Abstract
Aging is associated with a loss in muscle known as sarcopenia that is partially attributed to apoptosis. In aging rodents, caloric restriction (CR) increases health and longevity by improving mitochondrial function and the polyphenol resveratrol (RSV) has been reported to have similar benefits. In the present study, we investigated the potential efficacy of using short-term (6 weeks) CR (20%), RSV (50 mg/kg/day), or combined CR + RSV (20% CR and 50 mg/kg/day RSV), initiated at late-life (27 months) to protect muscle against sarcopenia by altering mitochondrial function, biogenesis, content, and apoptotic signaling in both glycolytic white and oxidative red gastrocnemius muscle (WG and RG, respectively) of male Fischer 344 × Brown Norway rats. CR but not RSV attenuated the age-associated loss of muscle mass in both mixed gastrocnemius and soleus muscle, while combined treatment (CR + RSV) paradigms showed a protective effect in the soleus and plantaris muscle (P < 0.05). Sirt1 protein content was increased by 2.6-fold (P < 0.05) in WG but not RG muscle with RSV treatment, while CR or CR + RSV had no effect. PGC-1α levels were higher (2-fold) in the WG from CR-treated animals (P < 0.05) when compared to ad-libitum (AL) animals but no differences were observed in the RG with any treatment. Levels of the anti-apoptotic protein Bcl-2 were significantly higher (1.6-fold) in the WG muscle of RSV and CR + RSV groups compared to AL (P < 0.05) but tended to occur coincident with elevations in the pro-apoptotic protein Bax so that the apoptotic susceptibility as indicated by the Bax to Bcl-2 ratio was unchanged. There were no alterations in DNA fragmentation with any treatment in muscle from older animals. Additionally, mitochondrial respiration measured in permeabilized muscle fibers was unchanged in any treatment group and this paralleled the lack of change in cytochrome c oxidase (COX) activity. These data suggest that short-term moderate CR, RSV, or CR + RSV tended to modestly alter key mitochondrial regulatory and apoptotic signaling pathways in glycolytic muscle and this might contribute to the moderate protective effects against aging-induced muscle loss observed in this study.
Keywords: Aging, Caloric restriction, Sarcopenia, Apoptosis, Biogenesis, Sirtuins
1. Introduction
Aging is a complex physiological process that is due, in part, to the accumulation of damage at the molecular, cellular, and organ levels that eventually manifests as impairments in whole body function. One of the hallmark features of aging in mammals is the progressive loss of muscle mass and strength known as sarcopenia (Rosenberg, 1997). This condition is characterized by a progressive loss of skeletal muscle and shown to be partially attributed to changes in mitochondrial ultrastructure and decrements in organelle function, biogenesis, and content (Calvani et al., 2013). While the specific molecular underpinnings of these changes continue to be enigmatic, much of the research has focused on the role of life-long exposure to oxidative stress and the steady accretion of intracellular damage due to the reactive oxygen species (ROS) produced largely by mitochondria (Siu et al., 2008). Chronic lifetime exposure of muscle to elevated levels of ROS has been postulated to result in a steady accumulation of damage to macromolecules such as DNA, proteins and lipids (Harman, 2003). This can subsequently lead to reduced bioenergetics and the activation of key mitochondrially-mediated apoptosis signaling pathways resulting in myonuclear loss. Since skeletal muscle is uniquely multi-nucleated, a reduced myonuclear number resulting from apoptosis reduces the myonuclear domain (nuclear to cytoplasmic ratio) and ultimately contributes to the sarcopenic phenotype observed in aging (Chabi et al., 2008; Dirks and Leeuwenburgh, 2002; Whitman et al., 2005). Interestingly, age-related muscle abnormalities and susceptibility to fiber loss appear to occur in a fiber-type specific manner with fast-twitch glycolytic fibers being more affected than slow-twitch oxidative fibers (Aspnes et al., 1997; Bua et al., 2002; McKenzie et al., 2002; Phillips and Leeuwenburgh, 2005; Pistilli et al., 2006; Rice and Blough, 2006).
Mitochondrial content in skeletal muscle is dynamic and unique since it can be altered in response to a wide variety of physiological stimuli which includes but is not limited to exercise, chronic contractile activity, chronic muscle disuse, and caloric restriction (Hood, 2001). To date, life-long caloric restriction (consumption of 20–40% fewer calories) remains the only recognized intervention capable of delaying age-related diseases and improving health and longevity in numerous species ranging from yeast to rodents (reviewed in Speakman and Mitchell, 2011). In muscle, caloric restriction delays the age-associated loss of muscle fibers, in part, by improving mitochondrial function, preventing the induction of apoptotic signaling pathways, and reducing the release of pro-apoptotic factors from the mitochondria that lead to myonuclear DNA fragmentation (Aspnes et al., 1997; Dirks and Leeuwenburgh, 2004; Lee et al., 1998). At a molecular level, caloric restriction exerts its beneficial effects by evoking a cellular state of energy deprivation which activates key signaling molecules such as the NAD+-dependent deacetylase sirtuin 1 (Sirt1), one of the seven mammalian sirtuin homologs of the yeast Sir2 gene (Cohen et al., 2004; Frye, 1999). In fact, proof-of-concept genetic experiments have shown that Sirt1-overexpressing mice display similar beneficial phenotypes as caloric restricted mice (Bordone et al., 2007), while knockout animals have a shorter lifespan compared to their wild-type counterparts (Guarente and Picard, 2005; Koubova and Guarente, 2003; McBurney et al., 2003). At a biochemical level, Sirt1 functions as a deacetylase and one of its prominent targets is the mitochondrial regulator peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) (Rodgers et al., 2005). Upregulation of PGC-1α in muscle activates a number of genes involved in substrate metabolism leading to elevated mitochondrial biogenesis, improved mitochondrial function, as well as a fiber type transition towards muscle with a more oxidative metabolic profile (Lin et al., 2002; Wu et al., 1999). Moreover, increased PGC-1α levels attenuate the muscle mass loss observed in aging animals (Wenz et al., 2009). The effects of PGC-1α on muscle mitochondrial biogenesis are also largely mediated by 5′ AMP-activated protein kinase (AMPK), a key metabolic sensor that regulates PGC-1α by increasing its expression levels, as well as directly phosphorylating the protein (Irrcher et al., 2008; Jager et al., 2007; Suwa et al., 2003). While a number of observations have linked these mitochondrial metabolism and biogenesis regulatory proteins to the caloric restriction-mediated protection observed in aging muscle, the molecular details of their involvement remain elusive.
