Abstract
Dengue virus (DENV) infection causes dengue fever, dengue haemorrhagic fever, or dengue shock syndrome. Mast cells have been speculated to play a role in DENV disease although their precise roles are unclear. In this study, we used mast cell-deficient KitW-sh/W-sh mice to investigate the involvement of mast cells after intradermal DENV infection. An approximately two- to three-fold higher level of DENV NS3 antigen was detected at the skin inoculation site in DENV-infected KitW-sh/W-sh mice than in DENV-infected wild-type (WT) mice (using a dose of 1 × 109 plaque-forming units/mouse). Moreover, as an indicator of heightened pathogenesis, a more prolonged bleeding time was observed in DENV-infected KitW-sh/W-sh mice than in WT mice. Monocytes/macrophages are considered to be important targets for DENV infection, so we investigated the susceptibility and chemokine response of DENV-infected peritoneal macrophages from KitW-sh/W-sh and WT mice both ex vivo and in vivo. There was a tendency for higher DENV infection and higher secretion of CCL2 (MCP-1) from peritoneal macrophages isolated from KitW-sh/W-sh mice than those from WT mice. In vivo studies using intradermal inoculation of DENV showed about twofold higher levels of infiltrating macrophages and CCL2 (MCP-1) at the inoculation site in both mock control and DENV-inoculated KitW-sh/W-sh mice than in corresponding WT mice. In summary, compared with WT mice, KitW-sh/W-sh mice show enhanced DENV infection and macrophage infiltration at the skin inoculation site as well as increased DENV-associated bleeding time. The results indicate an intriguing interplay between mast cells and tissue macrophages to restrict DENV replication in the skin.
Keywords: dengue virus, KitW-sh/W-sh mice, macrophage, mast cell
Introduction
Dengue virus (DENV) is a positive-sense single-stranded RNA virus. DENV belongs to the genus Flavivirus of the family Flaviviridae and consists of four serotypes DENV1, 2, 3 and 4. Patients infected by DENV may display a range of clinical syndromes from asymptomatic infection to a self-limiting febrile illness, dengue fever, to severe dengue disease, dengue haemorrhagic fever and dengue shock syndrome. The pathogenic mechanisms are not fully resolved.1,2 Dengue has now become a major international public health concern. There are about 390 million dengue infections each year, of which 96 million cases occur with clinical manifestations.3,4 Better understanding of dengue pathogenic mechanisms is of high importance for rational development of a protective vaccine or specific antiviral drug.
Mast cells arise from bone marrow-derived precursors that circulate in blood and become differentiated after entering tissues.5,6 Mast cells are resident in tissues throughout the body but are most common at sites that are exposed to the external environment, such as skin, airway and intestine.5 Mast cells are known to play critical roles in maintaining a healthy physiology, in wound healing and the defence of pathogens, participating in innate and adaptive immunity. Mast cells are also involved in the inflammatory process, recruiting different leucocytes to the site of injury and in allergic disease.7 Several studies showed that mast cells are involved in the mechanisms of antiviral defence and in viral disease pathogenesis. For example, mast cells could cause lung injury resulting from H5N1 influenza virus infection by releasing pro-inflammatory mediators.8 Mast cells are also involved in host defence at herpes simplex virus-infected sites through tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) production, which are induced by keratinocyte-derived IL-33.9
In the case of dengue, urinary and blood histamine levels are elevated in patients with dengue haemorrhagic fever.10 A recent report showed the elevation of mast cell-derived vascular endothelial growth factor and proteases in patients with dengue shock syndrome.11 Mast cell-derived chymase also promotes vascular leakage in DENV-infected mice.12 In vitro studies indicated that antibody-enhanced DENV infection of mast cells selectively induces production of chemokines CCL3 (MIP-1α), CCL4 (MIP-1β) and CCL5 (RANTES),13 as well as cytokines IL-6, IL-1β and TNF-α.14 The TNF-α produced from antibody-enhanced DENV infection of mast cells can trigger endothelial cell activation.15 Mast cells infected with several viruses including DENV produce a spectrum of chemokines that recruit specific leucocytes including natural killer (NK) cells.16–18 In a mouse model of DENV infection, mast cells were implicated in recruitment of NK and NKT cells, which may clear virus.18 RNA sensors, particularly RIG-I, enable human mast cell antiviral chemokine production and interferon-mediated protection in response to antibody-enhanced DENV infection.16 Localized responses of mast cells to DENV are protective through immune cell recruitment and viral clearance, whereas mast cell-induced vascular leakage systemically can contribute to DENV pathogenesis.19
Mast cell-deficient KitW-sh/W-sh mice are often used as a model to study the function of mast cells in human disease.20,21 Previous studies indicated that mast cells, at least in the human, develop from CD34+ CD117+ (c-Kit) progenitor cells originating in the bone marrow and mature within tissues.22 Mast cells at all stages of maturation express the receptor tyrosine kinase Kit. The ligand of Kit is stem cell factor, which can induce Kit dimerization and auto-phosphorylation in the progression of mast cell maturation. Hence, KitW-sh/W-sh mice, in which surface expression of Kit or Kit catalytic activity is defective, have significantly reduced mast cell numbers.23 In the present study, we used mast cell-deficient KitW-sh/W-sh mice to determine the involvement of mast cells in DENV infection and disease.
