Abstract
PTH is an osteoanabolic for treating osteoporosis but its potency wanes. Disabling the transcription factor nuclear matrix protein 4 (Nmp4) in healthy, ovary-intact mice enhances bone response to PTH and bone morphogenetic protein 2 and protects from unloading-induced osteopenia. These Nmp4−/− mice exhibit expanded bone marrow populations of osteoprogenitors and supporting CD8+ T cells. To determine whether the Nmp4−/− phenotype persists in an osteoporosis model we compared PTH response in ovariectomized (ovx) wild-type (WT) and Nmp4−/− mice. To identify potential Nmp4 target genes, we performed bioinformatic/pathway profiling on Nmp4 chromatin immunoprecipitation sequencing (ChIP-seq) data. Mice (12 w) were ovx or sham operated 4 weeks before the initiation of PTH therapy. Skeletal phenotype analysis included microcomputed tomography, histomorphometry, serum profiles, fluorescence-activated cell sorting and the growth/mineralization of cultured WT and Nmp4−/− bone marrow mesenchymal stem progenitor cells (MSPCs). ChIP-seq data were derived using MC3T3-E1 preosteoblasts, murine embryonic stem cells, and 2 blood cell lines. Ovx Nmp4−/− mice exhibited an improved response to PTH coupled with elevated numbers of osteoprogenitors and CD8+ T cells, but were not protected from ovx-induced bone loss. Cultured Nmp4−/− MSPCs displayed enhanced proliferation and accelerated mineralization. ChIP-seq/gene ontology analyses identified target genes likely under Nmp4 control as enriched for negative regulators of biosynthetic processes. Interrogation of mRNA transcripts in nondifferentiating and osteogenic differentiating WT and Nmp4−/− MSPCs was performed on 90 Nmp4 target genes and differentiation markers. These data suggest that Nmp4 suppresses bone anabolism, in part, by regulating IGF-binding protein expression. Changes in Nmp4 status may lead to improvements in osteoprogenitor response to therapeutic cues.
Patients with severe osteoporosis are often treated with PTH, a potent osteoanabolic agent (1); however, the bone-building ability of this drug or its “anabolic window” wanes, likely due to latent increases in bone resorption (2–4). This limits its effectiveness to treat a chronic degenerative disease. Recent advances in bone-forming agents have shown that one can increase the extent of bone mass accrual with antisclerostin treatment compared with PTH (5). However, there may be unique pathways triggered by PTH, which allows for sustained targeting of early osteogenesis as evidenced by serum markers of bone formation such as N-terminal propeptide of type 1 procollagen (P1NP) and osteocalcin (OCN) (also known as bone gamma carboxyglutamate protein [Bglap]) (6, 7). In contrast to PTH, antisclerostin antibodies may have a limited capacity for targeting osteoprogenitors as evidenced by a relatively transient up-regulation of collagen-based markers such as P1NP (5). Therefore, given PTH's unique mode of action, therapies that could enhance PTH-mediated recruitment of osteoprogenitors may add value to some patients. How to achieve this enhancement is not clear. For example, attempts to extend and enhance PTH efficacy by combining treatment with antiresorptive medications have met with mixed success and have generally been underwhelming (8–10).
Blocking the activity of nuclear matrix protein 4 (Nmp4)/Cas-interacting zinc finger protein in mice dramatically enhanced their response to anabolic doses of PTH (11–13), suggesting a potential strategy for an adjuvant therapy (14). Intermittent exogenous doses of hormone stimulated equivalent new bone formation in wild-type (WT) and Nmp4−/− mice during the first 2 weeks of challenge, but at 3 weeks of treatment, the null mice exhibited greater than a 2-fold increase in new trabecular bone compared with their WT littermates (11). This augmented skeletogenesis in the Nmp4−/− mice was extended to 7 weeks of treatment and was observed in the femur, tibia, and vertebra. Serum OCN continued to rise at this time point in the Nmp4−/− mice but had decreased in the WT animals (11). However, the PTH response of the cortical compartment was equivalent throughout treatment in the WT and null mice (13). This suggests that disabling Nmp4 accelerates and enhances the response of bone to intermittent PTH (11).
Nmp4−/− bone may have a generalized accelerated and heightened response to systemic or local anabolic cues. For example, these mice also exhibited augmented bone morphogenetic protein 2 (BMP2)-induced ectopic bone formation compared with their WT littermates (15). The Nmp4-null mice showed an accelerated osseous regeneration after marrow ablation (15) and did not lose bone during hind limb unloading, which appeared to derive from an enhanced osteoblast activity (16).
Prerequisite for an adjuvant therapy target, disabling Nmp4 has little impact on the health, longevity, or global baseline phenotype of the mouse, with a few exceptions. The Nmp4−/− baseline skeletal phenotype (ie, bone mineral density and/or content and trabecular architecture) is generally equivalent compared with WT animals; although we have occasionally observed an unprovoked increase in bone properties in Nmp4−/− mice (11–13, 15). Similarly, male Nmp4−/− mice exhibit variable degrees of spermatogenic cell degeneration resembling germinal-cell aplasia with focal spermatogenesis resulting in sporadic infertility (17).
Our recent work suggests that the cellular basis of the osteoanabolic repressor function of Nmp4 is due to its effect on the bone marrow (BM)-derived stromal stem/progenitor cells also known as mesenchymal stem progenitor cells (MSPCs) (12). Nmp4−/− mice have significantly more osteoprogenitor cells in their marrow, which lie in wait to be quickly mobilized to differentiate into active osteoblasts upon stimulation with various osteoanabolic stimuli (12). There was no difference between WT and Nmp4−/− BM cellularity or profiles of several blood elements; however, the null mouse exhibited a 4-fold increase in CD45−/CD105+/nestin+/CD146+ BM osteoprogenitor cells. These markers are a common hallmark to CFU-F cells with osteogenic potential (18, 19), and indeed, 4-fold more CFU-FAlk phos+ and CFU-FOb cells have been recovered from these mice compared with the WT animals (12, 15). A second, related phenomenon we have observed in Nmp4−/− mice is a 2-fold increase in the prevalence of CD8+ T cells in the femoral marrow, the lymphocyte population that provides potent input to induce MSPCs down the osteoblast differentiation pathway (12, 20–22). These blood cells express the parathyroid hormone 1 receptor and support the PTH anabolic response via the release of wingless-type MMTV integration site family, member 10B (Wnt10b) upon hormone challenge, which drives osteoprogenitor differentiation to preosteoblasts and mature matrix-producing bone cells (20–22).
There is little information on the molecular mechanisms and cellular pathways that mediate the antianabolic action of Nmp4. This transcription factor is a Cys2His2 zinc finger protein that primarily localizes to the nucleus although there is evidence for cytoplasmic activity (23, 24). The zinc fingers recognize the DNA minor groove of an AT-rich consensus sequence and 2 transactivation domains can suppress or activate transcription depending on the cellular context (24–28). The amino terminus of the rodent protein contains an src homology 3 domain-binding domain that associates with the adaptor signaling protein p130Crk-associated substrate (24), but the functional significance of this interaction remains unknown.
The Nmp4−/− progenitor cells and their progeny have an exaggerated stimulus response at the levels of transcription and cell signaling (29–31). Nmp4-null BM stromal cells show an enhanced transcriptional response to PTH and BMP2 (26, 29, 30). The Nmp4−/−-derived calvarial cells exhibit an increased load-induced phosphorylation of phosphatidylinositol 3-kinase and thymoma viral proto-oncogene (AKT) and β-catenin nuclear translocation (31). Analogous to heightened response to anabolic signals in Nmp4−/− osteolineage cells, osteoclast preparations from the null mice exhibited a heightened response to the remodeling signals of receptor activator of nuclear factor kappa-B ligand and macrophage colony-stimulating factor (11).
Two essential genotype-phenotype questions remaining to be addressed are 1) whether the Nmp4-null mouse is resistant to ovariectomy (ovx)-induced bone loss and 2) whether disabling Nmp4 improves PTH-based bone therapy in the ovx model. This is a focal preclinical extension of the Nmp4−/− phenotype necessary before this gene and its associated pathways can be considered potential targets for an adjuvant therapy. Additionally, we used expanded cultures of WT and Nmp4−/− MSPCs to probe the cell autonomous proliferative and mineralization activities of this cell population. To delineate the framework of the Nmp4 antianabolic network, we performed genome-wide chromatin immunoprecipitation sequencing (ChIP-seq) on MC3T3-E1 cells and combined these data with the data available for Nmp4 (also known as zinc finger protein 384 [ZFP384]) from the mouse Encyclopedia of DNA Elements (ENCODE) Consortium for transcription factors (32). Bioinformatic profiling, gene ontology (GO), and pathway analysis were performed on these datasets to infer a map of the negative regulation of bone anabolism under Nmp4 control. Interrogation of mRNA transcripts in nondifferentiating and osteogenic differentiating WT and Nmp4−/− MSPCs was performed on 90 Nmp4 target genes and differentiation markers to inaugurate validation of the Nmp4 antianabolic network.
Materials and Methods
Mice
Male and female Nmp4−/− mice, backcrossed onto a C57BL/6J background for 7 generations (11–13), and their WT littermates were produced and maintained in our colony at Indiana University Bioresearch Facility, Indiana University School of Dentistry. Our local Institutional Animal Care and Use Committee approved all husbandry practices and experimental procedures and regimens described in this investigation.
Bilateral ovx surgery
Twelve-week-old virgin mice were anesthetized using isoflurane inhalation followed by a mixture of xylazine and ketamine administered ip. A 1- to 2-cm dorsal incision was made in the midline below the level of the last rib and the skin bluntly dissected from the muscle on either side of the incision. Through the skin incision, the muscle wall was incised 1cm lateral to the midline 1–2cm below the last rib to enter the abdominal cavity. The periovarian fat pad was located and gently grasped and exteriorized. Care was taken not to directly handle the ovary to avoid abdominal implantation of ovarian tissue. Although holding the periovarian fat pad with forceps, the fallopian tube between the fat pad and uterus was clamped and crushed using mosquito hemostats. The crushed area was cut with scissors and the fat pad with ovary removed. The procedure was repeated on the contralateral side. The skin incision was closed with one or 2 surgical wound clips. The sham surgeries involved all the outlined steps except the crushing the fallopian tubes and the actual removal of the ovaries. To confirm the efficacy of ovx, uteri were weighed after euthanasia.
PTH treatment
At 16 weeks of age, ovx animals were sorted into 4 treatment groups based on equivalent mean-group-body weight. These 4 groups included 1) vehicle-treated WT; 2) PTH-treated WT; 3) vehicle-treated Nmp4−/−; and 4) PTH-treated Nmp4−/− mice. Mice were injected sc with synthetic human PTH (hPTH) 1–34 acetate salt (Bachem Bioscience, Inc) at 30 μg/kg · d, daily or vehicle control (0.2% BSA and 1.0μN HCl in saline; Abbott Laboratory) for the length of time indicated.
