Abstract
Background & Aims
Patients with colorectal cancer (CRC) have high circulating levels of macrophage inhibitory cytokine-1 (MIC1 or GDF15), a marker of inflammation that might be involved in carcinogenesis. We analyzed blood samples collected from individuals before they were diagnosed with CRC to determine whether levels of MIC1 were associated with mortality.
Methods
We collected data on survival of 618 participants diagnosed with CRC who provided pre-diagnosis blood specimens in 1990 (Nurses’ Health Study) and 1994 (Health Professionals’ Follow-up Study) and were followed through 2010. Levels of MIC1 were measured by ELISA and then were categorized into quartiles based upon the known distribution of MIC1 levels among previously matched individuals without CRC (controls) within each cohort. We then examined the association of MIC-1 levels with overall and CRC-specific mortality using Cox proportional hazards models, with adjustments for mortality-associated risk factors and other plasma markers of inflammation. We also assessed the relationship between levels of MIC1 and levels of prostaglandin-endoperoxide synthase 2 expression (PTGS2 or COX2), measured in 245 tumor samples by immunohistochemistry.
Results
Compared to participants in the lowest quartile for plasma level of MIC1, the multivariate hazard ratio (HR) for CRC-specific death for participants in the highest quartile of MIC1 level was 2.40 (95% confidence interval, 1.33–4.34; P for linear trend=.009). The association of MIC1 with survival varied with level of PTGS2 expression in tumor samples (Pinteraction=.04). For individuals with PTGS2-positive tumors, the HR for CRC-specific death among those with high levels of MIC1 (equal to or greater than the median) was 2.13 (95% CI, 0.99–4.58) compared to participants with low levels of MIC1 (below the median). In individuals with PTGS2-negative CRC, a high level of MIC1 was not associated with an increased risk of CRC-specific death (multivariate HR=0.61; 95% CI, 0.13–2.93).
Conclusions
Based on an analysis of blood and colorectal tumor samples from 2 large studies, high plasma levels of MIC1 (GDF15) before diagnosis of CRC are associated with greater CRC-specific mortality, particularly in individuals with PTGS2-positive tumors.
Keywords: cyclooxygenase, colon cancer risk factor, NHS, HPFS
Introduction
Chronic inflammation has been demonstrated to influence the pathogenesis and progression of colorectal cancer (CRC). This effect appears to be mediated in part by the enzyme PTGS2 (prostaglandin-endoperoxide synthase 2, also known as cyclooxygenase-2 [COX-2]).1 Observational and randomized clinical studies have suggested that regular use of aspirin and NSAIDs is associated with a lower risk of CRC incidence as well as CRC-specific mortality, particularly among tumors overexpressing PTGS2.2,3
The circulating inflammatory cytokine growth differentiation factor 15 (GDF15), also known as macrophage inhibitory cytokine-1 (MIC1), is a divergent member of the human transforming growth factor-β (TGFB1) superfamily and may play a specific role in carcinogenesis.4–8 We have recently shown in a prospective study that elevated prediagnostic levels of MIC1, independent of other markers of inflammation, are associated with an increased risk of incident CRC.9 In addition to CRC, MIC1 has been linked through cross-sectional studies to various cancers, including those of the prostate, pancreas, ovary, bile ducts, and brain, as well as recurrent adenoma among patients with a history of prior adenoma.4,10–13 Furthermore, experimental and epidemiological evidence suggests that high serum levels of MIC1 measured after diagnosis among patients with colorectal cancer, ovarian cancer, prostate cancer, and glioblastoma have been associated with shorter survival.11–15
Despite these prior data, prospective evidence relating prediagnostic circulating MIC1 levels and CRC-specific survival among patients with established CRC are limited. Therefore, within two large prospective cohorts we investigated the association between prediagnostic MIC1 plasma concentrations in relation to CRC-specific and overall survival among CRC patients. Given the important role of PTGS2 in inflammation-associated colorectal carcinogenesis, we also explored the association between MIC1 levels and CRC-specific survival according to the expression of PTGS2 in the primary tumor.
Methods
Study populations
We used data drawn from two ongoing cohorts, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). The NHS began in 1976 among 121,700 U.S. female registered nurses aged 30 to 55 at enrollment. The HPFS began in 1986 among 51,529 U.S. male podiatrists, dentists, osteopathic physicians, veterinarians, pharmacists, and optometrists aged 40 to 75 at enrollment. In both cohorts, participants have returned questionnaires every two years with follow-up rates to 2010 of 95.4% in the NHS and 95.9% in the HPFS to provide information about lifestyle and dietary factors, medication (including aspirin) use, and diagnoses of CRC and other diseases. The Institutional Review Board at the Brigham and Women’s Hospital and Harvard School of Public Health approved this study.
Blood collection
Blood samples were collected by mailing phlebotomy kits to participants. From 1989-90 and 1993-95, 32,826 NHS participants and 18,225 HPFS participants, respectively, returned a single blood sample on ice packs by overnight courier. When received, blood samples were immediately centrifuged, aliquoted into plasma, and stored in continuously monitored liquid nitrogen freezers (−130 °C or below). More than 95% of the blood samples arrived in our laboratory within 26 hours of phlebotomy. Additional details regarding blood collection, transportation of specimens, and processing and storage of plasma aliquots within these two cohorts has been previously described.16–17
Selection of colorectal cancer participants and ascertainment of death
Eligible men and women for this study were individuals diagnosed with incident CRC after blood draw through 2010 follow-up who provided a prediagnostic blood specimen, completed the baseline questionnaire, and did not have a history of inflammatory bowel disease or other cancer (except non-melanoma skin cancer) prior to diagnosis of CRC. Baseline characteristics of participants with colorectal cancer whose blood we collected were not materially different from those of participants with colorectal cancer whose blood we did not collect (mean age, 68.8 vs. 67.3 years; former or current smoker, 58% vs. 63%; mean body-mass index [the weight in kilograms divided by the square of the height in meters], 26.1 vs. 26.1 kg/m2; mean metabolic equivalent [MET; i.e., exercise intensity] score per week, 20.7 vs. 18.5 hours/week; cancer of colon or rectum, 75% vs. 72%).
