Abstract
Natural killer (NK) cells are considered critical components of the innate and adaptive immune responses. Deficiencies in NK cell activity are common, such as those that occur in cancer patients, and they can be responsible for dysfunctional immune surveillance. Persistent oxidative stress is intrinsic to many malignant tumours, and numerous studies have focused on the effects of reactive oxygen species on the anti-tumour activity of NK cells. Indeed, investigations in animal models have suggested that one of the most important thiol-dependent antioxidant enzymes, peroxiredoxin 1 (PRDX1), is essential for NK cell function. In this work, our analysis of the transcriptomic expression pattern of antioxidant enzymes in human NK cells has identified PRDX1 as the most prominently induced transcript out of the 18 transcripts evaluated in activated NK cells. The change in PRDX1 expression was followed by increased expression of two other enzymes from the PRDX-related antioxidant chain: thioredoxin and thioredoxin reductase. To study the role of thiol-dependent antioxidants in more detail, we applied a novel compound, adenanthin, to induce an abrupt dysfunction of the PRDX-related antioxidant chain in NK cells. In human primary NK cells, we observed profound alterations in spontaneous and antibody-dependent NK cell cytotoxicity against cancer cells, impaired degranulation, and a decreased expression of activation markers under these conditions. Collectively, our study pinpoints the unique role for the antioxidant activity of the PRDX-related enzymatic chain in human NK cell functions. Further understanding this phenomenon will prospectively lead to fine-tuning of the novel NK-targeted therapeutic approaches to human disease.
Keywords: degranulation, natural killer cells, oxidative stress, peroxiredoxin, tumour surveillance
Introduction
Natural killer (NK) cells constitute 10–15% of all peripheral blood lymphocytes. This fairly high frequency implicates an important role for NK cells in a variety of immune processes. Indeed, NK cells are considered to be essential components of innate antimicrobial and anticancer responses. The effector functions of NK cells include cytotoxicity, and cytokine and chemokine production.1 Upon interaction with target cells (i.e. altered, virus-infected or tumour-transformed cells), NK cells exert potent cytotoxicity via perforin/granzyme release or via the tumour necrosis factor-related apoptosis-inducing ligand or Fas ligand pathway. Additionally, the production of pro-inflammatory and regulatory cytokines and of chemokines allows NK cells to communicate with other immune cells.2,3 The triggering of effector functions is mediated via a large array of activating receptors, including NKp30, NKp44, NKp46, DNAM-1, NKG2D and FcγRIII (CD16). With the expression of the FcγRIII, NK cells act as potent effectors notably via antibody-dependent cell-mediated cytotoxicity (ADCC).4
To date, the impact of oxidative stress on NK cell function is not well understood. Some observations indicate that NK cell blood counts and activity are positively correlated with oxidative stress indicators.5 Moreover, intratumoral oxidative stress can change the immune recognition of cancer cells by up-regulating NKG2D ligand expression in primary human carcinoma.6 Nevertheless, oxidative stress is often exploited by tumour cells to impair immune responses. Hence, the conditions of local, though persistent, oxidative stress are often exaggerated at sites of tumour formation.7 Reactive oxygen species (ROS)-induced NK cell dysfunction is considered one of the mechanisms of ‘immune escape’ at sites of solid tumour progression.8 Furthermore, tumour-associated monocytes/macrophages9 or myeloid cells10 suppress NK cells in an oxidant-dependent manner. Oxidative stress is also responsible for the suppression of NK cell functions in non-malignant pathological conditions, such as uraemia11,12 or atherosclerosis.13 It is therefore important to elucidate the natural antioxidant mechanism(s) that can protect NK cells against these effects.
Various mechanisms are believed to protect the cells from oxidative stress. The maintenance of redox balance in the cell requires local and timely availability of the scavenging enzymes. Among these, a group of thiol-dependent enzymes, including peroxiredoxins (PRDXs) (for a comprehensive review, see ref. 14), offers such flexibility because they are not as compartmentalized in the cell as other enzymes, such as catalases. PRDXs are the most recently described family of antioxidant enzymes that are capable of reducing peroxides, the most prominent of which is hydrogen peroxide (H2O2). These enzymes are distributed throughout the cellular compartments and are therefore thought to be broad-range antioxidant defenders. Their catalytic function is to reduce peroxides by using the reactivity of their cysteine residues, and their presumed primary physiological role is to protect living organisms from peroxide toxicity. Moreover, compelling evidence suggests that typical 2-Cys PRDXs play fundamental signal regulatory roles in the multiple signalling networks by interacting with or residing near specific redox-sensitive molecules.15,16 Peroxiredoxin 1 (PRDX1), which was initially referred to as natural killer enhancing factor-A (NKEF-A),17 is a potent natural H2O2 and peroxynitrite scavenger that alleviates oxidative stress18 and regulates a variety of processes within mammalian cells.16 One of the most striking features in the Prdx1-deficient mice is a deep impairment of NK cell function. These mice present abnormalities in the number, phenotype and function of NK cells,19 accompanied by increased susceptibility to spontaneous19 or oncogene-induced20 tumour formation. It has also been shown that PRDX1 strongly increases antibody-dependent NK cell-mediated viral inhibition against simian immunodeficiency virus.21 These facts, which were all gathered mostly in animal models, suggest an important role for PRDXs, especially PRDX1, in NK cell biology. However, more detailed studies in the human system, particularly on the role of intracellular PRDXs in NK cell function, have been hampered until recently by the lack of an efficient inhibitor suitable for studies on the consequences of PRDX dysfunction in primary human NK cells.
Adenanthin (ADNT), a diterpenoid compound that is extracted from the herb Isodon adenantha,22 has been shown to inhibit the antioxidant activity of PRDX1 and, to a lesser extent, PRDX2.23 Our team24 and others25 have further described that such actions are mediated by the regulation of intramolecular or intermolecular disulphide bonds. The result of such actions can be detected in non-reducing SDS–PAGE, where enzymatically inactive PRDX monomers can be observed in lysates from ADNT-treated cells and this phenomenon is accompanied by a deep defect in H2O2 metabolism.26 ADNT has also been proposed to inhibit other enzymes of the PRDX-related chain, namely thioredoxin (TXN)24 and thioredoxin reductase (TXNRD),25 which can hamper the regeneration rate of PRDX. Hence, the effects of ADNT on PRDX metabolism can be in a direct or indirect manner. The effects of ADNT on glutathione (GSH) -related antioxidant defences have also been described, but only at high concentrations of ADNT.25 Hence, ADNT has arisen as a fairly potent research tool for interfering with thiol-dependent peroxidases. Because ADNT has been proposed to be a potential immunomodulatory27 or anti-tumour compound,27,28 it becomes important to identify the full spectrum of pharmacological actions of ADNT in biological systems, particularly those related to tumour surveillance. In this study, we assessed the effects of ADNT on the functions of primary human NK cells.