Cell culture
Cells from ATCC (MC3T3-E1 subclone 4) were maintained in α-MEM supplemented with 100-IU/mL penicillin, 100-μg/mL streptomycin, 25-μg/mL amphotericin, 2mM L-glutamine (Gibco BRL), ascorbic acid (50 μg/mL; Sigma-Aldrich), and 10% fetal bovine serum (Sigma-Aldrich). Expanded MSPC cultures were established as previously described (33). Briefly, long BM was isolated from euthanized mice 6–8 weeks of age, and the BM mononuclear cells were isolated using a Ficoll gradient. These cells were plated in Mesencult Media + Mesencult Stimulatory Supplement (StemCell Technologies) and maintained in culture for 3–4 weeks without passage and fed every 5–7 days by removing 50% of the old media and adding 50% fresh media, very gently so as not to disturb the cells. At approximately 80% confluence, the cells were passaged at 1:3 dilution for 2 more passages before use or were frozen for storage. Cells were used for experiments between passages 5–10. For comparing cell proliferation rates between WT and Nmp4−/− MSPCs, the cells were transferred to α-MEM without the ascorbic acid in 12-well plates at 5000 cells/well (d 0). Cells were counted on days 2, 4, and 6 after seeding before refreshing the medium for the remaining cells. To evaluate mineralizing capacity cells were transferred to osteogenic differentiation medium and after 48 hours (d 0), which was comprised of α-MEM supplemented with ascorbic acid (5–50 μg/mL; Sigma-Aldrich), dexamethasone (0nM–10nM; Sigma-Aldrich), and 10mM glycerol 2-phosphate disodium salt hydrate (BGP) (Sigma-Aldrich). For controls, cells were passaged into fresh MesenCult medium without the osteogenic/mineralization supplements. Cells were stained for alkaline phosphatase activity using naphthol AS-MX phosphate and fast red violet B salt following the manufacturer's instructions (catalog 85L3R-1KT; Sigma) or for mineralization using alizarin red.
To compare mRNA expression profiles of select genes in nondifferentiating and osteogenic-differentiating WT and Nmp4−/− MSPCs, cells were seeded into 12-well plates at either 10 000 or 25 000 cells/well in Mesencult Media + Mesencult Stimulatory Supplement. Those cells at the lower seeding density were harvested on day 3 after seeding (nondifferentiating). The remaining cells were transferred to osteogenic differentiation medium 48 hours after seeding and harvested on days 5, 7, 9, and 16.
Flow cytometry
Cellular surface marker profiles from BM and peripheral blood (PBL) were assessed as previously described (12). The antibodies employed for flow cytometry were obtained from BD Biosciences. Stained cells were analyzed on an FACSCalibur (BD Biosciences) and results were quantified using FlowJo Version 8.8.6 software (TreeStar, Inc).
Microcomputed tomography (μCT)
Trabecular bone architecture was analyzed as we have previously described (11, 12). Briefly, femurs and L5 vertebra were excised from the WT and Nmp4−/− mice after euthanasia, the muscle and connective tissue removed, and the bones transferred to 10% buffered formalin, 4°C for 48 hours, after which the bones were placed in 70% ethanol (4°C) until analyzed. For femur analysis, a 2.6-mm span (∼5 mm3 of medullary space) of the excised distal femoral metaphysis was scanned in 70% ethanol on a desktop μCT (μCT 35; Scanco Medical AG) at 10-μm resolution using 55-kVp tube potential and 400-millisecond integration time, to measure 3-dimensional morphometric properties. The entire vertebra (L5) were scanned using standard methods (Skyscan 1172). Bones were reconstructed and analyzed using the manufacturer's software. The trabecular bone between the 2 growth plates was isolated from the cortical shell via manual tracing and assessed for trabecular architecture. From the 3-dimensional reconstructions the next parameters were obtained using the Scanco and Skyscan software analyses: trabecular bone volume per total volume (BV/TV) (%), connectivity density (Conn.D) (mm−3), structure model index (SMI), trabecular number (Tb.N) (mm−1), trabecular thickness (Tb.Th) (mm), and trabecular spacing (Tb.Sp) (mm) (34).
Bone histomorphometry
All histomorphometric parameters were obtained as previously described (11) following the ASBMR guidelines (35). Briefly, mice were administered ip injections of calcein green (20 mg/kg; Sigma-Aldrich) and alizarin red (25 mg/kg; Sigma-Aldrich) 6 and 3 days before euthanasia, respectively. The femur marrow cavity was exposed via cutting the anterior face of the epiphyseal plate. Bones were embedded in methyl-methacrylate subsequent to dehydration with graded alcohols, sectioned (4 μm) with a Leica RM2255 microtome (Leica Microsystems), and mounted unstained on microscope slides and imaged under fluorescent light with a microscope system (11). Bone formation rate (BFR), mineral apposition rate (MAR), and mineralizing surface/bone surface (MS/BS) were obtained from a 0.03-mm2 metaphyseal region of interest from 250 to 1750 μm below the growth plate using ImagePro 3.1 software (Media Cybernetics).
Serum biochemistry
We analyzed serum P1NP to evaluate global bone formation in our experimental mice using the Rat/Mouse P1NP enzyme immuno-assay from IDS Immunodiagnostic Systems following the manufacturer's instructions. To follow bone resorption we analyzed serum C-terminal telopeptides (CTXs) with the RatLaps ELISA (Immunodiagnostic Systems, Inc) (11).
Quantitative real-time PCR (qRT-PCR) analysis
ChIP-quantitative PCR was used to authenticate select ChIP-seq profiles employing SYBR Green assays and SYBR Green Supermix (Bio-Rad). qRT-PCRs were carried out in triplicate on specific genomic regions. The resulting signals were normalized for primer efficiency by carrying out qRT-PCRs for each primer pair using Input DNA.
To evaluate gene expression in nondifferentiating and in osteogenic-differentiating WT and Nmp4−/− MPSCs, RNA was isolated with RNAeasy columns according to the manufacturer's instructions (QIAGEN). The RNA was reverse transcribed via the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). RNA expression profiling was performed on 3–4 replicates per time point for both genotypes over the 16-day culture period. Individual cDNAs were quantified by qRT-PCR using a custom TaqMan Low Density Array (TLDA) system (Format 96a; Applied Biosystems) designed for 96 genes, including Nmp4 target genes identified by our genome-wide ChIP-seq profiling, osteogenic differentiation marker genes, and candidate normalizer genes. All experiments were performed in biological quadruplicate or triplicate with TaqMan fast advanced master mix (Applied Biosystems) on a QuantStudio 7 Flex Real-Time PCR System. The probes used are listed in Supplemental Table 1. We used the ExpressionSuite v1.0.4 analysis software (Applied Biosystems) to analyze these data. This software uses the comparative threshold cycle (ΔΔCT) method to quantify relative gene expression across a large number of genes and samples. The software provides options to normalize expression data using either global normalization or endogenous controls and calculates fold changes with P values. Gene expression data were normalized to 5 endogenous controls (18S, Gusb, Rplp2, B2m, and Hprt), although we report Gusb and Rplp2 data here. In all experiments, the CT upper limit was set to 40, meaning that all mRNA detectors with a CT value greater than or equal to 40 were excluded. The multiple-comparisons correction (Benjamini-Hochberg method for false-discovery rate [FDR]) was applied to the data and P ≤ .05 was considered significant. Additionally, individual qRT-PCRs were performed to monitor the expressions of Sp7 (osterix, Mm00504574_m1) and Bglap (OCN, Mm03413826_mH) using Rplp2 as the normalizer (Mm03059047_gH). The prepared cDNA was used to set up qRT-PCRs using FastStart Universal Probe Master mix (Rox) (Roche Life Science).
ChIP-seq and ChIP analysis
Cells from ATCC (MC3T3-E1 subclone 4) were seeded into 21 150-mm plates at an initial density of 50 000 cells/plate (320 cells/cm2) and maintained in α-MEM complete medium + ascorbic acid. On day 14 after seeding, cells were treated with 25nM hPTH (1–34) or vehicle control for 1 hour before harvest. Subsequent to treatment cells were fixed with 1% formaldehyde for 15 minutes and quenched with 0.125M glycine. Cell pellets were frozen in an ethanol dry ice bath and shipped to Active Motif for FactorPath analysis. The chromatin was isolated from the pellets by adding lysis buffer followed by disruption with a Dounce homogenizer. Lysates were sonicated and the DNA sheared to an average length of 300–500 bp. Genomic DNA (Input) was prepared by treating aliquots of chromatin with ribonuclease, proteinase K and heat for decross-linking, followed by ethanol precipitation. Pellets were resuspended and the resulting DNA was quantified on a NanoDrop spectrophotometer. Extrapolation to the original chromatin volume allowed quantitation of the total chromatin yield. An aliquot of chromatin (30 μg) was precleared with protein A agarose beads (Invitrogen, Thermo Fisher Scientific). Genomic DNA regions of interest were isolated using 4-μg antibody against ZNF384 (lot A57874; Sigma HPA004051). Complexes were washed, eluted from the beads with SDS buffer, and subjected to ribonuclease and proteinase K treatment. Cross-links were reversed by incubation overnight at 65°C, and ChIP DNA was purified by phenol-chloroform extraction and ethanol precipitation.
ChIP-seq (Illumina)
ChIP and Input DNAs were prepared for amplification by converting overhangs into phosphorylated blunt ends and adding an adenine to the 3′-ends. Illumina genomic adapters were ligated and the sample was size-fractionated (200–300 bp) on an agarose gel. After a final PCR amplification step (18 cycles), the resulting DNA libraries were quantified and sequenced on HiSeq 2000. Sequences (50nt reads, single end) were aligned to the mouse genome (mm10) using the Burrows-Wheeler algorithm. Alignments were extended in silico at their 3′-ends to a length of 150 bp, which is the average genomic fragment length in the size-selected library, and assigned to 32-nt bins along the genome. The resulting histograms (genomic “signal maps”) were stored in BAR and bigWig files. ZFP384 peak locations were determined using the MACS algorithm (v1.4.2) with a cutoff of P = 1e-7 (36).
Bioinformatic profiling
In addition to generating our own Nmp4 ChIP-seq data from the MC3T3-E1 cells we used Nmp4 (Znf384) ChIP-seq data from murine embryonic stem cell line (ES-E14) and the B cell lymphoma cell lines Ch12 and MEL from the ENCODE Consortium for transcription factors 2011 Freeze datasets in NarrowPeak format (37). To assign an Nmp4 peak to a promoter region it had to be within −5 to +2 kb from a transcription start site (TSS). To assign a peak to an intragenic region it had to be located within the range defined by the TSS and the transcription end site, and not within the promoter range of the same gene. To assign a peak to an intergenic region, it had to be −10 000 kb from the TSS and +10 000 kb from the transcription end site, and not within the promoter range of the same gene. A peak could be assigned to multiple functional regions in an area of the genome harboring multiple genes. A common example of this is an area with genes on both strands. A peak may not fit any of these definitions and was assigned to the classification “other.” This methodology yielded 34 317 functional assignments for the peaks in the MC3T3-E1 cells.
Genome wide event finding and motif discovery (GEM) analysis
GEM (38) was used to derive the Nmp4 consensus sequence. The latest mouse genome build (mm10) was employed together with the GEM default ChIP-seq read distribution file and a minimal k-mer width of 6 and maximum of 20.
Gene ontology
GO analysis was conducted using Database for Annotation, Visualization, and Integrated Discovery (DAVID) (39), and terms summarized using REVIGO (40). The ENCODE ChIP-Seq Significance Tool was employed to identify enriched transcription factors in our Nmp4 gene target list (41). Additionally some functional analysis was also generated through the use of QIAGEN's ingenuity pathway analysis (QIAGEN Redwood City; www.qiagen.com/ingenuity).
Bone phenotype statistical analysis
Statistical evaluations were processed using the program JMP version 7.0.1 (SAS Institute). The animal studies employed a two-way ANOVA using genotype and treatment as the independent variables followed by either a Tukey's honest significant difference or LS Means post hoc test if a genotype × treatment interaction was indicated. Statistical significance was set at P ≤ .05. To compare growth rates of the WT and Nmp4−/− MSPCs derived from various experimental mice, we evaluated the slopes of log-transformed cell counts regressed onto experimental day using a t test. The numbers of mice per treatment group and replicates/treatment for the cell studies are indicated in the appropriate figures and tables.