Cases of CRC were self-reported by participants or next-of-kin and after obtaining consent confirmed with medical records or pathology reports. We identified deaths through next-of-kin and the National Death Index. For all deaths, we sought information to determine the cause, including death certificates and medical records. A study physician, blinded to exposure information, reviewed all records to confirm cases, as well as to extract data on histological type, anatomic location, and stage of the cancer. We excluded 33 cases that failed laboratory assays, thereby leaving 618 CRC cases for the analysis.
Laboratory Assays
In a core laboratory facility, technicians used sandwich enzyme-linked immunosorbent assays (ELISA) (R&D Systems, Minneapolis, MN) to measure MIC1 (GDF15) levels in the archived pre-diagnostic plasma specimens. Based on quality controls randomly interspersed among the case–control samples, the coefficients of variation were 9.0% in HPFS, 7.0% in NHS (1990–2004), and 11.0% in NHS (2006–2008). Because we measured NHS samples in two different batches (cases and controls from 1990–2004 follow up; cases and controls from 2006–2008 follow-up), duplicate samples were included to assess laboratory drift for the NHS. The estimated coefficient of reliability between the two drift batches was 0.96 (95% CI 0.87–0.99), suggesting excellent reproducibility in the assay’s performance. Furthermore, to assess the intra-individual variation of MIC1 levels over time, we also measured MIC1 among a subset of the controls enrolled in the NHS who also provided a second blood specimen in 2000–2001 (n=103). There appeared to be good correlation among MIC1 levels in these participants (Spearman’s rho = 0.81, p<0.0001), supporting the reproducibility of MIC1 levels within individuals over a ten-year period. We have previously described our measurements of MIC1 and the other biomarkers of inflammation in these cohorts, including high-sensitivity CRP and IL6.9,18,19
Assessment of PTGS2 (COX-2) Expression
Among cases of CRC in NHS and HPFS with available tumor specimens, we conducted immunohistochemical staining for PTGS2 as previously described.3,20 A pathologist (S.O.) who was unaware of any participant data scored the tumor epithelial PTGS2 expression according to the intensity of staining compared to surrounding normal epithelium using a standardized grading scheme. Tumors with moderate or strong staining intensity compared to normal colon were classified as PTGS2 positive, whereas tumors with weak or absent staining were classified as PTGS2 negative. For an agreement study, a random selection of 124 cases was examined by a second pathologist (T.M.) unaware of other data. There was good concordance between the 2 observers (kappa=0.69, p<0.001).
Statistical Analysis
We first examined heterogeneity for the association between MIC1 and CRC mortality among men in the HPFS compared with women in the NHS using the Cochran’s Q test.21 We found no significant heterogeneity (P for heterogeneity=0.41). Thus, we pooled data from the two cohorts and calculated means, medians, and proportions according to MIC1 levels for baseline characteristics of study participants for demographics and exposures at the time of blood draw as well as information on cancer stage, site, and grade at the time of diagnosis. To examine the association of MIC1 levels with overall and CRC-specific mortality, we categorized MIC1 into quartiles based upon the known distribution of MIC1 levels among previously matched controls without CRC within each cohort, with the lowest category used as reference group.9 The Kaplan-Meier method and log-rank test were performed for the survival analysis. In the time-to-event analysis, using CRC death as the outcome, patients dying from causes other than CRC were censored. To compare CRC-specific and overall mortality according to MIC1 levels, we estimated hazard ratios (HRs) for CRC with 95% confidence intervals (CIs) using Cox proportional hazards models. We stratified for age at the time of diagnosis, and conducted age and multivariate-adjusted analyses. Potential confounders adjusted in the model included cohort (sex); date of blood draw; race; body mass index (BMI) at the time of blood draw; physical activity (in MET-hours/week) at the time of blood draw; family history of CRC at time of blood draw; regular use of aspirin or NSAIDs at the time of blood draw; stage at diagnosis; history of polyps at the time of blood draw; grade (poor/unknown vs. well vs. moderate) at diagnosis; anatomic tumor location (colon vs. rectum) at diagnosis; and the markers of inflammation, CRP and IL6.
We assessed collinearity of the model by using collinearity diagnostics; variance inflation factors showed no significant collinearity between each of our included covariates (all variance inflation factors < 2.0). We tested for trend by assigning each participant to the median value of their quartile and using these values as continuous terms in the hazards model. The proportional hazards assumption was tested by the Harrell and Lee test, which detects correlations between the Schoenfeld residuals of each covariate and the ranking of individual failure times (p for correlation coefficient for age at diagnosis = 0.02; all other p for correlation coefficients >0.05).22 In our secondary analyses, we assessed statistical interaction of plasma MIC1 (as a dichotomous variable defined according to the median based on the distribution among the controls) with subgroups, including tumor PTGS2 expression, by including cross-product terms in our models and assessing their statistical significance using the likelihood ratio test. We used SAS version 9.3 (SAS Institute, Inc, Cary, NC) for all analyses. All statistical tests were two-sided and P< 0.05 was considered statistically significant.
