Abstract
To clarify the effect of secretory IgA (sIgA) deficiency on gut homeostasis, we examined intraepithelial lymphocytes (IELs) in the small intestine (SI) of polymeric immunoglobulin receptor-deficient (pIgR−/−) mice. The pIgR−/− mice exhibited the accumulation of CD8αβ+ T-cell receptor (TCR)-αβ+ IELs (CD8αβ+αβ-IELs) after weaning, but no increase of CD8αβ+γδ-IELs was detected in pIgR−/− TCR-β−/− mice compared with pIgR+/+ TCR-β−/− mice. When 5-bromo-2′-deoxyuridine (BrdU) was given for 14 days, the proportion of BrdU-labelled cells in SI-IELs was not different between pIgR+/+ mice and pIgR−/− mice. However, the proportion of BrdU-labelled CD8αβ+-IELs became higher in pIgR−/− mice than pIgR+/+ mice 10 days after discontinuing BrdU-labelling. Intravenously transferred splenic T cells migrated into the intraepithelial compartments of pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice to a similar extent. In contrast, in the case of injection of immature bone marrow cells, CD8αβ+αβ-IELs increased much more in the SI of pIgR−/− TCR-β−/− mice than pIgR+/+ TCR-β−/− mice 8 weeks after the transfer. αβ-IELs from pIgR−/− mice could produce more interferon-γ and interleukin-17 than those of pIgR+/+ mice, and intestinal permeability tended to increase in the SI of pIgR−/− mice with aging. Taken together, these results indicate that activated CD8αβ+αβ-IELs preferentially accumulate in pIgR−/− mice through the enhanced differentiation of immature haematopoietic precursor cells, which may subsequently result in the disruption of epithelial integrity.
Keywords: bone marrow cell, intestinal permeability, intraepithelial lymphocyte, polymeric immunoglobulin receptor, spleen cell
Introduction
A diverse array of cells and molecules are involved in the gut immune response, and secretory IgA (sIgA) constitutes the majority of antibody in the mucous layer of the intestinal tract. Comparative studies of healthy subjects and IgA-deficient patients suggest that sIgA may be responsible for the prevention of respiratory and gastrointestinal tract infections.1 It is reported that sIgA binds viral antigens penetrating across the epithelial layers and effectively protects the host against pathogenic microorganisms in naive mice.2–4 On the other hand, after IgA−/− mice and J-chain−/− mice, both of which are deficient in sIgA, have been immunized by pathogenic microorganisms, they exhibit resistance to mucosal infection by the same pathogens.5–8 These findings indicate that sIgA plays a critical role for neutralizing and inactivating microorganisms in the mucous layer of the naive host and is not necessarily required for the guard against pathogenic infection in the host where specific immunity has already been established.
The concentration of sIgA in the gut lumen is much lower in germ-free mice than conventional mice, and sIgA specific for commensal bacteria, Enterobacter cloacae, can be detected in gnotobiotic mice colonized by this bacterium but not in germ-free mice, showing that sIgA production is induced by bacteria residing in the gut lumen.9 Moreover, sIgA production was induced in response to live but not dead commensal bacteria and the bacteria-induced sIgA gradually declined according to the disappearance of the colonizing bacteria.10 These results demonstrate that sIgA production can be reversibly regulated corresponding to the bulk of commensal bacteria in the gut lumen.
Transport of IgA produced in the lamina propria across an epithelial layer is mediated by polymeric immunoglobulin receptors (pIgR) expressed on the basolateral side of intestinal epithelial cells.11 We and others have generated pIgR−/− mice to uncover the physiological functions of sIgA.12,13 Studies using pIgR−/− mice have supported the theory that sIgA inhibits colonization of Salmonella typhimurium or Mycobacterium bovis in the gut and nasal tracts of naive mice, and that sIgA induced by vaccination with influenza virus is effective in cross-protecting against different types of influenza viruses.14–16
It should be noted that pIgR−/− mice did not show any histological signs of disorders except for the enlargement of mesenteric lymph nodes and pIgR+/+ mice and pIgR−/− mice had equal lifespans (pIgR+/+ mice, 113 ± 17 weeks, n = 84; pIgR−/− mice, 110 ± 15 weeks, n = 89). Therefore, it is of interest to know whether gut homeostasis can be maintained even in the absence of sIgA.
Our previous report revealed that CD8αβ+ T-cell receptor (TCR)-αβ+ intraepithelial lymphocytes (CD8αβ+αβ-IELs) accumulate in the small intestine (SI) of pIgR−/− mice in response to commensal bacteria.17 This study shows that enhanced differentiation and activation of CD8αβ+αβ-IELs occur in the SI of pIgR−/− mice, subsequently resulting in the abnormalities of epithelial integrity. These results demonstrate the importance of sIgA in the maintenance of gut homeostasis.
Materials and methods
Mice
B6.129X1-Pigrtm1Ohw/Yit mice (pIgR−/− mice) were generated by backcrossing to C57BL/6 mice 10 times.13 B6.129P2-Tcabtm1Mon mice (TCR-β−/− mice) and B6.129P2-Tcadtm1Ito mice (TCR-δ−/− mice), both of which are from a C57BL/6 background, were kindly provided by Dr Susumu Tonegawa (Massachusetts Institute of Technology, Cambridge, MA). To obtain pIgR−/− mice lacking mature TCR-αβ+ cells, pIgR−/− mice and TCR-β−/− mice were cross-bred, and by brother–sister mating of the offspring, pIgR+/+ TCR-β+/− mice, pIgR−/− TCR-β+/− mice, pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice were generated. All experiments were approved by the ethics committee of the Yakult Central Institute.
Cell preparation
Small intestine was removed from the pyloric sphincter to the ileo–caecal junction. The SI-IELs were prepared as described elsewhere.17 Briefly, fragments of SI tissues approximately 1–2 cm in length were vigorously shaken in 5% fetal calf serum/25 mm HEPES/RPMI-1640 for 45 min to separate the cells from the intestinal wall. Cells were recovered by centrifugation in 30% Percoll solution (GE Healthcare Life Sciences Japan, Tokyo, Japan). Cell pellets were re-suspended and applied to a 44%/70% Percoll discontinuous density gradient. After centrifugation, the cells at the 44%/70% Percoll interface were recovered and counted as SI-IELs. Typically, more than 90% and 3–7% of SI-IELs were CD3+ cells and CD3− B220+ cells in wild-type mice, respectively.
