Abstract
BACKGROUND
Decisional conflict is a source of anxiety and stress for men diagnosed with prostate cancer given uncertainty surrounding myriad treatment options. Few data exist to help clinicians identify which patients are at risk for decisional conflict. The purpose of this study was to examine factors associated with decisional conflict in economically disadvantaged men diagnosed with prostate cancer before any treatment choices were made.
METHODS
A total of 70 men were surveyed at a Veterans Administration clinic with newly diagnosed localized prostate cancer enrolled in a randomized trial testing a novel shared decision-making tool. Baseline demographic, clinical, and functional data were collected. Independent variables included age, race, education, comorbidity, relationship status, urinary/sexual dysfunction, and prostate cancer knowledge. Tested outcomes were Decisional Conflict Scale, Uncertainty Subscale, and Perceived Effectiveness Subscale. Multiple linear regression modeling was used to identify factors associated with decisional conflict.
RESULTS
Mean age was 63 years, 49% were African American, and 70% reported an income less than $30,000. Poor prostate cancer knowledge was associated with increased decisional conflict and higher uncertainty (P < .001 and P = 0.001, respectively). Poor knowledge was also associated with lower perceived effectiveness (P = 0.003) whereas being in a relationship was associated with higher decisional conflict (P = 0.03).
CONCLUSIONS
Decreased patient knowledge about prostate cancer is associated with increased decisional conflict and lower perceived effective decision-making. Interventions to increase comprehension of prostate cancer and its treatments may reduce decisional conflict. Further work is needed to better characterize this relationship and identify effective targeted interventions.
Keywords: Decisional conflict, prostate cancer, low socioeconomic status, knowledge, patient education
INTRODUCTION
Men diagnosed with localized prostate cancer face myriad choices in the decision-making process. Management options include active treatment – i.e. surgery and radiation therapy – or active surveillance. While quality of life detriment has traditionally been ascribed to prostate cancer treatment1, recent studies illustrate the health related quality of life (HRQOL) impact in men undergoing active surveillance as well2,3. To that end, the decision-making process for men with localized prostate cancer is a challenging task for even the most well informed patients.
Shared decision making (SDM) is a process by which physicians share relevant risk and benefit information of all treatment options and patients share relevant personal information with the clinician4. Thereby, a truly patient-centered decision is reached. Decisional conflict is a central focus of the interactions that are a part of SDM. It is a measure of the uncertainty surrounding a treatment choice and patient confidence in making that decision5. Decisional conflict is especially important for choices complicated by competing risks and outcome uncertainty6 and may be a useful tool for measuring decision quality7. Decisional conflict is associated with decisional regret8 and a higher likelihood of blaming physicians for adverse effects9. Medical decision-making experts advocate for the use of decisional conflict assessment in the clinical setting to ensure provision of adequate patient support10.
The anxiety inherent in the prostate cancer decision-making process stems from the complexity surrounding treatment options and a lack of consensus on ideal management11. In men with prostate cancer participating in a SDM process, reduction in decisional conflict may be a good indicator of better decision quality7. Identifying men who have higher pre-treatment decisional conflict may allow for targeted SDM interventions. However, few data exist to guide physicians in determining which patients are at risk for decisional conflict, particularly in economically disadvantaged populations. To identify factors associated with decisional conflict, we conducted a cross-sectional study of economically disadvantaged men with newly diagnosed localized prostate cancer before any treatment choices were made. We hypothesized that men with lower knowledge about prostate cancer and lower educational achievement in general would have higher decisional conflict scores.
METHODS
Study Design
The institutional review board at the University of California, Los Angeles, and the Greater Los Angeles Veterans Health Administration approved this study, and informed consent was obtained from all subjects. Subjects with no prior history of prostate cancer undergoing biopsy were recruited into a multiarm randomized SDM trial. The trial sought to evaluate and improve parameters of SDM through application of a novel tool employing patient preference assessment. All subjects in the study completed baseline demographic questionnaires and survey instruments, which were used in this cross-sectional analysis of baseline data. Men were included in this analysis if they had a biopsy demonstrating prostate cancer and could be enrolled before their cancer consultation. Baseline demographic, clinical, and functional data were obtained as well as prostate cancer knowledge assessment and scores from the Decisional Conflict Scale (DCS).
