Tenofovir is now widely used by human immunodeficiency virus–infected pregnant women. This study demonstrates that infants with in utero tenofovir exposure had significantly lower bone mineral content than unexposed infants, supporting a long-standing concern about adverse fetal bone effects of maternal tenofovir.
Keywords: HIV, infant bone mineral content, tenofovir, intrauterine exposure
Abstract
Background. Fetal bone effects of maternal tenofovir use have not been well studied. We sought to compare whole-body bone mineral content (BMC) of newborns exposed vs not exposed to tenofovir in utero.
Methods. We enrolled participants from April 2011 to June 2013 at 14 US clinical sites. Singleton infants of women with human immunodeficiency virus (HIV) infection who took tenofovir in late pregnancy (tenofovir-exposed) or no tenofovir during pregnancy (tenofovir-unexposed) were enrolled during late pregnancy or within 72 hours of birth. Infants born before 36 weeks gestation or with confirmed HIV infection were excluded. Whole-body BMC was measured in the first month of life and compared with that of the tenofovir-exposed and tenofovir-unexposed newborns, unadjusted and adjusted for covariates.
Results. Seventy-four tenofovir-exposed and 69 tenofovir-unexposed infants had evaluable BMC measurements. Tenofovir-exposed mothers were more likely to be married (31% vs 22%; P = .04) and to use boosted protease inhibitors (84% vs 62%; P = .004). Tenofovir-exposed newborns did not differ from unexposed newborns on mean gestational age (38.2 vs 38.1 weeks) or mean length (−0.41 vs −0.18) or weight (−0.71 vs −0.48) Z-scores. The mean (standard deviation) BMC of tenofovir-exposed infants was 12% lower than for unexposed infants (56.0 [11.8] vs 63.8 [16.6] g; P = .002). The adjusted mean bone mineral content was 5.3 g lower (95% confidence interval, −9.5, −1.2; P = .013) in the tenofovir-exposed infants.
Conclusions. Maternal tenofovir use is associated with significantly lower neonatal BMC. The duration and clinical significance of this finding should be evaluated in longitudinal studies.
Clinical Trials Registration. ClinicalTrials.gov NCT01310023.
Tenofovir disoproxil fumarate (TDF), in combination with other antiretroviral drugs, is preferred initial therapy for adults with human immunodeficiency virus (HIV) infection because of its documented safety and efficacy [1, 2]. However, TDF has been associated with loss of bone mineral density (BMD) in adults [3–5] and children with HIV infection [6] and in adults taking TDF to prevent HIV infection [7, 8]. The clinical consequences of TDF-associated bone loss are less certain, but TDF use was a risk factor for bone fracture in adults with HIV infection in some studies [9, 10].
TDF has been increasingly prescribed for pregnant women [11]. Both the World Health Organization (WHO) and US guidelines recommend TDF as a preferred antiretroviral agent in pregnancy [1, 12]. However, primate studies have demonstrated impaired fetal growth and bone mineralization after maternal tenofovir administration during late pregnancy [13–15]. Some [16, 17], but not all [18], human studies have suggested a potential adverse effect of maternal TDF use on infant growth. However, studies evaluating infant bone outcomes after intrauterine tenofovir exposure have been limited [19].
In the present study, we aimed to evaluate the effects of maternal TDF use on infant bone mineral content (BMC).
METHODS
Study Participants
The source population for the present tenofovir substudy is the Surveillance Monitoring for ART Toxicities (SMARTT) study of the Pediatric HIV/AIDS Cohort Study. SMARTT was designed to study the effect of antenatal and postnatal antiretroviral exposure on childhood outcomes in HIV-exposed but uninfected (HEU) children. Since 2007, SMARTT has been enrolling HEU infants during pregnancy or within 72 hours of birth from 14 clinical sites in the mainland United States and Puerto Rico. The standard of care at all sites is formula-feeding. Infants with HIV infection are excluded.
SMARTT participants were eligible for this cross-sectional tenofovir substudy if they were singleton pregnancies born at ≥36 weeks gestation and met the following criteria for tenofovir exposure: maternal receipt of TDF for ≥8 weeks in the third trimester of pregnancy (tenofovir-exposed) or no maternal receipt of TDF during the pregnancy (tenofovir-unexposed). Infants not continuing to meet these eligibility criteria at birth were excluded.
Medical History and Anthropometrics
Maternal sociodemographic data and information on substance use during the current pregnancy were obtained by self-report. Maternal medical history abstracted from the medical chart included pregnancy complications, infections, antiretroviral drugs and dates received, first and last CD4+ T-lymphocyte (CD4) count/percent and HIV viral load during the current pregnancy, delivery mode, and gestational age. Infant medical history and birthweight were abstracted from the infant's chart.
Infant weight and length were measured at the entry visit by trained personnel using standard methods previously described [20]. Each measurement was obtained 3 times and the average computed. Weight and length Z-scores were calculated based on the Centers for Disease Control and Prevention 2000 normative data [21] for infants born ≥37 weeks gestation and based on preterm infant normative data for infants born <37 weeks gestation [22].
