Abstract
Special populations, including children and pregnant women, have been neglected in tuberculosis drug development. Patients in developing countries are inadequately represented in pharmacology research, and postmarketing pharmacovigilance activities tend to be rudimentary in these settings. There is an ethical imperative to generate evidence at an early stage to support optimal treatment in these populations and in populations with common comorbid conditions, such as diabetes and human immunodeficiency virus (HIV) infection. This article highlights the research needed to support equitable access to new antituberculosis regimens. Efficient and opportunistic pharmacokinetic study designs, typically using sparse sampling and population analysis methods, can facilitate optimal dose selection for children and pregnant women. Formulations suitable for children should be developed early and used in pharmacokinetic studies to guide dose selection. Drug–drug interactions between commonly coprescribed medications also need to be evaluated, and when these are significant, alternative approaches should be sought. A potent rifamycin-sparing regimen could revolutionize the treatment of adults and children requiring a protease inhibitor as part of antiretroviral treatment regimens for HIV infection. A sufficiently wide formulary of drugs should be developed for those with contraindications to the standard approaches. Because genetic variations may influence an individual's response to tuberculosis treatment, depending on the population being treated, it is important that samples be collected and stored for pharmacogenetic study in future clinical trials.
Keywords: tuberculosis, pharmacokinetic, safety, efficacy, special population, pregnant women, children, HIV, diabetes, pharmacogenetic
Decades after the introduction of antituberculosis drugs, information on their use in special populations remains inadequate and is commonly limited to small investigator-initiated studies. Mechanisms are needed to ensure that studies of new treatment and chemoprophylaxis regimens are funded and, at an early stage, conducted across the spectrum of patients who should benefit from their availability. Innovative methods are needed to maximize the available evidence and minimize the risks of investigations in understudied populations.
The scientific community, industry, regulators, and policymakers have a collective obligation to ensure that special populations have access to optimal antituberculosis treatment. Therefore, it is imperative that understudied populations who may have an altered response to antituberculosis drugs, including children, pregnant women, patients with comorbidities (such as human immunodeficiency virus infection [HIV] and diabetes), and patients in high-burden countries not engaged in drug development, are included in clinical investigations to support the safe and effective use of novel drug regimens. Regulatory guidelines address the need for equitable access to clinical investigation of drugs, without regard to age, sex, and end organ status, based on the likelihood of their use in these populations [1]. Moreover, recent provisions request evidence of safety and efficacy in children [2, 3]. However, information regarding the risks of altered drug exposure and drug response in these populations often becomes available only in the postmarketing setting.
The vast majority of patients with tuberculosis reside in low-income and middle-income countries. Providers of tuberculosis treatment in these settings have unique needs, such as well-tolerated regimens that have enduring efficacy, with simplified dosing schedules and fixed-dose combination formulations appropriate for decentralized provision of care. In addition, these populations have important nutritional, environmental, and genetic differences, compared with patients in developed countries. However, scant premarketing research takes place in developing countries. Moreover, pharmacovigilance systems, which can detect postmarketing problems in patients at increased risk of an altered drug response, are poorly developed or nonexistent. Africans have the highest degree of genetic diversity [4], and yet Africa is the least studied region. In some high-burden settings, 15%–20% of the tuberculosis cases are children, and tuberculosis remains an important cause of maternal and infant morbidity and mortality [5]. The specific dosing requirements for children and pregnant women have not been evaluated sufficiently in the past.
Patients with tuberculosis often have comorbidities. An estimated 13% of all individuals with incident tuberculosis in 2012 were HIV positive, while in Africa 43% of patients with tuberculosis were found to be HIV positive [6]. In addition, there is a rising incidence of diabetes in low-income and middle- income countries, which will likely exert an increasing burden on tuberculosis control programs. Both HIV-positive patients and diabetic patients are at considerably increased risk of tuberculosis and are chronic medication users. Since drug-disease interactions and drug–drug interactions (DDIs), along with factors such as nutritional status, genetics, age, and use of other medications, may alter pharmacokinetic (PK) and pharmacodynamic (PD) relationships, it is essential that DDIs between new drug regimens and commonly coprescribed drugs are evaluated in these patients at an early stage. Together, these data are necessary to guide modifications to existing regimens and to evaluate alternative drug combinations.
In the sections that follow, specific issues related to the development of new antituberculosis regimens in children, pregnant women, and patients with HIV infection or diabetes are considered. Pharmacogenetic (PG) issues that may cause altered drug responses are also reviewed.
DRUG DEVELOPMENT CONSIDERATIONS RELEVANT TO SPECIFIC POPULATIONS
Children With Tuberculosis
While estimates of the burden of childhood tuberculosis are imperfect, a recent analysis predicted a median tuberculosis burden during childhood of 650 977 cases (interquartile range, 424 871–983 118 cases) in 22 high-burden countries in 2010, varying by country from 4% to 21% of the tuberculosis case load [7]. The risk of a child developing tuberculosis following primary infection is greatest in infants and in children with HIV infection, 50% of whom are thought to progress to disease, with a 10%–20% incidence of tuberculosis meningitis or miliary disease [8]. After 1 year of age, the risk of developing tuberculosis declines to approximately 5% in children aged 2–5 years and to 2% in children aged 5–10 years [8]. It is imperative that children with tuberculosis are adequately treated. Young children and children living with HIV infection are vulnerable to severe tuberculosis, and inadequate treatment will allow later development of the disease and transmission.
Since the introduction of antituberculosis agents to the market 50 years ago, less than half of the approximately 56 clinical studies evaluating the PK of these agents in children were designed to include sensitive, chromatography-based, analytical assays with PK sampling schemes robust enough to characterize the disposition profile of the drugs being studied. Interpretation of data across studies has also been complicated by different PK methods, formulations, and routes of administration, as well as by genetic differences between study populations. In addition, studies evaluating the PK of antituberculosis drugs in children have included only small numbers of participants. PK data in infants are particularly limited, and there are almost no data to support dosing of antituberculosis medications in infants <3 months of age. Consequently, the doses of first-line agents used for more than a half century fail to attain therapeutic targets in the majority of children [9, 10]. Among the antituberculosis agents indicated for the treatment of drug-resistant tuberculosis, only a select few have pediatric PK data, and almost none of the data derive from children with tuberculosis [11].
Historically, drugs have been insufficiently studied in children for a multitude of reasons. There have been questions related to the ethics of conducting pharmaceutical investigations in children, references to small pediatric markets that do not justify the necessary financial investment to support drug labeling in that population, and assertions that pediatric studies are logistically more complex to conduct than comparable adult studies. Fortunately, academic and regulatory agencies have come to realize that the status quo of routinely administering medications to children that have not been evaluated in children is not ethically defensible [12, 13]. US and European legislation has prompted a major push to study medicines in children. Consequently, the questions surrounding the conduct of pediatric studies have shifted from if they should be done to when and how they should be performed.