Materials and methods
Cells
K562 and Raji cell lines were obtained from ATCC (American Type Culture Collection, Manassas, VA). Cells were cultured in RPMI-1640 medium (Sigma Aldrich, St Louis, MO) supplemented with 10% heat inactivated fetal bovine serum, 2 mm l-glutamine and 1% antibiotic antimycotic solution (Sigma Aldrich). Peripheral blood mononuclear cells (PBMC) were obtained by Histopaque-1077 separation from buffy coats from healthy volunteers, commercially obtained from the Regional Blood Centre in Warsaw. Ethics approval was not needed because the buffy coats were provided anonymously and could not be traced back to a specific individual. This is in line with Polish legislation section code §13 Dz.U.1997.106.681.
Reagents
Adenanthin was purchased from Faces Biochemical Com., Ltd (Wuhan, Hubei, China; catalogue no. CFN99215) and dissolved in DMSO. Rituximab was obtained from Roche (Basel, Switzerland). Propidium iodide (Sigma Aldrich) was dissolved in water and diluted in PBS. Carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) was dissolved in DMSO. N-acetyl l-cysteine (NAC) (Sigma Aldrich) was dissolved in sterile distilled water (stock solution) and diluted directly before use in experiments. The pH was adjusted to 7·4 by the addition of NaHCO3. Subsequently, 25 mm N-ethylmaleimide stock solution was obtained as a component of a GSH/GSSG (glutathione disulphide)-Glo™ Assay from Promega (Madison, WI).
Evaluation of ROS
Natural killer cells isolated from healthy donors were stimulated with 100 U interleukin-2 (IL-2) and 100 U interferon-α (IFN-α)/ml overnight and then loaded with CM-H2-DCFDA (Molecular Probes) according to the manufacturer’s protocol, using 1 μm dye concentration for 30 min at 37°. Next, labelled cells were incubated with 200 μm H2O2 or 4 μm ADNT in a culture medium at 37°, harvested and diluted in PBS, and the intensity of green fluorescence was analysed by flow cytometry using Accuri C6 (Becton Dickinson, Franklin Lakes, NJ).
Determination of glutathione levels
To measure total GSH and GSSG, we used a GSH/GSSG-Glo™ Assay (Promega). The experiment was performed as described in ref. 24. Briefly, NK cells that had been isolated from two different donors were seeded at 2 × 106/well in a 96-well plate and incubated with ADNT or DMSO (control group) in the cell culture medium for 2 hr at 37° in two independent experiments. The GSH-depleting agent N-ethylmaleimide (10 μm) was used as a control.
NK cell cytotoxicity assay
Human NK cells freshly isolated using Human CD56 Positive Selection Kit (Stemcell Technologies, Vancouver, BC) were stimulated overnight with human recombinant IL-2 (100 U/ml) (Proleukin; Novartis, Basel, Switzerland) and IL-15 (10 ng/ml; R&D Systems, Minneapolis, MN) or IFN-α (100 U/ml) (Roferon; Roche). K562 and Raji cells (target cells) were labelled with CFSE at a final concentration of 1·25 μm for 10 min at 37° 1 day before the cytotoxicity assay. Experiments were performed either in a pre-incubation or co-incubation model. In the pre-incubation model, NK cells were seeded into a 12-well plate at 4 × 106 cells/well in RPMI-1640 medium and were pretreated with 4 μm ADNT for 4 hr, washed three times and subsequently used in cytotoxicity assays. In the co-incubation, model target cells and previously untreated NK cells were incubated in ADNT (4 μm) for a 4-hr cytotoxicity assay. To study natural cytotoxicity, K562 cells were incubated in 96-well U-bottom plate with NK effector cells (at E : T ratio 6 : 1) for 4 hr at 37°. For the ADCC assay, Raji cells were incubated with anti-CD20 mAb rituximab (100 μg/ml) and NK cells for 4 hr (E : T 6 : 1). Upon incubation, ice-cold propidium iodide (final concentration 4 μg/ml) was added to all samples, and the cells were analysed using flow cytometry (FACScan; Becton Dickinson, San Jose, CA). NK cell cytotoxicity was calculated as a percentage of CFSE and propidium iodide-positive target cells.
Degranulation assay and cytokine secretion
For the degranulation and cytokine secretion assays, NK cells were isolated from PBMC using the EasySep™ Human NK cell Enrichment Kit (Stemcell Technologies) and stimulated overnight as described above. NK cells were incubated with K562 target cells (natural cytotoxicity) or rituximab-coated Raji cells (ADCC) in the presence of GolgiStop (BD Biosciences, San Jose, CA), anti-CD107a-FITC antibody (BD Biosciences) and ADNT (4 μm) (co-incubation model) for 4 hr at an E : T ratio of 1 : 1. Subsequently, NK cells were stained with phycoerythrin (PE)-Vio770-conjugated anti-CD56 (MACS; Miltenyi, Bergisch Gladbach, Germany), Peridinin chlorophyll protein-Cy5.5-conjugated anti-CD3 (BD Biosciences) and Fixable Viability Dye (eBioscience, San Diego, CA). NK cell degranulation was determined as a percentage of CD107a-positive cells within a CD56-positive and CD3-negative NK cell population using flow cytometry. To determine cytokine production after 4 hr of incubation with targets and monoclonal antibodies, NK cells were fixed and permeabilized with Cytoperm/Cytofix (BD Biosciences) and stained with Alexa Fluor®700-conjugated anti-IFN-γ antibody (BD Biosciences) and eFluor®450-conjugated anti-tumour necrosis factor-α (TNF-α) (eBioscience).
NAC treatment
The drug was dissolved as stock solution and diluted directly before experimentation. The pH was adjusted to 7·4 by the addition of NaHCO3. NAC toxicity on NK cells was evaluated upon overnight incubation with increasing concentrations of NAC using propidium iodide staining and flow cytometry analysis. Non-toxic concentrations of NAC were used for other purposes. NK cells that had been stimulated overnight as described previously were pretreated with NAC for 4 hr, washed and used for the degranulation assay. The degranulation assay was performed in the presence of NAC.
Flow cytometry annexin-V binding assay
NK cells alone or co-cultured with K562 cells (at E : T ratio 6 : 1) in a 96-well plate were incubated with increasing concentrations of ADNT (1, 2, 4 μm) for 4 hr. Upon incubation, cells were washed, stained with anti-CD56-PE-Vio770 antibody (MACS; Miltenyi), and then washed and stained with FITC-conjugated annexin-V (BD Biosciences). All staining procedures were performed on ice. Cells were analysed by flow cytometry (Aria III; Becton Dickinson). Annexin-V positive NK cells were calculated from the CD56-positive NK cell population.
