Abstract
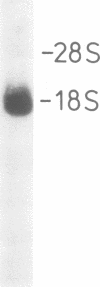
Immunological screening of a lambda gt11 library, constructed from HeLa mRNA, yielded several ribonuclease/angiogenin inhibitor (RAI) cDNA clones containing 900-bp inserts. Northern blot analysis revealed that the length of the RAI mRNA is approximately 1.9 kb. Construction and screening of a eukaryotic cDNA expression library (HeLa) containing preferentially complete cDNA inserts led to the isolation of a full length clone. The complete nucleotide sequence was determined. The C-terminal amino acid sequence deduced from the cDNA is identical to the peptide sequence obtained from a CNBr fragment of RAI, confirming the identity of the clone. The deduced primary structure of RAI consists of eight homologous tandem repeats with remarkable periodicity of leucine and cysteine residues. Each repeat is derived from the duplication of a leucine-rich 28-amino-acid module. This prototype module is closely related to a repetitive 24-amino-acid motif of unclear function, previously found in proteins involved in important biological processes such as blood coagulation, embryonic development, cell morphogenesis and signal transduction. Although homologous, the RAI modules show distinct differences in length and amino acid composition to the modules of this group of proteins, demonstrating their high potential of variability, necessary for adaptation to very diverse roles. Based on our results we propose that these repetitive modules are a common structural feature of a novel protein superfamily whose members exert their function by highly specific protein-protein interactions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn P. Ribonuclease inhibitor from human placenta: rapid purification and assay. J Biol Chem. 1979 Dec 25;254(24):12484–12487. [PubMed] [Google Scholar]
- Blackburn P., Wilson G., Moore S. Ribonuclease inhibitor from human placenta. Purification and properties. J Biol Chem. 1977 Aug 25;252(16):5904–5910. [PubMed] [Google Scholar]
- Brewer E. N., Foster L. B., Sells B. H. A possible role for ribonuclease in the regulation of protein synthesis in normal and hypophysectomized rats. J Biol Chem. 1969 Mar 25;244(6):1389–1392. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Day A. A., McQuillan C. I., Termine J. D., Young M. R. Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. Biochem J. 1987 Dec 15;248(3):801–805. doi: 10.1042/bj2480801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferencz A., Hidvégi E. J., Szabó L. D., Várterész V. The effect of whole-body x-irradiation of guinea pigs on the liver ribonuclease and ribonuclease-inhibitor system. Radiat Res. 1973 Aug;55(2):304–317. [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W., Willetts N. S. DNA sequence of the F traALE region that includes the gene for F pilin. J Bacteriol. 1984 Oct;160(1):395–401. doi: 10.1128/jb.160.1.395-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greif R. L., Eich E. F. Alkaline ribonuclease inhibitor in human thyroid. Metabolism. 1977 Aug;26(8):851–856. doi: 10.1016/0026-0495(77)90003-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Hudson K. L., Anderson K. V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988 Jan 29;52(2):269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Broek D., Wigler M. DNA sequence and characterization of the S. cerevisiae gene encoding adenylate cyclase. Cell. 1985 Dec;43(2 Pt 1):493–505. doi: 10.1016/0092-8674(85)90179-5. [DOI] [PubMed] [Google Scholar]
- Kershner R. M., Meyer W. L. A specific effect of glucocorticoids in decreasing neutral ribonuclease II in skeletal muscle. Biochem Biophys Res Commun. 1976 May 17;70(2):513–518. doi: 10.1016/0006-291x(76)91076-7. [DOI] [PubMed] [Google Scholar]
