Abstract
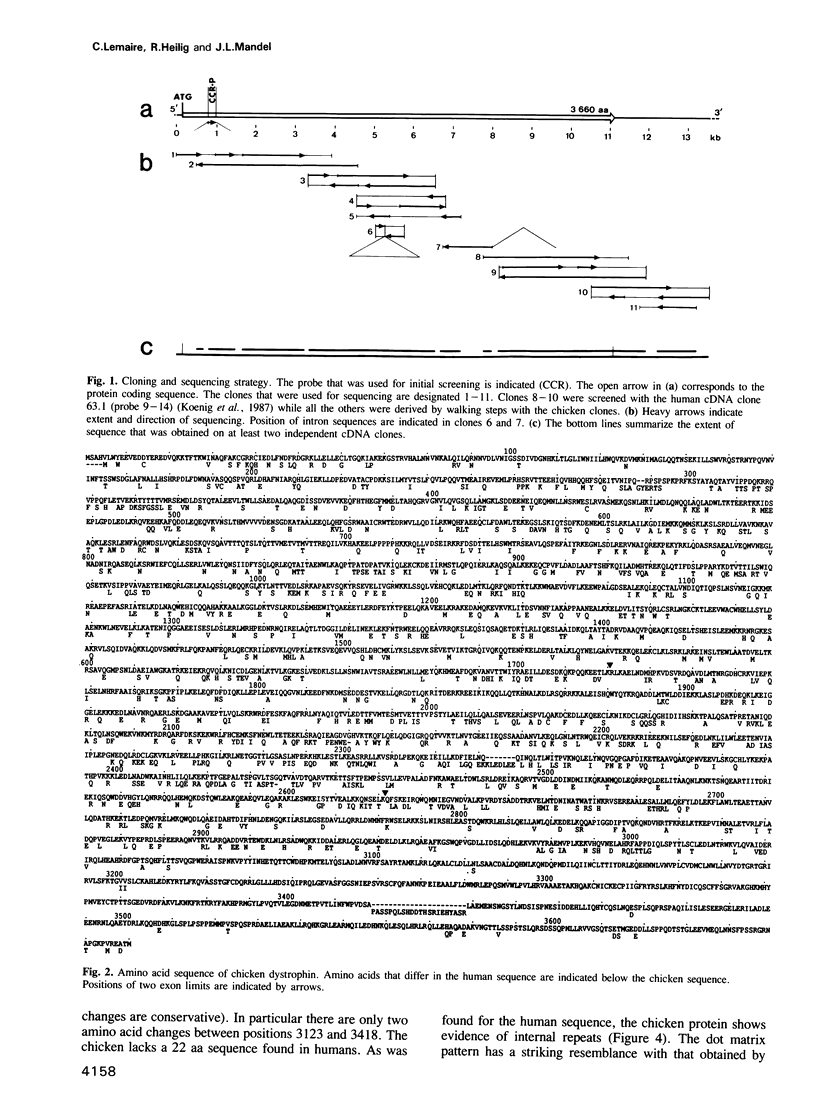
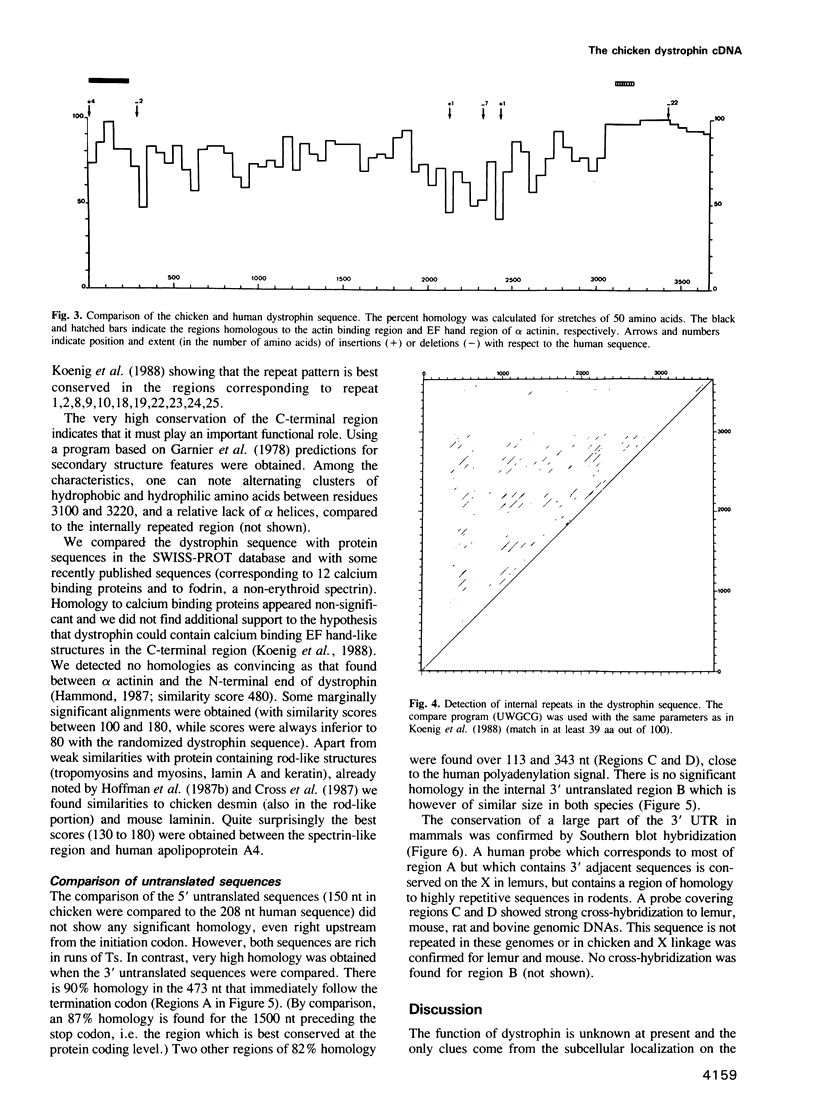
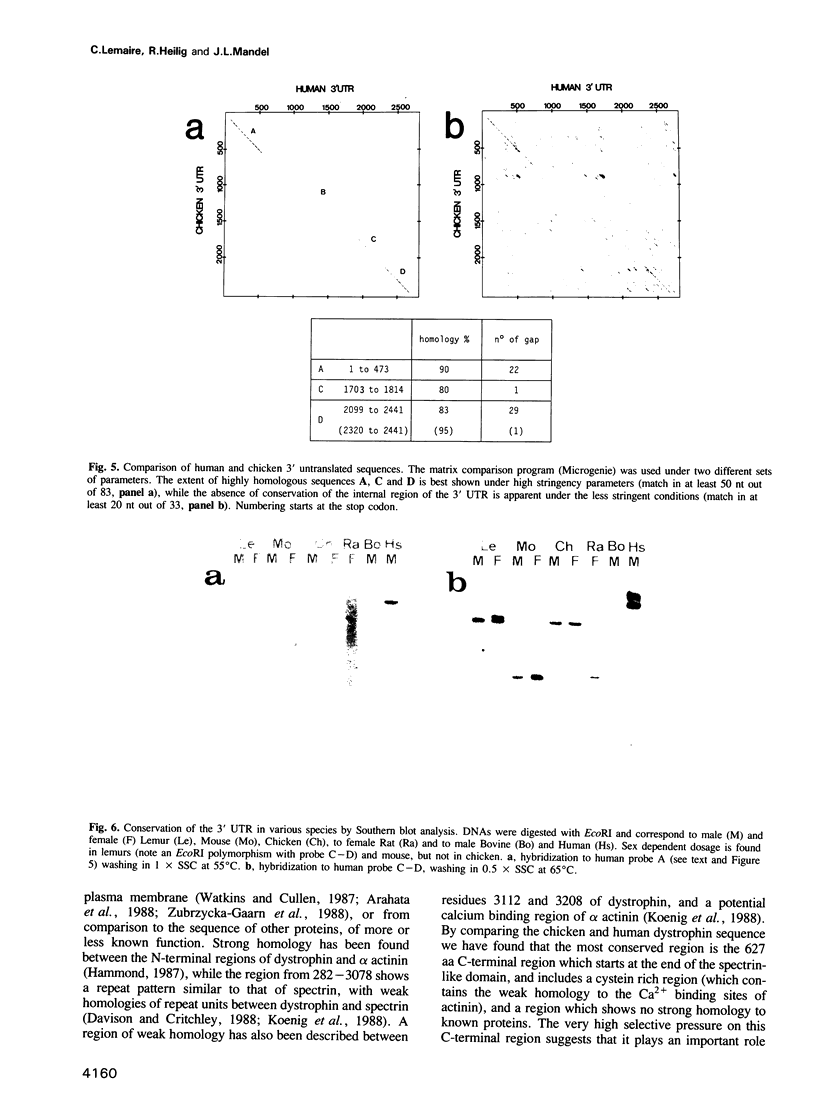
Dystrophin is a very large muscle protein (approximately 400 kd) the deficiency of which is responsible for Duchenne muscular dystrophy. Its function is unknown at present. In order to know whether different domains of the protein are differentially conserved during evolution, we have cloned and sequenced the chicken dystrophin cDNA. The protein coding sequence has almost the same size as in man. The N-terminal region that resembles the actin binding domain of alpha actinin, as well as the large spectrin like domain show 80% and 75% conservation respectively between chicken and man. In contrast, the C-terminal region shows 95% identity over 627 aa suggesting that it is an important region of interaction with other proteins. Comparison of the amino acid sequence of this C-terminal region to other protein sequences shows only marginally significant similarities. Finally we have found a striking conservation of three segments of the 3' untranslated sequence (85% homology over a total of 920 nt) between chicken and man. These also appear to be conserved in other mammals. This high conservation is not linked to open reading frames.