Guidance documents authored by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) serve as an appropriate starting point for discussions related to the timing of pediatric studies during antituberculosis drug development [14, 15]. There is a general consensus that there should exist, at a minimum, some adult data before pediatric studies are initiated. For new chemical entities or drug regimens in populations with drug-susceptible Mycobacterium tuberculosis where other treatment alternatives exist, it is recommended that pediatric trials begin after the completion of adult phase 3 studies. In populations with high rates of drug-resistant M. tuberculosis for which other therapeutic agents may not be available, pediatric trials can begin earlier when reasonable evidence of safety and modest evidence of efficacy in adults has been demonstrated. It is widely accepted that PK data should be the primary focus of pediatric drug studies to guide dose selection, with target PK profiles comparable to those that have demonstrated safety and efficacy in adults.
Ethical arguments in favor of evaluating medicines in children should be accompanied by the understanding that it is unethical to perform studies that have not been thoughtfully constructed to answer new questions about the population they are intended to serve [16]. It is mandatory that pediatric clinical trials are overseen by ethics committees and data safety monitoring boards whose members have pediatric expertise. These studies should be designed and conducted in collaboration with pediatric clinical trial experts and pediatric tuberculosis experts who can provide guidance on modifications to adult protocols. For instance, PK sampling strategies may be modified to ensure adequate characterization of the pediatric disposition profile owing to changes in body composition, absorption, metabolism, and elimination that occur with age. Pediatric experts should work closely with clinical laboratories to design assays to accommodate small sample volumes, ensuring the feasibility of studies in even the youngest of children. Finally, they provide the expertise to evaluate safety and tolerability, which may be challenging in nonverbal children or in circumstances where the training of healthcare providers is insufficient to objectively evaluate potential drug-related adverse events in children [16].
Tuberculosis is a global disease with marked variability in the genetic constitution of, comorbidities in, medications coadministered to, and underlying nutritional status of affected patients. Each of these factors may alter PK to a different extent, depending on the child's development, so that >1 study may be required to adequately characterize antituberculosis drug PK in children. A number of strategies can be used to optimize the efficiency of pediatric PK studies, including intensive sampling in small subsets of children with a classical analytical approach, sparse sampling over a broader range of patients followed by population PK analyses, and scavenged or opportunistic clinical sampling using population-based approaches. Adult PK data can be used to guide the design of studies in children. For example, approaches adjusting for changes in size and age [17] may allow more-accurate prediction of doses for early pediatric studies. However, differences in the maturation of metabolic proteins in children can be difficult to capture [18]. Physiologically based PK modeling, which incorporates known maturational changes in the anatomy and physiology of major organs of disposition, can also be considered. However, there are numerous examples in which the model's predictions differ from clinical results [19].
One consequence of the failure to integrate children early in the drug development process is the lack of age-appropriate dose formulations, which has the potential to compromise the care of children with tuberculosis. At present, the majority of tuberculosis treatment doses administered to children involve extemporaneous manipulation of adult formulations (eg, splitting, crushing, compounding, and admixing the formulation with food). The resultant problems that arise from the lack of a pediatric formulation impact both clinical trials and clinical practice, with the care of children with tuberculosis potentially compromised. If the only available formulation is a solid oral dose, titration of the dose for children of varying weights may be problematic. Moreover, when a suitable pediatric formulation is not available, extemporaneous manipulation of the adult form of the dose must be anticipated, and the consequences on the relative bioavailability of the compound should be carefully assessed. Examples of drugs affected by this manipulation include isoniazid, for which concentrations are reduced when adult formulations are admixed with food; rifamycin, for which concentrations drop when the adult tablet is crushed prior to administration; and fluoroquinolones, which become unpalatable when the tablet coating is compromised [20, 21].
Children remain underserved by clinical antituberculosis drug trials. In recognizing that control of the global tuberculosis epidemic requires that these studies must be conducted, the tuberculosis community has a unique opportunity to set the standard by which multidisciplinary collaborative initiatives targeted at global disease should be approached.
Pregnant Women With Tuberculosis
Influenced in large part by the outcry surrounding the exclusion of HIV-infected women from clinical trials of new antivirals [22], there has been a paradigm shift in the last 2 decades to include pregnant women in clinical drug trials. The FDA lifted their ban on the involvement of pregnant women in drug trials in 1993 [23], but these guidelines did not mandate the inclusion of women of childbearing potential in clinical trials. Thus, there is a limited pool of evidence to guide rational drug use during pregnancy, and the outcome of individual cases of drug exposure in pregnancy are seldom reported [24].
For tuberculosis, in which treatment of pregnant women will take place, clinical trials must be considered. The physiologic changes that occur during pregnancy preclude the prediction of PK/PD relationships by using data derived in nonpregnant populations. Acute physiologic changes in drug-disposition pathways occur throughout gestation, and a lack of knowledge about a drug's PK profile during pregnancy can result in inappropriate systemic exposures that place the mother and/or the fetus at risk. Furthermore, inadequate systemic exposure of drugs used for the treatment of infectious diseases has been shown to influence microbial resistance patterns in the larger community [25].
FDA guidelines can serve to frame discussions related to inclusion of pregnant women in phase 3 studies of antituberculosis drugs [26], depending on whether the drug will be used exclusively to treat conditions of pregnancy, will probably be used during pregnancy, or will be used to treat serious conditions without alternative effective therapies. Importantly, 45 CFR 46.204 stipulates that these clinical studies in pregnant women must be preceded by preclinical studies on pregnant animals and clinical studies on nonpregnant women. Moreover, the regulation asserts that the research should hold the prospect of direct benefit to the mother and/or the fetus or that the risk to the fetus should not be greater than minimal and the knowledge gained not be obtainable by any other means. In addition, the FDA guidance for industry on PK in pregnancy affirms the need for studies when the above criteria are met and when “pregnancy is likely to alter significantly the PK of a drug” [26].
For drugs that do not meet the above criteria, postmarketing studies may be more suitable. Analogous to the opportunistic study designs described for children, studies of drugs in pregnancy can capitalize on clinical environments where the pregnant woman is already receiving the drug, thus restricting the risks of the research. These settings are also suitable for registry studies in which data on the outcomes of the pregnancy and the health of the newborn are prospectively collected. With the exception of additional imaging that might be required for pregnancy monitoring, registry studies introduce essentially no added risks to the mother or fetus. Studies of drugs that merit evaluation of postmarketing efficacy should be designed in conjunction with obstetric, perinatal, and neonatal experts who can delineate criteria against which continuation of the trial and/or continued enrollment of its participants can be measured [25].
Surveys aimed at evaluating the concerns of pregnant women regarding their participation in clinical trials have been small, but results suggest that the majority of at-risk women would be willing to participate in interventional drug trials. The primary factors these women considered in their decision to participate included benefits to the fetus, benefits to the mother, and benefits to future mothers with a similar condition. Interestingly, the concern that they might be randomly assigned to receive placebo ranked behind their concern about the risk to the mother or child as a reason to decline participation [27, 28]. These data argue that the well-informed mother may be willing to accept the risks associated with medication administration during pregnancy.
Relevant legal precedent demonstrates that manufacturers, investigators, and prescribing clinicians can be vulnerable to litigation whether they choose to expose the fetus to an investigational drug or to restrict access to a putatively therapeutic agent [29]. Well-designed studies based on solid preclinical data and conducted with all of the protections delineated under existing regulations should mitigate the risk of either legal scenario.
