Abstract
We show that the key flowering regulators encoded by Phalaenopsis aphrodite FLOWERING LOCUS T1 (PaFT1) and PaFD share high sequence homologies to these from long-day flowering Arabidopsis and short-day flowering rice. Interestingly, PaFT1 is specifically up-regulated during flowering inductive cooling treatment but is not subjected to control by photoperiod in P. aphrodite. Phloem or shoot apex-specific expression of PaFT1 restores the late flowering of Arabidopsis ft mutants. Moreover, PaFT1 can suppress the delayed flowering caused by SHORT VEGATATIVE PHASE (SVP) overexpression as well as an active FRIGIDA (FRI) allele, indicating the functional conservation of flowering regulatory circuit in different plant species. PaFT1 promoter:GUS in Arabidopsis showed similar staining pattern to that of Arabidopsis FT in the leaves and guard cells but different in the shoot apex. A genomic clone or heat shock-inducible expression of PaFT1 is sufficient to the partial complementation of the ft mutants. Remarkably, ectopic PaFT1 expression also triggers precocious heading in rice. To further demonstrate the functional conservation of the flowering regulators, we show that PaFD, a bZIP transcription factor involved in flowering promotion, interacts with PaFT1, and PaFD partially complemented Arabidopsis fd mutants. Transgenic rice expressing PaFD also flowered early with increased expression of rice homologues of APETALA1 (AP1). Consistently, PaFT1 knock-down Phalaenopsis plants generated by virus-induced gene silencing exhibit delayed spiking. These studies suggest functional conservation of FT and FD genes, which may have evolved and integrated into distinct regulatory circuits in monopodial orchids, Arabidopsis and rice that promote flowering under their own inductive conditions.
Introduction
Plants have evolved mechanisms to integrate environmental and developmental signals and precisely control the transition terminating vegetative growth and initiating the formation of flowers.
In Arabidopsis, flowering is triggered by multiple pathways [1] converging on a few integrators such as FLOWERING LOCUS T (FT) [2]. FT mRNA expression is induced in the leaves and its protein moves systemically to the shoot apical meristem where flowers bloom in response to long summer days (LDs) [3,4] indicating the FT protein acts as a major flowering hormone ‘florigen’ [5,6]. In addition, FT-like genes are well conserved among flowering plants and were reported to play a role of flowering activators in plants other than Arabidopsis, including tomato [7], squash [8] and rice [9,10]. The FT movement from the leaves to meristems requires interaction with a partner called FTIP, or FT interacting protein [11]. On reaching the meristem, FT interacts with a bZIP domain transcription factor, FD and together they activate expression of downstream genes that change the identity of the meristem to that of a flower. One example target of the FT-FD complex is APETALA1 (AP1), a meristem identity gene; another target is SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), which promotes the expression of another meristem identity gene called LEAFY (LFY). Both AP1 and LFY expression are repressed during vegetative growth, and this repression is released upon arrival of FT in the meristem [12].
In rice, the expression of Heading date3a (Hd3a), a rice FT orthologue is up-regulated only under inductive short-day (SD) conditions [13] and 14-3-3 proteins act as intracellular receptors for Hd3a proteins. A hexameric florigen activation complex (FAC) composed of Hd3a, 14-3-3 proteins and OsFD1 activates OsMADS15, a rice homologue of Arabidopsis AP1 leading to floral induction [10]. Another model species used to study SD flowering response, morning glory (Pharbitis nil), possesses two FT orthologues, PnFT1 and PnFT2 [14]. The expression of the genes exhibits circadian rhythms that are set by the onset of darkness and are upregulated at the end of the night under SD only if the night is sufficiently long. Despite the conserved functions of FT homologues, their expression appears to be controlled by different systems in different plant species.
Phalaenopsis is an epiphytic and monopodial orchid with thick and succulent leaves. It is an ornamental crop species of great economic value. In general, orchids can be divided into two groups, sympodial and monopodial, based on their growth morphology. Sympodial orchids such as those from the genera Cattleya, Cymbidium, Dendrobium and Oncidium grow from a stem that is horizontal. Monopodial orchids on the other hand, including Phalaenopsis, Paphiopedilums and Vanda grow vertically as a single upright stem with one leaf following another on opposite sides of the center. Phalaenopsis aphrodite subsp. formosana, a Taiwanese native Phalaenopsis orchid, is considered to be a model Phalanopsis species [15, 16]. Like many other flowering plants, the flowering of orchids is affected by several environmental factors such as photoperiod and temperature [17–19]. Most Phalaenopsis species are native to areas close to the equator and thus they do not need a specific photoperiod to induce flowering although specifics depend on the orchid genus and can even differ according to species. Instead, low ambient temperature is one of the triggers for the flowering initiation of Phalaenopsis orchids including P. aphrodite subsp. formosana. Also, orchids including Phalaenopsisis are crassulacean acid metabolism (CAM) plants like pineapples and cacti that can tolerate high temperatures using well-adapted metabolic strategies for growth [20]. Commercially, orchid seedlings are produced through embryo culture in vitro [21] or clonal propagation [22].
Spike induction (initiation of inflorescences) of Phalaenopsis was significantly inhibited when it was grown under a constant temperature higher than 28°C [23]. Conversely, diurnal fluctuation of high day and low night temperature or cool temperature in the night promoted spike induction [24–26]. Currently, many growers use very expensive air-conditioning to cool temperatures down to 18–25°C inside greenhouses to spike Phalaenopsis during the warm period of the year [27]. Therefore, it is important to functionally characterize flowering genes such as FT and FD in the orchid for better understanding of flowering under inductive conditions. Such understanding would also be beneficial for the production of new varieties which do not require cooling for flowering in the future. In Arabidopsis, it was reported recently that the abundance of the SHORT VEGETATIVE PHASE (SVP)-FLOWERING LOCUS M (FLM) complex, as a floral repressor of FT expression is regulated by ambient temperature [28, 29]. Interestingly, two MADS-box floral repressors, FLM-ß and SVP were reported to be down-regulated transcriptionally and post-transcriptionally by high ambient temperature [28].
The Arabidopsis FLOWERING LOCUS C (FLC) gene encodes a MADS domain protein that acts as a repressor of flowering by suppressing FT and SOC1 [30, 31]. FRIGIDA (FRI) is a positive regulator of FLC [32]. Flowering of Arabidopsis is accelerated by prolonged exposure to cold (vernalization) and FLC levels progressively decline during the cold periods. Therefore, loss-of-function mutations in either FRI or FLC eliminate the vernalization requirement. Most commonly used lab strains of Arabidopsis such as Columbia lack active FRI and/or FLC alleles, and exhibit rapid-flowering behavior under inductive long days (LD) [32, 33]. In cereals, which lack FLC, the day length and vernalization are also likely to be interconnected through FT-like genes. In the cases of barley and wheat, the naturally occurring ‘vernalization genes’, VRN-H3 and VRN-B4 respectively, have been shown to encode FT orthologues [34]. In addition, OnFT, PhFT and CgFT, FT homologues from orchids such as Oncidium Gower Ramsey, Phalaenopsis hybrid Fortune Salzman and Cymbidium goeringii, respectively, were recently reported [35–37]. In Arabidopsis, distinct molecular mechanisms control FT expression for subsequent flowering under different ranges of environmental temperature. However, Phalaenopsis orchids including P. aphrodite subsp. formosana are known to originate from tropical and subtropical areas of the south pacific islands where photoperiod is almost constant throughout the year [23]. These orchids generally do not require vernalization despite low ambient temperature being necessary for flowering. Therefore, rather than photoperiod and/or vernalization, recognition and signaling systems for low environmental temperature are likely to be the major triggers for the induction of FT expression and consequently, the transition from vegetative to reproductive growth in Phalaenopsis orchids.
Here we demonstrated that PaFT1 that encodes an orthologue of Arabidopsis FT was accumulated during the low ambient temperature treatment required for floral induction of the monopodial orchid P. aphrodite subsp. formosana. We further demonstrated that the functional role of PaFT1 as a floral inducer is conserved in the orchid. Moreover, we showed that PaFT1-interacting protein, PaFD has a conserved floral activation function. Since plants have adapted themselves to various environments for successful reproduction, each plant has developed its own strategy to control the timing of floral transition. We provide evidence at least partially supporting the notion that distinct regulation of the FT and FD genes may have evolved for rapid flowering by inductive environmental cues in the monopodial orchid, P. aphrodite subsp. formosana.
Materials and Methods
Plant Materials and Growth Conditions
Phalaenopsis orchids (P. aphrodite subsp. formosana) at different developmental stages were purchased from Chain Port Orchid Nursery (Ping Tung, Taiwan). The orchids were adapted in the growth chamber environment (16 h light, 28°C/8 h dark, 25°C) for 2 weeks before starting each experiment. Wild-type Arabidopsis (A. thaliana ecotype Col) was used to generate transgenic plants. ft-10, fd-3 [38], soc1-2 [39] and FRI-Col [40] were described previously. SOC1:GUS and 35S:SVP seeds were kind gifts from Dr. Ilha Lee (Seoul National University, Korea) and Dr. Peter Huijer (MPIZ, Germany), respectively. Generally, Arabidopsis plants were grown in the growth chamber under LD conditions (16/8-h photoperiod at 100 μmol m-2 s-1) at 23°C. Two Japonica-type rice cultivars, Dongjin, a Korean cultivar and Tainung67 (TNG67), a Taiwanese cultivar were used to produce transgenic rice plants and the transgenic plants were grown in the growth chamber or in the outdoor GMO greenhouse of the Academia Sinica Biotechnology Center in Southern Taiwan. For Arabidopsis flowering time measurement, 8 to 12 plants per line were counted for total leaf numbers when their first flowers were at anthesis. Days of heading of 8–12 rice plants per each line were measured when panicles were emerged.
Cloning of PaFT1 Gene from P. aphrodite subsp. formosana
Total RNA extracted from young spikes (≤ 2 cm in length) of P. aphrodite subsp. formosana was used for cDNA synthesis as described by Su et al [16] and Bilgin et al [41]. Synthesized cDNAs and degenerated primers were used for the amplification of PaFT1. Degenerated primers for PaFT1 were as follows: forward primer (Deg F: 5′CHTTCTACACBCTYGTSATGGTAG3′) and reverse primer (Deg R: 5′CDGGSGCGTAMACYGTCTG3′). The amplified PCR product was cloned into pCR-Blunt II TOPO vector (Invitrogen) and the sequence of the partial PaFT1 was verified to have high similarity with PEBP genes. Internal gene-specific primers for PaFT1 were designed for the isolation of a full-length clone of PaFT1 by RACE (Rapid Amplification of cDNA Ends) using SMART RACE cDNA amplification kit (BD Biosciences Clontech). Gene-specific primers for 5′ and 3′-RACE of PaFT1 are 5′GAGGATCACTTGGACTTGGAGC3′ (GS-5′RACE) and 5′GTTGTTTCATCAACTAGGCCG3′ (GS-3′RACE), respectively. The cDNA of PaFT1 full open reading frame (ORF) was obtained by PCR with attB-linked gene-specific primers and the PaFT1 entry clone was constructed by BP reaction with pDONR201 (Invitrogen). The PaFT1Y86H form was generated by point mutagenesis using Stratagene’s QuikChange site-directed mutagenesis kit. The following two primers containing mismatched base pair (from T to C, bold type and underlined) were used for the mutagenesis: 5′CTCAACTTAGAGAACACTTACACTGGTTAG and 5'CTAACCAGTGTAAGTGTTCTCTAAGTTGAG.
Promoter Isolation and GUS Expression
Genomic DNA of P. aphrodite subsp. formosana was isolated by DNeasy Plant Mini kit (Qiagen). Genomic clone of PaFT1 was isolated by PCR with Phusion taq polymerase (NEB) and the exons and introns were annotated compared with the PaFT1 cDNA sequence. The promoter region of PaFT1 was isolated by inverse PCR (iPCR) together with the aid of Genome Walker Universal Kit (Clontech). The promoter entry clone was constructed and sequentially introduced into pMDC163 [42] (Curtis and Grossniklaus, 2003) to generate PaFT1 promoter:GUS construction. For the expression of PaFT1 genomic clone containing its own promoter and coding region, the pAlligator2 vector without 35S promoter and the triple HA was used for the destination vector. GUS staining with Arabidopsis seedlings was performed as described by An et al [43].
Expression Analyses
Total RNAs from various organs of P. aphrodite subsp. formosana were extracted by RNeasy Plant Mini Kit (Qiagen) and treated with RNase-free DNase (Invitrogen) following the manufacturer’s protocol to remove any residual genomic DNA. DNase-treated RNA was subjected to reverse transcriptase reactions using oligo-dT primer and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. The same procedure was applied to cDNA syntheses of Arabidopsis and rice after RNA extraction. Subsequent PCR was performed with the first-strand cDNA mixture and EX-Taq polymerase (Takara Biochemical, Japan). Quantitative real time-PCR (qPCR) was performed on the CFX96TM real-time system (Bio-Rad) using Maxima SYBR Green qPCR Master Mix (Thermo). The primers used for quantification are listed in S1 Table. For PCR, each sample was analyzed in triplicate. The running protocol was: denaturation at 95°C for 10 min, annealing/extension repeated 40 times (95°C for 15 s and 60°C for 30 s, data acquisition was performed). Gene expression data were normalized to the expression of housekeeping genes. For the expressional analyses of P. aphrodite subsp. formosana, PaACT [16] and PaUBQ [16] genes were used for normalization but only the figures using PaACT are shown as both sets of results were similar. The primers used in this study are listed in S2 Table. At least two independent experiments were performed for RNA extraction in expressional analyses.