Recently, resveratrol (3, 5, 4′-trihydroxystilbene), a natural polyphenol found in grape skins and red wine has gained much attention for its ability to induce Sirt1 activity and has been purported to exert anti-aging effects on various organisms (Howitz et al., 2003). Resveratrol is marketed as, and termed a “caloric restriction mimetic” since it can extend lifespan in lower organisms including yeast, drosophila, and small vertebrates and seems to operate via the same molecular machinery as caloric restriction (Howitz et al., 2003; Valenzano et al., 2006; Wood et al., 2004). The effects of resveratrol appear to be mediated through an AMPK-Sirt1-PGC-1α pathway but the mechanisms of this regulation are currently not well understood (Baur et al., 2006; Canto et al., 2009; Dasgupta and Milbrandt, 2007; Lagouge et al., 2006; Price et al., 2012; Um et al., 2010). Nonetheless, the benefits of resveratrol are not limited only to life extension properties since it has also been shown to improve the health decrements associated with diet-induced obesity. Specifically, in mice fed a high fat diet, resveratrol protected against aging diet-induced obesity, increased overall mitochondrial content, improved muscle strength and enhanced aerobic capacity, while importantly also extending lifespan in these rodents (Baur et al., 2006; Lagouge et al., 2006). Favorable metabolic improvements following resveratrol treatment have also been reported in humans (Brasnyo et al., 2011; Crandall et al., 2012; Timmers et al., 2011). Thus, resveratrol treatment appears to improve general health measures in both humans and animals and this seems to be partially mediated via mitochondrial adaptations. Interestingly, the health benefits derived from resveratrol treatment can be realized with short-term supplementation as opposed to the life-long commitment of caloric restriction which has significant clinical implications from an intervention standpoint. It is currently unclear whether short-term and/or late-life implementation of resveratrol supplementation can provide similar mitochondrial adaptations to improve overall health and suppress muscle wasting conditions such as sarcopenia. Some studies report improved mitochondrial protein expression profile, skeletal muscle function, and an attenuation of muscle mass loss during muscle disuse, cachexia, and aging (Jackson et al., 2010; Wyke et al., 2004), while other studies fail to show similar benefits. However, these contrasting results seem to be mainly attributed to differences in treatment dose and time (Jackson et al., 2011). Despite these inconsistent findings, the general consensus in the field is that due to its ability to modulate mitochondrial gene expression and its potential antioxidant properties, resveratrol may attenuate the oxidative stress burden imposed by aging to potentially protect and/or suppress the onset of sarcopenia (Jackson et al., 2010, 2011; Murase et al., 2009; Ryan et al., 2010). To date however, it is unclear whether the benefits of caloric restriction and resveratrol treatment occur via similar pathways, or whether there may be some independence between the intracellular pathways that activate mitochondrial biogenesis, improve mitochondrial function and/or suppress mitochondrially-mediated apoptotic signaling pathways. Furthermore, given the differential sarcopenic effect of aging on muscle fiber-types (preferentially affecting fast/glycolytic versus slow/oxidative), it is currently unknown whether these treatment paradigms induce fiber-type specific responses.
The purpose of the current study was to evaluate the efficacy of moderate short-term caloric restriction, resveratrol, or combined caloric restriction/resveratrol treatments to modulate mitochondrial function, biogenesis, and apoptotic susceptibility proteins to potentially delay sarcopenia in late-life aged animals. This is the first study to assess whether these short-term treatment paradigms implemented in late-life could provide beneficial mitochondrial and muscle adaptations. We hypothesized that 6 weeks of caloric restriction (20%) or resveratrol (50 mg/kg/day) supplementation would independently improve mitochondrial function and content, and reduce apoptotic susceptibility, and that combined treatment would exert an additive or potentially a synergistic protective effect in aging muscle. Additionally, we hypothesized that the regulation of these pathways would differ between the fast-twitch and slow-twitch gastrocnemius muscle.
2. Materials and methods
2.1. Animals
Muscle tissue from a total of thirty-one 27-month-old male Fischer 344 × Brown Norway Hybrid rats purchased from the National Institute on Aging was used in this study. These animals were part of a larger study (n = 64). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida (Study#:200902992) and performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals. The animals were acclimatized for 4 weeks before the start of intervention and housed in separate cages in a temperature (18–22 °C) and light-controlled environment with a 12-hour light/dark cycle. Animals were randomly assigned to one of four possible treatment groups: ad-libitum(AL), caloric restricted (CR) (20% reduced AL; 6 weeks), resveratrol (RSV) (50 mg/kg/day; 6 weeks), CR + RSV (20% CR and 50 mg/kg/day RSV for 6 weeks). All animals received a daily diet of food pellets (Custom Animal Diets, Bangor, PA). The average food intake of the AL group was determined and used to calculate the 20% reduction in caloric intake for the CR group. Animals in the RSV treated groups received a daily dose of one bacon flavored RSV fortified tablet (50 mg/kg/body weight), mixed in with their normal food pellets (Sigma Chemical Co., St. Louis, MO). The bioavailability of resveratrol and its conversion into dihydroresveratrol was confirmed by measuring resveratrol levels in plasma. At the end of the 6 weeks of treatment, animals were sacrificed, tibialis anterior, and red and white portions of the gastrocnemius muscle were separated, weighed, frozen in liquid nitrogen, and stored at −80 °C until further analysis.
2.2. Preparation of permeabilized muscle fibers
Portions of the tibialis anterior muscle were excised and immediately processed for permeabilized fiber respiration measurements as previously described (Joseph et al., 2012). Briefly, muscle from the tibialis anterior (~20–25 mg)was placed in ice-cold Buffer X containing 60 mM K-MES, 35 mM KCl, 7.23 mM K2EGTA, 2.77 CaK2EGTA, 20 mM imidazole, 0.5 mM DTT, 20 mM taurine, 5.7 mM ATP, 15 mM PCr, and 6.56 mM MgCl2·6 H2O (pH 7.1, 295 mosmol/kgH2O) and connective tissue removed. Muscle sections were cut into small bundles (~8–10 mg) and gently separated into single fibers. Myofibers were permeabilized in Buffer X with saponin (50 µg/ml) and incubated on a rotator for 30 min at 4 °C. Permeabilized fibers were washed in ice-cold Buffer Z containing 110 mM K-MES, 35 mM KCl, 1 mM EGTA, 5 mM K2HPO4, and 3 mM MgCl2·6H2O, 0.05 mM pyruvate, and 0.02 mM malate with 0.5 mg/ml BSA (pH 7.1, 295 mosmol/kgH2O) on a rotator at 4 °C for 45 min. Fiber bundles were subsequently washed in Buffer Z containing 5 mM pyrophosphate to deplete fibers of endogenous nucleotides.
2.3. Mitochondrial respiration in permeabilized muscle fibers
Respiration was measured using an Oroboros O2K Oxygraph (Inssbruck, Austria) according to methods previously described (Kuznetsov et al., 1998). The O2 flux rate was measured following the addition of a substrate and inhibitor protocol which consisted of 5 mM pyruvate and 2 mM malate, 2 mM ADP, 10 µM cytochrome c, 10 mM succinate, 2 µg/ml oligomycin and 0.5 µM carbonylcyanide p-trifluoromethoxyphenylhydrazone. Respiratory control ratios (state 3/state 4) were used as an indicator of well-coupled mitochondria in all groups measured (mean RCR = 5.07 ± 0.4).
2.4. Cytochrome c oxidase (COX) enzyme activity
Powdered tissues from white gastrocnemius, red gastrocnemius, and the tibialis anterior were diluted in a buffer (0.1 M KH2PO4 + 2 mM EDTA, pH 7.2) and sonicated (3 × 5 s) on ice. Following a brief spin, the supernatant was removed and enzyme activity determined by the maximal oxidation rate of completely reduced cytochrome c, evaluated as a change in absorbance at 550 nm using a multi-detection microplate reader (Synergy HT, Biotek Instruments, Winooski, VT) (Adhihetty et al., 2009).
2.5. Western blotting
Frozen white and red gastrocnemius muscles were pulverized and protein extracts made as previously described (Adhihetty et al., 2009). Muscle extracts (50 µg/lane) were separated by 4–15% SDS-PAGE and subsequently electroblotted to nitrocellulose membranes. After transfer, membranes were blocked by incubating at room temp in 5% skim milk in 1× TBST [Tris-buffered saline-Tween 20:25 mM Tris HCl (pH 7.5), 1 mM NaCl, and 0.1% Tween 20] solution. Blots were then incubated overnight at 4 °C in blocking buffer at a dilution of 1:3500 for AIF (Santa Cruz, sc-9416), 1:1000 for Bax and Bcl-2 (Santa Cruz, sc-493 and sc-7382), 1:1500 for cytochrome c (Santa Cruz sc-8385), 1:1500 for MnSOD (Santa Cruz, sc-30080), PGC-1α (Calbiochem, 516557), phospho-AMPKα and total AMPKα (Cell Signaling 2531 and 2532, respectively) and Sirt1 (Cell Signaling, 8469). After overnight incubation, blots were washed in TBST (3 × 5 min), incubated at room temperature for 45 min with appropriate secondary antibody, and washed again in TBST (3 × 5 min). Antibody binding was detected with the use of secondary antibodies conjugated to horseradish peroxidase and an enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology, Santa Cruz, CA). Films were scanned and images analyzed using the Kodak ID Imaging Software. Target bands were normalized to the amount of protein loaded in each lane, as determined by densitometric analysis of the corresponding Ponceau S-stained (Marzetti et al., 2009a).
