantly, FAAH inhibitors do not produce behaviors associated with a central activation of CB1 receptors (Kathuria et al., 2003; Jayamanne et al., 2006) and do not appear to behave like THC in drug-discrimination tests (Gobbi et al., 2005), although there may be some issues with respect to alcohol consumption (Vinod et al., 2008; but see Cippitelli et al., 2008).
It should be emphasized that the notion that FAAH inhibitors produce all their effects via a potentiation of endocannabinoid signaling may be an oversimplification. FAAH inhibitors also increase the bioavailability of endogenous fatty-acid amides that are biologically active, but that do not bind to cannabinoid receptors, such as palmitoylethanolamide (PEA). PEA blocks both phases of the formalin response in rats and mice, in addition to carrageenan-induced hyperalgesia in rats, when applied locally or systemically (Calignano et al., 1998, 2001; Jaggar et al., 1998; Conti et al., 2002); an effect that was blocked by SR144528 (Calignano et al., 1998). PEA has also been shown to block acetic acid-induced visceral pain (Calignano et al., 2001), nerve growth factor (NGF)-induced hyperalgesia, and accumulation of immune cells at the site of NGF injection; CB2 receptor-, but not CB1 receptor-selective antagonists blocked both effects (Farquhar-Smith and Rice, 2003). Given that PEA lacks direct effects upon CB1 and CB2 receptors, it is possible that the effects of the compound are either indirect (via entourage effects, that increase the bioavailability of anandamide by competing with the same enzymes for hydrolysis), or via CB2-like receptor (review, see Lambert et al., 2002). However, recent data has suggested that peroxisome-proliferator activated receptor α (PPARα) may mediate at least some of the effects of PEA, and that the ability of SR144528 to block PEA may be related to an off-target action of this compound upon PPARα (LoVerme et al., 2006). In this respect, Sagar et al. (in press) recently reported that the responsiveness of wide dynamic range neurons following carrageenan-injection into the hind paws is attenuated by local injection of URB597 in a manner blocked by concomitant local injection of a PPAR-α, but not a CB1 receptor antagonist. Indeed, the observation that the effects of URB597 in the CFA model are partially blocked by rimonabant and SR144528 (the combination producing a complete block) (Jayamanne et al., 2006) may reflect involvement of PPAR-α rather than CB2 receptors in addition to CB1 receptors.
Inhibitors of endocannabinoid uptake are also effective in models of inflammatory and neuropathic pain (see Maione et al., in press, for a recent article investigating the efficacy and degree of CB-receptor involvement in a series of compounds with different relative potencies towards FAAH and AEA uptake). The most potent compound so far described is LY2318912, which produces good effects in the formalin model (Moore et al., 2005). This compound, however, is a potent FAAH inhibitor (Alexander and Cravatt, 2006), and so it is unclear as to whether its effects in the formalin test are due to blockade of AEA accumulation or its subsequent metabolism. Indeed, the uncertainty concerning the mechanism(s) by which AEA is accumulated into cells remains an important issue that in the worst case could prevent development of a potentially useful class of drugs.
4. Endocannabinoid modulating agents as a component of new pharmacotherapies for pain
The approaches outlined in section 3 have all been considered per se, but a useful approach may be the combination of these actions with other drugs, with the aim of either improving efficacy or providing a better safety profile than that seen with currently available analgesics. An obvious combination is that of cannabinoids and opioids, given that some of the antinociceptive effects of cannabinoids involve activation of the opioid system and vice versa (Ibrahim et al., 2005; da Fonseca Pacheco et al., 2008). Moreover, CB receptor agonists enhance the effect of μ-opioid receptor agonists in a variety of models of analgesia (Reche et al., 1996; Yesilyurt et al., 2003; Finn et al., 2004; Tham et al., 2005; Cox et al., 2007). Activation of CB receptors either directly or indirectly also increases the analgesic effect of non-steroidal anti-inflammatory drugs (NSAIDs) (Guindon et al., 2006a,b; Ulugöl et al., 2006; Naidu and Lichtman, 2007), raising the possibility that a combination of suitable agents could, by reducing the NSAID dose needed, provide an efficacious treatment strategy while minimizing the potential for NSAID-induced gastrointestinal and cardiovascular disturbances. Other potential “partners” for endocannabinoid modulatory agents include α2-adrenoceptor, peroxisome proliferator-activated receptor α agonists and TRPV1 antagonists (see e.g. Yoon and Choi, 2003; Tham et al., 2005; Russo et al., 2007a,b).