Dual-Energy X-Ray Absorptiometry
Infant dual-energy X-ray absorptiometry (DXA) scans were attempted within 4 (allowed up to 5) weeks of birth on a Hologic scanner (Delphi A, Discovery A, Discovery W, QDR4500A) using infant whole-body mode. DXA results included whole-body and whole-body less head measures of BMC, BMD, and total mass. To generate whole-body less head results, a custom subregion was created to divide the head from the rest of the body. Acceptable images were free of artifacts, contained the entire body of the infant, and had minimal movement distortion. A European Spine Phantom was circulated to all sites to ensure calibration among scanners. All scans were analyzed centrally by an International Society for Clinical Densitometry–certified bone densitometry technologist at the Tufts University Body Composition Analysis Center using Hologic APEX software version 13.4.
One DXA trainer traveled to sites that did not have personnel with prior experience in performing infant DXA scans (13/14) to train site technicians. Detailed instructions for performing infant whole-body DXA scans were provided in a manual of operations. The undressed infant (cotton shirt and diaper allowed) was swaddled and not sedated. Up to 3 scan attempts were permitted.
Outcome and Exposure Measures
The primary objective was to compare the mean BMC of tenofovir-exposed infants to that of tenofovir-unexposed infants. The primary BMC outcome was whole-body BMC (with head), but whole body BMC less head was also evaluated.
Potential Confounders
Potential confounding maternal factors included age, race/ethnicity, tobacco use during pregnancy, delivery in summer/fall vs winter/spring [23], CD4% and HIV viral load before starting antiretroviral regimen, and other antiretroviral medications received during the pregnancy. Infant factors considered as risk factors for the outcome were sex, race/ethnicity, body length and weight, gestational age, age at DXA, whole-body mass with and less head (by DXA), and whole-body lean mass with and less head (by DXA). The gestational age at birth and age at DXA were included because BMC accrues rapidly during late fetal and neonatal periods.
Statistical Analyses
Target enrollment was set at 75 per group to ensure at least 63 evaluable participants per group. A sample size of 63 per group would allow detection of a difference of 0.5 standard deviations (SD) of mean whole-body BMC between groups, assuming 80% power and a 2-sided alpha of 0.05.
Differences in continuous variables and categorical variables between the groups were compared using Wilcoxon and χ2 tests, respectively. For unadjusted BMC analyses, we compared mean BMC (with and less head) between groups. We then performed multivariable linear regression analysis to evaluate differences in BMC between groups, adjusted for potential confounders. All models were adjusted for clinical site, infant length, gestational age, age at DXA, and maternal tobacco use anytime during pregnancy. In addition, we considered the following potential covariates: infant whole-body mass with and less head from DXA; whole-body lean mass with and less head from DXA; and maternal height, prepregnancy weight, and body mass index. These potential confounders were included if the univariate association with BMC was P < .2 and retained in the model if the effect estimate changed by >10%. We fit models separately for whole-body BMC with and less head. We also conducted subgroup and sensitivity analyses to explore potential effects of other drugs in the maternal antiretroviral regimen.
We were unable to adjust for confounding by indication because maternal CD4 and viral load values before antiretroviral therapy (ART) initiation were missing for many women, and reasons for maternal regimen choice were not collected. We did not include CD4 and viral load values from the third trimester of pregnancy in the models because these might be the result of the exposure and on the causal pathway between maternal TDF use and infant BMC. However, we evaluated the associations of CD4% and viral load with the BMC outcomes.
All analyses were performed using SAS 9.2.
RESULTS
Study Population
Of 195 enrolled infants, 21 were no longer eligible at birth for the following reasons: gestational age <36 weeks (N = 16, including 1 fetal demise), born at another hospital (N = 1), stillborn (N = 1), loss of maternal custody (N = 1), and disqualifying change in maternal antiretroviral regimen during pregnancy (N = 2; Figure 1). No infant was excluded because of HIV infection. Of the 174 who were eligible, 126 enrolled before delivery, 37 were enrolled by age 2 days, 24 between ages 3 and 14 days, and 8 between ages 15 and 22 days. Evaluable DXA scans were obtained on the first scan attempt in 81 infants, the second attempt in 38 infants, and the third attempt in 24 infants. DXA scans were performed at a median age of 15 days overall, but at a median of 13 days in tenofovir-exposed infants and 19 days in unexposed infants (P < .001).
Figure 1.