2.6. Cell death ELISA assay
DNA fragmentation in both white and red gastrocnemius muscle samples was measured using the Cell Death ELISA DetectionPLUS kit (Roche Diagnostics, Indianapolis, IN). This assay has commonly been used to accurately assess apoptosis in skeletal muscle of both young and old animals (Pistilli and Alway, 2008). Briefly, tissue was homogenized in lysis buffer followed by centrifugation at 200 g for 10 min to obtain the cytosolic fraction. Subsequently, 20 µg of supernatant was added into the wells of a streptavidin-coated plate and incubated with 80 µl anti-histone-biotin and anti-DNAPOD for 2 h on a shaker at room temperature. The wells were then washed and incubated with the substrate, 2,2′-azino-di (3-ethylbenzthiazoline sulfate) (ABTS) on a shaker for 10–20 min. Absorbance was measured at 405 nm with a multi-detection microplate reader (Synergy HT, Biotek Instruments, Winooski, VT). Positive and negative controls were run with each assay and values adjusted for background. The resulting OD was recorded as the apoptotic index (OD405/mg protein).
2.7. Statistical analysis
One-way analysis of variance (ANOVA) was performed to determine whether there was a main effect of treatment followed by a Tukey's post-hoc test to identify differences within groups. Statistical differences were considered significant if P < 0.05. Data are expressed as means ± SE.
3. Results
3.1. Animal characteristics
Animals used in this study were a subset from a larger study and therefore the descriptive animal characteristics in Table 1 represent a summary of the total animals in the study (n = 64). Animal weights were taken prior to and following 6 weeks of the dietary intervention. Animals were given a daily resveratrol dose of 50 mg/kg/day of body weight. This dose has been previously shown to be within the range that induces changes in mitochondrial protein expression pathways with long-term treatment but well below the threshold that can potentially lead to apoptosis (Jackson et al., 2011). Body weights indicated in Table 1 were taken prior to and following 6 weeks of intervention, immediately before the animals were sacrificed. As expected, animal weights at baseline were not different between groups, but caloric restriction (CR), and caloric restriction with resveratrol (CR + RSV) groups, had a significantly lower body mass compared to ad-libitum (AL)-fed animals (P < 0.05) at the end of the 6 weeks of treatment. Also, following the intervention, animals in the CR and CR + RSV groups had similar percent losses in body weight, while reduced compared to AL and RSV (P < 0.05), respectively. There was no protection observed by any treatment on gross muscle weights although a reduction in the gastrocnemius muscle was detected in the CR + RSV group compared to AL and CR (P < 0.05). However, since body masses differ significantly between groups, gross muscle weights were corrected for body weight to obtain a more accurate depiction of changes. After normalizing muscle weights by mean body weight, we found that both the gastrocnemius and soleus muscle were higher (P < 0.05) with CR compared to AL (Table 1). Additionally, CR + RSV treatment resulted in a protective effect in the soleus, as well as the plantaris muscle (P < 0.05).
Table 1.
Descriptive characteristics of animals.
| Characteristic | AL | CR | RSV | CR + RSV |
|---|---|---|---|---|
| Number of animals | 23 | 9 | 8 | 24 |
| BW (pre) (g) | 567 ± 13.2 | 564.6 ± 16.3 | 559 ± 13.0 | 567.3 ± 17 |
| BW (post) (g) | 581 ± 8.0 | 529 ± 15.1* | 565 ± 16.6 | 521 ± 7.1*# |
| % BW (g) | 1.2 ± 0.47 | −6% ± 0.73*# | 1.0 ± 1.18 | −8% ± 1.56*# |
| Gastrocnemius muscle wet weight (g) | 1.89 ± 0.03 | 1.95 ± 0.05 | 1.88 ± 0.06 | 1.77 ± 0.03*† |
| Gastrocnemius muscle wet weight/BW | 3.30 ± 0.04 | 3.7 ± 0.16*#¶ | 3.33 ± 0.09 | 3.40 ± 0.05 |
| Soleus muscle wet weight (g) | 0.17 ± 0.004 | 0.18 ± 0.003 | 0.17 ± 0.004 | 0.16 ± 0.003 |
| Soleus muscle wet weight/BW | 0.29 ± 0.007 | 0.32 ± 0.007* | 0.30 ± 0.008 | 0.31 ± 0.004* |
| Plantaris muscle wet weight (g) | 0.47 ± 0.02 | 0.43 ± 0.02 | 0.42 ± 0.02 | 0.47 ± 0.04 |
| Plantaris muscle wet weight/BW | 0.80 ± 0.02 | 0.82 ± 0.03 | 0.74 ± 0.04 | 0.90 ± 0.02*# |
| Food intake (g/day) | 2.54 ± 0.03 | 2.07 ± 0.03* | 2.60 ± 0.09† | 1.97 ± 0.01*# |
Animals were weighed prior to (pre) and following the 6-week intervention (post) and body weights (BW) represented in grams (g) and as percent changes (%Δ). Muscle weights were taken immediately following excision and data represented as a ratio to body weight (mg/g). Animals were randomly assigned to 4 groups which included ad-libitum (AL, regular chow), Caloric Restricted (CR; 20%), Resveratrol (RSV; 50 mg/kg/day), or combined Caloric Restricted and Resveratrol (CR + RSV; 20% CR and 50 mg/kg/day RSV).
Significantly different (P < 0.05) than AL animals.
Significantly different (P < 0.05) than CR.
Significantly different (P < 0.05) than RSV.
Significantly different (P < 0.05) than CR + RSV. All data are represented as mean ± SE.
3.2. Mitochondrial function and enzyme activity
Mitochondrial function was measured by directly assessing oxygen consumption in permeabilized muscle fibers from the tibialis anterior muscle (Table 2). Respiratory control ratios for all groups were between the range of 3.5–5.1 indicating well coupled mitochondria. Mitochondrial respiration rates were unchanged by any of the interventions, even when corrected for COX activity (common marker of mitochondrial content) in the same tissue (data not shown) (Adhihetty et al., 2007). This was also substantiated, and consistent with COX activity measured in white-gastrocnemius (WG) and red gastrocnemius (RG) muscles (Table 2).
Table 2.
Mitochondrial respiration and enzyme activity.
| Measurement | AL | CR | RSV | CR + RSV |
|---|---|---|---|---|
| Oxygen consumption (pmol/s/mg wet weight) | ||||
| State 4 | 16.81 ± 0.29 | 15.78 ± 1.44 | 15.65 ± 1.25 | 15.79 ± 1.35 |
| State 3 | 59.80 ± 5.32 | 64.33 ± 9.15 | 56.43 ± 8.09 | 68.57 ± 11.5 |
| RCR | 3.54 ± 0.25 | 4.65 ± 0.94 | 4.00 ± 0.96 | 5.07 ± 0.4 |
| Cytochrome c oxidase activity (U/g) | ||||
| White | 8.76 ± 0.74 | 9.08 ± 1.47 | 9.67 ± 1.27 | 8.60 ± 0.31 |
| Gastrocnemius | ||||
| Red | 24.0 ± 1.34 | 21.2 ± 1.84 | 25.0 ± 2.97 | 20.5 ± 1.84 |
| Gastrocnemius | ||||
Oxygen consumption was performed in permeabilized muscle fibers from the tibialias anterior. Rate of mitochondrial oxygen consumption in the presence (state 3) or absence (state 4) of ADP. Respiratory control ratio (RCR). Values are means ± SEM of 4–8 mice. There were no statistical differences between groups (P < 0.05).
