Abstract
Inactivation of target of rapamycin complex 1 (TORC1) signaling is considered important for the beneficial effects of caloric restriction (CR) on metabolism and health span. It is however not fully elucidated which cellular processes downstream of TORC1 are the main regulators of metabolic health. In this issue of EMBO Reports, Zidek et al 1 describe that inhibition of mammalian TORC1 (mTORC1) leads to decreased translation of CCAAT/enhancer-binding protein β (C/EBPβ)-liver inhibitory protein (LIP). Moreover, loss of C/EBPβ-LIP in mice improves metabolic health, similar to the effects of CR. Zidek et al 1 thus report that reduced C/EBPβ-LIP translation is a novel mTORC1-regulated process that could play a major role in mediating the beneficial metabolic effects of caloric restriction.
See also: LM Zidek et al (August 2015)
Obesity is a strong risk factor for the development of several diseases, such as diabetes, cardiovascular diseases and even cancer. Moreover, in the last decades, the prevalence of obesity has dramatically increased worldwide 2. There is thus a strong need for novel therapeutic options aimed at treating obesity. It is well known that CR, defined as a reduction in caloric intake without malnutrition, is an effective strategy to improve health span and life span in many organisms, including yeast, worms, flies and mice (reviewed in 3). Hence, using therapeutic agents that mimic the metabolic effects of CR could be an efficient way to treat obesity and decrease the risk of obesity-associated diseases. However, to generate CR-mimetic compounds, it is important to understand the molecular signaling pathways that mediate the CR-induced effects on metabolism. A crucial mechanism for how CR can increase organismal life span and health span is through inhibition of TORC1 signaling. Importantly, several TORC1-regulated processes, such as protein synthesis and autophagy, have been implicated in the metabolic effects of CR (reviewed in 3). However, there might still be additional processes downstream of TORC1 that are important for the increased life span and health span observed upon CR. In this issue of EMBO Reports, Zidek et al 1 identify a novel mTORC1-regulated process that might be involved in mediating the beneficial effects of CR on metabolism 1.
“reduced C/EBPβ-LIP translation […] could play a major role in mediating the beneficial metabolic effects of caloric restriction”
Fifteen years ago, Calkhoven et al 4 demonstrated that mTORC1 signaling stimulates eIF4E-mediated translation of C/EBPβ-LIP. However, the role of mTORC1-mediated translation of C/EBPβ-LIP in metabolism and metabolic health was not examined. C/EBPβ, a member of the C/EBP transcription factor family, regulates transcription of genes involved in proliferation, inflammation and metabolism 5. Due to the presence of alternative translation initiation sites in C/EBPβ mRNA, C/EBPβ exists in three different isoforms, C/EBPβ-liver activating protein (LAP), C/EBPβ-LAP* and C/EBPβ-LIP. While the C/EBPβ-LAP and C/EBPβ-LAP* isoforms are transcription activators, the C/EBPβ-LIP isoform lacks the activation domain and can thus act as competitive inhibitor of LAP and LAP*. Consequently, the LAP(*)/LIP ratio is crucial for C/EBPβ function 5. Zidek et al 1 demonstrate now that inhibition of mTORC1 in cultured cells by rapamycin treatment or by knockdown of the essential mTORC1 component raptor decreases C/EBPβ-LIP translation without altering C/EBPβ-LAP levels. Furthermore, the authors show that inactivation of mTORC1 signaling in mice also decreases C/EBPβ-LIP levels in various metabolic tissues, such as liver and white adipose tissue (WAT). They found that decreased mTORC1 activity results in reduced C/EBPβ-LIP protein levels due to 4EBP-mediated inhibition of eIF4E-regulated translation, which is in agreement with the study by Calkhoven et al 4 (Fig1A). It is known that translation of C/EBPβ-LIP is dependent on the presence of an upstream open reading frame (uORF) in the 5′ untranslated region (UTR) of the C/EBPβ mRNA 6. Consequently, lack of this uORF results in loss of C/EBPβ-LIP. Zidek et al 1 demonstrate that the uORF in the 5′ UTR of C/EBPβ mRNA is necessary for mTORC1-4EBP-eIF4E-mediated stimulation of C/EBPβ-LIP translation.
Figure 1.
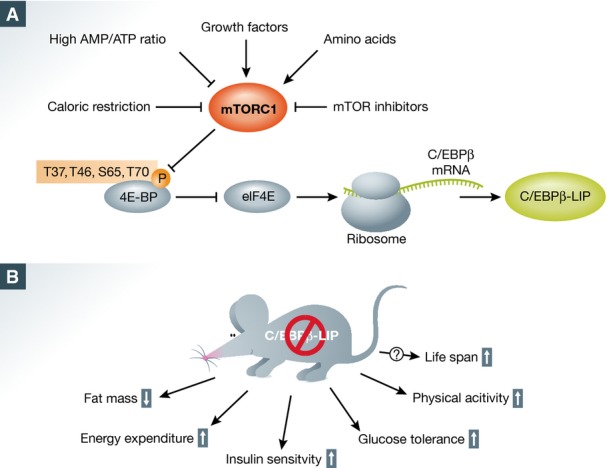
C/EBPβ-LIP translation is regulated by mTORC1 and affects metabolism
(A) Regulation of C/EBPβ-LIP translation by mTORC1-4EBP-eIF4E signaling. (B) Loss of C/EBPβ-LIP in mice improves metabolic health and might increase life span. See text for details.