In Situ Hybridization
Young spikes of Phalaenopsis orchid were collected and fixed, dehydrated, embedded, sliced (10 ìm thickness), and performed hybridization as previously described by Lin et al [44] with slight modification. For preparation of digoxigenin (DIG)-labeled RNA probes, we amplified gene-specific 261 bp fragment of PaFD using the following primers: 5'GTTCGTCCAACAGTCTTC3' (forward) and 5'GTTTCCAGACTTCTTCCATAC3' (reverse). The DNA fragment was cloned into pGEM-T vector (Promega) and each sense and antisense probe was synthesized by T7 and SP6 RNA polymerases, respectively. Hybridization was performed at 63°C or 66°C with 20 ng of DIG-labeled RNA probe.
Yeast Two-Hybrid Screening for PaFD
PaFT1 full-length ORF was cloned in-frame in the pBD-GAL4 Cam vector (Stratagene) to generate a pBD: PaFT1 vector as a bait. For the cDNA library construction, total RNA was extracted from young spikes (≤ 2 cm in length) of P. aphrodite subsp. formosana and poly (A) + RNA was isolated using a PolyATract mRNA purification kit (Promega). The GAL4 AD vector library was constructed using a GAL4 Two-Hybrid Phagemid Vector Kit (Stratagene) according to manufacturer’s instructions. Screening and X-gal filter assay were performed as described previously [45].
The PaFDΔ1–53 clone was generated by PCR using the following attB-linked primers: 5'GGGGACAAGTTTGTACAAAAAAGCAGGCTGCATGGAAGAAGTCTGGAAACACATTGAC and 5’GGGGACCACTTTGTACAAGAAAGCTGGGTGTTAAAATGGCGCGGATGAAGTTCTCTGAAG. PaFD T225A, S226A, S227A and triple (PaFD T225A, S226A, S227A) clones were also generated by PCR using the following primers: 5' GGGGACAAGTTTGTACAAAAAAGCAGGCTGCATGTGGCTCCTATCTCCTGC (forward), 5' GGGGACCACTTTGTACAAGAAAGCTGGGTGTTAAAATGGCGCGGATGAAGCTCTCTGAAG (reverse for T225A), 5' GGGGACCACTTTGTACAAGAAAGCTGGGTGTTAAAATGGCGCGGATGCAGTTCTCTGAAG (reverse for S226A), 5' GGGGACCACTTTGTACAAGAAAGCTGGGTGTTAAAATGGCGCGGCTGAAGTTCTCTG (reverse for S227A), 5' GGGGACCACTTTGTACAAGAAAGCTGGGTGTTAAAATGGCGCGGCTGCAGCTCTCTGAAG (reverse for triple). Amplified PCR products were cloned into pDNONR201 via BP reaction (Invitrogen).
Particle Bombardment and BiFC Assays
For cellular localization of AtFT, AtFD, AtFDP, PaFT1 and PaFD in Arabidopsis, either YFP:GW or CFP:GW vector was used for the florescence fusion as described previously [46]. For BiFC assays in Arabidopsis, Arabidopsis FT and PaFT1 cDNA were cloned into pVYCE vector for AtFT:cYFP and PaFT1:cYFP fusions. AtFD and AtFDP cDNAs were cloned into pVYNE vector for nYFP:AtFD and nYFP:AtFDP fusions, respectively [47]. Bombardment on Arabidopsis leaves was carried out as described by Shirasu et al [48]. For BiFC in Phalaenopsis, cDNAs encoding the PaFT1 and PaFD genes were introduced into pE3136 and pE3130, respectively [49] (http://www.bio.purdue.edu/people/faculty/gelvin/nsf/protocols_vectors.htm). Bombardment-mediated transient transformation of Phalaenopsis and generation of images were performed as described by Su et al [16].
VIGS Assays
Each specific fragment of PaFT1, PaFD and GUS genes was cloned into pCymMV vector [50] by in vitro recombination with BP Clonase II (Invitrogen) to generate pCymMV-GUS, pCymMV-PaFT1 and pCymMV-PaFD, respectively. For growth of Agrobacterium and leaf injection, we followed the procedure described by Hsieh et al [51, 52].
Plant Transformation and Analyses of Transgenic Plants
For the pSUC2:PaFT1 and pKNAT1:PaFT1 constructs, the PaFT1 entry clone was inserted into the pSUC2:Gateway (GW) and pKNAT1:GW destination vectors, respectively [43, 46]. For the pFD:PaFT1 construct, the 35S promoter and the triple HA of the pAlligator2 vector was exchanged for the 3.1-kb FD promoter and then PaFT1 was introduced by LR reaction. With the same strategy, PaFT1 was also fused to Arabidopsis heat shock protein (HSP) 18.2 promoter [53] to generate pHSP18.2:PaFT1. All plasmids for plant transformation were introduced into Agrobacterium strain GV3101 (pMP90RK) [54] and transformed into Columbia wild-type, ft-10 or ft-10 soc1-2 double homozygous plants by the floral-dip method [55]. For overexpression of target genes in rice, binary vector, pGA3426 was used and each transgene construct was introduced into rice genome by Agrobacterium-mediated transformation [56].
Results
Isolation and Molecular Characterization of PaFT1
We isolated PaFT1 by a combined reverse transcription PCR (RT-PCR) and RACE strategy from young spikes of P. aphrodite subsp. formosana. Degenerated primers were designed based on the conserved regions of the FT sequences from Oncidium orchids, rice and barley (see the materials and methods).
The PaFT1 cDNA encodes a 178 amino acid protein with a calculated molecular mass of 20.02 kDa and a theoretical pI of 6.83. The PaFT1 protein showed 70%, 76% and 89% identity to Arabidopsis FT, rice Hd3a and Oncidium orchid OnFT, respectively. The key amino acid residues Tyr and Gln that are conserved among the FT homologues were located at positions 86 and 141 of the PaFT1 protein (S1 Fig) [57–59].
The PaFT1 and other FT protein sequences from various plant species were used to construct a phylogenetic tree. The PaFT1 isolated from P. aphrodite subsp. formosana belonged to the FT family of monocotyledonous plants and interestingly, it was grouped with two other orchid FTs, Oncidium FT (OnFT) and Cymbidium FT (CgFT) (S1 Fig).
Expression Pattern of PaFT1
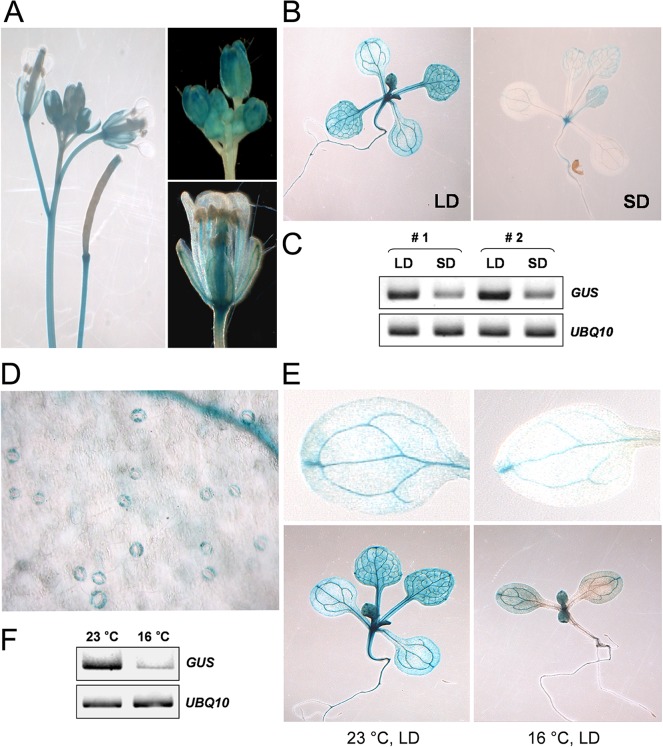
The spatial expression pattern of PaFT1 was investigated by quantitative RT-PCR analyses. The PaFT1 transcript accumulated to high levels in developing inflorescences (spikes) and developing floral buds, and was also detected in vegetative organs such as leaves and roots as well as reproductive organs such as lips, columns, pedicels. However, PaFT1 mRNA was hardly detectable in sepals and petals (Fig 1).
Fig 1. Growth and flowering of P.aphrodite subsp. formosana with the expression of PaFT1.
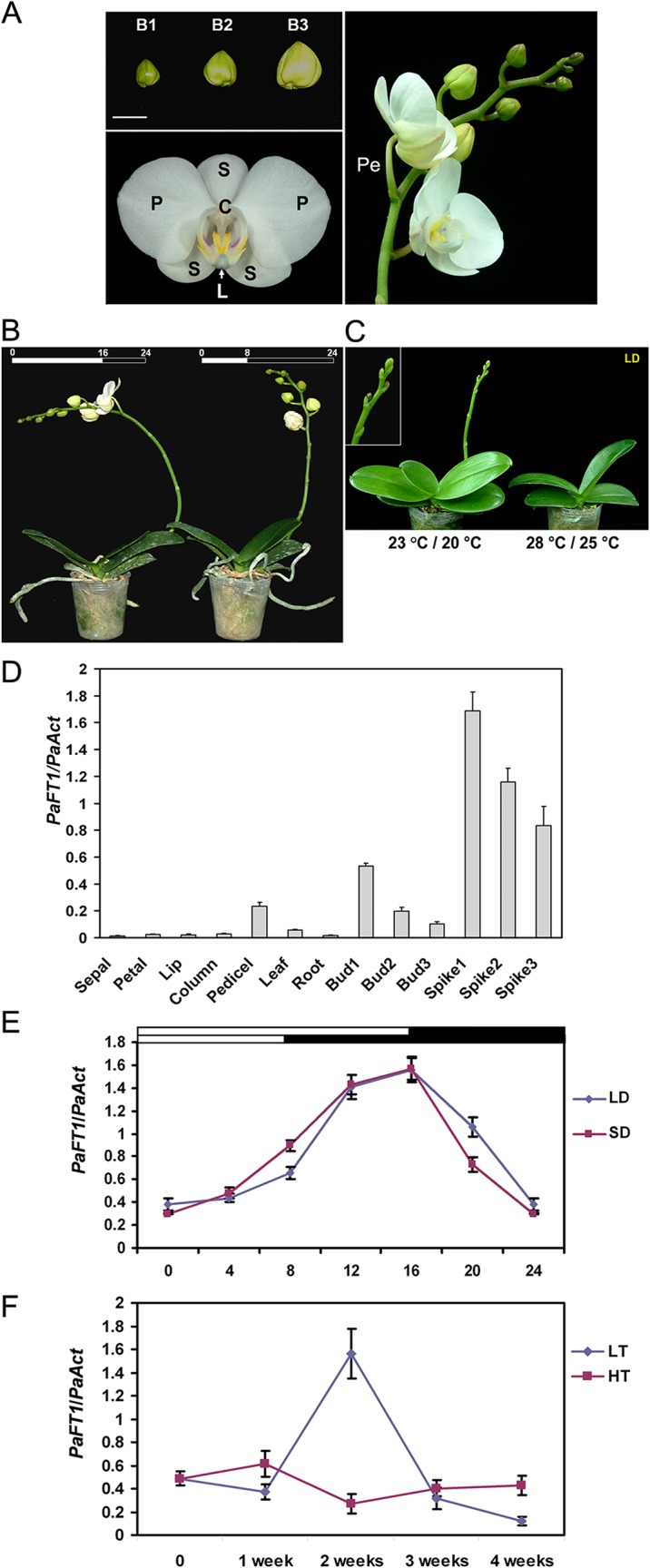
A, Floral buds at different developmental stages and the structure of flower. S: sepal, P: petal, L: lip, C: column, Pe: pedicel. Bar is 1 cm. B, Spiking and flowering of P. aphrodite subsp. formosana under LD and SD conditions at constant 23°C. C, Under LD conditions, low temperature treatment is essential to induce inflorescence of P.aphrodite subsp. formosana. Day and night temperatures are shown in parenthesis. D, Spatial expression of PaFT1 in P. aphrodite subsp. formosana. Materials for RNA extraction were harvested from six to eight plants. Bud 1, bud 2 and bud 3 indicate the B1, B2 and B3, respectively in A. Spike 1, spike 2 and spike 3 indicate ≤ 3 cm, 3–10 cm and ≥ 10 cm in length, respectively. The 3rd, 4th and 5th leaves were used for the leaf RNA extraction. E, Daily oscillation of PaFT1 expression under LD and SD conditions. In each time point, leaves of 4 plants (18 months old) were harvested for RNA extraction. F, The effect of ambient temperature on PaFT1 expression. LT; low temperature (23°C/20°C), HT; high temperature (28°C/25°C). Thirty six mature plants (34-month old as the stage 4) were grown at HT and then sixteen plants were transferred to the LT conditions. All leaves of four plants were used for the analysis of PaFT1 expression, at each time point. All the samples were harvested at the end of light (ZT 16). Two independent experimental results showed similar expression patterns.