Evaluation of NK cell activation markers by flow cytometry
To evaluate the expression of surface activation markers, NK cells were isolated from PBMC using an EasySep™ Human NK cell Enrichment Kit (Stemcell Technologies) and stimulated overnight as described above. After 24 hr of incubation with increasing doses of ADNT, NK cells were washed and incubated for 20 min with the following antibodies: PE-Vio770 conjugated anti-CD56 (clone AF12-7H3; Miltenyi Biotec), allophycocyanin-Cy7 conjugated anti-CD16 (clone 3G8; BD Biosciences), PE-conjugated anti-CD25 (clone M-A251; BD Biosciences), PE-conjugated anti-CD69 (clone TP1.55.3; Beckman Coulter, Brea, CA), PE-conjugated anti-NKp30 (clone AF29-4D12; Miltenyi Biotec), PE-conjugated anti-NKp44 (clone Z231; Beckman Coulter), allophycocyanin-conjugated anti-NKG2D (clone BAT221; Miltenyi), PE-conjugated anti-DNAM1 (clone DX11; BD Biosciences) and Fixable Viability Dye eFluor 506 (eBioscience). After washing with PBS, cells were analysed using flow cytometry (FACSCanto; Becton Dickinson).
Western blotting
Natural killer cells were seeded onto a 12-well plate at 4 × 106 cells/well in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mm l-glutamine, and 1% Antibiotic Antimycotic Solution. After 24 hr, cells were incubated with increasing concentrations of ADNT (0, 2, 4 μm) for either 4 or 8 hr. Cells were pelleted, washed twice in PBS and lysed using RIPA buffer with protease and phosphatase inhibitor cocktail followed by centrifugation at 20 817 g (14 000 rpm) at 4°. The supernatants were collected, and the protein concentrations were determined using the Bradford method. Then, 30 μg of total protein was loaded per lane and separated on an SDS–PAGE in non-reducing conditions and transferred to a nitrocellulose membrane. Membrane was then incubated for 1 hr at 25° in 10% low-fat dry milk in TBS-Tween 20 (TBST). After a 4° overnight incubation in the primary antibody [1 : 1000 anti-PRDX1 (Atlas Antibodies, Stockholm, Sweden) or 1 : 50 000 anti-β-actin (Sigma)], the membrane was washed thrice for 10 min in TBST, incubated for 1 hr at 25° with a horseradish peroxidase-conjugated secondary antibody, and again washed thrice in TBST. The proteins were detected using SuperSignal West Femto Chemiluminescent Substrate (Thermo Scientific, Waltham, MA). Chemiluminescence was detected using the Stella Imaging System (Raytest Isotopenmessgeraete GmbH, Straubenhardt, Germany).
Results
PRDX1, TXN and TXNRD1 transcripts are highly up-regulated in activated NK cells
Given pre-existing information on the role of oxidative stress in NK function, we decided to investigate whether the activation of NK cells is related to detectable changes in the expression of antioxidant enzymes. To this end, we reanalysed the publically available transcriptomic data derived from the gene expression profiling in NK cells by Hanna et al.29 We focused on the expression of 18 transcripts that encode natural antioxidant-related proteins in three subsets of purified peripheral-blood-derived NK cells: CD56dim CD16+ NK, CD56bright CD16− NK and in vitro stimulated CD56+ CD16+ NK (Fig.1). Our analysis revealed a drastic change in the expression of several enzymes upon long-term NK cell stimulation with IL-2 and phytohaemagglutinin.29 In particular, in activated CD56dim CD16+ NK cells, the PRDX1 transcripts increased 18·4-fold compared with the unstimulated NK subset. This phenomenon was accompanied by a stark increase in the transcripts of two other PRDX-related antioxidant enzymes, TXN [fold change (FC) = 14·4] and TXNRD1 (FC = 11). Altogether, this microarray analysis reveals the specific up-regulation of the elements of the PRDX1-related enzymatic chain in the process of NK cell activation. Increases (FC > 2) in the PRDX2-5, GPX4, GLRX, GSR, CAT and SOD1 transcripts could also be observed between unstimulated and stimulated NK cells. Taken together, these results indicate a potent mobilization of the antioxidant defence systems in activated NK cells.
Figure 1.
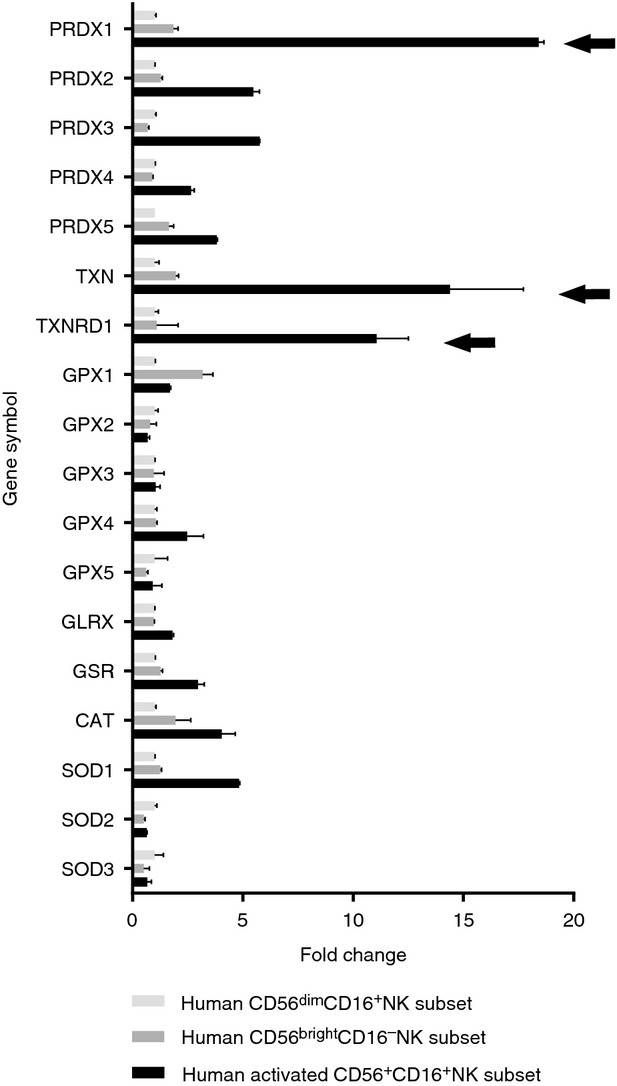
Peroxiredoxin 1 (PRDX1)-encoding transcript is markedly up-regulated in activated natural killer (NK) cells. Reanalysis of changes in antioxidant gene expression pattern in transcriptomic profiling in the pooled purified peripheral blood-derived CD56dim CD16+ NK, CD56bright CD16− NK and in-vitro activated (interleukin-2 + phytohaemagglutinin) CD56+ CD16+ NK subsets obtained from nine healthy donors29 (GEO accession number: GSE1511). The expression level for each gene in CD56dimCD16+ subset was set as 1, and the levels in the remaining two subsets are presented as the relative fold change. PRDX1, thioredoxin (TXN) and thioredoxin reductase (TXNRD1) relative expression bars in the activated NK cells are indicated with arrows. Data are presented as the averages ± SD for two technical replicates.