“decreasing C/EBPβ-LIP in patients with mTORC1-driven tumors could potentially slow cancer growth”
To study the effects of C/EBPβ-LIP depletion on systemic metabolism, the authors use ΔuORF mice that are deficient for the C/EBPβ-LIP isoform, without alteration in C/EBPβ-LAP 6. Interestingly, ΔuORF mice display improved metabolic health, such as reduced adiposity, increased energy expenditure, improved glucose tolerance, enhanced insulin sensitivity and increased physical activity, which are similar to the effects elicited by CR (Fig1B). Taken together, the authors propose that CR might mediate beneficial effects on metabolic health by reducing mTORC1 activity and subsequently decreasing C/EBPβ-LIP levels.
In search for a mechanism for how reduced C/EBPβ-LIP levels lead to an improved metabolic phenotype, Zidek et al 1 examined gene expression profiles in liver and adipose tissue of ΔuORF mice. The authors found that mice lacking C/EBPβ-LIP display increased transcription of genes involved in β-oxidation in liver and increased transcription of lipogenic, lipolytic and β-oxidation genes in WAT. Zidek et al 1 speculate that these transcriptional effects could account for the increased lipid oxidation, energy expenditure and metabolic health observed in ΔuORF mice. Since the authors investigated the phenotype of mice with a whole body deletion of C/EBPβ-LIP, it would be of interest in future studies to use liver- and adipose tissue-specific ΔuORF mice to determine whether the absence of C/EBPβ-LIP only in these tissues is sufficient to confer the observed beneficial effects on systemic metabolism. Interestingly, C/EBPβ knockout mice, which display a complete loss of all C/EBPβ isoforms, also display reduced adiposity and increased energy expenditure 7. Moreover, whole body complete deletion of C/EBPβ has been shown to improve the metabolic phenotype of db/db mice 8. Since mice with ablation of all C/EBPβ isoforms display a metabolic phenotype similar to that of mice deficient only for C/EBPβ-LIP, this suggests that C/EBPβ-LIP could be the main isoform responsible for metabolic regulation. Hence, it would be of interest to determine the role of the individual C/EBPβ isoforms in systemic metabolic regulation.
While the authors did not investigate the effect of increased mTORC1 activity on C/EBPβ-LIP translation in the current study, this would also be an interesting area for further research, especially in the context of mTORC1-driven tumors where mTORC1 signaling is chronically elevated. Interestingly, C/EBPβ-LIP levels have been shown to be increased in highly proliferative breast cancers, and mice lacking C/EBPβ-LIP display decreased cell proliferation 6,9. Hence, C/EBPβ-LIP seems to promote a proliferative phenotype. Based on the findings in the current study by Zidek et al 1, increased translation of C/EBPβ-LIP could potentially drive cell proliferation in tumors with elevated mTORC1 signaling. It is thus tempting to speculate that decreasing C/EBPβ-LIP in patients with mTORC1-driven tumors could potentially slow cancer growth and tumor progression.
In summary, Zidek et al 1 demonstrate that loss of C/EBPβ-LIP results in metabolic alterations that are reminiscent of the metabolic effects observed upon CR. Importantly, the authors show that reduction of mTORC1 activity, both in vitro and in vivo, decreases C/EBPβ-LIP translation. These findings suggest that the beneficial metabolic effects elicited by CR are at least in part due to the inhibition of mTORC1-mediated C/EBPβ-LIP translation. Thus, a future interesting area of research would be to identify compounds that target C/EBPβ-LIP and to test whether these compounds act as CR-mimetic and anti-cancer drugs.
References
- Zidek LM, Ackermann T, Hartleben G, et al. EMBO Rep. 2015;16:1022–1036. doi: 10.15252/embr.201439837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, et al. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cabo R, Carmona-Gutierrez D, Bernier M, et al. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkhoven CF, Muller C, Leutz A. Genes Dev. 2000;14:1920–1932. [PMC free article] [PubMed] [Google Scholar]
- Ramji DP, Foka P. Biochem J. 2002;365(Pt 3):561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethmar K, Begay V, Smink JJ, et al. Genes Dev. 2010;24:15–20. doi: 10.1101/gad.557910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward CA, Heaney JD, Sinasac DS, et al. Diabetes. 2007;56:161–167. doi: 10.2337/db06-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder-Gloeckler JM, Rahman SM, Janssen RC, et al. J Biol Chem. 2007;282:15717–15729. doi: 10.1074/jbc.M701329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milde-Langosch K, Loning T, Bamberger AM. Breast Cancer Res Treat. 2003;79:175–185. doi: 10.1023/a:1023929504884. [DOI] [PubMed] [Google Scholar]