3.3. AMPK activation and sirtuin 1 (Sirt1) protein content
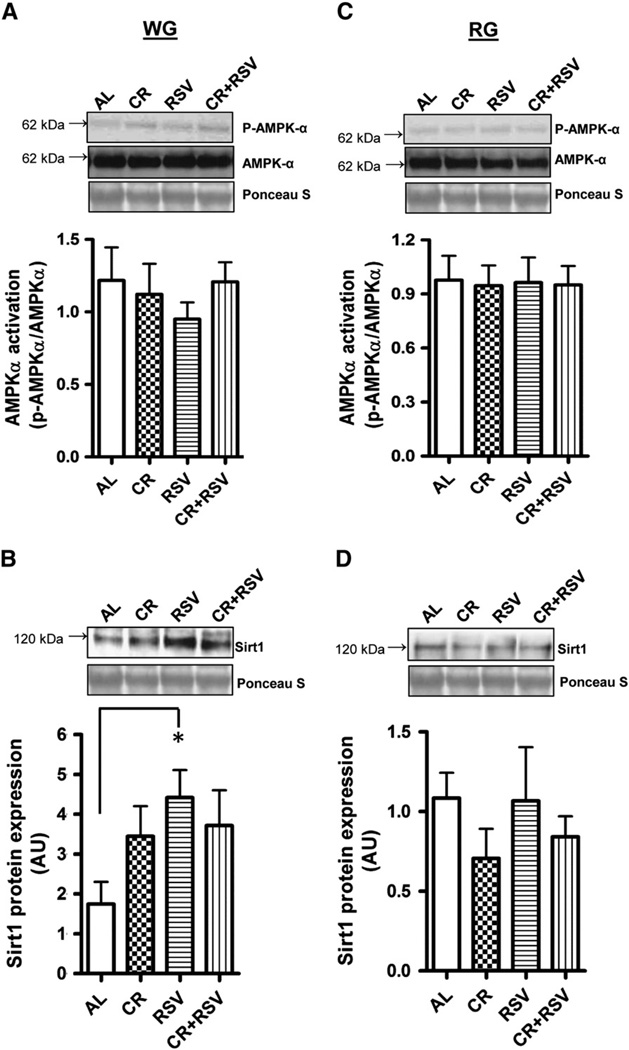
Activation of the upstream metabolic sensor AMPK was not different in any of the groups in the WG (Fig. 1A) or RG (Fig. 1C). However, Sirt1 protein was 2.6-fold higher (P < 0.05) in RSV-treated WG muscle when compared to muscle from AL-fed animals (Fig. 1B). There were no detectable differences in Sirt1 protein content in RG muscle (Fig. 1D). Additionally, activation of the mammalian target of rapamycin (mTOR) kinase, a downstream target of AMPK was not different between any groups (data not shown).
Fig. 1.
AMPK activation and Sirtuin 1 (Sirt1) protein content. AMPK activation was measured in white gastrocnemius (WG; A) and red gastrocnemius (RG; C) muscle in 27 month old AL = ad-libitum fed (n = 11), CR = caloric restricted (n = 4), RSV = resveratrol (n = 4), and CR + RSV = caloric restricted + resveratrol (n = 12). Kinase activation status was determined as the ratio of the phosphorylated form (p-AMPKα) of the protein compared to the total protein (AMPKα) content for each sample. Sirt1 protein in WG (B) and RG (D) muscle. Representative protein blots are shown along with the corresponding Ponceau S-stained membrane used to correct for loading. A graphical summary of the data is shown below (n = 4–11). Significance was set at P < 0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). *P < 0.05 vs. AL.
3.4. Mitochondrial biogenesis
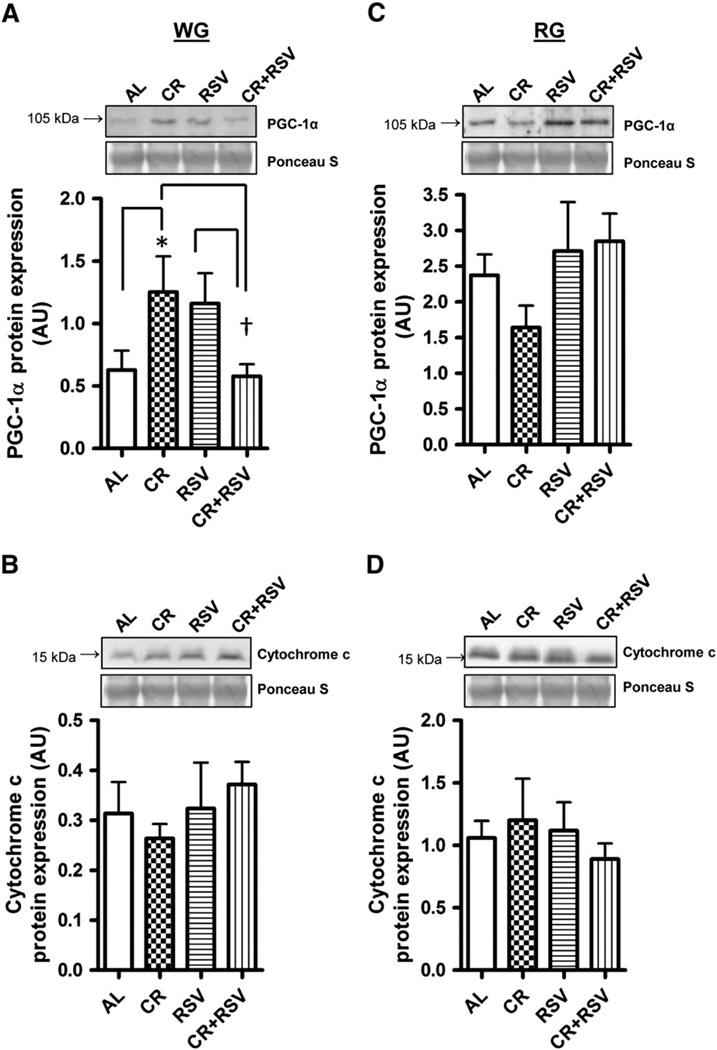
Protein levels of peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) were 2-fold higher in the WG following CR treatment when compared to AL (P < 0.05; Fig. 2A), and reduced in the CR + RSV group when compared to both CR and RSV groups (P < 0.05; Fig. 2A). In contrast, no changes were detected in PGC-1α protein in the RG muscle (Fig. 2C). Cytochrome c protein content was not affected by any treatments in the WG (Fig. 2B) or RG (Fig. 2D) muscles.
Fig. 2.
PGC-1α and cytochrome c protein content. PGC-1α protein was measured in white gastrocnemius (WG; A) and red gastrocnemius (RG; C) muscles and cytochrome c protein in WG (B) and RG (D) in 27 month old AL = ad-libitum, CR = caloric restricted, RSV = resveratrol, and CR + RSV = caloric restricted + resveratrol. Representative Western blots and corresponding Ponceau S-stained membranes, along with a graphical summary of the data is shown (n = 4–11). Significance was set at P < 0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). *P < 0.05 vs. AL; †P < 0.05 vs. CR group and RSV group.
