Abstract
The overall mortality of hepatitis C virus (HCV)-infected patients has not been fully elucidated. The aim of this study was to analyze mortality in subjects positive for antibody to HCV (anti-HCV) in a community-based, prospective cohort study conducted in an HCV hyperendemic area of Japan. During a 10-year period beginning in 1995, 1,125 anti-HCV seropositive residents of Town C were enrolled into the study and followed for mortality through 2005. Cause of death was assessed by death certificates. Subjects with detectable HCV core antigen (HCVcAg) or HCV RNA were considered as having hepatitis C viremia and were classified as HCV carriers; subjects who were negative for both HCVcAg and HCV RNA (i.e., viremia-negative) were considered as having had a prior HCV infection and were classified as HCV noncarriers. Among the anti-HCV-positive subjects included in the analysis, 758 (67.4%) were HCV carriers, and 367 were noncarriers. A total of 231 deaths occurred in these subjects over a mean follow-up of 8.2 years: 176 deaths in the HCV carrier group and 55 in the noncarrier group. The overall mortality rate was higher in HCV carriers than in noncarriers, adjusted for age and gender (hazard ratio [HR], 1.53; 95% confidence interval [CI], 1.13–2.07). Although liver-related deaths occurred more frequently among the HCV carriers (HR, 5.94; 95% CI, 2.58–13.7), the rates of other causes of death did not differ between HCV carriers and noncarriers. Among HCV carriers, a higher level of HCVcAg (≥100 pg/ml) and persistently elevated alanine aminotransferase levels were important predictors of liver-related mortality.
Conclusions
The presence of viremia increases the rate of mortality, primarily due to liver-related death, among anti-HCV seropositive persons in Japan.
Keywords: HCV, cause of death, cohort study, hyperendemic area, hepatocellular carcinoma
INTRODUCTION
Hepatitis C virus (HCV) was identified 20 years ago. It is now known that between 50% and 85% of acute HCV infections become chronic1–3; after developing chronic infection, spontaneous HCV clearance is very rare. Approximately 170 million people worldwide are infected with HCV, and chronic HCV infection is a major health problem. HCV is a common cause of fatal liver disease, including liver cirrhosis and hepatocellular carcinoma (HCC). However, the liver-related mortality rate associated with chronic HCV infection is highly variable across different populations. In patients that have been infected for more than 20 years, the occurrence of liver cirrhosis, HCC, and liver disease-related mortality are reported to be 10–50%, 1–23%, and 4–15%, respectively4–6.
The range in published HCV-related mortality rates is related in part to the variability in the natural history of HCV infection as well as in the subjects studied. Some HCV-positive individuals have persistently normal alanine aminotransferase (ALT) levels and exhibit no clinical symptoms. Persons with this phenotype were often not included in previous hospital-based studies that focused on liver-related mortality in patients with HCV-associated liver disease/cirrhosis4–5. A few studies have systematically examined the risk of causes of death after HCV infection in a community-based setting7–9; however, the status of HCV viremia was not clear in these studies. In addition, the age range of HCV-infected subjects followed for mortality can vary considerably, with some cohort studies conducted in subjects whose average age was younger than 45 years1, 10–13 and others among older individuals9, 14, 15. To overcome some of these limitations, we analyzed mortality in 1,125 HCV-antibody-positive subjects with data on viremia status, who were enrolled in a population-based cohort study in an HCV hyperendemic area of Japan between 1995 and 2005. These subjects were followed prospectively until death or until the end of the study in December 2005.
MATERIALS AND METHODS
Study population
Since 1993, we have been following anti-HCV seropositive residents in a hyperendemic area (Town C) of Japan. The overall prevalence of anti-HCV positivity is higher (20.6%) in this region than in the surrounding area16. Town C is a small town in midwestern Miyazaki Prefecture, Japan, and the Town C HCV Study is a cohort study examining the natural course of HCV infection17–20. A general health examination program, begun in 1993, has been conducted annually for residents over 20 years of age. An ultrasonography-based liver disease screening program was initiated in 1994 to detect HCC in Town C residents who were identified as positive for anti-HCV. A total of 1,321 anti-HCV-positive residents were enrolled into the cohort from 1994 through the last liver disease screening in 2006. Informed consent was obtained from subjects at the time of enrollment. The study was approved by the human subjects committees of the Harvard School of Public Health, the University of Miyazaki Faculty of Medicine, the Boston University School of Public Health, and the Kagoshima University Graduate School of Medical and Dental Sciences.
