Synopsis
Staphylococcus aureus infections pose a significant health burden. The emergence of community-associated methicillin-resistant S. aureus has resulted in an epidemic of skin and soft tissue infections (SSTI), and many patients experience recurrent SSTI. As S. aureus colonization is associated with subsequent infection, decolonization is recommended for patients with recurrent SSTI or in settings of ongoing transmission. S. aureus infections often cluster within households and asymptomatic carriers serve as reservoirs for transmission; therefore, a household approach to decolonization is more effective than measures performed by individuals alone. Other factors, such as environmental surface contamination, may also be considered. Novel strategies for the prevention of recurrent SSTI are needed.
Keywords: Staphylococcus aureus, MRSA, skin infection, prevention, decolonization, staphylococcal vaccine, pediatrics
INTRODUCTION
“Once the organisms gain a foothold, they may be very difficult to eradicate; sometimes boil after boil appears and these lesions may continue to develop in crops for months. The scalp, face, and shoulders are favorite sites but any part of the body may be involved; in some instances, the entire body is covered with furuncles.” These prescient words, found in the chapter on skin infections from the 11th Edition (1940) of Holt’s Diseases of Infancy and Childhood [1], are as true today of Staphylococcus aureus skin and soft tissue infections (SSTI) as they were in the pre-antibiotic era. SSTI are among the most common reasons for healthcare visits in the United States, accounting for over 14 million outpatient and emergency department visits annually [2]. Moreover, these infections frequently recur, leading to substantial morbidity in the pediatric population and provoking frustration for both patients and clinicians. In this review, we will describe the epidemiology of S. aureus SSTI, with a focus on recurrent SSTI; delineate the current paradigm of SSTI pathogenesis; and provide evidence-based recommendations for treatment and prevention of these infections.
THE EMERGENCE OF COMMUNITY-ASSOCIATED METHICILLIN-RESISTANT S. AUREUS
Staphylococcus aureus is a Gram-positive commensal bacterium that colonizes the anterior nares, as well as other anatomic sites, of approximately one third of the human population [3–7]. Upon leaving the site of colonization, S. aureus can infect virtually any body site, making it the most prevalent pathogen isolated from SSTIs, a leading cause of food-borne illness, the second leading cause of infectious endocarditis [8], and an important cause (~2%) of all hospital admissions [9]. With an estimated incidence of 32 infections per 100,000 persons, S. aureus has surpassed Streptococcus pneumoniae and Haemophilus influenzae to become the most common invasive bacterial pathogen in the United States [10].
A major challenge posed by S. aureus is antimicrobial resistance. Soon after the β-lactam antibiotics penicillin and methicillin were introduced into clinical practice, strains of antibiotic-resistant S. aureus were identified [11]. Over the next several decades, methicillin-resistant S. aureus (MRSA) became an important healthcare-associated pathogen, complicating the care of post-surgical and dialysis patients and the chronically ill [12–15]. Treatment was challenging, owing to resistance to multiple antibiotics, and by the turn of the century, MRSA accounted for nearly 60% of all S. aureus isolates recovered from hospital intensive care units [16]. At present, it is projected that MRSA infections account for >100,000 hospitalizations each year in the U.S. [17].