Results
Nmp4−/− mice are not protected from ovx-induced bone loss
To determine whether genetically disabling Nmp4 activity protects mice from ovx-induced bone loss as it does from unloading-associated osteopenia (16), we removed the ovaries or performed sham operations on both WT and Nmp4−/− mice (Figure 1). Both the ovx WT and ovx Nmp4−/− mice experienced significant weight gain at 4-week postoperation (postop) (Table 1) consistent with previous mouse studies (42). Additionally, ovx resulted in a significant decrease in uterine weight in both genotypes (Table 1). There was no genotype × treatment interaction in either of these parameters.
Figure 1.
Schematic of treatment regimen for WT and Nmp4−/− mice; group 1 mice were subjected to ovx or sham operation at 12 weeks of age and evaluated for bone loss 4-week postop (16 wk of age). Group 2 mice were ovx at 12 weeks of age and began PTH or vehicle therapy at 16 weeks of age for a duration of 4 and 8 weeks. Endpoint analyses included μCT, serum analysis for P1NP and CTXs, and dynamic histomorphometry.
Table 1.
Bone Loss Data
| WT |
Nmp4−/− |
Two-Way ANOVA P Values |
|||||
|---|---|---|---|---|---|---|---|
| Sham | ovx | Sham | ovx | Genotype | Treatment | Gene × Treat | |
| %Δ body weight | 2.48 ± 7.73 | 8.65 ± 5.48 | 4.14 ± 4.70 | 5.66 ± 2.94 | .69 | 0.03 | .17 |
| Uterine weight (g) | 0.10 ± 0.05 | 0.04 ± 0.02 | 0.10 ± 0.02 | 0.05 ± 0.02 | .30 | <0.0001 | .34 |
| Distal femur | |||||||
| BV/TV | 0.019 ± 0.004 | 0.012 ± 0.004 | 0.038 ± 0.011 | 0.021 ± 0.010 | <.0001 | <0.0001 | .06 |
| SMI | 3.818 ± 0.250 | 4.055 ± 0.357 | 3.387 ± 0.263 | 3.810 ± 0.294 | .0008 | 0.0011 | .32 |
| Tb.N (mm−1) | 2.554 ± 0.239 | 2.165 ± 0.385 | 3.128 ± 0.218 | 2.797 ± 0.276 | <.0001 | 0.0004 | .76 |
| Tb.Th (mm) | 0.040 ± 0.005 | 0.041 ± 0.005 | 0.039 ± 0.003 | 0.037 ± 0.004 | .08 | 0.87 | .23 |
| Tb.Sp (mm) | 0.393 ± 0.036 | 0.477 ± 0.097 | 0.317 ± 0.026 | 0.359 ± 0.037 | <.0001 | 0.0012 | .25 |
| L5 vertebra | |||||||
| BV/TV | 0.189 ± 0.028 | 0.177 ± 0.013 | 0.253 ± 0.019 | 0.212 ± 0.019 | <.0001 | 0.0004 | .05 |
| Tb.N (mm−1) | 3.797 ± 0.513 | 3.580 ± 0.285 | 4.491 ± 0.345 | 4.022 ± 0.254 | <.0001 | 0.0091 | .32 |
| Tb.Th (mm) | 0.050 ± 0.003 | 049 ± 0.002 | 0.056 ± 0.002 | 0.053 ± 0.002 | <.0001 | 0.0032 | .02 |
| Tb.Sp (mm) | 0.227 ± 0.023 | 0.229 ± 0.013 | 0.202 ± 0.020 | 0.214 ± 0.012 | .0013 | 0.25 | .44 |
| Serum P1NP | WT: Nmp4−/− |
P Values [G; T; G × T]4 wks | P Values [G; T; G × T]12 wks | ||
|---|---|---|---|---|---|
| Preop | Postop4 wks | Postop12 wks | |||
| 5.62 ± 1.21: 6.02 ± 1.41 | 5.83 ± 1.41: 4.77 ± 1.22 | 3.15 ± 0.65: 2.81 ± 0.76 | .34; .16; .05 | .95; <.0001; .36 | |
| Serum CTX | WT: Nmp4−/− |
P Values [G; T; G × T]4 wks | P Values [G; T; G × T]12 wks | ||
|---|---|---|---|---|---|
| Preop | Postop4 wks | Postop12 wks | |||
| 13.66 ± 2.43: 13.37 ± 1.88 | 12.96 ± 3.04: 13.53 ± 2.58 | 11.47 ± 2.24: 9.36 ± 1.22 | .92; .46; .96 | .14; .0003; .18 | |
The % change body weight, uterine weight, and μCT of distal femur and L5 vertebra from WT and Nmp4−/− mice after ovx or sham operation 4-week postop. Serum bone formation (P1NP) and bone resorption (CTX) marker levels were compared in mice previous to the operation (preop) and 4 and 12 weeks after surgery (postop). The 12-week postop data were obtained from the vehicle-control treatment groups. Data are average ± SD, number of mice/experimental group = 8–14 (4 mice in WT sham uterine weight). Statistical significance was set at P ≤ .05, and differences were determined using a two-way ANOVA.
Both WT and Nmp4−/− mice exhibited significant bone loss 4 weeks after ovx surgery as measured in the trabecular bone compartment of the distal femur and the L5 vertebra (Table 1). The Nmp4−/− mice exhibited a trend towards enhanced loss of bone that neared significance in the distal femur (BV/TV, genotype × treatment interaction = 0.06) (Table 1) and reached significance in the L5 vertebra (BV/TV, genotype × treatment interaction < 0.05) (Table 1). Despite this enhanced (or nearly enhanced) rate of bone loss the Nmp4−/− animals maintained more trabecular bone compared with WT mice during the first 4 weeks after ovx. The Nmp4−/− mice exhibited a decrease in the serum bone formation marker P1NP at 4-week postop and both genotypes showed significant decreases in this marker at 12-week postop in the vehicle-treated mice. A small decrease in the serum bone resorption marker CTX was observed at 12-week postop in the vehicle-treated mice.
Ovx Nmp4−/− mice show an enhanced bone gain response to PTH therapy
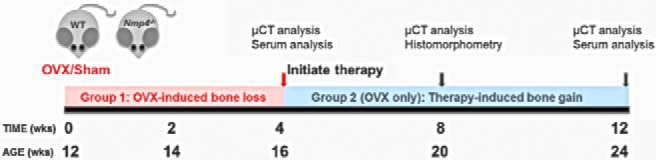
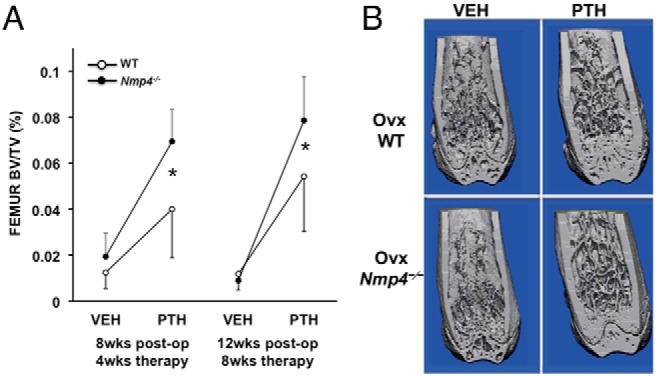
With a separate group of ovx mice we initiated treatment of both WT and Nmp4-null ovx animals with PTH (30 μg/kg · d) or vehicle control 4 weeks after surgery. The duration of hormone therapy lasted 4 weeks (8-wk postop) and 8 weeks (12-wk postop). The ovx Nmp4−/− mice showed an enhanced PTH-induced gain in femoral BV/TV and Conn.D at 4 and 8 weeks of therapy compared with their ovx WT littermates as well as an augmented gain in Tb.Th at 8 weeks (Figure 2 and Table 2). The null mice also showed an enhanced PTH response at the L5 vertebra at 8 weeks of treatment (Figure 3 and Table 2). Specifically the two-way ANOVA indicated strong genotype × treatment effects for the distal femur for both 4 and 8 weeks of therapy and for the L5 vertebra for 8 weeks of therapy (see Figures 2A and 3A); the post hoc tests concluded that the difference between the genotypes was within the hormone-treated groups. The vehicle-treated ovx WT and ovx Nmp4−/− groups showed no difference in BV/TV (Figures 2 and 3) at the end of the treatment regimens indicating that the modest enhanced loss in bone in the Nmp4−/− mice was stabilized by 4 weeks of therapy. PTH significantly elevated MAR, MS/BS, and BFR at the end of 4 weeks of treatment as shown by strong treatment effects (Table 3). However, there was no genotype effect or genotype × treatment interaction for any of these parameters (Table 3). Hormone significantly elevated serum levels of the bone formation marker P1NP and the resorption marker CTX at 8 weeks of therapy, but there was no treatment × genotype interaction for either of these parameters (Table 3).
Figure 2.
Disabling Nmp4 enhances PTH restorative therapy in the distal femur of ovx Nmp4−/− mice. A, Interaction plots of femoral trabecular BV/TV of ovx WT and ovx Nmp4−/− mice as determined by μCT at 4 and 8 weeks of treatment. Data are average ± SD, number of mice/experimental group = 8–9. Statistical differences were determined using a two-way ANOVA, and significance was set at P ≤ .05. The Tukey's honest significant difference post hoc test was used to determine differences between the treatment groups. There were genotype, treatment and genotype × treatment interaction at both time points. There was no difference between the vehicle-treated WT and Nmp4−/− mice. B, μCT images showing PTH-induced improvements in distal femur trabecular architecture in ovx WT and Nmp4−/− mice after 8 weeks of treatment (12-wk postop, 24 wk of age).
Table 2.