To purify TCR-αβ+ cells or TCR-γδ+ cells from SI-IELs, they were incubated with biotinylated anti-TCR-δ or biotinylated anti-TCR-β monoclonal antibodies (mAbs; both from BD Biosciences, San Diego, CA), and then mixed with streptavidin microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The cells bound to the magnetic microbeads were removed using an LS column (Miltenyi Biotec) and the passing-through cells were collected as either TCR-αβ+ or TCR-γδ+ cell fractions, respectively. Purity of both fractions was higher than 95%.
The spleen was loosened between gauze using a pincette to obtain a single-cell suspension. Spleen cells were incubated with a cocktail of biotinylated anti-B220, biotinylated anti-CD11b and biotinylated anti-CD11c mAbs (all from BD Biosciences), and then mixed with streptavidin microbeads. Cells were magnetically separated using an LS column and the passing-through cells (CD3+ cell-enriched fraction) were recovered. The proportion of CD3+ cells in this fraction was higher than 95%.
Bone marrow cells were obtained by pushing out the contents of the femur with Hanks’ balanced salt solution using a syringe equipped with a 26-gauge needle. After the cells were treated with haemolytic buffer (0·144 m NH4Cl/0·017 m Tris–HCl, pH 7·65), the remaining cells were incubated with a cocktail of biotinylated anti-CD3, biotinylated anti-B220, biotinylated anti-CD11b and biotinylated anti-CD11c mAbs (all from BD Biosciences), followed by incubation with streptavidin microbeads. The cells were magnetically separated using an LS column and the passing-through cells were collected. CD3− B220− CD11b− CD11c− cells occupied more than 95% of this cell preparation.
Flow cytometry analysis
Cells were stained with antibodies and analysed using an EPICS Altra flow cytometer (Beckman Coulter, Inc., Brea, CA). The following antibodies were used at a concentration of 10–20 μg/ml: phycoerythrin (PE)-conjugated anti-TCR-β (H57-597), FITC-conjugated anti-TCR-δ (GL3), FITC-conjugated or PE-conjugated anti-CD4 (RM4-5), PE-conjugated or biotinylated anti-CD8α (53-6.7), and FITC-conjugated or PE-conjugated anti-CD8β (53-5.8) (all from BD Biosciences). Biotinylated antibodies were detected by Tri-Color-conjugated streptavidin (Caltag Laboratories, Burlingame, CA). Dead cells and debris were gated out, and gating of the lymphocyte fraction was used for analysis.
Bromodeoxyuridine staining
Mice were given ad libitum access to distilled water containing 5-bromo-2′-deoxyuridine (BrdU; 0·8 mg/ml; Sigma-Aldrich, St Louis, MO) for 14 days. Thereafter, distilled water without BrdU was given to mice for an additional 10 days. Bone marrow cells, thymocytes and SI-IELs were prepared 14 days after starting the exposure to BrdU-containing water and 10 days after discontinuing the exposure to BrdU-containing water. To analyse which subsets of SI-IELs incorporated BrdU, cells were stained with PE-conjugated anti-CD4, PE-conjugated anti-CD8α, or PE-conjugated anti-CD8β mAbs; cell suspensions were in 0·5 ml of saline, mixed with 1·2 ml of 95% cold ethanol, and kept for 30 min at room temperature. Thereafter, cells were suspended in 2 ml of PBS and mixed with 1 ml of 1% paraformaldehyde/0·01% Tween-20 in PBS. Cells were stained with FITC-conjugated anti-BrdU mAb (BD Biosciences) and analysed with a flow cytometer.
Adoptive transfer experiments
Aliquots of the CD3+ cell-enriched fraction prepared from the spleens of wild-type mice were intravenously injected into pIgR+/+ TCR-β−/− mice or pIgR−/− TCR-β−/− mice (5 × 106 cells/mouse), and the splenic cells and SI-IELs in the recipient mice were analysed 2 weeks after the transfer. CD3− B220− CD11b− CD11c− cell-enriched fractions prepared from the bone marrow of TCR-δ−/− mice were intravenously injected into pIgR+/+ TCR-β−/− mice or pIgR−/− TCR-β−/− mice (1 × 106 cells/mouse), and the SI-IELs in the recipient mice were analysed at 4, 8 and 12 weeks after the transfer.
Measurement of cytokines
Purified αβ-IELs and γδ-IELs were stimulated with immobilized anti-CD3 mAb for 48 hr, after which the culture supernatants were collected and kept at −30° until the measurement. The concentrations of interferon-γ (IFN-γ), interleukin-17 (IL-17), IL-4 and IL-10 were determined using a sandwich ELISA. The following combinations of mAbs or an assay kit were used: for IFN-γ, clone R4-6A2 and clone XMG1.2; for IL-17, clone TC11-18H10 and clone TC11-8H4; for IL-4, clone BVD4-1D11 and clone BVD6-24G2 (all from Biosource International, Camarillo, CA); for IL-10, Opt EIA set (BD Biosciences).
Polymerase chain reaction
Total RNA was extracted from αβ-IELs and γδ-IELs using an RNAqueous® kit (Applied Biosystems, Foster City, CA). Cells were treated with Lysis/Binding solution and RNA was purified. PCR was conducted using the following TaqMan® primer sets (Applied Biosystems): IFN-γ (Assay ID: Mm00801778_m1, amplicon size: 101 bp), IL-17 (Assay ID: Mm00439619_m1, amplicon size: 91 bp), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Assay ID: Mm99999915_g1, amplicon size: 107 bp).
Intracellular cytokine staining
To detect IFN-γ-producing cells, SI-IELs were treated with PMA and ionomycin in the presence of brefeldin A (Leukocyte Activation Cocktail; BD Biosciences) for 4 hr. Cells were stained with FITC-conjugated anti-CD4 or FITC-conjugated anti-CD8β mAbs and then treated with Cytofix/Cytoperm Buffer, Perm/Wash Buffer and Stain Buffer (BD Biosciences). Next, cells were stained with PE-conjugated anti-IFN-γ mAb (clone XMG1.2; Immunotech, Marseille, France) and analysed using flow cytometry.
Assessment of intestinal permeability
Mice were anaesthetized with isoflurane and loops with a length of about 3 cm were generated at both the duodenum and the ileum per mouse. FITC-dextran solution (Sigma-Aldrich, 60 mg/ml) or rhodamine B isothiocyanate-dextran solution (Sigma-Aldrich, 60 mg/ml) was injected at the same time into the duodenal loop or the ileal loop, respectively, at 0·2 ml per loop. Peripheral blood was collected from the tail vein at intervals of 10 min during the 60 min after the injection of fluorescence dye solutions. Serum was prepared from the blood and fluorescence intensity was measured. Intestinal permeability was assessed from the change over time of fluorescence dye concentration in the serum (expressed as ng/ml/min).