Materials and methods
Mice
Wild-type (WT) C57BL/6 mice were obtained from Charles River Breeding Laboratories (Wilmington, MA) and maintained on standard laboratory food and water in the Laboratory Animal Centre of the National Cheng Kung University Medical College. STOCK KitW-sh/HNihrJaeBsmJ (KitW-sh/W-sh) mice were obtained from the Jackson Laboratory (Bar harbor, ME) and maintained at the National Laboratory Animal Centre, Tainan facility. The animal-use protocol was reviewed and approved by the Institutional Animal Care and Use Committee. Six-week-old progeny of WT C57BL/6 and KitW-sh/W-sh mice were used for the DENV infection experiments. In this study, the 6-week-old KitW-sh/W-sh mice had approximately 12% of the number of skin mast cells compared with WT mice. This percentage is very similar to that reported in a previous study.24
Virus culture
DENV2 16681 was propagated in C6/36 cells. Briefly, monolayers of C6/36 cells were inoculated with DENV at a multiplicity of infection (MOI) of 0·01 and incubated at 28° in 5% CO2 for 5 days. The culture medium was harvested and cell debris was removed by centrifugation at 2000 g for 5 min. The virus supernatant was collected and stored at −70° until use in experiments. In some experiments, high-titre DENV was prepared by concentrating virus supernatant using Centrifugal Filters (30-kDa cut off) (Amicon; Millipore, Billerica, MA) by centrifugation. Virus titre was determined by plaque assay using the BHK-21 cell line as described below.
Plaque assay
BHK-21 cells were plated into 12-well plates (2 × 105 cells/well) and cultured overnight in Dulbecco’s modified Eagle’s medium (DMEM) at 37° in a humidified atmosphere of 5% CO2. After adsorption with a serially diluted virus inoculum for 1 hr, the inoculum was replaced with fresh DMEM containing 2% fetal bovine serum (FBS) and 0·5% methyl cellulose. At 5 days post-infection (p.i.), the medium was removed, and the cells were fixed and stained with crystal violet solution (1% crystal violet, 0·64% NaCl and 2% formalin).
Animal model
Mice were intradermally (i.d.) inoculated with 200 µl DENV [109 plaque-forming units (PFU)/mouse] at four sites (50 µl/site) on the upper back as previously described.25 Mice were killed 2 or 3 days p.i.
Bleeding time
Bleeding time was measured by a 3-mm tail-tip transection. Blood droplets were collected on filter paper every 30 seconds. Bleeding time was recorded when the blood spot was smaller than 0·1 mm in diameter.26,27
Immunohistochemistry staining
Skin sections were embedded in paraffin and sliced on slides. Slides were deparaffinized using xylene and an alcohol gradient (100%, 95%, 85%, 70% and 50%). The sections were incubated in 2 m HCl solution for 20 min and then treated with 20 µg/ml proteinase K in TE buffer (50 mm Tris Base, 1 mm EDTA, and 0·5% Triton X-100, pH 8·0) for another 20 min at room temperature. The sections were incubated with 3·5% H2O2 in PBS for 15 min to inhibit endogenous peroxidase activity and blocked by 5% BSA in PBST for 30 min.
The primary and secondary antibodies were appropriately diluted in antibody diluents (Dako Corporation, Glostrup, Denmark). Primary antibodies including rabbit anti-DENV2 NS3 (1 : 500, GeneTex, Irvine, CA), rat anti-mouse F4/80 (1:50, Serotec, Raleigh, NC), and rabbit anti-mouse CCL2 (MCP-1) (1 : 300, Abcam, Cambridge, UK) were incubated overnight at 4°, followed by biotin-labelled donkey anti-rabbit antibody (Jackson Immunoresearch Laboratories, West Grove, PA) or donkey anti-rat antibody (Jackson Immunoresearch Laboratories), respectively, for 2 hr at room temperature. After washing twice with PBST, the sections were incubated with horseradish peroxidase-conjugated streptavidin (Dako Corporation) for 15 min at room temperature. The skin sections were developed with the AEC substrate kit (Vector Laboratories, Burlingame, CA) and nuclei were stained with haematoxylin for 3 min. The sections were analysed using a TissueFAXS (TissueGnostics Vienna, Austria) image cytometer. Cells were counted in 15 regions per mouse field and the average numbers were calculated by HistoQuest software (TissueGnostics). HistoQuest separated the antibody-mediated chromogen (AEC) stain and the counterstain (haematoxylin). The positive cells were detected by signals of AEC in the nucleated cells. Results were displayed as dot plots with each dot representing a single cell in the tissue sample. The cut-off threshold was determined by the AEC signal intensity based on AEC-negative cells in the same section. The forward/backward gating tool of HistoQuest software was used for quality control of measurements. By clicking on high or low AEC staining intensity cells in the image, the forward gating tool showed the individual staining intensities of selected cells in the scattergram. Backward gating was used to verify data by visual inspection on the original image.28
Isolation of murine peritoneal macrophages
Mice were killed by cervical dislocation, and cleaned with 70% ethanol. Mice were injected intraperitoneally with 5 ml 4° PBS and the peritoneum was gently massaged to collect any attached cells. Recovered cells were centrifuged at 1000 g for 10 min at 4°. Cells were resuspended in 5 ml of RPMI-1640 medium (CAISSON) containing 10% FBS and antibiotics and plated in 6-cm Petri dishes. After 30 min of incubation at 37° with 5% CO2, non-adherent cells were removed by PBS washing. The peritoneal macrophages were cultured with RPMI-1640 medium before DENV infection studies, as described below.
Ex vivo DENV infection
Mouse peritoneal macrophages were aliquoted into 24-well plates (2 × 105 cells/well). Cells were incubated for 1·5 hr at 4° in RPMI-1640 with 2% FBS (mock) or DENV at MOI of 10 or 50. At 1·5 hr p.i., the virus was removed. Cells and cell supernatants were collected at 48 and 72 hr p.i.
Detection of infection
Peritoneal macrophages were washed briefly in PBS and fixed with 1% formaldehyde at room temperature for 10 min, and then washed again with PBS. Cells were incubated with normal human serum (1 : 10) at 4° for 1 hr. After washing with PBS and permeabilization buffer (1% BSA, 0·1% saponin and 15 mm NaN3), cells were incubated with rabbit anti-DENV2 NS4B antibody (1 : 500, GeneTex) at 4° for 1 hr. After washing twice with permeabilization buffer, the cells were incubated with Alexa-488-conjugated donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA) at 4° for 30 min. DENV infection (DENV2 NS4B-positive cells) was analysed by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA) with excitation set at 488 nm. The percentage of positive cells was determined by comparison with the mock-infected group.