PTH-Induced Bone Gain Data
| WT |
Nmp4−/− |
Two-Way ANOVA P Values |
|||||
|---|---|---|---|---|---|---|---|
| VEH | PTH | VEH | PTH | Genotype | Treatment | Gene × Treat | |
| Distal femur | |||||||
| 4 wk | |||||||
| Conn.D (mm−3) | 3.180 ± 3.870 | 33.230 ± 26.730 | 9.681 ± 15.979 | 67.533 ± 14.111 | .0018 | <.0001 | .03 |
| SMI | 3.752 ± 0.437 | 3.013 ± 0.384 | 3.472 ± 0.327 | 2.514 ± 0.113 | .0025 | <.0001 | .36 |
| Tb.N (mm−1) | 2.100 ± 0.519 | 2.441 ± 0.281 | 2.712 ± 0.241 | 2.833 ± 0.224 | .0002 | .06 | .36 |
| Tb.Th (mm) | 0.039 ± 0.010 | 0.042 ± 0.007 | 0.033 ± 0.003 | 0.044 ± 0.003 | .54 | .004 | .09 |
| Tb.Sp (mm) | 0.510 ± 0.157 | 0.409 ± 0.051 | 0.370 ± 0.036 | 0.342 ± 0.032 | .0019 | .04 | .24 |
| 8 wk | |||||||
| Conn.D (mm−3) | 3.123 ± 5.307 | 38.658 ± 14.910 | 0.982 ± 1.103 | 58.128 ± 13.570 | .03 | <.0001 | .0064 |
| SMI | 3.808 ± 0.479 | 2.470 ± 0.284 | 3.589 ± 0.218 | 2.262 ± 0.141 | .05 | <.0001 | .96 |
| Tb.N (mm−1) | 2.132 ± 0.297 | 2.164 ± 0.431 | 2.286 ± 0.145 | 2.552 ± 0.277 | .02 | .17 | .28 |
| Tb.Th (mm) | 0.037 ± 0.006 | 0.048 ± 0.005 | 0.030 ± 0.004 | 0.049 ± 0.003 | .12 | <.0001 | .02 |
| Tb.Sp (mm) | 0.476 ± 0.072 | 0.471 ± 0.109 | 0.438 ± 0.033 | 0.378 ± 0.045 | .01 | .20 | .27 |
| L5 vertebra | |||||||
| 4 wk | |||||||
| Tb.N (mm−1) | 3.453 ± 0.451 | 4.875 ± 0.587 | 3.891 ± 0.504 | 5.518 ± 0.381 | .0049 | <.0001 | .56 |
| Tb.Th (mm) | 0.051 ± 0.002 | 0.049 ± 0.002 | 0.054 ± 0.004 | 0.051 ± 0.001 | .04 | .03 | .60 |
| Tb.Sp (mm) | 0.246 ± 0.021 | 0.224 ± 0.030 | 0.229 ± 0.021 | 0.197 ± 0.021 | .02 | .0036 | .52 |
| 8 weeks | |||||||
| Tb.N (mm−1) | 4.046 ± 0.917 | 5.648 ± 1.191 | 3.627 ± 0.235 | 5.906 ± 0.754 | .79 | <.0001 | .26 |
| Tb.Th (mm) | 0.053 ± 0.003 | 0.049 ± 0.004 | 0.055 ± 0.001 | 0.054 ± 0.001 | .0018 | .0044 | .09 |
| Tb.Sp (mm) | 0.239 ± 0.021 | 0.206 ± 0.037 | 0.256 ± 0.020 | 0.186 ± 0.023 | .86 | <.0001 | .05 |
μCT (distal femur and L5 vertebra) from ovx WT and ovx Nmp4−/− mice after 4 and 8 weeks of PTH/VEH therapy. Data are average ± SD, number of mice/experimental group = 8–9. Statistical significance was set at P ≤ .05, and differences were determined using a two-way ANOVA.
Figure 3.
The exaggerated response to anabolic PTH persists in the L5 vertebra of ovx Nmp4−/− mice. A, Interaction plots of L5 vertebra BV/TV of ovx WT and ovx Nmp4−/− mice as determined by μCT at 4 and 8 weeks of treatment. Data are average ± SD, number of mice/experimental group = 8–9. Statistical differences were determined using a two-way ANOVA, and significance was set at P ≤ .05. The LS Means Student's t post hoc test was used to determine differences between the treatment groups. There were genotype, treatment effects at both time points, and a genotype × treatment interaction at 8 weeks of therapy. There was no difference between the vehicle-treated WT and Nmp4−/− mice. B, μCT images showing PTH-induced improvements in L5 trabecular architecture in ovx WT and Nmp4−/− mice after 8 weeks of treatment (12-wk postop, 24 wk of age).
Table 3.
Histomorphometry and Serum Analyses
| WT |
Nmp4−/− |
Two-Way ANOVA P Values |
|||||
|---|---|---|---|---|---|---|---|
| VEH | PTH | VEH | PTH | Genotype | Treatment | Gene × Treat | |
| Dynamic histo | |||||||
| MAR (μm/d) | 2.28 ± 0.37 | 3.80 ± 0.73 | 2.29 ± 0.37 | 3.61 ± 0.40 | .70 | <.0001 | .66 |
| MS/BS (%) | 0.41 ± 0.09 | 0.55 ± 0.05 | 0.44 ± 0.10 | 0.52 ± 0.06 | .98 | .01 | .45 |
| BFR (μm2/μm · d) | 0.95 ± 0.28 | 2.09 ± 0.52 | 1.01 ± 0.25 | 1.86 ± 0.22 | .60 | <.0001 | .37 |
| Serum | |||||||
| WT |
Nmp4−/− |
Two-Way ANOVA P Values |
|||||
|---|---|---|---|---|---|---|---|
| VEH8 wks | PTH8 wks | VEH8 wks | PTH8 wks | Genotype | Treatment | Gene x Treat | |
| P1NP (ng/mL) | 3.147 ± 0.653 | 10.066 ± 2.659 | 2.806 ± 0.760 | 8.042 ± 3.304 | .19 | <.0001 | .34 |
| CTX (ng/mL) | 11.466 ± 2.239 | 15.147 ± 3.518 | 9.361 ± 1.222 | 14.157 ± 1.532 | .12 | .0002 | .56 |
Dynamic bone histomorphometry data of the distal femur from WT and Nmp4−/− mice treated with intermittent PTH or vehicle for 4 weeks (8-wk postop). Sera data were collected at the end of 8 weeks of treatment (12-wk postop). The parameters include MAR, MS/BS, and BFR. Data are average ± SD, number of mice/experimental group = 4–7. A two-way ANOVA was used to determine statistical differences, and significance was set at P ≤ .05.
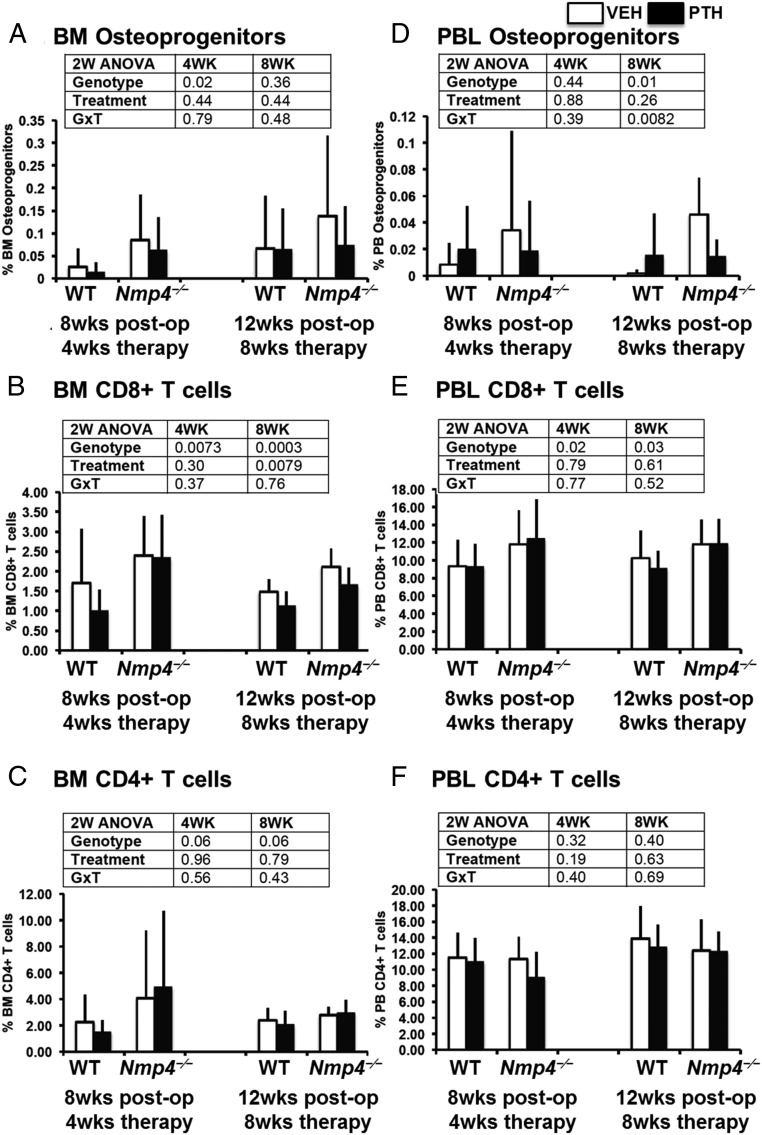
FACS analysis of the BM CD45−/CD105+/CD146+/nestin+ osteoprogenitors revealed a significant elevation in the number of these cells in the BM obtained from the Nmp4−/− mice at the end of 4 weeks of therapy, irrespective of treatment (Figure 4A). This is consistent with our previous observation in the ovary-intact null mice (12). By the end of 8 weeks of treatment (12-wk postop) the observed increase in the number of these Nmp4−/− cells in the BM failed to reach statistical significance, but there was a significant elevation in the number of the PBL Nmp4−/− osteoprogenitors in the vehicle-treated mice (Figure 4D). The Nmp4−/− mice showed a significant elevation in CD8+ T cells in both the BM and the PBL throughout the entire therapy regimen (Figure 4, B and E). PTH significantly decreased the numbers of these cells in the BM at 8 weeks of therapy in both genotypes (Figure 4B) but had no impact on the number of these cells in the PBL (Figure 4E). Disabling Nmp4 had little to no effect on CD4+ T cells, nor did treatment with PTH (Figure 4, C and F). The modest increase in BM CD4+ T cells approached significance (P < .06) but this was not reflected in the PBL, just as we previously observed in the ovary-intact mice (12).
Figure 4.
Ovx does not abrogate the expanded population of osteoprogenitors and CD8+ T cells in Nmp4−/− mice. FACS analysis of BM and PBL osteoprogenitors, CD8+ T cells, and CD4+ T cells. A and D, The frequency of femoral BM and PBL CD45−/CD105+/CD146+/CD105+/nestin+ osteoprogenitor cells in WT and Nmp4−/− mice at the end of 4 and 8 weeks of treatment with intermittent PTH or vehicle control. B and E, The frequency of BM and PBL CD8+ T cells from the WT and Nmp4−/− mice. C and F, The frequency of BM and PBL CD4+ T cells from the WT and null mice. Data are average ± SD, number of mice/experimental group = 8–9. Statistical differences were determined using a two-way ANOVA, and significance was set at P ≤ .05.
To determine whether the enhanced osteogenic potential of the BM could be reliably and reproducibly maintained in vitro in MSPC cultures over several passages and in the absence of supporting cells (eg, T cells), we established expanded WT and Nmp4−/− MSPCs from ovary-intact mice. The expanded Nmp4−/− MSPCs from ovary-intact mice exhibited modest but significantly enhanced proliferation compared with the WT cells (Figure 5A). Both the null and WT expanded MSPCs showed strong alkaline phosphatase expression (Figure 5B). However, the expanded Nmp4−/− MSPCs typically showed an accelerated and enhanced mineralization compared with WT cells under various concentrations of dexamethasone and ascorbic acid (Figure 5B). Finally, the expanded Nmp4−/− and WT MSPCs exhibited varying degrees of alkaline phosphatase staining while maintained in MesenCult medium, depending on the confluence of the cells and time in culture (3–9 d); however, no mineralization was observed in these control cultures (data not shown).
Figure 5.
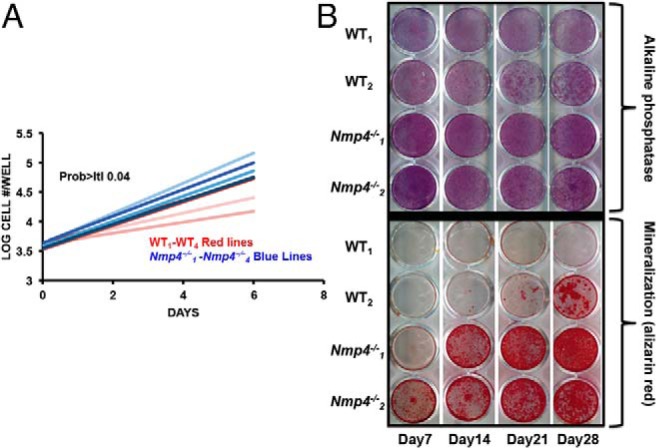
Expanded Nmp4−/− MSPCs exhibit enhanced proliferation and mineralization in culture. A, Comparative growth rates of expanded WT and Nmp4−/− MSPCs. Cell counts/d (n = 4 lines per genotype log10 cells/well, 3 wells/sample, average ± SD, t test, t < 0.05). Note: each “line” is derived from a single mouse. B, Alkaline phosphatase (alk phos) and alizarin red staining of a WT and Nmp4−/− MSPC cultures from day 7 to 28. See text for details.