Statistical analysis
The statistical significance of differences was determined using the two-tailed Student’s t-test. Differences with a P-value < 0·05 were considered significant.
Results
Accumulation of SI-IELs begins after weaning in pIgR−/− mice
When sera obtained from pIgR+/+ mice and pIgR−/− mice were transferred intravenously into Rag-2−/− mice, IgA was detected in the faeces in the same time course (S. Shimada, unpublished data). Moreover, serum IgA from naive pIgR−/− mice bound a variety of antigens, including food component (ovalbumin), bacteria (Pneumococcus) and cell-derived antigens (sheep red blood cell) (S. Shimada, unpublished data), indicating that IgA with broad antigen specificity produced by gut plasma cells is circulating in the blood of pIgR−/− mice because it is not secreted into the gut lumen. These results confirm that serum IgA in pIgR−/− mice has properties similar to natural polyreactive sIgA.18 Therefore, we consider that pIgR−/− mice can be used to reveal the abnormalities caused by the lack of secretory antibody.
As reported previously,17 there were more αβ-IELs in the SI of adult pIgR−/− mice with an age of 11–12 weeks compared with age-matched pIgR+/+ mice (Fig.1a). To see when αβ-IELs begin to accumulate in a growth process of pIgR−/− mice, we cross-bred female pIgR−/− mice and male pIgR+/− mice and examined the change of SI-IELs of neonatal mice from 3 to 7 weeks after the birth. As nursing mothers are pIgR−/− mice and their breast milk contains the least amount of sIgA, the effect of mother-derived sIgA on SI-IEL development can be excluded. As shown in Fig.1(b), the number of αβ-IELs and γδ-IELs was not different between pIgR+/− mice and pIgR−/− mice until 5 weeks after the birth. SI-IELs increased in number between 5 and 7 weeks after the birth, and the number of αβ-IELs, but not of γδ-IELs, was significantly greater in pIgR−/− mice compared with pIgR+/− mice 7 weeks after the birth (Fig.1b). These results demonstrate that αβ-IELs do not accumulate in the SI of suckling pIgR−/− mice and increase after the weaning, and the higher level of αβ-IELs continues in the adult pIgR−/− mice.
Figure 1.
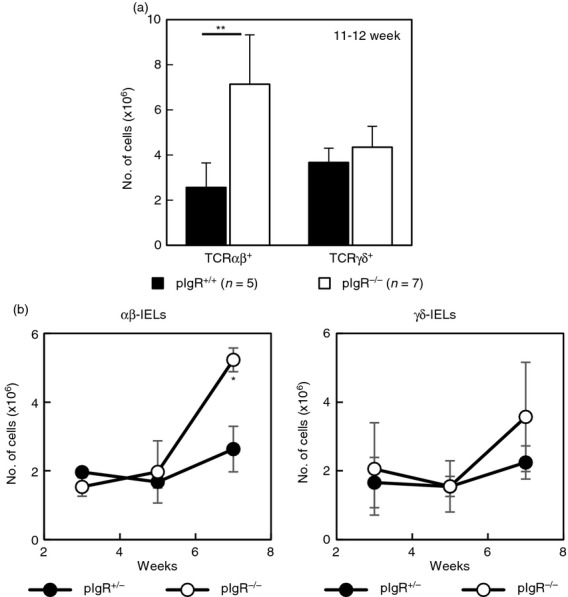
Small intestine intraepithelial lymphocytes (SI-IELs) in polymeric immunoglobulin receptor-positive (pIgR+/+ or pIgR+/−) mice and pIgR−/− mice. (a) SI-IELs were prepared from pIgR+/+ mice (11–12 weeks old, n = 5, ■) and pIgR−/− mice (11–12 weeks old, n = 7, □). By multiplying the total number of SI-IELs by the proportion of T-cell receptor-αβ-positive (TCR-αβ+) cells or TCR-γδ+ cells, the numbers of αβ-IELs or γδ-IELs were calculated. (b) Change of αβ-IELs and γδ-IELs in the newborn pIgR+/− and pIgR−/− mice. Male pIgR+/− mice and female pIgR−/− mice were crossbred and the newborn pIgR+/− mice (●) or pIgR−/− mice (○) were nursed by pIgR−/− mice. Numbers of αβ-IELs and γδ-IELs were examined 3, 5 and 7 weeks after the birth. Data are shown as mean ± SD of two to three mice. *P < 0·05, **P < 0·01.
Increase of SI-IELs does not occur in pIgR−/− mice without TCR-αβ+ cells
To clarify whether accumulation of SI-IELs in sIgA-deficiency is restricted to the αβ-IEL subset, we generated pIgR−/− mice lacking mature TCR-αβ+ cells by cross-breeding pIgR−/− mice and TCR-β−/− mice. Although the total number of SI-IELs increased in pIgR−/− TCR-β+/− mice in association with an expansion of CD8αβ+αβ-IELs compared with pIgR+/+ TCR-β+/− mice, the number of SI-IELs in pIgR−/− TCR-β−/− mice was not different from that of pIgR+/+ TCR-β−/− mice (Fig.2a). More than 85% of the SI-IELs in the pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice were TCR-γδ+ cells, and their relative composition and the expression of surface molecules (B220, CD69, CD103) on the γδ-IELs were similar in pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice (data not shown). Although CD8αβ+γδ-IELs were detected in pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice, their proportion was very low (1–2% among γδ-IELs) and not different between the two types of mice (Fig.2b).
Figure 2.
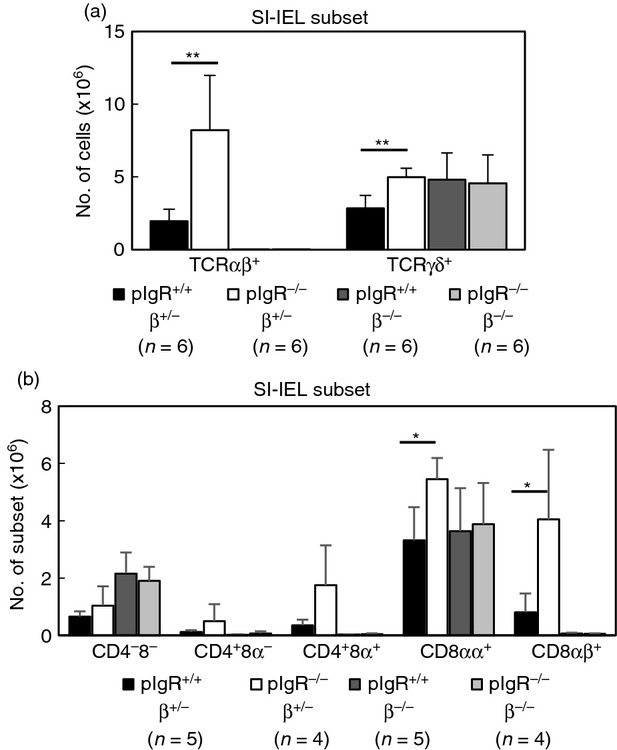
Cellular composition of small intestine intraepithelial lymphocytes (SI-IELs) in polymeric immunoglobulin receptor-deficient (pIgR−/−) mice with and without mature T-cell receptor-αβ-positive (TCR-αβ+) cells. (a) Number of αβ-IELs and γδ-IELs in the SI from pIgR+/+ TCR-β+/− (pIgR+/+β+/−) mice, pIgR−/−β+/− mice, pIgR+/+β−/− mice, and pIgR−/−β−/− mice. Data are represented as mean ± SD of six mice. **P < 0·01. (b) Cell constitution of SI-IELs from pIgR+/+β+/− mice, pIgR−/−β+/− mice, pIgR+/+β−/− mice, and pIgR−/−β−/− mice. Data are mean ± SD of four to five mice. *P < 0·05.