Adenanthin dysregulates redox homeostasis in NK cells
To study the role of PRDX-related antioxidants in human NK cell function, we chose to chemically inhibit PRDX. First, we evaluated the effects of ADNT on the accumulation of ROS in NK cells. As presented in Fig.2(a), the incubation of primary NK cells with 4 μm ADNT for 4 hr resulted in a significant increase in intracellular ROS, which indicates that ADNT treatment induces exaggerated oxidative stress in these cells. Indeed, ADNT has been reported to interfere with PRDX1 dimer formation in human cells, which correlates with the impairment of H2O2 metabolism.26 Accordingly, in this study, we observed that 4 μm ADNT produced a detectable decrease in PRDX1 dimer content that was accompanied by the appearance of PRDX1-monomers in primary human NK cells (Fig.2b), which corresponds to our previous observations26 and suggests the suitability of ADNT as a tool for the rapid impairment of PRDX-related antioxidant defences in NK cells.
Figure 2.
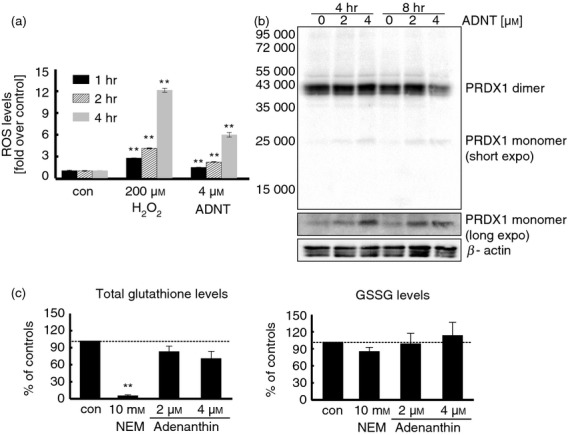
Adenanthin (ADNT) affects the redox balance in natural killer (NK) cells. (a) Relative reactive oxygen species (ROS) levels in NK cells incubated with hydrogen peroxide or ADNT measured by CM-H2-DCFDA fluorescence. (b) Dose- and time-dependent effects of ADNT on peroxiredoxin 1 (PRDX1) dimer/monomer presence in human NK cells, as assessed by electrophoresis in denaturing, non-reducing conditions, followed by immunoblotting. (c) Effects of N-ethylmaleimide (NEM) or ADNT on levels of glutathione (left-hand panel), glutathione disulphide (GSSG) (right-hand panel) in human NK cells. Data presented as the averages from two independent donors ± SEM. **P < 0·01.
Because the shift from the dimeric to monomeric form of PRDX1 can be caused by a decrease in the PRDX reduction rate due to the inhibition of the TXN-TXNRD enzymatic system, we evaluated the effects of ADNT on TXN-TXNRD activity using an insulin reduction assay, as previously described.24 As shown in the Supplementary material (Fig. S1), incubating the NK cells with ADNT resulted in a statistically significant, though very modest decrease in TXN-TXNRD activity. Therefore, although the indirect component of ADNT actions towards PRDX1 cannot be excluded, it is unlikely that this is the only considerable route for ADNT-induced effects.
Given the previous report suggesting that high concentrations of ADNT are capable of depleting GSH levels in biological systems,25 we assessed this phenomenon using the ADNT concentrations applied in this work. As shown in Fig.2(c), 2 and 4 μm ADNT only slightly reduced the total glutathione in NK cells compared with the GSH-depleting agent, N-ethylmaleimide. In general, our data confirm the applicability of ADNT as a tool for inducing intracellular oxidative stress in human NK cells, with greater effects towards PRDX-related antioxidants rather than GSH-related defences.
Adenanthin impairs the natural cytotoxicity and degranulation of NK cells in a pre-incubation model
We next assessed the impact of ADNT on NK cell natural cytotoxicity against the NK-sensitive target cell line K562 (Fig.3a, left-hand panel). Overnight-activated NK cells were first pre-treated with ADNT for 4 hr and were then subsequently co-incubated with K562 cells. Treating NK cells by ADNT impaired cytotoxicity compared with the cytotoxicity mediated by untreated NK cells (8·35% versus 54·48%, respectively, P ≤ 0·01). Of note, the killing process of NK cells relies primarily on the release of perforin and granzyme B granules towards the target cell, a process that is called degranulation,30 followed by CD107a expression at the NK cell surface.31 Consistent with cytotoxicity, degranulation against K562 was significantly decreased after pre-treating NK cells with ADNT (Fig.3a, right-hand panel).
Figure 3.
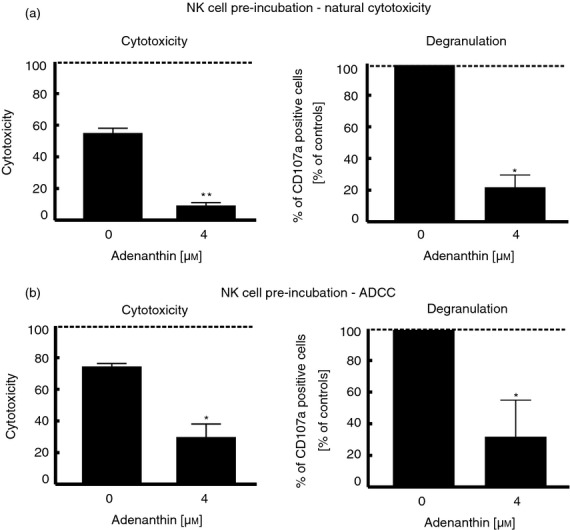
Adenanthin (ADNT) affects natural killer (NK) cell cytotoxicity and degranulation in the pre-incubation model. To study cytotoxicity and degranulation, NK cells (isolated from three healthy donors) were stimulated overnight with cytokines, then pretreated with ADNT for 4 hr, washed three times and used as effector cells in the assays. (a) To determine the influence of ADNT on natural cytotoxicity of NK cells, CFSE-labelled K562 target cells were incubated with NK effector cells (pre-treated with ADNT) for 4 hr at an effector : target ratio of 6 : 1. Upon incubation, cells were stained with propidium iodide (PI) and analysed using flow cytometry. NK cell cytotoxicity was determined as the percentage of CFSE and PI double-positive target cells. Data are presented as the averages ± SD from three donors (left-hand graph). For the degranulation assay (right-hand graph), unstained targets were incubated for 4 hr with NK cells (pretreated with ADNT), anti-CD107a antibody and GolgiStop (at E : T ratio 1 : 1). Cells were washed, stained with anti-CD56, anti-CD3 antibodies and Flexible Viability Dye and analysed by flow cytometry. For every donor, CD107a expression in ADNT-treated groups was calculated as a percentage of CD107a-positive NK cells in the control sample of every donor. The results represent the averages of normalized results ± SD. (b) In experiments determining the influence of ADNT on NK cell cytotoxicity and degranulation in antibody-dependent cell-mediated cytotoxicity (ADCC), NK cells were incubated with Raji cells and rituximab (100 μg/ml) as described in (a). Statistical analysis was performed using the paired Student’s t-test. *P < 0·05; **P < 0·01.