Genome-wide ChIP-seq/GO analysis reveals Nmp4 target genes and potential pathways of the antianabolic axis
Nmp4 is expressed in nearly all cells, yet the most singular consequence of globally disabling this protein is the enhanced mobilization of bone cells upon osteoanabolic induction (11–13, 15). As a first step in understanding the origins of this phenotype, which may have clinical significance, we needed the next information: 1) the identity of the Nmp4 target genes including “core” target genes common to multiple cell types; 2) identify common functions of these core genes to distinguish pathways that make osteoprogenitors particularly vulnerable to the effects of Nmp4; and 3) experimental confirmation of some of these pathways. To begin to understand how Nmp4 works, we set out to understand 4) whether Nmp4 targets functional regions of the genome; 5) if it binds directly to DNA or via other proteins; and 6) whether osteoanabolic agents, eg, PTH, alter Nmp4 DNA-binding along target genes.
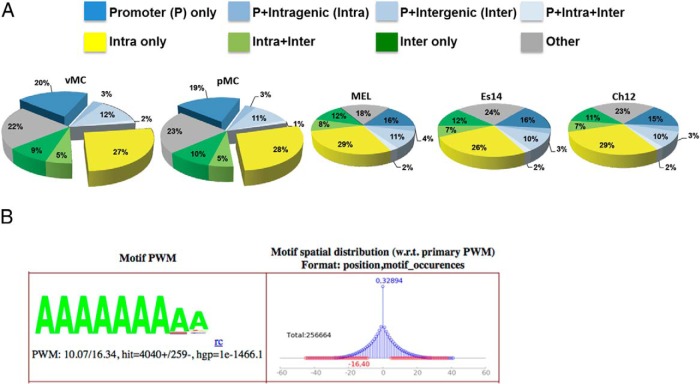
The potential Nmp4 target genes identified by ChIP-seq in the MC3T3-E1 (vehicle-treated) cells and those established in the 3 ENCODE cell lines were compared using those genes that had 1 or more peaks associated with the TSS. A Venn diagram of these genes showed that 2114 Nmp4 core target genes were common to the 4 cell lines (Figure 6A and Supplemental Table 2). These core target genes were classified into functionally related categories using GO analysis with the DAVID tool (39). The functional annotation-clustering algorithm was applied to the target list, which is able to give a more insightful view of the relationships between annotation categories and terms compared with other analytic modules (39). The significance of group classification was defined by enrichment scores based on Fisher exact statistics (FDR P < .05). The DAVID-derived biological profile was further summarized using REVIGO (40). GO analysis of the core target genes designated Nmp4 as a negative regulator of cellular biosynthetic processes showing significant enrichment for genes involved in the regulation of transcription, chromatin modification, protein catabolic processes, regulation of the cell cycle, and mRNA processing/splicing (Figure 6B). Interestingly, the genes specific to any one particular cell line or specific to vehicle-treated or PTH-treated MC3T3-E1 cells did not yield a distinct biological process profile that reached statistical significance as obtained with the core target genes (data not shown). However, peak-associated genes common to the vehicle- and PTH-treated MC3T3-E1 cells yielded a profile nearly identical to that obtained with the core target genes.
Figure 6.
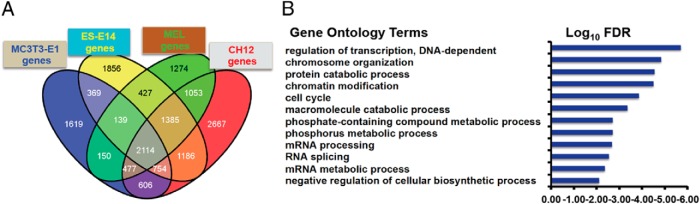
Nmp4 associates with core target genes common to multiple cell types and acts as a negative regulation of cellular biosynthetic processes. A, Venn diagram illustrating the shared Nmp4 target genes in the MC3T3-E1 osteoblast-like cells (vehicle treated), and the 3 ENCODE cells lines, ES-E14 (embryonic stem cells), MEL, and CH12 cells (B cell lymphomas). B, DAVID/REVIGO GO profile of Nmp4 core target genes.
Next, we probed existing datasets for enriched transcription factors within our Nmp4 core target gene list using the ENCODE ChIP-seq Significance Tool (Table 4) (41). This profile shows that Nmp4 binding in the promoter regions of its target genes predominantly cooccurs with proteins that regulate chromatin organization and with proteins that contribute to maintaining stem/progenitor pluripotency/multipotency and the poised gene state, eg, chromodomain helicase DNA binding protein 2, Swi-independent 3a, and K(lysine) acetyltransferase 2A (KAT2a) (43–45).
Table 4.
ENCODE ChIP-Seq Significance Tool Profile for Enriched TFs Within the Nmp4 Target Core Gene List
| Factor | Q Value | Factor | Q Value |
|---|---|---|---|
| Nmp4 | 0.00E + 00 | Max | 0.00E + 00 |
| CHD2 | 0.00E + 00 | Mxi1 | 0.00E + 00 |
| CTCF | 0.00E + 00 | NELFe | 0.00E + 00 |
| GCN5 | 0.00E + 00 | Pol2 | 0.00E + 00 |
| HCFC1 | 0.00E + 00 | SIN3A | 0.00E + 00 |
| MAZ | 0.00E + 00 | TBP | 0.00E + 00 |
| p300 | 0.00E + 00 | c-Myc | 7.352e-317 |
TF, transcription factor; Q value, hypergeometric test; Benjamini-Hochberg (select TFs from 72 entries).
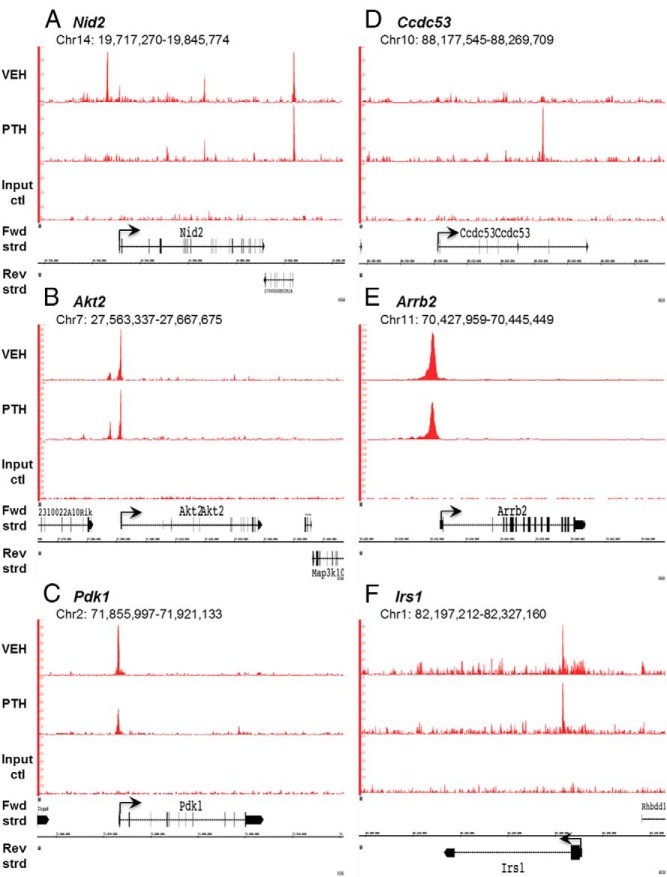
In an effort to gain further understanding of how Nmp4 regulates gene expression, we prepared a genome-wide functional region map of the Nmp4 binding sites for all 4 cell types as described in Materials and Methods. Most the occupancy peaks were located in or near the TSS or in intragenic regions, areas typically associated with regulatory functions (Figure 7A). To determine whether Nmp4 binds directly to DNA or can associate with the genome via other proteins we used the discovery algorithm GEM to derive the Nmp4 consensus-binding site from the MC3T3-E1 data. In support of previous studies by our lab and others the derived binding site matched the unusual homopolymeric (dA · dT) consensus sequence previously derived by cyclic amplification and electrophoretic mobility shift assay (Figure 7B) (24, 25). No other consensus sequences were identified suggesting a single and direct mode of genome association, mediated by the Cys2His2 DNA-binding domain (28). To determine whether PTH challenge altered Nmp4 DNA-binding along target genes we generated genome-wide Nmp4 ChIP-seq profiles using the preosteoblast cell line MC3T3-E1 treated with hPTH (1–34) or vehicle control for 1 hour. We used the 1-hour time point because we observed the most significant differences in femoral mRNA expression profiles between WT and Nmp4−/− mice 1 hour after injection (11). Hormone reduced Nmp4 genome-wide occupancy from a total of 15 446 to 13 109 binding sites. However, at the level of the single gene there was a diversity of changes in Nmp4 occupancy, ie, PTH was observed to remove (eg, Nid2), induce (eg, Ccdc53) or have no effect on Nmp4-DNA association (eg, Akt2 and Arrb2) (Figure 8; also see ChIP-quantitative PCR confirmation of Nmp4 binding; Supplemental Figure 1).
Figure 7.
Nmp4 binds to AT-rich DNA typically proximal to TSS sites or within intragenic regions. A, Genome-wide mapping of the Nmp4 binding sites show that most sites are distributed in the TSS and intragenic regions of the genome. ChIP-seq analysis included vehicle-treated and PTH-treated MC3T3-E1 osteoblast-like cells (vMC and pMC, respectively) and 3 murine cell lines from the ENCODE Consortium, including ES-E14 (Es14), which are E14 undifferentiated mouse embryonic stem cells, and 2 mouse erythroleukemia cell lines (Ch12 and MEL) derived from B cell lymphomas. B, GEM analysis for the Nmp4 consensus sequence derived from MC3T3-E1 cells. A minimal k-mer width of 6 and maximum of 20 was used. The optimal position weight matrix (PWM) score for the MC3T3-E1 data was 10.07. The hypergeometric P value (hgp) was 1e-1466.1.
Figure 8.
ChIP-seq reveals Nmp4 binding profiles at specific gene loci. Mouse MC3T3-E1 cells were seeded into 21 150-mm plates at an initial density of 50 000 cells/plate (320 cells/cm2) and maintained in α-MEM complete medium + ascorbic acid for 14 days. Before harvest cells were treated with 25nM hPTH (1–34) or vehicle control for 1 hour. Processing for ChIP-seq analysis was performed as described in Materials and Methods. Sequences (50-nt reads, single end) were aligned to the mouse genome (mm10) using the Burrows-Wheeler algorithm. Alignments were extended in silico at their 3′-ends to a length of 150 bp, which is the average genomic fragment length in the size-selected library, and assigned to 32-nt bins along the genome. Nmp4 (Znf384) peak locations were determined using the MACS algorithm (v1.4.2) with a cutoff of P = 1e-7. The genomic loci including the chromosome number and nucleotide interval are indicated. Read scales are indicated on the y-axis. An arrow indicates the transcriptional start sites and direction of transcription for each of the genes; vertical boxes within the gene indicate exons. The Nmp4 ChIP-seq gene profiles include (A) Nid2 (B) Akt2, (C) Pdk1, (D) ccdc53, (E) Arrb2, and (F) Irs1. The input DNA profiles were devoid of peaks.