The present analysis focuses on the 1,125 subjects with hepatitis C viremia data between 1995 and February of 2005, who were followed for mortality from the beginning of 1996 through the end of 2005. Anti-HCV seropositive subjects with detectable HCV core antigen (HCVcAg) or HCV RNA were considered to be persistently infected with HCV and were classified as HCV carriers. HCV antibody-positive subjects who were negative for HCVcAg and HCV RNA were assumed to have had a prior HCV infection and were classified as noncarriers. Subjects who underwent oral or intravenous administration of medical herbs or other palliative therapies or who had received interferon therapy were not excluded from the analyses. A sub-group analysis was conducted on HCV carrier subjects with at least three independent alanine aminotransferase (ALT) measurements obtained at an annual general health examination or liver disease screening; ALT levels greater than or equal to 35 were considered as abnormal.
Follow-up
For the present analysis, follow-up started at the date of first HCV viremia measurement (baseline) and ended at date of death or December 31, 2005. During the course of the study, 12 residents moved to other areas, and their follow-up time was censored at that point; no other subjects were lost to follow-up. Cause of death was based on the information from the death certificate and was classified into one of seven categories: HCC, liver disease excluding HCC, neoplasms excluding HCC (i.e., other neoplasms), stroke, heart disease, pulmonary disease excluding lung cancer, and other/unknown causes.
Laboratory methods
Serum anti-HCV antibodies were detected using second-generation enzyme immunoassay testing (Immunocheck F-HCV Ab, International Reagents Co., Kobe, Japan) or third-generation chemiluminescence enzyme immunoassays (Lumipulse Ortho II, Ortho-Clinical Diagnostics, Tokyo, Japan). In the anti-HCV-positive residents, serum levels of HCVcAg were tested with a fluorescence enzyme immunoassay (Immunocheck F-HCV Ag Core, International Reagents Co., Kobe, Japan)21, with a detection threshold of 8 pg/ml. The presence of HCV RNA was determined by reverse transcription-polymerase chain reaction (Amplicore HCV Monitor v1.0 [Nippon Roche, Tokyo, Japan] or v2.0 [Nippon Roche or Roche Diagnostics K.K., Tokyo, Japan]) in study subjects whose HCVcAg levels were below the detection threshold.
Serologically defined HCV genotype (HCV serotype) was determined with a serological genotyping assay kit (Immunocheck F-HCV Grouping, International Reagents Co., Tokyo, Japan). If the HCV serotype could not be determined, the HCV genotype was examined (HCV Core Genotype, SRL, Tokyo, Japan). HCV genotype 1b was included with serotype I, and genotypes 2a and 2b with serotype II. No other HCV genotype was detected in this study population.
Statistical analysis
One-factor ANOVA, χ2-tests, Fisher’s exact tests, and the Mann-Whitney U tests were used, when appropriate, for statistical comparisons of the baseline characteristics of the HCV carrier and noncarrier groups of subjects. Cox proportional hazards regression was used to obtain hazards ratios (HRs) and 95% confidence intervals (CIs) that were adjusted for age and gender; for the analyses of cause-specific mortality, subjects who died from a different cause were censored at the time of death. The cumulative incidence of death was analyzed by the Kaplan-Meier method, and differences in the survival curves were evaluated by the log-rank test. Statistical analyses were performed using Statistical Analysis System (version 9.1; SAS Institute, Cary, NC), STATVIEW (version 5.0; Abacus Concepts, Berkeley, CA), or SPSS (SPSS Inc., Chicago, IL) software programs. A P value less than 0.05 was considered to be statistically significant.
RESULTS
Demographic characteristics of study subjects
As shown in Table 1, 758 (67.4%) of the anti-HCV-positive subjects were HCV carriers (i.e., positive for HCVcAg or HCV RNA), with a mean age at enrollment of 64.9 years. The HCV noncarrier group, who were considered to have had a prior HCV infection, included 367 subjects whose mean age at enrollment was 62.6 years. On average, the HCV carriers were older and had higher levels of ALT and gamma-glutamyltransferase (γ-GTP) than the noncarriers, at baseline. In contrast, there were no significant differences between the two groups with respect to gender, alcohol intake, or history of blood transfusions. The number of subjects positive for hepatitis B surface antigen (HBsAg) was small and not significantly different between the two groups. Sixty-seven subjects reported that they had previously received interferon (IFN) therapy, all of whom were categorized as HCV carriers when they entered the study. Fifteen of these subjects were treated prior to entering the study, 5 were treated during the study, and 1 was treated both prior to and during the study; for the other 46 subjects, the timing of interferon treatment was unknown. Although the results of interferon therapy could not be fully determined for these 67 subjects, 41 of 44 with available data in 2005 were HCV RNA-positive at that time and only 3 (7%) were HCV RNA-negative.
Table 1.
