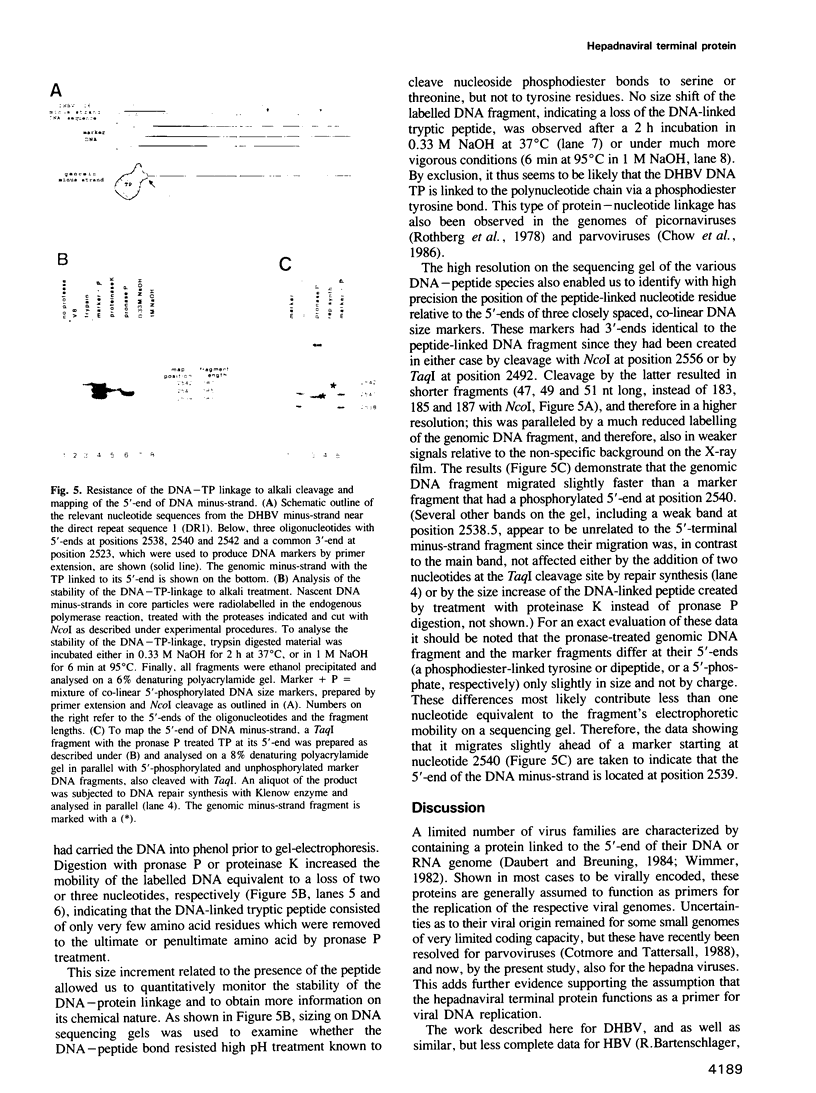
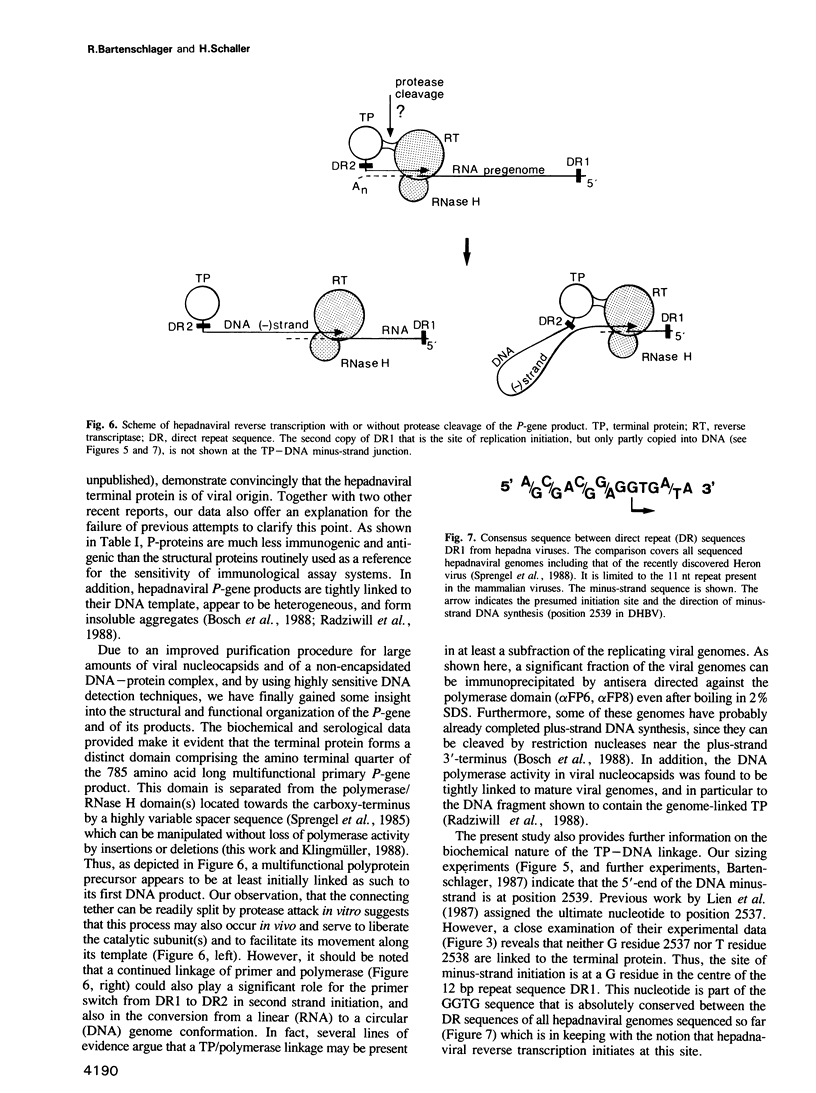
Abstract
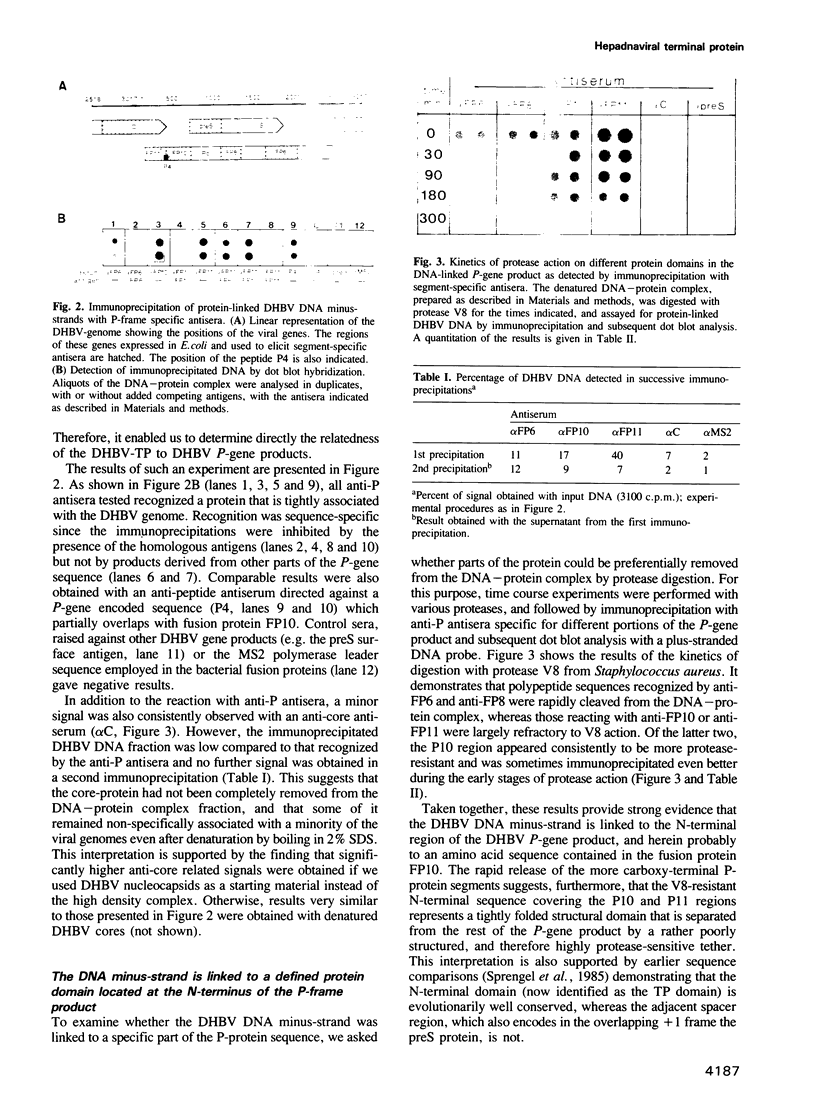
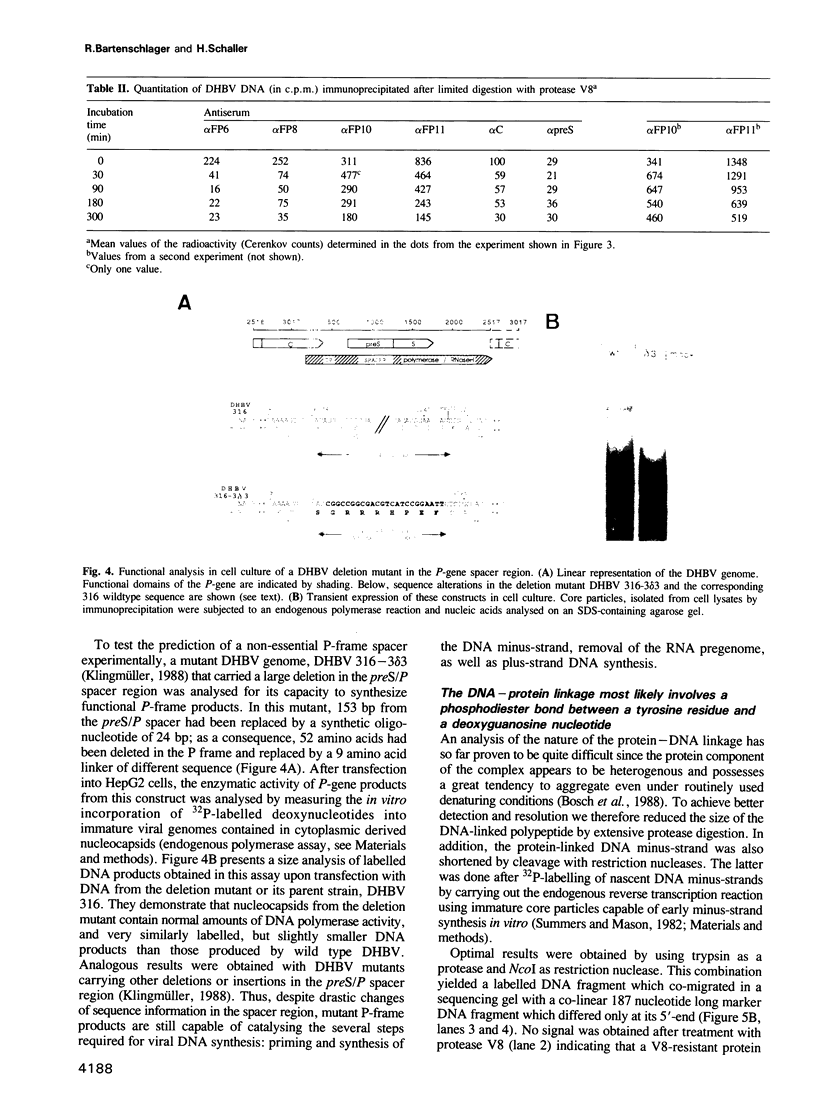
A series of antisera directed against amino acid sequences from different segments of the duck hepatitis B virus (DHBV) P-gene were shown to immunoprecipitate DHBV DNA molecules that were covalently linked to the DHBV DNA terminal protein. Restriction analysis and sizing after protease treatment demonstrated that the P-gene proteins were bound to the 5'-end of the DHBV DNA minus-strand which was mapped to a G-residue in the centre of the repeat sequence DR1. Resistance to alkali treatment indicated a phosphodiester linkage to tyrosine between protein and DNA. Limited protease treatment prior to immunoprecipitation cleaved C-terminal P-proteins from the viral DNA, indicating that the terminal protein forms a separate domain encoded in the N-terminal part of the P-gene. Functional analysis of a deletion mutant confirmed the notion that a non-essential spacer separates the terminal protein from the polymerase domain residing in the C-terminal half of the P-gene. Thus, the major proteins required for hepadnaviral reverse transcription, namely the primer, DNA polymerase, and possibly also RNase H, appear to be synthesized as a polyprotein precursor