As a first step in the validation of the ChIP-seq-derived antianabolic map we interrogated 90 mRNA transcripts in nondifferentiating and osteogenic differentiating WT and Nmp4−/− expanded MSPCs at 5 different time points. The accelerated and enhanced mineralization of the Nmp4−/− MSPCs (Figure 5) is consistent with our previous observation that in response to PTH Nmp4−/− mice add more bone and add it faster than WT mice (11). Our choice of Nmp4 target genes (Supplemental Table 1) was based on our DAVID analysis. We chose both core target genes and Nmp4 target genes identified in the MC3T3-E1 preosteoblasts. DAVID also uses Kyoto Encyclopedia of Genes and Genomes (KEGG) database to map large gene lists to signaling pathways (39). For example the DAVID/KEGG profile of the Nmp4 core target genes included the target of rapamycin and insulin/IGF-1 signaling pathways (Table 5) and indeed the insulin/IGF-1→insulin receptor substrate 1→PI3K→3-phosphoinositide dependent protein kinase-1→AKT signaling response limb is common to many of the pathways listed. This is also consistent with our ingenuity pathway analysis (Supplemental Table 3). Also included were Nmp4 target genes coding for proteins involved in the ubiquitin-proteasome system, chromatin remodeling, transcription regulation, and RNA processing. Finally, we analyzed the expression of osteogenic differentiation markers.
Table 5.
DAVID Profile of KEGG Pathway Mapping
| GO Term Pathways | FDR |
|---|---|
| Target of rapamycin signaling pathway | 0.003 |
| Insulin signaling pathway | 0.004 |
| Chronic myeloid leukemia | 0.026 |
| JAK-STAT signaling pathway | 0.026 |
| Neurotrophin signaling pathway | 0.034 |
Only pathways with an FDR of P < .05 are listed.
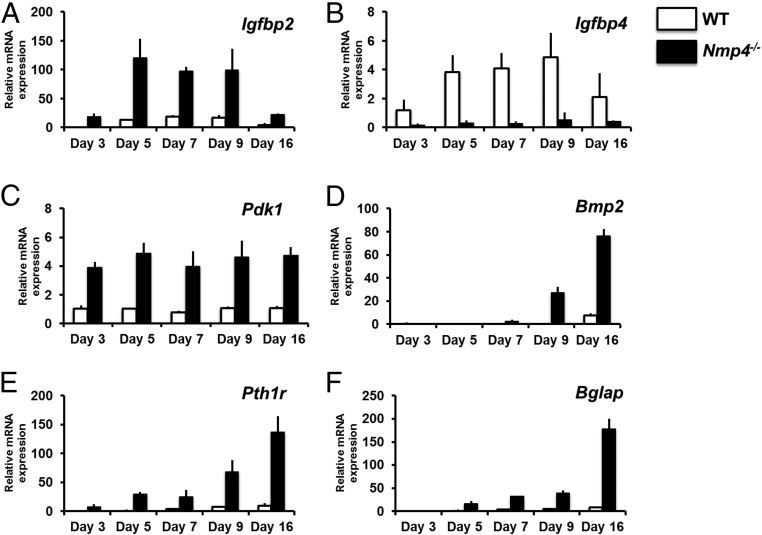
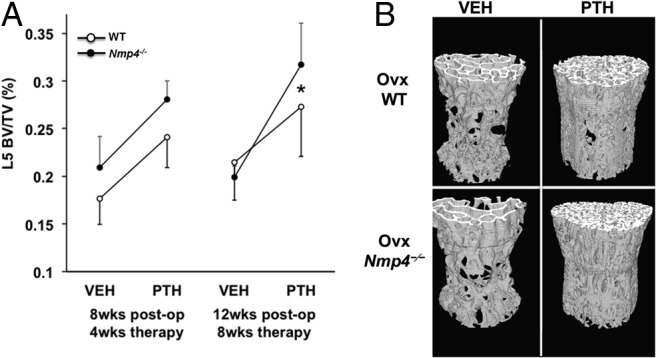
Volcano plots (Supplemental Figure 2) identify genes that were statistically significantly up-regulated or down-regulated by at least 2-fold in the Nmp4−/− cells compared with the WT cohort on the same day of culture. Figures 9 and 10 compare the relative mRNA expression of select genes with the level of the transcript in WT cells on day 3 of culture thus providing a time-course view. The Nmp4−/− cells showed a strikingly elevated expression of the Nmp4 target gene insulin-like growth factor binding protein 2 (Igfbp2) mRNA and down-regulation of the target gene Igfbp4 mRNA during the early differentiation period (Figure 9, A and B). IGFBP2 stimulates osteoblast differentiation, whereas the IGFBP4 is a potent inhibitor of Igf actions (46, 47). 3-phosphoinositide dependent protein kinase-1 (Pdk1, target gene) a key component of the IGF1/insulin signaling pathway (48) was up-regulated in the Nmp4−/− cells throughout the developmental time course (Figure 9C). Interestingly, neither the target genes Igf1 nor its receptor Igf1r exhibited striking differences in gene expression between the 2 genotypes (data not shown). The Nmp4−/− cells exhibited an enhanced anabolic profile during the latter differentiation period as evidenced by elevated expression levels of the nontarget genes Bmp2 (Figure 9D), Pth1r (Figure 9E), and Bglap (OCN) (Figure 9F).
Figure 9.
Comparison of mRNA expression profiles derived from nondifferentiating (d 3) and osteogenic-differentiating (d 5–16) WT and Nmp4−/− cells. All transcript levels are compared with WT day 3 providing a time course of expression. mRNA profiles (A) Igfbp2, (B) Igfbp4, (C) Pdk1, (D) Bmp2, and (E) Pth1r were derived from the TLDA system (Format 96a; Applied Biosystems) performed on a QuantStudio 7 Flex Real-Time PCR System and normalized with GusB. F, Bglap mRNA profile qRT-PCRs were performed on an Eppendorf Mastercycler RealPlex2 using Rplp2 Mm03059047_gH) as the normalizer, as previously described (Robling et al [13]). Comparison of profiles using GusB and Rplp2 as the normalizer showed no differences in the shape of the expression profiles.
Figure 10.
Comparison of mRNA expression profiles derived from nondifferentiating (d 3) and osteogenic-differentiating (d 5–16) WT and Nmp4−/− cells. All transcript levels are compared with WT day 3 providing a time course of expression. mRNA profiles (A) Cxcl12, (B) Plaur, (C) Spp1, (D) Thbs2, (E) Col1a1 were derived from the TLDA system (Format 96a; Applied Biosystems) performed on a QuantStudio 7 Flex Real-Time PCR System and normalized with GusB. F, Sp7 mRNA profile qRT-PCRs were performed on an Eppendorf Mastercycler RealPlex2 using Rplp2 (Mm03059047_gH) as the normalizer, as previously described (Robling et al [13]). The day-16 WT sample is the average of 2 replicates. Comparison of profiles using GusB and Rplp2 as the normalizer showed no differences in the shape of the expression profiles.
Chemokine (C-X-C motif) (CXC) ligand 12 (Cxcl12) expression (target gene), also known as stromal-derived factor 1, was dramatically down-regulated in the Nmp4−/− cells throughout development (Figure 10A) and the target gene Plaur (urokinase plasminogen activator receptor [uPAR]) was up-regulated in the null cells (Figure 10B). Both genes play roles in MSPC osteogenic lineage commitment (49–52). Spp1 (osteopontin, target gene) and thrombospondin 2 (Thbs2) (target gene) regulate aspects of mineralization (53, 54); the former was up-regulated in our null cell, whereas the latter was down-regulated (Figure 10, C and D). Type I collagen (Col1a1, target gene) expression was elevated in the Nmp4−/− cells throughout the developmental period (Figure 10E). Interestingly, we observed no substantial difference in the expression profiles of Sp7 (osterix) (Figure 10F) or the target gene Runx2 (data not shown), essential transcription factors for osteoblast differentiation (55).
Discussion
Our findings that the Nmp4−/− mice are not protected from ovx-induced bone loss yet maintain the amplified response to PTH therapy is a key advance necessary for further consideration of Nmp4-based treatment strategies. The ovx Nmp4−/− mice displayed an enhanced hormone-induced recovery of femoral and L5 trabecular BV/TV despite delaying treatment until 4-week postop to allow for significant bone loss. Both the ovx WT and ovx Nmp4−/− mice showed strong responses to PTH therapy. After 4 and 8 weeks of treatment the WT mice displayed a 3.2- and 4.6-fold increase in femoral BV/TV over vehicle-treated mice, respectively. However, the Nmp4−/− mice showed a 3.6- and 8.8-fold increase over the same time period resulting in a very strong genotype × treatment interaction. Differences in PTH-mediated BV/TV restoration efficacy between the WT and Nmp4−/− mice in the L5 vertebra and was less striking although statistically significant (1.3- vs 1.6-fold at 8 wk in the WT and Nmp4−/− mice, respectively). We observed similar PTH-responsive femoral and L5 profiles between younger, ovary-intact WT and Nmp4-null mice (11–13). The histomorphometry and serum data reported here tracked the PTH-induced increases in bone mass in the ovx animals showing strong treatment effects for bone formation parameters MAR, BFR, and MS/BS (at 4 wk of treatment) as well as strong increases in bone remodeling serum P1NP and CTX (at 8 wk of treatment). However, these parameters did not distinguish the genotypes in regards to the amount of bone formed over this time period as was achieved with the μCT data. Interestingly, the histomorphometry data did not distinguish the differences in PTH-induced bone formation in ovary-intact WT and Nmp4−/− mice (11). Our present observation that Nmp4−/− MSPCs exhibit an accelerated and enhanced mineralization in response to anabolic cues, ie, osteogenic medium suggests that the augmented bone formation is an early event. Similarly, we did not observe the expected ovx-induced small increase in serum CTX. Instead, the serum data of the vehicle-treated mice showed a large decrease in P1NP and a smaller decrease in CTX over the time course of the entire experiment, ie, preop vs 12-week postop. This suggests a decrease in bone remodeling in the untreated mice predominantly in the bone formation arm. A more extensive time course with earlier harvest points for histomorphometry and serum samples is required to more fully characterize the anticipated differences in WT and Nmp4−/− dynamic bone remodeling.
The most robust phenotypic characteristic of Nmp4 ablation is the exaggerated bone formation response to PTH or BMP2, which suggests that the adult mice harbor more BM MSPCs with heightened sensitivity to osteoanabolic signals. Disabling Nmp4 has no observable impact on embryonic or perinatal skeletal development. Adult MSPCs are a heterogeneous population of multipotent stem, progenitor, and stromal cells that contribute to BM homeostasis (56). In mouse BM, much of the CFU-F activity is in the nestin+ cell population and in the human marrow the CD146+ population (56, 57). In ovary-intact, Nmp4−/− mice, we observed a 4-fold increase in the frequency of CD45−/CD105+/CD146+/nestin+ cells irrespective of treatment (PTH vs vehicle control), which paralleled the magnitude increase in CFU-F and CFU-Falk phos+ cell number in culture (12). Similarly, the ovx Nmp4−/− mice exhibited an approximate 3-fold increase in the CD45−/CD105+/CD146+/nestin+ cells at 8-week postop compared with the ovx WT animals.
