Abstract
BACKGROUND
The objective of this systematic review and meta-analysis was to assess the clinical outcomes of Clostridium difficile infection (CDI) in patients with chronic kidney diseases (CKD) and end stage renal disease (ESRD).
METHODS
A literature search was performed from inception through February 2015. Studies that reported relative risks, odds ratios, or hazard ratios comparing the clinical outcomes of CDI in patients with CKD or ESRD and those without CKD or ESRD were included. Pooled risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using a random-effect, generic inverse variance method.
RESULTS
19 studies (a case-control and 18 cohort studies) with 116,875 patients assessing clinical outcomes of CDI were included in the meta-analysis. Pooled RR of severe or complicated CDI in CKD patients was 1.51 (95% CI 1.00–2.28). The risk of recurrent CDI is significant higher in patients with a pooled RR of 2.73 (95% CI, 1.36–5.47). The pooled RR of mortality risk of CDI in patients with CKD, ESRD, and CKD or ESRD were 1.76 (95% CI, 1.26–2.47), 1.58 (1.37–1.83) and 1.76 (1.32–2.34), respectively.
CONCLUSION
This meta-analysis demonstrates poor outcomes of CDI including severe and recurrent CDI in CKD patients. History of CKD and ESRD are both associated with increased mortality risk in patients with CDI.
Keywords: Clostridium, Clostridium difficile, c difficile, Clostridium difficile–associated colitis, c. diff infection, chronic kidney disease, diarrhea, dialysis, end stage kidney disease, Infection, Infectious disease, Meta-analysis, outcome, severity
INTRODUCTION
Clostridium difficile infection (CDI) or Clostridium difficile associated diarrhea (CDAD) is the most identifiable pathogen accountable for 12% of health care–associated infection in the United States [1]. During the last decade, its incidence and severity have been markedly increasing worldwide [2–7]. When patients develop CDI, they encounter increased risk of mortality, morbidity, prolonged hospitalization and hospital readmission [8, 9]. Therefore, previous studies have attempted to identify risk factors for poor outcomes including recurrence, complications, and mortality in CDI.
Recently, Abou Chakra et al [8] performed a comprehensive review of risk factors for CDI outcomes (recurrent, treatment failure, complicated infection and mortality). Among several risk factors, co-morbidities were identified as a risk of complicated CDI and increased mortality. Chronic kidney disease (CKD) is a common disease estimated to effect 8–16% worldwide [10–12]. However, the correlation of CDI outcome and CKD and end-stage renal disease (ESRD) are still inconclusive. Several studies have shown significant increased mortality risk in CKD or ESRD patients with CDI [13–18]. Conversely, a number of studies have shown no significant increased risk of incident and recurrent CDI in patients with CKD or ESRD [19–23]. A study even found that with CDI, CKD patients had lower mortality risk compared with patients without CKD [24].
Thus the objective of this systematic review and meta-analysis was to assess the risks of poor clinical outcomes including recurrence, complications, and mortality in CKD or ESRD patients with CDI.
MATERIALS AND METHODS
Search Strategy
Two investigators (CT and WC) independently searched published studies and conference abstracts indexed in EMBASE, MEDLINE and the Cochrane database from inception to February, 2015 using the search strategy described in online supplementary data. A manual search for additional relevant studies using references from retrieved articles was also performed.
Inclusion Criteria
The inclusion criteria were as follows: (1) randomized controlled trials (RCTs) or observational studies (case-control, cross-sectional or cohort studies) published as original studies or conference abstracts that evaluated the clinical outcomes of CDI in patients with CKD and ESRD, (2) studies that provided data to calculate odds ratios (ORs), relative risks, hazard ratios or standardized incidence ratios with 95% confidence intervals (CIs), and (3) a reference group composed of patients without CKD or ESRD.
Study eligibility was independently determined by the 2 investigators noted previously. Differing decisions were resolved by mutual consensus. The quality of each study was evaluated by using the Jadad quality-assessment scale [25] for RCTs and the Newcastle-Ottawa quality assessment scale [26] for observational studies. No limits were applied for language and foreign papers were translated.
Data Extraction
A standardized data collection form was used to extract the following information: last name of first author, country of origin, study design, year of publication, sample size, definition of CDI, definition of severe/complicated CDI, definition of CKD and ESRD, confounder adjustment, and adjusted effect estimate with 95% CI.