Adenanthin impairs ADCC in a pre-incubation model
Natural killer cells are important mediators of anti-tumour activity mediated by therapeutic monoclonal antibodies, for example, rituximab, which binds the CD20 surface antigen.32 Therefore, we next assessed whether ADNT would affect rituximab-dependent cell-mediated (R-ADCC) NK cell cytotoxicity and degranulation. CD20-expressing Raji cells incubated with rituximab were used as targets for the R-ADCC assays. ADNT pre-treatment significantly impaired R-ADCC, NK cell cytotoxicity and degranulation (Fig.3b, left- and right-hand panels, respectively). Notably, ADNT was not toxic to NK cells within the range of tested concentrations because the viability of NK cells was not affected upon 16 hr of incubation with ADNT (see Supplementary material, Fig. S2). Collectively, our results indicate that pre-treatment with ADNT significantly suppresses the cytotoxic functions of NK cells.
Adenanthin impairs the functions of NK cells in a co-incubation model
In the following studies, we used a co-incubation model33 that reflected more physiological conditions where both target and effector cells were co-incubated for 4 hr in the presence of ADNT. In the co-incubation model, ADNT potently inhibited NK cell degranulation in spontaneous NK cell cytotoxicity and R-ADCC assays (Fig.4a, left- and right-hand panels, respectively), as determined by anti-CD107a staining.
Figure 4.
Adenanthin (ADNT) decreases natural killer (NK) cell degranulation, annexin-V binding and cytokine secretion in the co-incubation model. (a) NK cell degranulation is inhibited in the presence of ADNT in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) assays. NK cells stimulated with cytokines were seeded with target cells and ADNT and incubated for 4 hr in the presence of anti-CD107a antibody and GolgiStop (at an effector : target ratio of 1 : 1). Upon incubation, cells were washed and stained as described previously. The results represent the averages of normalized results ± SD. (b) Effect of ADNT on NK cell degranulation is reversed by N-acetylcysteine (NAC) supplementation. Freshly isolated NK cells were stimulated overnight with cytokines. The next day, cells were pre-treated with NAC for 4 hr, washed and used for the degranulation assay in the presence of ADNT (4 μm) and NAC. The results represent averages of normalized results from two donors ± SD (c) In the annexin-V binding assay, NK cells isolated from the peripheral blood mononuclear cells of seven different donors were incubated alone (con) or in the presence of K562 cells for 4 hr and subsequently stained with anti-CD56 antibody and annexin-V (left graph). At right, ADNT modulates annexin-V binding to NK cells stimulated with K562 targets. NK cells were incubated alone or with K562 cells in the presence of ADNT (0, 1, 2, 4 μm) for 4 hr. Then, cells were washed and stained as described above with anti-CD56 antibody and annexin-V. The results are shown for one representative donor. Bars represent percentage of annexin-V-positive NK cells ± SD for two technical replicates. (d) Target K562 (natural cytotoxicity) or Raji cells with rituximab (100 μg/ml) (ADCC), were seeded with stimulated NK cells and ADNT for 4 hr as described in the Materials and methods section. Upon incubation, cells stained with anti-CD56, anti-CD3 and Flexible Viability Dye, were subsequently fixed, permeabilized and stained with anti-tumour necrosis factor-α (TNF-α) and anti-interferon-γ (IFN-γ) antibodies. Data are presented as a percentage of TNF-α or IFN-γ positive cells within the whole NK cell population. The experiment was performed using NK cells isolated from three different donors. Statistical analysis was performed using the paired Student’s t-test. *P < 0·05; **P < 0·01.
Interestingly, in this model, natural NK cell cytotoxicity remained roughly unchanged (see Supplementary material, Fig. S3, left-hand panel), and only a modest decrease in R-ADCC-mediated NK cell cytotoxicity was observed (see Supplementary material, Fig. S3, right-hand panel). The difference between the pre-incubation and co-incubation results can be explained by either the shorter time course between the start of ADNT treatment and the read-out of the assay or the sensitization of target cells to the cytotoxic effects, which can compensate for NK cell dysfunction. Therefore, we assessed the effects of pre-incubation of the target cells with ADNT before the NK cytotoxicity assay. As shown in the Supplementary material (Fig. S4), ADNT-pretreated K562 or Raji cells were similarly or even less susceptible to the cytotoxic effects of NK cells compared with the control. Hence, we conclude that the difference in cytotoxicity between the pre-incubation model and the co-incubation model is most likely associated with the differences in time course between these models.
To assess whether the effects of ADNT on degranulation of NK cells depend on the induction of oxidative stress conditions, we performed a degranulation assay in the presence of non-toxic concentrations (see Supplementary material, Fig. S2b) of a potent antioxidant, NAC. Indeed, as presented in Fig.4(b), 0·1 mm NAC was capable of almost completely alleviating the effects of ADNT, which confirms the role of oxidative stress in our experimental model.
To confirm our finding on the impact of ADNT on NK cell degranulation, we adapted an assay based on the binding of exogenously added annexin-V to cell surface secretory granules in proportion to the degree of degranulation.34 Specifically, the externalization of phosphatidylserine (PS) has already been proven to be a marker of degranulation of mast cells.34 We assumed that in NK cells, the release of cytolytic granules upon interaction with target cells is accompanied by the flip-flop and expression of PS on the surface of NK cells. Hence, using annexin-V staining (see Supplementary material, Fig. S5, for gating strategy), we observed significantly higher annexin-V binding following interaction between NK cells and K562 cells (P < 0·001, compared with NK cells alone), which strongly suggests the applicability of the annexin-V-based assay as an indicator of the NK cell degranulation process (Fig.4c, left-hand panel). Consistent with these results, increasing concentrations of ADNT resulted in a dose-dependent reduction of PS externalization (Fig.4c, right-hand panel, grey bars). Importantly, ADNT had no significant effects on the basal phosphatidylserine levels (Fig.4c, right-hand panel, black bars).
In addition to cell cytotoxicity, upon target cell stimulation, NK cells are potent producers of pro-inflammatory cytokines, including IFN-γ and TNF-α.35 Using intracellular staining, we then determined the effects of ADNT on the production of IFN-γ and TNF-α by NK cells (see Supplementary material, Fig. S6, for gating strategy). The incubation of NK cells with target cells (K562 and rituximab-coated Raji cells, Fig.4d,e) in the presence of ADNT resulted in a severe reduction in cytokine production compared with the controls.
Collectively, our results demonstrate that interfering with redox-dependent systems by treating the NK cells with ADNT significantly affects the cytotoxic and cytokine production capabilities of NK cells.