To explore whether PaFT1 expression oscillated over 24 hours, the PaFT1 transcript was analyzed every 4 hours over a 24-hour period under both LD and SD conditions using leaves of 18-month-old orchids. The highest peak of PaFT1 expression was detected at zeitgeber time (ZT) 16 irrespective of the photoperiod. The lowest level of expression of PaFT1 was observed at dawn (Fig 1). Since cooling treatment is necessary for spiking of P. aphrodite subsp. formosana, the expression of PaFT1 was investigated under two different conditions (Fig 1). There was no clear difference in spiking induction time between the plants grown under SD and LD (Fig 1).
When the mature plants (34 months old) grown at high temperature (28°C light/25°C dark, LD) were transferred to a cooler temperature (23°C light/20°C dark, LD), spike sprouting initiated 4 weeks after the transfer (Fig 1). However, the plants maintained at the high temperature did not develop spikes. During this period, we measured mRNA levels of PaFT1 and three putative SOC1 homologues from the orchid at weekly intervals. The experiment revealed that PaFT1 expression was increased at the two-week point in the transferred plants but was not changed in the plants that remained at high temperature (Fig 1). The three putative SOC1 homologues, PaSOC1-1, PaSOC1-2 and PaSOC1-3, were all highly expressed at the spiking stage under low ambient temperature although they showed distinct expression patterns during the temperature shift (S2 Fig).
PaFT1 Promotes Flowering in Arabidopsis and Rice
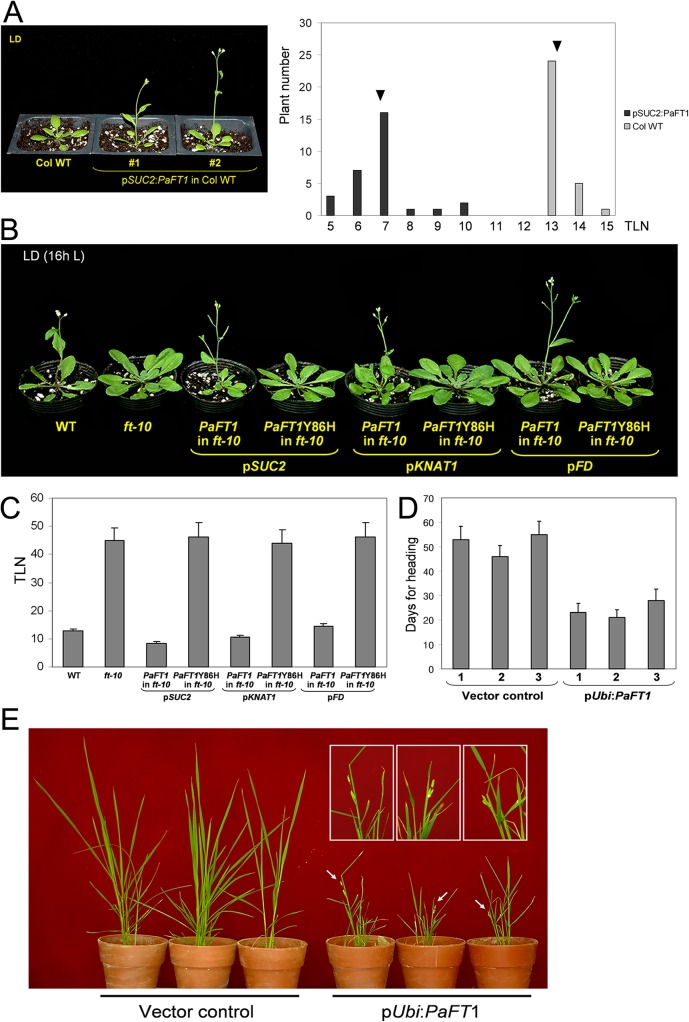
To test the activity of PaFT1 as a flowering regulator, the pSUC2:PaFT1 transgene, which overexpresses PaFT1 in the phloem companion cells, was introduced into Arabidopsis Columbia wild type (WT) plants. The transgenic plants exhibited early flowering with an average of 6.3 fewer leaves than the control WT (Fig 2).
Fig 2. Phloem-specific expression of PaFT1 in Arabidopsis and overexpression of PaFT1 in rice drive early flowering.
A, Comparison of flowering time between transgenic plants containing pSUC2:PaFT1 and wild type plants. Thirty independent T1 plants were used for each genotype. Arrows represent the mean value of total leaf number in each genotype. P ≤ 0.0001 (Student’s t-test). B and C, PaFT1 driven by phloem-specific or shoot apex-specific promoters rescues the late flowering phenotype of Arabidopsis ft null mutant, ft-10. This activity is at least dependent on Tyr-86 residue, one of the conserved amino acids among FT proteins from various plant species. D and E, Ectopic expression of PaFT1 in rice also caused early flowering (Dongjin cultivar, grown under SD condition). Magnified panicles are shown in the box of E and flowering time data is shown in D. TLN means total leaf number.
In order to investigate whether PaFT1 was able to rescue the late flowering phenotype caused by the ft mutation, the pSUC2:PaFT1 construct was introduced into the ft-10, ft null mutant [60]. The transgenic ft mutant plants expressing PaFT1 in the phloem companion cells flowered much earlier than the parental ft mutant plants with a similar total number of leaves to the WT (Fig 2). Expressing PaFT1 under shoot apical meristem (SAM)-specific promoters such as pKNAT1 or pFD also rescued the delayed flowering phenotype in the ft mutant (Fig 2). These results indicated that expression of PaFT1 in phloem companion cells or SAM is sufficient to promote flowering in Arabidopsis plants that completely lack endogenous FT. However, a single amino acid change from the conserved Tyr-86 to His of PaFT1 resulted in a loss of the flowering promotion capability (Figs 2 and 3) [57–59].
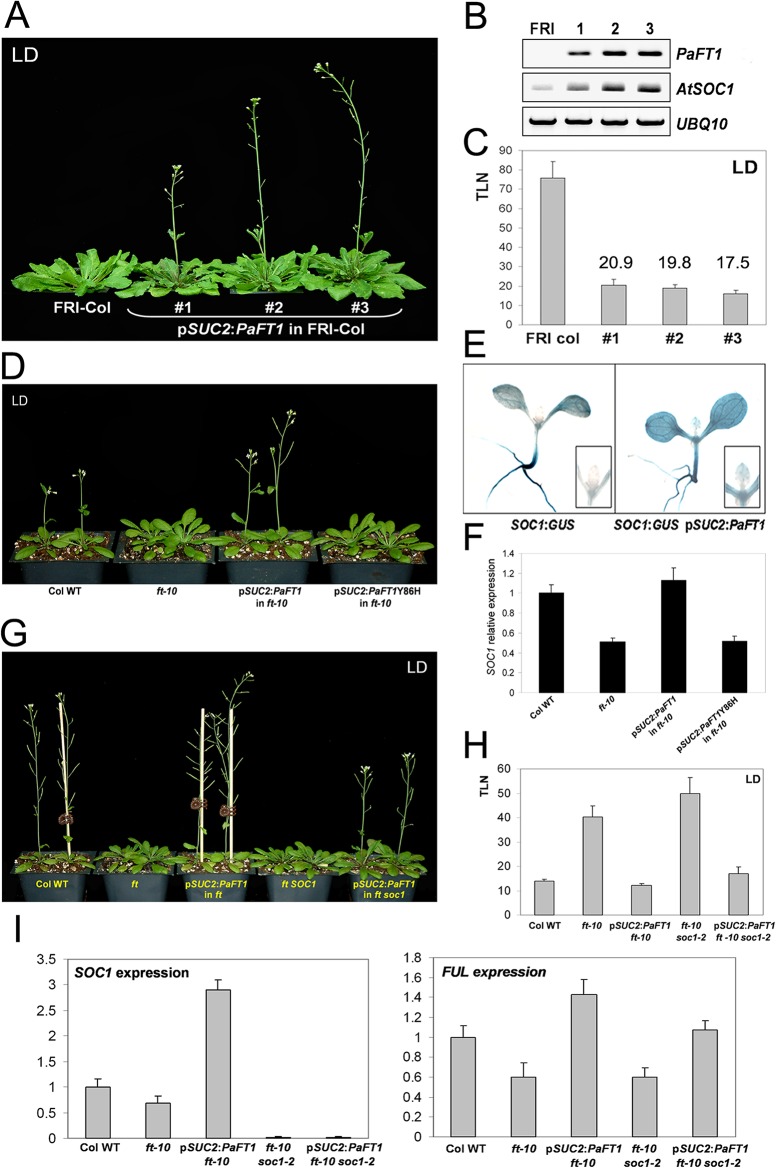
Fig 3. Effect of phloem-specific expression of PaFT1 in FRI Col, ft-10 and ft-10 soc1-2 double mutant background.
A and C, Phloem-specific expression of PaFT1 overcomes the effect of FRI, a positive regulator of central floral repressor, FLC. C, Average total leaf numbers of three independent homozygous lines of pSUC2:PaFT1 in FRI-Col were presented above each bar. B and E, Arabidopsis SOC1 expression is increased in plants expressing PaFT1 in phloem companion cells. D and F, The mutant form of PaFT1 loses the activity to increase the SOC1 expression and induce flowering. G, H and I, The pSUC2:PaFT1 ft-10 soc1-2 plants flower earlier than the double, ft-10 soc1-2 implying that other factors are still affected by PaFT1 in floral induction and FUL is one of the candidates. F and I, Relative expression was presented compared with that of Col WT.
We also introduced the PaFT1 gene into Dongjin rice. PaFT1 expression was under the control of maize ubiqutin promoter, a strong constitutive promoter in monocot plants [56]. Three independent transgenic lines containing pUbi:PaFT1 showed early heading compared with control plants containing the empty vector (Fig 2). Under SD condition, transgenic rice plants produced panicles within one month after sowing although they did not produce many healthy grains. This early flowering phenotype correlated with increased expression of two rice AP1-like genes, OsMADS14 and OsMADS15 (S3 Fig).
Expression of PaFT1 Overcomes the Late Flowering Phenotype of FRI-Col Plants
To test the contribution of PaFT1 to the flowering of FRI-Col that contains an active FRI locus of San feliu-2 accession [61], the pSUC2:PaFT1 construct was introduced into the FRI-Col.
Transgenic plants expressing PaFT1 flowered earlier than control FRI-Col plants without cold treatment (Fig 3). As expected, SOC1 expression was increased in the transgenic plants and the increase in SOC1 transcripts was also observed in the ft-10 mutants containing the pSUC2:PaFT1 construct (Fig 3). However, PaFT1Y86H, a mutant form of PaFT1 could not induce SOC1 expression (Fig 3).
To confirm whether PaFT1 positively regulates SOC1, we employed a β-glucuronidase (GUS) reporter assay. We used pSOC1:GUS [60] plants to visualize the expression of SOC1. pSUC2:PaFT1 pSOC1:GUS plants were produced to examine whether SOC1 promoter-driven expression of GUS was affected by pSUC2:PaFT1. A histochemical GUS assay showed stronger GUS staining in the shoot apex, cotyledons and the first true leaf in the pSUC2:PaFT1 pSOC1:GUS plants compared with pSOC1:GUS plants (Fig 3). This suggests that SOC1 expression was reinforced by PaFT1 and the increased SOC1 expression contributed to the early flowering.
The pSUC2:PaFT1 transgene was introduced into ft-10 soc1-2 double mutants to evaluate the effect of the transgene in flowering of the double mutants. The pSUC2:PaFT1 ft-10 soc1-2 plants flowered earlier with approximately 32 fewer leaves than the ft-10 soc1-2 double mutants, whereas they flowered later with approximately 5 more leaves than the pSUC2:PaFT1 ft-10 plants under LDs (Fig 3). This implies that SOC1 is not a unique target of PaFT1 for promotion of flowering in Arabidopsis. Recently, soc1 ful was reported to be epistatic to pSUC2:FT in flowering [62]. Analyses of FRUITFULL (FUL) transcript levels revealed that the gene expression was higher in pSUC2:PaFT1 ft-10 soc1-2 plants compared to that in ft-10 soc1-2 but lower than that of pSUC2:PaFT1 ft-10 plants (Fig 3).This indicates that PaFT1 induces FUL expression and sequentially promotes flowering in Arabidopsis lacking endogenous FT and/or SOC1.