Statistical Analysis
Review Manager 5.2 software (The Cochrane Collaboration, Oxford, UK) was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird [27]. Given the high likelihood of between study variances, a random-effect model was used rather than a fixed-effect model. Statistical heterogeneity was assessed using Cochran’s Q test. This statistic was complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. An I2 of 0%–25% represents insignificant heterogeneity, 26%–50% low heterogeneity, 51%–75% moderate heterogeneity and >75% high heterogeneity [28]. The presence of publication bias was assessed by funnel plots of the logarithm of odds ratios vs their standard errors [29]. Forest plots were demonstrated in order by weight of each study.
RESULTS
The search strategy yielded 1674 potentially relevant articles: 1477 were excluded based on the title and abstract indicating that they clearly did not fulfill inclusion criteria on the basis of article type, study design, population, or outcome of interest (Online supplement data). The remaining 197 articles underwent full-length review, with 178 excluded because they did not report outcomes of interest (n=143) or were not RCTs or observational studies (n=35). 19 studies (a case-control [30] and 18 cohort studies [13–24, 31–36]) with 116,875 patients assessing clinical outcomes of CDI were identified. No RCT met our inclusion criteria. Of 19 studies, 12 studies [13–24] with 115,113 patients were included in the meta-analysis of mortality risk of CDI in patients with CKD or ESRD. Four studies [14, 31, 32, 34] with 1,283 patients and five studies [13, 21, 30, 35, 36] with 1,512 patients were included in the meta-analyses assessing the risks of severe CDI and recurrent CDI in patients with CKD, respectively. The data on the risk of severe CDI and recurrent CDI in patients with ESRD were limited. Tables 1 contains detailed characteristics and quality assessment of all included studies.
Table 1.
Main characteristics of the studies included in this meta-analysis
| Cunney et al [19] | Do et al [30] | Morris et al [20] | Yousuf et al [13] | Henrich et al [31] | |
| Country | Ireland | Canada | USA | USA | USA |
| Study design | Cohort study | Case-control study | Cohort study | Cohort study | Cohort study |
| Year | 1998 | 1998 | 2002 | 2002 | 2009 |
| Total number | 32 | 59 | 147 | 77 | 336 |
| Study sample | Hospital-based; inpatients with CDI admitted in a nephrology unit | Hospital-based; inpatients with CDI | Hospital-based; inpatients with CDI | Hospital-based; inpatients and outpatients with CDI | Hospital-based; inpatients with CDI |
| CDI detection | C. difficile toxin A in stool using EIA, stool culture of C. difficile, histologic examination of colonic biopsies and diarrhea | C. Difficile-positive stool culture and diarrhea | Positive stool test for toxin A and/or B using EIA and hospital discharge code for CDI | C. difficile toxin A in stool using EIA and diarrhea | Positive stool test for C. difficile toxin using Cytotoxic assay or toxin A and B ELISA |
| Chronic kidney disease definition and ascertainment | Chronic renal failure (not defined); medical record review | Chronic renal insufficiency, defined as baseline SCr of 1.5 mg/dL | Renal disease and/or diabetes mellitus; medical record review | Chronic renal insufficiency, defined as persistently elevated SCr of ≥1.5 mg/dL for ≥ 3 months | Renal disease; physician-documented medical condition |
| Definition of severe/complicated CDI | N/A | N/A | N/A | N/A | CDI that resulted in death within 30 days after diagnosis, required ICU admission, colectomy or other surgery or led to intestinal perforation |
| OR for severe/complicated CDI | N/A | N/A | N/A | N/A | Renal disease 0.84 (0.37–1.91) |
| OR for mortality | 5 (0.99–25.3) | N/A | 0.82 (0.26–2.61) | 6.15 (2.14–17.66) | N/A |
| OR for recurrence | N/A | 6.5 (1.4–32.3) | N/A | 12.75 (2.45–66.26) | N/A |