3.5. Apoptosis regulatory proteins
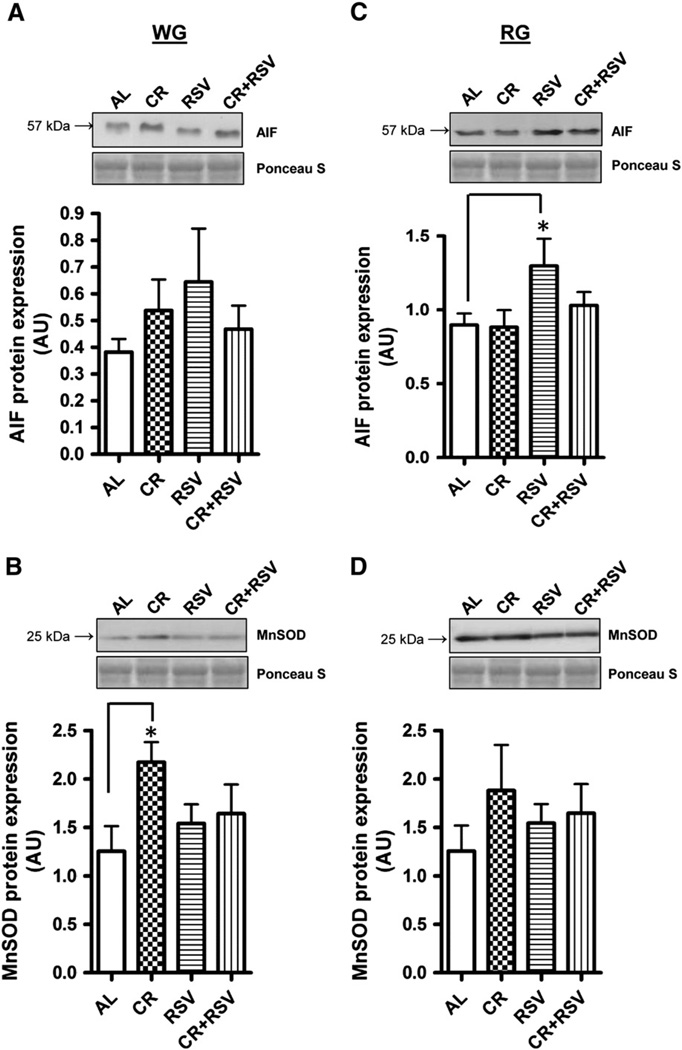
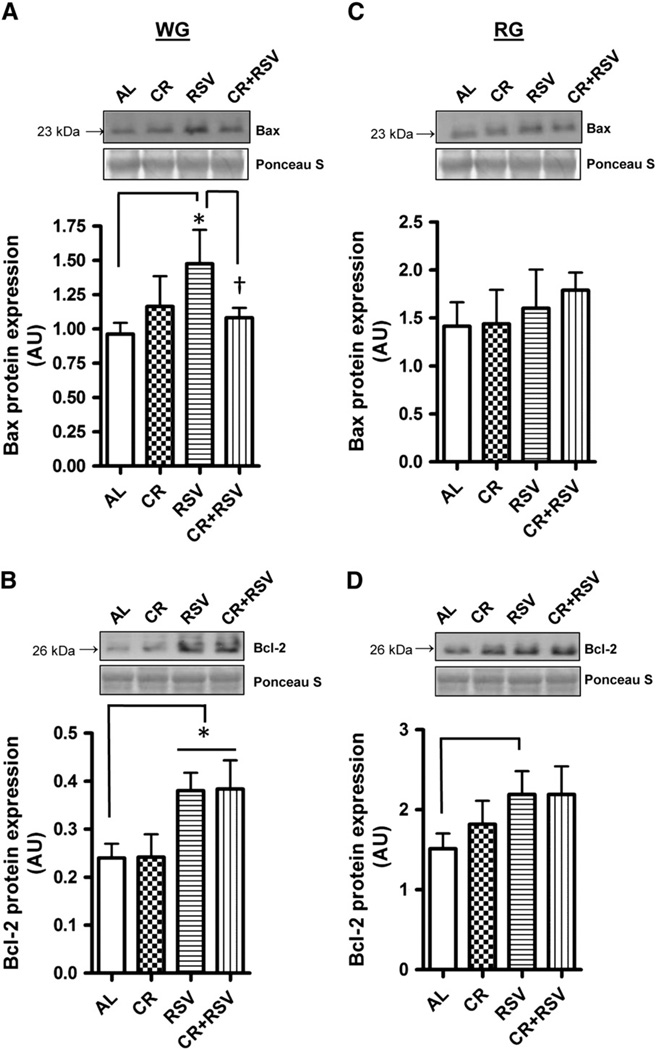
To investigate whether apoptotic potential is modulated in aged muscle following supplementation, several apoptosis regulatory proteins were measured. Apoptosis Inducing Factor (AIF) protein content was markedly elevated in RSV treated RG muscle (1.5-fold) (P < 0.05; Fig. 3C) compared to AL-fed animals but not altered with CR or CR + RSV. No differences were detected in the levels of this protein in WG muscle (Fig. 3A). The antioxidant enzyme manganese superoxide dismutase (MnSOD) was 1.7-fold higher in the WG muscle of CR-treated animals (P < 0.05; Fig. 3B) but was not statistically different in the RG (Fig. 3D). Next we wanted to measure levels of the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 which are crucial factors in the susceptibility to apoptosis. RSV increased the abundance of Bax protein in WG compared to muscle from AL-fed animals (P < 0.05; Fig. 4A), an effect that was attenuated by combined CR + RSV treatment (P < 0.05). Levels of the anti-apoptotic protein Bcl-2 were also elevated in WG muscle of RSV treated animals (P < 0.05), as well as in animals treated with combined CR + RSV (P < 0.05) compared to AL-fed animals (Fig. 4C). In contrast to WG muscle, there were no significant changes in the levels of these apoptosis regulators in RG (Fig. 4B and D). The Bax/Bcl-2 ratio, commonly used as an indicator of apoptotic susceptibility was not different between any of the groups in either muscle.
Fig. 3.
Apoptosis Inducing Factor (AIF) and Manganese Superoxide Dismutase (MnSOD) protein content. AIF protein was measured via immunoblotting in white gastrocnemius (WG; A) and red gastrocnemius (RG; C) muscles and MnSOD protein in WG (B) and RG (D). AL = ad libitum, CR = caloric restricted, RSV = resveratrol, and CR + RSV = caloric restricted + resveratrol. Representative blots are shown above with the corresponding Ponceau S-stained membrane below. A summary of repeated experiments is graphically illustrated (n = 4–11). Significance was set at P < 0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). *P < 0.05 vs. AL.
Fig. 4.
Protein levels of pro-apoptotic Bax and anti-apoptotic Bcl-2. Bax protein was measured via immunoblotting in white gastrocnemius (WG; A) and red gastrocnemius (RG; C) muscles and Bcl-2 protein in WG (B) and RG(D). AL = ad-libitum, CR = caloric restricted, RSV = resveratrol, and CR + RSV = caloric restricted + resveratrol. Representative Western blots, and the corresponding Ponceau S-stained membranes are shown above with a graphical summary of the data illustrated below (n = 4–11). Significance was set at P < 0.05 and all data are represented as mean ± SE. Data are expressed as arbitrary units (AU). *P < 0.05 vs. AL; †P < 0.05 vs. RSV group.