Results
Baseline cohort characteristics
Among the 618 eligible participants with CRC, we documented 266 total deaths among which 178 were due to CRC. For participants who were alive through the end of follow-up, the median time of follow-up from date of diagnosis was 9.2 years (interquartile range, 6.0–12.3). Plasma collection was performed at a median of 9.8 years for NHS and 6.3 years for HPFS (standard deviation, 5.0 years for NHS and 3.6 years for HPFS) before CRC diagnosis. Table 1 shows the baseline characteristics of the 618 CRC participants according to quartiles of MIC1 defined by the distribution among control individuals without CRC in our previous study.9 The proportion of colon versus rectal cancer, the distribution of stage at diagnosis, and the median age at diagnosis (including the proportion of postmenopausal women) appeared to vary significantly across quartiles of MIC1.
Table 1.
Baseline characteristics of study participants according to MIC1 levels.a
| Quartiles of MIC1b |
||||
|---|---|---|---|---|
| Characteristic | 1 (n=119) | 2 (n=160) | 3 (n=174) | 4 (n=165) |
| Age at diagnosis, y, mean (SD) | 63.7 (8.0) | 68.7 (7.2) | 71.4 (7.6) | 74.4 (7.1) |
| Sex | ||||
| Female, % | 55 | 63 | 57 | 48 |
| Body mass index, kg/m2, mean (SD) | 26.1 (4.1) | 26.2 (4.5) | 26.0 (4.4) | 25.8 (3.8) |
| Race | ||||
| White, % | 95 | 96 | 97 | 95 |
| Other, % | 5 | 4 | 3 | 5 |
| Smoking status | ||||
| Current or past, % | 51 | 43 | 45 | 38 |
| Never, % | 49 | 58 | 55 | 62 |
| Physical activity, METs, mean (SD) | 20.3 (20.1) | 20.9 (22.2) | 24.5 (25.7) | 24.4 (22.7) |
| Postmenopausalc, % | 69 | 88 | 95 | 96 |
| Menopausal hormone usec | ||||
| Current or past, % | 42 | 54 | 71 | 53 |
| Never, % | 58 | 46 | 29 | 48 |
| Regular use of aspirin or NSAIDsd, % | 40 | 45 | 50 | 52 |
| Site of CRC, % | ||||
| Colon | 83 | 86 | 90 | 93 |
| Rectum | 17 | 14 | 10 | 7 |
| Stage of Disease, % | ||||
| I | 37 | 26 | 21 | 29 |
| II | 23 | 24 | 27 | 18 |
| III | 18 | 23 | 21 | 18 |
| IV | 11 | 15 | 15 | 16 |
| Other/unknown | 12 | 16 | 16 | 19 |
| Grade of differentiation, % | ||||
| Well | 13 | 11 | 11 | 7 |
| Moderate | 61 | 53 | 56 | 55 |
| Poor/undifferentiated | 13 | 18 | 13 | 14 |
| Unknown | 13 | 19 | 20 | 24 |