Ultimately, decisions about the inclusion of pregnant women with tuberculosis in clinical trials need to be considered on a case-by-case basis. These decisions will be influenced by evaluation of the risks of teratogenicity against the morbidity and/or mortality of the infection or infection-associated complications that are targeted by the drug.
Patients With Tuberculosis and HIV Coinfection
An estimated 12% of individuals with incident tuberculosis globally have HIV coinfection. The incidence is markedly higher in settings such as southern Africa, where >50% of patients with a diagnosis of tuberculosis have HIV infection [6]. Historically, the fear of complications based on large pill burdens, complex dosing schedules, overlapping drug toxicities, DDI, and development of immune reconstitution syndrome discouraged many clinicians from concurrently treating HIV infection and tuberculosis. However, several large studies conducted in low-income and middle-income settings have shown that the current standard of care, introducing antiretroviral treatment (ART) during tuberculosis treatment, substantially reduces mortality [30–33].
Most studies evaluating tuberculosis treatment regimens exclude patients with severe or disseminated disease. There is a critical need for research to include patients with advanced immunosuppression to assess the regimens in patients with high mycobacterial loads and to address the specific management challenges they present [34]. The prospect of potently bactericidal antituberculosis regimens with the potential to rapidly reduce the mycobacterial load gives rise to optimism that novel regimens might reduce immunological complications in such patients. The interruption of antituberculosis treatment or ART due to the appearance of serious skin rashes or hepatotoxicity can seriously jeopardize recovery in patients with advanced immunosuppression. Therefore, switching to a new regimen would be preferable to rechallenge with drugs in the existing regimen. However, this necessitates a broad formulary of drugs from which alternative effective regimens can be constructed.
DDIs are an important consideration when combining the multidrug regimens used to treat tuberculosis and HIV infection [35]. However, the clinical consequences of DDIs may be difficult to predict. In vitro experiments do not provide accurate estimates of the magnitude of PK interactions, and PK studies in small groups of volunteers or patients fail to capture the variability of interactions. In addition, DDIs between antituberculosis agents and ART may be complex [36], are not always unidirectional [37], do not always necessitate dose adjustments [30, 38, 39], and may be modified by the patient's genetic constitution [40], age [41], and other coadministered drugs, among other factors.
The interactions between the rifamycins and key antiretrovirals are of particular concern. Rifampicin is a potent activator of pregnane X receptor (PXR). It reduces systemic concentrations of many concomitantly administered antiretrovirals that serve as substrates for CYP2B6 (nonnucleoside reverse transcriptase inhibitors [NNRTIs]), CYP3A4 (NNRTIs, protease inhibitors [PIs], and maraviroc), P-glycoprotein (PIs and maraviroc), and UGT1A1 (raltegravir). Particularly problematic are the extensive interactions between PIs and rifamycins. Unanticipated hepatotoxicity resulted from the combination of rifampicin and ritonavir-boosted PI [42]. The severity of the toxicity was heightened in healthy volunteers preinduced with rifampicin [43, 44] and may be modified by preexisting hepatic disease, HIV status [45], companion drugs such as isoniazid, and age [46]. Rifabutin, an alternative rifamycin, requires dose adjustments when given with PIs, but optimal doses are not well understood. Moreover, rifabutin is associated with important dose-related toxicities and is not available in formulations suitable for children [47, 48].
Advances in metabolomics have the potential to identify patients at risk and devise safer cotreatment strategies by enhancing our understanding of interaction-mediated toxicities [49]. With the number of patients requiring PI-based regimens expected to increase dramatically in low-income and middle-income countries as antiretroviral programs mature, management of the impact of the extensive interactions between rifamycins and PIs on the exposure, safety, and toxicity profiles of the drugs becomes critical. Development of alternative combined treatment approaches for patients with tuberculosis who need second-line ART regimens is also critical. In particular, a potent rifamycin-sparing antituberculosis regimen, compatible with PIs and other antiretrovirals, would offer an opportunity to improve treatment options for adults and children requiring non-NNRTI-based ART regimens. Additionally, the introduction of novel antituberculosis drugs in the context of concurrent HIV infection requires that special attention be paid to assessments of safety.
Agents for the treatment of drug-resistant tuberculosis also need to be addressed. The currently available second-line, multidrug regimens are poorly tolerated. In patients with HIV infection, adverse effects may be exacerbated by comorbidities such as long-standing illness and nutritional depletion. There is considerable overlap in the adverse effect profiles of second-line agents for drug-resistant tuberculosis and ART (Table 1). However, there are limited data evaluating the risk of combined treatment in which relatively high rates of neuropathy, hypokalemia, hypothyroidism, and renal impairment have been reported in large cohorts of patients with drug-resistant tuberculosis and HIV infection [50, 51].
Table 1.
Overlapping Syndromes and Combined Toxicity of Drugs Used to Treat Tuberculosis and Human Immunodeficiency Virus (HIV) Infection
| Adverse Event | Potential Causes |
|---|---|
| Febrile, Generally Unwell | IRIS, Paradoxical IRIS, Drug-Resistant Tuberculosis |
| Rash | Isoniazid, pyrazinamide, rifampicin, PAS, abacavir, nevirapine, efavirenz, stavudine, others,a HIV infection |
| Hepatotoxicity | Isoniazid, ethionamide, prothionamide, rifampicin, pyrazinamide, thiacetazone, nevirapine, efavirenz, ritonavir, and other PIs, NRTIs, paradoxical IRIS, hepatitis virus flares; rarely: fluoroquinolones, PAS |
| Neuropathy | Isoniazid, linezolid, ethionamide, prothionamide, cycloserine, terizidone, stavudine, didanosine, zalcitabine, HIV infection |
| Ophthalmologic problems | Ethambutol, rifabutin, linezolid, ethionamide, prothionamide, didanosine |
| CNS toxicity | Efavirenz, cycloserine, terizidone, ethionamide, prothionamide, isoniazid |
| QT interval prolongation | Lopinavir, atazanavir, moxifloxacin, gatifloxacin, levofloxacin, clarithromycin |
| Arthropathies/arthralgia | Pyrazinamide, fluoroquinolones, PAS, HIV infection |
| Gastrointestinal disturbance | Ethionamide, prothionamide, PAS, clofazimine, isoniazid, ethambutol, pyrazinamide, ritonavir, stavudine, nevirapine, others |
| Pancreatitis | Stavudine, didanosine, zalcitabine, linezolid |
| Lactic acidosis | Stavudine, didanosine, zidovudine, linezolid |
| Renal impairment, electrolyte disturbance | Aminoglycosides, capreomycin, tenofovir, HIV infection |
| Hematological abnormalities | Linezolid, rifabutin, rifampicin, isoniazid, zidovudine, trimethoprim-sulfamethoxazole, HIV infection, tuberculosis |
| Hypothyroidism | Ethionamide, prothionamide, PAS, stavudine, HIV infection |
| Dysglycemia | Protease inhibitors, fluoroquinolones, ethionamide, prothionamide, isoniazid |
Reproduced with permission of S. Karger AG, Basel (McIlleron H, Khoo SH. Interactions between antituberculosis and antiretroviral agents, in Progress in Respiratory Research 2011 doi: 10.1159/000324217).