CD8αβ+ IELs originate from the proliferating cells in pIgR−/− mice
The proliferative state of SI-IELs was determined by assessing the incorporation of BrdU. Mice were given ad libitum access to distilled water containing BrdU for 14 days (pulse), after which they were given access to distilled water without BrdU for an additional 10 days (chase). By day 14, almost all of the bone marrow cells and thymocytes had incorporated BrdU, and SI-IELs in both pIgR+/+ mice (13·7 ± 3·1%, n = 5) and pIgR−/− mice (13·6 ± 4·4%, n = 5) were equally labelled with BrdU. Ten days after the supply of BrdU-containing water was discontinued, BrdU-labelled bone marrow cells and BrdU-labelled thymocytes were almost undetectable. In contrast, BrdU-labelled cells were still detected among SI-IELs 10 days after the BrdU supply was ended, and the proportion of BrdU-labelled cells was higher in the SI-IELs of pIgR−/− mice than in those of pIgR+/+ mice (Fig.3). Although the proportion of BrdU-labelled cells among CD4+ SI-IELs was not different between pIgR+/+ mice and pIgR−/− mice, a significantly higher proportion of BrdU-labelled CD8β+ SI-IELs was detected in pIgR−/− cells than pIgR+/+ mice 10 days after having discontinued BrdU supply (Fig.3).
Figure 3.
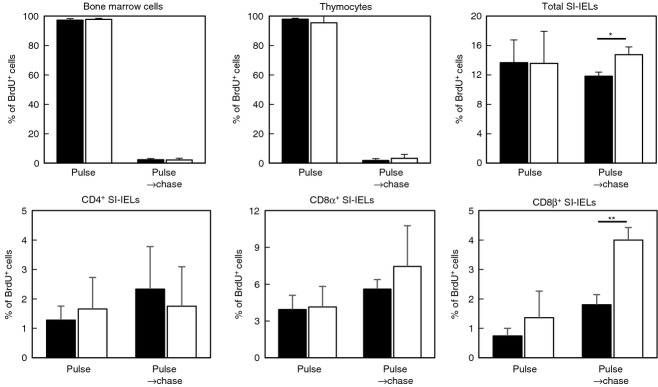
Proliferative status of small intestine intraepithelial lymphocytes (SI-IELs) in polymeric immunoglobulin receptor-positive (pIgR+/+) mice and pIgR−/− mice. Mice were given distilled water containing 5-bromo-2′-deoxyuridine (BrdU; 0·8 mg/ml) for 14 days (pulse), after which they received distilled water without BrdU for an additional 10 days (pulse → chase). Bone marrow cells, thymocytes and SI-IELs were prepared from pIgR+/+ mice (■) or pIgR−/− mice (□) just after the ending of pulse and 10 days after the starting of chase. Cells were stained with FITC-conjugated anti-BrdU monoclonal antibody (mAb), or in the case of SI-IELs they were stained with phycoerythrin-conjugated mAbs specific for CD4, CD8α and CD8β, after which they were incubated with FITC-conjugated anti-BrdU mAb. Data are shown as mean ± SD of two to five mice. **P < 0·05, *P < 0·01.
Migration of splenic TCR-αβ+ cells into the intestine is not augmented in pIgR−/− TCR-β−/− mice
The finding that the proportion of BrdU-labelled CD8β+ SI-IELs increased during the chase period in pIgR−/− mice suggests that CD8αβ+ cells are derived from the cells proliferating outside the intestine. It is reported that chemokine receptor CCR9 on intestinal T cells, and its ligand CCL25 produced by intestinal epithelial cells, play a central role for trafficking of CD8αβ+ T cells into the intestine.19 We therefore examined the expression of CCR9 on SI-IELs and CCL25 mRNA through the intestinal tract. The expression patterns of the CCR9 molecule on the SI-IELs from pIgR+/+ mice and pIgR−/− mice were similar, and CCL25 mRNA expression in the intestine was not different between these mice (see Supplementary material, Fig. S1a,b).
To analyse the migration of mature TCR-αβ+ cells to the intestinal epithelium, CD3+ cells purified from spleens of wild-type mice were intravenously transferred into pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice. Ninety-five per cent of splenic CD3+ cells expressed TCR-αβ and they contained CD4+ cells and CD8αβ+ cells in 65·5 ± 4·5% and 31·6 ± 3·1%, respectively. As the recipient mice do not have TCR-αβ+ cells, all of the TCR-αβ+ cells in the chimeric mice are donor-derived. Splenic TCR-αβ+ cells migrated to the spleens of pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice to a similar extent, and the proportions of CD4+ TCR-αβ+ cells and CD8αβ+ TCR-αβ+ cells in the recipient spleens were 54·9–74·7% and 12·5–17·4% 2–4 weeks after the transfer, respectively (data not shown). TCR-αβ+ cells could also be detected within 2 weeks after the transfer in the intestinal epithelia of both pIgR+/+ TCR-β−/− and pIgR−/− TCR-β−/− recipient mice. There was no difference between both recipient mice with respect to the relative ratio of αβ-IELs (Table1). Moreover, αβ-IELs detected in pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice strongly expressed CD69 and CD103 antigens, although the transferred splenic CD3+ cells expressed neither CD69 nor CD103 (data not shown). In addition, there was no difference in the relative composition of CD4+αβ-IELs and CD8αβ+αβ-IELs in pIgR+/+ TCR-β−/− and pIgR−/− TCR-β−/− recipient mice, although there were more CD8αβ+αβ-IELs than CD4+αβ-IELs in both recipients (Table1). Taken together, these results demonstrate that mature CD8αβ+ TCR-αβ+ cells can migrate to the intestine and are activated in the gut environment to a similar extent, regardless of the presence or absence of sIgA.