Expression of PaFT1 Reduces the Effect of p35S:SVP in Flowering
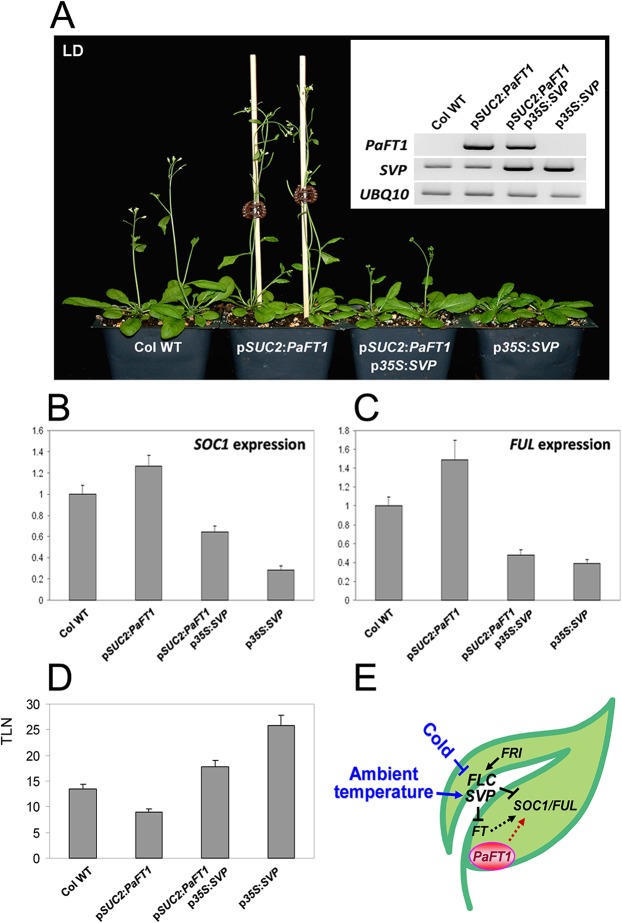
Since SVP has been identified as a flowering repressor mediated by ambient temperature, we generated pSUC2:PaFT1 p35S:SVP plants by introducing the transgene pSUC2:PaFT1 into the p35S:SVP background to investigate whether phloem-specific expression of PaFT1 is able to overcome the flowering repression effect of p35S:SVP. We observed that the expression level of SOC1 and FUL was higher in the plants expressing PaFT1 than in the plants expressing SVP alone (Fig 4). The pSUC2:PaFT1 p35S:SVP plants flowered with an average of 8 fewer leaves than p35S:SVP plants under LD condition demonstrating that the phloem-specific expression of PaFT1 partially suppresses SVP overexpression in flowering induction (Fig 4) although aberrant floral morphology was observed both in p35S:SVP [63] and pSUC2:PaFT1 p35S:SVP plants (data not shown).
Fig 4. Phloem-specific expression of PaFT1 reduces the late flowering effect by overexpression of SVP encoding an ambient temperature-dependent floral repressor.
A and D, pSUC2:PaFT1 retards the effect of late flowering by p35S:SVP. B and C, The expression of SOC1 and FUL in each genotype shown in A. Relative expression was presented compared with that of Col WT. E, A model showing the effect of PaFT1 expression in Arabidopsis flowering.
Genomic Clone of PaFT1 Partially Rescues the Late Flowering Phenotype of ft-10
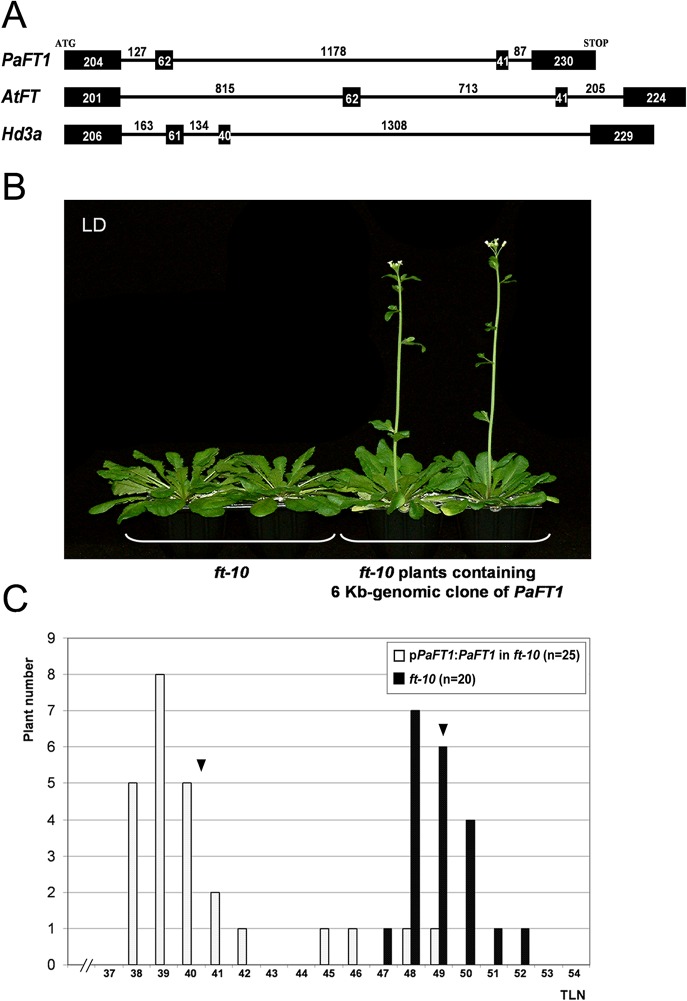
To evaluate whether the PaFT1 genomic clone is functional in Arabidopsis, we isolated an approximate 6-kb fragment of PaFT1 containing a 2-kb coding region and a 4-kb promoter region. The PaFT1 gene consists of four exons and three introns in the ORF region similar to the Arabidopsis FT and rice Hd3a genes (Fig 5). The isolated genomic clone was introduced into ft-10 and the flowering time of 25 individual T1 plants was measured (Fig 5). The transgenic plants produced flowers with an average of 9 fewer leaves than ft-10 mutants under LD conditions. This indicates that the 4 kb-PaFT1 promoter is, at least partially, functional and the orchid introns are properly spliced in Arabidopsis to produce functional PaFT1 proteins.
Fig 5. Genomic PaFT1 partially complements Arabidopsis ft mutants.
A, Genomic structure of PaFT1. AtFT is an Arabidopsis FT and Hd3a is a rice FT homologue. Filled boxes indicate coding sequences and the lines between boxes represent introns. Numbers represent the length of nucleotides in coding regions and introns (bp). B and C, 6-kb genomic clone of PaFT1 containing its 4-kb promoter region partially rescues the late flowering phenotype of Arabidopsis ft null mutants. C, Flowering time was measured with twenty five individual T1 plants containing the genomic clone in the ft mutant background and twenty ft-10 plants. Arrows represent the mean value of total leaf number in each genotype. P ≤ 0.0005 (Student’s t-test).
Expression Pattern of pPaFT1:GUS in Arabidopsis
Because the PaFT1 promoter is functional in Arabidopsis, we created a PaFT1 promoter reporter line, pPaFT1:GUS using the 4-kb promoter of PaFT1. The GUS expression was detected in vasculature and guard cells in the same way as observed in Arabidopsis FT (Fig 6) [64, 65]. However, strong GUS signals were also detectable in the shoot apex, which is distinguishable from the Arabidopsis FT expression pattern (Fig 6) [64]. The GUS signals were not present in the petals and mature anthers (Fig 6). The GUS expression was regulated by photoperiod and growth temperature. Under LDs, GUS transcripts were highly accumulated and the GUS signal was also stronger than those from plants grown under SDs (Fig 6). Plants grown at 16°C showed reduced GUS expression compared with plants grown at 23°C under LDs (Fig 6). Thus, the response of GUS expression to different photoperiod and growth temperature displayed similar patterns to those of the Arabidopsis FT gene implying the existence of transacting factors acting on the both FT promoters from Arabidopsis and orchid.
Fig 6. A homozygous line expressing a PaFT1-promoter GUS fusion that showed a characteristic staining pattern was chosen for histochemical analysis of pPaFT1:GUS expression in different organs of Arabidopsis.
A, Inflorescence, floral buds, flower and siliques. B, 8-day-old seedlings grown under LD and 14-day-old seedlings grown under SD. C, GUS mRNA expression under different photoperiods. D, GUS expression in guard cells. E and F, Different levels of GUS expression are detectable under different temperature. 8-day-old seedlings grown under constant 23°C and 16°C, respectively were used for GUS staining or mRNA analysis.
Induced Expression of PaFT1 by High Temperature Causes Early Flowering in Arabidopsis
Since Phalaenopsis orchids did not respond to chemicals such as gibberellic acids in spiking when it was sprayed [66], other methods rather than chemicals were explored for the use in the inducible gene expression system in the orchid.
To establish heat-inducible flowering in orchid, we created pHSP18.2:PaFT1 construction and introduced it into the Arabidopsis ft-10 mutants. The transgenic plants did not display an early flowering phenotype under normal temperatures. However, heat treatment induced early flowering of the transgenic plants (S4 Fig) which resembled Arabidopsis FT [67]. A similar phenotype was observed in wild-type Arabidopsis containing pHSP18.2:PaFT1 under SD condition with heat treatment (S4 Fig). This indicates that PaFT1 is a floral activator and the HSP18.2 promoter has the potential to be used for heat-induced flowering in the orchid.
PaFD Is a bZIP Domain Protein that Interacts with PaFT1 in Orchids
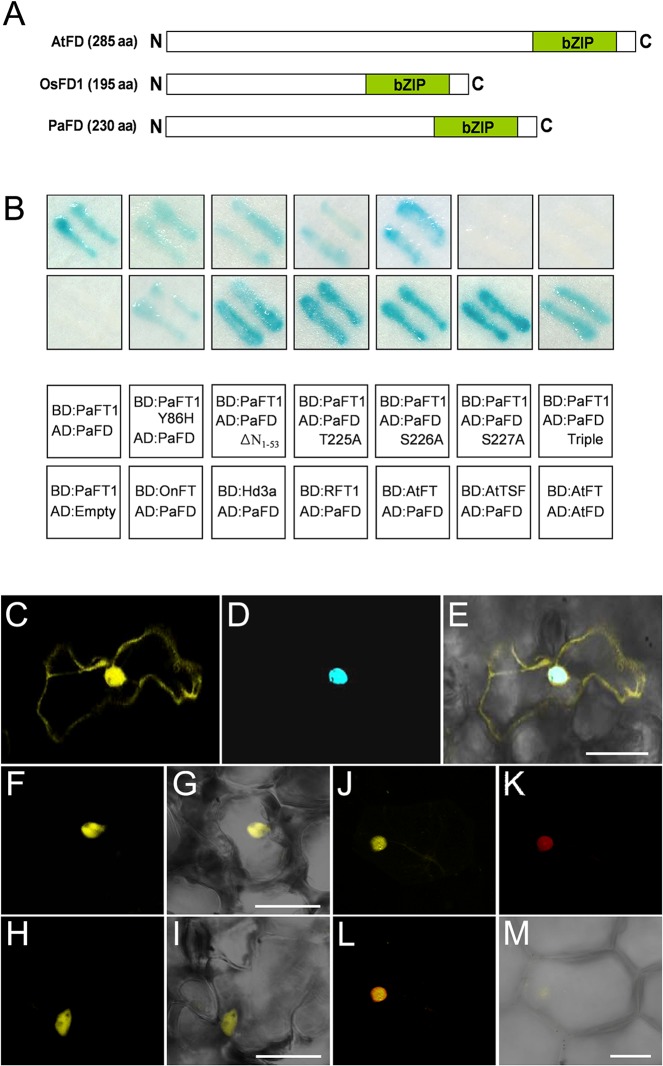
To investigate whether PaFT1 interacts with bZIP domain proteins in orchids in the same way as Arabidopsis FT or rice Hd3a, we performed a yeast two-hybrid screen using a young spike cDNA library to isolate a P. aphrodite homologue of Arabidopsis FD, as an interacting protein of PaFT1. Three partial and two full-length PaFD ORF clones were isolated. For verification of the interaction, we generated an entry clone for PaFD and sequentially produced a pAD:PaFD, a prey construction via LR recombination [38]. We observed that PaFT1 interacted with PaFD that consists of 230 amino acids containing the conserved bZIP domain at its carboxyl terminal similar to AtFD and OsFD1 (Fig 7). Interestingly, PaFD was also able to interact with FT proteins from other plant species such as Arabidopsis, rice and Oncidium orchid (Fig 7). Additionally, removal of the serine-rich amino terminal of PaFD (PaFDΔN1-53) had no effect on the interaction with PaFT1, while change of a single amino acid (S227A) or triple amino acids (T225A, S226A, S227A) prevented the interaction (Fig 7). In AtFD protein, the threonine residue at the 282nd position is important for AtFT-AtFD interaction: changing the 282nd threonine residue to alanine prevents its interaction with AtFT but changing it to serine does not affect the interaction [68]. When the 227th serine residue of PaFD, a positional equivalent of the 282nd threonine of AtFD was mutated to alanine, the PaFT1-PaFD interaction was also abolished (S9 Fig) indicating phosphorylation at the residue, threonine or serine may be critical for the interaction.
Fig 7. PaFD, a PaFT1-interacting bZIP protein.