which is at least initially linked as such to its first DNA product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch V., Bartenschlager R., Radziwill G., Schaller H. The duck hepatitis B virus P-gene codes for protein strongly associated with the 5'-end of the viral DNA minus strand. Virology. 1988 Oct;166(2):475–485. doi: 10.1016/0042-6822(88)90518-1. [DOI] [PubMed] [Google Scholar]
- Büscher M., Reiser W., Will H., Schaller H. Transcripts and the putative RNA pregenome of duck hepatitis B virus: implications for reverse transcription. Cell. 1985 Mar;40(3):717–724. doi: 10.1016/0092-8674(85)90220-x. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Bodnar J. W., Polvino-Bodnar M., Ward D. C. Identification and characterization of a protein covalently bound to DNA of minute virus of mice. J Virol. 1986 Mar;57(3):1094–1104. doi: 10.1128/jvi.57.3.1094-1104.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5' termini of duplex replicative-form DNA and progeny single strands. J Virol. 1988 Mar;62(3):851–860. doi: 10.1128/jvi.62.3.851-860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galle P. R., Schlicht H. J., Fischer M., Schaller H. Production of infectious duck hepatitis B virus in a human hepatoma cell line. J Virol. 1988 May;62(5):1736–1740. doi: 10.1128/jvi.62.5.1736-1740.