| Confounder adjusted | None | None | none | None | Renal disease: none Hemodialysis: Age, sex, antimicrobial use, malignancy, chemotherapy, steroid use, WBC, glucose, ALT, albumin, SCr |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 2 Comparability: 0 Outcome: 2 |
Selection: 2 Comparability: 0 Exposure: 2 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 2 Outcome: 3 |
| Dudukgian et al [14] | Pepin et al [23] | Wilson et al [22] | Fujitani et al [32] | Bauer et al [33] | |
| Country | USA | Canada | UK | USA | Europe |
| Study design | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study |
| Year | 2009 | 2009 | 2010 | 2011 | 2011 |
| Total number | 398 | 130 | 128 | 184 | 496 |
| Study sample | Hospital-based; inpatients with CDC | Multi-center; inpatients with fulminant CDI requiring an emergency colectomy | Hospital-based; inpatients with CDI | Multi-center; inpatients with CDI | Multi-center; outpatient and inpatients with CDI |
| CDI detection | Discharge diagnosis of CDC (ICD-9 008.45) and positive toxin ELISA or biopsy consistent with pseudomembranous colitis | Positive C. difficile cytotoxin assay, endoscopic or histopathologic evidence of pseudomembranous colitis | Positive stool test for toxin A or B using ELISA and diarrhea | Positive stool test for C. difficile toxin A and B and diarrhea | positive stool test for toxin A, B or both using EIA, cytotoxicity test or PCR or stool culture for toxin-producing C. difficile and diarrhea |
| Chronic kidney disease definition and ascertainment | Renal insufficiency (not defined); medical record review | Chronic renal failure, defined as baseline SCr ≥1.5 mg/dL | Renal failure (not clearly defined); medical record review | Chronic renal insufficiency/end-stage renal disease (not defined); medical record review | Chronic dialysis; APACHE II |
| Definition of severe/complicated CDI | CDI requiring surgery or resulting in death | N/A | N/A | CDI that required ICU admission, surgery for toxic megacolon, large-bowel perforation or refractory colitis or resulted in death within 30 days after diagnosis | CDI that contributed or caused ICU admission or death or led to colectomy |
| OR for severe/complicated CDI | 1.90 (0.98–3.69) | N/A | N/A | 1.78 (0.64–4.97) | 0.29 (0.04–2.35) |
| OR for mortality | 2.64 (1.32–5.27) | 1.75 (0.85–3.60) | 1.65 (0.77–3.54) | N/A | N/A |
| OR for recurrence | N/A | N/A | N/A | N/A | 2.23 (0.59–8.37) |
| Confounder adjusted | None | None | None | None | Complicated: none Recurrence: Age, health-care association, pulmonary disease, previous antibiotics use, recent CDI, C. difficile strain |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 4 Comparability: 0 Outcome: 3 |
Selection: 4 Comparability: 2 Outcome: 3 |
| Manek et al [34] | Stewart et al [24] | Welfare et al [15] | Kim et al [36] | Pant et al [18] | |
| Country | Canada | USA | UK | Korea | USA |
| Study design | Cohort study | Cohort study | Cohort study | Cohort study | Cohort study |
| Year | 2011 | 2011 | 2011 | 2012 | 2012 |
| Total number | 365 | 41207 | 2761 | 198 | 64944 |
| Study sample | Hospital-based; inpatients with CDI | Nationwide Inpatient Sample (NIS) 2007 database; inpatients with CDC in US hospitals | Hospital-based; inpatients with CDI | Hospital-based; inpatients who recovered from CDI | Nationwide Inpatient Sample (NIS) 2009 database; inpatients with CDC in US hospitals |
| CDI detection | Positive stool test for C. difficile toxin A and B using EIAand diarrhea, visualization of pseudomembrane on endoscopy or histopathology | ICD-9 code of 008.45 | Positive stool test for C. difficile toxin A and B immunoassay and diarrhea | Positive stool test for C. difficile toxin A and B and diarrhea | ICD-9 code of 008.45 |