ELISA of CCL2 (MCP-1) levels
CCL2 (MCP-1) levels were measured by a sandwich ELISA technique with commercial ELISA development kits (R&D Systems, Minneapolis, MN). The 96-well plates were coated with diluted capture antibodies in PBS and incubated overnight at room temperature. After washing three times with wash buffer (0·05% Tween-20 in PBS) and blocking with blocking buffer (1% BSA in PBS) for 1 hr, cell culture supernatants and standards were diluted with reagent diluents (1% BSA in PBS) and then added into plates for 2 hr incubation at room temperature. After washing, the detection antibodies in reagent diluents (1% BSA in PBS) were added and incubated for 2 hr. After washing, streptavidin-horseradish peroxidase (1 : 200) was added for 20 min. Finally, the substrate solution tetramethylbenzidine (TMB) (Clinical Science Products, Mansfield, MA) was added and the reaction was stopped with 1 m H2SO4. The optical density was determined using a microplate reader set to 450 nm.
Statistics
Comparisons between various treatments were performed by unpaired t-test with GraphPad Prism version 5.0 (GraphPad, San Diego, CA). Statistical significance was set at P < 0·05.
Results
DENV antigen and viral RNA expression at the skin inoculation site in WT and KitW-sh/W-sh mice
We sought to determine possible differences between KitW-sh/W-sh and WT mice with respect to DENV infection at the skin inoculation site. Mice were infected with 1 × 109 PFU/mouse of DENV and killed on day 2 or 3 (Fig.1a). We detected the expression of DENV antigen NS3, a marker for viral replication, at the skin inoculation site by immunohistochemical staining. Immunohistochemical staining showed that few cells in the dermis layer expressed NS3 on day 2 (Fig.1b). Immunohistochemical stained images were further quantified by HistoQuest software. The results of HistoQuest analysis showed that the percentage of NS3-positive cells was significantly higher in DENV-infected KitW-sh/W-sh mice compared with that in DENV-infected WT mice (1·03 ± 0·23% versus 0·38 ± 0·11%, about a 2·7-fold change; P < 0·05) (Fig.1c). By day 3, we found that there were more NS3-positive cells in the dermis layer (Fig.1d). We further quantified these immunohistochemical stained images by HistoQuest software. The results of HistoQuest analysis also showed that the percentage of NS3-positive cells was higher, although not statistically significantly, in DENV-infected KitW-sh/W-sh mice compared with that in DENV-infected WT mice (1·17 ± 0·26% versus 0·58 ± 0·14%, about a twofold change; P = 0·0665) (Fig.1e). Using rabbit IgG as negative control antibody for the NS3 staining, the results showed that there was no significant difference between those four groups (see Supplementary material, Fig. S1). The results therefore indicate that DENV infection does not lead to an increase in autofluorescence or non-specific staining. We further performed in situ hybridization to confirm a higher viral RNA level in DENV-infected KitW-sh/W-sh mice compared with that in DENV-infected WT mice (see Supplementary material, Fig. S2).
Figure 1.
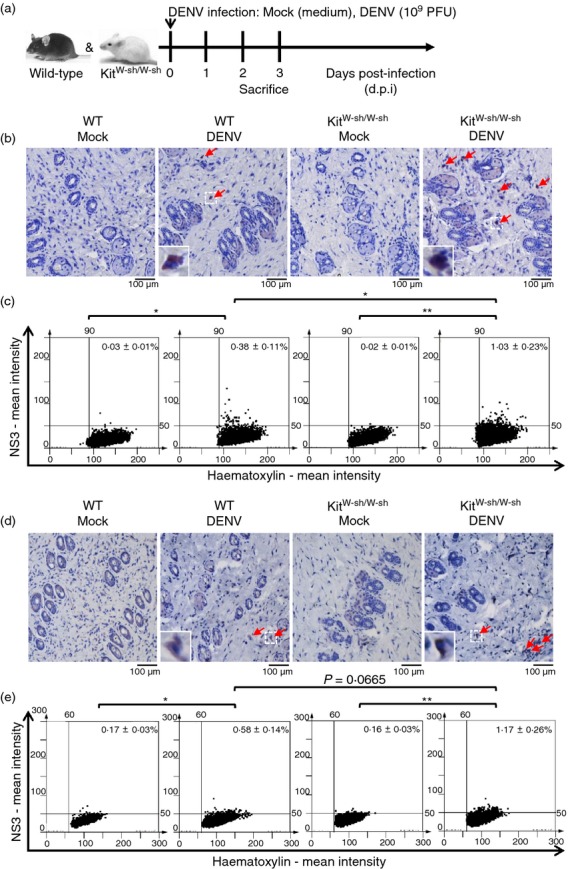
NS3 antigen expression at the dengue virus (DENV) inoculation site in skin is higher in DENV-infected KitW-sh/W-sh than in wild-type (WT) mice. (a) Experimental design of the DENV-infection mouse model. WT and KitW-sh/W-sh mice were intradermally (i.d.) inoculated with medium (Mock) or DENV (1 × 109 plaque-forming units/mouse) at four sites on the upper back and were killed on 2 (b, c) or 3 (d, e) days post-infection (d.p.i.) The skin inoculation site sections were stained with anti-NS3 antibody (red) and nuclei were stained with haematoxylin (blue). Red arrows indicate NS3-positive cells (magnification: ×200). The NS3-positive cells were counted in 15 regions per mouse field and the average numbers of NS3-positive cells were calculated using HistoQuest software. (b, c) n = 5/group; (d, e) n = 8/group. *P < 0·05; **P < 0·01.