A, Comparison of PaFD with other FD proteins from Arabidopsis and rice. Green boxes indicate bZIP domain and the number in the parenthesis is the length of each polypeptide. B, PaFT1 interacts with PaFD in the yeast system. PaFD also interacts with FT proteins from other species such as Arabidopsis (AtFT, AtTSF), rice (Hd3a, RFT1) and Oncidium orchid (OnFT). PaFT1Y86H indicates mutant form of PaFT1 and PaFDΔN1-53, PaFD T225A, S226A, S227A indicates N-terminal deletion and mutant forms of PaFD, respectively. The interaction between AtFT and AtFD is a positive control. C, YFP:PaFT1 fusion proteins in Arabidopsis cell. D, CFP:PaFD fusion proteins in Arabidopsis cell. E, Merged image of YFP:PaFT1 and CFP:PaFD proteins in Arabidopsis cell. F, G, H and I, BiFC assays in Arabidopsis cells. Plasmids for YFPn:PaFT1 and YFPc:PaFD expression were introduced into Arabidopsis cells, simultaneously. J, L and M, BiFC assays in Phalaenopsis cells. K, NLS:RFP was used for nuclear localization marker. Bar is 40 ìm in E, G, I and 20 ìm in M.
PaFT1 was localized both in the nucleus and cytoplasm which is similar to the subcellular localization of AtFT in plant cells (Fig 7). PaFD was exclusively localized in the nucleus as a putative bZIP transcription factor (Fig 7). Positive interaction was observed between PaFT1 and PaFD in Phalaenopsis cells as well as Arabidopsis cells via BiFC assays (Fig 7). A higher interaction affinity of AtFT to AtFD was observed than that of PaFT1 to AtFD and/or AtFDP based on florescence intensities in BiFC assays (S5 Fig).
Expression of PaFD in the Shoot Apex Partially Complements Arabidopsis fd Mutants
PaFD transcripts were detectable in almost all organs in the orchid and they were gradually accumulated as plants become mature under LDs at high growth temperature (28/25°C, light/dark) (Fig 8 and S7 Fig). In particular, in situ hybridization results showed PaFD transcripts were accumulated in the emerging floral meristems in young spikes (Fig 8).
Fig 8. Spatiotemporal expression pattern of PaFD and its functional activity in Arabidopsis.
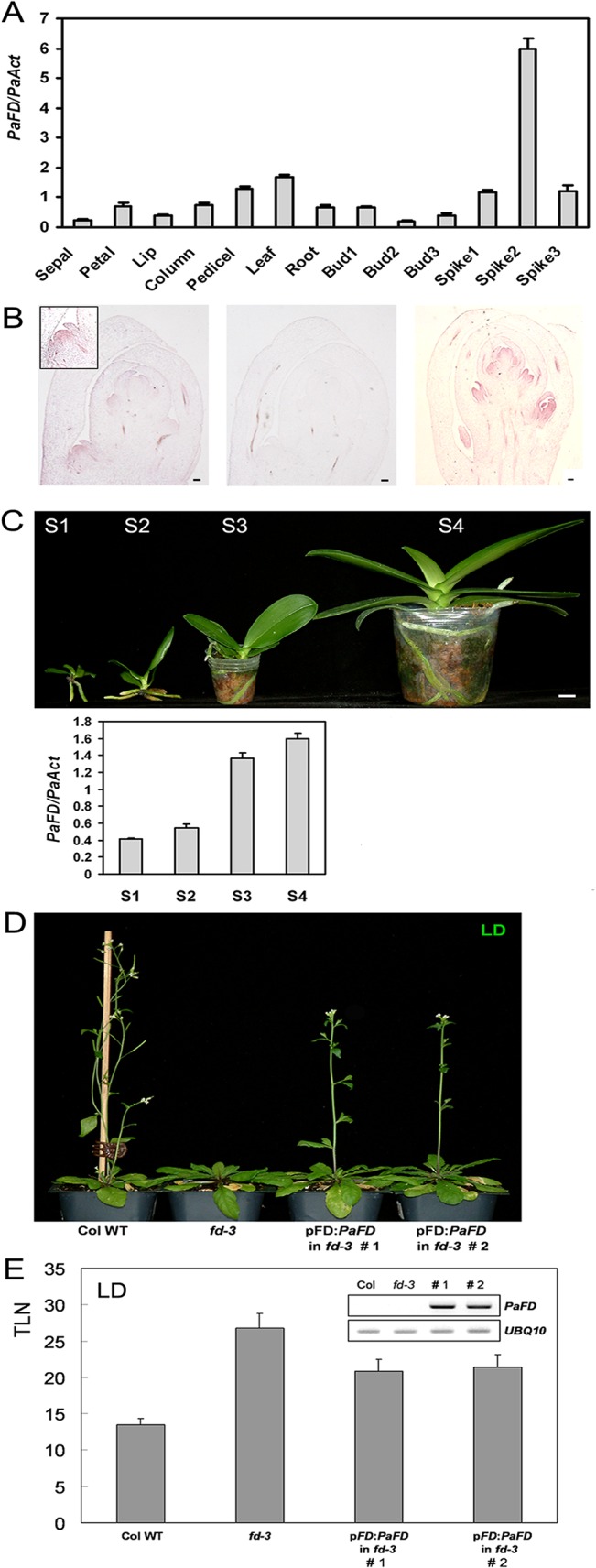
A, Developmental stages of floral buds and spikes are described in Fig 1. B, PaFD expression in a developing spike by in situ hybridization. The magnified axillary floral meristem in the emerging spike (≤ 3 cm in length) is in the box (left panel) and the middle panel is a negative control with a sense probe. PaFD transcript was detected in the floral meristems in the developing spike (3–10 cm in length, right panel). Bar is 100 ìm. C, Expression pattern of PaFD in different developmental stages. The stage 1 (S1) is 16-month and the S2 is 20-month old stages of the orchid in the flasks. S3 and S4 show 26-month and 34-month old stages of the orchid in the pots, respectively. Bar is 1 cm. D, Flowering phenotypes of wild type, fd mutant (fd-3), and two independent homozygous transgenic plants expressing PaFD under the control of Arabidopsis FD promoter in fd-3 background. E, Flowering time of the each genotype shown in D. Twelve individuals for each genotype were used for flowering time measurement and PaFD expression is detectable only in the transgenic plants.
To test whether PaFD is a functional homologue of Arabidopsis FD, we constructed pFD:PaFD for the expression of PaFD under the control of Arabidopsis FD promoter and the pFD:PaFD transgene was introduced into Arabidopsis fd-3 mutants via an Agrobacterum-mediated dipping procedure. Transgenic fd-3 plants expressing PaFD driven by Arabidopsis FD promoter flowered with 5–6 fewer leaves than Arabidopsis fd-3 under LD conditions demonstrating that PaFD plays a positive role, at least weakly, in Arabidopsis flowering in a similar manner to Arabidopsis FD (Fig 8). A higher level of Arabidopsis AP1 and SOC1 expression was also detected in the transgenic plants than in fd-3 (S8 Fig).
Ectopic Expression of PaFD Causes Early Heading in Rice
To examine the effect of PaFD in a monocotyledonous, SD plant, transgenic rice plants overexpressing PaFD were generated. Compared with control plants containing an empty vector, PaFD overexpressors showed early heading and the expression of rice AP1 homologues, OsMADS14 and OsMADS15 was also increased in the transgenic rice plants (Fig 9). This observation implies that our orchid FD is able to induce flowering in rice through the increased expression of rice AP1 genes. Overexpression of the rice AP1 genes such as OsMADS14 and OsMADS15 has been reported to cause extremely early flowering in rice [69, 70]. It is noteworthy that two rice FD genes, OsFD1 and OsFD2 which belong to the poaceae FD group (S9 Fig), did not show alterations in heading time when their expression was increased or decreased indicating functional divergence between FDs that belong to different groups [10, 71].
Fig 9. Ectopic expression of PaFD causes early heading in rice.
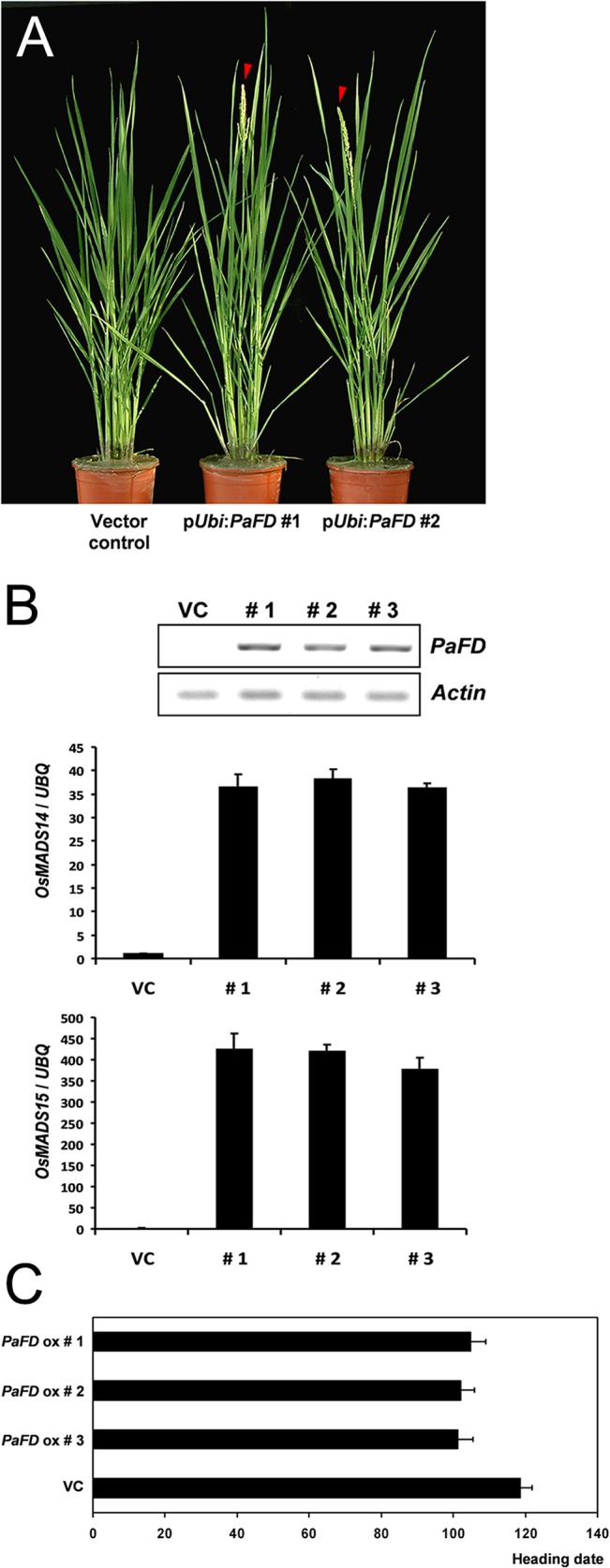
A and B, The vector control plant is a transgenic rice plant containing an empty vector. Expression level of two rice AP1 homologues, OsMADS14 and OsMADS15 is high compared with the control. VC indicates vector control. C, Flowering time (heading date) of the transgenic rice plants overexpressing PaFD compared with the control containing empty vector. Eight to twelve individual plants per each line were used for heading date measurement.
Reduced Expression of PaFT1 in P. aphrodite subsp. formosana Causes Delayed Spiking
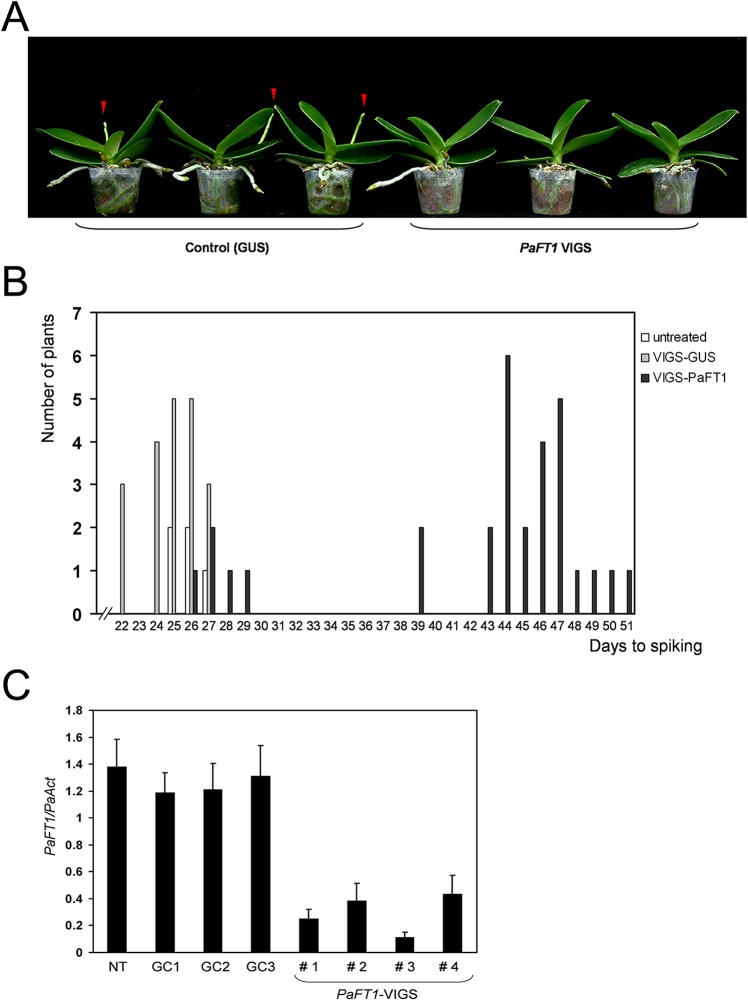
Because a transformation system for P. aphrodite subsp. formosana has not been established, we applied the virus induced gene silencing (VIGS) method to study the PaFT1 gene function in the orchid. We used the GUS gene as a control for VIGS and twenty individual orchids treated with VIGS of GUS flowered synchronously together with untreated plants under LDs with constant 23°C. However, orchids treated with VIGS of PaFT1 exhibited significantly delayed spiking under the same growth conditions (Fig 10). The level of endogenous PaFT1 expression in the VIGS of PaFT1 orchids was reduced compared to that of controls (Fig 10). Thus, the reduced PaFT1 expression contributes to the late spiking phenotype of the PaFT1 VIGS orchids at spike-inducing temperature. Sequentially, the elongation of inflorescences, floral bud formation and opening of the flowers in the PaFT1 VIGS orchids were all delayed compared with non-treated and GUS VIGS orchids (S10 Fig).