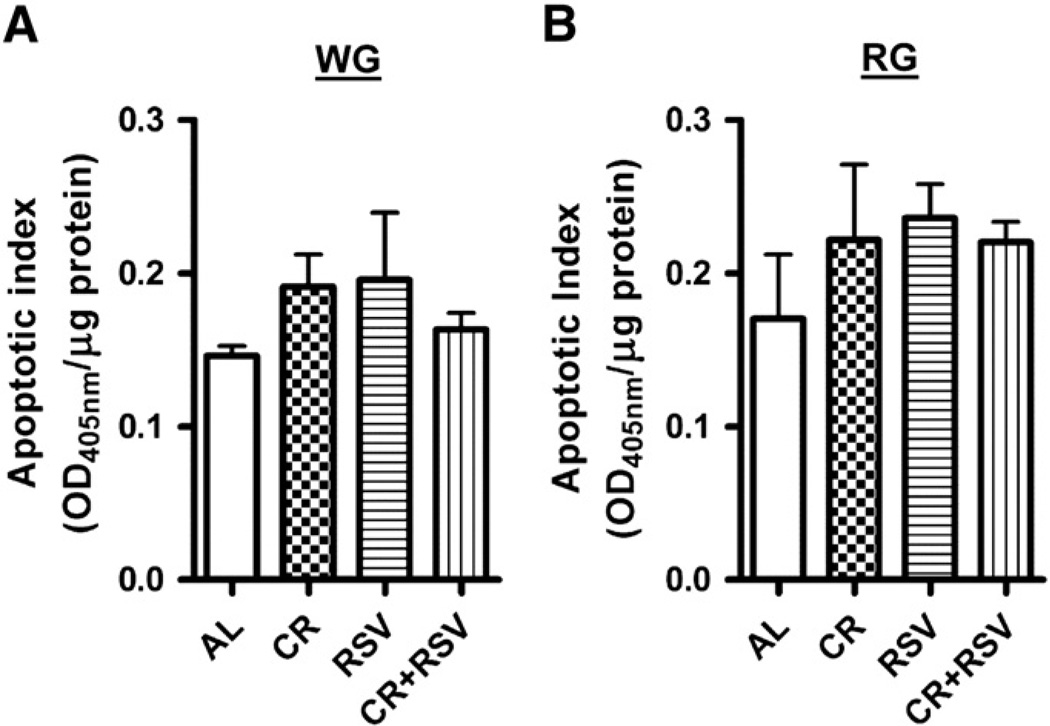
3.6. Efficacy of CR and RSV supplementation on cell death in aging muscle
To determine whether cell death was affected by the treatment regimens, we used a cell death ELISA to measure DNA fragmentation in both WG and RG (indicated as apoptotic index). CR, RSV or combined CR + RSV was not different when compared to AL-treated animals in either the WG (Fig. 5A) or RG muscles (Fig. 5B) supporting the lack of change observed in the Bax/Bcl-2 ratio.
Fig. 5.
Cell death in aged gastrocnemius muscle. DNA fragmentation was measured in cytosol from WG (A) and RG (B) using a Cell death ELISA kit. The data are presented as an apoptotic index. All data are represented as mean ± SE (n = 4–8).
4. Discussion
Oxidative stress beyond a certain threshold is a well-established inducer of cellular dysfunction since this can lead to the dysregulation of a number of key cellular pathways including cell growth, proliferation and survival (Migliore and Coppede, 2002; Ryter et al., 2007). Aging muscle is particularly susceptible to oxidative stress due to age-induced reductions in overall antioxidant and repair pathways which are responsible for managing and/or neutralizing the levels of stress imposed by elevated oxidants (Pansarasa et al., 1999; Proctor et al., 1995). This elevation in oxidative stress in muscle throughout life can cause cumulative detrimental effects which may eventually lead to increased inflammatory responses, reduced mitochondrial bioenergetics, as well as higher apoptotic potential culminating in myonuclear cell death and ultimately muscle loss (Calvani et al., 2013; Harman, 2003). Thus, disturbances in the balance between oxidants and reductants are believed to be a significant contributor towards sarcopenia. The present study is the first to determine whether short-term interventions of moderate caloric restriction, resveratrol, or combined caloric restriction and resveratrol treatment could improve key mitochondrial and apoptotic pathways to suppress the signaling events that typically lead to muscle loss in late-life aging.
Previous studies report that long-term (initiated at late middle age) caloric restriction of greater than 40% preserves fiber number and fiber type composition in rodents (Aspnes et al., 1997; Lee et al., 1998; Phillips and Leeuwenburgh, 2005). However, this strict dietary restriction is not tolerated well by older animals and is certainly not feasible and/or easily translatable to humans (Dirks and Leeuwenburgh, 2006). Thus, we elected to use a much more tolerable 20% dietary restriction to be a more moderate and applicable treatment regimen that could potentially be implemented in humans, in particular if used in conjunction with resveratrol treatment. Although we did not anticipate large changes in muscle mass given the brevity of the treatment time and the moderate dose, we did observe what appeared to be a modest protective effect in both the slow-twitch soleus and mixed gastrocnemius muscle following caloric restriction when muscle mass was normalized for body weight. Additionally, we found that the combination of caloric restriction and resveratrol positively affected muscle mass in the soleus and plantaris muscle but not the mixed gastrocnemius muscle. The lack of change observed in the mixed gastrocnemius muscle is due to the loss of absolute muscle mass with the combination of treatments. At present, we are unsure of whether fiber mass loss is more detectable due to the larger overall size of the muscle compared to smaller muscles such as the soleus and/or plantaris or whether this represents a physiologically relevant finding unique to the mixed gastrocnemius muscle. However, it is important to note that muscle weight measurements can be affected by a number of variables including dissection and fluid retention, and therefore without performing additional analyses of fiber size and number this cannot be confirmed at the present time.
Decrements in mitochondrial function and biogenesis have commonly been associated with aging in skeletal muscle (Joseph et al., 2012; Rooyackers et al., 1996; Short et al., 2005). The ability of caloric restriction and resveratrol treatment to attenuate mitochondrial deficits and improve tissue function in various animal models is thought to occur through an AMPK-Sirt1-PGC-1α-dependent pathway (Baur et al., 2006; Canto et al., 2009; Dasgupta and Milbrandt, 2007; Egan et al., 2011; Lagouge et al., 2006; Price et al., 2012). While the mechanistic details are unclear, it has recently been proposed that activation of AMPK by resveratrol increases intracellular levels of NAD+ and enhances Sirt1 activity, leading to the deacetylation and activation of PGC-1α. Additionally, phosphorylation of PGC-1α by AMPK also promotes the deacetylation action of Sirt1. Thus, PGC-1α can co-activate itself, to enhance its own transcription, and positively regulate the expression of other nuclear genes-encoding mitochondrial proteins to upregulate mitochondrial biogenesis (Baur et al., 2006; Canto et al., 2009; Egan et al., 2011; Lagouge et al., 2006; Nemoto et al., 2005; Price et al., 2012; Rodgers et al., 2005; Suwa et al., 2003). In the present study, total PGC-1α protein was increased with caloric restriction in white, glycolytic gastrocnemius muscle fibers but not in red, oxidative muscle fibers. Additionally, no changes were detected with caloric restriction in AMPK activation or Sirt1 protein. While there is considerable evidence pointing to a key role of AMPK and Sirt1 in promoting the beneficial effects of caloric restriction, several studies do not support this, reporting no change in the activation of these factors following this intervention (Gonzalez et al., 2004; To et al., 2007). Moreover, we expected that the combination of caloric restriction and resveratrol would produce an additive or even a synergistic effect on these mitochondrial regulatory proteins since global gene expression profiling comparing the specific intracellular effects of both caloric restriction and resveratrol in aging mice revealed a partial overlap between the two treatments (Barger et al., 2008; Pearson et al., 2008). Unexpectedly, our data suggest a blunted response of combined caloric restriction and resveratrol treatment on PGC-1α protein, and at present we are unable to explain this except by speculating that combined treatment regimens may potentially counteract one another but further work is required to confirm these findings.