Data on lifestyle characteristics from the time of the blood draw. Data on tumor characteristics from the time of cancer diagnosis.
Quartiles of MIC1 were defined by the distribution among control individuals without CRC in our previous study.9
The percentages of postmenopausal participants as well as hormone use is among women only.
Regular use of aspirin or NSAIDs defined as ≥2 tablets/week
Abbreviations: CRC = colorectal cancer; METs = metabolic equivalent task score hours per week; MIC1 = macrophage inhibitory cytokine-1; NSAIDs = nonsteroidal anti-inflammatory drugs; SD = standard deviation.
Plasma MIC1 and risk of CRC-specific and overall mortality
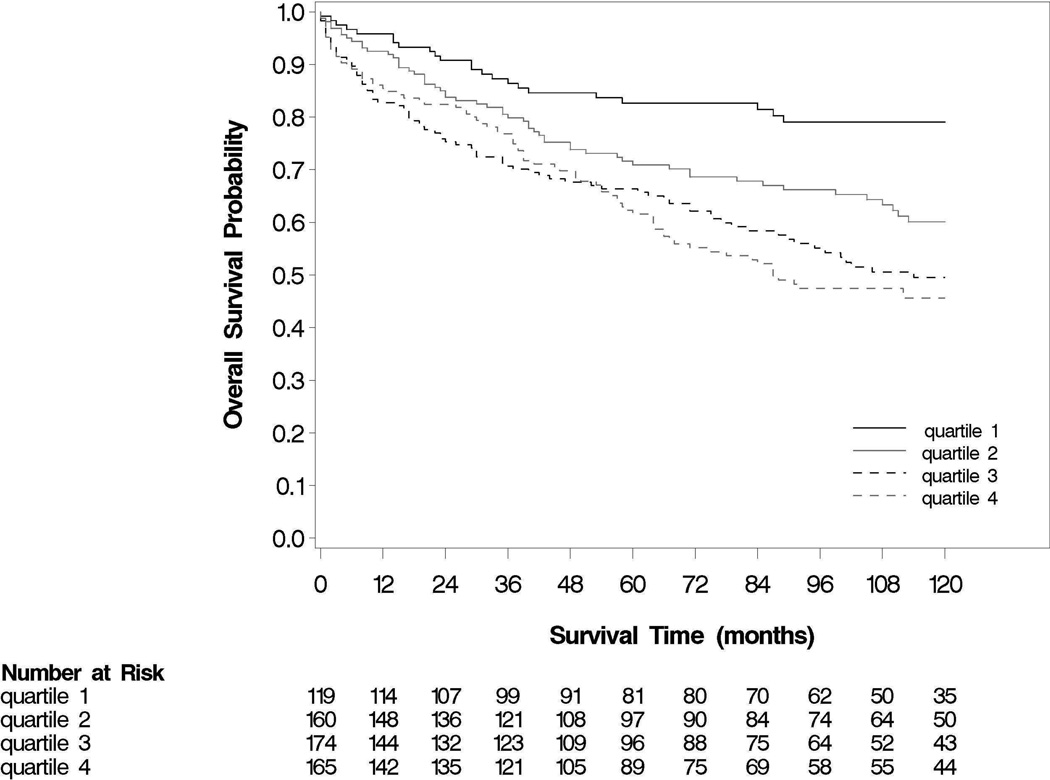
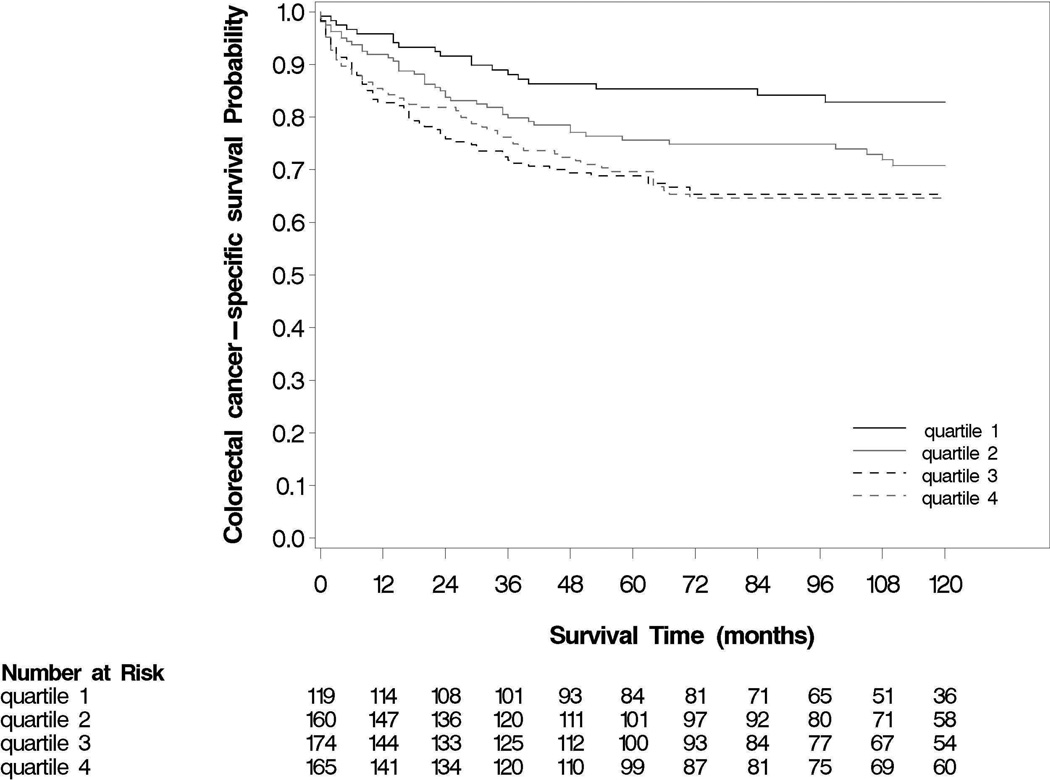
We examined the association of MIC1 with risk of CRC-specific and all-cause death according to quartile categories defined by the distribution among control individuals without CRC in our previous study (Table 2). Elevated levels of MIC1 were associated with a statistically significant higher in risk of overall mortality (log-rank p<0.0001; Figure 1) and CRC-specific mortality (log-rank p=0.003; Figure 2). For both cohorts together, the overall 5-year survival was 83% for those participants with the lowest levels of MIC1 compared with 62% for those with the highest levels of MIC1. The corresponding 10-year survival rates were 79% and 46%. Compared to men and women with levels of plasma MIC1 in the lowest quartile (Q1), the multivariate HRs for CRC were 1.67 (95% CI, 0.93 – 3.00) for those with MIC1 levels in the second quartile; 2.67 (95% CI, 1.49 – 4.80) for the third quartile; and 2.40 (95% 1.33 – 4.34) for the highest quartile after adjustment for other prognostic factors, including age, sex, stage at diagnosis, tumor grade, anatomic site, BMI, and family history of CRC as well as plasma CRP and IL6 (p for linear trend = 0.009). In contrast, there was no association observed with CRC-specific death when comparing extreme quartiles for CRP (multivariate HR, 1.09; 95% CI, 1.09 – 1.72; p for linear trend = 0.32) or IL6 (multivariate HR, 1.15; 95% CI, 0.72 – 1.84; p for linear trend = 0.59).
Table 2.
Colorectal cancer-specific mortality and overall mortality according to MIC1 levels.