Fig 10. The VIGS of PaFT1 exhibits delayed spiking.
A and B, Compared with control lines for the VIGS of GUS gene, the VIGS of PaFT1 lines show late flowering. B, Spiking time was measured in thirty independent lines for VIGS of PaFT1 together with twenty lines for VIGS of GUS and five non-treated lines. We counted the first day of transferring the orchids to the low temperature condition (23°C / 20°C) after VIGS treatment as day one for spiking. C, Reduced expression of endogenous PaFT1 in the VIGS lines of PaFT1. NT and GC mean non-treated and GUS control (VIGS of GUS gene), respectively.
Discussion
Functional study of the FT and FD genes of P.aphrodite subsp. formosana was conducted to understand floral induction of the orchid under its inductive environmental conditions. The diurnal expression pattern of PaFT1 is similar under both LD and SD conditions (peaks at ZT 16) correlating with the similar flowering time of the orchid under the two different photoperiodic conditions. These results demonstrate that P. aphrodite subsp. formosana has a day-neutral flowering characteristics under our conditions. In the day-neutral plant tomato, expression of the FT orthologue SINGLE FLOWER TRUSS (SFT) is not regulated by the photoperiod, although overexpression of SFT promotes early flowering and sft mutations delay flowering [7]. Amino acid comparison and the construction of a phylogenetic tree suggest that PaFT1 is potentially an orthologue of FTs from various plant species that may control floral initiation in P. aphrodite subsp. formosana. The induction of expression of PaFT1 during low ambient temperature treatment also supports this notion since flowering of the orchid was observed only under low ambient temperature. The regulation of FT expression by ambient temperature has been reported in other plant species such as Arabidopsis and rice. For example, Arabidopsis flowers earlier when grown at 23°C than at 16°C and rice flowers earlier at 27°C than at 23°C. Both plant species flower earlier and their FT expression is also higher when they are grown at high ambient temperature [72, 73] indicating a direct relationship between the level of FT expression and flowering time at a certain temperature. However, PaFT1 expression is induced under low ambient temperature that mimics a mild winter season in the original habitat of the orchid. Recently, Hsu et al. demonstrated that FT1 of poplar induces flowering in response to vernalization rather than photoperiod [74]. Indeed, in a naturally growing poplar tree, FT1 transcript is abundant only in winter. Another example showing the relation between reduced temperature and accumulated FT transcripts was demonstrated in citrus stem as a result of floral induction by low temperature [75]. On the contrary, NtFT a homologue of the FT gene from Narcissus tazetta was reported to be expressed under high temperature and the floral transition of the plant was shown to be affected by high temperature but not by photoperiod or vernalization [76]. Thus, change in environmental temperature is sufficient to trigger floral initiation. However, whether known floral repressors such as SVP and FLM-ß regulate PaFT1 expression in the orchid remains to be seen. The highest expression level of PaFT1 in the young spikes may indicate that PaFT1 accumulated in the sprouting inflorescence and the expression pattern of PaFT1 in floral buds was similar to that of OnFT, a FT homologue from Oncidium Gower Ramsey [35] but different from that of Arabidopsis FT which, in contrast, gradually increased during flower maturation. Interestingly, PaFT1 mRNA was relatively abundant in the pedicel, the connecting organ between the inflorescence and flower suggesting that PaFT1 may play a pivotal role either in the initiation of the inflorescence (spiking) or in the formation of floral buds along the inflorescence or have a role in both steps. However, the expression level of PaFT1 in roots was relatively low, similar to OnFT from Oncidium and CgFT from Cymbidium, but different from PhFT, a FT homologue of Phalaenopsis hybrid Fortune Salzman [35, 36, 37]. These results show that FT genes have distinct spatial expression among different orchid species. AtFT is closely related to the floral repressor, TERMINAL FLOWER1 (TFL1). Tyrosine (Tyr) at position 86 of PaFT1 is conserved in FT proteins from other plant species and important for the structural feature of FT as a floral activator [57–59]. Since substitution of Tyr at position 86 to His abolished its function as a floral activator and phloem-specific as well as shoot apex-specific expression of PaFT1 in the ft mutant background complemented the mutant phenotype in flowering, PaFT1 is also likely to move from companion cells to the shoot apex where AtFD is expressed to induce flowering in Arabidopsis. Mild flowering phenotype of Arabidopsis plants expressing PaFT1 is likely to be due to weaker interaction affinity between PaFT1 and AtFD than that of the AtFT-AtFD complex resulting in partial activation of down-stream genes or lack of recruitment of co-factors required for the activation of down stream targets for floral induction [59]. Early heading observed in transgenic rice plants overexpressing PaFT1 demonstrates that PaFT1 acts as a floral activator both in dicot LD plant, Arabidopsis and monocot SD plant, rice. The effect of PaFT1 overexpression on rice heading was weaker than that of rice Hd3a overexpression since overexpression of Hd3a caused extremely early flowering during rice transformation (S6 Fig). The difference in flowering phenotype can also be explained by the reasons given for the mild flowering phenotype of Arabidopsis by PaFT1 expression. In addition, the examination of the effect of phloem-specific PaFT1 expression in Arabidopsis backgrounds where temperature-linked floral repressors are highly expressed showed that PaFT1 at least partially overcomes the activity of the repressors such as FRI/FLC and SVP by up-regulating the expression of common target genes such as SOC1 and FUL for floral induction indicating molecular functional conservation of PaFT1 as a floral activator in Arabidopsis. Complementation of ft mutants by the PaFT1 genomic clone also suggests that the promoter and coding region of PaFT1 contribute to floral promotion in Arabidopsis. Furthermore, the GUS expression pattern of pPaFT1:GUS in Arabidopsis, except in the shoot apex, was reminiscent of Arabidopsis FT expression raising the possibility that parts of the promoter are also recognized by trans-acting factors acting on cis-regulatory regions of Arabidopsis FT. However, distinct expression patterns of PaFT1 by low temperature and photoperiod in the orchid also suggest that ‘orchid-specific’ or ‘P. aphrodite subsp. formosana-specific’ trans-acting factors may control PaFT1 expression under certain conditions. In rice, for instance, Ehd1 is a strong regulator of the expression of Hd3a, a rice FT, but has no homologue in the Arabidopsis genome and its upstream regulators such as Ehd2 and Ehd4 are also known to be ‘monocot-specific’ or ‘Oryza-genus-specific’ [77–79]. Inducible expression systems other than chemical sprays are likely to be useful in orchid flowering manipulation. We, therefore, tested heat-shock inducible expression of PaFT1 in Arabidopsis and confirmed the activity of PaFT1 as a floral activator (S4 Fig).
PaFD that encodes a protein that interacts with PaFT1 was expressed in almost all the organs examined in the orchid. In particular, PaFD transcripts were relatively abundant in the developing inflorescences and the level of expression in leaves gradually increased with plant growth. Interestingly, rice FD genes such as OsFD1, OsFD2 and OsFD3 are also expressed in the leaves and stems and the interaction between Hd3a and OsFD1 is mediated by 14-3-3 proteins [10, 71]. PaFD was able to interact with other FT proteins from several plant species including Arabidopsis, rice and Oncidium orchid. Of note, the mutant form of PaFT1, PaFT1Y86H could also interact with PaFD indicating that a putative PaTFL1 may compete with PaFT1 for the interaction with PaFD as is the case in Arabidopsis [80]. Thus, the regulation of the FT and FD genes for floral induction is likely to be conserved in the orchid although the expression pattern of PaFD is different from that of AtFD which is shoot apex-specific in Arabidopsis. PaFD belongs to the ‘eudicot and non-Poaceae FD group’ together with AtFD [71]. Closer analysis of the FD protein sequence indicates that PaFD has a TSSAPF motif at its carboxyl end and most members of the group have a conserved T(S)SS(T)APF motif at the same position. Abe et al [68] showed the threonine (Thr) residue at position 282 plays a critical role in the interaction of AtFD with AtFT and the interaction is also mediated by 14-3-3 proteins through the phosphorylation of AtFD at the Thr 282 [81]. Our analyses of the interaction between PaFT1 and PaFD mutant forms showed the serine (Ser) residue at position 227 of PaFD, the positional equivalent of Thr 282 of AtFD is important for the PaFT1-PaFD interaction. Meanwhile, OsFD1 and the members of the ‘Poaceae FD1 group’ have a conserved VL(MP)SAPF motif at their carboxyl ends (S9 Fig). Since the site is similar to the recognition site of the 14-3-3 proteins, the functional difference among various FD proteins through the interactions with other proteins including FT-like proteins may be due to the two amino acids in front of the S(T)AP motif at the carboxyl terminals. For example, both serine and threonine in the T(S)SS(T)APF motif of FDs in the eudicot and non-Poaceae FD group can be phosphorylated and this post-translational modification may cause functional diversity through multiple phosphorylations at the14-3-3 recognition site. Partial complementation of the Arabidopsis fd mutant using pAtFD:PaFD construct implies that PaFD is able to replace the functional activity of Arabidopsis FD in floral promotion. Furthermore, transgenic rice plants expressing PaFD exhibited early heading with increased expression of two rice AP1 homologues, OsMADS14 and OsMADS15, which is distinct from the results with rice FD overexpressors. Interestingly, neither rice FD1 (OsFD1) nor FD2 (OsFD2) overexpressors cause early heading although the proteins interact with Hd3a through 14-3-3 proteins [10, 71]. Since PaFD belongs to the ‘eudicot and non-Poaceae monocot FD group’ which is distinct from the Poaceae FD groups, the different activity or diverged function of the FDs may contribute to rice development at various steps (S9 Fig). Indeed, a recent report showed that OsFD2 controls rice leaf development [71]. Although VIGS of PaFD was also applied to the orchid, we did not observe plants showing significantly delayed spiking with reduced expression level of PaFD (data not shown). In the case of PaFT1, however, more than 80% of the orchids treated with VIGS of PaFT1 showed significant delayed spiking under inductive temperature compared with the control and the endogenous PaFT1 expression level was also reduced suggesting that PaFT1 at the very least plays a role in the initiation of florescence in the orchid. Recently, it was shown that increased level of TaFT, a FT from wheat could overcome the necessity of vernalization in flowering of wheat by a transgenic approach [82]. Thus, it will definitely be worthwhile examining whether transgenic orchids with increased level of PaFT1 will initiate spiking under non-inductive high temperature.
Conclusions
In conclusion, the present study demonstrates the potential roles of PaFT1 as a floral activator and its interacting protein PaFD in orchid flowering. Low ambient temperature is absolutely necessary, while the photoperiodic signal is not necessary for flowering of P. aphrodite subsp. formosana. The level of PaFT1 expression correlates with the inductive environmental conditions and the flowering phenotype of P. aphrodite subsp. formosana. PaFD that encodes a PaFT1-interacting protein also shows flower-promoting activity in both Arabidopsis and rice. We, therefore, suggest the possibility that regulation of FT and FD genes in plants may have evolved and integrated into the distinct flowering circuits of plants to promote flowering under conditions favorable to each plant species. These findings broaden our understanding of various flowering processes of plants and provide potential tools for molecular breeding of orchid.
Supporting Information
A, Phylogenetic tree of the deduced amino acid sequences of PaFT1 and FT sequences of other plant species. The tree was created with MEGA 5.2 using the neighbor-joining method and clustalW [83]. The accession numbers of the sequences are as follows: Arabidopsis thaliana (FT, BAA77838; TSF, BAA77840), Citrus unshiu (CiFT, AB027456), Cymbidium goeringii (CgFT, ADI58462), Carica papaya (CpFT, ACX85427), Hordeum vulgare (HvFT1, ABJ97441), Ipomoea nil (PnFT1, ABW73562), Lactuca sativa (LsFT, BAK14369), Malus x domestica (MdFT1, AB161112), Oncidium Gower Ramsey (OnFT, ACC59806), Oryza sativa (Hd3a, BAB61030; RFT1, BAO03187), Populus nigra (PnFT2a, AB109804), Solanum lycopersicum (SP3D, AY186735), Solanum tuberosum (StFT, ADA77529), Triticum aestivum (TaFT, ACA25437) and Vitis vinifera (VvFT, ACZ26523). The numbers at nodes represent the bootstrap values (with 1000 replicates) and the scale bar displays branch length. FTs from orchids are in a dotted box. B, PaFT1 has two conserved key amino acids 86-Tyr (▼) and 141-Gln (▼) which are believed to be important residues in FT proteins that act as floral activators [57–59].