Baseline Characteristics of Anti-HCV Antibody-Positive Subjects in Town C HCV Study
| Characteristics | All Patients (n = 1125) |
HCV Carriers (n = 758) |
HCV Noncarriers (n = 367) |
P Value |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (±SD) | 64.2 (±11.1) | 64.9 (±10.6) | 62.6 (±11.9) | 0.007 |
| Range | 28–97 | 32–97 | 28–90 | |
| Sex | ||||
| Male | 456 (40.5%) | 313 (41.3%) | 143 (39%) | 0.46 |
| Female | 669 (59.5%) | 445 (58.7%) | 224 (61%) | |
| ALT (IU/L) | 40 ± 42.8 (1062) | 47 ± 47.5 (719) | 25.3 ± 25 (343) | <0.001 |
| GGT (IU/L) | 35.8 ± 46 (912) | 39.1 ± 50.7 (612) | 29.2 ± 33.6 (300) | <0.001 |
| HCV core antigen level (pg/mL) | ||||
| Mean (±SD) | 207.5 (±208.4) | – | ||
| Median | 140 | – | ||
| Range | 20–1445 | – | ||
| HCV serotype | ||||
| I | 463 (64.5%) | – | ||
| II | 220 (30.6%) | – | ||
| Indeterminate | 35 (4.9%) | – | ||
| HBs antigen | ||||
| Positive | 6 (0.6%) | 4 (0.6%) | 2 (0.6%) | 0.99 |
| Negative | 948 (99.4%) | 638 (99.4%) | 310 (99.4%) | |
| History of alcohol intake | ||||
| Daily | 365 (34.3%) | 236 (32.9%) | 129 (37.2%) | |
| Occasionally | 206 (19.4%) | 140 (19.5%) | 66 (19.0%) | 0.37 |
| None | 493 (46.3%) | 341 (47.6%) | 152 (43.8%) | |
| History of blood transfusion | ||||
| Yes | 165 (15.7%) | 101 (14.3%) | 64 (18.6%) | 0.07 |
| No | 885 (84.3%) | 605 (85.7%) | 280 (81.4%) |
Abbreviations: ALT, alanine aminotransferase; GGT, gamma-glutamyltranspeptidase; HBs antigen, hepatitis B surface antigen; HCV, hepatitis C virus.
Overall and cause-specific mortality
Over an average of 8.2 years of follow-up, 231 deaths occurred among the 1,125 subjects (Table 2). The overall mortality rate was 25.0 per 1,000 person-years in this study population. Most deaths were liver-related, with 45 due to HCC and 31 to other liver diseases including cirrhosis, hepatic failure, and ruptured esophageal varix. The next most frequent cause of death was other neoplasms (n=41), followed by pulmonary disease excluding lung cancer (n=32), stroke (n=30), other/unknown causes (n=30), and heart disease (n=22).
Table 2.
Cause of Death in Subjects Positive for Anti-HCV Antibody
| Cause of Death | All Patients | HCV Carriers | HCV Noncarriers |
|---|---|---|---|
| All causes | 231 | 176 | 55 |
| 1. All liver-related deaths | 76 | 70 | 6 |
| a. HCC | 45 | 41 | 4 |
| b. Non-HCC | 31 | 29 | 2 |
| 2. Neoplasms excluding HCC | 41 | 28 | 13 |
| 3. Stroke | 30 | 20 | 10 |
| 4. Heart disease | 22 | 13 | 9 |
| 5. Pulmonary disease excluding lung cancer | 32 | 22 | 10 |
| 6. Other/unknown | 30 | 23 | 7 |
Abbreviations: HCC, hepatocellular carcinoma; HCV, hepatitis C virus.
Of the 231 deaths, 176 were in the HCV carrier group, and 55 in the noncarrier group (Table 2). After adjusting for age and gender, HCV carriers had a significantly higher overall mortality rate (HR, 1.53; 95% CI, 1.13–2.07), compared to noncarriers (Table 3). The elevated mortality rate among the subjects with evidence of HCV viremia was due to a much higher occurrence of liver-related deaths (HR, 5.94; 95% CI, 2.58–13.7). In contrast, HCV viremia was not significantly associated with death from other malignancies, stroke, heart disease, or pulmonary disease. The cumulative risk of death, based on Kaplan-Meier estimates, was 28.0% for the HCV carrier group and 17.9% for the HCV noncarrier group over 10.3 years (Figure 1), a statistically significant difference (P<0.001).
Table 3.
The Association of HCV Viremia with Causes of Mortality Among Anti-HCV Antibody-Positive Subjects in Town C HCV Study
| Cause of Death | HR | 95% CI |
|---|---|---|
| All causes | 1.53 | (1.13, 2.07) |
| 1. All liver-related deaths | 5.94 | (2.58, 13.7) |
| a. HCC | 4.85 | (1.73, 13.5) |
| b. Non-HCC | 8.11 | (1.94, 33.8) |
| 2. Neoplasms excluding HCC | 1.04 | (0.54, 2.02) |
| 3. Stroke | 0.89 | (0.41, 1.90) |
| 4. Heart disease | 0.68 | (0.29, 1.60) |
| 5. Pulmonary disease excluding lung cancer | 1.05 | (0.50, 2.22) |
| 6. Other/unknown | 1.59 | (0.68, 3.71) |
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio.