| Quartile of plasma MIC1a |
|||||
|---|---|---|---|---|---|
| Analysis | 1 | 2 | 3 | 4 | Ptrendb |
| All-cause mortality (n=618) | |||||
| No. of events/No. at risk | 25/119 | 62/160 | 83/174 | 96/165 | |
| Age-adjusted HR (95% CI) | 1 (referent) | 1.88 (1.17–3.03) | 2.51 (1.57–3.99) | 2.85 (1.78–4.58) | <0.0001 |
| MV-adjusted HR (95% CI)c | 1 (referent) | 1.77 (1.07–2.91) | 2.55 (1.55–4.19) | 2.63 (1.60–4.32) | 0.0002 |
| MV-adjusted HR (95% CI)d | 1 (referent) | 1.74 (1.06–2.88) | 2.54 (1.54–4.19) | 2.63 (1.60–4.32) | 0.0002 |
| CRC-specific mortality (n=618) | |||||
| No. of events/No. at risk | 19/119 | 43/160 | 59/174 | 57/165 | |
| Age-adjusted HR (95% CI) | 1 (referent) | 1.94 (1.12–3.37) | 2.64 (1.54–4.51) | 2.73 (1.57–4.75) | 0.003 |
| MV-adjusted HR (95% CI)c | 1 (referent) | 1.65 (0.92–2.94) | 2.60 (1.45–4.67) | 2.34 (1.30–4.21) | 0.01 |
| MV-adjusted HR (95% CI)d | 1 (referent) | 1.67 (0.93–3.00) | 2.67 (1.49–4.80) | 2.40 (1.33–4.34) | 0.009 |
Quartiles of plasma MIC1 were defined by the distribution among control individuals without CRC in our previous study.9 We assigned participants to quartile categories based upon cohort-specific cutpoints (NHS: Q1 <565; Q2 565–747, Q3 748–953, Q4 >953 pg/mL) (HPFS: Q1 <610; Q2 610–825; Q3 826–1060; Q4 >1060 pg/mL).
Tests for linear trend were conducted using the median values for each quartile of MIC1.
Additionally adjusted for the following potential confounders: cohort (sex); date of blood draw; race; body mass index (BMI) at the time of blood draw; physical activity (in MET-hours/week) at the time of blood draw; family history of CRC at time of blood draw; regular use of aspirin or NSAIDs at the time of blood draw; stage at diagnosis; history of polyps at the time of blood draw; grade (poor/unknown vs. well vs. moderate) at diagnosis; and anatomic tumor location (colon vs. rectum) at diagnosis
Multivariate models additionally adjusted for the plasma inflammatory markers CRP and IL6 as continuous measures as well as the factors listed in c
Figure 1.
All-cause survival according to levels of MIC1 (in quartiles). Log rank test: p<0.0001.
Figure 2.
CRC-specific survival according to levels of MIC1 (in quartiles). Log rank test: p = 0.003.
To assess if the association between MIC1 and CRC-specific death differed according to selected mortality-associated risk factors, we performed secondary analyses according to subgroups defined by age at diagnosis, sex, BMI, tumor stage, tumor grade, and smoking status (Table 3). No statistically significant interactions were identified between MIC1 levels and these factors. We also did not observe material differences between the association of MIC1 and risk of CRC-specific death according to anatomic site of tumor (colon vs. rectum) (p=0.92). Finally, we did not observe differences between the association of MIC1 and risk of CRC-specific death according to history of polyps at the time of blood draw (pint=0.16).
Table 3.
Colorectal cancer-specific mortality according to plasma MIC1 within selected subgroups
| Quartiles of plasma MIC1 |
|||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Ptrend | Pint | ||
| Younger Agea (n = 308) | No. of events/No. at risk | 17/94 | 29/92 | 32/79 | 14/43 | ||
| MV HR (95% CI) | 1 (referent) | 1.71 (0.93 – 3.16) | 3.08 (1.66 – 5.69) | 1.67 (0.81 – 3.45) | 0.11 | 0.92 | |
| Older Ageb (n = 310) | No. of events/No. at risk | 2/25 | 14/68 | 27/95 | 43/122 | ||
| MV HR (95% CI) | 1 (referent) | 1.35 (0.30 – 6.06) | 2.38 (0.56 – 10.1) | 2.94 (0.70 – 12.3) | 0.003 | ||
| Male (n=274) | No. of events/No. at risk | 9/54 | 11/60 | 23/75 | 28/85 | ||
| MV HR (95% CI) | 1 (referent) | 1.56 (0.62 – 3.94) | 2.08 (0.89 – 4.86) | 2.16 (0.94–4.95) | 0.11 | 0.96 | |
| Female (n=344) | No. of events/No. at risk | 10/65 | 32/100 | 36/99 | 29/80 | ||
| MV HR (95% CI) | 1 (referent) | 1.76 (0.83 – 3.69) | 3.06 (1.45 – 6.49) | 2.46 (1.14 – 5.28) | 0.03 | ||
| Stage I/II/III tumors (n=533) | No. of events/No. at risk | 9/106 | 27/141 | 34/148 | 31/138 | ||
| MV HR (95% CI) | 1 (referent) | 2.87 (1.31–6.32) | 3.54 (1.59–7.87) | 3.23 (1.43– 7.28) | 0.04 | 0.42 | |
| Stage IV tumors (n=85) | No. of events/No. at risk | 10/13 | 16/19 | 25/26 | 26/27 | ||
| MV HR (95% CI) | 1 (referent) | 0.88 (0.38–2.06) | 2.05 (0.91–4.60) | 1.77 (0.80–3.89) | 0.08 | ||
| Lower BMIc (n = 251) | No. of events/No. at risk | 8/46 | 24/65 | 26/73 | 27/67 | ||
| MV HR (95% CI) | 1 (referent) | 2.40 (1.03 – 5.57) | 2.28 (0.98 – 5.30) | 2.38 (1.02 – 5.55) | 0.18 | 0.78 | |
| Higher BMId (n = 367) | No. of events/No. at risk | 11/73 | 19/95 | 33/101 | 30/98 | ||
| MV HR (95% CI) | 1 (referent) | 1.20 (0.55 – 2.60) | 2.88 (1.37 – 6.05) | 2.27 (1.07 – 4.84) | 0.03 | ||
| Well or moderate grade (n = 409) | No. of events/No. at risk | 10/89 | 17/101 | 34/117 | 31/102 | ||
| MV HR (95% CI) | 1 (referent) | 2.17 (0.94 – 5.01) | 3.27 (1.49 – 7.20) | 2.90 (1.30 – 6.46) | 0.02 | 0.46 | |
| Poor or undifferentiated Grade (n = 88) | No. of events/No. at risk | 4/15 | 8/28 | 7/22 | 7/23 | ||
| MV HR (95% CI) | 1 (referent) | 1.15 (0.43 – 3.05) | 1.69 (0.57– 4.96) | 1.55 (0.53 – 4.56) | 0.36 | ||
| Current/Past Smoker (n = 349) | No. of events/No. at risk | 13/58 | 23/92 | 30/96 | 36/103 | ||
| MV RR (95% CI) | 1 (referent) | 1.39 (0.67 – 2.87) | 2.18 (1.08 – 4.38) | 2.35 (1.17 – 4.72) | 0.02 | 0.84 | |
| Never Smoker (n = 269) | No. of events/No. at risk | 6/61 | 20/68 | 29/78 | 21/62 | ||
| MV RR (95% CI) | 1 (referent) | 2.15 (0.83–5.56) | 3.49 (1.35–9.00) | 2.45 (0.92– 6.53) | 0.16 | ||
Younger age defined as below the median at diagnosis (<68 for women and < 74 for men)