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Kaplan P. M., Greenman R. L., Gerin J. L., Purcell R. H., Robinson W. S. DNA polymerase associated with human hepatitis B antigen. J Virol. 1973 Nov;12(5):995–1005. doi: 10.1128/jvi.12.5.995-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien J. M., Petcu D. J., Aldrich C. E., Mason W. S. Initiation and termination of duck hepatitis B virus DNA synthesis during virus maturation. J Virol. 1987 Dec;61(12):3832–3840. doi: 10.1128/jvi.61.12.3832-3840.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Kimber K. L., Summers J., Taylor J. M., Mason W. S. Protein covalently bound to minus-strand DNA intermediates of duck hepatitis B virus. J Virol. 1983 Jan;45(1):165–172. doi: 10.1128/jvi.45.1.165-172.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwill G., Zentgraf H., Schaller H., Bosch V. The duck hepatitis B virus DNA polymerase is tightly associated with the viral core structure and unable to switch to an exogenous template. Virology. 1988 Mar;163(1):123–132. doi: 10.1016/0042-6822(88)90239-5. [DOI] [PubMed] [Google Scholar]
- Rothberg P. G., Harris T. J., Nomoto A., Wimmer E. O4-(5'-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Kuhn C., Guhr B., Mattaliano R. J., Schaller H. Biochemical and immunological characterization of the duck hepatitis B virus envelope proteins. J Virol. 1987 Jul;61(7):2280–2285. doi: 10.1128/jvi.61.7.2280-2285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Salfeld J., Schaller H. The duck hepatitis B virus pre-C region encodes a signal sequence which is essential for synthesis and secretion of processed core proteins but not for virus formation. J Virol. 1987 Dec;61(12):3701–3709. doi: 10.1128/jvi.61.12.3701-3709.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandau D. F., Lee C. H. trans-activation of viral enhancers by the hepatitis B virus X protein. J Virol. 1988 Feb;62(2):427–434. doi: 10.1128/jvi.62.2.427-434.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R., Kaleta E. F., Will H. Isolation and characterization of a hepatitis B virus endemic in herons. J Virol. 1988 Oct;62(10):3832–3839. doi: 10.1128/jvi.62.10.3832-3839.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengel R., Kuhn C., Will H., Schaller H. Comparative sequence analysis of duck and human hepatitis B virus genomes. J Med Virol. 1985 Apr;15(4):323–333. doi: 10.1002/jmv.1890150402. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]
- Zahm P., Hofschneider P. H., Koshy R. The HBV X-ORF encodes a transactivator: a potential factor in viral hepatocarcinogenesis. Oncogene. 1988 Aug;3(2):169–177. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]