Figure 1.
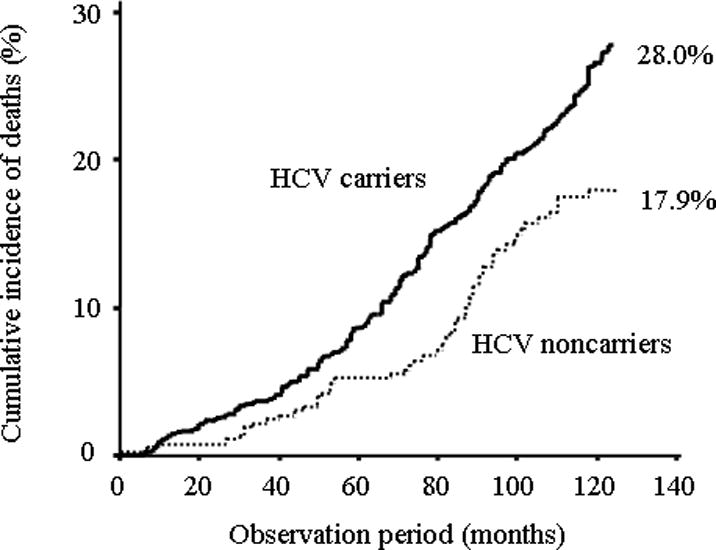
Cumulative incidence of all-cause deaths in HCV carriers and noncarriers.
Predictors of mortality among HCV carriers
The age- and gender-adjusted association between HCV serotype and HCVcAg level and mortality was examined among the subjects with HCV viremia. Compared to HCV serotype II, those with serotype I infection did not have a higher rate of overall (HR, 1.04) or liver-related mortality (HR, 1.12); however, having an indeterminate HCV serotype was related to both overall (HR, 3.59; 95% CI, 2.1–6.1) and liver-related death (HR, 2.12; 95% CI, 0.78–5.75). Of note, both serotype I infection (HR, 2.21; 95% CI, 0.91–5.33) and indeterminate HCV infection (HR, 3.89; 95% CI, 0.97–15.7) appeared to increase HCC mortality. In addition, a significant increased rate of liver-related death was associated with a higher level (≥100 pg/ml) of HCVcAg (HR, 1.81; 95% CI, 1.08–3.06); the effect of higher HCVcAg level was stronger with respect to other liver-related death (HR, 2.58; 95% CI, 1.04–6.41) than to HCC death (HR, 1.48; 95% CI, 0.77–2.82). HCVcAg level had no effect on overall mortality among the HCV carriers (HR, 1.06).
In a sub-group analysis of 719 HCV carrier subjects who had data for at least three separate ALT measurements, 173 had persistently normal ALT levels while 141 had persistently abnormal levels. Subjects whose ALT levels fluctuated were not included in the analysis. Adjusting for age and gender, overall mortality (HR, 2.23; 95% CI, 1.37–3.61) and liver-related death (HR, 11.0; 95% CI, 4.35–27.9) were significantly higher for HCV carriers with persistently elevated ALT than for those with persistently normal ALT. The strongly elevated rate of liver-related mortality was evident for death due to both HCC (HR, 11.1) as well as other liver-related disease (HR, 14.5).
DISCUSSION
Our study indicated that liver-related mortality is strongly associated with the presence of HCV viremia among persons who are seropositive for anti-HCV antibodies and that HCVcAg and ALT levels were predictors of liver-related mortality in HCV carriers. In this study population, the age distribution of anti-HCV-positive subjects, the prevalence of viremia, and the frequency of HCV serotype I were similar to previously reported data in Japan22–25. Japan has the highest incidence rate of HCC attributed to HCV infection among developed countries. Tanaka et al. estimated that HCV infection was spread in Japan during the 1920s, whereas HCV was widely disseminated in the United States in the 1960s26. The authors suggested that the HCC burden in the United States will likely increase in the next two or three decades, possibly to a level equal to that currently experienced in Japan.