Older age defined as above the median at diagnosis(≥ 68 for women and ≥ 74 for men)
Lower BMI defined as below the median at blood draw(<24.0 kg/m2 for women and < 25.0 kg/m2for men)
Higher BMI defined as above the median at blood draw (≥ 24.0 kg/m2for women and ≥ 25.0 kg/m2 for men)
To address the possibility that occult or subclinical CRC could influence levels of MIC1, we excluded cases diagnosed up to four years after blood draw from our analysis and observed similar results (multivariate HR for CRC-specific death 2.51, 95% CI, 1.20–5.24), comparing extreme quartiles of MIC1 (ptrend = 0.02). We also explored if the association between plasma MIC1 and colorectal cancer-specific mortality varied according to time between blood draw and diagnosis, but did not find material differences (pinteraction=0.44). Finally, given the measurement of MIC1 pre-diagnostically, we explored if stratification by tumor stage and adjustment for grade may have attenuated our associations if the association of MIC1 and survival was mediated by differences in these prognostic factors at diagnosis. Comparing extreme quartiles, we observed a stronger association without additional adjustment for stage or grade (multivariate HR for CRC-specific death 3.10, 95% CI, 1.74–5.51) (ptrend = 0.0003).
Association between MIC1 levels and colorectal cancer-specific mortality according to PTGS2 status
In an exploratory analysis, we considered the possibility that MIC1 may be differentially associated with risk of CRC-specific death according to tumoral PTGS2 expression among the 245 cases in which there was available tumor tissue for analysis. We found evidence suggestive of an interaction between plasma MIC1 and tumor PTGS2 expression in survival analysis (pinteraction=0.04). Among individuals with PTGS2 positive cancers (n=161), we found that those with levels of plasma MIC1 at or above the median had a multivariate HR for CRC-specific mortality of 2.13 (95% CI 0.99 – 4.58) compared to those with levels of MIC1 below the median. In contrast, the corresponding HR for CRC-specific mortality among participants with PTGS2 negative cancer (n=84) was 0.61 (95% CI 0.13–2.93).
Association of aspirin/NSAIDs use and risk of CRC-specific death according to MIC1 levels
In an exploratory analysis, we also examined if aspirin/NSAIDs use may be differentially associated with the risk of CRC-specific mortality according to baseline levels of MIC1. Consistent with previous findings in these cohorts, regular use of aspirin compared to non-use after diagnosis was associated with a multivariate HR for CRC-specific death of 0.36 (95% CI, 0.22–0.60).3 However, there did not appear to be a differential association of aspirin with CRC-specific survival according to prediagnostic levels of MIC1.
Discussion
In this prospective study, we observed that men and women with CRC in the highest two quartiles of prediagnostic plasma MIC1 had a nearly three-fold higher risk of CRC-specific mortality. Results were similar in men and women and persisted after adjusting for known prognostic factors, lifestyle risk factors for CRC, as well as other plasma markers of inflammation, including CRP and IL6. The associations did not materially change according to subgroups defined by age, sex, BMI, tumor site, history of colonic polyps, tumor grade, or tumor stage. Our results, which included a large number of incident cases of CRC (n=618), are consistent with and significantly extend upon a prior smaller cross-sectional investigation demonstrating an association between MIC1 and overall survival and between MIC1 and relapse-free survival (n=224).14 Furthermore, they build upon our recent findings reporting an association between MIC1 and incident CRC.9
Our results support the role of chronic inflammation in the development and progression of CRC. This is supported by considerable evidence demonstrating a specific mechanistic role for MIC1 in tumor development. MIC1, as a member of the TGFB1 superfamily, appears to have pleiotropic roles in carcinogenesis, showing anti-tumorigenic behavior in early stages but contributing to enhanced invasive and metastatic abilities by the cancer in later stages.23 In early stages of neoplasia, in vitro evidence shows that MIC1 expression is up-regulated by several tumor suppressor genes, such as TP53 and early growth response protein 1 (EGR1) and may mediate endoplasmic reticulum-stress-induced apoptosis.4,24 In animal models, MIC1-overexpressing transgenic mice bred to the Apcmin mouse (a model for human familial adenomatous polyposis) had a significantly reduced number of colonic polyps.6
However, MIC1 may promote neoplasia in other contexts. Experimental evidence shows enhancement of tumorigenic activity by MIC1 via the AKT and MAPK3/MAPK1 (ERK1/ERK2) pathways in human breast and gastric cancer cells.25 In mouse xenograft models, MIC1 enhances tumor growth, stimulates cell proliferation, and promotes distant metastases.4 Furthermore, in both mice and humans, MIC1 (GDF15) has been shown to be involved in cancer-related cachexia.26 Alternatively, it has been hypothesized that cancer cells may become resistant to the initial tumor suppressive effects of MIC1; thus, as these cells proliferate, ongoing expression of MIC1 may contribute to declining tumor immunity, leading to greater dissemination and/or earlier death.4,15 Nonetheless, regardless of the specific role of MIC1 in either inhibiting or stimulating carcinogenesis, our findings provide evidence that elevated prediagnostic circulating levels of MIC1 may have potential as a biomarker for risk of CRC death.