Previous studies have shown the efficacy of resveratrol treatment to protect against sarcopenia. Jackson et al. (2010) reported that low doses of resveratrol (12.5 mg/kg/day for 21 days) attenuates muscle loss following a more potent, short term muscle disuse model of 7 days of hind-limb suspension in aged 34 month old Fischer 344 × Brown Norway animals. Similar benefits in muscle were also observed when mice on a high-fat diet were supplemented with slightly higher doses of resveratrol (22 mg/kg/day) (Baur et al., 2006). However, in another study, 10 months of resveratrol treatment (46 mg/kg/day) started at middle-age (18 months) was incapable of attenuating the loss in muscle mass that occurred with aging (Jackson et al., 2011).We found that resveratrol treatment induced significant changes in the longevity-related protein Sirt1 but that this was not associated with improvements in muscle mass. Similar to our caloric restriction results, changes in Sirt1 protein induced with resveratrol did not coincide with detectable changes in AMPK or PGC-1α. Such incongruent findings in these regulatory proteins have previously been reported following resveratrol treatment (Jackson et al., 2011; Um et al., 2010). However, the activity of PGC-1α is tightly controlled by post-translational modifications such as acetylation (Rodgers et al., 2005). Unfortunately, we did not assess PGC-1α acetylation levels in this study and are unable to conclude with certainty at the present time whether PGC-1α activity is altered with these treatment paradigms.
As mentioned above, there are variable accounts regarding the beneficial effects of resveratrol in skeletal muscle which have primarily been attributed to differences in the dose and time of treatment. In fact, recent dose and time course experiments with resveratrol clearly indicate differential effects of moderate and high doses on intracellular signaling pathways and downstream mitochondrial adaptations (Price et al., 2012). Therefore, it appears that the efficacy of resveratrol treatment, at least in muscle, is largely dependent on the dose and time of treatment, as well as the type of physiological perturbation being investigated (i.e. aging, muscle disuse, high-fat diet). Inconsistent findings based on differences in dose and treatment times might also be related to resveratrol's relatively poor bioavailability and rapid metabolism, which likely affects its protective functions (Das et al., 2008). Additionally, it is currently unknown whether inherent differences exist in the bioavailability of resveratrol in aged versus young tissues or between oxidative and glycolytic fibers. This may further help to explain our modest effects in aged animals and some of the fiber-type specific effects observed in our study. Thus, it is clear that a more detailed characterization of the absorption and bioavailability of resveratrol are needed in order to provide an accurate dosage for resveratrol supplementation. Lastly, the lack of response in mitochondrial proteins observed in the more oxidative gastrocnemius muscle points towards a fiber-specific action of these treatment regimens. Although there is limited data investigating these differential fiber type-specific effects, resveratrol treatment has been shown to cause a shift towards more oxidative fibers, and preferentially increases mitochondrial content and biogenesis in non-oxidative glycolytic fibers compared to oxidative fibers (Lagouge et al., 2006; Price et al., 2012). Collectively, we interpret these caloric restriction and resveratrol responses in muscle as being not only fiber type-specific but also likely dependent on the dose and time of treatment.
It is well established that the apoptotic potential is higher in aged muscle when compared to young muscle (Chabi et al., 2008; Dirks and Leeuwenburgh, 2005) and that the efficacy of both caloric restriction and resveratrol to deter muscle loss, is largely due to higher levels of endogenous antioxidants, as well as reduced mitochondrially-mediated apoptotic susceptibility and cell death (Knutson and Leeuwenburgh, 2008; Marzetti et al., 2009b). Resveratrol supplementation increased levels of the pro-apoptotic factor Bax, as well as the anti-apoptotic protein Bcl-2 in white gastrocnemius muscle. We also note that combined caloric restriction and resveratrol treatment attenuated the increase in Bax observed with resveratrol alone. Similar inductions of Bcl-2 protein content with resveratrol have been confirmed by other studies (Brito et al., 2008; Jackson et al., 2010). Changes in these pro- and anti-apoptotic proteins however, were not sufficient to alter the apoptotic potential of the muscle (Bax to Bcl-2 ratio).Moreover, the mitochondrial antioxidant enzyme, MnSOD was higher with caloric restriction, which is consistent to previous reports (Qiu et al., 2010). There is also evidence suggesting that MnSOD is upregulated following resveratrol supplementation through a Sirt1-dependent pathway (Tanno et al., 2010) but this was not observed in our study or by others (Jackson et al., 2011).
We also observed differential apoptosis-related protein expression patterns between muscle fiber-types following caloric restriction and resveratrol treatment. Similar to other mitochondrial-associated proteins discussed above, white gastrocnemius muscle was significantly more responsive than red gastrocnemius muscle. Type II glycolytic fibers are more susceptible than Type I oxidative fibers to age-associated decrements in mitochondrial function and apoptotic susceptibility and this likely contributes to the greater loss of muscle mass that is characteristic of these fibers (Lexell, 1999; Muller et al., 2006; Phillips and Leeuwenburgh, 2005). Therefore, it is certainly noteworthy in our study that the muscle fibers most susceptible to age-related stress and damage (Type II glycolytic fibers) are the most responsive to our treatment paradigms (McKiernan et al., 2004). Moreover, the fact that muscle mass changes and mitochondrial adaptations were not associated with improvements in mitochondrial content or function may suggest the involvement of alternate mitochondrial quality control processes. This is supported by recent evidence in human cells that resveratrol activates autophagy cell death pathways through a Sirt1-dependent mechanism, and that this is essential for the lifespan-prolonging effects of resveratrol (Morselli et al., 2010). Interestingly, under nutrient-limited conditions, AMPK activation can modulate autophagy through Sirt1, as well as by phosphorylating Ulk1, the mammalian homolog of the yeast protein kinase Atg1 (Egan et al., 2011; Kim et al., 2011; Lee et al., 2008, 2010). Finally, the importance of autophagy in maintaining muscle quality control is supported with the observation that mice lack the critical autophagy gene Atg7. Muscle from these null animals exhibits a number of abnormalities including mitochondrial dysfunction, myofiber degeneration, as well as muscle atrophy and weakness (Masiero and Sandri, 2010). Whether autophagy is required for the beneficial effects of resveratrol and other similar interventions in skeletal muscle is currently not known and will be the focus of future studies.
5. Conclusions
The current data suggests that short-term moderate caloric restriction (20%) and resveratrol (50 mg/kg/day) supplementation initiated in late-life, modestly alter key mitochondrial biogenesis regulators and apoptotic signaling proteins in aged muscle in a fiber-type specific manner with glycolytic muscle being more responsive to treatment than oxidative muscle. Furthermore, despite our original hypothesis, combined moderate caloric restriction and resveratrol treatment did not lead to any further additional benefits as evidenced by the lack of change in most proteins measured suggesting perhaps a divergence in the molecular pathways through which these two regimens act. It is clear from this study that further work in this area investigating the appropriate dose and time of resveratrol treatment in older animals is warranted. Additionally, studies investigating the effects of these treatments in different fiber types will be important in understanding their specific modes of action in muscle and the potential development of therapeutic treatments for sarcopenia.
6. Limitations of study
One limitation of the current study is that due to low tissue availability, we were unable to assess all mitochondrial markers in numerous tissues exhibiting differential fiber type distributions. However, we utilized tissues that provided a wide spectrum of fiber types representing a high proportion of slow- and fast- twitch muscle composition. Additionally, the absorption and bioavailability of resveratrol were not measured in each of the muscle fiber types investigated. Thus, we are unable to draw conclusions on whether any of the fiber type effects in this study are related to inherent differences in the absorption and metabolism of resveratrol in different fiber types. This possibility is being addressed in future studies.
Acknowledgments
We would like to thank Marvin Louie Servanez Dirain for his assistance with the animals and tissue preparation. This work was supported by start-up funds allocated to Dr. Peter Adhihetty from the College of Health and Human Performance and the Department of Applied Physiology and Kinesiology at the University of Florida (UF), as well as funding to Dr. Christiaan Leeuwenburgh from the UF, Claude D. Pepper Older Americans Independence Center (OAIC) supported by the National Institutes of Health/National Institute on Aging (1P30AG028740; NIH AG17994).
Footnotes
Conflict of interest
The authors have no conflicts of interests.