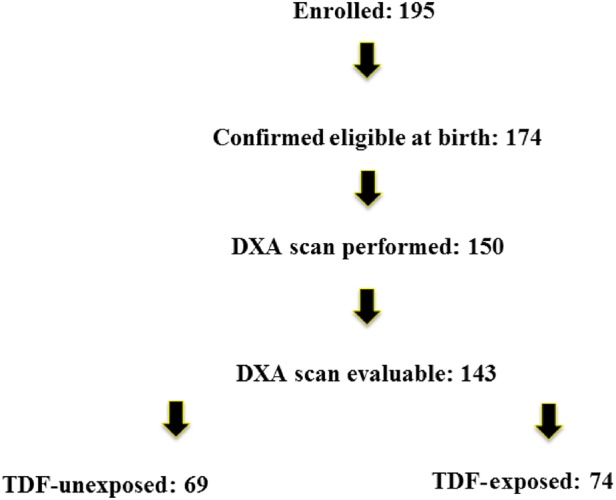
Participants were enrolled 2 April 2011 to 4 June 2013 during pregnancy (gestation age ≥23 weeks) through 2 weeks after birth. Eligibility was confirmed at birth for those enrolled before birth; 21 infants were excluded for the following reasons: gestational age less than 36 weeks (N = 16, 1 of whom was fetal demise), born at another hospital (N = 1), stillborn (N = 1), mother lost custody of child (N = 1), disqualifying change in maternal antiretroviral regimen during pregnancy (N = 2). Abbreviations: DXA, dual-energy X-ray absorptiometry; TDF, tenofovir disoproxil fumarate.
Characteristics of Mothers and Infants
Mothers who used TDF were more likely to be married (31% vs 22%; P = .035) and use a boosted protease inhibitor (PI) (84% vs 62%; P = .004). There were significant differences in the distribution of participants by tenofovir exposure across clinical sites (P < .001). The 2 groups of mothers did not differ on race/ethnicity, summer/fall delivery, high school education, income, or substance use during pregnancy (Table 1). Mothers received diverse combinations of antiretroviral drugs, including 13 combinations for women who used TDF and 18 combinations for women who did not use TDF (Table 2).
Table 1.
Characteristics of Mothers and Infants by Tenofovir Exposure Group
| Characteristica | Tenofovir Unexposed (N = 69) | Tenofovir Exposed (N = 74) | P Value |
|---|---|---|---|
| Mothers | |||
| Median age, y | 28 | 30 | .10 |
| Married | 15 (22%) | 23 (31%) | .035 |
| High school or higher education | 47 (70%)b | 51 (69%) | .87 |
| Annual income ≤$10 000 | 33 (49%) | 46 (62%) | .12c |
| Substance use during pregnancy | 25 (37%) | 19 (26%) | .19 |
| Black non-Hispanic | 40 (59%) | 51 (70%) | .17 |
| Summer/fall delivery | 39 (56%) | 43 (58%) | .85 |
| Boosted protease inhibitor use | 43 (62%) | 62 (84%) | .004 |
| 3rd trimester CD4 ≥500 cells/mm3 | 25/44 (57%) | 26/63 (41%) | .11 |
| 3rd trimester viral load ≥400 copies/mL | 5/50 (10%) | 6/62 (10%) | .95 |
| Infants | |||
| Mean gestational age at birth, weeks | 38.1 | 38.2 | .60 |
| Mean length Z-score at birth (SD) | −0.18 (1.13) | −0.41 (1.02) | .26 |
| Mean weight Z-score at birth (SD) | −0.48 (0.96) | −0.71 (0.78) | .38 |
| Black non-Hispanic | 40 (58%) | 52 (70%) | .13 |
| Female sex | 34 (49%) | 28 (38%) | .17 |
| Median age at dual-energy X-ray absorptiometry, days | 19 | 13 | <.001 |
Abbreviation: SD, standard deviation.
a Distribution of clinical research sites also differed significantly (P < .001) between the tenofovir-unexposed and tenofovir-exposed participant groups.
b Missing data on educational level for 2 women in tenofovir-unexposed group.
c Missing income data for 9 tenofovir-unexposed and 2 tenofovir-exposed groups.
Table 2.
Most Recent Antiretroviral Drug Regimens During Pregnancy by Study Arm
| Study Group | Maternal Antiretroviral Regimen | N (% Within Tenofovir Exposure Group) |
|---|---|---|
| Tenofovir exposed (n = 74)a | TDF-FTC-ATVr | 39 (53) |
| TDF-FTC-DRVr | 12 (16) | |
| TDF-FTC-RAL | 6 (8) | |
| TDF-FTC-LPVr | 4 (5) | |
| TDF-FTC-RAL-ATVr | 3 (4) | |
| TDF-FTC-RPV | 2 (3) | |
| TDF-FTC-ZDV-RPV | 2 (3) | |
| Otherb | 6 (8) | |
| Tenofovir unexposed (n = 69)c | ZDV-3TC-LPVr | 27 (39) |
| ABC-3TC-ZDV | 16 (23) | |
| ZDV-3TC-DRVr | 5 (7) | |
| ABC-3TC-ATVr | 3 (4) | |
| ZDV-3TC-NFV | 3 (4) | |
| ABC-3TC-DRVr | 2 (3) | |
| ZDV-3TC-RAL | 2 (3) | |
| Otherd | 11 (16) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ATV, atazanavir; DRV, darunavir; ETR, etravirine; FPV, fosamprenavir; FTC, emtricitabine; LPV, lopinavir; MVC, maraviroc; NFV, nelfinavir; NVP, nevirapine; r, ritonavir (boosting dose); RAL, raltegravir; RPV, rilpivirine; SQV, saquinavir; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
a In the tenofovir-exposed group, 29 (39%) women were already receiving their TDF-containing antiretroviral regimen when they became pregnant, while 11 (15%), 30 (41%), and 4 (5%) started that regimen in the first, second, and third trimesters, respectively.