Instruments and Psychometric Properties
We used the Decisional Conflict Scale (Appendix 1) and its subscales, Uncertainty and Perceived Efficacy, to determine decisional conflict 6. The DCS is well validated and has been used in a variety of populations, including men facing decisions about treatment for benign prostatic hypertrophy12. The Perceived Efficacy subscale measures the extent to which decisions would be informed, consistent with personal values, and would be implemented6. Measured variables included age, race, relationship status, education, Charlson Comorbidity Index13, prostate cancer knowledge score14, and EPIC urinary incontinence and sexual functioning scores15.
Statistical Analysis
We used multiple linear regression modeling to identify factors associated with decisional conflict. Demographic, medical, and baseline functional variables were selected a priori. Adjusted means were calculated as predicted means from the estimated linear regression equation with all other covariates set to their mean values. Statistical significance was defined a priori as 0.05. P-values >0.1 are omitted from the tables for clarity.
Conceptual Framework
This study was guided by a broad conceptual framework (Figure 1) – adapted from Fishbein’s Integrative Model of behavior16,17 – that captures the working elements of the prostate cancer decision-making process. The context into which a man enters the process is framed by his demographic, medical and psychosocial backgrounds. His unique set of health beliefs direct the behavioral intention, which in turn frames the decision-making process with the physician. This study explores the unique role decisional conflict plays toward the end of the decision-making process.
Figure 1.
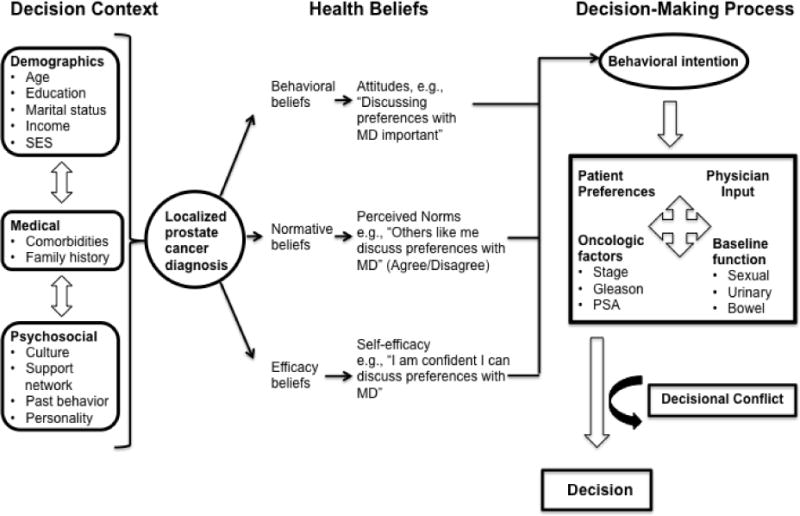
Conceptual framework of decision-making process in localized prostate cancer.
RESULTS
Data from all men with newly diagnosed localized prostate cancer (n=70) enrolled in the SDM trial trial between January 2011 and October 2013were for used for this analysis. Cohort characteristics are shown in Table 1. Mean age was 63 years and nearly half of the cohort was African-American. Seventy percent reported an annual income less than $30,000 and 68% were either retired or unemployed.
Table 1.
Cohort characteristics.