Adenanthin affects the immunophenotype of NK cells
The triggering of NK cell function is mediated by a wide range of surface activating receptors, including CD16, NKp30, NKp44, NKp46, DNAM-1 and NKG2D. Moreover, activation of NK cells is followed by the up-regulation of surface markers such as CD25 and CD69 (widely known as activation markers).1,36,37 We therefore studied the effects of ADNT on the expression of activating receptors and activation markers on the NK cell surface. A 24-hr incubation with ADNT induced profound changes in the expression of most of these molecules on NK cells and had the most distinct effect on CD25, CD69 and NKG2D expression (Fig.5).
Figure 5.
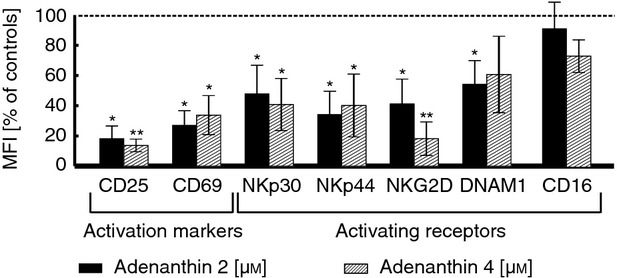
Adenanthin (ADNT) down-regulates expression of natural killer (NK) cell surface molecules. NK cells isolated from three healthy donors, stimulated and incubated with ADNT for 24 hr were subsequently stained with fluorochrome-conjugated antibodies and analysed using flow cytometry. The expression of each antigen was calculated as a ratio of mean fluorescence intensity (MFI) to isotype control MFI. Data are presented as the percentage of control cells. Bars indicate the averages of normalized results ± SD. Statistical analysis was performed using a paired Student’s t-test. *P < 0·05; **P < 0·01.
Discussion
In the last decade, significant efforts have been made to improve our understanding of NK cell function and to propose therapeutic strategies based on NK cell properties, especially in oncology (reviewed in ref. 38). Nevertheless, many aspects of human NK cell biology still remain obscure. One of the outstanding issues is to fully understand the role and mechanism(s) of actions of the antioxidant defence system in NK cell function. Hence, there is a constant need for developing the proper tools for studies of redox-related changes in NK cells.
Many studies have shown that tumour-associated NK cells display alterations of activating NK cell receptor expression, which may be deleterious for immune surveillance (reviewed in ref. 39). Similar observations have been made in peripheral blood NK cells in cancer patients, where NK cells presented decreased activity and IFN-γ production.40,41 It is most probable that cancers have developed numerous pathways to suppress NK cells.42,43 For instance, multiple pro-oncogenic signalling pathways result in an increase in ROS production, including H2O2.44 Hence, persistent oxidative stress conditions are characteristic for many malignant tumours, and immune cells infiltrating the tumour must cope with this adverse environment.
Mammalian cells possess several metabolic systems to alleviate the effects of high levels of H2O2, including the PRDX-related system that has been directly linked to NK cell function.45 Indeed, our analysis of transcriptomic data showed that transcripts encoding components of the PRDX1→TXN→TXNRD enzymatic chain are prominently up-regulated in activated, compared with resting, human NK cells. It remains unclear, however, how exactly PRDXs contribute to NK cell function. It was shown that the genetic loss of this H2O2 scavenger in mice leads to an elevation of ROS levels46 and tumorigenesis19 in various tissues, which is accompanied by abnormalities in number, phenotype and function of NK cells.19 Studies by Riddell et al.47 have suggested that extracellular PRDX1 might act in the immune system via binding to and stimulating Toll-like receptor 4 (TLR4), although this remains to be confirmed in NK cells.21 Notably, however, the role of TLR4-mediated signalling in human NK cells is considered minor; the recent study by Kanevskiy et al.48 found that approximately 0·2% of the NK cells were shown to be positive for surface TLR4 and that intracellular staining revealed only modest amounts of TLR4 inside the NK cell population. Similar results were obtained in the study by Souza-Fonseca-Guimaraes et al.49 Therefore, although paracrine or intracrine PRDX1–TLR4 interactions cannot be excluded, it seems reasonable to expect that PRDX1 also plays TLR4-independent roles in NK cells. To address that assumption, we used the chemical inhibition of an enzymatic activity of PRDX1 with a novel thiol-targeted compound, ADNT. To our knowledge, our study is the first to assess how inhibiting the intracellular PRDX-related antioxidant system influences human NK cell function and the first to study the applicability of ADNT as a pro-oxidant research tool in NK cells. Despite obvious considerations about the complete specificity of ADNT towards PRDX1 (indeed, ADNT also inhibits the functions of PRDX2, TXN and TXNRD, to some extent),24,25 we find this approach favourable over the virus-mediated genetic knockdown of PRDX1, a technique that requires a long-term culture of the primary NK cells in vitro or the use of NK cell lines.50 Importantly, previous data from our group have shown that the application of ADNT mimics the impairment in H2O2 catabolism achieved by PRDX1-knockdown.26 Under such conditions, we observed that ADNT induces deep alterations in degranulation and cytokine production by human primary NK cells upon interacting with cancer cells and that it reduces the expression of several NK cell activating receptors and activation markers. Hence, we provide evidence that dysfunction of the PRDX-related system in human cells is sufficient to potently inhibit the effector functions of NK cells. This study substantiates previous findings from animal models, as studies performed with Prdx1-deficient mice indicated that this molecule is critically important for the biology of NK cells.19 We further conclude that the H2O2-degrading capability of the PRDX-related enzymatic system is crucial for NK cell effector activities.
Because PRDX activity has been shown to undergo multilevel regulation in mammalian cells,51–53 our results indicate that PRDX-related enzymes may serve as one of the mechanisms regulating NK cell function and can potentially be explored therapeutically. Indeed, our study raises hope that targeting the thiol-dependent antioxidants can be one of the modalities to regulate the effector functions of NK cells. This is of special importance in light of novel strategies using ex vivo expanded, activated and/or genetically manipulated NK cells for adoptive anticancer therapies (reviewed in refs 54,55). Collectively, our study provides new information that opens new avenues for studies on NK cells in healthy or pathological conditions.
Acknowledgments
This work was supported by grants from the European Commission 7th Framework Programme (FP7-REGPOT-2012-CT2012-316254-BASTION), Polish National Science Center (2012/07/B/NZ7/04183; RZ, 2012/07/B/NZ6/03498, MW; 2014/13/N/NZ6/02081, MS) and Polish Ministry of Science and Higher Education (IP2011 012671, MF). MS and MBo are supported by the stipend from the Postgraduate School of Molecular Medicine (Warsaw, Poland). DO, and CF is supported by the Institut National du Cancer (InCa) and the Fondation pour la recherche médicale (FRM).