Although we had a limited number of cases with available tissue for PTGS2 analysis, we found evidence in exploratory analyses suggesting that individuals with PTGS2 positive tumors and elevated plasma levels of MIC1 had a particularly higher risk of CRC-specific death compared to individuals with PTGS2 negative tumors. These findings offer a mechanistic corroboration of our results, suggesting that MIC1 is primarily associated with progression among cancers that arise and progress through a proinflammatory milieu. Our findings are also consistent with the interactive effect of physical activity and tumor PTGS2 expression in colorectal cancer survival,27 supporting a potential immuno-modulatory role of physical activity in reducing systemic tumor-promoting inflammation. Furthermore, our exploratory results are supported by our recent findings that the association between MIC1 and incident CRC appeared more evident among PTGS2-positive tumors compared with PTGS2-negative tumors.9
There are several strengths of our study, including its large size, its prospective design, and high follow-up rate. By measuring MIC1 levels before diagnosis of CRC, we were uniquely able to examine the association of an individual’s pro-inflammatory status prior to diagnosis in relation to survival, minimizing the potential bias related to elevation of this marker by the cancer itself. Second, our findings were consistent between two independent cohorts. Third, we were able to examine a potential interaction between MIC1 levels and tumor expression of PTGS2 in our molecular pathological epidemiology database.28 Finally, we were able to examine associations after accounting for other markers of inflammation.
There are also some limitations to this study. Information on cancer treatment was limited. However, it is unlikely that differential receipt of chemotherapy could account for our findings. During the time period of the study, there was little variation in the type of chemotherapy available and the receipt of chemotherapy was likely defined by stage of disease for which we were able to adjust. Further, data on cancer recurrence and relapse are not available in these cohorts. However, because median survival for recurrent colorectal cancer was approximately 10–12 months during the time period of this study, colorectal-cancer specific survival should be a reasonable surrogate for recurrence.29 Also, we only had a single measurement of MIC1. However, we found minimal intra-individual variation in MIC1 levels over a 10 year period among a subset of NHS participants, supporting MIC1 as a stable biomarker over time. Moreover, we found that the time between blood draw and diagnosis may have little effect on the association between MIC1 and CRC survival. Finally, because our participants were all health professionals and are largely Caucasian, our study may not be generalizable to other populations.
In summary, our data shows a higher risk of CRC-specific mortality associated with elevated prediagnostic MIC1 (GDF15) levels, even after accounting for other clinicopathological (e.g. stage) and lifestyle risk factors and other markers of inflammation. This association appeared to differ by CRC tumor PTGS2 status. These findings suggest that MIC1 may be a specific prognostic marker for risk of CRC death, and provide additional evidence for the role of chronic inflammation in the development and progression of CRC. Additional studies are needed to further explore the mechanistic role for MIC1 in the development and progression of colorectal cancer as well as a prognostic role of MIC1 after diagnosis.
Acknowledgements
We acknowledge the technical assistance of Gary Bradwin and Dr. Nadir Rifai for conducting the measurements of the MIC1, IL6, and CRP. We would also like to thank the participants and staff of the NHS and HPFS for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Grant support: This work was supported by the National Institutes of Health (P01 CA87969, UM1 CA186107, UM1 CA167552, R01 CA137178, K24 DK 098311, K07 CA190673, K07 CA148894, R35 CA197735, R01 CA151993). R.S.M. is a Howard Hughes Medical Institute Medical Research Fellow and an AGA–Eli and an Edythe Broad Student Research Fellow.
Abbreviations used in this paper
- CI
Confidence Interval
- COX-2
cyclooxygenase-2
- CRC
colorectal cancer
- CRP
C-reactive protein
- ELISA
Enzyme-linked Immunosorbent Assay
- HPFS
Health Professionals Follow-up Study
- HR
hazards ratio
- IL6
interleukin 6
- MIC1
macrophage inhibitory cytokine-1
- NHS
Nurses’ Health Study
- PTGS2
prostaglandin-endoperoxide synthase 2
- TGF
transforming growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: A. T. C. previously served as a consultant for Bayer Healthcare, Millennium Pharmaceuticals, Pozen Inc, and Pfizer Inc. This study was not funded by Bayer Healthcare, Millennium Pharmaceuticals, Pozen Inc., or Pfizer Inc. No other conflict of interest exists.
Author Contributions: Study concept and design (RSM ELG CSF SO ATC); acquisition of data (JAM KN RN ZQ TM KW ELG CSF ATC); analysis and interpretation of data (RSM DQC MS ATC); drafting of the manuscript (RSM ATC); critical revision of the manuscript for important intellectual content (CSF ELG ATC SO); obtained funding (CSF SO ATC); study supervision (ATC).