Bleeding times of DENV-infected WT and KitW-sh/W-sh mice
Our previous study showed that DENV infection caused prolonged bleeding time in WT mice.29 As increased bleeding time is an important indicator of DENV pathogenesis, we inoculated WT and KitW-sh/W-sh mice with 1 × 109 PFU/mouse of DENV and determined the bleeding time on 2 and 3 days p.i. The results showed that DENV infection caused prolonged bleeding time, both in WT and KitW-sh/W-sh mice. However, the bleeding time was longer in DENV-infected KitW-sh/W-sh mice than in WT mice (Fig.2).
Figure 2.
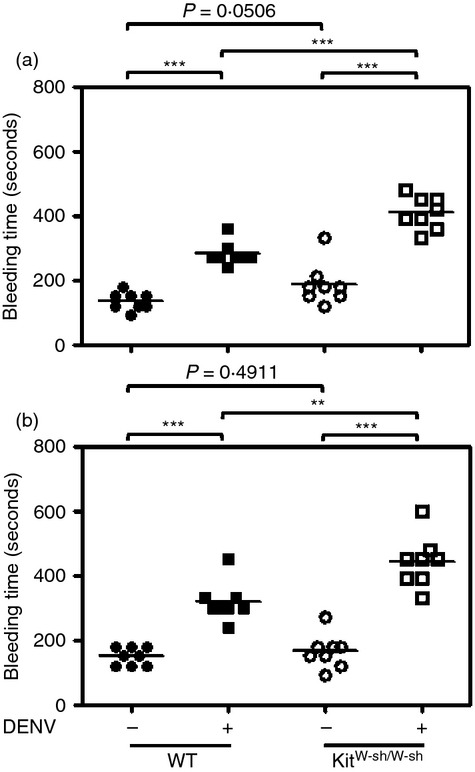
Mouse tail bleeding time is increased in dengue virus (DENV)-inoculated KitW-sh/W-sh mice. Wild-type (WT) and KitW-sh/W-sh mice (n = 8/group) were intradermally (i.d.) inoculated with medium (Mock) or DENV (1 × 109 plaque-forming units/mouse) at four sites on the upper back and the mouse tail bleeding time was determined at 2 (a) and 3 (b) days post-infection (d.p.i.). **P < 0·01; ***P < 0·001.
Infection of DENV and production of CCL2 (MCP-1) in ex vivo peritoneal macrophages isolated from WT and KitW-sh/W-sh mice
CCL2 (MCP-1) is a highly expressed chemokine in patients with dengue haemorrhagic fever/dengue shock syndrome that may act on endothelial cells, causing permeability changes and contributing to plasma leakage.30–33 In addition, CCL2 (MCP-1) contributes to the migration and infiltration of monocytes/macrophages, memory T cells and NK cells, which are involved in dengue disease pathogenesis. Isolated peritoneal macrophages from WT and KitW-sh/W-sh mice were infected with DENV at an MOI of 10 or 50. At 48 or 72 hr p.i., we used flow cytometry to analyse the expression of NS4B, a DENV replication marker. Results showed that at both 48 and 72 hr p.i., DENV infection of peritoneal macrophages was higher, although not statistically significantly, in those from KitW-sh/W-sh than in WT mice (Fig.3a). A set of representative dot plots and histograms of each group is shown in the Supplementary material (Fig. S3). We further detected the production of CCL2 (MCP-1) in the culture supernatants from DENV-infected peritoneal macrophages from WT and KitW-sh/W-sh mice. The results showed that the levels of CCL2 (MCP-1) were higher, although not statistically significantly, in supernatants from peritoneal macrophages isolated from KitW-sh/W-sh mice compared with those isolated from WT mice (Fig.3b). A tendency for higher infection of peritoneal macrophages isolated from KitW-sh/W-sh mice than those from WT mice was also observed using a lower MOI (see Supplementary material, Fig. S4).
Figure 3.
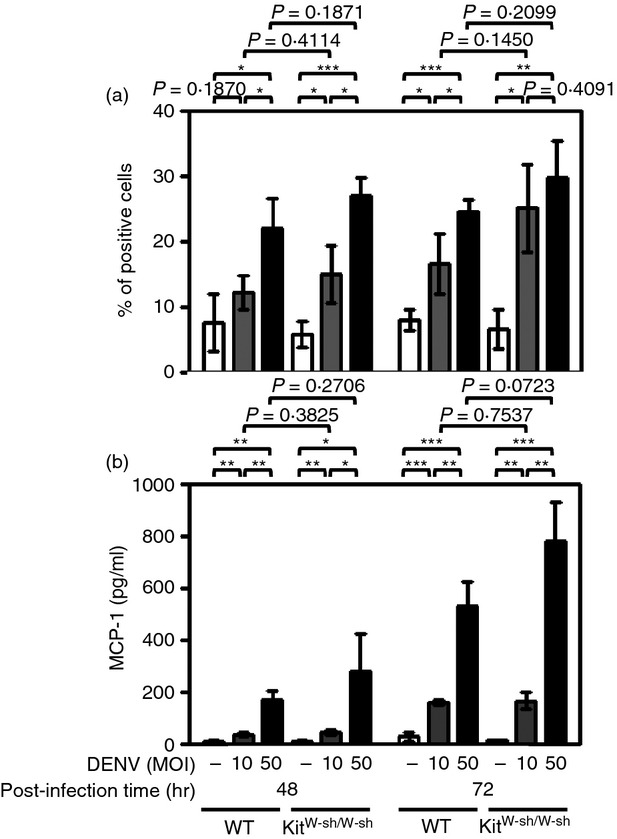
Infection by dengue virus (DENV) and production of CCL2 (MCP-1) are higher in peritoneal macrophages isolated from KitW-sh/W-sh mice than that from wild-type (WT) mice. Cells were incubated for 1·5 hr at 4° with medium alone (RPMI-1640 containing 2% fetal bovine serum; Mock) or with DENV (multiplicity of infection 10 or 50). After washing, cells were cultured in fresh medium and incubated for 48 or 72 hr followed by flow cytometric analysis. (a) The DENV-infected cells were detected by anti-NS4B antibody. The averages of triplicate cultures ± SD are shown. (b) The culture supernatants were assayed for the production of CCL2 (MCP-1) by ELISA. The averages of triplicate cultures ± SD are shown. *P < 0·05; **P < 0·01; ***P < 0·001.