Glossary
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADNT
adenanthin
- CAT
catalase
- CSFE
carboxyfluorescein succinimidyl ester
- FC
fold change
- GLRX
glutaredoxin
- GPX
glutathione peroxidase
- GSH
glutathione
- GSR
glutathione reductase
- GSSG
glutathione disulphide
- IL
interleukin
- NAC
N-acetylcysteine
- NKEF
natural killer enhancing factor
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- PRDX
peroxiredoxin
- PS
phosphatidylserine
- ROS
reactive oxygen species
- SOD
superoxide peroxidase
- TLR
toll-like receptor
- TXNRD
thioredoxin reductase
- TXN
thioredoxin
Authors’ contribution
MS, MW, MBa, MF, AM, MBo, CF performed the experiments, analysed the data, and contributed to the writing of the manuscript. JG and DO provided unique research materials and contributed to the writing of the manuscript; RZ conceived and designed the study, supervised the project, analysed the data, and wrote the manuscript. All authors participated in the critical revision of the manuscript.
Disclosures
No potential conflicts of interest were disclosed.
Supporting Information
Figure S1. Effects of adenanthin on the activity of thioredoxin–thioredoxin reductase system in natural killer (NK) cells.
Figure S2. Viability of natural killer (NK) cells is not affected by adenanthin or N-acetylcysteine (NAC).
Figure S3. Natural killer (NK) cell cytotoxicity in the presence of adenanthin (co-incubation model).
Figure S4. Natural killer (NK) cell cytotoxicity in target cell pre-incubation model.
Figure S5. Gating strategy for annexin-V staining.
Figure S6. Gating strategy for the degranulation assay and cytokine detection.
References
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Van Elssen CH, Oth T, Germeraad WT, Bos GM, Vanderlocht J. Natural killer cells: the secret weapon in dendritic cell vaccination strategies. Clin Cancer Res. 2014;20:1095–103. doi: 10.1158/1078-0432.CCR-13-2302. [DOI] [PubMed] [Google Scholar]
- Crome SQ, Lang PA, Lang KS, Ohashi PS. Natural killer cells regulate diverse T cell responses. Trends Immunol. 2013;34:342–9. doi: 10.1016/j.it.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol. 2013;4:76. doi: 10.3389/fimmu.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaszubowska L, Kaczor JJ, Hak L, Dettlaff-Pokora A, Szarynska M, Kmiec Z. Sensitivity of natural killer cells to activation in the process of ageing is related to the oxidative and inflammatory status of the elderly. J Physiol Pharmacol. 2011;62:101–9. [PubMed] [Google Scholar]
- Vantourout P, Willcox C, Turner A, et al. Immunological visibility: posttranscriptional regulation of human NKG2D ligands by the EGF receptor pathway. Sci Transl Med. 2014;6:231ra49. doi: 10.1126/scitranslmed.3007579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- Mellqvist UH, Hansson M, Brune M, Dahlgren C, Hermodsson S, Hellstrand K. Natural killer cell dysfunction and apoptosis induced by chronic myelogenous leukemia cells: role of reactive oxygen species and regulation by histamine. Blood. 2000;96:1961–8. [PubMed] [Google Scholar]
- Zheng MZ, Pan HD, Pan JX, Guo JX. Monocyte-induced NK cell inactivation: role of reactive oxygen and nitrogen metabolites. Immunopharmacol Immunotoxicol. 2011;33:150–6. doi: 10.3109/08923973.2010.489051. [DOI] [PubMed] [Google Scholar]
- Akhiani AA, Werlenius O, Aurelius J, Movitz C, Martner A, Hellstrand K, Thoren FB. Role of the ERK pathway for oxidant-induced parthanatos in human lymphocytes. PLoS ONE. 2014;9:e89646. doi: 10.1371/journal.pone.0089646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraldi MN, Berrou J, Dulphy N, et al. Oxidative stress mediates a reduced expression of the activating receptor NKG2D in NK cells from end-stage renal disease patients. J Immunol. 2009;182:1696–705. doi: 10.4049/jimmunol.182.3.1696. [DOI] [PubMed] [Google Scholar]
- Peraldi MN, Berrou J, Metivier F, Toubert A. Natural killer cell dysfunction in uremia: the role of oxidative stress and the effects of dialysis. Blood Purif. 2013;35(Suppl. 2):14–9. doi: 10.1159/000350839. [DOI] [PubMed] [Google Scholar]
- Li W, Johnson H, Yuan XM, Jonasson L. 7β-hydroxycholesterol induces natural killer cell death via oxidative lysosomal destabilization. Free Radic Res. 2009;43:1072–9. doi: 10.1080/10715760903176919. [DOI] [PubMed] [Google Scholar]
- Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH. Thioredoxins, glutaredoxins, and peroxiredoxins – molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 2013;19:1539–605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Karplus PA, Poole LB. Typical 2-Cys peroxiredoxins – structures, mechanisms and functions. FEBS J. 2009;276:2469–77. doi: 10.1111/j.1742-4658.2009.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee S, Lee S, Kang SW. 2-cys peroxiredoxins: emerging hubs determining redox dependency of mammalian signaling networks. Int J Cell Biol. 2014;2014:715867. doi: 10.1155/2014/715867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauri H, Ashjian PH, Kim AT, Shau H. Recombinant natural killer enhancing factor augments natural killer cytotoxicity. J Leukoc Biol. 1996;59:925–31. doi: 10.1002/jlb.59.6.925. [DOI] [PubMed] [Google Scholar]
- Poole LB, Hall A, Nelson KJ. Overview of peroxiredoxins in oxidant defense and redox regulation. Curr Protoc Toxicol. 2011 doi: 10.1002/0471140856.tx0709s49. Chapter 7:Unit7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CA, Krause DS, Carman CV, et al. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–5. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- Cao J, Schulte J, Knight A, et al. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–17. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmal M, Letvin NL, Geiben-Lynn R. Natural killer cell-dependent and non-dependent anti-viral activity of 2-Cys peroxiredoxin against HIV. Int Trends Immun. 2013;1:69–77. [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Yang H, Li ML, Hou AJ, Han QB, Wang SJ, Li SH, Sun HD. Diterpenoids from Isodon adenantha. J Nat Prod. 2002;65:1111–6. doi: 10.1021/np020084k. [DOI] [PubMed] [Google Scholar]
- Liu CX, Yin QQ, Zhou HC, et al. Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemic cells. Nat Chem Biol. 2012;8:486–93. doi: 10.1038/nchembio.935. [DOI] [PubMed] [Google Scholar]
- Muchowicz A, Firczuk M, Chlebowska J, et al. Adenanthin targets proteins involved in the regulation of disulphide bonds. Biochem Pharmacol. 2014;89:210–6. doi: 10.1016/j.bcp.2014.02.022. [DOI] [PubMed] [Google Scholar]
- Soethoudt M, Peskin AV, Dickerhof N, Paton LN, Pace PE, Winterbourn CC. Interaction of adenanthin with glutathione and thiol enzymes: selectivity for thioredoxin reductase and inhibition of peroxiredoxin recycling. Free Radic Biol Med. 2014;77:331–9. doi: 10.1016/j.freeradbiomed.2014.09.025. [DOI] [PubMed] [Google Scholar]