| Chronic kidney disease definition and ascertainment | Renal disease (not defined); medical record review | Renal failure (not defined; AHRQ comorbidity indicators | Renal disease; ICD-10 code of N00–N28 | Renal disease (not defined); medical record review | End-stage renal disease; discharge diagnosis based on ICD-9 code of 585.6 |
| Definition of severe/complicated CDI | CDI that caused severe hypokalemia, toxic megacolon, bowel perforation, lower GI bleeding requiring blood transfusion, ICU transfer or death before treatment completion | N/A | N/A | - | N/A |
| OR for severe/complicated CDI | 1.76 (0.71–4.39) | N/A | N/A | - | N/A |
| OR for mortality | N/A | 0.93 (0.91–0.95) | 1.96 (1.62–2.38) | - | 1.56 (1.43–1.70) |
| OR for recurrence | N/A | N/A | N/A | 2.14 (0.45–10.26) | N/A |
| Confounder adjusted | none | Yes but not clearly specified | Age and comorbidities | Age, sex, treatment, additional use of antibiotics, underlying disease | None |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 3 Comparability: 0 Outcome: 3 |
Selection: 4 Comparability: 1 Outcome: 3 |
Selection: 3 Comparability: 2 Outcome: 3 |
Selection: 4 Comparability: 2 Outcome: 3 |
Selection: 4 Comparability: 0 Outcome: 3 |
| Halabi et al [16] | Mullane et al [21] | Samie et al [35] | Lee et al [17] | |
| Country | USA | United, Canada and Europe | Germany | USA |
| Study design | Cohort study | Cohort study | Cohort study | Cohort study |
| Year | 2013 | 2013 | 2013 | 2014 |
| Total number | 3900 | 1054 | 124 | 335 |
| Study sample | Nationwide Inpatient Sample (NIS) 2001–2010 database; inpatients with CDC who underwent total or subtotal colectomy for CDC in US hospitals | Multi-center; patients with CDI enrolled in randomized controlled trial | Hospital-based; inpatients with CDI | ACS-NSQIP database 2005–2010; inpatients underwent emergent open colectomy for CDC |
| CDI detection | ICD-9 code of 008.45 | Positive stool test for C. difficile toxin A and/or B and diarrhea | positive stool test for toxin A, B or positive stool culture for C. difficile and diarrhea | ICD-9 code of 008.45 |
| Chronic kidney disease definition and ascertainment | Chronic kidney disease (not defined); NIS dataset | Chronic kidney disease stage 3–4, defined as CrCl < 60 ml/min/1.73 m2; Estimated CrCl was calculated using Cockcroft-Gault formula | Chronic kidney disease stage 3–4 defined as a GFR < 60 ml/min; GFR was calculated using Cockcroft-Gault formula | Dialysis dependence, defined as acute or chronic within 2 weeks before surgery; ACS-NSQIP database |
| Definition of severe/complicated CDI | N/A | N/A | N/A | N/A |
| OR for severe/complicated CDI | N/A | N/A | N/A | N/A |
| OR for mortality | 1.63 (1.50–1.76) | 1.72 (0.75–3.97) | N/A | 2.3 (1.1–4.8) |
| OR for recurrence | N/A | 1.80 (1.08–2.98) | 1.52 (0.51–4.53) | N/A |
| Confounder adjusted | none | Age, treatment, fever WBCs, albumin, concomitant antibiotics | C-reactive protein, leukocytosis, PPI use, DM, glucocorticoid therapy, cerebral insult, cirrhosis | Age, preoperative septic shock, severe COPD, postoperative cardiac arrest, would classification, preoperative platelet, INR and BUN |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 4 Comparability: 0 Outcome: 3 |
Selection: 4 Comparability: 2 Outcome: 3 |
Selection: 3 Comparability: 2 Outcome: 3 |
Selection: 4 Comparability: 2 Outcome: 3 |
Abbreviations: ALT, alanine transaminase; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; CDI, Clostridium difficile infection; ICU, intensive care unit; N/A, not available; SCr, serum creatinine; WBC, white blood cell.
Abbreviations: CDC, Clostridium difficile colitis; AHRQ, Agency for Health Care Research and Quality
Abbreviations: ICU, intensive care unit; ACS-NSQIP, American College of Surgeons – National Surgical Quality Improvement Program.
Abbreviations: BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; INR, international normalized ratio; K, Potassium; PPI, proton pump inhibitor; WBC, white blood cell.