Table 1.
Migration of T-cell receptor-αβ-positive (TCR-αβ+) cells into the intestinal epithelia of polymeric immunoglobulin receptor-positive (pIgR+/+) and polymeric immunoglobulin receptor-deficient (pIgR−/−) TCR-β−/− mice
| Subset | HBSS→pIgR+/+β−/− (n = 3) | HBSS→pIgR−/−β−/− (n = 3) | SpT→pIgR+/+β−/− (n = 3) | SpT→pIgR−/−β−/− (n = 5) |
|---|---|---|---|---|
| No. of small intestine intraepithelial lymphocytes (SI-IEL) (×106) | 7·7 ± 3·1 | 7·0 ± 1·9 | 17·6 ± 7·9 | 12·3 ± 4·4 |
| % of αβ-IEL | 0·3 ± 0·2 | 0·3 ± 0·1 | 24·4 ± 15·9 | 21·2 ± 4·1 |
| % of γδ-IEL | 86·0 ± 2·3 | 82·0 ± 5·8 | 67·9 ± 13·7 | 68·9 ± 7·9 |
| % of CD4− CD8− cells in αβ-IEL | Not tested | Not tested | 1·8 ± 1·2 | 1·5 ± 0·4 |
| % of CD4+ CD8− cells in αβ-IEL | Not tested | Not tested | 21·1 ± 10·7 | 18·5 ± 7·2 |
| % of CD8αα+ cells in αβ-IEL | Not tested | Not tested | 1·4 ± 0·6 | 1·6 ± 2·1 |
| % of CD8αβ+ cells in αβ-IEL | Not tested | Not tested | 66·2 ± 16·9 | 68·9 ± 11·1 |
| % of CD4+ CD8+ cells in αβ-IEL | Not tested | Not tested | 9·5 ± 4·9 | 9·5 ± 4·3 |
Differentiation of bone marrow cells into αβ-IELs is enhanced in pIgR−/− TCR-β−/− mice
Adoptive transfer of bone marrow cells was conducted to examine the influence of sIgA-deficiency on the differentiation from immature haematopoietic cells to αβ-IELs. CD3− B220− CD11b− CD11c− cells were prepared from the bone marrow of TCR-δ−/− mice and intravenously transferred into pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice, and thereafter SI-IELs in the recipients were analysed. In these chimeric mice, all the TCR-αβ+ cells are donor-derived and the TCR-γδ+ cells originate from the host. Very few αβ-IELs were detected in both recipient mice 4 weeks after the transfer, but αβ-IELs could be detected 8 weeks later and the number of CD8αβ+αβ-IELs was greater in pIgR−/− TCR-β−/− mice than pIgR+/+ TCR-β−/− mice (Fig.4a). The number of CD8αβ+αβ-IELs further increased in pIgR−/− TCR-β−/− mice from 8 to 12 weeks after the transfer, although there was no further increase in the number of CD8αβ+αβ-IELs in pIgR+/+ TCR-β−/− mice during the same period (Fig.4b). These results indicate that sIgA-deficiency enhances the differentiation of immature haematopoietic cells to CD8αβ+αβ-IELs.
Figure 4.
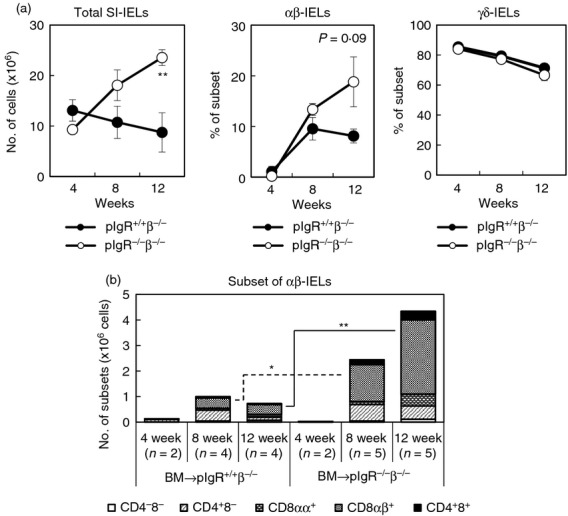
Reconstitution of αβ-intraepithelial lymphocytes (IELs) in polymeric immunoglobulin receptor-positive T-cell receptor-αβ-positive (pIgR+/+ TCRβ−/−) mice and pIgR−/− TCR-β−/− mice by adoptive transfer of immature bone marrow cells. (a) Bone marrow cells were obtained from TCR-δ−/− mice, and CD3− B220− CD11b− CD11c− cells were prepared and transferred intravenously into pIgR+/+ TCR-β−/− mice (pIgR+/+β−/−, ●) and pIgR−/−β−/− mice (○) by 1 × 106 cells/mouse. Total number of small intestine (SI)-IELs and the relative constitution of αβ-IELs and γδ-IELs in the recipient mice were examined at 4, 8 and 12 weeks after the transfer. Data are presented as mean ± SD of two to five mice. (b) Cellular constitution of SI-IELs in the recipient mice was analysed by a flow cytometer and the numbers of αβ-IEL subsets were calculated by multiplying the total number of SI-IELs by the relative proportion of each subset. *P < 0·05, **P < 0·01.
Cytokine production of SI-IELs is skewed towards cellular immune response in pIgR−/− mice
To determine whether functions of SI-IELs are different between pIgR+/+ mice and pIgR−/− mice, αβ-IELs and γδ-IELs were purified from SI-IELs of both mice and their cytokine-producing abilities were compared. They were stimulated with immobilized anti-CD3 mAb for 48 hr, and the cytokine level in the supernatants was measured. αβ-IELs and γδ-IELs from pIgR−/− mice produced more abundant IFN-γ than those from pIgR+/+ mice, and γδ-IELs produced less IFN-γ than αβ-IELs did (Fig.5a). αβ-IELs from pIgR−/− mice also produced more IL-17 than αβ-IELs of pIgR+/+ mice, although the difference between both mice did not reach significance (Fig.5a). Interleukin-4 and IL-10 were not detected in these culture supernatants. RT-PCR analysis also showed that IFN-γ and IL-17 mRNA expression level was higher in αβ-IELs from pIgR−/− mice than in those from pIgR+/+ mice (Fig.5b). Finally, intracellular cytokine staining analysis revealed that more IFN-γ+ CD4+ cells and IFN-γ+ CD8αβ+ cells were detected in pIgR−/− mice than pIgR+/+ mice (Fig.5c).