| Characteristic | Mean ± SD, Range or n (%) |
|---|---|
|
| |
| Age | 63 ± 6, range 45 to 78 |
|
| |
| Race/ethnicity | |
| White (non-Hispanic) | 24 (34%) |
| Black/African American | 34 (49%) |
| Hispanic/Latino | 8 (11%) |
| Other or mixed race/ethnicity | 4 (6%) |
|
| |
| Partnership status | |
| In significant relationship | 41 (59%) |
| Not in a significant relationship | 29 (41%) |
|
| |
| Employment status | |
| Employed | 22 (31%) |
| Unemployed | 12 (17%) |
| Retired | 36 (51%) |
|
| |
| Educational attainment | |
| High school graduate or less | 21 (30%) |
| Some college | 29 (42%) |
| College graduate | 19 (28%) |
|
| |
| Household income | |
| Less than $10,000 | 13 (19%) |
| $10,000 to $30,000 | 35 (51%) |
| More than $30,000 | 20 (29%) |
|
| |
| Current smoker | 19 (27%) |
|
| |
| Medical conditions (ever had) | |
| Diabetes | 11 (16%) |
| Heart attack | 9 (13%) |
| Stroke | 7 (10%) |
| Amputation | 2 (3%) |
| Circulation problems | 12 (17%) |
| Asthma, emphysema or breathing problems | 12 (17%) |
| Stomach ulcer or irritable bowel | 11 (16%) |
| Kidney disease | 3 (4%) |
| Major depression | 18 (26%) |
| Seizures | 4 (6%) |
| Alcoholism or alcohol problems | 17 (24%) |
| Drug problems | 12 (17%) |
|
| |
| Problems in last 4 weeks | |
| Urinary function | 17 (24%) |
| Sexual function | 31 (44%) |
| Bowel habits | 7 (10%) |
| Hot flashes | 1 (1%) |
| Breast tenderness/enlargement | 0 (0%) |
| Depressed | 10 (14%) |
|
| |
| Lack of energy | 9 (13%) |
| Change in body weight | 6 (9%) |
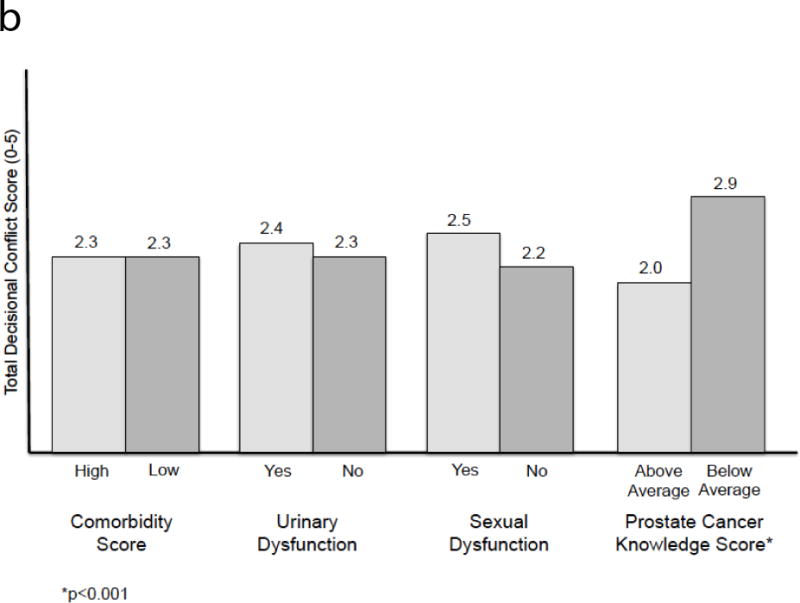
The linear regression analysis is presented in Table 2. Older age was associated with lower perceived efficacy in decision-making (p=0.005). Poor prostate cancer knowledge was associated with increased overall decisional conflict and more uncertainty (p<0.001 and p=0.001, respectively). Poor knowledge was also associated with lower perceived efficacy (p=0.003). Being in a relationship was associated with more decisional conflict (p=0.03). Adjusted R2 values ranged from 0.13 to 0.19. Adjusted means, calculated from the regression model, are provided in Table 3. Figure 2 also displays the adjusted means from total DCS score graphically. Unadjusted means were omitted, as the results were largely similar. Higher prostate cancer knowledge scores were associated with lower decisional conflict across all domains.
Table 2.