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Burmeister M., Monaco A. P., Gillard E. F., van Ommen G. J., Affara N. A., Ferguson-Smith M. A., Kunkel L. M., Lehrach H. A 10-megabase physical map of human Xp21, including the Duchenne muscular dystrophy gene. Genomics. 1988 Apr;2(3):189–202. doi: 10.1016/0888-7543(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Cross G. S., Speer A., Rosenthal A., Forrest S. M., Smith T. J., Edwards Y., Flint T., Hill D., Davies K. E. Deletions of fetal and adult muscle cDNA in Duchenne and Becker muscular dystrophy patients. EMBO J. 1987 Nov;6(11):3277–3283. doi: 10.1002/j.1460-2075.1987.tb02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M. D., Critchley D. R. alpha-Actinins and the DMD protein contain spectrin-like repeats. Cell. 1988 Jan 29;52(2):159–160. doi: 10.1016/0092-8674(88)90503-x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hammonds R. G., Jr Protein sequence of DMD gene is related to actin-binding domain of alpha-actinin. Cell. 1987 Oct 9;51(1):1–1. doi: 10.1016/0092-8674(87)90002-x. [DOI] [PubMed] [Google Scholar]
- Heilig R., Lemaire C., Mandel J. L. A 230kb cosmid walk in the Duchenne muscular dystrophy gene: detection of a conserved sequence and of a possible deletion prone region. Nucleic Acids Res. 1987 Nov 25;15(22):9129–9142. doi: 10.1093/nar/15.22.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Monaco A. P., Feener C. C., Kunkel L. M. Conservation of the Duchenne muscular dystrophy gene in mice and humans. Science. 