- O’Leary PC, Terrile M, Bajor M, et al. Peroxiredoxin-1 protects estrogen receptor α from oxidative stress-induced suppression and is a protein biomarker of favorable prognosis in breast cancer. Breast Cancer Res. 2014;16:R79. doi: 10.1186/bcr3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin QQ, Liu CX, Wu YL, et al. Preventive and therapeutic effects of adenanthin on experimental autoimmune encephalomyelitis by inhibiting NF-κB signaling. J Immunol. 2013;191:2115–25. doi: 10.4049/jimmunol.1203546. [DOI] [PubMed] [Google Scholar]
- Hou JK, Huang Y, He W, et al. Adenanthin targets peroxiredoxin I/II to kill hepatocellular carcinoma cells. Cell Death Dis. 2014;5:e1400. doi: 10.1038/cddis.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Bechtel P, Zhai Y, Youssef F, McLachlan K, Mandelboim O. Novel insights on human NK cells’ immunological modalities revealed by gene expression profiling. J Immunol. 2004;173:6547–63. doi: 10.4049/jimmunol.173.11.6547. [DOI] [PubMed] [Google Scholar]
- Atkinson EA, Gerrard JM, Hildes GE, Greenberg AH. Studies of the mechanism of natural killer (NK) degranulation and cytotoxicity. J Leukoc Biol. 1990;47:39–48. doi: 10.1002/jlb.47.1.39. [DOI] [PubMed] [Google Scholar]
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Winiarska M, Glodkowska-Mrowka E, Bil J, Golab J. Molecular mechanisms of the antitumor effects of anti-CD20 antibodies. Front Biosci (Landmark Ed) 2011;16:277–306. doi: 10.2741/3688. [DOI] [PubMed] [Google Scholar]
- Bojarczuk K, Siernicka M, Dwojak M, et al. B-cell receptor pathway inhibitors affect CD20 levels and impair antitumor activity of anti-CD20 monoclonal antibodies. Leukemia. 2014;28:1163–7. doi: 10.1038/leu.2014.12. [DOI] [PubMed] [Google Scholar]
- Demo SD, Masuda E, Rossi AB, et al. Quantitative measurement of mast cell degranulation using a novel flow cytometric annexin-V binding assay. Cytometry. 1999;36:340–8. doi: 10.1002/(sici)1097-0320(19990801)36:4<340::aid-cyto9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Fauriat C, Long EO, Ljunggren HG, Bryceson YT. Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood. 2010;115:2167–76. doi: 10.1182/blood-2009-08-238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard AL, Spirig R, Mueller NJ, Seebach JD, Rieben R. Inhibition of direct and indirect TLR-mediated activation of human NK cells by low molecular weight dextran sulfate. Mol Immunol. 2010;47:2349–58. doi: 10.1016/j.molimm.2010.05.284. [DOI] [PubMed] [Google Scholar]
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10:230–52. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy EM, Roberti MP, Mordoh J. Natural killer cells in human cancer: from biological functions to clinical applications. J Biomed Biotechnol. 2011;2011:676198. doi: 10.1155/2011/676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konjevic G, Mirjacic Martinovic K, Jurisic V, Babovic N, Spuzic I. Biomarkers of suppressed natural killer (NK) cell function in metastatic melanoma: decreased NKG2D and increased CD158a receptors on CD3–CD16+ NK cells. Biomarkers. 2009;14:258–70. doi: 10.1080/13547500902814658. [DOI] [PubMed] [Google Scholar]
- Mamessier E, Pradel LC, Thibult ML, et al. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J Immunol. 2013;190:2424–36. doi: 10.4049/jimmunol.1200140. [DOI] [PubMed] [Google Scholar]
- Lee JC, Lee KM, Ahn YO, Suh B, Heo DS. A possible mechanism of impaired NK cytotoxicity in cancer patients: down-regulation of DAP10 by TGF-β1. Tumori. 2011;97:350–7. doi: 10.1177/030089161109700316. [DOI] [PubMed] [Google Scholar]
- Mamessier E, Sylvain A, Thibult ML, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121:3609–22. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–47. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- Shau H, Gupta RK, Golub SH. Identification of a natural killer enhancing factor (NKEF) from human erythroid cells. Cell Immunol. 1993;147:1–11. doi: 10.1006/cimm.1993.1043. [DOI] [PubMed] [Google Scholar]
- Rani V, Neumann CA, Shao C, Tischfield JA. Prdx1 deficiency in mice promotes tissue specific loss of heterozygosity mediated by deficiency in DNA repair and increased oxidative stress. Mutat Res. 2012;735:39–45. doi: 10.1016/j.mrfmmm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell JR, Wang XY, Minderman H, Gollnick SO. Peroxiredoxin 1 stimulates secretion of proinflammatory cytokines by binding to TLR4. J Immunol. 2010;184:1022–30. doi: 10.4049/jimmunol.0901945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanevskiy LM, Telford WG, Sapozhnikov AM, Kovalenko EI. Lipopolysaccharide induces IFN-γ production in human NK cells. Front Immunol. 2013;4:11. doi: 10.3389/fimmu.2013.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza-Fonseca-Guimaraes F, Parlato M, Philippart F, Misset B, Cavaillon JM, Adib-Conquy M Captain Study Group. Toll-like receptors expression and interferon-γ production by NK cells in human sepsis. Crit Care. 2012;16:R206. doi: 10.1186/cc11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy AK, Campbell KS. Introduction of shRNAs into human NK-like cell lines with retrovirus. Methods Mol Biol. 2010;612:223–31. doi: 10.1007/978-1-60761-362-6_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CA, Cao J, Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8:4072–8. doi: 10.4161/cc.8.24.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman R, Weisman-Shomer P, Ziv T, Xu J, Arner ES, Benhar M. Multilevel regulation of 2-Cys peroxiredoxin reaction cycle by S-nitrosylation. J Biol Chem. 2013;288:11312–24. doi: 10.1074/jbc.M112.433755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KL, Lew QJ, Rajasegaran V, et al. Regulation of PRDX1 peroxidase activity by Pin1. Cell Cycle. 2013;12:944–52. doi: 10.4161/cc.23916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs RW, Berg M. Bringing natural killer cells to the clinic: ex vivo manipulation. Hematology Am Soc Hematol Educ Program. 2013;2013:234–46. doi: 10.1182/asheducation-2013.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. 2014;3:e28147. doi: 10.4161/onci.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effects of adenanthin on the activity of thioredoxin–thioredoxin reductase system in natural killer (NK) cells.
Figure S2. Viability of natural killer (NK) cells is not affected by adenanthin or N-acetylcysteine (NAC).
Figure S3. Natural killer (NK) cell cytotoxicity in the presence of adenanthin (co-incubation model).
Figure S4. Natural killer (NK) cell cytotoxicity in target cell pre-incubation model.
Figure S5. Gating strategy for annexin-V staining.
Figure S6. Gating strategy for the degranulation assay and cytokine detection.