An extension of the polypharmacological approach above is to combine the desired pharmacological properties of the treatment within a single molecule. Although this lacks the flexibility of dosing that is possible when separate drugs are given, a major advantage is that the potential for variability of response due to inter-individual variations in the metabolism of the two components relative to each other is eliminated (for a review of the concept of “designed multiple ligands” see Morphy and Rankovic, 2005). Such compounds are beginning to appear in the literature. For example, N-arachidonoylserotonin is a TRPV1 (transient receptor potential vanilloid type 1) antagonist with FAAH inhibitory properties that is active in a number of models of inflammatory and neuropathic pain (Maione et al., 2007). The ibuprofen analog N-(3-methylpyridin-2-yl)-2-(4′-isobutylphenyl)propionamide, a compound active in models of visceral pain (Cocco et al., 2003), is 2-3 orders of magnitude more potent an inhibitor of FAAH than ibuprofen, while retaining its cyclooxygenase-inhibitory potency (Holt et al., 2007) and may be useful as a template for the design of potent FAAH/cyclooxygenase-2 inhibitors. Pravadoline, which has both cyclooxygenase inhibitory and cannabinoid receptor agonist properties (D’Ambra et al., 1992), has greater analgesic efficacy than NSAIDs such as zomepirac in several different tests for analgesia (Haubrich et al., 1990).
5. Conclusions
The aim of this review has been to highlight different approaches whereby the endocannabinoid system can be harnessed to produce novel analgesic drugs that lack the psychotropic effects that place a limit upon the usefulness of THC. Lead discovery and lead optimization activities at numerous industrial and academic pharmacology/chemistry laboratories has led to the identification of a number of novel, selective and peripherally restricted modulators of the endocannabinoid system highlighted here. The preclinical profile of a number of these proof-of-concept compounds highlighted here is extremely encouraging, and it is hoped that these potent, selective and efficacious compounds will translate to the clinic.
REFERENCES
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JP, Cravatt BF. The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J. Am. Chem. Soc. 2006;128:9699–9704. doi: 10.1021/ja062999h. [DOI] [PubMed] [Google Scholar]
- Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, Birch R, Benham C, Bountra C, Chessell IP, Anand P. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–680. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Arévalo-Martin N, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J. Neurosci. 2003;23:2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MM, Griffin G, Huffman JW, Wu MJ, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend. 2000;60:133–140. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- Barann M, Molderings G, Brüss M, Bönisch H, Urban BW, Göthert M. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 2002;137:589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Bernardini N, Bertorelli R, Campanella M, Nicolussi E, Fredduzzi S, Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur. J. Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. Br. J. Pharmacol. 2005;144:459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ. Novel cannabinoid receptors. Br. J. Pharmacol. 2007;152:567–575. doi: 10.1038/sj.bjp.0707481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Piomelli D. Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide. Eur. J. Pharmacol. 2001;419:191–198. doi: 10.1016/s0014-2999(01)00988-8. [DOI] [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Luo L, Palmer JA, Sutton S, Wilson SJ, Barbier AJ, Breitenbucher JG, Chaplan SR, Webb M. Inhibition of fatty acid amide hydrolase produces analgesia by multiple mechanisms. Br. J. Pharmacol. 2006;148:102–113. doi: 10.1038/sj.bjp.0706699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology. 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. (Berl.) [DOI] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O’Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Cocco MT, Congiu C, Onnis V, Morelli M, Cauli O. Synthesis of ibuprofen heterocyclic amides and investigation of their analgesic and toxicological properties. Eur. J. Med. Chem. 2003;38:513–518. doi: 10.1016/s0223-5234(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Conti S, Costa B, Colleoni M, Parolaro D, Giagnoni G. Antiinflammatory action of endocannabinoid palmitoylethanolamide and the synthetic cannabinoid nabilone in a model of acute inflammation in the rat. Br. J. Pharmacol. 2002;135:181–187. doi: 10.1038/sj.bjp.0704466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP. Synergy between Δ9-tetrahydrocannabinol and morphine in the arthritic rat. Eur. J. Pharmacol. 2007;567:125–130. doi: 10.1016/j.ejphar.2007.04.010. [DOI] [PubMed] [Google Scholar]