b Other regimens used by 1 (1%) woman in tenofovir-exposed group included: TDF-FTC-ABC-RAL, TDF-FTC-FPVr, TDF-FTC-NVP, TDF-FTC-SQVr, TDF-ZDV-ATVr, TDF-FTC-RAL-DRVr.
c In the tenofovir-unexposed group, 18 (26%) women were already receiving their antiretroviral regimen prior to pregnancy, while 17 (25%), 31 (45%), and 3 (4%) started their pregnancy regimen in the first, second, and third trimesters, respectively.
d Other regimens used by 1 (1%) woman in tenofovir-unexposed group included: ABC-3TC-ATV, ZDV-3TC-ATVr, ZDV-3TC-NVP, ZDV-3TC-MVC, ETR-RAL-DRVr, ABC-3TC-ZDV-NVP, ABC-3TC-ZDV-RAL, ZDV-3TC RAL-LPVr, ZDV-3TC-RAL-MVC-DRVr, ZDV-3TC-ETR-DRVr, 3TC-LPVr.
Gestational age at birth was 36 to <37 weeks for 5 (7%) tenofovir-exposed infants and 4 (6%) tenofovir-unexposed infants. Tenofovir-exposed infants did not differ from unexposed infants with respect to race/ethnicity, gestational age at birth, or length or weight Z-scores (Table 1).
Mean Bone Mineral Content by Tenofovir Exposure
The mean (SD) whole-body BMC of infants exposed to tenofovir was 56.0 (11.8) g compared with 63.8 (16.6) g for unexposed infants (P = .002; Table 3). This unadjusted difference of 7.8 g in BMC represents a difference of 12.2% and 0.5 SD between exposure groups. For whole-body BMC less head, the mean (SD) was 33.3 (7.3) g in the tenofovir-exposed group compared with 36.3 (9.7) g in the unexposed group (P = .038), representing a difference of 8.3% and 0.4 SD between exposure groups.
Table 3.
Unadjusted Mean Whole-Body Bone Mineral Content (BMC), Whole-Body BMC Per Body Weight, Whole-Body Bone Mineral Density, and Whole-Body Body Mass From Dual-Energy X-Ray Absorptiometry by Tenofovir Exposure
| Whole-Body Dual-Energy X-Ray Absorptiometry Measure | Tenofovir Unexposed (N = 69) | Tenofovir Exposed (N = 74) | P Value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Bone mineral content (g) | |||
| With head | 63.8 (16.6) | 56.0 (11.8) | .002 |
| Less head | 36.3 (9.7) | 33.3 (7.3) | .038 |
| Bone mineral content per body weight (g/kg) | |||
| With head | 17.0 (2.3) | 16.1 (1.9) | .02 |
| Less head | 9.7 (1.5) | 9.5 (1.2) | .86 |
| Bone mineral density (g/cm2) | |||
| With head | 0.19 (0.02) | 0.18 (0.02) | .047 |
| Less head | 0.15 (0.02) | 0.15 (0.02) | .51 |
| Whole body mass (g) | |||
| With head | 3755.2 (678.5) | 3497.3 (531.4) | .038 |
| Less head | 2757.9 (521.9) | 2605.7 (452.3) | .12 |
Abbreviation: SD, standard deviation.
Adjusted Difference in Whole-Body Bone Mineral Content by Tenofovir Exposure
We fit a linear regression model to determine the mean difference in whole-body BMC between the tenofovir-exposed and tenofovir-unexposed infants, adjusted for potential cofactors. Our final model was adjusted for maternal age at delivery, use of tobacco during pregnancy, infant race (black vs non-black), gestational age, length, age at DXA scan, and clinical site (Table 4). The adjusted mean whole-body BMC (with head) was 5.3 g lower (95% confidence interval [CI], −9.5, −1.2; P = .013) in the tenofovir-exposed compared with unexposed infants. When we fit the model for whole-body BMC less head, adjusted for the same factors, the mean whole-body BMC was 1.9 g lower (95% CI, −4.5, .7; P = .15) in the tenofovir-exposed compared with the unexposed infants.
Table 4.