Figure 5.
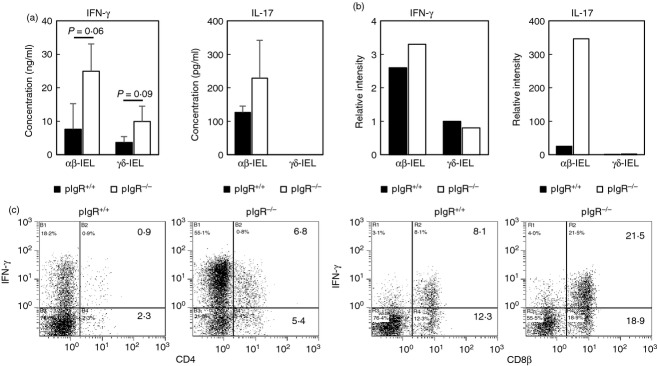
Cytokine production by small intestine intraepithelial lymphocytes (SI-IELs) of polymeric immunoglobulin receptor-positive (pIgR+/+) mice and pIgR−/− mice. (a) Purified αβ-IELs and γδ-IELs from pIgR+/+ mice (■) and pIgR−/− mice (□) were stimulated with immobilized anti-CD3 monoclonal antibody (mAb) for 48 hr, after which the concentrations of interferon-γ (IFN-γ) and interleukin-17 (IL-17) in the culture supernatants were measured using ELISA. Results were expressed as mean ± SD of data from three independent experiments. (b) Total RNA was extracted from purified αβ-IELs and γδ-IELs from pIgR+/+ mice (■) and pIgR−/− mice (□), and mRNA levels of IFN-γ and IL-17 were examined by quantitative PCR. Relative density to GAPDH mRNA was expressed. (c) Intracellular IFN-γ was detected in CD4+ and CD8β+ subsets in SI-IELs isolated from pIgR+/+ mice and pIgR−/− mice.
Intestinal integrity tends to be disrupted after SI-IEL accumulation in pIgR−/− mice
Taking into consideration that CD8αβ+αβ-IELs expressing IFN-γ preferentially accumulate in pIgR−/− mice, they may have influences on the epithelial integrity.20 Therefore, we compared the intestinal permeability of SI of pIgR+/+ mice and pIgR−/− mice. In young mice (6 weeks old), SI of pIgR+/+ mice and pIgR−/− mice exhibited similar levels of permeability. In contrast, the permeability of SI tended to increase in older pIgR−/− mice (20 weeks old), although the difference of permeability between pIgR+/+ mice and pIgR−/− mice did not reach significance because of inter-individual variation (Fig.6).
Figure 6.
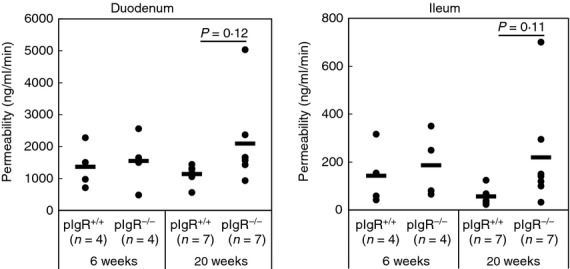
Intestinal permeability of small intestine (SI) of polymeric immunoglobulin receptor-positive (pIgR+/+) mice and pIgR−/− mice. Loops were generated at duodenum and ileum of mice under isoflurane-anaesthesia, and FITC-dextran or rhodamine B isothiocyanate-dextran solutions was injected into the loops, respectively. By measuring the fluorescence intensity in the serum collected at intervals, permeability of SI was evaluated. Closed circles show the data of each mouse and the bars represent the mean value.
Discussion
We have previously reported that Thy-1+ CD8αβ+αβ-IELs preferentially accumulate in the SI of pIgR−/− mice in response to the gut microbiota.17 In this study, we reconfirmed the accumulation of CD8αβ+αβ-IELs in adult pIgR−/− mice and furthermore found that this happened after weaning (older than 5 weeks) (Fig.1b). Intestinal bacteria start to colonize just after delivery and the infant gut microbiota is established during the suckling stage. It has been reported that the gut microbiota changes with aging and the compositions of intestinal bacteria in infants and adults are different.21,22 The accumulation of CD8αβ+αβ-IELs may be triggered in response to the adult-type gut microbiota.
Although the increase of SI-IELs was marked in pIgR−/− mice, the number and constitution of SI-IELs in pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice were similar (Fig.2a,b). These results demonstrate that SI-IEL subsets mainly affected by sIgA-deficiency were αβ-IELs but not γδ-IELs. TCR-αβ+ cells and TCR-γδ+ cells play different roles in the defence against pathogenic infection and the control of intestinal epithelial cell differentiation.23–28 αβ-IELs expand and are activated to become cytolytic in response to gut microbiota, but the number and cytolytic activity of γδ-IELs are similar in conventional mice and germ-free mice.29,30 Judging from the fact that cytolytic CD8αβ+ TCR-αβ+ cells accumulate on the intraepithelial space of the intestine after viral infection,31,32 αβ-IELs are considered to be a sentry against pathogenic infection in the gut. In contrast, it is known that γδ-IELs recognize the antigens on intestinal epithelial cells and play central roles in their homeostasis.27,28 These results indicate that the expansion of CD8αβ+αβ-IELs in response to the intestinal bacteria due to sIgA-deficiency occurs independently of γδ-IELs.
To understand how αβ-IELs expand the pool size in pIgR−/− mice, we examined the incorporation of BrdU into SI-IELs of pIgR+/+ mice and pIgR−/− mice. Although the proportion of BrdU-labelled SI-IELs was comparable in pIgR+/+ mice and pIgR−/− mice 14 days after supplying BrdU, a relative ratio of BrdU-labelled cells among CD8αβ+ SI-IELs was higher in pIgR−/− mice than pIgR+/+ mice 10 days after BrdU administration was discontinued (Fig.3). These results suggest that sIgA-deficiency may promote the migration of CD8β− BrdU-labelled precursor cells into the intestinal epithelia and their differentiation to CD8αβ+ SI-IELs. It has been reported that CD8αβ+αβ-IELs are derived from recent thymic emigrants.33 Therefore, it is likely that CD8β− precursors having completed DNA synthesis migrate and differentiate to CD8αβ+ TCR-αβ+ cells in the intestinal epithelia more efficiently in sIgA-deficiency.