- D Ambra TE, Estep KG, Bell MR, Eissenstat MA, Josef KA, Ward SJ, Haycock DA, Baizman ER, Casiano FM, Beglin NC, Chippari SM, Grego JD, Kullnig RK, Daley GT. Conformationally restrained analogues of pravadoline: nanomolar potent, enantioselective, (aminoalkyl) indole agonists of the cannabinoid receptor. J. Med. Chem. 1992;35:124–135. doi: 10.1021/jm00079a016. [DOI] [PubMed] [Google Scholar]
- da Fonseca Pacheco D, Klein A, de Castro Perez A, da Fonseca Pacheco CM, de Francischi JN, Duarte ID. The 1-opioid receptor agonist morphine, but not agonists at d- or j-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br. J. Pharmacol. 2008;154:1143–1149. doi: 10.1038/bjp.2008.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004;3(9):771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Dziadulewicz EK, Bevan SJ, Brain CT, Coote PR, Culshaw AJ, Davis AJ, Edwards LJ, Fisher AJ, Fox AJ, Gentry C, Groarke A, Hart TW, Huber W, James IF, Kesingland A, La Vecchia L, Loong Y, Lyothier I, McNair K, O’Farrell C, Peacock M, Portmann R, Schopfer U, Yaqoob M, Zadrobilek J. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl) methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J. Med. Chem. 2007;50:3851–3856. doi: 10.1021/jm070317a. [DOI] [PubMed] [Google Scholar]
- Elmes SJR, Jhaveri MD, Smart D, Kendall DA, Chapman V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naïve rats and in rat models of inflammatory and neuropathic pain. Eur. J. Neurosci. 2004;20:2311–2320. doi: 10.1111/j.1460-9568.2004.03690.x. [DOI] [PubMed] [Google Scholar]
- Elmes SJ, Winyard LA, Medhurst SJ, Clayton NM, Wilson AW, Kendall DA, Chapman V. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118(3):327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Farquhar-Smith WP, Rice AS. A novel neuroimmune mechanism in cannabinoid-mediated attenuation of nerve growth factor-induced hyperalgesia. Anesthesiology. 2003;99(6):1391–1401. doi: 10.1097/00000542-200312000-00024. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V. Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur. J. Neurosci. 2004;19:678–686. doi: 10.1111/j.0953-816x.2004.03177.x. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. “The tools of the trade”—an overview of the pharmacology of the endocannabinoid system. Curr. Pharm. Des. 2008;14:2254–2265. doi: 10.2174/138161208785740126. [DOI] [PubMed] [Google Scholar]
- Fride E, Feigin C, Ponde DE, Breuer A, Hanus L, Arshavsky N, Mechoulam R. (+)-Cannabidiol analogues which bind cannabinoid receptors but exert peripheral activity only. Eur. J. Pharmacol. 2004;506:179–188. doi: 10.1016/j.ejphar.2004.10.049. [DOI] [PubMed] [Google Scholar]
- Gallant M, Dufresne C, Gareau Y, Guay D, Leblanc Y, Prasit P, Rochette C, Sawyer N, Slipetz DM, Tremblay N, Metters KM, Labelle M. New class of potent ligands for the human peripheral cannabinoid receptor. Bioorg. Med. Chem. Lett. 1996;6:2263–2268. [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, O’Shaughnessy C, Disney G, Marshall F. Reporter assays for human cannabinoid CB1 and CB2 receptors for the identification of novel agonists. Br. J. Pharmacol. 1999;126(Proc. Suppl.):112P. [Google Scholar]
- Guindon J, De Léan A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006a;121:85–93. doi: 10.1016/j.pain.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Guindon J, LoVerme J, De Léan A, Piomelli D, Beaulieu P. Synergistic antinociceptive effects of anandamide, an endocannabinoid, and nonsteroidal anti-inflammatory drugs in peripheral tissue: a role for endogenous fatty-acid ethanolamides? Eur. J. Pharmacol. 2006b;550:68–77. doi: 10.1016/j.ejphar.2006.08.045. [DOI] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. Br. J. Pharmacol. 2007;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br. J. Pharmacol. 2007;150:153–163. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanuš L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E. HU-308: a specific agonist for CB2, a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. U. S. A. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrich DR, Ward SJ, Baizman E, Bell MR, Bradford J, Ferrari R, Miller M, Perrone M, Pierson AK, Saelens JK, Luttinger D. Pharmacology of pravadoline: a new analgesic agent. J. Pharmacol. Exp. Ther. 1990;255:511–522. [PubMed] [Google Scholar]
- Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem. Phys. Lipids. 2002;121:173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90(3):923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435(7045):1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A. Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin. J. Pharmacol. Exp. Ther. 2004;308:446–453. doi: 10.1124/jpet.103.060079. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL., 2nd. Endocannabinoid mechanisms of pain modulation. AAPS J. 2006;8(4):E693–E708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S, Paylor B, Boldrup L, Alajakku K, Vandevoorde S, Sundström A, Cocco MT, Onnis V, Fowler CJ. Inhibition of fatty acid amide hydrolase, a key endocannabinoid metabolizing enzyme, by analogues of ibuprofen and indomethacin. Eur. J. Pharmacol. 2007;565:26–36. doi: 10.1016/j.ejphar.2007.02.051. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, Martin BR. 3-(1′,1′.Dimethylbutyl)-1-deoxy-Δ8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg. Med. Chem. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng HF, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, Porreca F, Buckley NE, Makriyannis A, Malan TP. CB2 cannabinoid receptor mediation of nociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Jaggar SI, Hasnie FS, Sellaturay S, Rice ASC. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain. 1998;76:189–199. doi: 10.1016/s0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br. J. Pharmacol. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Simone DA. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain. 2004;109:432–442. doi: 10.1016/j.pain.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, Simone DA. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–186. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- LaBuda CJ, Koblish M, Little PJ. Cannabinoid CB2 receptor agonist activity in the hindpaw incision model of postoperative pain. Eur. J. Pharmacol. 2005;527:172–174. doi: 10.1016/j.ejphar.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Vandevoorde S, Jonsson K-O, Fowler CJ. The palmitoylethanolamide family: a new class of anti-inflammatory agents? Curr. Med. Chem. 2002;9:663–674. doi: 10.2174/0929867023370707. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J. Pharmacol. Exp. Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor- J. Pharmacol. Exp. Ther. 2006;319:1051–1061. doi: 10.1124/jpet.106.111385. [DOI] [PubMed] [Google Scholar]
- Maione S, De Petrocellis L, de Novellis V, Moriello AS, Petrosino S, Palazzo E, Rossi FS, Woodward DF, Di Marzo V. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br. J. Pharmacol. 2007;150:766–781. doi: 10.1038/sj.bjp.0707145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Morera E, Marabese I, Ligresti A, Luongo L, Ortar G, Di Marzo V. Antinociceptive effects of tetrazole inhibitors of endocannabinoid inactivation: cannabinoid and non-cannabinoid receptor-mediated mechanisms. Br. J. Pharmacol. 155(5):775–782. doi: 10.1038/bjp.2008.308. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng HF, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001a;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ibrahim MM, Deng H, Makriyannis A, Vanderah TW. Anti-inflammatory effects of the CB2 cannabinoid receptor-selective agonist AM1241. Anesthesiology. 2001b;95:A894. [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. In: The Pharmacohistory of Cannabis Sativa in Cannabinoids as Theapeutic Agents. Mechoulam R, editor. CRC; Boca Raton: 1986. pp. 1–19. [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Δ9-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Nomikos GG, Dickason-Chesterfield AK, Schober DA, Schaus JM, Ying BP, Xu YC, Phebus L, Simmons RM, Li D, Iyengar S, Felder CC. Identification of a high-affinity binding site involved in the transport of endocannabinoids. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17852–17857. doi: 10.1073/pnas.0507470102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005;48:6523–6543. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Adams M, Whiteaker K, Daza A, Kage K, Cassar S, Meyer M, Yao BB. Species comparison and pharmacological characterization of rat and human CB2 cannabinoid receptors. Eur. J. Pharmacol. 2004;505:1–9. doi: 10.1016/j.ejphar.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Suplita RL, Hohmann AG. A peripheral cannabinoid mechanism suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003a;117:659–670. doi: 10.1016/s0306-4522(02)00870-9. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003b;119(3):747–757. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J. Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Lichtman AH. Synergistic antinociceptive effects of URB597 and diclofenac in a mouse visceral pain model; 17th Annual Symposium on the Cannabinoids; Burlington, Vermont, International Cannabinoid Research Society. 2007; #172. Available online at http://cannabinoidsociety.org/SYMPOSIUM.2007/2007.ICRS.Program.and.Abstracts.pdf. [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Perchuk A, Meozzi PA, Myers L, Mora Z, Tagliaferro P, Gardner E, Brusco A, Akinshola BE, Liu QR, Hope B, Iwasaki S, Arinami T, Teasenfitz L, Uhl GR. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N.Y. Acad. Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- Patel HJ, Birrell MA, Crispino N, Hele DJ, Venkatesan P, Barnes PJ, Yacoub MH, Belvisi MG. Inhibition of guinea-pig and human sensory nerve activity and the cough reflex in guinea-pigs by cannabinoid (CB2) receptor activation. Br. J. Pharmacol. 2003;140:261–268. doi: 10.1038/sj.bjp.0705435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J, Ribera MV. Managing neuropathic pain with SativexR: a review of its pros and cons. Expert Opin. Pharmacother. 2008;9:1189–1195. doi: 10.1517/14656566.9.7.1189. [DOI] [PubMed] [Google Scholar]
- Price TJ, Helesic G, Parghi D, Hargreaves KM, Flores CM. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neuroscience. 2003;120(1):155–162. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A, Malan TP. Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- Reche I, Fuentes JA, Ruiz-Gayo M. Potentiation of Δ9-tetrahydrocannabinol-induced analgesia by morphine in mice: involvement of μ- and κ-opioid receptors. Eur. J. Pharmacol. 1996;318:11–16. doi: 10.1016/s0014-2999(96)00752-2. [DOI] [PubMed] [Google Scholar]
- Ross RA, Coutts AA, McFarlane CM, Anavi-Goffer S, Irving AJ, Pertwee RG, MacEwan DJ, Scott RH. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001;40(2):221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Russo R, LoVerme J, La Rana G, Compton TR, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, Piomelli D. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3′-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. J. Pharmacol. Exp. Ther. 2007a;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- Russo R, LoVerme J, La Rana G,D, Agostino G, Sasso O, Calignano A, Piomelli D. Synergistic antinociception by the cannabinoid receptor agonist anandamide and the PPAR-α receptor agonist GP7647. Eur. J. Pharmacol. 2007b;566:117–119. doi: 10.1016/j.ejphar.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar DR, Kelly S, Millns PJ, O’Shaughnessey CT, Kendall DA, Chapman V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur. J. Neurosci. 2005;22:371–379. doi: 10.1111/j.1460-9568.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- Sagar DR, Kendall DA, Chapman V. Inhibition of fatty acid amide hydrolase produces PPAR-α-mediated analgesia in a rat model of inflammatory pain. Br. J. Pharmacol. 2008;155(8):1297–1306. doi: 10.1038/bjp.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Wright CE, Angus JA. Evidence that CB-1 and CB-2 cannabinoid receptors mediate antinociception in neuropathic pain in the rat. Pain. 2004;109:124–131. doi: 10.1016/j.pain.2004.01.020. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- Smith FL, Fujimori K, Lowe J, Welch SP. Characterization of Δ9-tetrahydrocannabinol and anandamide antinociception in nonarthritic and arthritic rats. Pharmacol. Biochem. Behav. 1998;60:183–191. doi: 10.1016/s0091-3057(97)00583-2. [DOI] [PubMed] [Google Scholar]