Adjusted Differences in Whole-Body Bone Mineral Content in Tenofovir-Exposed Compared With Tenofovir-Unexposed Infants
| Characteristic | Mean Difference (g) in Whole Body Bone Mineral Content (With Head) |
|||
|---|---|---|---|---|
| Unadjusted |
Adjusteda |
|||
| Mean Difference (95% CI) | P Value | Mean Difference (95% CI) | P Value | |
| Primary exposure | ||||
| Tenofovir vs no tenofovir exposure | −7.8 (−12.6, −3.1) | .001 | −5.3 (−9.5, −1.2) | .013 |
| Maternal characteristics | ||||
| Age, per year | 0.08 (−.3, .5) | .69 | 0.04 (−.24, .33) | .77 |
| Did not smoke in pregnancy | 2.5 (−3.8, 8.7) | .43 | 1.1 (−3.4, 5.7) | .62 |
| CD4 count ≥500 cells/mm3 in 3rd trimester | 1.7 (−4.4, 7.8) | .58 | … | |
| Viral load ≥400 copies/mL in 3rd trimester | 0.4 (−9.1, 9.8) | .94 | … | |
| Infant characteristics | ||||
| Female sex | −2.6 (−7.5, 2.3) | .30 | −0.20 (−3.4, 3.8) | .91 |
| Gestational age at birth, per week | 3.8 (1.8, 5.9) | .0003 | 2.1 (.50, 3.7) | .013 |
| Age at dual-energy X-ray absorptiometry, days | 0.5 (.2, .9) | .004 | 0.53 (.23, .82) | .0006 |
| Non-black vs black, non Hispanic | 8.7 (3.8, 13.6) | .0006 | 3.2 (−1.2, 7.6) | .16 |
| Body length (cm) | 3.0 (2.3, 3.8) | <.0001 | 2.4 (1.7, 3.2) | <.0001 |
Abbreviation: CI, confidence interval.
a Model also adjusted for clinical site in addition to age, smoking, CD4 count, viral load, sex, gestational age, age at dual-energy X-ray absorptiometry, race, and body length.
Including boosted PI exposure in the models had a minimal impact on the estimate of the tenofovir effect. Duration of maternal TDF use was not associated with mean whole-body BMC in a multivariate model limited to tenofovir-exposed infants (P = .81).
Because triple nucleoside reverse transcriptase inhibitor therapy was a nonconventional ART regimen and was limited to the tenofovir-unexposed infants, we refit our model by adjusting for covariates without these 16 participants. In this model, BMC with head was 6.5 g lower in the tenofovir exposed infants (95% CI, −10.9; −2.2, P = .004). To exclude the possibility that atazanavir, a drug often prescribed with TDF, might drive the observed negative effect on BMC, we also performed a subgroup analysis that included only infants without atazanavir exposure (n = 91). In this smaller sample, the tenofovir effect persisted with an even larger effect size of −7.5 g (95% CI, −13.3, −1.7; P = .012).
Maternal CD4 Values and Viral Load
CD4 values were available for 107 women and viral loads were available for 112 women in the third trimester. There were no differences between the groups in the proportion with CD4 ≥ 500 cells/mm3 (57% vs 41%; P = .11) or with viral load ≥400 copies/mL (10% vs 10%; P = .95; Table 1). There was also no significant correlation between CD4% or viral load in the third trimester and BMC. For whole-body BMC with head, the correlations were r = 0.12 (P = .21) for CD4% and r = 0.15 (P = .13) for viral load. For whole-body BMC less head, the correlations were r = 0.12 (P = .21) for CD4% and r = 0.09 (P = .33) for viral load.
DISCUSSION
Infants with in utero tenofovir exposure had a significantly lower BMC than infants without in utero tenofovir exposure. The magnitude and significance of this difference remained robust despite adjustment for factors expected to affect neonatal BMC. This finding is consistent with observations in animal studies and strongly supports a long-standing concern about potential adverse effects of maternal TDF on offspring bone mineral status [24].
The magnitude of the mean BMC difference (12% or 0.5 SD) between tenofovir-exposed and tenofovir-unexposed infants should be considered relative to other studies of bone effects of tenofovir and other exposures. In a double-blind, placebo-controlled randomized clinical trial of TDF–emtricitabine in HIV-uninfected adults, spine BMD declined 0.13 SD in the TDF–emtricitabine arm but increased by 0.13 SD in the placebo arm by week 24, for a net difference of 0.26 SD [7]. In a trial that randomized HIV-infected adults to initiate a TDF-containing regimen vs an abacavir-containing regimen (without TDF), spine BMD declined by 0.22 SD in the TDF arm but only by 0.11 SD in the abacavir arm at week 48 [4]. In a trial that randomized preterm infants to receive preterm formula vs standard term-infant formula, whole-body BMC was 18% and 19% higher in the preterm formula-fed infants after 2 and 4 months, respectively; the corresponding differences when BMC was corrected for body weight were 9% and 7%, respectively [25]. Thus, the magnitude of difference in the present study appears to be larger than the effect sizes reported for adults who take TDF and in infants with nutritional interventions.
While the association of maternal TDF during pregnancy with lower infant BMC in our study is concerning, the need to modify current WHO and US recommendations for TDF in pregnancy will depend on whether the effect persists and whether there are clinical consequences. The present study provides strong evidence of a biologic effect of maternal TDF on infant bone. However, the lack of infant BMC reference standards makes it difficult to determine if the lower BMC in tenofovir-exposed infants is abnormal. In a large study of healthy, full-term (37–42 weeks gestation) Canadian infants in whom whole-body BMC measurements were obtained within 2–4 weeks of birth using Hologic scanners, the mean (SD) whole-body BMC (with head) was 76.0 (14.2) g and mean (SD) whole-body BMC per body weight was 20.7 (2.5) g/kg [23], substantially greater than the corresponding unadjusted measurements for both exposure groups in the present study (Table 3). These relatively large differences are unlikely the result of the lower gestational age in the present study (mean 38.2 weeks vs 39.3 weeks in the Canadian study); in our study, each additional week of gestational age was associated with an adjusted mean BMC increase of 2.1 g (Table 4). The difference in participant racial composition between these studies is also not likely to explain the differences since BMC is similar for black and white young infants [26].