Macrophage infiltration and production of CCL2 (MCP-1) in WT and KitW-sh/W-sh mice
A previous study using C57BL/6 mice showed that there were only infiltrating macrophages (Mac-1+/F4/80+/CD14+) at the site of DENV inoculation in the skin at day 3, but no appreciable numbers of NK cells (NK1.1+), T cells (CD3+), dendritic cells (CD11c+), Langerhans cells (CD207+), or neutrophils (Gr.1+).25 We further determined the numbers of infiltrating macrophages (F4/80-positive cells) and expression of CCL2 (MCP-1) at the skin inoculation site in WT and KitW-sh/W-sh mice infected in vivo with DENV (1 × 109 PFU/mouse) or medium as mock control. At 3 days p.i., we detected F4/80-positive macrophage infiltration in the dermis layer at the skin inoculation site by immunohistochemical staining (Fig.4a). Immunohistochemical staining images were further quantified using HistoQuest analysis software (Fig.4b). Interestingly, we found that the basal numbers of infiltrating macrophages were higher in mock-infected KitW-sh/W-sh mice than in WT mice (5·50 ± 0·47% versus 2·97 ± 0·37%, about a twofold change; P < 0·001). After DENV infection, the numbers of infiltrating macrophages were significantly increased in KitW-sh/W-sh mice and were higher than in WT mice (9·69 ± 1·36% versus 6·64 ± 0·59%, about a 1·5-fold change; P < 0·05). For CCL2 (MCP-1) production, we also found that CCL2 (MCP-1)-positive cells in the dermis layer (Fig.5a) and the basal levels of CCL2 (MCP-1) were higher in KitW-sh/W-sh mice than in WT mice (4·54 ± 0·54% versus 1·90 ± 0·24%, about a 2·4-fold change; P < 0·001) (Fig.5b). After DENV infection, the levels of CCL2 (MCP-1) were significantly increased in KitW-sh/W-sh mice and were higher than in WT mice (10·87 ± 1·34% versus 5·59 ± 0·86%, about a twofold change; P < 0·01) (Fig.5b). There was no significant difference when comparing the mock group with the non-injected group with regard to macrophage infiltration or MCP-1 production (see Supplementary material, Fig. S5), which excludes the possibility that macrophage infiltration and MCP-1 production might be induced by the intradermal injection itself.
Figure 4.
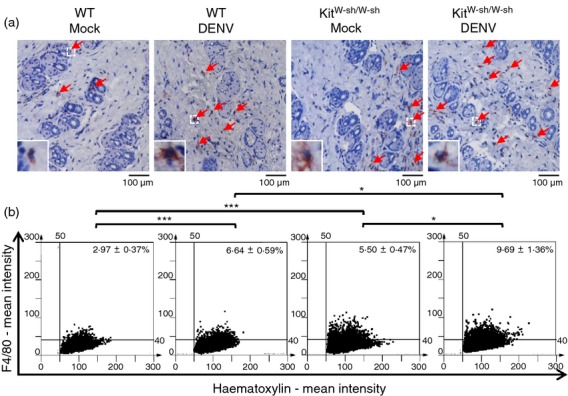
The numbers of infiltrating macrophages in skin are higher in dengue virus (DENV)-inoculated KitW-sh/W-sh than in wild-type (WT) mice. WT and KitW-sh/W-sh mice (n = 8/group) were intradermally (i.d.) inoculated with medium (Mock) or (DENV) (1 × 109 plaque-forming units/mouse) at four sites on the upper back and were killed 3 days post-infection (d.p.i.). (a) The skin inoculation site sections were stained with anti-F4/80 antibody (red) and nuclei were stained with haematoxylin (blue). Red arrows indicate F4/80-positive macrophages (magnification: ×200). (b) The F4/80-positive macrophages were counted in 15 regions per mouse field and the average numbers of macrophages were calculated using HistoQuest software. *P < 0·05; ***P < 0·001.
Figure 5.
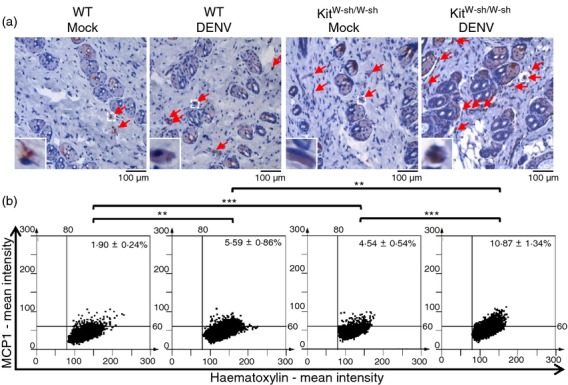
Expression levels of CCL2 (MCP-1) at the skin inoculation site are higher in KitW-sh/W-sh than in wild-type (WT) mice. WT and KitW-sh/W-sh mice (n = 8/group) were intradermally (i.d.) inoculated with medium (Mock) or dengue virus (DENV) (1 × 109 plaque-forming units/mouse) at four sites on the upper back and were killed 3 days post-infection (d.p.i.). (a) The skin inoculation site sections were stained with anti-CCL2 (MCP-1) antibody (red) and nuclei were stained with haematoxylin (blue). Red arrows indicate CCL2 (MCP-1)-positive cells (magnification: ×200). (b) The CCL2 (MCP-1)-positive cells were counted in 15 regions per mouse field and the average numbers of CCL2 (MCP-1)-positive cells were calculated using HistoQuest software. **P < 0·01; ***P < 0·001.