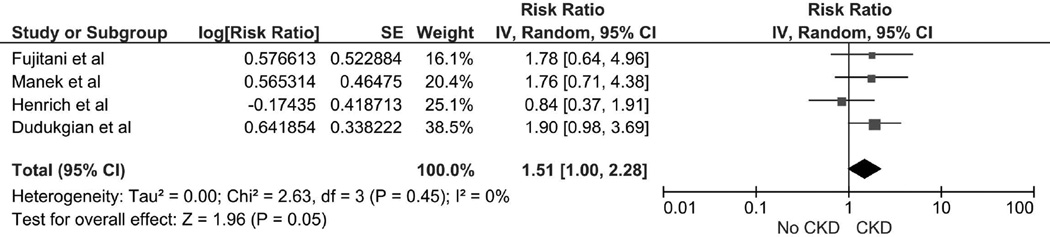
The Risk of Severe or Complicated Clostridium Difficile Infection in patients with CKD or ESRD
The pooled risk ratio (RR) of severe or complicated CDI in patients with CKD was 1.51 (95% CI 1.00–2.28). There was no significant statistical heterogeneity with an I2 of 0% (Figure 1). The data on the risk of severe CDI in patients with ESRD were limited. A study by Bauer et al [33] found no significant increased risk of severe or complicated CDI in patients with ESRD with OR of 0.29 (95% CI 0.04–2.35) (Table 1).
Figure 1.
Forest plot of the all included studies comparing the risk of severe or complicated CDI in patients in CKD vs. without CKD; square data markers represent risk ratios (RRs); horizontal lines, the 95% CIs with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall RR and 95% CI for the outcome of interest. IV, inverse variance; SE, standard error.
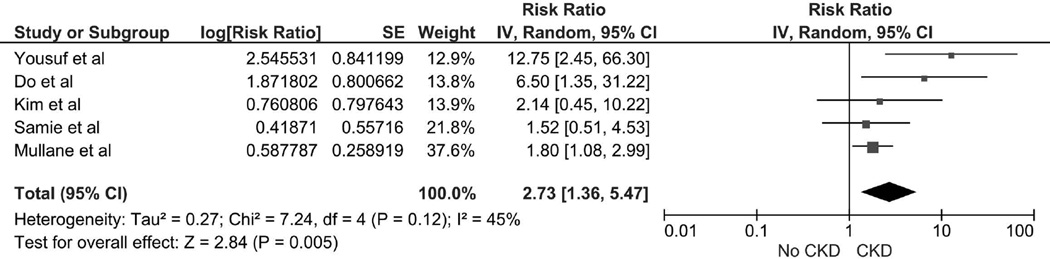
The Risk of Recurrent Clostridium Difficile Infection in patients with CKD
The pooled RR of recurrent CDI in patients with CKD was 2.73 (95% CI, 1.36–5.47, I2 =45%) (Figure 2). The data on the risk of recurrent CDI in patients with ESRD was limited. Bauer et al [33] found no significant increased risk of recurrent CDI in patients with ESRD with OR of 2.23 (95% CI 0.59–8.37).
Figure 2.
Forest plot of the all included studies comparing the risk of recurrent CDI in patients in CKD vs. without CKD; square data markers represent risk ratios (RRs); horizontal lines, the 95% CIs with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall RR and 95% CI for the outcome of interest. IV, inverse variance; SE, standard error.
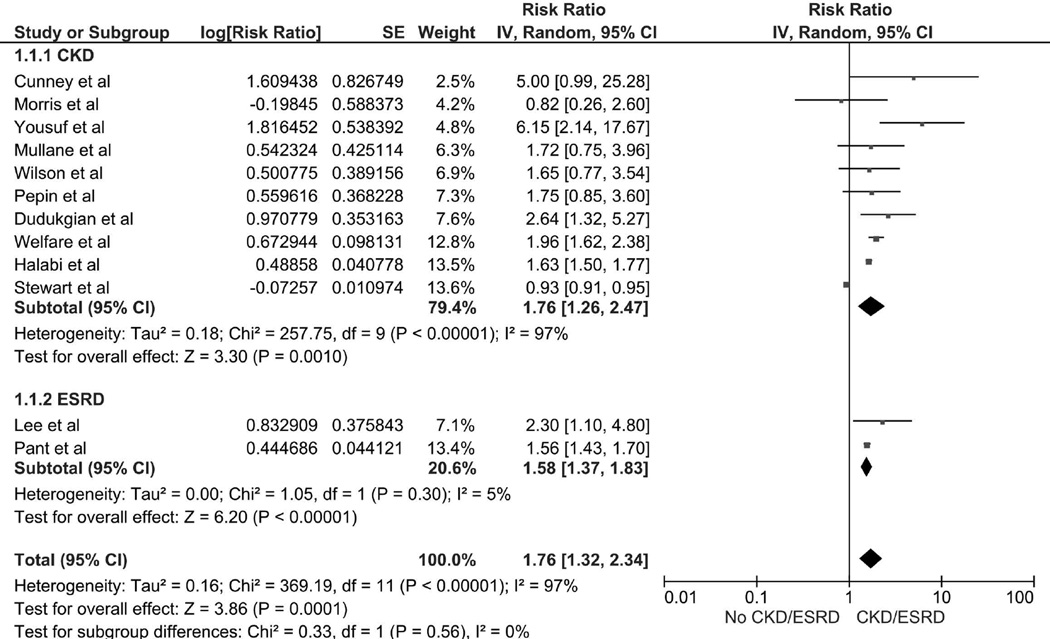
The Mortality Risk of Clostridium Difficile Infection and CKD/ESRD
The pooled RRs of mortality of CDI in patients with a history of CKD, ESRD and CKD or ESRD were 1.76 (95% CI 1.26–2.47, I2 =97%), 1.58 (95% CI 1.37–1.83, I2 =5%) and 1.76 (95% CI 1.32–2.34, I2 =97%). respectively (Figure 3).