(PDF)
HT; high temperature (28°C/25°C as day and night temperature under LDs), LT; low temperature (23°C/20°C). The same RNAs used for Fig 1F were utilized for the analyses of gene expression. Recently, transcriptomic analyses using petals and lips of P. amabilis, a species that is closely related to P. aphrodite, identified eight SOC1 genes [84]. Although the number of SOC1 homologues that exist in P. aphrodite has not yet been reported, the expression of three reported SOC1 homologues, PaSOC1-1 (PATC136427), PaSOC1-2 (PATC150808) and PaSOC1-3 (PATC 154491) [85] was examined during the temperature shift.
(PDF)
Control is a transgenic rice plant containing an empty vector.
(PDF)
A, Heat treated transgenic ft-10 containing pHSP18.2:PaFT1 showed earlier flowering than untreated plants. Sixteen-day-old seedlings of the plants were heat treated (2 hours from ZT 14 to ZT 16 under LDs at 37°C) for 3 weeks. PaFT1 transcripts only highly accumulated in plants with heat treatment (in the box). B, Flowering time of plants with and without heat treatment. Three independent homozygous lines (14 to 22 individuals for each line) were tested for flowering time measurement. C and D, Heat treated transgenic plants (Col WT background) containing pHSP18.2:PaFT1 showed earlier flowering than untreated plants. Three weeks old seedlings of the plants grown under SD (10 h light) were heat treated (2 hours from ZT 8 to ZT 10 at 37°C) for 3 weeks. Two independent homozygous lines (14 and 17 individuals for each line) were tested for flowering time measurement. The asterisk indicates that heat-treated plants flowered earlier than untreated plants or control. P ≤ 0.005 (Student’s t-test).
(PDF)
A and B in the left panel, CFP:AtFT in an Arabidopsis cell. C and D in the left panel, CFP:PaFT1 in an Arabidopsis cell. E and F in the left panel, YFP:AtFD in an Arabidopsis cell. G and H in the left panel, YFP:AtFDP in an Arabidopsis cell. A and B in the right panel, BiFC assay between YFPn:AtFT and YFPc:AtFD in an Arabidopsis cell. C and D in the right panel, BiFC assay between YFPn:PaFT1 and YFPc:AtFD in an Arabidopsis cell. E and F, BiFC assay between YFPn:PaFT1 and YFPc:AtFDP in an Arabidopsis cell. G, Measurement of florescence intensity in each BiFC assay. For the evaluation of the relative fluorescence intensities of nuclei in the BiFC experiments the hardware values of gain, offset and zoom on the Leica SP2 AOBS instrument were adjusted image the nuclei of the positive control (AtFT:cYFP + YFPn:AtFD) such that the values of the 8 bit color scale included 255 (brightest level). Imaging of the other BiFC pairs were under the the same hardware values.
(PDF)
Transgenic rice plants containing pUbi:Hd3a produce flowers in the callus during transformation. Bars = 5 mm.
(PDF)
Leaf numbers of orchids used in this study (upper) and the expression of PaFT1 and PaFD in each leaf.
(PDF)
Nine-day-old seedlings grown under LDs were used for RNA extraction.
(PDF)
A, A phylogenetic tree (by MEGA5.2) [83]showing PaFD (*) belongs to eudicots and non-Poaceae FD group. B, Alignment of FD proteins used for the construction of the tree. The SAP motif [71] is marked with a dotted line.
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Dr. George Coupland, Dr. Seth Davis, Dr. Peter Huijer, Dr. Ilha Lee and Dr. Hsin-Hung Yeh for materials ft-10, FRI-Col, 35S:SVP, SOC1:GUS and pCymMV vector, respectively. We would also like to thank Ms. Pei-Chun Liao and Mr. Rainer Franzen for technical assistance in expression analyses and plant transformation, Dr. Wen-Huei Chen for comments on orchid experimental design and Dr. Ji Hoon Ahn and Dr. Jan Sheen for their careful reading of the manuscript and valuable suggestions that greatly improved the manuscript, and Ms. Miranda Loney for her help in English editing.
Data Availability
The nucleotide sequences reported in this paper have been submitted to GenBank database under the accession numbers KJ609179 (PaFT1) and KJ609180 (PaFD).
Funding Statement
Funded by Development Program of Industrialization for Agricultural Biotechnology (DPIAB, Taiwan 099S0030086-AA to SJ), http://dpiab.sinica.edu.tw/index_en.php. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Amasino R. Seasonal and developmental timing of flowering. Plant J. 2010; 61: 1001–1013. 10.1111/j.1365-313X.2010.04148.x [DOI] [PubMed] [Google Scholar]
- 2. Song YH, Ito S, Imaizumi T. Flowering time regulation: photoperiod- and temperature-sensing in leaves. Trends Plant Sci. 2013; 18: 575–583. 10.1016/j.tplants.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Ann Rev Plant Biol. 2008; 59: 573594. [DOI] [PubMed] [Google Scholar]
- 4. Andrés F, Coupland G. The genetic basis of flowering responses to seasonal cues. Nat Rev Genet. 2012; 13: 627–639. 10.1038/nrg3291 [DOI] [PubMed] [Google Scholar]
- 5. Zeevaart JAD. Leaf-produced floral signals. Curr Opin Plant Biol. 2008; 11: 541–547. 10.1016/j.pbi.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 6. Turnbull C. Long-distance regulation of flowering time. J Exp Bot. 2011; 62: 4399–4413. 10.1093/jxb/err191 [DOI] [PubMed] [Google Scholar]
- 7. Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z. et al. The tomato FT orthologue triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA. 2006; 103: 6398–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin MK, Belanger H, Lee YJ, Varkonyi-Gasic E, Taoka K, Miura E. et al. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. 2007; Plant Cell. 19:1488–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007; 316: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 10. Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T. et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature. 2011; 476: 332–335. 10.1038/nature10272 [DOI] [PubMed] [Google Scholar]
- 11. Liu L, Liu C, Hou X, Xi W, Shen L, Tao Z. et al. FTIP1 is an essential regulator required for florigen transport. PLOS Biol. 2012; 10: e1001313 10.1371/journal.pbio.1001313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jaeger KE, Pullen N, Lamzin S, Morris RJ, Wigge PA. Interlocking feedback loops govern the dynamic behavior of the floral transition in Arabidopsis . Plant Cell. 2013; 25: 820–833. 10.1105/tpc.113.109355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Izawa T. Daylength measurements by rice plants in photoperiodic short-day flowering. Int Rev Cytol. 2007; 256: 191–222. [DOI] [PubMed] [Google Scholar]
- 14. Hayama R, Agashe B, Luley E, King R, Coupland G. A circadian rhythm set by dusk determines the expression of FT homologs and the short-day photoperiodic flowering response in Pharbitis . Plant Cell; 2007; 19: 2988–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. An FM, Hsiao SR, Chan MT. Sequencing-based approaches reveal low ambient temperature-responsive and tissue-specific microRNAs in Phalaenopsis orchid. PLoS ONE. 2011; 6: e18937 10.1371/journal.pone.0018937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su CL, Chao YT, Alex Chang YC, Chen WC, Chen CY, Lee AY. et al. De novo assembly of expressed transcripts and global analysis of the Phalaenopsis aphrodite transcriptome. Plant Cell Physiol. 2011; 52: 1501–1514. 10.1093/pcp/pcr097 [DOI] [PubMed] [Google Scholar]
- 17. Chen WS, Liu HY, Yang L, Chen WH. Gibberellin and temperature influence carbohydrate content and flowering in Phalaenopsis . Physiol Plant. 1994; 90: 391–395. [Google Scholar]
- 18. Lopez RG, Runkle ES. Environmental Physiology of growth and flowering of orchids. HortScience. 2005; 40: 1969–1973. [Google Scholar]
- 19. Sakanishi Y, Imanishi H, Ishida G. Effect of temperature on growth and flowering of Phalaenopsis amabilis . Bull Univ Osaka Pref Ser B. 1980; 32: 1–9. [Google Scholar]
- 20. Endo M, Ikusima I. Diurnal rhythm and characteristics of photosynthesis and respiration in the leaf and root of a Phalaenopsis plant. Plant Cell Physiol. 1989; 30: 43–47. [Google Scholar]
- 21. Lee N. Embryo culture of orchids. J Chinese Soc Hort Sci. 1990; 36: 223–244. [Google Scholar]
- 22. Sinha P, Jahan MAA, Munshi JL, Khatun R . High frequency regeneration of Phalaenopsis amabilis (L.) Bl. cv. Lovely through In vitro culture. Plant Tissue Cul Biotechnol. 2010; 20: 185–193. [Google Scholar]
- 23. Yoneda K, Momose H, Kubota S. Effects of daylength and temperature on flowering in juvenile and adult Phalaenopsis plants. J Jpn Soc Hortic Sci. 1991; 60: 651–657. [Google Scholar]
- 24. Lee N, Lin GM. Effect of temperature on growth and flowering of Phalaenopsis White Hybrid. J Chin Soc Hort Sci. 1984; 30: 223–231. [Google Scholar]
- 25. Blanchard MG, Runkle ES. Temperature during the day, but not during the night, controls flowering of Phalaenopsis orchids. J Exp Bot. 2006; 57: 4043–4049. [DOI] [PubMed] [Google Scholar]
- 26. Chen WH, Tseng YC, Liu YC, Chuo CM, Chen PT, Tseng KM. et al. Cool-night temperature induces spike emergence and affects photosynthetic efficiency and metabolizable carbohydrate and organic acid pools in Phalaenopsis aphrodite . Plant Cell Rep. 2008; 27: 1667–1675. 10.1007/s00299-008-0591-0 [DOI] [PubMed] [Google Scholar]
- 27.Lee N, Lin GM. Control the flowering of Phalaenopsis. In: Proc Symp Forcing Culture Hort Crops. Chang LR, Editor. Taichung District Agr Improv Sta, Taiwan; 1987 pp. 27–44.