Linear regression models.
| DCS total score | DCS Uncertainty subscale | DCS Perceived Efficacy subscale | ||||
|---|---|---|---|---|---|---|
| Adjusted R2: 0.18 | Adjusted R2: 0.13 | Adjusted R2: 0.19 | ||||
| Coef (SE) | p | Coef (SE) | p | Coef (SE) | p | |
| Age (10-year increase) | −.28 (.21) | .05 (.29) | −.59 (.20) | .005 | ||
| Black (ref: White) | .25 (.26) | .56 (.36) | −.07 (.26) | |||
| Hispanic (ref: White) | −.14 (.39) | .30 (.55) | −.59 (.39) | |||
| Other race (ref: White) | −.11 (.49) | −.06 (.70) | −.69 (.49) | |||
| In significant relationship (ref: not) | .54 (.24) | .03 | .67 (.34) | .05 | .44 (.24) | .08 |
| Some college (ref: high school or less) | .09 (.30) | −.05 (.42) | .18 (.30) | |||
| College (ref: high school or less | .04 (.32) | −.06 (.44) | .16 (.31) | |||
| Charlson Comorbidity Index (1-pt increase) | −.02 (.10) | .05 (.14) | −.02 (.10) | |||
| Urinary functioning problem | −.01 (.28) | −.01 (.40) | −.10 (.28) | |||
| Sexual functioning problem | .20 (.24) | .25 (.34) | .26 (.24) | |||
| Prostate cancer knowledge score | −1.9 (0.5) | <.001 | −2.4 (0.7) | .001 | −1.5 (0.5) | .003 |
P-values >0.1 not provided.
DCS – Decisional Conflict Scale
Table 3.
Adjusted means as derived from the regression model.
| DCS total score | DCS Uncertainty subscale | DCS Perceived Efficacy subscale | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Adjusted mean (SE) | p | Adjusted mean (SE) | p | Adjusted mean (SE) | p | |
|
| ||||||
| Age | ||||||
| <65 years | 2.4 (0.2) | 2.4 (0.2) | 2.4 (0.2) | |||
| ≥65 years | 2.2 (0.2) | 2.4 (0.2) | 2.0 (0.2) | |||
|
| ||||||
| Race/ethnicity | ||||||
| White | 2.2 (0.2) | 2.2 (0.3) | 2.3 (0.2) | |||
| Black | 2.5 (0.2) | 2.7 (0.2) | 2.3 (0.2) | |||
| Hispanic | 2.0 (0.3) | 2.3 (0.5) | 1.8 (0.3) | |||
| Other | 2.2 (0.5) | 2.2 (0.6) | 1.8 (0.5) | |||
|
| ||||||
| In significant relationship | .02 | .04 | .06 | |||
| Yes | 2.6 (0.2) | 2.7 (0.2) | 2.4 (0.2) | |||
| No | 2.0 (0.2) | 2.0 (0.2) | 1.9 (0.2) | |||
|
| ||||||
| Education | ||||||
| HS or less | 2.4 (0.2) | 2.7 (0.3) | 2.2 (0.2) | |||
| Some college | 2.3 (0.2) | 2.3 (0.2) | 2.3 (0.2) | |||
| College graduate | 2.2 (0.2) | 2.3 (0.3) | 2.2 (0.2) | |||
|
| ||||||
| Comorbidity Score | ||||||
| High | 2.3 (0.1) | 2.4 (0.3) | 2.2 (0.2) | |||
| Low | 2.3 (0.2) | 2.4 (0.2) | 2.2 (0.1) | |||
|
| ||||||
| Urinary functioning problem | ||||||
| Yes | 2.4 (0.2) | 2.5 (0.3) | 2.2 (0.2) | |||
| No | 2.3 (0.1) | 2.4 (0.2) | 2.2 (0.1) | |||
|
| ||||||
| Sexual functioning problem | ||||||
| Yes | 2.5 (0.2) | 2.7 (0.2) | 2.4 (0.2) | |||
| No | 2.2 (0.2) | 2.2 (0.2) | 2.1 (0.2) | |||
|
| ||||||
| Prostate cancer knowledge score | <.001 | .001 | .003 | |||
| Above average | 2.0 (0.1) | 2.0 (0.2) | 1.9 (0.1) | |||
| Below average | 2.9 (0.2) | 3.1 (0.3) | 2.7 (0.2) | |||
P-values >0.1 not provided.
DCS – Decisional Conflict Scale
Figure 2.