Several studies have examined mortality in patients with HCV. Seeff et al provided mortality data for 222 transfusion-associated hepatitis C cases and 377 control patients after approximately 25 years of follow-up27. Kamitsukasa et al also reported mortality data for 302 HCV-infected patients with tuberculosis sequelae who had received a blood transfusion15. Although both studies showed that liver-related mortality was significantly higher in the disease groups than in the control groups, liver-related mortality was not the main cause of death. Kamitsukasa et al. reported that the main cause of death for approximately 45% of the patients in their study was tuberculosis sequelae15. Similar results were obtained in patients with inherited bleeding disorders and hepatitis C, where the main cause of death was HIV/AIDS28. Moreover, there was no significant difference between patients with and without hepatitis C in the overall mortality rates in the study by Seeff et al. In contrast, our study showed that all-cause mortality and liver-related mortality with or without HCC were significantly higher in the HCV carrier group than in the noncarrier group. The incidence of HCC in Caucasian patients with HCV-related cirrhosis has been reported to be 1.2% in the United States29, whereas the incidence in Japanese patients is reportedly between 6 and 7%30. Furthermore, HCV-related cases in some studies included subjects with previous HCV infections15, 27. Ethnic- and racial-dependent variation in the rates of HCC, the composition of the comparisons groups, and/or complications unrelated to liver disease, such as tuberculosis sequelae or HIV/AIDS, may have resulted in differences in the patient prognoses between our study and previous studies.
It has been reported that HCC was the main cause of liver-related death in patients with compensated cirrhosis due to HCV infection31, 32. Kasahara et al. found that 81% of HCC of liver-related death in patients with chronic hepatitis C who had not received interferon therapy was due to HCC33. Although HCC was more frequently observed than other liver-related deaths in our study, the proportion of HCC among all liver disease deaths (64% in the HCV carrier group) was relatively low compared to that study33. This occurrence may have been because the causes of death were obtained from death certificates in our study and cases of severe hepatic failure due to HCC may have been classified as liver disease excluding HCC.
A large community-based linkage study that included 78,438 people with hepatitis C indicated that the risk of dying from drug-related causes was significantly greater than from liver-related causes; however, the incidence of liver-related deaths was greater than that of drug-related deaths in patients older than 45 years7. In addition, other studies have shown that age appears to be an important risk factor that affects HCC development14 and that the risk of cirrhosis is related to the patient’s age at the time of infection and to disease activity34, 35. These reports, which focused on patients with transfusion-associated chronic hepatitis C, suggest that the younger the patients are at the time of infection, the lower the rate of progression. Although the exact dates of infection and HCC diagnosis were not clear in our study population, the median age at enrollment was older than 60 years. Thus, the incidence of liver-related deaths might be expected to be greater than that of other cause of deaths.
In our study, HCV serotype I, which included HCV genotypes 1a and 1b, was found in 64.5% of the HCV carrier subjects in whom serotype was measured; whereas serotype 2, which included genotypes 2a and 2b, was detected in 30.6% of patients. These results agree with the overall distributions of HCV genotypes and serotypes in the entire Japanese population, which show that genotype 1b is the most prevalent genotype at 70%36. Several studies have demonstrated that genotype 1b is associated with severe liver disease, including cirrhosis and HCC37, 38. In the current study, there was an apparent association between HCV serotype I infection and mortality due to HCC. Other studies, however, have not found an effect of HCV genotype on liver disease development39, 40. In addition, although an association of indeterminate serotype with mortality was observed (HR=3.6), the reason for this finding is not clear. A larger study is needed to elucidate the role of genotype in the prognosis of HCV infection.
HCV RNA levels have also been reported to be associated with the progression of chronic hepatitis C41, 42. Although the level of HCV RNA was not quantified in the current study, HCVcAg levels, which are known to correlate with HCV RNA levels21, were assessed by fluorescence enzyme immunoassay. We observed that high HCVcAg levels were predictive of liver-related mortality, including death due to HCC, in the HCV carriers. The precise mechanism underlying HCV infection-dependent hepatocarcinogenesis is not clear. However, a study of transgenic mice that express the HCV core protein demonstrated that this protein was important in HCC development43. Of interest, Moucari et al. reported that insulin resistance is a specific feature of chronic hepatitis C and associated with high serum HCV RNA levels44. A significant increase in the incidence of diabetes also has been seen in subjects with high titer of HCV core compared to anti-HCV-negative subjects45. Moreover, significant fibrosis is associated with insulin resistance44, and diabetes mellitus is known to increase the risk of primary liver cancer in the presence of other risk factors such as hepatitis C46. Thus, HCVcAg levels might be associated with liver-related mortality through the development of HCV-induced insulin resistance or diabetes mellitus.
We have previously shown that elevated ALT levels are an important predictor of HCC among HCV carriers in this study population19. In the current analysis, ALT, AST, and γ-GTP levels at enrollment were significantly higher in subjects who died due to a liver-related disease compared with subjects who died from other causes (data not shown). In addition, after adjusting for age and gender, overall mortality (HR, 2.23) and liver-related death (HR, 11.0) were significantly higher for HCV carriers with persistently elevated ALT than for those with persistently normal ALT.