The enhanced osteogenic potential of the Nmp4−/− BM as measured by the frequency of cells capable of becoming osteoprogenitors persists in expanded Nmp4−/− MSPC cultures over 5–10 passages and removed from the supporting CD8+ T cells. In culture these cells displayed a modest increase in proliferative activity and perhaps this aspect of the phenotype contributes to the observed expanded pool of osteoprogenitors in vivo. In an earlier study, Noda and coworkers (15) demonstrated that Nmp4−/− BM yielded significantly more CFU-FOb mineralizing colonies at passage P0 than WT BM. Our present data extend these observations and show that the serially passaged Nmp4−/− MSPCs maintain a strikingly enhanced capacity for mineralization compared with the capacity of the WT cultures. Taken together these observations suggest that there is a cell autonomous role of Nmp4 for regulating MSPC osteogenesis.
Our -omics data combined with our low-density array results suggest that upon challenge with an anabolic cue Nmp4−/− MSPCs produce autocrine/paracrine factors that enhance the replication and differentiation of neighboring osteoprogenitors, a key early event driving the PTH anabolic response (58). We observed that once Nmp4−/− cells were transferred to osteogenic medium they expressed strikingly elevated levels of the Nmp4 target gene Igfbp2, a strong autocrine/paracrine factor that enhances osteogenesis (47). Consistent with our observations, overexpression of Igfbp2 in MC3T3-E1 cells accelerated the time course of differentiation and mineralization as well as increased the total number of differentiating cells. By day 6, in this previous study, Igfbp2-overexpressing cells expressed twice as much OCN as control cultures and this difference persisted (47). This is a strikingly similar phenotype to the Nmp4−/− cells. Interestingly, the expression of the Nmp4 target gene Igfbp4 was decreased in the null cells. This binding protein is a strong inhibitor of osteoblast differentiation (46), and thus its suppression may further accelerate and enhance the differentiation of the null cells. Igf1 is a key mediator of the PTH anabolic response (59, 60), and although there was no notable alteration in the expression profiles of Igf1 or Igf1r in the null cells, the expression of Pdk1, a target of Nmp4 and a key kinase component of the IGF1/insulin signaling pathway, was elevated. This may enhance the sensitivity of the Nmp4−/− cells to this growth factor. At the end of the 16-day culture period the Nmp4−/− cells exhibited an enhanced anabolic profile as evidenced by the elevated expressions of the nontarget Nmp4 genes Bmp2, Pth1r, and Bglap. Perhaps this is an autocrine/paracrine response to the earlier surge in Igfbp2 expression. Indeed the Igf1 pathway plays a significant role in MSPC proliferation and mineralization (61, 62) and the null cells exhibited alterations in the expression not only of Bglap but the Nmp4 target extracellular matrix proteins Col1a1, Spp1, and Thbs2. Although the molecular mechanisms underlying mineralization remain to be elucidated, Spp1 is an anionic phosphoprotein expressed in mineralizing tissues that appears to regulate crystal size, shape, and location (54). Thbs2 is an extracellular matrix glycoprotein that has pleiotropic effects on bone phenotype. This protein appears to suppress the MSPC osteoprogenitor pool but also supports mineralization (53, 63, 64). Therefore, whether the decrease in Thbs2 expression in the Nmp4−/− cells impacts the observed alteration in the number of osteoprogenitors, alterations in mineralization or impacts cell phenotype in other ways remains to be determined. Finally, we observed no striking differences in the expression of the transcription regulators Sp7 and Runx2 between the genotypes. This suggests that disabling Nmp4 alters select aspects of the developing osteoblast phenotype.
The dramatic decrease in Cxcl12 expression in the Nmp4−/− cells raises the question as to whether this plays a role in the observed increase in CFU-Falk phos+ osteoprogenitors in the null mice (12, 15). CXCL12 and its receptor CXC receptor 4 (CXCR4) play key roles in maintaining the BM niche and CXCL12 is expressed by BM stromal cells and cells of the osteoblast lineage (52, 65). Ablation of the receptor CXCR4 in mature osteoblasts increased the number of CFU-Falk phos+ osteoprogenitors recovered from these mice although the phenotype also included a decrease in BV/TV (51). Our results suggest that suppressing the expression of the CXCR4 ligand CXCL12 results in a similar impact on osteoprogenitor number but a different bone phenotype. The up-regulation of Plaur expression in the Nmp4−/− MSPCs may potentially contribute to the increased number of CFU-Falk phos+ cells because abrogating the activity of this GPI-anchored receptor suppressed MSPC osteogenic differentiation (50). Finally, mutagenesis and rescuing experiments to determine whether the potential targets are truly functionally significant is the next required step for authenticating this new antianabolic network.
Further parsing of the enhanced Nmp4−/− BM osteogenic potential implicates the elevated frequency of CD8+ T cells in both ovary-intact and ovx Nmp4−/− mice, although this requires functional confirmation in these models. The ovx null animals exhibited elevated numbers of CD8+ T cells in both BM and PBL compartments throughout the entire treatment regimen, similar to what we previously observed in the younger ovary-intact Nmp4−/− mice, although this increase was limited to the BM (12). The elevated number of CD8+ T cells is intriguing, because these cells are documented to amplify the PTH anabolic response (20, 22). MSPCs regulate T-cell proliferation and survival (66), and perhaps disabling Nmp4 derepresses this aspect of the cell-cell interaction, although this apparent alteration in proliferation/survival may be a cell autonomous feature of the Nmp4−/− T-cell phenotype. Although the elevated number of Nmp4−/− osteoprogenitors declined at 12-week postop, the higher number of Nmp4−/− CD8+ T cells did not decrease. Therefore, although the augmented response to PTH might be weakened in the Nmp4−/− mice, it may not disappear because the persistent increased lymphocyte number might provide extra Wnt10b as a potent osteoprogenitor differentiation factor.
Why is there typically no difference between the amount of baseline trabecular bone in WT and Nmp4−/− mice despite the presence of an expanded pool of osteoprogenitors and CD8+ T cells in the null BM? This phenomenon differs from the results of recent clinical trials in which neutralizing sclerostin, an inhibitor of the Wnt signaling pathway and osteoblast differentiation, significantly increases baseline bone mineral density (67). Apparently disabling Nmp4 is not sufficient for driving excess bone formation but instead primes the aforementioned cells for activation by an anabolic cue. The occasionally observed elevated trabecular volume in untreated Nmp4−/− mice may be due to the sporadic local release of growth factors, eg, IGF1, IGFBP2 or BMP2. However, exogenous pharmacological doses of PTH provide a strong stimulus for triggering the response leading to the enhanced bone formation. Once activated by the anabolic cue, the Nmp4−/− cells produce the autocrine/paracrine factors that enhance the anabolic response.
Consistent with the requirement for a strong anabolic cue to trigger enhanced bone formation in the Nmp4−/− mice, disabling this transcription factor did not protect the animals from ovx-induced bone loss, indeed the initial rate of loss during the first 4 weeks after ovx was higher (L5) or nearly higher (distal femur) in the Nmp4−/− mice. These animals harbor a modestly elevated number of osteoclast progenitors (CFU-GM) (12) that upon differentiation exhibit an enhanced bone-resorbing activity in vitro (11). Therefore, a decrease in estrogen might accentuate this aspect of the phenotype. Moreover, differences in sex steroid levels may underlie why intact male Nmp4−/− mice did not lose bone under hind limb suspension (16). As mentioned, the Nmp4−/− baseline phenotype includes an occasional unprovoked enhancement in trabecular architecture, which we observed in the present study. That is to say, despite the elevated initial bone loss, the cohort of sham and ovx Nmp4−/− mice had more femoral and L5 trabecular bone compared with WT at the time of harvest (Table 3). However, there was no statistical difference between vehicle-treated animals in either the 4 or 8-week hormone therapy cohorts (Figures 2 and 3). Longitudinal studies for serum turnover markers coupled with peripheral quantitative computed tomography in live mice could be used to track the real-time dynamics of ovx-induced bone loss and subsequent therapy-induced bone gain between the WT and Nmp4−/− mice. In lieu of this, we employed a two-way ANOVA, which incorporates differences in control groups, to evaluate whether there is an interaction between genotype and treatment.
The present data also contribute to our knowledge as to how Nmp4 works at the molecular level. Nmp4 binds throughout the genome but is primarily localized to regions near the TSS and within the gene, consistent with mediating a regulatory role. GEM analysis confirmed the AT-rich homopolymeric-binding site and did not identify other consensus sequences expected only if Nmp4 also interacted with the genome indirectly via other DNA-binding proteins. Nmp4 association with the genome is responsive to PTH because hormone decreased genome-wide occupancy in the MC3T3-E1 cells after 1 hour of exposure. However, the impact of PTH on Nmp4 occupancy was gene and site-specific and hormone stimulation was observed to induce, remove, or have no effect on Nmp4 genomic occupancy. This may further augment the fine control that this transcription factor has over the regulation of osteoprogenitor and/or bone-forming capacity.
There is a critical need for osteoanabolic agents (68). We have taken a 2-pronged approach in our research to serve this clinical demand: 1) identify molecular and cellular mechanisms that could be used, for example in an adjuvant setting to promote enhanced efficacy or less frequent dosing with current osteoanabolic agents; and 2) identify innovative approaches to identify new drug targets/pathways or mechanisms of action that would provide needed substrate for the future drug discovery initiatives in bone disease, including osteoporosis. Our discovery-driven approaches have mapped a global network of Nmp4-regulated pathways potentially comprising a bone antianabolic axis. Further functional studies charting the hierarchy and interactions of theses network pathways will provide a novel integrated mechanism underlying the natural constraints on bone formation. We postulate that the Nmp4 antianabolic network may constitute a novel strategy to identify and reveal pharmacologically accessible pathways for adding new bone to the old skeleton.
Acknowledgments
This work was supported in part by the Department of Defense Grant PR120563 (to J.P.B.), the Eli Lilly Grant 062079-00002B (to J.P.B.), and the National Institutes of Health Clinical and Translational Science Institute Predoctoral Fellowship TL1 000162 (to P.C.).
Disclosure Summary: P.C., M.B.A., Z.W., Y.S., S.H.-B., Y.H., D.H., F.M.P., A.G.R., S.J.W., F.-C.Y., and Y.L. have nothing to disclose. Eli Lilly and Company has awarded research funds to J.P.B. and M.R.A. Eli Lilly and Company funded part of this work. V.K. is an employee of Eli Lilly and Company and owns stock in this company. K.R.S., J.K.M., and Z.R.G. are employees of Eli Lilly and Company.
Footnotes
- AKT
- thymoma viral proto-oncogene
- BFR
- bone formation rate
- Bglap
- bone gamma carboxyglutamate protein
- BM
- bone marrow
- BMP2
- bone morphogenetic protein 2
- BV/TV
- trabecular bone volume per total volume
- ChIP-seq
- chromatin immunoprecipitation sequencing
- Conn.D
- connectivity density
- ΔΔCT
- comparative threshold cycle
- μCT
- microcomputed tomography
- CTX
- C-terminal telopeptide
- CXC
- chemokine (C-X-C motif)
- CXCR4
- CXC receptor 4
- DAVID
- Database for Annotation, Visualization, and Integrated Discovery
- ENCODE
- Encyclopedia of DNA Elements
- FDR
- false-discovery rate
- FACS
- fluorescence-activated cell sorting
- GEM
- genome wide event finding and motif discovery
- GO
- gene ontology
- hPTH
- human parathyroid hormone
- Igfbp
- Igf binding protein
- KEGG
- Kyoto Encyclopedia of Genes and Genomes
- MAR
- mineral apposition rate
- MS/BS
- mineralizing surface/bone surface
- MSPC
- mesenchymal stem progenitor cell
- Nmp4
- nuclear matrix protein 4
- OCN
- osteocalcin
- ovx
- ovariectomy
- PBL
- peripheral blood
- P1NP
- N-terminal propeptide of type 1 procollagen
- preop
- pre-operation
- postop
- postoperation
- qRT-PCR
- quantitative real-time PCR
- SMI
- structure model index
- Tb.N
- trabecular number
- Tb.Sp
- trabecular spacing
- Tb.Th
- trabecular thickness
- Thbs2
- thrombospondin 2
- TLDA
- TaqMan Low Density Array
- TSS
- transcription start site
- Wnt10b
- wingless-type MMTV integration site family
- WT
- wild-type
- ZFP384
- zinc finger protein 384 (mouse)
- ZNF384
- zinc finger protein 384 (human).