In the late 1990s, a shift in MRSA epidemiology occurred. After the alarming deaths of four previously healthy Midwestern children following infection with MRSA [18], it was realized that MRSA infections were no longer restricted to those with chronic illnesses or frequent hospitalizations; rather MRSA had emerged as a community pathogen, capable of infecting healthy hosts, and thus was termed community-associated (CA) MRSA. Initially thought to represent a feral strain of healthcare-associated (HA) MRSA that had “escaped” into the community, it was soon determined that CA-MRSA strains were fundamentally different from traditional HA-MRSA strains. Compared to HA-MRSA strains, CA-MRSA strains exhibit a faster bacterial doubling time in vitro [19], possess a smaller gene cluster (Staphylococcal Cassette Chromosome mec) conferring resistance mainly to β-lactam antibiotics (although resistance to other antimicrobials has recently emerged) [20–36], and exhibit altered regulation of exotoxins and other virulence factors [37–41], characteristics which are thought to correlate with the aggressive clinical behavior and transmissibility of CA-MRSA. CA-MRSA causes a broad spectrum of disease entities, ranging from asymptomatic colonization to SSTI (particularly purulent abscesses) to invasive infections (e.g., fulminant necrotizing pneumonia, musculoskeletal infections, fatal bacteremia) [37, 42–47]. The host and bacterial determinants driving this spectrum are not well understood. Recently, highly virulent strains of methicillin-susceptible S. aureus (MSSA) belonging to the same genetic lineage as the current CA-MRSA epidemic strains (USA300) have been described [48, 49]. These strains share phenotypic similarities with MRSA USA300 strains, leading to SSTI, recurrent abscesses, and invasive, necrotizing infections.
EPIDEMIOLOGY OF PEDIATRIC S. aureus SSTI
The most common manifestation of CA-MRSA infection is SSTI. Although many SSTIs are superficial, they carry significant morbidity, including pain and subsequent scarring caused by drainage procedures and time lost from school and work by patients and their families. While many patients with CA-MRSA SSTIs are treated as outpatients, patients with moderate to severe SSTI often require hospitalization. SSTI now ranks among the top 10 reasons for pediatric hospital admission [50].
The epidemiology of staphylococcal SSTI changes rapidly and in the past 15 years, the landscape has been dominated by CA-MRSA. In 2005, Purcell et al. demonstrated a substantial rise in MRSA infection incidence, increasing from <10 cases annually in the 1990s to nearly 500 cases annually by 2003 [51]. By 2005, several centers across the U.S. reported that CA-MRSA accounted for nearly 75% of all staphylococcal infections [43]. These high rates of CA-MRSA necessitated changes in empiric antibiotic therapy when MRSA was suspected [52], particularly for SSTI in which well over 50% of infections in most centers were due to CA-MRSA [43, 44, 51, 53, 54]. More recently, Gerber et al. performed a retrospective, observational study using the Pediatric Health Information System (PHIS), a database of clinical and financial data from >40 tertiary care children’s hospitals in the U.S. Over the 6-year study period, the investigators identified nearly 60,000 children with S. aureus infections, 51% of whom had infection with MRSA; SSTI comprised 61% of these infections [55].
MRSA colonizes the anterior nares, throat, rectum, and skin (axilla, inguinal area, and perineum) [4–7, 56–59]. MRSA carriage is a risk factor for the development of subsequent infections [3, 6, 60–64]. Colonized individuals also are important sources for transmission [65]. Up to 10% of healthy individuals in the U.S. are colonized with MRSA [45, 46]. The prevalence of MRSA colonization has significantly increased over the past decade [46, 47], accompanied by a rise in MRSA infection incidence. Two studies, utilizing data from the National Ambulatory Medical Care Survey and a large integrated health plan in Northern California, have identified children and African-Americans as being disproportionally affected by the current CA-MRSA epidemic [2, 66, 67]. In addition to race and age, there are specific populations who have experienced a substantial increase in SSTI due to CA-MRSA (Box 1). These include military personnel, in whom MRSA colonization significantly increases the risk of developing SSTI (compared to MSSA colonization) [63]; prisoners [68–70]; and athletes, in whom colonization can be detected frequently within sports teams, though outbreaks are sporadic [71–74]. In each of these high-risk groups, risk factors for infection include close contact, compromised skin integrity, and increased prevalence of colonization; in addition, outbreaks are often linked to periods of increased colonization or exposure to specific strains of S. aureus, e.g., USA300 CA-MRSA.
Box 1. Risk Factors for S. aureus SSTI.