Abbreviations: CNS, central nervous system; HIV, human immunodeficiency virus; IRIS, immune reconstitution inflammatory syndrome; NRTI, nucleoside reverse transcriptase inhibitor; PAS, para-aminosalicylic acid; PI, protease inhibitor.
a Thiacetazone is contraindicated in HIV-infected patients because of severe reactions and is no longer readily available.
Because adverse event surveillance mechanisms are not widely established in high-burden countries, support of clinical centers to prospectively monitor safety, efficacy, and drug resistance could provide valuable data on cotreatment approaches. Better understanding of the mechanisms of adverse effects, together with elucidation of the clearance pathways of drugs and their metabolites, has the potential to enhance prediction of toxicity and allow more-efficient identification of potentially important DDIs. Physiologically based PK models, although not a substitute for clinical trials, hold promise for quantitative prediction of DDIs. However, there is currently insufficient knowledge to support models accounting for the complex control of enzyme expression and activity, particularly with respect to non-CYP enzymes and drug transporters. Moreover, the effects of disease, nutritional, environmental, and genetic factors that contribute to the wide variability in DDIs in patients with tuberculosis and HIV infection are incompletely understood.
PK interactions and safety should be studied in patients with tuberculosis and HIV infection, including young children, as early as possible during the drug development process. PK evaluation as part of early bactericidal activity in phase 2 and phase 3 studies should be considered together with population PK analysis, which could be used to pool data across studies.
Patients With Tuberculosis and Diabetes
A systematic review of 13 observational studies demonstrated a 3-fold increased risk of active tuberculosis in patients with diabetes mellitus [52]. The incidence was higher in younger patients, in populations with a higher background incidence of tuberculosis, and in non–North American populations. In one study, diabetes was a risk factor for tuberculosis in 25% of Hispanic patients with tuberculosis aged 25–54 years, suggesting that ethnicity may play a role [53]. A major limitation of the systematic review quoted above was that studies from Africa were not included, even though Africa harbors a significant burden of the global tuberculosis pandemic. Data on the number of African patients with tuberculosis and diabetes is sparse.
Diabetes has been shown to impair chemotaxis [54] and phagocytosis [55–57], as well as intracellular killing of M. tuberculosis. Studies suggest that, when compared to nondiabetic patients, diabetics with pulmonary tuberculosis have more cavitary lesions, are more likely to be sputum negative, and have fewer symptoms and signs at diagnosis [58].
There are conflicting data regarding tuberculosis treatment outcome in patients with diabetes. Diabetics have been reported to have worse treatment outcomes [59], with a 2-fold risk of death [60], a higher risk of treatment failure [61], and a greater risk of developing multidrug resistant tuberculosis [62, 63]. However, other studies have not confirmed these findings [64, 65]. Interestingly, diabetic patients receiving insulin have been reported to have a greater risk of developing tuberculosis than those not receiving insulin [66, 67], and patients with poorly controlled diabetes also have a greater risk of tuberculosis [68].
The impact of M. tuberculosis on glucose homeostasis and diabetes is also not well understood. However, it is well established that, as part of a stress response, bacterial infection can cause hyperglycemia, but this usually improves once the bacterial infection has been cleared. Two studies, from Nigeria and Turkey, failed to show persistent hyperglycemia 3 months after initiation of tuberculosis treatment [69, 70]. Although the number of patients in these studies was small, all patients were euglycemic after 3 months of treatment, indicating that the hyperglycemia present in the acute setting was most likely due to the stress response.
Antituberculosis treatment may complicate glucose control in some patients. Fluoroquinolones can cause dysglycemia, and pyrazinamide and ethionamide have been reported to be associated with poor glycemic control in diabetics. Furthermore, DDIs may alter the PK of antidiabetic agents. Small studies have demonstrated marked reductions in the average concentrations of sulphonylureas, meglitinide analogues, and thiazolidinediones when they are given with rifampicin [71]. However, there is substantial individual variability in these effects, and further studies are needed to evaluate appropriate monitoring and therapeutic strategies.
The present and future impact of diabetes on tuberculosis treatment outcomes cannot be ignored. The growing problem of obesity among poor populations in urban areas of countries such as South Africa, Brazil, and Mexico needs to be considered. In South Africa, an estimated 73.6% of women are overweight and 42.8% are obese, while 62.0% of men are estimated to be overweight and 23.2% are obese [72]. In addition, the risk of metabolic complications from ART may contribute to increasing numbers of patients developing diabetes [73–76]. Globally, the number of patients with diabetes has increased from 177 million in 2000 to 382 million in 2013 and is estimated to increase further to 592 million in 2035 [77], with the brunt of this increase from developing countries. Furthermore, current literature on the association of diabetes and tuberculosis does not adequately represent developing country populations, which have unique environmental, genetic, socioeconomic, and nutritional conditions.
It is therefore important that studies evaluate the prevalence of diabetes in patients with tuberculosis, as well as the drug-disease and DDIs in diabetics with tuberculosis with and without HIV infection in large cross-sectional and longitudinal studies in at-risk communities. In addition, predictors of outcome and the effect of various drugs on both disease processes and DDIs need to be determined within the high-burden contexts. These studies will help guide policy on when and whom to screen, optimal drug regimens and doses, appropriate monitoring, and allocation of resources at various levels of care for both diabetes and tuberculosis.
Other Special Populations
In addition to the special patient populations addressed above, separate assessments are required for elderly individuals and across sexes, owing to the potential effects of altered physiology in these populations. It will also be important to characterize the impact of renal and hepatic impairment on the PK (and PD, when appropriate) and on the recommended doses of the drugs in question and to include possible dose adjustments in the product label. Both the FDA and the EMA have issued general guidance for assessing the effects of renal and hepatic impairment on drug disposition [78, 79].
PG ISSUES
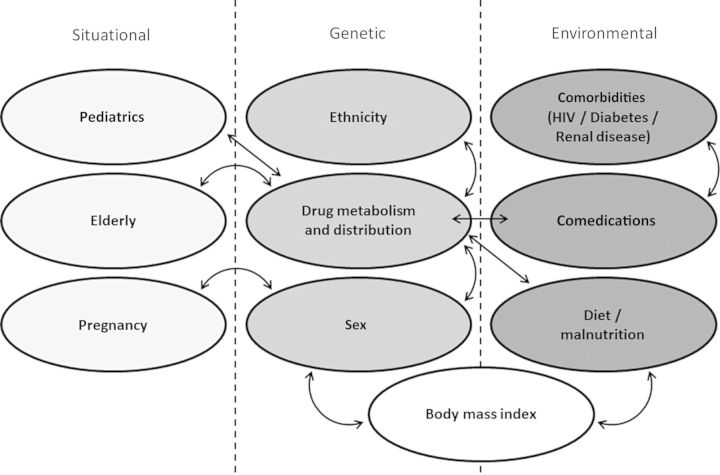
At standard medication doses, interindividual variation in both desired and toxic effects is often observed. Contributing factors include age, sex, ethnicity, body mass index, physiologic status, comorbidities, diet, and coprescribed medications (Figure 1). However, PG variation also contributes to interindividual variability at rates ranging between 20% and 95% [80]. If the impact of functionally significant polymorphisms is not addressed during drug development, recommended treatment regimens that target the average patient may not result in the desired outcomes.
Figure 1.