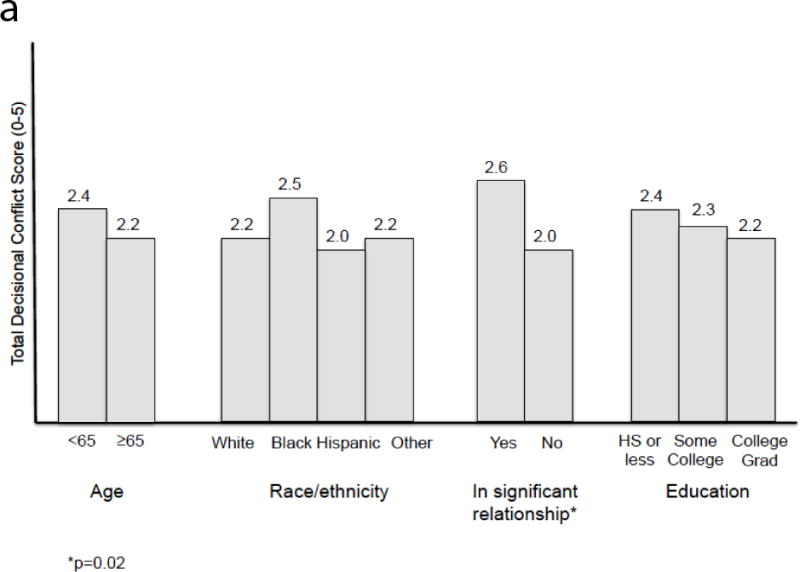
a. Adjusted means of total DCS score for age, race/ethnicity, relationship status, and education.
b. Adjusted means of total DCS score for comorbidity, urinary and sexual dysfunction, and prostate cancer knowledge score.
DISCUSSION
In the prostate cancer decision-making process, men must weigh the risks and benefits of complex treatment modalities in the face of outcome uncertainty and lack of consensus among physicians regarding the best decision. Men with pre-treatment decisional conflict are important targets for SDM interventions. Few data exist however to guide clinicians in identifying these patients early in the decision-making process. Our cross-sectional study, identifying factors associated with decisional conflict in economically disadvantaged men, has several important findings.
First, poor prostate cancer knowledge was associated with increased overall decisional conflict and more uncertainty. In a separate prostate cancer SDM trial, Kim et al found that poor prostate cancer knowledge corresponded to lower literacy in men of low socioeconomic status (SES)18. An estimated 36% of adults in the United States have “basic” or “below basic” health literacy19. Men from economically disadvantaged backgrounds have alarmingly low prostate health literacy19,20, making them susceptible to poor prostate cancer knowledge and decisional conflict. This literacy-knowledge deficit is an ideal target for educational intervention to improve decision-making in economically disadvantaged men with prostate cancer.
Although the literature examining decisional conflict is relatively immature, previous authors identified other mediators of decisional conflict in prostate cancer patients. Berry et al showed that men with localized prostate cancer that exhibited less uncertainty were more satisfied with their decision21. This cohort comprised predominantly white men from non-disadvantaged backgrounds. The effect of poor knowledge and uncertainty is likely more pronounced in the currently described cohort. In our analysis, we used a 14-item prostate cancer knowledge questionnaire14, which may represent a practical and effective means of screening for pre-treatment decisional conflict.
Second, poor prostate cancer knowledge and older age was associated with lower perceived efficacy in the decision-making process. Perceived efficacy is the belief in one’s own ability to complete tasks. In prostate cancer decision-making, perceived efficacy represents a man’s belief in his ability to make a good decision regarding the course of management. We found that men with poor prostate cancer knowledge, as well as older men, had lower perceived efficacy.
In a cross-sectional study of men on active surveillance for localized prostate cancer, Goh et al found that men with higher self-efficacy experienced less decisional conflict 22. Heckman et al showed that among disadvantaged men with localized prostate cancer those with low self-efficacy suffered worse quality of life across all domains23. Likewise, perceived- or self-efficacy may also be important for preparedness in decision-making. In a study evaluating cancer patients’ preparedness for clinical trials, Manne et al determined that assessing self-efficacy might be as important as examining knowledge or attitudinal beliefs24.