Our study had several limitations. First, data regarding liver histology were lacking. It is likely that HCV carriers had more cirrhosis than did HCV noncarriers, given that more HCV carriers died of HCC and non-HCC liver deaths (Table 2). However, we were unable to examine this possibility directly. Information on platelet counts, which are generally inversely correlated with hepatic fibrosis, was available for a subset of subjects. Based on data obtained in 1996, mean platelet counts were significantly lower in HCV carriers (n=539; 18.4 [×104/μl] ±5.6) than in HCV noncarriers (n=277; 21.3±6.0). In addition, data from the last examination attended after 2001 showed that the PAALT group had lower mean platelet counts (n=94; 14.5 [×104/μl] ±5.5) than did the PNALT group (n=123; 21.8±7.3). These findings suggest that the presence of viremia may increase the rate of hepatic fibrosis, especially in HCV carriers with high ALT levels.
Second, although the effect of interferon therapy may have implications with respect to the overall death rates in the study population, information on treatment was limited. However, the proportion of treated subjects with an observed sustained viral response to interferon was small (7%). Data on socioeconomic factors, which are strongly related to mortality outcomes47, also were not available in this study. We would not expect much variation in socioeconomic status in the study population, since the cohort included only Japanese subjects who resided in a small rural community where farming is the principal occupation. In addition, all subjects in the study population had health insurance. Thus, we believe that socioeconomic factors and interferon therapy likely did not greatly affect the rate of mortality in our study population.
In conclusion, the results of this prospective 10-year follow-up study showed a strong effect of HCV carrier status on liver-related mortality among anti-HCV seropositive individuals. Moreover, high HCVcAg and ALT levels were important predictors of liver-related death in this population. Monitoring HCV load and ALT level in HCV carriers may be important for identifying those individuals at increased risk for HCC or other liver disease, particularly among older carriers who are less likely to respond to HCV treatment.
Acknowledgments
Grant support and other assistance. This work was supported by a grant (No. CA87982) from the United States National Institutes of Health, a grant-in-aid (Research on Hepatitis and BSE) from the Ministry of Health, Labour and Welfare of Japan, and a grant from the Miyazaki Prefecture Collaboration of Regional Entities for the Advancement of Technological Excellence (Japan Science and Technology Corporation). We thank Keiko Toyama, Yuriko Kuwabara, and Erika Edwards for their technical assistance.
Abbreviations-List
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- HCVcAg
hepatitis C virus core antigen
- ALT
alanine aminotransferase
Footnotes
Financial Disclosures: All authors disclose no financial arrangements.
References
- 1.Kenny-Walsh E, for the Irish hepatology research group Clinical outcomes after hepatitis C infection from contaminated Anti-D immune globulin. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 2.Paraná R, Vitvitski L, Andrade Z, Trepo C, Cotrim H, Bertillon P, et al. Acute sporadic Non-A Non-B hepatitis in Northeastern Brazil: Etiology and natural history. Hepatology. 1999;30:289–293. doi: 10.1002/hep.510300143. [DOI] [PubMed] [Google Scholar]
- 3.Alberti A, Chemello L, Benvegnu L. Natural history of hepatitis C. J Hepatol. 1999;31(Suppl.1):17–24. doi: 10.1016/s0168-8278(99)80369-9. [DOI] [PubMed] [Google Scholar]
- 4.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 5.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 6.Casiraghi MA, De Paschale M, Romanò L, Biffi R, Assi A, Binelli G, Zanetti AR. Long-term outcomes (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology. 2004;39:90–96. doi: 10.1002/hep.20030. [DOI] [PubMed] [Google Scholar]
- 7.Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. Cause of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet. 2006;368:938–945. doi: 10.1016/S0140-6736(06)69374-4. [DOI] [PubMed] [Google Scholar]
- 8.Guiltinan AM, Kaidarova Z, Custer B, Orland J, Strollo A, Cyrus S, et al. Increased all-cause, liver, and cardiac mortality among hepatitis C virus-seropositive blood donors. Am J Epidemiol. 2008;167:743–750. doi: 10.1093/aje/kwm370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boschi-Pinto C, Stuver S, Okayama A, et al. A follow-up study of morbidity and mortality associated with hepatitis C virus infection and its interaction with human T lymphotropic virus type I in Miyazaki, Japan. J Infect Dis. 2000;181:35–41. doi: 10.1086/315177. [DOI] [PubMed] [Google Scholar]