- Sokal DM, Elmes SJR, Kendall DA, Chapman V. Intraplantar injection of anandamide inhibits mechanically-evoked responses of spinal neurons via activation of CB2 receptors in anaesthetised rats. Neuropharmacology. 2003;45:404–411. doi: 10.1016/s0028-3908(03)00195-3. [DOI] [PubMed] [Google Scholar]
- Staton PC, Hatcher JP, Walker DJ, Morrison AD, Shapland EM, Hughes JP, Chong E, Mander PK, Green PJ, Billinton A, Fulleylove M, Lancaster HC, Smith JC, Bailey LT, Wise A, Brown AJ, Richardson JC, Chessell IP. The putative cannabinoid receptor GPR55 plays a role in mechanical hyperalgesia associated with inflammatory and neuropathic pain. Pain. 2008;139:225–236. doi: 10.1016/j.pain.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Suplita RL, Farthing JN, 2nd, Gutierrez T, Hohmann AG. Inhibition of fatty-acid amide hydrolase enhances cannabinoid stress-induced analgesia: sites of action in the dorsolateral periaqueductal gray and rostral ventromedial medulla. Neuropharmacology. 2005;49(8):1201–1209. doi: 10.1016/j.neuropharm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with μ opioid receptor and α2-adrenoceptor agonists in acute pain models in mice. Br. J. Pharmacol. 2005;144:875–884. doi: 10.1038/sj.bjp.0706045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokanovic S, Malone DT, Ventura S. Stimulation of epithelial CB1 receptors inhibits contractions of the rat prostate gland. Br. J. Pharmacol. 2007;150:227–234. doi: 10.1038/sj.bjp.0706952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulugöl A, Ozyigit F, Yesilyurt O, Dogrul A. The additive antinociceptive interaction between WIN 55,212-2, a cannabinoid agonist, and ketorolac. Anesth. Analg. 2006;102:443–447. doi: 10.1213/01.ane.0000194587.94260.1d. [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, Mark L, Pearson MS, Miller W, Shan S, Rabadi L, Rotshteyn Y, Chaffer SM, Turchin PI, Elsemore DA, Toth M, Koetzner L, Whiteside GT. Pharmacological and pharmakokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Vinod KY, Sanguino E, Yalamanchili R, Manzanares J, Hungund BL. Manipulation of fatty acid amide hydrolase functional activity alters sensitivity and dependence to ethanol. J. Neurochem. 2008;104:233–243. doi: 10.1111/j.1471-4159.2007.04956.x. [DOI] [PubMed] [Google Scholar]
- Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb. Exp. Pharmacol. 2005;(168):509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- Walter L, Stella N. Cannabinoids and neuroinflammation. Br. J. Pharmacol. 2004;141:775–785. doi: 10.1038/sj.bjp.0705667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sañudo-Peña MC. Pain modulation by release of the endogenous cannabinoid anandamide. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12198–12203. doi: 10.1073/pnas.96.21.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside GT, Gottshall SL, Boulet JM, Chaffer SM, Harrison JE, Pearson MS, Turchin PI, Mark L, Garrison AE, Valenzano KJ. A role for cannabinoid receptors, but not endogenous opioids, in the antinociceptive activity of the CB2-selective agonist, GW405833. Eur. J. Pharmacol. 2005;528:65–72. doi: 10.1016/j.ejphar.2005.10.043. [DOI] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Yao BB, Mukherjee S, Fan Y, Garrison TR, Daza AV, Grayson GK, Hooker BA, Dart MJ, Sullivan JP, Meyer MD. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? Br. J. Pharmacol. 2006;149:145–154. doi: 10.1038/sj.bjp.0706838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, Grayson GK, Zhu CZ, Pai M, Chandran P, Salyers AK, Wensink EJ, Honore P, Sullivan JP, Dart MJ, Meyer MD. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br. J. Pharmacol. 2008;153:390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilyurt O, Dogrul A, Gul H, Seyrek M, Kusmez O, Ozkan Y, Yildiz O. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303–308. doi: 10.1016/s0304-3959(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati R, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon MH, Choi JI. Pharmacologic interaction between cannabinoid and either clonidine or neostigmine in the rat formalin test. Anesthesiology. 2003;99:701–707. doi: 10.1097/00000542-200309000-00027. [DOI] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- Zias J, Stark H, Sellgman J, Levy R, Werker E, Breuer A, Mechoulam R. Early medical use of cannabis. Nature. 1993;363:215. doi: 10.1038/363215a0. [DOI] [PubMed] [Google Scholar]