Finally, being in a relationship predicted more decisional conflict. In a study of low-income, uninsured men with prostate cancer, Gore et al identified an association between partnership status and improved quality of life25. Conversely, Bergman et al demonstrated comparable physical and mental health scores between partnered and unpartnered men with prostate cancer, although this cohort was comprised of men with a homogeneously high SES26. Prostate cancer is often referred to as a “couple’s disease” because the impact of treatment on the patient can decrease his ability to be a part of an ongoing sexual relationship with his partner27. Discordant preferences between patient and partner might increase decisional conflict since the partner’s views are especially salient in the face of a potential decrease in their own sexual QOL. Further work into the impact of discordant preferences between patient and partner in this setting is indicated.
For men with newly diagnosed prostate cancer, their clinical consultation is the point of interaction with the health care system28 but the impact of their diagnosis will extend further than the physician’s office. Our study identifies factors that may be used to distinguish which men are at high-risk for decisional conflict. These data are also hypothesis generating in that certain factors – i.e. prostate cancer knowledge – may represent a modifiable target to reduce decisional conflict. Widespread implementation of decision support interventions in clinical practice has been slow owing to lack of physician time and resources as well as information systems that are incapable of tracking patients through the SDM process29,30. However, individual reports indicate that systematic approaches to reducing decisional conflict are feasible and effective. In a cohort of primary care patients, Ferron Parayre et al validated a four-item checklist (SURE) to detect decisional conflict 31; although this has not been studied in men with prostate cancer. A Scottish randomized controlled trial using a “decision navigation” intervention in men with newly diagnosed prostate cancer found less decisional conflict and lower decisional regret32. Likewise, interventions using health coaches in low-income patients in California has shown promise33. Through early identification of men at high-risk for decisional conflict, clinicians may be able to guide the patients most in-need toward effective decision support interventions.
The potential for shared decision-making interventions to reduce decisional conflict via potential targets identified here may have benefits beyond those experienced by the patient. Reductions in decisional conflict are associated with decreases in patient delay in making a choice as well as measures of ‘fretting’ and ‘nervousness’34. Improvements in decisional conflict may result in more confidence in patients’ overall decision-making ability. Such confident patients have been termed ‘activated’. Patient activation, as measured by an individual’s knowledge, skill and confidence in managing their own healthcare, correlates with improved health economic outcomes35,36. Patient activation is also associated with higher compliance, an important consideration for men considering active surveillance36.
The results of this study must be interpreted in the context of its limitations. First, the size of the cohort is relatively small. Internal review data show dropping referral rates to our clinic for abnormal PSA – likely reflecting new VA health system screening practice guidelines – which has resulted in fewer de novo cancer diagnoses. Nonetheless, we were able to detect significant differences and the factors associated with pre-treatment decisional conflict were similar when studied at interim analysis. Second, decisional conflict measured after the prostate cancer decision has been made is not available in this analysis. We felt that identifying clinical factors associated with pre-treatment decisional conflict was important to gain an understanding of the decision-making process in economically disadvantaged men with newly diagnosed disease.
CONCLUSIONS
In this cross-sectional study of men with newly diagnosed localized prostate cancer, we found that poor prostate cancer knowledge is associated with increased decisional conflict and lower perceived efficacy in decision-making. Through early identification of men at high-risk for decisional conflict, targeted interventions aimed at increasing comprehension of prostate cancer and its treatments may reduce decisional conflict. Further work is needed to better characterize this relationship and identify strategies to improve the decision-making process in economically disadvantaged men with this disease.