References
- 1. Kraenzlin ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011;7:647–656. [DOI] [PubMed] [Google Scholar]
- 2. Baron R, Hesse E. Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab. 2012;97:311–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cipriani C, Capriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res. 2012;27:2419–2428. [DOI] [PubMed] [Google Scholar]
- 4. Yu EW, Neer RM, Lee H, et al. Time-dependent changes in skeletal response to teriparatide: escalating vs. constant dose teriparatide (PTH 1–34) in osteoporotic women. Bone. 2011;48:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. [DOI] [PubMed] [Google Scholar]
- 6. Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. [DOI] [PubMed] [Google Scholar]
- 7. Saag KG, Zanchetta JR, Devogelaer JP, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–3355. [DOI] [PubMed] [Google Scholar]
- 8. Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. [DOI] [PubMed] [Google Scholar]
- 9. Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26:503–511. [DOI] [PubMed] [Google Scholar]
- 10. Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Childress P, Philip BK, Robling AG, et al. Nmp4/CIZ suppresses the response of bone to anabolic parathyroid hormone by regulating both osteoblasts and osteoclasts. Calcif Tissue Int. 2011;89:74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He Y, Childress P, Hood M, Jr, et al. Nmp4/CIZ suppresses the parathyroid hormone anabolic window by restricting mesenchymal stem cell and osteoprogenitor frequency. Stem Cells Dev. 2013;22:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robling AG, Childress P, Yu J, et al. Nmp4/CIZ suppresses parathyroid hormone-induced increases in trabecular bone. J Cell Physiol. 2009;219:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krane SM. Identifying genes that regulate bone remodeling as potential therapeutic targets. J Exp Med. 2005;201:841–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morinobu M, Nakamoto T, Hino K, et al. The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med. 2005;201:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hino K, Nakamoto T, Nifuji A, et al. Deficiency of CIZ, a nucleocytoplasmic shuttling protein, prevents unloading-induced bone loss through the enhancement of osteoblastic bone formation in vivo. Bone. 2007;40:852–860. [DOI] [PubMed] [Google Scholar]
- 17. Nakamoto T, Shiratsuchi A, Oda H, et al. Impaired spermatogenesis and male fertility defects in CIZ/Nmp4-disrupted mice. Genes Cells. 2004;9:575–589. [DOI] [PubMed] [Google Scholar]
- 18. Isern J, Martín-Antonio B, Ghazanfari R, et al. Self-renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep. 2013;3:1714–1724. [DOI] [PubMed] [Google Scholar]
- 19. Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bedi B, Li JY, Tawfeek H, et al. Silencing of parathyroid hormone (PTH) receptor 1 in T cells blunts the bone anabolic activity of PTH. Proc Natl Acad Sci USA. 2012;109:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li JY, Walker LD, Tyagi AM, Adams J, Weitzmann MN, Pacifici R. The sclerostin-independent bone anabolic activity of intermittent PTH treatment is mediated by T-cell-produced Wnt10b. J Bone Miner Res. 2014;29:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terauchi M, Li JY, Bedi B, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bidwell JP, Childress P, Alvarez MB, et al. Nmp4/CIZ closes the parathyroid hormone anabolic window. Crit Rev Eukaryot Gene Expr. 2012;22:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakamoto T, Yamagata T, Sakai R, et al. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol Cell Biol. 2000;20:1649–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alvarez M, Thunyakitpisal P, Morrison P, Onyia J, Hock J, Bidwell JP. PTH-responsive osteoblast nuclear matrix architectural transcription factor binds to the rat type I collagen promoter. J Cell Biochem. 1998;69:336–352. [DOI] [PubMed] [Google Scholar]
- 26. Shah R, Alvarez M, Jones DR, et al. Nmp4/CIZ regulation of matrix metalloproteinase 13 (MMP-13) response to parathyroid hormone in osteoblasts. Am J Physiol Endocrinol Metab. 2004;287:16. [DOI] [PubMed] [Google Scholar]
- 27. Thunyakitpisal P, Alvarez M, Tokunaga K, et al. Cloning and functional analysis of a family of nuclear matrix transcription factors (NP/NMP4) that regulate type I collagen expression in osteoblasts. J Bone Miner Res. 2001;16:10–23. [DOI] [PubMed] [Google Scholar]
- 28. Torrungruang K, Alvarez M, Shah R, Onyia JE, Rhodes SJ, Bidwell JP. DNA binding and gene activation properties of the Nmp4 nuclear matrix transcription factors. J Biol Chem. 2002;277:16153–16159. [DOI] [PubMed] [Google Scholar]
- 29. Alvarez MB, Childress P, Philip BK, et al. Immortalization and characterization of osteoblast cell lines generated from wild-type and Nmp4-null mouse bone marrow stromal cells using murine telomerase reverse transcriptase (mTERT). J Cell Physiol. 2012;227:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen ZJ, Nakamoto T, Tsuji K, et al. Negative regulation of bone morphogenetic protein/Smad signaling by Cas-interacting zinc finger protein in osteoblasts. J Biol Chem. 2002;277:29840–29846. [DOI] [PubMed] [Google Scholar]
- 31. Yang Z, Bidwell JP, Young SR, Gerard-O'Riley R, Wang H, Pavalko FM. Nmp4/CIZ inhibits mechanically induced β-catenin signaling activity in osteoblasts. J Cell Physiol. 2010;223:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stamatoyannopoulos JA, Snyder M, Hardison R, et al. An encyclopedia of mouse DNA elements (mouse ENCODE). Genome Biol. 2012;13:2012–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu X, Estwick SA, Chen S, et al. Neurofibromin plays a critical role in modulating osteoblast differentiation of mesenchymal stem/progenitor cells. Hum Mol Genet. 2006;15:2837–2845. [DOI] [PubMed] [Google Scholar]
- 34. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. [DOI] [PubMed] [Google Scholar]
- 35. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenbloom KR, Sloan CA, Malladi VS, et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res. 2013;41:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo Y, Mahony S, Gifford DK. High resolution genome wide binding event finding and motif discovery reveals transcription factor spatial binding constraints. PLoS Comput Biol. 2012;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- 40. Supek F, Bosnjak M, Skunca N, Smuc T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One. 2011;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Auerbach RK, Chen B, Butte AJ. Relating genes to function: identifying enriched transcription factors using the ENCODE ChIP-Seq significance tool. Bioinformatics. 2013;29:1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vieira Potter VJ, Strissel KJ, Xie C, et al. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology. 2012;153:4266–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harada A, Okada S, Konno D, et al. Chd2 interacts with H3.3 to determine myogenic cell fate. EMBO J. 2012;31:2994–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin W, Srajer G, Evrard YA, Phan HM, Furuta Y, Dent SY. Developmental potential of Gcn5(−/−) embryonic stem cells in vivo and in vitro. Dev Dyn. 2007;236:1547–1557. [DOI] [PubMed] [Google Scholar]
- 45. Nascimento EM, Cox CL, MacArthur S, et al. The opposing transcriptional functions of Sin3a and c-Myc are required to maintain tissue homeostasis. Nat Cell Biol. 2011;13:1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miyakoshi N, Richman C, Qin X, Baylink DJ, Mohan S. Effects of recombinant insulin-like growth factor-binding protein-4 on bone formation parameters in mice. Endocrinology. 1999;140:5719–5728. [DOI] [PubMed] [Google Scholar]
- 47. Xi G, Wai C, DeMambro V, Rosen CJ, Clemmons DR. IGFBP-2 directly stimulates osteoblast differentiation. J Bone Miner Res. 2014;29:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Calleja V, Laguerre M, de Las Heras-Martinez G, et al. Acute regulation of PDK1 by a complex interplay of molecular switches. Biochem Soc Trans. 2014;42:1435–1440. [DOI] [PubMed] [Google Scholar]
- 49. Furlan F, Galbiati C, Jorgensen NR, et al. Urokinase plasminogen activator receptor affects bone homeostasis by regulating osteoblast and osteoclast function. J Bone Miner Res. 2007;22:1387–1396. [DOI] [PubMed] [Google Scholar]
- 50. Kalbasi Anaraki P, Patecki M, Larmann J, et al. Urokinase receptor mediates osteogenic differentiation of mesenchymal stem cells and vascular calcification via the complement C5a receptor. Stem Cells Dev. 2014;23:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shahnazari M, Chu V, Wronski TJ, Nissenson RA, Halloran BP. CXCL12/CXCR4 signaling in the osteoblast regulates the mesenchymal stem cell and osteoclast lineage populations. FASEB J. 2013;27:3505–3513. [DOI] [PubMed] [Google Scholar]
- 52. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. [DOI] [PubMed] [Google Scholar]
- 53. Alford AI, Terkhorn SP, Reddy AB, Hankenson KD. Thrombospondin-2 regulates matrix mineralization in MC3T3-E1 pre-osteoblasts. Bone. 2010;46:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int. 2013;93:348–354. [DOI] [PubMed] [Google Scholar]
- 55. Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011;112:750–755. [DOI] [PubMed] [Google Scholar]
- 56. Mizoguchi T, Pinho S, Ahmed J, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. [DOI] [PubMed] [Google Scholar]
- 58. Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bikle DD, Wang Y. Insulin like growth factor-I: a critical mediator of the skeletal response to parathyroid hormone. Curr Mol Pharmacol. 2012;5:135–142. [PMC free article] [PubMed] [Google Scholar]
- 60. Elis S, Courtland HW, Wu Y, et al. Elevated serum IGF-1 levels synergize PTH action on the skeleton only when the tissue IGF-1 axis is intact. J Bone Miner Res. 2010;25:2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kumar S, Ponnazhagan S. Mobilization of bone marrow mesenchymal stem cells in vivo augments bone healing in a mouse model of segmental bone defect. Bone. 2012;50:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hankenson KD, Bain SD, Kyriakides TR, Smith EA, Goldstein SA, Bornstein P. Increased marrow-derived osteoprogenitor cells and endosteal bone formation in mice lacking thrombospondin 2. J Bone Miner Res. 2000;15:851–862. [DOI] [PubMed] [Google Scholar]
- 64. Hankenson KD, Bornstein P. The secreted protein thrombospondin 2 is an autocrine inhibitor of marrow stromal cell proliferation. J Bone Miner Res. 2002;17:415–425. [DOI] [PubMed] [Google Scholar]
- 65. Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. [DOI] [PubMed] [Google Scholar]
- 66. Wang L, Zhao Y, Shi S. Interplay between mesenchymal stem cells and lymphocytes: implications for immunotherapy and tissue regeneration. J Dent Res. 2012;91:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Becker CB. Sclerostin inhibition for osteoporosis–a new approach. N Engl J Med. 2014;370:476–477. [DOI] [PubMed] [Google Scholar]
- 68. Lewiecki EM. New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol. 2011;7:631–638. [DOI] [PubMed] [Google Scholar]