- Kraft N., Shortman K. A suggested control function for the animal tissue ribonuclease-ribonuclease inhibitor system, based on studies of isolated cells and phytohaemagglutinin-transformed lymphocytes. Biochim Biophys Acta. 1970 Sep 17;217(1):164–175. doi: 10.1016/0005-2787(70)90133-4. [DOI] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Little B. W., Meyer W. L. Ribonuclease-inhibitor system abnormality in dystrophic mouse skeletal muscle. Science. 1970 Nov 13;170(3959):747–749. doi: 10.1126/science.170.3959.747. [DOI] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Davie E. W., Roth G. J. The alpha and beta chains of human platelet glycoprotein Ib are both transmembrane proteins containing a leucine-rich amino acid sequence. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2135–2139. doi: 10.1073/pnas.85.7.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J. A., Chung D. W., Fujikawa K., Hagen F. S., Papayannopoulou T., Roth G. J. Cloning of the alpha chain of human platelet glycoprotein Ib: a transmembrane protein with homology to leucine-rich alpha 2-glycoprotein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5615–5619. doi: 10.1073/pnas.84.16.5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Patthy L. Detecting homology of distantly related proteins with consensus sequences. J Mol Biol. 1987 Dec 20;198(4):567–577. doi: 10.1016/0022-2836(87)90200-2. [DOI] [PubMed] [Google Scholar]
- Patthy L. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell. 1985 Jul;41(3):657–663. doi: 10.1016/s0092-8674(85)80046-5. [DOI] [PubMed] [Google Scholar]
- Quirin-Stricker C., Gross M., Mandel P. Influence d'une carence en protéines sur l'activité des ribonucleases et sur celle de leur inhibiteur dans le foie de rat. Biochim Biophys Acta. 1968 Apr 24;159(1):75–80. doi: 10.1016/0005-2744(68)90245-3. [DOI] [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. V. Studies on the properties and distribution of ribonuclease inhibitor in the rat. Biochim Biophys Acta. 1956 Jul;21(1):34–43. doi: 10.1016/0006-3002(56)90091-9. [DOI] [PubMed] [Google Scholar]
- Reinke R., Krantz D. E., Yen D., Zipursky S. L. Chaoptin, a cell surface glycoprotein required for Drosophila photoreceptor cell morphogenesis, contains a repeat motif found in yeast and human. Cell. 1988 Jan 29;52(2):291–301. doi: 10.1016/0092-8674(88)90518-1. [DOI] [PubMed] [Google Scholar]
- SHORTMAN K. Studies on cellular inhibitors of ribonuclease. III. The levels of ribonuclease and ribonucleases inhibitor during the regeneration of rat liver. Biochim Biophys Acta. 1962 Jul 9;61:50–55. doi: 10.1016/0926-6550(62)90028-2. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska E. M., Marotta C. A. Alzheimer's disease brain: alterations in RNA levels and in a ribonuclease-inhibitor complex. Science. 1984 Aug 31;225(4665):947–949. doi: 10.1126/science.6206567. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. K. The variation in the activities of hepatic ribonuclease and ribonuclease inhibitor in chicken livers as a function of age. Life Sci II. 1970 Jun 22;9(12):675–681. doi: 10.1016/0024-3205(70)90275-4. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Human placental ribonuclease inhibitor abolishes both angiogenic and ribonucleolytic activities of angiogenin. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2238–2241. doi: 10.1073/pnas.84.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1906–1910. doi: 10.1073/pnas.82.7.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Takio K., Handa M., Ruggeri Z. M. Amino acid sequence of the von Willebrand factor-binding domain of platelet membrane glycoprotein Ib. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5610–5614. doi: 10.1073/pnas.84.16.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Zillig W., Millette R. L., Schweiger M. Untersuchungen zur Biosynthese der Proteine. VII. Aktivität verschiedener Desoxyribonucleinsäuren und eines Ribonucleaseinhibitors aus Kaninchenreticulocyten in einem zellfreien Proteinsynthese-System aus Escherichia coli. DNA-abhängige in vitro-Synthese "früher Proteine" des E.-coli-Phagen T4. Hoppe Seylers Z Physiol Chem. 1965;343(4):261–275. [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Weiner L. H., Swain J. L. Tissue distribution and developmental expression of the messenger RNA encoding angiogenin. Science. 1987 Jul 17;237(4812):280–282. doi: 10.1126/science.2440105. [DOI] [PubMed] [Google Scholar]