Appendix 1. Decisional Conflict Scale
| Strongly Agree [0] |
Agree [1] |
Neither Agree Nor Disagree [2] |
Disagree [3] |
Strongly Disagree [4] |
|
|---|---|---|---|---|---|
| 1. I know which options are available to me. | □ | □ | □ | □ | □ |
| 2. I know the benefits of each option. | □ | □ | □ | □ | □ |
| 3. I know the risks and side effects of each option. | □ | □ | □ | □ | □ |
| 4. I am clear about which benefits matter most to me. | □ | □ | □ | □ | □ |
| 5. I am clear about which risks and side effects matter most to me. | □ | □ | □ | □ | □ |
| 6. I am clear about which is more important to me (the benefits or the risks and side effects). | □ | □ | □ | □ | □ |
| 7. I have enough support from others to make a choice. | □ | □ | □ | □ | □ |
| 8. I am choosing without pressure from others. | □ | □ | □ | □ | □ |
| 9. I have enough advice to make a choice. | □ | □ | □ | □ | □ |
| 10.I am clear about the best choice for me, | □ | □ | □ | □ | □ |
| 11. I feel sure about what to choose. | □ | □ | □ | □ | □ |
| 12. This decision is easy for me to make. | □ | □ | □ | □ | □ |
| 13. I feel I have made an informed choice. | □ | □ | □ | □ | □ |
| 14. My decision shows what is important to me. | □ | □ | □ | □ | □ |
| 15. I expect to stick with my decision. | □ | □ | □ | □ | □ |
| 16. I am satisfied with my decision. | □ | □ | □ | □ | □ |
Footnotes
Disclosures: None
References
- 1.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 2.Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790–796. doi: 10.1056/NEJMoa021483. [DOI] [PubMed] [Google Scholar]
- 3.Vasarainen H, Lokman U, Ruutu M, et al. Prostate cancer active surveillance and health-related quality of life: results of the Finnish arm of the prospective trial. BJU Int. 2012;109:1614–1619. doi: 10.1111/j.1464-410X.2011.10677.x. [DOI] [PubMed] [Google Scholar]
- 4.King JS, Moulton B. Rethinking informed consent: the case for shared medical decision-making. Am J Law Med. 2006;32:429–501. doi: 10.1177/009885880603200401. [DOI] [PubMed] [Google Scholar]
- 5.Carpenito LJ. Nursing Diagnosis: application to clinical practice. 8th. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- 6.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 7.Song MK, Sereika SM. An evaluation of the Decisional Conflict Scale for measuring the quality of end-of-life decision making. Patient Educ Couns. 2006;61:397–404. doi: 10.1016/j.pec.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281–292. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 9.Gattelari M, Ward JE. Will men attribute fault to their GP for adverse effects arising from controversial screening tests? An Australian study using scenarios about PSA screening. J Med Screen. 2004;11:165–169. doi: 10.1258/0969141042467386. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor AM, Légaré F, Stacey D. Risk communication in practice: The contribution of decision aids. BMJ. 2003;327:736–740. doi: 10.1136/bmj.327.7417.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeliadt SB, Ramsey SD, Penson DF, et al. Why do men choose one treatment over another? A review of patient decision making for localized prosate cancer. Cancer. 2006;106:1865–1874. doi: 10.1002/cncr.21822. [DOI] [PubMed] [Google Scholar]
- 12.Murray E, Davis H, Tai SS, et al. Randomised controlled trial of an interactive multimedia decision aid on benign prostatic hypertrophy in primary care. BMJ. 2001;323:493–496. doi: 10.1136/bmj.323.7311.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales K, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Deibert CM, Maliski S, Kwan L, et al. Prostate cancer knowledge among low income minority men. J Urol. 2007;177:1851–1855. doi: 10.1016/j.juro.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 15.Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the expanded prostate cancer composite index (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 16.Fishbein M. The role of theory in HIV prevention. AIDS Care. 2000;12:273–278. doi: 10.1080/09540120050042918. [DOI] [PubMed] [Google Scholar]
- 17.Frosch DL, Légaré F, Fishbein M, et al. Adjuncts or adversaries to shared decision-making? Applying the Integrative Model of behavior to the role and design of decision support interventions in healthcare interactions. Implement Sci. 2009;4:73. doi: 10.1186/1748-5908-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SP, Knight SJ, Tomori C, et al. Health literacy and shared decision making for prostate cancer patients with low socioeconomic status. Cancer Invest. 2001;19:684–691. doi: 10.1081/cnv-100106143. [DOI] [PubMed] [Google Scholar]