To examine whether the migration of mature TCR-αβ+ cells is enhanced in sIgA-deficiency, we carried out the adoptive transfer experiments. When splenic TCR-αβ+ cells were transferred to pIgR+/+ TCR-β−/− mice or pIgR−/− TCR-β−/− mice, CD8αβ+αβ-IELs accumulated to a similar extent in both recipients, suggesting that sIgA-deficiency does not affect the migration of mature CD8αβ+ TCR-αβ+ cells to the intestinal epithelia. It is known that CCL25 secreted by intestinal epithelial cells promotes the migration of mature CCR9+ CD8αβ+ TCR-αβ+ cells to the intestinal epithelia in wild-type mice,19 but neither CCL25 mRNA expression in the intestine nor the density of CCR9 molecules on SI-IELs were different in pIgR+/+ mice and pIgR−/− mice (see Supplementary material, Fig. S1). Therefore, it is considered that CCR9/CCL25 is not involved in the increase of CD8αβ+αβ-IELs due to sIgA-deficiency.
In the next experiment, we transferred immature haematopoietic cells into pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice, and examined the change of SI-IELs in the recipients from 4 to 12 weeks after the transfer. As a result, donor-derived αβ-IELs could be detected 8 weeks after the transfer and the absolute number of CD8αβ+αβ-IELs was greater in pIgR−/− TCR-β−/− mice than pIgR+/+ TCR-β−/− mice (Fig.4b). These results demonstrate that the differentiation of immature haematopoietic cells to CD8αβ+αβ-IELs was enhanced in sIgA-deficiency. As the numbers of SI-IELs were not different between pIgR+/+ TCR-β−/− mice and pIgR−/− TCR-β−/− mice (Fig.2b), it is assumed that the expansion of CD8αβ+ SI-IELs in pIgR−/− mice requires the expression of functional TCR-αβ. If this is the case, CD8αβ+αβ-IELs may expand the pool size by recognizing gut antigens in pIgR−/− mice.
It is widely known that sIgA plays an important role in preventing pathogenic microorganisms from invading the body through mucosal surfaces.1,14,34 Sait et al. detected a significantly greater number of bacteria in mesenteric lymph nodes of pIgR−/− mice than pIgR+/+ mice.35 We therefore examined the bacterial translocation into mesenteric lymph nodes of pIgR+/+ mice and pIgR−/− mice. In our studies, bacterial translocation was observed in pIgR−/− TCR-β+/− mice and pIgR−/− TCR-β−/− mice, but not in pIgR+/+ TCR-β+/− mice and pIgR+/+ TCR-β−/− mice (see Supplementary material, Fig. S2), supporting the theory that sIgA may inhibit the penetration of intestinal bacteria into the body. It should be noted that the proportion of pIgR−/− TCR-β+/− mice exhibiting bacterial translocation was much lower in our study compared with the results reported previously.35,36 The reason for this discrepancy is not clear, but the extent of bacterial translocation may be influenced by the breeding condition in animal facilities.
We tried to find the functional difference of SI-IELs between pIgR+/+ mice and pIgR−/− mice by examining the cytokine-producing abilities. As a result, αβ-IELs in pIgR−/− mice could produce more IFN-γ than those in pIgR+/+ mice (Fig.5), and therefore it is assumed that functions of αβ-IELs are skewed toward cellular immune responses in sIgA-deficiency. It has been reported that IFN-γ-producing CD8αβ+αβ-IELs disrupt the barrier of epithelial cells in vitro.20 We here found that the intestinal permeability tends to increase in pIgR−/− mice after CD8αβ+αβ-IELs have already expanded (Fig.6). These findings suggest that sIgA works to maintain the epithelial integrity by suppressing the excessive activation of CD8αβ+αβ-IELs.
Gut microbiota has been reported to differ in the presence or absence of sIgA,36,37 so we compared bacterial composition in the small and large intestine contents of pIgR+/+ and pIgR−/− mice using a pyrosequencing method and quantitative PCR method with primers recognizing the bacterial 16S rRNA gene. We found that there was no significant difference in the diversity of gut microbiota between wild-type and pIgR−/− mice at 10–11 weeks, but the composition of bacterial community in SI seemed to be different (see Supplementary material, Fig. S3a). Furthermore, the number of Clostridium coccoides and segmented filamentous bacteria in the SI was greater in pIgR−/− mice compared with pIgR+/+ mice at 20 weeks (see Supplementary material, Fig. S3b). It is likely that colonization by higher numbers of segmented filamentous bacteria may be connected with the tendency to produce more IL-17 production by αβ-IELs in pIgR−/− mice.38,39 Therefore, it is considered that IFN-γ-producing CD8αβ+αβ-IELs expand in response to commensal bacteria after weaning, thereafter the gut microbiota change in composition, and finally the epithelial integrity may be disrupted in association with the increase of IL-17 later in life. It has been recently reported that a fibre-free diet reduces the expression of pIgR in the large intestine of rats.40 These findings suggest that an unbalanced diet disturbs the secretory immune response by reducing pIgR expression, which may result in the disruption of epithelial integrity.
In conclusion, our results indicate that activated CD8αβ+αβ-IELs accumulate due to sIgA-deficiency and may evoke the disruption of epithelial integrity. The harmonized mucosal immune system made up of sIgA and a huge number of lymphoid cells in the intestinal mucosa,41 seems to be very efficient in the defence against the unexpectedly diverse antigens constantly coming into the gut lumen and for maintaining gut homeostasis.
Acknowledgments
We greatly appreciate the staff members of the Yakult Central Institute Animal Facility in breeding the mice. We also thank Drs Hiromichi Ishikawa and Yoshinori Umesaki for their valuable advice and critical reading of the manuscript. N K-N, YY, ST, TN, HS and YW performed the experiments, SS and MN designed the study, and KS and SM have written the paper together with MN.
Glossary
- IEL
intraepithelial lymphocyte
- pIgR
polymeric immunoglobulin receptor
- SI
small intestine
- sIgA
secretory IgA
Disclosures
The authors declare no commercial or financial conflict of interest to disclose.
Supporting Information
Figure S1. Expression of CCR9 on small intestine intraepithelial lymphocyte (SI-IELs) and CCL25 mRNA in the intestinal wall of polymeric immunoglobulin receptor-positive (pIgR+/+) mice and pIgR−/− mice.
Figure S2. Bacterial translocation into mesenteric lymph nodes and IgA level binding commensal Lactobacillus in polymeric immunoglobulin receptor-deficient (pIgR−/−) mice.
Figure S3. Comparison of gut microbiota in wild-type mice and polymeric immunoglobulin receptor-deficient (pIgR−/−) mice.