Lower BMC and BMD are well-established risk factors for bone fracture in adults [27] and children [28, 29]. Low BMC at birth may increase fracture risk in infancy and early childhood and also have longer-term implications. Failure to reach expected peak bone mass in early adulthood is a risk factor for osteoporosis in later life [30]. Preterm birth and low birthweight increase the risk of lower adult bone mass and fracture, suggesting that intrauterine exposures may have lifelong bone consequences [31]. Maternal sun exposure, vitamin D status, magnesium intake, and other dietary factors during pregnancy correlate with BMC and BMD outcomes in offspring 9 years later [32], providing evidence that time-limited exposures during pregnancy can lead to long-term bone consequences. The potential clinical consequences of lower BMC in the present study will require longitudinal evaluation.
Although tenofovir was the primary exposure of interest, the potential effect on neonatal bones of other antiretroviral drugs was considered. As a class, PIs have been associated with low BMD in some, but not all, studies of children [33, 34] and adults [5, 35] with HIV infection. In addition, higher tenofovir levels (but lower PI levels) occur when tenofovir is co-administered with atazanavir or other PIs [36, 37]. However, adjustment for PIs as a class did not affect the relationship of tenofovir exposure to infant BMC in the present study. Furthermore, subgroup analysis provided evidence that atazanavir alone, the PI most commonly used with TDF in this study, did not explain the association between maternal TDF and lower infant BMC. The relatively small sample size, heterogeneity of antiretroviral drug regimens, and imbalance in antiretroviral drugs and drug classes by concurrent tenofovir exposure prevented further analysis of the effect of these other drugs on infant BMC outcomes. More data are needed about the potential for PIs in pregnancy to affect neonatal BMC.
Vitamin D, parathyroid hormone, and diet were not assessed in the mothers in the present study. Vitamin D insufficiency is common in people with HIV infection, and TDF leads to parathyroid hormone elevations that are mitigated by vitamin D supplementation [38]. Recent data suggest that vitamin D supplementation in adults initiating ART can prevent ART-associated BMD loss [39]. The role of vitamin D levels in the association between maternal TDF and infant bone outcomes should be explored in future studies. Maternal or infant vitamin D supplementation may be a strategy to counteract the infant bone effects of intrauterine tenofovir exposure, though infant vitamin D supplementation has not improved bone outcomes in trials of preterm infants or healthy breastfeeding infants [40, 41]. Infant physical therapy interventions may be another option for improving postnatal bone growth and bone mass accrual [42].
Limitations of this study include the potential for residual confounding and the lack of criteria for defining abnormal and normal BMC in infants. While preterm birth has not been associated with tenofovir exposure and therefore would not be on the causal pathway, we excluded preterm infants because preterm birth results in lower BMC. Thus, findings may not be generalizable to preterm infants. Very few women in this study used non-nucleoside reverse transcriptase inhibitors (NNRTIs), preventing evaluation of potentially different effects of TDF when used in a regimen with NNRTIs. This cross-sectional study does not provide information about longitudinal BMC changes or later risk of bone fragility. However, since infants in this substudy are part of the ongoing SMARTT cohort study, further follow-up will be possible as the infants grow and develop. The International Maternal Pediatric AIDS Adolescent Clinical Trials network has completed enrollment in the multisite study (P1084s) in Africa that measures BMC at birth and again at age 26 weeks in infants whose mothers were randomized to receive ART regimens with or without tenofovir during pregnancy. Results from this study are expected in 2016 [43].
TDF remains an important part of highly efficacious antiretroviral regimens for treating women with HIV infection and for preventing infant HIV. The association of maternal TDF with lower infant BMC in the present study will need confirmation in other studies that can also evaluate persistence and clinical implications of lower infant BMC before recommendations for TDF use in pregnancy are modified.
Notes
Acknowledgments. We thank the children and families for their participation in Pediatric HIV/AIDS Cohort Study (PHACS) and the individuals and institutions involved in the conduct of PHACS.