Figure 3.
Forest plot of the all included studies comparing the mortality risk of CDI in patients in CKD or ESRD vs. without CKD or ESRD; square data markers represent risk ratios (RRs); horizontal lines, the 95% CIs with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall RR and 95% CI for the outcome of interest. IV, inverse variance; SE, standard error.
Evaluation for Publication Bias
Funnel plots to evaluate publication bias for the risks of complicated CDI, recurrent CDI and mortality of CDI in CKD (Figure S1, Figure S2 and Figure S3) and ESRD patients (Figure S3) are fairly symmetric and suggest no significant publication bias.
DISCUSSIONS
In this current meta-analysis, we demonstrated significant increased risks of poor clinical outcomes of CDI including complicated CDI and recurrent CDI in patients with CKD, with 1.51-fold and 2.73-fold increased risks, respectively. CKD and ESRD are both associated with 1.76-fold and 1.58-fold increased risks of mortality in CDI.
The findings of increased risks of poor clinical outcomes in patients with CKD and mortality risk in both CKD and ESRD is likely explained by impaired immune system function to fight against infection [37–39]. A reduction in the number and function of lymphoid cells has been described in patients with reduced kidney function and uremia [38]. When CKD and ESRD patients develop CDI, therefore, they may have higher risk of developing complications from CDI such as toxic megacolon requiring colectomy [40]. Studies have also found higher morbidities and lengths of hospital stay in CKD and ESRD patients with CDI resulting in increased long-term mortality [18].
Interestingly, despite increased risk of mortality in both patient with CKD and ESRD, those with ESRD have a lower risk than CKD. Our finding is also consistent with the finding in a recent study by Keddis et al. [40] which found lower rate of colectomy and mortality in patients with ESRD requiring dialysis compared with patients with less severe stages of CKD. It was speculated that the lower mortality risk in ESRD with CDI could be due to more frequent admissions, regular nephrology care, and close monitoring.
There are some limitations in our current meta-analysis. First, all included studies were observational studies. Therefore, our meta-analysis can best demonstrate an association but not a causal relationship. Second, there are statistical heterogeneities in the complete analysis in CKD patients with CDI. The potential sources of these heterogeneities include the differences in the definitions of CKD, diagnostic methodology of CDI, and the differences in confounder adjustment methods. The available data in included studies was limited. Therefore, it prevented us from further investigation for these potential sources of heterogeneities.
In summary, this meta-analysis shows significant increased risk of poor clinical outcomes of CDI in patients with CKD. Patients with CKD and ESRD, who develop CDI, have a significant mortality risk. Patients with CKD and ESRD need careful monitoring to prevent CDI. In addition, these patients may require more aggressive management since they carry poorer clinical outcomes of CDI.
Supplementary Material
What’s already known about this topic?
-
-
Clostridium difficile infection (CDI) or Clostridium difficile associated diarrhea (CDAD) is the most identifiable pathogen accountable for 12% of health care–associated infection in the United States.
-
-
Its incidence and severity have been markedly increasing worldwide.
-
-
Chronic kidney disease (CKD) is a common disease estimated to effect 8–16% worldwide.
-
-
However, the correlation of CDI outcome and CKD and end-stage renal disease (ESRD) are still inconclusive.
What does this article add?
-
-
This meta-analysis demonstrates poor outcomes of CDI including severe and recurrent CDI in CKD patients.