1987 Oct 16;238(4825):347–350. doi: 10.1126/science.3659917. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Lin H. C., Lei S. P., Wilcox G. An improved DNA sequencing strategy. Anal Biochem. 1985 May 15;147(1):114–119. doi: 10.1016/0003-2697(85)90016-8. [DOI] [PubMed] [Google Scholar]
- Molina M. I., Kropp K. E., Gulick J., Robbins J. The sequence of an embryonic myosin heavy chain gene and isolation of its corresponding cDNA. J Biol Chem. 1987 May 15;262(14):6478–6488. [PubMed] [Google Scholar]
- Munnich A., Daegelen D., Besmond C., Marie J., Dreyfus J. C., Kahn A. Cell-free translation of messenger RNAs from human muscle biopsies: a miniaturized tool for investigation of neuromuscular diseases. Pediatr Res. 1982 May;16(5):335–339. doi: 10.1203/00006450-198205000-00001. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Schönthal A., Angel P., Litfin M., Rüther U., Herrlich P. Posttranscriptional regulation of c-fos mRNA expression. Nucleic Acids Res. 1987 Feb 25;15(4):1643–1659. doi: 10.1093/nar/15.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Watkins S. C., Cullen M. J. A qualitative and quantitative study of the ultrastructure of regenerating muscle fibres in Duchenne muscular dystrophy and polymyositis. J Neurol Sci. 1987 Dec;82(1-3):181–192. doi: 10.1016/0022-510x(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]