- 19.Kutner M, Greenberg E, Jin Y, et al. US Department of Education. Washington, DC: National Center for Education Statistics; 2006. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy. [Google Scholar]
- 20.Wang DS, Jani AB, Tai CG, et al. Severe lack of comprehension of common prostate health terms among low-income inner-city men. Cancer. 2013;119:3204–3211. doi: 10.1002/cncr.28186. [DOI] [PubMed] [Google Scholar]
- 21.Berry DL, Ellis WJ, Russell KJ, et al. Factors that predict treatment choice and satisfaction with the decision in men with localized prostate cancer. Clin Genitourin Cancer. 2006;5:219–226. doi: 10.3816/CGC.2006.n.040. [DOI] [PubMed] [Google Scholar]
- 22.Goh AC, Kowalkowski MA, Bailey DE, Jr, et al. Perception of cancer and inconsistency in medical information are associated with decisional conflict: a pilot study of men with prostate cancer who undergo active surveillance. BJU Int. 2012;110:e50–56. doi: 10.1111/j.1464-410X.2011.10791.x. [DOI] [PubMed] [Google Scholar]
- 23.Heckman JE, Chamie K, Maliski SL, et al. The role of self-efficacy in quality of life for disadvantaged men with prostate cancer. J Urol. 2011;186:1855–1861. doi: 10.1016/j.juro.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 24.Manne S, Kashy D, Albrecht T, et al. Knowledge, attitudes, and self-efficacy as predictors of preparedness for oncology clinical trials: A meditational model. Med Decis Making. 2013 Nov 18; doi: 10.1177/0272989X13511704. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gore JL, Krupski T, Kwan L, et al. Partnership status influences quality of life in low-income, uninsured men with prostate cancer. Cancer. 2005;104:191–198. doi: 10.1002/cncr.21139. [DOI] [PubMed] [Google Scholar]
- 26.Bergman JB, Gore JL, Saigal CS, et al. Partnership and outcomes in men with prostate cancer. Cancer. 2009;115:4688–4694. doi: 10.1002/cncr.24544. [DOI] [PubMed] [Google Scholar]
- 27.Soloway CT, Soloway MS, Kim SS, et al. Sexual, psychological and dyadic qualities of the prostate cancer ‘couple’. BJU Int. 2005;95:780–785. doi: 10.1111/j.1464-410X.2005.05400.x. [DOI] [PubMed] [Google Scholar]
- 28.Legare F, Witteman HO. Shared decision making: Examining key elements and barriers to adoption in routine clinical practice. Health Aff (Millwood) 2013;32:276–284. doi: 10.1377/hlthaff.2012.1078. [DOI] [PubMed] [Google Scholar]
- 29.Taylor KL, Williams RM, Davis K, et al. Decision making in prostate cancer screening using decision aids vs usual care: A randomized clinical trial. JAMA Intern Med. 2013 Jul 29; doi: 10.1001/2103.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedberg MW, Van Busum K, Wexler R, et al. A demonstration of shared decision making in primary care highlights barriers to adoption and potential remedies. Health Aff (Millwood) 2013;32:286–275. doi: 10.1377/hlthaff.2012.1084. [DOI] [PubMed] [Google Scholar]
- 31.Ferron Parayre A, Labrecque M, Rousseau M, et al. Validation of SURE a four-item clinical checklist for decisional conflict in patients. Med Decis Making. 2014;34:54–65. doi: 10.1177/0272989X13491463. [DOI] [PubMed] [Google Scholar]
- 32.Hacking B, Wallace L, Scott S, et al. Testing the feasibility, acceptability and effectiveness of a ‘decision navigation’ intervention for early stage prostate cancer patients in Scottland – a randomised controlled trial. Psychooncology. 2013;22:1017–1024. doi: 10.1002/pon.3093. [DOI] [PubMed] [Google Scholar]
- 33.Bennett H, Laird K, Margolius D, et al. The effectiveness of health coaching, home blood pressure monitoring, and home titration in controlling hypertension among low-income patients: protocol for a randomized controlled trial. BMC Public Health. 2009;9:456. doi: 10.1186/1471-2458-9-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knops AM, Goossens A, Ubbinik DT, et al. Interpreting patient decisional conflict scores: behavior and emotions in decisions about treatment. Med Decis Making. 2013;33:78–84. doi: 10.1177/0272989X12453500. [DOI] [PubMed] [Google Scholar]
- 35.Hibbard JH, Greene J, Overton V. Patients with lower activation associated with higher costs; Delivery systems should know their patients’ ‘scores’. Health Aff (Millwood) 2013;32:216–222. doi: 10.1377/hlthaff.2012.1064. [DOI] [PubMed] [Google Scholar]
- 36.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