Summary of interrelated factors influencing pharmacokinetics/treatment response in tuberculosis therapy. Abbreviation: HIV, human immunodeficiency virus.
There is currently a paucity of information regarding PG contributions to antituberculosis therapy. For established drugs there is some information regarding the mechanisms influencing variability in PK. For example, variability in the urinary excretion of isoniazid was reported in the 1950s and later attributed to variability in acetylation mediated by the N-acetyltransferase 2 gene (NAT2) [81]. Furthermore, homozygosity for variants within NAT2, cytochrome P450 2E1 (CYP2E1), and glutathione S-transferase M1 (GSTM1) have been identified as significant predictors of hepatotoxicity associated with antituberculosis therapy in a meta-analysis [82]. It is important to note that most of these studies were performed in Asian populations receiving varying antituberculosis medication with unstandardized definitions of hepatotoxicity and uncharacterized environmental factors. Most recently, polymorphisms within the SLCO1B1 gene have been related to rifampicin PK, with the genetic variants occurring at different frequencies in patients of African and European ancestry [83, 84]. It is imperative that the frequency and functional consequences of variants be characterized across ethnic groups.
Before clinical application of PG tests can be justified, careful consideration should be given to the optimum strategy for deploying a PG test. Across all populations, the most commonly considered use of PG is in predicting adverse drug reactions, suboptimal drug exposure, and/or a loss of efficacy. These data could impact choice of drugs, choice of regimens, or duration of therapy at individual or population levels. Since genetic variants may also influence absorption, clearance, or distribution into specific compartments and cells (eg, the central nervous system or macrophages), there is a need for further study in this area for both established and emerging tuberculosis drugs.
Some PG tests may be useful for predicting which individuals are more susceptible to DDIs. For example, the aforementioned association of SLCO1B1 polymorphisms with rifampicin PK [83, 84] may be of interest with regard to the complications associated with the choice of second-line antiretroviral therapy in patients with tuberculosis and HIV coinfection. One variant of SLCO1B1 (rs4149056) is associated with higher plasma concentrations of lopinavir, while another variant (rs11045819) is associated with lower plasma concentrations of lopinavir [85]. Further studies are required to determine the usefulness of SLCO1B1 PG for identifying individuals with less marked interaction between these agents.
Most of the DDIs associated with rifampicin are mediated through its activation of nuclear receptor type transcription factors, such as the PXR, which subsequently increases the expression of numerous hepatic and intestinal drug disposition genes [86]. It is interesting to note that a polymorphism within the PXR gene has been shown to be important in constitutive and inducible PXR activity [87] and the PK of the downstream substrates such as atazanavir [88]. The use of PXR variants in predicting the magnitude of DDIs with rifampicin could be ubiquitous, and further study in this area is required.
It is important to recognize that, in contrast to adolescents and adults, many drug disposition proteins are immature in infants and children [89, 90]. There is also evidence that compensatory pathways may exist when primary pathways have yet to mature (eg, CYP3A4 vs CYP3A7 [77]). Of particular interest to tuberculosis therapy is the observation that the enzyme maturation profile for isoniazid acetylation is dependent on NAT2 polymorphisms in infants [91].
In contrast to children, the usefulness of PG analysis in pregnancy may be similar to that in the general population. However, assessment of the exposure to tuberculosis therapy in utero is of great interest and is likely to be influenced by drug disposition proteins present within the placenta. Furthermore, there is emerging evidence that functional polymorphisms within certain transporter genes may influence the expression of these proteins in the placenta [92]. Further work is required to determine the consequences to fetal drug exposure.
Host genetic factors can also influence susceptibility to tuberculosis. Several polymorphisms in genes such as human leukocyte antigen and nonhuman leukocyte antigen genes [93–96], solute carrier family 11A member 1 (formerly known as natural resistance-associated macrophage protein 1), cytokines and their receptors, chemokines and their receptors, pattern-recognition receptors (eg, Toll-like receptors, mannose binding lectin, and dendritic cell–specific intercellular adhesion molecule 3 grabbing nonintegrin) [97–101], and purinergic P2X7 receptor [102] have been associated with differential susceptibility to infection in various populations. However, it is important to note that in several cases associations have not been found across studies, and this may be influenced by host-pathogen and gene-environment interactions, evolutionary selection pressures, or lack of statistical power to detect differences. These associations have not yet had a direct impact upon prevention or treatment, but the identification of susceptibility markers is likely to help clarify underlying mechanisms that influence susceptibility to infection and pathogenesis.
It is important to recognize that not all PG associations will translate into clinically worthwhile tests. For example, the magnitude of the association may not be of sufficient predictive power, the genetic variant may be too rare to be of clinical usefulness, or testing may not be cost-effective. However, thorough analysis of genetic associations will enable algorithms to be developed for assessing the combined influence of multiple genetic and demographic factors. This knowledge can inform future drug development and novel pharmacological strategies, along the lines of maraviroc development from knowledge of CCR5 Δ32 [103].
It is also important to stress that PG associations should be interpreted in the context of the PK/PD relationship. For example, there is a clear relationship between isoniazid exposure and early bactericidal activity, which is strongly influenced by NAT2 polymorphisms [104]. However, for rifampicin, the effect on sterilizing activity associated with genetically determined changes in PK is less well understood. Furthermore, for ethambutol and pyrazinamide, PG determinants of exposure have not been identified, and definition of the PK/PD relationship in patients is limited.
It is clear that there are a number of research needs to accelerate implementation of PG to optimize tuberculosis regimens, especially in special populations. As in other areas of PG [105], it is imperative that samples be collected and stored for this purpose in future clinical trials. PG associations need to be underpinned by biologically plausible mechanisms to facilitate rational deployment, uncover new research avenues, and facilitate drug development. Central to effective PG test deployment will be appropriately characterized phenotypes in exploratory studies and sufficient resource availability to properly define clinical usefulness.
CONCLUDING REMARKS
Historically, special populations have been neglected in the drug development process. In this article, we have highlighted the needs of children, pregnant women, persons living with HIV infection, and diabetics, all of whom are susceptible to tuberculosis and contribute substantial numbers to the population of patients with tuberculosis, particularly in high-burden settings. Innovative methods are emerging to efficiently obtain and analyze data, thus minimizing the risk and reducing the cost of research in understudied populations. Recognition of the need for evidence to optimize treatment in special populations has led to ethical and regulatory review of the perceived barriers to including some vulnerable populations in research. Ethicists and drug regulators should continue to be engaged in the specific challenges of ensuring that special populations have equitable access to novel regimens. Ensuring that appropriate studies of new regimens are funded and conducted in time to benefit the spectrum of patients represents the greater challenge. To this end, it is essential to support centers and associated laboratories in high-burden countries that are capable of excellent clinical research. Platforms for the pooling and sharing of data would maximize the use of relatively scarce data from special populations. Furthermore, expertise, training, and funding is necessary to support platforms for standardized and structured monitoring and evaluation of new drug combinations in special populations.