The following institutions, clinical site investigators, and staff participated in conducting the PHACS Surveillance Monitoring for ART Toxicities Tenofovir Substudy, in alphabetical order: Ann & Robert H. Lurie Children's Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris, Shelley Buschur; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Anna Cintron, Mildred Maldonado; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Pam Henry, Stephanie Nelson; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera; State University of New York Downstate Medical Center: Hermann Mendez, Ava Dennie, Susan Bewley; Tulane University Health Sciences Center: Chi Dola, Robert Maupin, Karen Craig, Patricia Sirois; Ochsner Hospital: Shantrice Joseph; University of Alabama, Birmingham: Marilyn Crain, Newana Beatty, Dan Marullo, Paige Hickman; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Jenna Wallace, Carrie Chambers, Christine Reed, Lilian Millan; University of Florida/Jacksonville: Mobeen Rathore, Kristi Stowers, Ann Usitalo; University of Illinois, Chicago: Kenneth Rich, Lourdes Richardson, Renee Smith; University of Southern California: Toni Frederick, Mariam Davtyan, Maribel Mejia; University of California–Los Angeles: Paul A. Krogstad, Violeta Crow; University of Puerto Rico Medical Center: Zoe Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
We thank Mary Sherman of the University of California–San Francisco for conducting on-site training in the performance of infant dual-energy X-ray absorptiometry (DXA) scans and Andrea Miller and Roger A. Fielding at the Tufts University Body Composition Analysis Center for centralized DXA services.
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services.
Author contributions. D. L. J. has had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: G. K. S., D. L. J., J. W. W. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: D. L. J., J. W. W. Administrative, technical, or material support: all authors. Study supervision: all authors.
Financial support. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (U01 HD052102; principal investigator: G. R. S.; project director: Julie Alperen) and the Tulane University School of Medicine (U01 HD052104; principal investigator: R. B. V-D.; co-principal investigator: K. C. R.; project director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (principal investigator: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (principal investigator: Julie Davidson). Centralized DXA management and reading services were provided by Tufts DXA Center (principal investigator: Roger A. Fielding, PhD, Senior Scientist and Director, Nutrition, Exercise Physiology, and Sarcopenia Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging Tufts University, Professor of Nutrition and Medicine, Friedman School of Nutrition Science and Policy Tufts University School of Medicine; Associate Director, Boston Claude D. Pepper Older Americans Independence Center).
Role of funders/sponsors: The funding source was the US federal government. Federal government employees and academic investigators participated together in all aspects of study design and supervision of the conduct of the study; interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Data collection, management, and analysis were performed by academic investigators. Gilead Inc provided a grant to Tulane University to support infant DXA training; Gilead representatives did not participate in the study conduct, analysis, interpretation or manuscript preparation.
Potential conflicts of interest. All authors: No potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Collaborators: for the Pediatric HIV/AIDS Cohort Study, Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter, William Shearer, Mary Paul, Norma Cooper, Lynnette Harris, Shelley Buschur, Murli Purswani, Emma Stuard, Anna Cintron, Mildred Maldonado, Katherine Knapp, Kim Allison, Megan Wilkins, Pam Henry, Stephanie Nelson, Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera, Hermann Mendez, Ava Dennie, Susan Bewley, Chi Dola, Robert Maupin, Karen Craig, Patricia Sirois, Shantrice Joseph, Marilyn Crain, Newana Beatty, Dan Marullo, Paige Hickman, Elizabeth McFarland, Jenna Wallace, Carrie Chambers, Christine Reed, Lilian Millan, Mobeen Rathore, Kristi Stowers, Ann Usitalo, Kenneth Rich, Lourdes Richardson, Renee Smith, Toni Frederick, Mariam Davtyan, Maribel Mejia, Paul A. Krogstad, Violeta Crow, Zoe Rodriguez, Ibet Heyer, and Nydia Scalley Trifilio
References
- 1.World Health Organization consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/. Accessed 15 March 2015. [PubMed]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 15 March 2015. [Google Scholar]
- 3.Gallant JE, Staszewski S, Pozniak AL et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 2004; 292:191–201. [DOI] [PubMed] [Google Scholar]
- 4.Stellbrink H-J, Orkin C, Arribas JR et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study . Clin Infect Dis 2010; 51:963–72. [DOI] [PubMed] [Google Scholar]
- 5.McComsey GA, Kitch D, Daar ES et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a Substudy of ACTG A5202. J Infect Dis 2011; 203:1791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Purdy JB, Gafni RI, Reynolds JC, Zeichner S, Hazra R. Decreased bone mineral density with off-label use of tenofovir in children and adolescents infected with human immunodeficiency virus. J Pediatr 2008; 152:582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thigpen MC, Kebaabetswe PM, Paxton LA et al. TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367:423–34. [DOI] [PubMed] [Google Scholar]
- 8.Liu AY, Vittinghoff E, Sellmeyer DE et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One 2011; 6:e23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 2008; 93:3499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young B, Dao CN, Buchacz K, Baker R, Brooks JT; HIV Outpatient Study (HOPS) Investigators. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis 2011; 52:1061–8. [DOI] [PubMed] [Google Scholar]
- 11.Griner R, Williams PL, Read JS et al. Pediatric HIV/AIDS Cohort Study. In utero and postnatal exposure to antiretrovirals among HIV-exposed but uninfected children in the United States. AIDS Patient Care STDs 2011; 25:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed 15 March 2015.