Discussion
The present study offers new insights into the roles of mast cells in dengue infection. Most importantly, we show higher levels of DENV replication at the site of intradermal inoculation, as well as increased bleeding time, an important indicator of pathogenesis, in mast cell-deficient KitW-sh/W-sh mice compared with WT mice. Furthermore, the observed heightened macrophage infiltration and CCL2 (MCP-1) expression in mast cell-deficient mice indicates a collaborative relationship between mast cells and macrophages, which may contribute to restricting DENV infection at the skin inoculation site.
Mouse models for DENV disease are imperfect surrogates for human DENV disease and generally require higher titres of DENV inoculation than is the case for humans.34 Previous studies showed that intradermal inoculation of a wide range of DENV doses (4 × 107 to 3 × 109 PFU/mouse) in mice caused different levels of haemorrhage and thrombocytopenia.25 Using this model in our study (1 × 109 DENV PFU/mouse), we found that KitW-sh/W-sh mice are more susceptible to intradermal DENV infection than WT mice, as indicated by increased NS3 staining at the skin inoculation site. Our results suggest a role for both mast cells and macrophages in modulating DENV infection in skin and accompanying pathological manifestations, including a more prolonged bleeding time and a higher level of F4/80-positive macrophage infiltration in KitW-sh/W-sh mice.
Our results reveal an intriguing dynamic between mast cells and skin macrophages. The basal levels of infiltrating macrophages and CCL2 (MCP-1) were higher in uninfected KitW-sh/W-sh mice than in WT mice. DENV infection induced significantly increased CCL2 (MCP-1) production and macrophage infiltration in KitW-sh/W-sh mice (Fig.6). These results may reflect an antiviral protective role for mast cells, or an increased number of virus-susceptible target cells in KitW-sh/W-sh mice. The identity of such virus-susceptible cells is unknown but could include macrophages that are considered to be a major target of DENV infection.35 As we found that the basal numbers of infiltrating macrophages were higher in KitW-sh/W-sh than in WT mice, it is likely that the greater availability of macrophages in mast cell-deficient mice accounts for the observed higher DENV infection in these mice.
Figure 6.
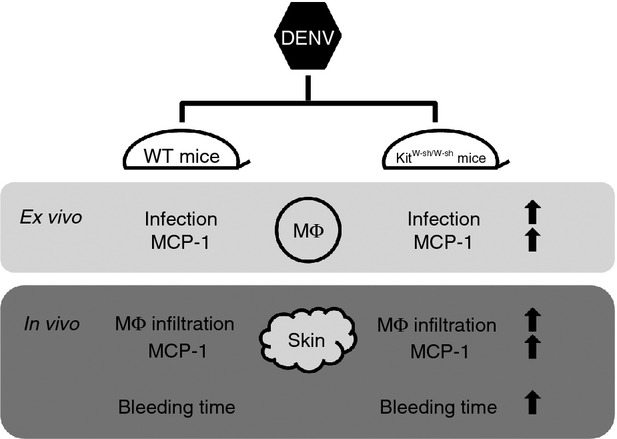
Schematic diagram showing features involved in enhanced dengue virus (DENV) infection in mast cell-deficient mice compared to wild-type (WT) mice both ex vivo and in vivo. Mast cell-deficient KitW-sh/W-sh mice are more susceptible to intradermal DENV infection than WT mice as indicated by a more prolonged bleeding time in KitW-sh/W-sh mice. The ex vivo experiments indicate that peritoneal macrophages isolated from KitW-sh/W-sh mice show higher DENV infection and higher levels of CCL2 (MCP-1) after DENV infection compared with WT mice. In addition, DENV infection induces significantly increased CCL2 (MCP-1) production and macrophage infiltration in KitW-sh/W-sh mice than in WT mice.
Interestingly, our results indicated an intrinsic (i.e. mast cell-independent) difference in macrophages from WT and KitW-sh/W-sh mice. The ex vivo experimental results showed that peritoneal macrophages isolated from KitW-sh/W-sh mice expressed more DENV NS4B antigen and secreted higher levels of CCL2 (MCP-1) after DENV infection compared with WT mice. The mechanism underlying the differential macrophage sensitivity to DENV remains unknown and is worthy of further investigation.
The role of mast cells in DENV infection is complex. Previous in vitro studies indicated that antibody-enhanced DENV infection of mast cells induces chemokines CCL3 (MIP-1α), CCL4 (MIP-1β) and CCL5 (RANTES)13 as well as cytokines IL-6, IL-1β and TNF-α production.14 Furthermore, the TNF-α produced from antibody-enhanced DENV infection of mast cells can trigger endothelial cell activation.15 These results suggest that mast cells may play a pathogenic role in DENV infection. However, specific chemokines, such as CCL4 (MIP-1β), CCL5 (RANTES) and CXCL10 (IP-10) released from mast cells could also contribute to the recruitment of T cells and NK cells, both of which are important for suppressing viral infection.16–18 Mast cells also contribute to immune surveillance, responding to DENV by activating host antiviral responses and releasing chemokines CX3CL1 (Fractalkine), CXCL12 (SDF-1) and CCL5 (RANTES) that recruit additional immune cells.17,18
Mast cells produce TNF-α, which activates endothelial cells15 as well as chymase which can increase vascular permeability.12 Coupled with the well-known chemokine responses of DENV-infected mast cells, these findings suggest that the response of mast cells to DENV can be both beneficial as well as detrimental. During DENV infection, mast cells can also trigger an antiviral response by releasing granules and by up-regulating intracellular antiviral molecules (e.g. RIG-I and MDA5).16,18,19 The activated mast cells also produce chemokines, which recruit NK, NKT and T cells and then help to clear the virus. However, if local control mechanisms fail, the virus will enter the blood and be carried to other organs. The activated mast cells in these organs undergo degranulation to release proteases and inflammatory mediators. These mediators further increase the permeability of endothelial cells, leading to vascular leakage. However, the factors that determine whether the overall or net mast cell response is beneficial or harmful to the host are still unclear.36
In our study, we found significant differences in several pathological features of DENV-infected mast cell-deficient KitW-sh/W-sh mice compared with WT mice. Although previous studies showed that the numbers of immune cells such as macrophages, B cells, T cells, dendritic cells and NK cells are normal in KitW-sh/W-sh mice,24 the tissue distributions of these cells are less clear. We found that the basal levels of CCL2 (MCP-1) and local skin macrophages were higher in KitW-sh/W-sh mice. CCL2 (MCP-1) is produced by many cell types, including macrophages, fibroblasts, epithelial cells and endothelial cells.37 However, monocytes/macrophages are likely major sources of CCL2 (MCP-1).38 Moreover, CCL2 (MCP-1) is also a potent chemotactic factor for monocytes/macrophages.39 In patients with severe dengue, increased levels of CCL2 (MCP-1) have been observed. The CCL2 (MCP-1) levels were also associated with marked thrombocytopenia and hypotension.30,40 Our findings indicate that macrophages from DENV-infected KitW-sh/W-sh mice were more infectable and produced more CCL2 (MCP-1) than those from WT mice. DENV-associated pathogenesis was also heightened in KitW-sh/W-sh mice, as indicated by increased bleeding times.