Notes
Acknowledgments. We thank Graeme Meinjies, MD, PhD (Institute of Infectious Diseases and Molecular Medicine, University of Cape Town), and the cochairs of the Critical Path to Tuberculosis Drug Regimens Preclinical and Clinical Sciences Workgroup, Eric Nuermberger, MD (Johns Hopkins University School of Medicine), Debra Hanna, PhD (Critical Path Institute), and Thorir Bjornsson, MD, PhD (Critical Path Institute), for their input and support with writing this article; and the Bill and Melinda Gates Foundation, for supporting the Critical Path to Tuberculosis Drug Regimens (CPTR) Initiative.
This article is one of several sponsored by the Regulatory Science Consortium, which is part of the CPTR Initiative (available at: http://www.cptrinitiative.org).
Financial support. This work was supported by the National Institutes of Health, through the Human Heredity and Health in Africa Initiative (1U01HG007046-01 to A. O.); and the National Research Foundation of South Africa (grant 90729 to H. M.).
Potential conflicts of interest. M. B. is a member of the South African National Essential Medicines List Committee, the Western Cape Provincial Pharmacy and Therapeutics Committee and its executive committee, and the Medicines Control Council (MCC) and its central clinical committee; chair of the Tertiary/Quaternary Essential Medicines List Committee and the MCC's Pharmacovigilance Committee; and a consultant for various private sector drug and therapeutics committees, including those for Liberty Health and Metropolitan Health Risk Management. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.FDA action plan to enhance the collection and availability of demographic subgroup data. August 2014. http://www.fda.gov/downloads/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/UCM410474.pdf Accessed 11 September 2014.
- 2.Pediatric Research Equity Act of 2007. Pub L No 110-85, 121 Stat 823. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM049870.pdf Accessed 10 September 2014.
- 3.Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use. 2006.
- 4.Tishkoff SA, Reed FA, Friedlaender FR, et al. The genetic structure and history of Africans and African Americans. Science 2009; 324:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Nayak U, Ram M, et al. ; Byramjee Jeejeebhoy Medical College-Johns Hopkins University Study Group. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis 2007; 45:241–9. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Global tuberculosis report 2013. WHO/HTM/TB/2013 http://www.who.int/tb/publications/global_report/en/ Accessed 3 June 2013.
- 7.Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modeling study. Lancet Glob Health 2014; 2:453–9. [DOI] [PubMed] [Google Scholar]
- 8.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004; 8:392–402. [PubMed] [Google Scholar]
- 9.Abdel-Rahman SM, Kearns GL. Technical study report prepared for the World Health Organization (WHO). CPMT project 08-010. Pharmacokinetic analyses of fixed-dose drug combinations for pediatric tuberculosis. Geneva: WHO, 2009. [Google Scholar]
- 10.Abdel-Rahman SM, Kearns GL. Technical study report prepared for the World Health Organization (WHO). CPMT project 09-010. Pharmacokinetic simulations of a fixed-dose ethambutol formulation for pediatric tuberculosis. Geneva: WHO, 2009. [Google Scholar]
- 11.Goldman JL, Kearns GL, Abdel-Rahman SM. Pharmacological considerations of antitubercular agents in children. Prog Respir Res 2011; 40:161–75. [Google Scholar]
- 12.Shaddy RE, Denne SC. The Committee on Drugs and Committee of Pediatric Research. Guidelines for the ethical conduct of studies to evaluate drugs in pediatric populations. Pediatrics 2010; 125:850–60. [DOI] [PubMed] [Google Scholar]
- 13.Royal College of Paediatrics and Child Health (RCPCH) Ethics Advisory Committee. Guidelines for the ethical conduct of medical research involving children. Arch Dis Child 2000; 82:117–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Conference on Harmonization. Guidance on E11 clinical investigation of medicinal products in the pediatric population. Fed Regist 2000; 65:19777–81. [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services, Food and Drug Administration (FDA). Draft guidance for industry. General consideration for pediatric pharmacokinetic studies for drugs and biological products. Rockville, MD: FDA, 1998. [Google Scholar]
- 16.Abdel-Rahman SM, Wells T, Reed M, Kearns GL. Considerations in the rational design and conduct of pediatric clinical pharmacology trials: avoiding the problems and pitfalls. Clin Pharmacol Ther 2007; 81:483–94. [DOI] [PubMed] [Google Scholar]
- 17.Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet 2009; 24:25–36. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg G, Hattis D, Sonawane B, et al. Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci 2002; 66:185–200. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez W, Selen A, Avant D, et al. Improving Pediatric Dosing Through Pediatric Initiatives: What We Have Learned. Pediatrics 2008; 121:530–9. [DOI] [PubMed] [Google Scholar]
- 20.Notterman DA, Nardi M, Saslow JG. Effect of dose formulation on isoniazid absorption in two young children. Pediatrics 1986; 77:850–2. [PubMed] [Google Scholar]
- 21.Weiner M, Savic RM, Mac Kenzie WR, et al. Tuberculosis Trials Consortium PREVENT TB Pharmacokinetic Group. Rifapentine pharmacokinetics and tolerability in children and adults treated once weekly with rifapentine and isoniazid for latent tuberculosis infection. J Ped Infect Dis 2014; 3:132–45. [DOI] [PubMed] [Google Scholar]
- 22.Gorenberg H, White A. Off the pedestal and into the arena: toward including women in experimental protocols. Rev Law Soc Change 1991; 19:205–46. [PubMed] [Google Scholar]
- 23.National Institutes of Health Revitalization Act of 1993. Pub L No. 103-43, 107 Stat 122.