- 28. Lee JH, Ryu HS, Chung KS, Posé D, Kim S, Schmid M. et al. Regulation of temperature-responsive flowering by MADS-box transcription factor repressors. Science. 2013; 342: 628–632. 10.1126/science.1241097 [DOI] [PubMed] [Google Scholar]
- 29. Posé D, Verhage L, Ott F, Yant L, Mathieu J, Angenent GC. et al. Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature. 2013; 503: 414–417. 10.1038/nature12633 [DOI] [PubMed] [Google Scholar]
- 30. Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S. et al. The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis . Genes Dev; 2006; 20: 898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. Plant J. 2006; 46: 183–192. [DOI] [PubMed] [Google Scholar]
- 32. Michaels SD, Amasino RM. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell. 2001; 13: 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simpson GG, Dean C. Arabidopsis, the Rosetta stone of flowering time? Science, 2002; 296: 285–289. [DOI] [PubMed] [Google Scholar]
- 34. Cockram J, Jones H, Leigh FJ, O'Sullivan D, Powell W, Laurie DA. et al. Control of flowering time in temperate cereals: genes, domestication, and sustainable productivity. J Exp Bot. 2007; 58: 1231–1244. [DOI] [PubMed] [Google Scholar]
- 35. Hou CJ, Yang CH. Functional analysis of FT and TFL1 orthologs from orchid (Oncidium Gower Ramsey) that regulate the vegetative to reproductive transition. Plant Cell Physiol. 2009; 50: 1544–1557. 10.1093/pcp/pcp099 [DOI] [PubMed] [Google Scholar]
- 36. Li DM, L FB, Zhu GF, Sun YB, Liu HL, Liu JW. et al. Molecular characterization and functional analysis of a Flowering locus T homolog gene from a Phalaenopsis orchid. Genet Mol Res. 2014; 13:5982–5994. 10.4238/2014.August.7.14 [DOI] [PubMed] [Google Scholar]
- 37. Xiang L, Li X, Qin D, Guo F, Wu C, Miao L. et al. Functional analysis of FLOWERING LOCUS T orthologs from spring orchid (Cymbidium goeringii Rchb. f.) that regulates the vegetative to reproductive transition. Plant Physiol Biochem. 2012; 58: 98–105. 10.1016/j.plaphy.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 38. Jang S, Torti S, Coupland G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis . Plant J. 2009; 60: 614–625. 10.1111/j.1365-313X.2009.03986.x [DOI] [PubMed] [Google Scholar]
- 39. Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG. et al. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis . Genes Dev. 2000; 14: 2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee I, Michaels SD, Masshardt AS, Amasino RM. The late-flowering phenotype of FRIGIDA and LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis . Plant J. 1994; 6: 903–909. [Google Scholar]
- 41. Bilgin DD, DeLucia EH, Clough SJ. A robust plant RNA isolation method suitable for Affymetrix GeneChip analysis and quantitative real-time RT-PCR. Nat Protoc. 2009; 4: 333–340. 10.1038/nprot.2008.249 [DOI] [PubMed] [Google Scholar]
- 42. Curtis MD, Grossniklaus U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003; 133: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M. et al. CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis . Development. 2004; 131: 3615–3626. [DOI] [PubMed] [Google Scholar]
- 44. Lin HY, Chen JC, Wei MJ, Lien YC, Li HH, Ko SS. et al. Genome-wide annotation, expression profiling, and protein interaction studies of the core cell-cycle genes in Phalaenopsis aphrodite . Plant Mol Biol. 2014; 84: 203–226. 10.1007/s11103-013-0128-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jang S, An K, Lee S, An G. Characterization of tobacco MADS-box genes involved in floral initiation. Plant Cell Physiol. 2002; 43: 230–238. [DOI] [PubMed] [Google Scholar]
- 46. Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, Deng XY. et al. Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27: 1277–1288. 10.1038/emboj.2008.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 2008; 56: 505–516. 10.1111/j.1365-313X.2008.03612.x [DOI] [PubMed] [Google Scholar]
- 48. Shirasu K, Lahaye T, Tan MW, Zhou F, Azevedo C, Schulze-Lefert P. A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in C. elegans . Cell. 1999; 99: 355–366. [DOI] [PubMed] [Google Scholar]
- 49. Citovsky V, Lee LY, Vyas S, Glick E, Chen MH, Vainstein A. et al. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J Mol Biol. 2006; 362: 1120–1131. [DOI] [PubMed] [Google Scholar]
- 50. Lu HC, Chen HH, Tsai WC, Chen WH, Su HJ, Chang DC. et al. Strategies for functional validation of genes involved in reproductive stages of orchids. Plant Physiol. 2007; 143: 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hsieh MH, Lu HC, Pan ZJ, Yeh HH, Wang SS, Chen WH. et al. Optimizing virus-induced gene silencing efficiency with Cymbidium mosaic virus in Phalaenopsis flower. Plant Sci. 2013; 201–202: 25–41. 10.1016/j.plantsci.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 52. Hsieh MH, Pan ZJ, Lai PH, Lu HC, Yeh HH, Hsu CC. et al. Virus-induced gene silencing unravels multiple transcription factors involved in floral growth and development in Phalaenopsis orchids. J Exp Bot. 2013; 64: 3869–3884. 10.1093/jxb/ert218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Takahashi T, Komeda Y. Characterization of two genes encoding small heat-shock proteins in Arabidopsis thaliana. Mol Gen Genet. 1989; 219: 365–372. [DOI] [PubMed] [Google Scholar]
- 54. Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986; 204: 383–396. [Google Scholar]
- 55. Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J. 1998; 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 56. Kim SR, Lee DY, Yang JI, Moon S, An G. Cloning vectors for rice. J Plant Biol. 2009; 52: 73–78. [Google Scholar]
- 57. Hanzawa Y, Money T, Bradley D. A single amino acid converts a repressor to an activator of flowering. Proc Natl Acad Sci USA. 2005; 102: 7748–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY. et al. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006; 25: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ho WW, Weigel D. Structural features determining flower-promoting activity of Arabidopsis FLOWERING LOCUS T. Plant Cell. 2014; 26: 552–564. 10.1105/tpc.113.115220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ. et al. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis . Plant Physiol. 2005; 139: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee I, Amasino RM. Effect of vernalization, photoperiod and light quality on the flowering phenotype of Arabidopsis plants containing the FRIGIDA gene. Plant Physiol. 1995; 108: 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Torti S, Fornara F, Vincent C, Andres F, Nordstrom K, Gobel U. et al. Analysis of the Arabidopsis shoot meristem transcriptome during floral transition identifies distinct regulatory patterns and a leucine-rich repeat protein that promotes flowering. Plant Cell. 2012; 24: 444–462. 10.1105/tpc.111.092791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu C, Zhou J, Bracha-Drori K, Yalovsky S, Ito T, Yu H. Specification of Arabidopsis floral meristem identity by repression of flowering time genes. Development. 2007; 134, 1901–1910. [DOI] [PubMed] [Google Scholar]
- 64. Farrona S, Thorpe FL, Engelhorn J, Adrian J, Dong X, Sarid-Krebs L. et al. Tissue-specific expression of FLOWERING LOCUS T in Arabidopsis is maintained independently of polycomb group protein repression. Plant Cell. 2011; 23: 3204–3214. 10.1105/tpc.111.087809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kinoshita T, Ono N, Hayashi Y, Morimoto S, Nakamura S, Soda M. et al. FLOWERING LOCUS T regulates stomatal opening. Curr Biol. 2011; 21: 1232–1238. 10.1016/j.cub.2011.06.025 [DOI] [PubMed] [Google Scholar]
- 66. Wang YT. Gibberellic acid on Phalaenopsis . Am Orchid Soc Bull. 1995; 64: 744. [Google Scholar]
- 67. Notaguchi M, Abe M, Kimura T, Daimon Y, Kobayashi T, Yamaguchi A. et al. Long-distance, graft-transmissible action of Arabidopsis FLOWERING LOCUS T protein to promote flowering. Plant Cell Physiol. 2008; 49: 1645–1658. 10.1093/pcp/pcn154 [DOI] [PubMed] [Google Scholar]
- 68. Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y. et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005; 309: 1052–1056. [DOI] [PubMed] [Google Scholar]
- 69. Jeon JS, Lee S, Jung KH, Yang WS, Yi GH, Oh BG. et al. Production of transgenic rice plants showing reduced heading date and plant height by ectopic expression of rice MADS-box genes. Mol Breed. 2000; 6: 581–592. [Google Scholar]
- 70. Lu SJ, Wei H, Wang HM, Yang RF, Zhang XB, Tu JM. Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (Oryza sativa L.). Plant Mol Biol Rep. 2012; 30: 1461–1469. [Google Scholar]
- 71. Tsuji H, Nakamura H, Taoka K, Shimamoto K. Functional diversification of FD transcription factors in rice, components of florigen activation complexes. Plant Cell Physiol. 2013; 54: 385–397. 10.1093/pcp/pct005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis . Genes Dev. 2007; 21: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Luan W, Chen H, Fu Y, Si H, Peng W, Song S. et al. The effect of the crosstalk between photoperiod and temperature on the heading-date in rice. PLOS ONE. 2009; 4: e5891 10.1371/journal.pone.0005891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hsu CY, Adams JP, Kim H, No K, Ma C, Strauss SH. et al. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proc Natl Acad Sci USA. 2011; 108: 10756–10761. 10.1073/pnas.1104713108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nishikawa F, Endo T, Shimada T, Fujii H, Shimizu T, Omura M. et al. Increased CiFT abundance in the stem correlates with floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.). J Exp Bot. 2007; 58: 3915–3927. [DOI] [PubMed] [Google Scholar]
- 76. Noy-Porat T, Cohen D, Mathew D, Eshel A, Kamenetsky R, Flaishman MA. Turned on by heat: differential expression of FT and LFY-like genes in Narcissus tazetta during floral transition. J Exp Bot. 2013; 64: 3273–3284. 10.1093/jxb/ert165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z. et al. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1 . Genes Dev. 2004; 18: 926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M. Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1 . Plant Physiol. 2008; 148: 1425–1435. 10.1104/pp.108.125542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gao H, Zheng XM, Fei G, Chen J, Jin M, Ren Y. et al. Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLOS Genet. 2013; 9: e1003281 10.1371/journal.pgen.1003281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hanano S, Goto K. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell. 2011; 23: 3172–3184. 10.1105/tpc.111.088641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kawamoto N, Sasabe M, Endo M, Machida Y, Araki T. Calcium-dependent protein kinases responsible for the phosphorylation of a bZIP transcription factor FD crucial for the florigen complex formation. Sci Rep. 2015; 5: 8341 10.1038/srep08341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M. et al. The wheat and barley vernalization gene VRN3 is an orthologue of FT . Proc Natl Acad Sci USA. 2006; 103: 19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 2011; 28: 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li YX, Song XM, Wang Z, Lv SW, Zhang CW. Transcriptome analysis of differential expression genes from petals and lips of Phalaenopsis amabilis to identify genes associated with floral development. Plant Omics 2014; 7: 328–336. [Google Scholar]
- 85. Su CL, Chen WC, Lee AY, Chen CY, Chang YC, Chao YT, Shih MC. A modified ABCDE model of flowering in orchids based on gene expression profiling studies of the moth orchid Phalaenopsis aphrodite . PLoS One 2013; 8: e80462 10.1371/journal.pone.0080462 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, Phylogenetic tree of the deduced amino acid sequences of PaFT1 and FT sequences of other plant species. The tree was created with MEGA 5.2 using the neighbor-joining method and clustalW [83]. The accession numbers of the sequences are as follows: Arabidopsis thaliana (FT, BAA77838; TSF, BAA77840), Citrus unshiu (CiFT, AB027456), Cymbidium goeringii (CgFT, ADI58462), Carica papaya (CpFT, ACX85427), Hordeum vulgare (HvFT1, ABJ97441), Ipomoea nil (PnFT1, ABW73562), Lactuca sativa (LsFT, BAK14369), Malus x domestica (MdFT1, AB161112), Oncidium Gower Ramsey (OnFT, ACC59806), Oryza sativa (Hd3a, BAB61030; RFT1, BAO03187), Populus nigra (PnFT2a, AB109804), Solanum lycopersicum (SP3D, AY186735), Solanum tuberosum (StFT, ADA77529), Triticum aestivum (TaFT, ACA25437) and Vitis vinifera (VvFT, ACZ26523). The numbers at nodes represent the bootstrap values (with 1000 replicates) and the scale bar displays branch length. FTs from orchids are in a dotted box. B, PaFT1 has two conserved key amino acids 86-Tyr (▼) and 141-Gln (▼) which are believed to be important residues in FT proteins that act as floral activators [57–59].
(PDF)
HT; high temperature (28°C/25°C as day and night temperature under LDs), LT; low temperature (23°C/20°C). The same RNAs used for Fig 1F were utilized for the analyses of gene expression. Recently, transcriptomic analyses using petals and lips of P. amabilis, a species that is closely related to P. aphrodite, identified eight SOC1 genes [84]. Although the number of SOC1 homologues that exist in P. aphrodite has not yet been reported, the expression of three reported SOC1 homologues, PaSOC1-1 (PATC136427), PaSOC1-2 (PATC150808) and PaSOC1-3 (PATC 154491) [85] was examined during the temperature shift.
(PDF)
Control is a transgenic rice plant containing an empty vector.
(PDF)
A, Heat treated transgenic ft-10 containing pHSP18.2:PaFT1 showed earlier flowering than untreated plants. Sixteen-day-old seedlings of the plants were heat treated (2 hours from ZT 14 to ZT 16 under LDs at 37°C) for 3 weeks. PaFT1 transcripts only highly accumulated in plants with heat treatment (in the box). B, Flowering time of plants with and without heat treatment. Three independent homozygous lines (14 to 22 individuals for each line) were tested for flowering time measurement. C and D, Heat treated transgenic plants (Col WT background) containing pHSP18.2:PaFT1 showed earlier flowering than untreated plants. Three weeks old seedlings of the plants grown under SD (10 h light) were heat treated (2 hours from ZT 8 to ZT 10 at 37°C) for 3 weeks. Two independent homozygous lines (14 and 17 individuals for each line) were tested for flowering time measurement. The asterisk indicates that heat-treated plants flowered earlier than untreated plants or control. P ≤ 0.005 (Student’s t-test).
(PDF)
A and B in the left panel, CFP:AtFT in an Arabidopsis cell. C and D in the left panel, CFP:PaFT1 in an Arabidopsis cell. E and F in the left panel, YFP:AtFD in an Arabidopsis cell. G and H in the left panel, YFP:AtFDP in an Arabidopsis cell. A and B in the right panel, BiFC assay between YFPn:AtFT and YFPc:AtFD in an Arabidopsis cell. C and D in the right panel, BiFC assay between YFPn:PaFT1 and YFPc:AtFD in an Arabidopsis cell. E and F, BiFC assay between YFPn:PaFT1 and YFPc:AtFDP in an Arabidopsis cell. G, Measurement of florescence intensity in each BiFC assay. For the evaluation of the relative fluorescence intensities of nuclei in the BiFC experiments the hardware values of gain, offset and zoom on the Leica SP2 AOBS instrument were adjusted image the nuclei of the positive control (AtFT:cYFP + YFPn:AtFD) such that the values of the 8 bit color scale included 255 (brightest level). Imaging of the other BiFC pairs were under the the same hardware values.
(PDF)
Transgenic rice plants containing pUbi:Hd3a produce flowers in the callus during transformation. Bars = 5 mm.
(PDF)
Leaf numbers of orchids used in this study (upper) and the expression of PaFT1 and PaFD in each leaf.
(PDF)
Nine-day-old seedlings grown under LDs were used for RNA extraction.
(PDF)
A, A phylogenetic tree (by MEGA5.2) [83]showing PaFD (*) belongs to eudicots and non-Poaceae FD group. B, Alignment of FD proteins used for the construction of the tree. The SAP motif [71] is marked with a dotted line.
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
The nucleotide sequences reported in this paper have been submitted to GenBank database under the accession numbers KJ609179 (PaFT1) and KJ609180 (PaFD).