In addition to increased levels of CCL2 (MCP-1), we also found that CCL5 (RANTES) and CXCL10 (IP-10) were higher in DENV-infected KitW-sh/W-sh mice compared with WT mice (see Supplementary material, Figs S6–S8). These results were unexpected since mast cells infected with DENV in vitro produce increased levels of several chemokines including CCL5 (RANTES) and CXCL10 (IP-10)13,16. The present in vivo study showing higher levels of these chemokines in mast cell-deficient mice may reflect the complex interactions among various cell types that occur in vivo. This needs to be further investigated. Taken together, the results indicate a cooperative interplay between mast cells and macrophages to modulate DENV infection in the skin following intradermal inoculation.
Acknowledgments
YTC, SWW, RA and YSL conceived and designed the experiments. YTC and SWW performed the experiments and analysed the data. YTC, RA and YSL wrote the paper. We are grateful to Dr Jean S. Marshall and Dr Shau-Ku Huang for stimulating discussions. We also thank Dr K. J. Tsai and Y. C. Hsiao for the technical assistance of imaging and analysing from the FACS-like Tissue Cytometry in the Centre of Clinical Medicine, National Cheng Kung University Hospital, Tainan, Taiwan. This work was supported by Grants MOST/NSC101-2325-B-006-006 and MOST/NSC102-2325-B-006-006 from Ministry of Science and Technology, Taiwan.
Glossary
- BSA
bovine serum albumin
- DENV
dengue virus
- FBS
fetal bovine serum
- i.d.
intradermally
- MOI
multiplicity of infection
- NK
natural killer cells
- p.i.
post-infection
- WT
wild-type
Disclosures
The authors have no conflict of interest to declare.
Supporting Information
Figure S1. Dengue virus (DENV) infection does not lead to an increase in autofluorescence and nonspecific staining.
Figure S2. The expression levels of viral RNA are higher in dengue virus (DENV)-infected KitW-sh/W-sh than in wild-type mice.
Figure S3. Dengue virus (DENV) infection rates are higher in peritoneal macrophages isolated from KitW-sh/W-sh mice than those from wild-type mice.
Figure S4. Dengue virus (DENV) infection rates are slightly higher in peritoneal macrophages isolated from KitW-sh/W-sh mice than those from wild-type mice.
Figure S5. The numbers of infiltrating macrophages and CCL2 (MCP-1) show no difference between non-injected and mock-infected KitW-sh/W-sh mice.
Figure S6. Levels of CCL2 (MCP-1) at the skin inoculation site and in serum.
Figure S7. Levels of CXCL10 (IP-10) at the skin inoculation site and in serum.
Figure S8. Levels of CCL5 (RANTES) at the skin inoculation site and in serum.