- 24.Berlin JA, Ellenberg SS. Inclusion of women in clinical trials. BMC Med 2009; 7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldkind SF, Sahin L, Gallauresi B. Enrolling pregnant women in research— lessons from the H1N1 influenza pandemic. N Engl J Med 2010; 362:2241–3. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services, Food and Drug Administration (FDA), Center for Drug Evaluation and Research. Guidance for industry. Pharmacokinetics in pregnancy—study design, data analysis and impact on dosing and labelling. Rockville, MD: FDA, 2004: 5. [Google Scholar]
- 27.Rodger MA, Makropoulos D, Walker M, Keely E, Karovitch A, Wells PS. Participation of Pregnant Women in Clinical Trials:Will They Participate and Why? Am J Perinatol 2003; 20:69–76. [DOI] [PubMed] [Google Scholar]
- 28.Mohanna K, Tunna K. Withholding consent to participate in clinical trials: decisions of pregnant women. Br J Obstet Gynaecol 1999; 106:892–7. [DOI] [PubMed] [Google Scholar]
- 29.Kass NE, Taylor HA, King PA. Harms of excluding pregnant women from clinical research: the case of HIV-infected pregnant women. J Law Med Ethics 1996; 24:36–46. [DOI] [PubMed] [Google Scholar]
- 30.Boulle A, Van Cutsem G, Cohen K, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA 2008; 300:530–9. [DOI] [PubMed] [Google Scholar]
- 31.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365:1471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Havlir DV, Kendall MA, Ive P, et al. AIDS Clinical Trials Group Study A5221. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365:1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis 2008; 8:516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIlleron H, Khoo SH. Interactions between antituberculosis and antiretroviral agents. In: Donald PR, Van Helden PD, eds. Progress in respiratory research: antituberculosis chemotherapy. Vol 40 Basel, Switzerland: Karger Publishing, 2011: 191–202. [Google Scholar]
- 36.Zhang C, Denti P, Decloedt E, et al. Model-based approach to dose optimization of lopinavir/ritonavir when co-administered with rifampicin. Br J Clin Pharmacol 2012; 73:758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ren Y, Nuttall JJ, Eley BS, et al. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr 2009; 50:439–43. [DOI] [PubMed] [Google Scholar]
- 38.Cohen K, Grant A, Dandara C, et al. Effect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516G>T polymorphism on efavirenz concentrations in adults in South Africa. Antivir Ther 2009; 14:687–95. [PMC free article] [PubMed] [Google Scholar]
- 39.Manosuthi W, Kiertiburanakul S, Sungkanuparph S, et al. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. AIDS 2006; 20:131–2. [DOI] [PubMed] [Google Scholar]
- 40.Ngaimisi E, Mugusi S, Minzi O, et al. Effect of rifampicin and CYP2B6 genotype on long-term efavirenz autoinduction and plasma exposure in HIV patients with or without tuberculosis. Clin Pharmacol Ther 2011; 90:406–13. [DOI] [PubMed] [Google Scholar]
- 41.McIlleron H, Ren Y, Nuttall J, et al. Lopinavir exposure is insufficient in children given double doses of lopinavir/ ritonavir during rifampicin-based treatment for tuberculosis. Antivir Ther 2011; 16:417–21. [DOI] [PubMed] [Google Scholar]
- 42.La Porte CJ, Colbers EP, Bertz R, et al. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother 2004; 48:1553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nijland HM, L'homme RF, Rongen GA, et al. High incidence of adverse events in healthy volunteers receiving rifampicin and adjusted doses of lopinavir/ritonavir tablets. AIDS 2008; 22:931–5. [DOI] [PubMed] [Google Scholar]
- 44.Haas DW, Koletar SL, Laughlin L, et al. ; A5213 StudyTeam. Hepatotoxicity and gastrointestinal intolerance when healthy volunteers taking rifampin add twice-daily atazanavir and ritonavir. J Acquir Immune Defic Syndr 2009; 50:290–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decloedt EH, McIlleron H, Smith P, Merry C, Orrell C, Maartens G. Pharmacokinetics of lopinavir in HIV-infected adults receiving rifampin with adjusted doses of lopinavir-ritonavir tablets. Antimicrob Agents Chemother 2011; 55:3195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frohoff C, Moodley M, Fairlie L, et al. Antiretroviral therapy outcomes in HIV-infected children after adjusting protease inhibitor dosing during tuberculosis treatment. PLoS One 2011; 6:e17273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davies G, Cerri S, Richeldi L. Rifabutin for treating pulmonary tuberculosis. Cochrane Database Syst Rev 2007: CD005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moultrie H, McIlleron H, Sawry S, et al. Pharmacokinetics and safety of rifabutin in young HIV-infected children receiving rifabutin and lopinavir/ritonavir. J Antimicrob Chemother 2014:pii:dku382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov 2002; 1:153–61. [DOI] [PubMed] [Google Scholar]
- 50.O'Donnell MR, Padayatchi N, Master I, Osburn G, Horsburgh CR. Improved early results for patients with extensively drug-resistant tuberculosis and HIV in South Africa. Int J Tuberc Lung Dis 2009; 13:855–61. [PMC free article] [PubMed] [Google Scholar]
- 51.Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, Satti H. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PLoS One 2009; 4:e7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pablos-Mendez A, Blustein J, Knirsch CA. The role of diabetes mellitus in the higher prevalence of tuberculosis among Hispanics. Am J Public Health 1997; 87:574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moutschen MP, Scheen AJ, Lefebvre PF. Impaired immune responses in diabetes mellitus: analysis of the factors and mechanisms involved. Relevance to the increased susceptibility of diabetic patients to specific infections. Diabete Metab 1992; 18:187–201. [PubMed] [Google Scholar]
- 55.Chang FY, Shaio MF. Decreased cell-mediated immunity in patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 1995; 28:137–46. [DOI] [PubMed] [Google Scholar]
- 56.Chang FY, Shaio MF. Respiratory burst activity of monocytes from patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 1995; 29:121–7. [DOI] [PubMed] [Google Scholar]
- 57.Saiki O, Negoro S, Tsuyuguchi I, Yamamura Y. Depressed immunological defence mechanisms in mice with experimentally induced diabetes. Infect Immun 1980; 28:127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banerjee S, Banerjee M. Diabetes and tuberculosis interface. J Indian Med Assoc 2005; 103:318., 320, 322 passim. [PubMed] [Google Scholar]
- 59.Wang CS, Yang CJ, Chen HC, et al. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol Infect 2009; 137:203–10. [DOI] [PubMed] [Google Scholar]
- 60.Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 2009; 80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 61.Morsy AM, Zaher HH, Hassan MH, Shouman A. Predictors of treatment failure among tuberculosis patients under DOTS strategy in Egypt. East Mediterr Health J 2003; 9:689–701. [PubMed] [Google Scholar]
- 62.Bashar M, Alcabes P, Rom WN, Condos R. Increased incidence of multidrug-resistant tuberculosis in diabetic patients on the Bellevue Chest Service, 1987 to 1997. Chest 2001; 120:1514–9. [DOI] [PubMed] [Google Scholar]
- 63.Fisher-Hoch SP, Whitney E, McCormick JB, et al. Type 2 diabetes and multidrug-resistant tuberculosis. Scand J Infect Dis 2008; 40:888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banu Rekha VV, Balasubramanian R, Swaminathan S, et al. Sputum conversion at the end of intensive phase of category-1 regimen in the treatment of pulmonary tuberculosis patients with diabetes mellitus or HIV infection: An analysis of risk factors. Indian J Med Res 2007; 126:452–8. [PubMed] [Google Scholar]
- 65.Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis 2006; 10:74–9. [PubMed] [Google Scholar]
- 66.Swai AB, McLarty DG, Mugusi F. Tuberculosis in diabetic patients in Tanzania. Trop Doct 1990; 20:147–50. [DOI] [PubMed] [Google Scholar]
- 67.Olmos P, Donoso J, Rojas N, et al. Tuberculosis and diabetes mellitus: a longitudinal-retrospective study in a teaching hospital. Rev Med Chil 1989; 117:979–83. [PubMed] [Google Scholar]
- 68.Leung CC, Lam TH, Chan WM, et al. Diabetic control and risk of tuberculosis: a cohort study. Am J Epidemiol 2008; 167:1486–94. [DOI] [PubMed] [Google Scholar]
- 69.Başoğlu OK, Bacakoğlu F, Cok G, Sayiner A, Ateş M. The oral glucose tolerance test in patients with respiratory infections. Monaldi Arch Chest Dis 1999; 54:307–10. [PubMed] [Google Scholar]
- 70.Oluboyo PO, Erasmus RT. The significance of glucose intolerance in pulmonary tuberculosis. Tubercle 1990; 71:135–8. [DOI] [PubMed] [Google Scholar]
- 71.Ruslami R, Van Crevel R. Diabetes mellitus and tuberculosis treatment. In: Donald PR, van Helden PD, eds. Antituberculosis chemotherapy. Vol 40 Basel, Switzerland: Karger Publishing, 2011:203–12. [Google Scholar]
- 72.World Health Organisation. Overweight and obesity. http://www.who.int/gho/ncd/risk_factors/overweight/en/index.htm Accessed 1 November 2011.