-
-
History of CKD and ESRD are both associated with increased mortality risk in patients with CDI.
Acknowledgments
Funding
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Conflict of interest statement for all authors:
We do not have any financial or non-financial potential conflicts of interest.
Authors' contributions
All authors had access to the data and a role in writing the manuscript.
REFERENCES
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65–S70. doi: 10.1093/cid/cis319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald LC, Owings M, Jernigan DB. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996–2003. Emerg Infect Dis. 2006;12(3):409–415. doi: 10.3201/eid1203.051064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg Infect Dis. 2007;13(9):1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burckhardt F, Friedrich A, Beier D, Eckmanns T. Clostridium difficile surveillance trends, Saxony, Germany. Emerg Infect Dis. 2008;14(4):691–692. doi: 10.3201/eid1404.071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravel D, Miller M, Simor A, Taylor G, Gardam M, McGeer A, Hutchinson J, Moore D, Kelly S, Boyd D, et al. Health care-associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program Study. Clin Infect Dis. 2009;48(5):568–576. doi: 10.1086/596703. [DOI] [PubMed] [Google Scholar]
- 7.Minino AM, Xu J, Kochanek KD. Deaths: preliminary data for 2008. Natl Vital Stat Rep. 2010;59(2):1–52. [PubMed] [Google Scholar]
- 8.Abou Chakra CN, Pepin J, Sirard S, Valiquette L. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. 2014;9(6):e98400. doi: 10.1371/journal.pone.0098400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thongprayoon C, Cheungpasitporn W, Phatharacharukul P, Mahaparn P, Bruminhent J. High Mortality Risk in Chronic Kidney Disease and End Stage Kidney Disease Patients with Clostridium Difficile Infection: A Systematic Review and Meta-analysis. J Nat Sci. 2015;1(4) [PMC free article] [PubMed] [Google Scholar]
- 10.(CDC) CfDCaP. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2014. National Chronic Kidney Disease Fact Sheet: general information and national estimates on chronic kidney disease in the United States, 2014. [Google Scholar]
- 11.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 12.Cheungpasitporn W, Thongprayoon C, O'Corragain OA, Edmonds PJ, Kittanamongkolchai W, Erickson SB. Associations of sugar-sweetened and artificially sweetened soda with chronic kidney disease: a systematic review and meta-analysis. Nephrology. 2014;19(12):791–797. doi: 10.1111/nep.12343. [DOI] [PubMed] [Google Scholar]
- 13.Yousuf K, Saklayen MG, Markert RJ, Barde CJ, Gopalswamy N. Clostridium difficile-associated diarrhea and chronic renal insufficiency. Southern medical journal. 2002;95(7):681–683. [PubMed] [Google Scholar]
- 14.Dudukgian H, Sie E, Gonzalez-Ruiz C, Etzioni DA, Kaiser AM. C. difficile colitis--predictors of fatal outcome. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2010;14(2):315–322. doi: 10.1007/s11605-009-1093-2. [DOI] [PubMed] [Google Scholar]
- 15.Welfare MR, Lalayiannis LC, Martin KE, Corbett S, Marshall B, Sarma JB. Co-morbidities as predictors of mortality in Clostridium difficile infection and derivation of the ARC predictive score. J Hosp Infect. 2011;79(4):359–363. doi: 10.1016/j.jhin.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Halabi WJ, Nguyen VQ, Carmichael JC, Pigazzi A, Stamos MJ, Mills S. Clostridium difficile colitis in the United States: a decade of trends, outcomes, risk factors for colectomy, and mortality after colectomy. Journal of the American College of Surgeons. 2013;217(5):802–812. doi: 10.1016/j.jamcollsurg.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 17.Lee DY, Chung EL, Guend H, Whelan RL, Wedderburn RV, Rose KM. Predictors of mortality after emergency colectomy for Clostridium difficile colitis: an analysis of ACS-NSQIP. Ann Surg. 2014;259(1):148–156. doi: 10.1097/SLA.0b013e31828a8eba. [DOI] [PubMed] [Google Scholar]
- 18.Pant C, Deshpande A, Anderson MP, Sferra TJ. Clostridium difficile infection is associated with poor outcomes in end-stage renal disease. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2012;60(2):529–532. doi: 10.2310/JIM.0b013e318242b313. [DOI] [PubMed] [Google Scholar]