References
- Whitehorn J, Farrar J. Dengue. Clin Med. 2011;11:483–7. doi: 10.7861/clinmedicine.11-5-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons CP, Farrar JJ, Nguyen VV, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–99. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–52. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller CL, Collington SJ, Williams T, Lamb JR. Mast cells in health and disease. Clin Sci. 2011;120:473–84. doi: 10.1042/CS20100459. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jin Y, Han D, et al. Mast cell-induced lung injury in mice infected with H5N1 influenza virus. J Virol. 2012;86:3347–56. doi: 10.1128/JVI.06053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki R, Kawamura T, Goshima F, et al. Mast cells play a key role in host defense against herpes simplex virus infection through TNF-α and IL-6 production. J Invest Dermatol. 2013;133:2170–9. doi: 10.1038/jid.2013.150. [DOI] [PubMed] [Google Scholar]
- Tuchinda M, Dhorranintra B, Tuchinda P. Histamine content in 24-hour urine in patients with dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health. 1977;8:80–3. [PubMed] [Google Scholar]
- Furuta T, Murao LA, Lan NT, et al. Association of mast cell-derived VEGF and proteases in Dengue shock syndrome. PLoS Negl Trop Dis. 2012;6:e1505. doi: 10.1371/journal.pntd.0001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John AL, Rathore AP, Raghavan B, Ng ML, Abraham SN. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. Elife. 2013;2:e00481. doi: 10.7554/eLife.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, Anderson R, Marshall JS. Dengue virus selectively induces human mast cell chemokine production. J Virol. 2002;76:8408–19. doi: 10.1128/JVI.76.16.8408-8419.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, Marshall JS, Alshurafa H, Anderson R. Release of vasoactive cytokines by antibody-enhanced dengue virus infection of a human mast cell/basophil line. J Virol. 2000;74:7146–50. doi: 10.1128/jvi.74.15.7146-7150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MG, Hermann LL, Issekutz AC, Marshall JS, Rowter D, Al-Afif A, Anderson R. Dengue virus infection of mast cells triggers endothelial cell activation. J Virol. 2011;85:1145–50. doi: 10.1128/JVI.01630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MG, McAlpine SM, Huang YY, Haidl ID, Al-Afif A, Marshall JS, Anderson R. RNA sensors enable human mast cell anti-viral chemokine production and IFN-mediated protection in response to antibody-enhanced dengue virus infection. PLoS ONE. 2012;7:e34055. doi: 10.1371/journal.pone.0034055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–76. doi: 10.1182/blood-2007-10-118547. [DOI] [PubMed] [Google Scholar]
- St John AL, Rathore AP, Yap H, Ng ML, Metcalfe DD, Vasudevan SG, Abraham SN. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc Natl Acad Sci USA. 2011;108:9190–5. doi: 10.1073/pnas.1105079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John AL. Influence of mast cells on dengue protective immunity and immune pathology. PLoS Pathog. 2013;9:e1003783. doi: 10.1371/journal.ppat.1003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallen-St Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest. 2004;113:628–34. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin Exp Allergy. 2005;35:82–8. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34+, c-kit+, and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–42. [PubMed] [Google Scholar]
- Gilfillan AM, Austin SJ, Metcalfe DD. Mast cell biology: introduction and overview. Adv Exp Med Biol. 2011;716:2–12. doi: 10.1007/978-1-4419-9533-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Hofman FM, Kung JT, Lin YD, Wu-Hsieh BA. Both virus and tumor necrosis factor α are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J Virol. 2007;81:5518–26. doi: 10.1128/JVI.02575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Lin CF, Lei HY, Lin SC, Liu HS, Yeh TM, Anderson R, Lin YS. Deletion of the C-terminal region of dengue virus nonstructural protein 1 (NS1) abolishes anti-NS1-mediated platelet dysfunction and bleeding tendency. J Immunol. 2009;183:1797–803. doi: 10.4049/jimmunol.0800672. [DOI] [PubMed] [Google Scholar]
- Séverin S, Gratacap MP, Lenain N, et al. Deficiency of Src homology 2 domain-containing inositol 5-phosphatase 1 affects platelet responses and thrombus growth. J Clin Invest. 2007;117:944–52. doi: 10.1172/JCI29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlederer M, Mueller KM, Haybaeck J, et al. Reliable quantification of protein expression and cellular localization in histological sections. PLoS ONE. 2014;9:e100822. doi: 10.1371/journal.pone.0100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan SW, Lu YT, Huang CH, et al. Protection against dengue virus infection in mice by administration of antibodies against modified nonstructural protein 1. PLoS ONE. 2014;9:e92495. doi: 10.1371/journal.pone.0092495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Liu MT, Lei HY, et al. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol. 2006;87:3623–30. doi: 10.1099/vir.0.82093-0. [DOI] [PubMed] [Google Scholar]
- Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116:4615–28. doi: 10.1242/jcs.00755. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra B, Perez AB, Vogt K, et al. MCP-1 and MIP-1α expression in a model resembling early immune response to dengue. Cytokine. 2010;52:175–83. doi: 10.1016/j.cyto.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Zellweger RM, Shresta S. Mouse models to study dengue virus immunology and pathogenesis. Front Immunol. 2014;5:151. doi: 10.3389/fimmu.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E, Flacher V, Papageorgiou V, Decossas M, Fauny JD, Krämer M, Mueller CG. Dermal CD14+ dendritic cell and macrophage infection by dengue virus is stimulated by interleukin-4. J Invest Dermatol. 2015;135:1743–51. doi: 10.1038/jid.2014.525. [DOI] [PubMed] [Google Scholar]
- Avirutnan P, Matangkasombut P. Unmasking the role of mast cells in dengue. Elife. 2013;2:e00767. doi: 10.7554/eLife.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Ueda A. Monocyte chemoattractant protein-1. In: Aggarwal BB, Gutterman JU, editors. Human Cytokines: Handbook for Basic and Clinical Research, II. Cambridge: Blackwell Science; 1996. pp. 198–221. [Google Scholar]
- Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–93. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- Gu L, Rutledge B, Fiorillo J, Ernst C, Grewal I, Flavell R, Gladue R, Rollins B. In vivo properties of monocyte chemoattractant protein-1. J Leukoc Biol. 1997;62:577–80. doi: 10.1002/jlb.62.5.577. [DOI] [PubMed] [Google Scholar]
- Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT, Kubelka CF. Multiplex cytokine profile from dengue patients: MIP-1β and IFN-γ as predictive factors for severity. BMC Infect Dis. 2008;8:86. doi: 10.1186/1471-2334-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dengue virus (DENV) infection does not lead to an increase in autofluorescence and nonspecific staining.
Figure S2. The expression levels of viral RNA are higher in dengue virus (DENV)-infected KitW-sh/W-sh than in wild-type mice.
Figure S3. Dengue virus (DENV) infection rates are higher in peritoneal macrophages isolated from KitW-sh/W-sh mice than those from wild-type mice.
Figure S4. Dengue virus (DENV) infection rates are slightly higher in peritoneal macrophages isolated from KitW-sh/W-sh mice than those from wild-type mice.
Figure S5. The numbers of infiltrating macrophages and CCL2 (MCP-1) show no difference between non-injected and mock-infected KitW-sh/W-sh mice.
Figure S6. Levels of CCL2 (MCP-1) at the skin inoculation site and in serum.
Figure S7. Levels of CXCL10 (IP-10) at the skin inoculation site and in serum.
Figure S8. Levels of CCL5 (RANTES) at the skin inoculation site and in serum.