References
- Cunningham-Rundles C. Physiology of IgA and IgA deficiency. J Clin Immunol. 2001;21:303–9. doi: 10.1023/a:1012241117984. [DOI] [PubMed] [Google Scholar]
- Bomsel M, Heyman M, Hocini H, et al. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity. 1998;9:277–87. doi: 10.1016/s1074-7613(00)80610-x. [DOI] [PubMed] [Google Scholar]
- Corthésy B, Benureau Y, Perrier C, et al. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J Virol. 2006;80:10692–9. doi: 10.1128/JVI.00927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya Y, Kirimanjeswara GS, Roberts S, Metzger DW. Increased susceptibility of IgA-deficient mice to pulmonary Francisella tularensis live vaccine strain infection. Infect Immun. 2013;81:3434–41. doi: 10.1128/IAI.00408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson BA, Guo J, Brown I, et al. Decreased vaginal disease in J-chain-deficient mice following herpes simplex type 2 genital infection. Virology. 2000;271:155–62. doi: 10.1006/viro.2000.0303. [DOI] [PubMed] [Google Scholar]
- Benton KA, Misplon JA, Lo C-Y, et al. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or γδ T cells. J Immunol. 2001;166:7437–45. doi: 10.4049/jimmunol.166.12.7437. [DOI] [PubMed] [Google Scholar]
- Maaser C, Housley MP, Iimura M, et al. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect Immun. 2004;72:3315–24. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uren TK, Wijburg OL, Simmons C, et al. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur J Immunol. 2005;35:180–8. doi: 10.1002/eji.200425492. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Gatto D, Sainsbury E, et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–6. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MA, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–9. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- Johansen FE, Pekna M, Norderhaug IN, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–22. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Kawaguchi-Miyashita M, Kushiro A, et al. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol. 1999;163:5367–73. [PubMed] [Google Scholar]
- Wijburg OLC, Uren TK, Simpfendorfer K, et al. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–6. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjärnlund A, Rodríguez A, Cardona PJ, et al. Polymeric IgR knockout mice are more susceptible to mycobacterial infections in the respiratory tract than wild-type mice. Int Immunol. 2006;18:807–16. doi: 10.1093/intimm/dxl017. [DOI] [PubMed] [Google Scholar]
- Asahi Y, Yoshikawa T, Watanabe I, et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168:2930–8. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Shimada S, Kato-Nagaoka N, et al. Accumulation of intestinal intraepithelial lymphocytes in association with lack of polymeric immunoglobulin receptor. Eur J Immunol. 2005;35:1211–9. doi: 10.1002/eji.200425627. [DOI] [PubMed] [Google Scholar]
- Quan CP, Berneman A, Pires R, et al. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect Immun. 1997;65:3997–4004. doi: 10.1128/iai.65.10.3997-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenstad H, Svensson M, Cucak H, et al. Differential homing mechanisms regulate regionalized effector CD8αβ+ T cell accumulation within the small intestine. Proc Natl Acad Sci USA. 2007;104:10122–7. doi: 10.1073/pnas.0700269104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey C, Erhart D, Saurer L, Mueller M. Production of interferon-γ by activated T-cell receptor-αβ CD8αβ intestinal intraepithelial lymphocytes is required and sufficient for disruption of the intestinal barrier integrity. Immunology. 2009;128:351–9. doi: 10.1111/j.1365-2567.2009.03110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Itoh T, Kuwahara T, et al. Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res. 2007;14:169–81. doi: 10.1093/dnares/dsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorne J, Mombaerts P, Tonegawa S. αβ and γδ T cells in the immune response to the erythrocytic stages of malaria in mice. Int Immunol. 1995;7:1005–11. doi: 10.1093/intimm/7.6.1005. [DOI] [PubMed] [Google Scholar]
- Ladel CH, Hess J, Daugelat S, et al. Contribution of αβ and γδ T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette–Guérin: studies with T cell receptor-deficient mutant mice. Eur J Immunol. 1995;25:838–46. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- Roberts SJ, Smith AL, West AB, et al. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–9. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanno M, Shiohara T, Yamamoto H, et al. γδ T cells: firefighters or fire boosters in the front lines of inflammatory responses. Immunol Rev. 2007;215:103–13. doi: 10.1111/j.1600-065X.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- Witherden DA, Havran WL. Cross-talk between intraepithelial γδ T cells and epithelial cells. J Leukoc Biol. 2013;94:69–76. doi: 10.1189/jlb.0213101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisa SI, Kari MS, Shipra V, et al. γδ intraepithelial lymphocytes are essential mediators of host–microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci USA. 2011;108:8743–8. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira A, Mota-Santos T, Itohara S, et al. Localization of γδ T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172:239–44. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Nanno M, Umesaki Y, et al. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing γδ T-cell antigen receptors. Proc Natl Acad Sci USA. 1993;90:8591–4. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–56. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staton TL, Habtezion A, Winslow MM, et al. CD8+ recent thymic emigrants home to and efficiently repopulate the small intestine epithelium. Nat Immunol. 2006;7:482–8. doi: 10.1038/ni1319. [DOI] [PubMed] [Google Scholar]
- Sun K, Johansen FE, Eckmann L, Metzger DW. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J Immunol. 2004;173:4576–81. doi: 10.4049/jimmunol.173.7.4576. [DOI] [PubMed] [Google Scholar]
- Sait LC, Galic M, Price JD, et al. Secretory antibodies reduce systemic antibody responses against the gastrointestinal commensal flora. Int Immunol. 2007;19:257–65. doi: 10.1093/intimm/dxl142. [DOI] [PubMed] [Google Scholar]
- Reikvam DH, Derrien M, Islam R, et al. Epithelial–microbial crosstalk in polymeric Ig receptor deficient mice. Eur J Immunol. 2012;42:2959–70. doi: 10.1002/eji.201242543. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Meek B, Doi Y, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101:1981–6. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–89. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Sonoyama K. Reduced expression of polymeric immunoglobulin receptors in the intestine of young rats fed a fiber-free diet. Biosci Microbiota Food Health. 2012;31:51–8. doi: 10.12938/bmfh.31.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurashima Y, Goto Y, Kiyono H. Mucosal innate immune cells regulate both gut homeostasis and intestinal inflammation. Eur J Immunol. 2013;43:3108–15. doi: 10.1002/eji.201343782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of CCR9 on small intestine intraepithelial lymphocyte (SI-IELs) and CCL25 mRNA in the intestinal wall of polymeric immunoglobulin receptor-positive (pIgR+/+) mice and pIgR−/− mice.
Figure S2. Bacterial translocation into mesenteric lymph nodes and IgA level binding commensal Lactobacillus in polymeric immunoglobulin receptor-deficient (pIgR−/−) mice.
Figure S3. Comparison of gut microbiota in wild-type mice and polymeric immunoglobulin receptor-deficient (pIgR−/−) mice.