References
- 1.Wang D, DuBois RN. The Role of COX-2 in Intestinal Inflammation and Colorectal Cancer. Oncogene. 2010;29:781–788. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 3.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Baek SJ, Eling TE. The diverse roles of nonsteroidal anti-inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem Pharmacol. 2013;85:597–606. doi: 10.1016/j.bcp.2012.11.025. Author names in bold designate shared co-first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Kingsley PJ, Marnett LJ, et al. The role of NAG-1/GDF15 in the inhibition of intestinal polyps in APC/Min mice by sulindac. Cancer Prev Res. 2011;4:150–160. doi: 10.1158/1940-6207.CAPR-10-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baek SJ, Okazaki R, Lee S-H, et al. Nonsteroidal Anti-Inflammatory Drug-Activated Gene-1 Over Expression in Transgenic Mice Suppresses Intestinal Neoplasia. Gastroenterology. 2006;131:1553–1560. doi: 10.1053/j.gastro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Breit SN, Johnen H, Cook AD, et al. The TGF-β superfamily cytokine, MIC-1/GDF15: a pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors. 2011;29:187–195. doi: 10.3109/08977194.2011.607137. Author names in bold designate shared co-first authors. [DOI] [PubMed] [Google Scholar]
- 8.Zimmers TA, Gutierrez JC, Koniaris LG. Loss of GDF-15 abolishes sulindac chemoprevention in the ApcMin/+ mouse model of intestinal cancer. J Cancer Res Clin Oncol. 2010;136:571–576. doi: 10.1007/s00432-009-0691-4. Author names in bold designate shared co-first authors. [DOI] [PubMed] [Google Scholar]
- 9.Mehta RS, Song M, Bezawada N, et al. A prospective study of macrophage inhibitory cytokine-1 (MIC-1/GDF15) and risk of colorectal cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju016. dju016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koopmann J, Buckhaults P, Brown DA, et al. Serum Macrophage Inhibitory Cytokine 1 as a Marker of Pancreatic and Other Periampullary Cancers. Clin Cancer Res. 2004;10:2386–2392. doi: 10.1158/1078-0432.ccr-03-0165. Author names in bold designate shared co-first authors. [DOI] [PubMed] [Google Scholar]
- 11.Shnaper S, Desbaillets I, Brown DA, et al. Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. Int J Cancer. 2009;125:2624–2630. doi: 10.1002/ijc.24639. [DOI] [PubMed] [Google Scholar]
- 12.Staff A, Bock A, Becker C, et al. Growth differentiation factor-15 as a prognostic biomarker in ovarian cancer. Gynecol Oncol. 2010;118:237–243. doi: 10.1016/j.ygyno.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Brown DA, Hance KW, Rogers CJ, et al. Serum Macrophage Inhibitory Cytokine-1 (MIC-1/GDF15): A Potential Screening Tool for the Prevention of Colon Cancer? Cancer Epidem Biomar Prev. 2012;21:337–346. doi: 10.1158/1055-9965.EPI-11-0786. Author names in bold designate shared co-first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown DA, Ward RL, Buckhaults P, et al. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin Cancer Res. 2003;9:2642–2650. [PubMed] [Google Scholar]
- 15.Brown DA, Lindmark F, Stattin P, et al. Macrophage inhibitory cytokine 1: a new prognostic marker in prostate cancer. Clin. Cancer Res. 2009;15:6658–6664. doi: 10.1158/1078-0432.CCR-08-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hankinson SE, Willett WC, Manson JE, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87(17):1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 17.Wei EK, Ma J, Pollak MN, et al. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidem Biomar Prev. 2006;15(4):750–755. doi: 10.1158/1055-9965.EPI-05-0820. [DOI] [PubMed] [Google Scholar]
- 18.Wei EK, Giovannucci E, Fuchs CS, et al. Low Plasma Adiponectin Levels and Risk of Colorectal Cancer in Men: A Prospective Study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 19.Chan AT, Ogino S, Giovannucci EL, et al. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011;140:799–808. doi: 10.1053/j.gastro.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogino S, Brahmandam M, Kawasaki T, et al. Combined Analysis of COX-2 and p53 Expressions Reveals Synergistic Inverse Correlations with Microsatellite Instability and CpG Island Methylator Phenotype in Colorectal Cancer. Neoplasia. 2006;6:458–464. doi: 10.1593/neo.06247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 22.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. 3rd ed. London: Springer; 2010. pp. 585–588. [Google Scholar]
- 23.Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J Cell Phys. 2010;224(3):626–635. doi: 10.1002/jcp.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SH, Choi HJ, Yang H, et al. Two in-and-out modulation strategies for endoplasmic reticulum stress-linked gene expression of pro-apoptotic macrophage-inhibitory cytokine 1. J Biol Chem. 2012;287:19841–19855. doi: 10.1074/jbc.M111.330639. Author names in bold designate shared co-first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KK, Lee JJ, Yang Y, et al. Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells. Carcinogenesis. 2008;29:704–712. doi: 10.1093/carcin/bgn031. [DOI] [PubMed] [Google Scholar]
- 26.Johnen H, Lin S, Kuffner T, et al. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat Med. 2007;13:1333–1340. doi: 10.1038/nm1677. Author names in bold designate shared co-first authors. [DOI] [PubMed] [Google Scholar]
- 27.Yamauchi M, Lochhead P, Imamura Y, Kuchiba A, et al. Physical Activity, Tumor PTGS2 Expression, and Survival in Patients with Colorectal Cancer. Cancer Epidemiol. Biomarkers Prev. 2013;22:1142–1152. doi: 10.1158/1055-9965.EPI-13-0108. Author names in bold designate shared co-first authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular Pathologic Epidemiology of Colorectal Neoplasia: An Emerging Transdisciplinary and Interdisciplinary Field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyerhardt JA, Mayer RJ. Systemic Therapy for Colorectal Cancer. N Eng J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]