- 73.Samaras K. Metabolic consequences and therapeutic options in highly active antiretroviral therapy in human immunodeficiency virus-1 infection. J Antimicrob Chemother 2008; 61:238–45. [DOI] [PubMed] [Google Scholar]
- 74.Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 1999; 353:2093–9. [DOI] [PubMed] [Google Scholar]
- 75.Dagogo-Jack S. HIV therapy and diabetes risk. Diabetes Care 2008; 31:1267–8. [DOI] [PubMed] [Google Scholar]
- 76.Dave JA, Lambert EV, Badri M, West S, Maartens G, Levitt NS. Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV-infected patients. J Acquir Immune Defic Syndr 2011; 57:284–9. [DOI] [PubMed] [Google Scholar]
- 77.International Diabetes Federation. Diabetes: facts and figures. http://www.idf.org/worlddiabetesday/toolkit/gp/facts-figures Accessed 23 June 2014.
- 78.US Department of Health and Human Services, Food and Drug Administration (FDA). Guidance for industry. Pharmacokinetics in patients with impaired hepatic function: study design, data analysis, and impact on dosing and labeling. Rockville, MD: FDA, 2003. [Google Scholar]
- 79.US Department of Health and Human Services, Food and Drug Administration. Draft guidance for industry. Pharmacokinetics in patients with impaired renal function: study design, data analysis, and impact on dosing and labeling. Rockville, MD: FDA, 2010. [Google Scholar]
- 80.Kalow W, Tang BK, Endrenyi L. Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics 1998; 8:283–9. [DOI] [PubMed] [Google Scholar]
- 81.Ellard GA, Gammon PT. Pharmacokinetics of isoniazid metabolism in man. J Pharmacokinet Biopharm 1976; 4:83–113. [DOI] [PubMed] [Google Scholar]
- 82.Sun F, Chen Y, Xiang Y, Zhan S. Drug-metabolising enzyme polymorphisms and predisposition to anti-tuberculosis drug-induced liver injury: a meta-analysis. Int J Tuberc Lung Dis 2008; 12:994–1002. [PubMed] [Google Scholar]
- 83.Chigutsa E, Visser ME, Swart EC, et al. The SLCO1B1 rs4149032 Polymorphism Is Highly Prevalent in South Africans and Is Associated with Reduced Rifampin Concentrations: Dosing Implications. Antimicrob Agents Chemother 2011; 55:4122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weiner M, Peloquin C, Burman W, et al. Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob Agents Chemother 2010; 54:4192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hartkoorn RC, Kwan WS, Shallcross V, et al. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics 2010; 20:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martin P, Riley R, Back DJ, Owen A. Comparison of the induction profile for drug disposition proteins by typical nuclear receptor activators in human hepatic and intestinal cells. Br J Pharmacol 2008; 153:805–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lamba J, Lamba V, Strom S, Venkataramanan R, Schuetz E. Novel single nucleotide polymorphisms in the promoter and intron 1 of human pregnane X receptor/NR1I2 and their association with CYP3A4 expression. Drug Metab Dispos 2008; 36:169–81. [DOI] [PubMed] [Google Scholar]
- 88.Schipani A, Siccardi M, D'Avolio A, et al. Population pharmacokinetic modeling of the association between 63396C->T pregnane X receptor polymorphism and unboosted atazanavir clearance. Antimicrob Agents Chemother 2010; 54:5242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver--evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem 1997; 247:625–34. [DOI] [PubMed] [Google Scholar]
- 90.Vyhlidal CA, Gaedigk R, Leeder JS. Nuclear receptor expression in fetal and pediatric liver: correlation with CYP3A expression. Drug Metab Dispos 2006; 34:131–7. [DOI] [PubMed] [Google Scholar]
- 91.Zhu R, Kiser JJ, Seifart HI, et al. The pharmacogenetics of NAT2 enzyme maturation in perinatally HIV exposed infants receiving isoniazid. J Clin Pharmacol 2012; 52:511–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, Patrikeeva SL, Hankins GD, Ahmed MS. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol 2010; 79:921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dheenadhayalan V, Shanmugalakshmi S, Vani S, et al. Association of interleukin-10 cytokine expression status with HLA non-DRB1*02 and Mycobacterium bovis BCG scar-negative status in south Indian pulmonary tuberculosis patients. Infect Immun 2001; 69:5635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim HS, Park MH, Song EY, et al. Association of HLA-DR and HLA-DQ genes with susceptibility to pulmonary tuberculosis in Koreans: preliminary evidence of associations with drug resistance, disease severity, and disease recurrence. Hum Immunol 2005; 66:1074–81. [DOI] [PubMed] [Google Scholar]
- 95.Shi GL, Hu XL, Yang L, Rong CL, Guo YL, Song CX. Association of HLA-DRB alleles and pulmonary tuberculosis in North Chinese patients. Genet Mol Res 2011; 10:1331–6. [DOI] [PubMed] [Google Scholar]
- 96.Yuliwulandari R, Sachrowardi Q, Nakajima H, et al. Association of HLA-A, -B, and -DRB1 with pulmonary tuberculosis in western Javanese Indonesia. Hum Immunol 2010; 71:697–701. [DOI] [PubMed] [Google Scholar]
- 97.Denholm JT, McBryde ES, Eisen DP. Mannose-binding lectin and susceptibility to tuberculosis: a meta-analysis. Clin Exp Immunol 2010; 162:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol 1999; 163:3920–7. [PubMed] [Google Scholar]
- 99.Morahan G, Kaur G, Singh M, et al. Association of variants in the IL12B gene with leprosy and tuberculosis. Tissue Antigens 2007; 69(suppl 1):234–6. [DOI] [PubMed] [Google Scholar]
- 100.Shibasaki M, Yagi T, Yatsuya H, et al. An influence of Interferon-gamma gene polymorphisms on treatment response to tuberculosis in Japanese population. J Infect Dis 2009; 58:467–9. [DOI] [PubMed] [Google Scholar]
- 101.Xue Y, Zhao ZQ, Wang HJ, et al. Toll-like receptors 2 and 4 gene polymorphisms in a southeastern Chinese population with tuberculosis. Int J Immunogenet 2010; 37:135–8. [DOI] [PubMed] [Google Scholar]
- 102.Denholm JT, McBryde ES. P2X7 A1513 polymorphisms and tuberculosis susceptibility. Respirology 2012; 17:191. [DOI] [PubMed] [Google Scholar]
- 103.Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med 1996; 2:1240–3. [DOI] [PubMed] [Google Scholar]
- 104.Donald PR, Parkin DP, Seifart HI, et al. The influence of dose and N-acetyltransferase-2 (NAT2) genotype and phenotype on the pharmacokinetics and pharmacodynamics of isoniazid. Eur J Clin Pharmacol 2007; 63:633–9. [DOI] [PubMed] [Google Scholar]
- 105.Burns DK, Hughes AR, Power A, Wang SJ, Patterson SD. Designing pharmacogenomic studies to be fit for purpose. Pharmacogenomics 2010; 11:1657–67. [DOI] [PubMed] [Google Scholar]