- 13.Tarantal AF, Castillo A, Ekert JE, Bischofberger N, Martin RB. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta). J Acquir Immune Defic Syndr 2002; 29:207–20. [DOI] [PubMed] [Google Scholar]
- 14.Van Rompay KK, Brignolo LL, Meyer DJ et al. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob Agents Chemother 2004; 48:1469–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Rompay KKA, Durand-Gasselin L, Brignolo LL et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother 2008; 52:3144–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siberry GK, Williams PL, Mendez H et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS 2012; 26:1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ransom CE, Huo Y, Patel K et al. Infant growth outcomes after maternal tenofovir disoproxil fumarate use during pregnancy. J Acquir Immune Defic Syndr 2013; 64:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibb DM, Kizito H, Russell EC et al. DART Trial Team. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med 2012; 9:e1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viganò A, Mora S, Giacomet V et al. In utero exposure to tenofovir disoproxil fumarate does not impair growth and bone health in HIV-uninfected children born to HIV-infected mothers. Antivir Ther 2011; 16:1259–66. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson DL, Patel K, Siberry GK et al. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: outcomes from the Pediatric HIV/AIDS Cohort Study (PHACS). Am J Clin Nutr 2011; 94:1485–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM et al. CDC growth charts: United States. Adv Data 2000; 314:1–27. [PubMed] [Google Scholar]
- 22.Fenton TR, Sauve RS. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr 2007; 61:1380–5. [DOI] [PubMed] [Google Scholar]
- 23.Gallo S, Vanstone CA, Weiler HA. Normative data for bone mass in healthy term infants from birth to 1 year of age. J Osteoporos 2012; 2012:672403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Kourtis AP, Ellington S, Legardy-Williams J, Bulterys M. Safety of tenofovir during pregnancy for the mother and fetus: a systematic review. Clin Infect Dis 2013; 57:1773–81. [DOI] [PubMed] [Google Scholar]
- 25.Picaud JC, Decullier E, Plan O et al. Growth and bone mineralization in preterm infants fed preterm formula or standard term formula after discharge. J Pediatr 2008; 153:616–21. [DOI] [PubMed] [Google Scholar]
- 26.Kalkwarf HJ, Zemel BS, Yolton K, Heubi JE. Bone mineral content and density of the lumbar spine of infants and toddlers: influence of age, sex, race, growth, and human milk feeding. J Bone Miner Res 2013; 28:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelstein JS, Brockwell SE, Mehta V et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab 2008; 93:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res 2006; 21:1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark EM, Tobias JH, Ness AR. Association between bone density and fractures in children: a systematic review and meta-analysis. Pediatrics 2006; 117:e291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int 2003; 14:843e7. [DOI] [PubMed] [Google Scholar]
- 31.Gale CR, Martyn CN, Kellingray S, Eastell R, Cooper C. Intrauterine programming of adult body composition. J Clin Endocrinol Metab 2001; 86:267–72. [DOI] [PubMed] [Google Scholar]
- 32.Winsloe C, Earl S, Dennison EM, Cooper C, Harvey NC. Early life factors in the pathogenesis of osteoporosis . Curr Osteoporos Rep 2009; 7:140–4. [DOI] [PubMed] [Google Scholar]
- 33.Puthanakit T, Saksawad R, Bunupuradah T et al. Prevalence and risk factors of low bone mineral density among perinatally HIV-infected Thai adolescents receiving antiretroviral therapy. J Acquir Immune Defic Syndr 2012; 61:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimeglio LA, Wang J, Siberry GK et al. Bone mineral density in children and adolescents with HIV infection. AIDS 2013; 27:21120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr 2009; 51:554–61. [DOI] [PubMed] [Google Scholar]
- 36.Hill A, Khoo S, Back D, Pozniak A, Boffito M. Should the dose of tenofovir be reduced to 200–250 mg/day, when combined with protease inhibitors? J Int AIDS Soc 2014; 17(4 suppl 3):19583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirochnick M, Best BM, Stek AM et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr 2011; 56:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Havens PL, Stephensen CB, Hazra R et al. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis 2012; 54:1013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Overton ET, Chan ES, Brown TT et al. ACTG A5280 Study Team. 21st Conference on Retroviruses and Opportunistic Infections (CROI), 3–6 March 2014, Boston, MA, Abstract 133. [Google Scholar]
- 40.Natarajan CK, Sankar MJ, Agarwal R et al. Trial of daily vitamin D supplementation in preterm infants. Pediatrics 2014; 133:e628–34. [DOI] [PubMed] [Google Scholar]
- 41.Gallo S, Comeau K, Vanstone C et al. Effect of different dosages of oral vitamin D supplementation on vitamin D status in healthy, breastfed infants: a randomized trial. JAMA 2013; 309:1785–92. [DOI] [PubMed] [Google Scholar]
- 42.Moyer-Mileur LJ, Ball SD, Brunstetter VL, Chan GM. Maternal-administered physical activity enhances bone mineral acquisition in premature very low birth weight infants. J Perinatol 2008; 28:432–7. [DOI] [PubMed] [Google Scholar]
- 43.Study of effects of tenofovir on bone health and kidneys during pregnancy and breastfeeding. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01066858?term=siberry&rank=4. Accessed 15 March 2015.