- 19.Cunney RJ, Magee C, McNamara E, Smyth EG, Walshe J. Clostridium difficile colitis associated with chronic renal failure. Nephrol Dial Transplant. 1998;13(11):2842–2846. doi: 10.1093/ndt/13.11.2842. [DOI] [PubMed] [Google Scholar]
- 20.Morris AM, Jobe BA, Stoney M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Archives of surgery. 2002;137(10):1096–1100. doi: 10.1001/archsurg.137.10.1096. [DOI] [PubMed] [Google Scholar]
- 21.Mullane KM, Cornely OA, Crook DW, Golan Y, Louie TJ, Miller MA, Josephson MA, Gorbach SL. Renal impairment and clinical outcomes of Clostridium difficile infection in two randomized trials. American journal of nephrology. 2013;38(1):1–11. doi: 10.1159/000351757. [DOI] [PubMed] [Google Scholar]
- 22.Wilson V, Cheek L, Satta G, Walker-Bone K, Cubbon M, Citron D, Gerding DN, Llewelyn MJ. Predictors of death after Clostridium difficile infection: a report on 128 strain-typed cases from a teaching hospital in the United Kingdom. Clin Infect Dis. 2010;50(12):e77–e81. doi: 10.1086/653012. [DOI] [PubMed] [Google Scholar]
- 23.Pepin J, Vo TT, Boutros M, Marcotte E, Dial S, Dube S, Vasilevsky CA, McFadden N, Patino C, Labbe AC. Risk factors for mortality following emergency colectomy for fulminant Clostridium difficile infection. Diseases of the colon and rectum. 2009;52(3):400–405. doi: 10.1007/DCR.0b013e31819a69aa. [DOI] [PubMed] [Google Scholar]
- 24.Stewart DB, Hollenbeak CS. Clostridium difficile colitis: factors associated with outcome and assessment of mortality at a national level. J Gastrointest Surg. 2011;15(9):1548–1555. doi: 10.1007/s11605-011-1615-6. [DOI] [PubMed] [Google Scholar]
- 25.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 30.Do AN, Fridkin SK, Yechouron A, Banerjee SN, Killgore GE, Bourgault AM, Jolivet M, Jarvis WR. Risk factors for early recurrent Clostridium difficile-associated diarrhea. Clin Infect Dis. 1998;26(4):954–959. doi: 10.1086/513952. [DOI] [PubMed] [Google Scholar]
- 31.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15(3):415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32(3):220–228. doi: 10.1086/658336. [DOI] [PubMed] [Google Scholar]
- 33.Bauer MP, Notermans DW, van Benthem BH, Brazier JS, Wilcox MH, Rupnik M, Monnet DL, van Dissel JT, Kuijper EJ, Group ES. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377(9759):63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 34.Manek K, Williams V, Callery S, Daneman N. Reducing the risk of severe complications among patients with Clostridium difficile infection. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2011;25(7):368–372. doi: 10.1155/2011/153020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rouleau JL, Warnica WJ, Baillot R, Block PJ, Chocron S, Johnstone D, Myers MG, Calciu CD, Dalle-Ave S, Martineau P, et al. Effects of angiotensin-converting enzyme inhibition in low-risk patients early after coronary artery bypass surgery. Circulation. 2008;117(1):24–31. doi: 10.1161/CIRCULATIONAHA.106.685073. [DOI] [PubMed] [Google Scholar]
- 36.Kim YG, Graham DY, Jang BI. Proton pump inhibitor use and recurrent Clostridium difficile-associated disease: a case-control analysis matched by propensity score. J Clin Gastroenterol. 2012;46(5):397–400. doi: 10.1097/MCG.0b013e3182431d78. [DOI] [PubMed] [Google Scholar]
- 37.Pardi DS. Recurrent Clostridium difficile infection: an immunodeficiency state? Clin Gastroenterol Hepatol. 2007;5(6):672–673. doi: 10.1016/j.cgh.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 38.Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013;9(5):255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 39.Johnson S. Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58(6):403–410. doi: 10.1016/j.jinf.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Keddis MT, Khanna S, Noheria A, Baddour LM, Pardi DS, Qian Q. Clostridium difficile infection in patients with chronic kidney disease. Mayo Clin Proc. 2012;87(11):1046–1053. doi: 10.1016/j.mayocp.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.