References
- Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J. Appl. Physiol. 2007;102:1143–1151. doi: 10.1152/japplphysiol.00768.2006. [DOI] [PubMed] [Google Scholar]
- Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle. Am. J. Physiol. Cell Physiol. 2009;297:C217–C225. doi: 10.1152/ajpcell.00070.2009. [DOI] [PubMed] [Google Scholar]
- Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997;11:573–581. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh C, Allison DB, Saupe KW, Cartee GD, Weindruch R, Prolla TA. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le CD, Shaw RJ, Navas P, Puigserver P, Ingram DK, de CR, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van VE, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, Mikolas E, Szijarto IA, Merei A, Halmai R, Meszaros LG, Sumegi B, Wittmann I. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011;106:383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- Brito PM, Simoes NF, Almeida LM, Dinis TC. Resveratrol disrupts peroxynitrite-triggered mitochondrial apoptotic pathway: a role for Bcl-2. Apoptosis. 2008;13:1043–1053. doi: 10.1007/s10495-008-0235-4. [DOI] [PubMed] [Google Scholar]
- Bua EA, McKiernan SH, Wanagat J, McKenzie D, Aiken JM. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J. Appl. Physiol. 2002;92:2617–2624. doi: 10.1152/japplphysiol.01102.2001. [DOI] [PubMed] [Google Scholar]
- Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C, Bernabei R, Marzetti E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013;394:393–414. doi: 10.1515/hsz-2012-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de CR, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Crandall JP, Oram V, Trandafirescu G, Reid M, Kishore P, Hawkins M, Cohen HW, Barzilai N. Pilot study of resveratrol in older adults with impaired glucose tolerance. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:1307–1312. doi: 10.1093/gerona/glr235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Lin HS, Ho PC, Ng KY. The impact of aqueous solubility and dose on the pharmacokinetic profiles of resveratrol. Pharm. Res. 2008;25:2593–2600. doi: 10.1007/s11095-008-9677-1. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic. Biol. Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. The role of apoptosis in age-related skeletal muscle atrophy. Sports Med. 2005;35:473–483. doi: 10.2165/00007256-200535060-00002. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech. Ageing Dev. 2006;127:1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem. Biophys. Res. Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1032–E1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Harman D. The free radical theory of aging. Antioxid. Redox Signal. 2003;5:557–561. doi: 10.1089/152308603770310202. [DOI] [PubMed] [Google Scholar]
- Hood DA. Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001;90:1137–1157. doi: 10.1152/jappl.2001.90.3.1137. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS One. 2008;3:e3614. doi: 10.1371/journal.pone.0003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JR, Ryan MJ, Hao Y, Alway SE. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R1572–R1581. doi: 10.1152/ajpregu.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Adhihetty PJ, Buford TW, Wohlgemuth SE, Lees HA, Nguyen LM, Aranda JM, Sandesara BD, Pahor M, Manini TM, Marzetti E, Leeuwenburgh C. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–809. doi: 10.1111/j.1474-9726.2012.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson MD, Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1: effects on aging and age-related diseases. Nutr. Rev. 2008;66:591–596. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Wiedemann FR, Winkler K, Kunz WS. Use of saponin-permeabilized muscle fibers for the diagnosis of mitochondrial diseases. Biofactors. 1998;7:221–223. doi: 10.1002/biof.5520070312. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee CM, Aspnes LE, Chung SS, Weindruch R, Aiken JM. Influences of caloric restriction on age-associated skeletal muscle fiber characteristics and mitochondrial changes in rats and mice. Ann. N. Y. Acad. Sci. 1998;854:182–191. doi: 10.1111/j.1749-6632.1998.tb09901.x. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J. Effects of strength and endurance training on skeletal muscles in the elderly. New muscles for old! Lakartidningen. 1999;96:207–209. [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Marzetti E, Carter CS, Wohlgemuth SE, Lees HA, Giovannini S, Anderson B, Quinn LS, Leeuwenburgh C. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech. Ageing Dev. 2009a;130:272–280. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. Biofactors. 2009b;35:28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiero E, Sandri M. Autophagy inhibition induces atrophy and myopathy in adult skeletal muscles. Autophagy. 2010;6:307–309. doi: 10.4161/auto.6.2.11137. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Bieman M, Th'ng J, Lemieux M. The absence of SIR2alpha protein has no effect on global gene silencing in mouse embryonic stem cells. Mol. Cancer Res. 2003;1:402–409. [PubMed] [Google Scholar]
- McKenzie D, Bua E, McKiernan S, Cao Z, Aiken JM. Mitochondrial DNA deletion mutations: a causal role in sarcopenia. Eur. J. Biochem. 2002;269:2010–2015. doi: 10.1046/j.1432-1033.2002.02867.x. [DOI] [PubMed] [Google Scholar]
- McKiernan SH, Bua E, McGorray J, Aiken J. Early-onset calorie restriction conserves fiber number in aging rat skeletal muscle. FASEB. J. 2004;18:580–581. doi: 10.1096/fj.03-0667fje. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Genetic and environmental factors in cancer and neurodegenerative diseases. Mutat. Res. 2002;512:135–153. doi: 10.1016/s1383-5742(02)00046-7. [DOI] [PubMed] [Google Scholar]
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ, Csete M, Faulkner JA, Van RH. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Murase T, Haramizu S, Ota N, Hase T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. 2009;10:423–434. doi: 10.1007/s10522-008-9177-z. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J. Biol. Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Pansarasa O, Bertorelli L, Vecchiet J, Felzani G, Marzatico F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic. Biol. Med. 1999;27:617–622. doi: 10.1016/s0891-5849(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Pistilli EE, Alway SE. Systemic elevation of interleukin-15 in vivo promotes apoptosis in skeletal muscles of young adult and aged rats. Biochem. Biophys. Res. Commun. 2008;373:20–24. doi: 10.1016/j.bbrc.2008.05.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli EE, Jackson JR, Alway SE. Death receptor-associated pro-apoptotic signaling in aged skeletal muscle. Apoptosis. 2006;11:2115–2126. doi: 10.1007/s10495-006-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, Hubbard BP, Varela AT, Davis JG, Varamini B, Hafner A, Moaddel R, Rolo AP, Coppari R, Palmeira CM, de Cabo R, Baur JA, Sinclair DA. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J. Appl. Physiol. 1995;78:2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Rice KM, Blough ER. Sarcopenia-related apoptosis is regulated differently in fast- and slow-twitch muscles of the aging F344/N × BN rat model. Mech. Ageing Dev. 2006;127:670–679. doi: 10.1016/j.mad.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J. Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Jackson JR, Hao Y, Williamson CL, Dabkowski ER, Hollander JM, Alway SE. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:815–831. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid. Redox Signal. 2007;9:2157–2173. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Alway SE. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J. Appl. Physiol. 2008;105:1695–1705. doi: 10.1152/japplphysiol.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Mol. Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl. Physiol. 2003;95:960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- Tanno M, Kuno A, Yano T, Miura T, Hisahara S, Ishikawa S, Shimamoto K, Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J. Biol. Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K, Yamaza H, Komatsu T, Hayashida T, Hayashi H, Toyama H, Chiba T, Higami Y, Shimokawa I. Down-regulation of AMP-activated protein kinase by calorie restriction in rat liver. Exp. Gerontol. 2007;42:1063–1071. doi: 10.1016/j.exger.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Whitman SA, Wacker MJ, Richmond SR, Godard MP. Contributions of the ubiquitin-proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch. 2005;450:437–446. doi: 10.1007/s00424-005-1473-8. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Wyke SM, Russell ST, Tisdale MJ. Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF-kappaB activation. Br. J. Cancer. 2004;91:1742–1750. doi: 10.1038/sj.bjc.6602165. [DOI] [PMC free article] [PubMed] [Google Scholar]