- 10.Vogt M, Lang T, Frösner G, Klingler C, Sendl AF, Zeller A, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999;341:866–870. doi: 10.1056/NEJM199909163411202. [DOI] [PubMed] [Google Scholar]
- 11.Rodger AJ, Roberts S, Lanigan A, Bowden S, Brown T, Crofts N. Assessment of long-term outcomes of community-acquired hepatitis C in a cohort with sera stored from 1971 to 1975. Hepatology. 2000;32:582–587. doi: 10.1053/jhep.2000.9714. [DOI] [PubMed] [Google Scholar]
- 12.Wiese M, Berr F, Lafrenz M, Porst H, Oesen U. Low frequency of cirrhosis in a hepatitis C (Genotype 1b) single-source outbreak in Germany: A 20-year multicenter study. Hepatology. 2000;32:91–96. doi: 10.1053/jhep.2000.8169. [DOI] [PubMed] [Google Scholar]
- 13.Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, et al. The natural history of hepatitis C virus infection: Host, viral, and environmental factors, The natural history of hepatitis C virus infection. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 14.Hamada H, Yatsuhashi H, Yano K, Daikoku M, Arisawa K, Inoue O, et al. Impact of aging on the development of hepatocellular carcinoma in patients with posttransfusion chronic hepatitis C. Cancer. 2002;95:331–9. doi: 10.1002/cncr.10662. [DOI] [PubMed] [Google Scholar]
- 15.Kamitsukasa H, Harada H, Tanaka H, Yagura M, Tokita H, Ohbayashi A. Late liver-related mortality from complications of transfusion-acquired hepatitis C. Hepatology. 2005;41:819–825. doi: 10.1002/hep.20648. [DOI] [PubMed] [Google Scholar]
- 16.Uto H, Hayashi K, Kusumoto K, Hasuike S, Nagata K, Kodama M, et al. Spontaneous elimination of hepatitis C virus RNA in individuals with persistent infection in a hyperendemic area of Japan. Hepatol Res. 2006;34:28–34. doi: 10.1016/j.hepres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K, Hasuike S, Kusumoto K, Ido A, Uto H, Kenji N, et al. Usefulness of a new immuno-radiometric assay to detect hepatitis C core antigen in a community-based population. J Viral Hepat. 2005;12:106–110. doi: 10.1111/j.1365-2893.2005.00558.x. [DOI] [PubMed] [Google Scholar]
- 18.Kusumoto K, Uto H, Hayashi K, Takahama Y, Nakao H, Suruki R, et al. Interleukin-10 or tumor necrosis factor-alpha polymorphisms and the natural course of hepatitis C virus infection in a hyperendemic area of Japan. Cytokine. 2006;34:24–31. doi: 10.1016/j.cyto.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Suruki R, Hayashi K, Kusumoto K, Uto H, Ido A, Tsubouchi H, Stuver SO. Alanine aminotransferase level as a predictor of hepatitis C virus-associated hepatocellular carcinoma incidence in a community-based population in Japan. Int J Cancer. 2006;119:192–195. doi: 10.1002/ijc.21796. [DOI] [PubMed] [Google Scholar]
- 20.Kashiwakuma T, Hasegawa A, Kajita T, Takata A, Mori H, Ohta Y, et al. Detection of hepatitis C virus specific core protein in serum of patients by a sensitive fluorescence enzyme immunoassay (FEIA) J Immunol Methods. 1996;190:79–89. doi: 10.1016/0022-1759(95)00261-8. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka T, Lau JY, Mizokami M, Orito E, Tanaka E, Kiyosawa K, et al. Simple fluorescent enzyme immunoassay for detection and quantification of pepatitis C viremia. J Hepatol. 1995;23:742–745. doi: 10.1016/0168-8278(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 22.Ohkoshi S, Tawaraya H, Kuwana K, Harada T, Watanabe M, Higuchi S, et al. A retrospective study of hepatitis C virus carriers in a local endemic town in Japan. A possible presence of asymptomatic carrier. Dig Dis Sci. 1995;40:465–71. doi: 10.1007/BF02065436. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi S, Sata M, Suzuki H, Mizokami M, Tanikawa K. Routes of transmission of hepatitis C virus in an endemic rural area of Japan. Molecular epidemiologic study of hepatitis C virus infection. Scand J Infect Dis. 1997;29:23–8. doi: 10.3109/00365549709008659. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi J, Kishihara Y, Yamaji K, Furusyo N, Yamamoto T, Pae Y, et al. Hepatitis C viral quasispecies and liver damage in patients with chronic hepatitis C virus infection. Hepatology. 1997;25:697–701. doi: 10.1002/hep.510250334. [DOI] [PubMed] [Google Scholar]
- 25.Koyama T, Tsuda F, Ishikawa K, Oishi H, Tazawa M, Yoshizawa H, et al. Antibodies to hepatitis C virus and elevated transaminase levels in a town of hyperendemicity in Iwate, Japan. J Gastroenterol Hepatol. 1997;12:67–72. doi: 10.1111/j.1440-1746.1997.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka Y, Kurbanov F, Mano S, Orito E, Vargas V, Esteban JI, et al. Molecular tracing of the global hepatitis C virus epidemic predicts regional patterns of hepatocellular carcinoma mortality. Gastroenterology. 2006;130:703–714. doi: 10.1053/j.gastro.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 27.Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, et al. Long-term mortality and morbidity of transfusion-associated Non-A, Non-B, and Type C hepatitits: A national heart, lung, and blood institute collaborative study. Hepatology. 2001;33:455–463. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 28.Posthouwer D, Makris M, Yee TT, Fischer K, van Veen JJ, Griffioen A, et al. Progression to end-stage liver disease in patients with inherited bleeding disorders and hepatitis C: an international, multicenter cohort study. Blood. 2007;109:3667–3671. doi: 10.1182/blood-2006-08-038349. [DOI] [PubMed] [Google Scholar]
- 29.Colombo M, Franchis RD, Ninno ED. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 30.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 31.Degos F, Christidis C, Ganne-Carrie N, Farmachidi JP, Degott C, Guettier C, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut. 2000;47:131–136. doi: 10.1136/gut.47.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 33.Kasahara A, Tanaka H, Okanoue T, Imai Y, Tsubouchi H, Yoshioka K, et al. Interferon treatment improves survival in chronic hepatitis C patients showing biochemical as well as virological responses by preventing liver-related death. J Viral Hepat. 2004;11:148–156. doi: 10.1046/j.1365-2893.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 34.Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, et al. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C, The American Society of Hepatoligy. Blood. 2002;99:4588–4591. doi: 10.1182/blood-2001-12-0192. [DOI] [PubMed] [Google Scholar]
- 35.Poynard T, Bedossa P, Opolon P, et al. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto H, Mishiro S. Genetic heterogeneity of hepatitis C virus. Intervirology. 1994;37:68–76. doi: 10.1159/000150360. [DOI] [PubMed] [Google Scholar]
- 37.Silini E, Bottelli R, Asti M, Bruno S, Candusso ME, Brambilla S, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: A case-control study. Gastroenterology. 1996;111:199–205. doi: 10.1053/gast.1996.v111.pm8698200. [DOI] [PubMed] [Google Scholar]
- 38.Pozzato G, Kaneko S, Moretti M, Crocè LS, Franzin F, Unoura M, et al. Different genotypes of hepatitis C virus are associated with different severity of chronic liver disease. J Med Virol. 1994;43:291–296. doi: 10.1002/jmv.1890430318. [DOI] [PubMed] [Google Scholar]
- 39.Yamada M, Kakumu S, Yoshioka K, Higashi Y, Tanaka K, Ishikawa T, Takayanagi M. Hepatitis C virus genotypes are not responsible for development of serious liver deisease. Dig Dis Sci. 1994;39:234–239. doi: 10.1007/BF02090191. [DOI] [PubMed] [Google Scholar]
- 40.Yotsuyanagi H, Koike K, Yasuda K, Moriya K, Hino K, Kurokawa K, Iino S. Hepatitis C virus genotypes and development of hepatocellular carcinoma. Cancer. 1995;76:1352–1355. doi: 10.1002/1097-0142(19951015)76:8<1352::aid-cncr2820760809>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara H, Hayashi N, Mita E, Naito M, Kasahara A, Fusamoto H, Kamada T. Quantitation of hepatitis C virus RNA in serum of asymptmatic blood donors and patients with type C chronic liver disease. Hepatology. 1993;17:545–550. doi: 10.1002/hep.1840170404. [DOI] [PubMed] [Google Scholar]
- 42.Kato N, Yokosuka O, Hosoda K, Ito Y, Ohto M, Omata M. Quantification of hepatitis C virus by competitive reverse Transcription-polymerase chain reaction: Increase of the virus in advanced liver disease. Hepatology. 1993;18:16–20. [PubMed] [Google Scholar]
- 43.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, et al. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 44.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–23. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Kawaguchi T, Nagao Y, Tanaka K, Ide T, Harada M, Kumashiro R, Sata M. Causal relationship between hepatitis C virus core and the development of type 2 diabetes mellitus in a hepatitis C virus hyperendemic area: a pilot study. Int J Mol Med. 2005;16:109–14. [PubMed] [Google Scholar]
- 46.El-Serag HB, Richardson PA, Everhart JE. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol. 2001;96:2462–7. doi: 10.1111/j.1572-0241.2001.04054.x. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: Biological, cultural, or socioeconomic factors. Hepatology. 2008;47:1058–66. doi: 10.1002/hep.22223. [DOI] [PubMed] [Google Scholar]