S. aureus colonization
Injection drug use
Diabetes mellitus
Chronic dermatologic conditions (e.g., eczema)
Recent use of antimicrobial agents
African-American race
Previous SSTI
Close contact with an SSTI patient
Participation in contact sports
Military personnel
Prisoners
PATHOGENESIS OF S. AUREUS SSTI
In the development of SSTI in an otherwise healthy individual, the site of symptomatic infection is first colonized with a relatively low number of bacteria. Staphylococci easily accomplish this, since over 80% of humans are intermittently colonized at some point with S. aureus, including 10–15% of humans who are persistently colonized [3, 75, 76]. Since colonization alone is insufficient to initiate disease, S. aureus must then reach the deeper portions of the epidermis and dermis through microabrasions of the skin, traumatic injury, or skin disruption due to inflammatory lesions (e.g., eczema). S. aureus possess a number of virulence determinants responsible for initiation and maintenance of infection [77–90], representatives of which are listed in Table 1. Upon invasion, the host inflammatory response leads to microvascular leak, production of inflammatory cytokines/chemokines, and recruitment of leukocytes (in particular, neutrophils).
Table 1.
Staphylococcus aureus virulence determinants involved in SSTI pathogenesis
| Activity | Mechanism | Virulence Determinant |
|---|---|---|
| Adherence to host tissue | MSCRAMMs [77] | |
| Tissue Destruction | Alpha toxin [78] | |
| Nutrient Acquisition | Essential Metal Acquisition | Isd system [79] |
| Disruption of Host Defense | Impaired Chemotaxis | ChIPS, Eap [80, 81] |
| Phagocyte Destruction | Leukocidins (LukAB, LukDE, PVL), PSM [82–84] | |
| Impaired Opsonization | Protein A, Polysaccharide capsule [85] | |
| Impaired Intracellular Killing | Staphyloxanthin, Catalase, Superoxide dismutase [86–90] |
SSTI CHARACTERISTICS AND INITIAL MANAGEMENT
SSTI are best characterized by depth of infection and associated skin structures as described in Box 2. The Infectious Diseases Society of America (IDSA) asserts that incision and drainage (I/D) represents primary treatment for purulent staphylococcal skin abscesses [91]. The procedural approach to abscess drainage has been recently reviewed [92]. It is important to note that sufficient drainage, with disruption of loculations and facilitation of ongoing drainage, is challenging in the pediatric population, often requiring sedation and post-procedural pain management. What is gained by this approach, however, is nearly immediate pain relief from large abscesses, faster wound healing and access to material for bacterial culture.
Box 2. Manifestations of skin infections.
Erysipelas
Superficial skin infection characterized by well-demarcated, intensely erythematous lesions. Nearly universally due to S. pyogenes.
Cellulitis
Painful infection of the dermis and subcutaneous tissues, often occurring near breaks in the skin.
Impetigo
Relatively superficial infection leading to bullous or non-bullous lesions.
Folliculitis
Superficial or deep inflammation of the hair follicle leading to papulopustular lesions.
Furuncle
Extension of suppurative infection from the hair follicle, leading to infection of the deeper skin structures (typical abscess)
Carbuncle
An aggregate of infected follicles leading to a deep, painful mass
Traditionally, drainage has been the mainstay of therapy for the majority of staphylococcal abscesses [93]; this has held true in the contemporary era of CA-MRSA in several studies. In a prospective cohort study by Lee et al. of children presenting to a large emergency department with SSTI, >75% of children experienced clinical cure following I/D, even if prescribed an antibiotic that was ineffective (based on eventual susceptibility results) [94]. Similarly, Chen et al. determined that among children with SSTI who experienced spontaneous drainage or had I/D performed, despite MRSA being the causative agent in 69%, no differences were evident in clinical cure between those receiving cephalexin vs. clindamycin [95]. Two randomized trials, one in adults [96] and one in children [97], have compared trimethoprim-sulfamethoxazole (TMP/SMX) to placebo after I/D of suspected staphylococcal abscesses. While both trials showed that the clinical cure rate did not differ between treatment groups, receipt of antimicrobial therapy resulted in a lower incidence of early recurrent disease. While current data are reassuring that antimicrobial agents are not required in all children, definitive data are not yet available in this population. An NIH-sponsored clinical trial [98] is expected to answer this question more completely, comparing the effectiveness of clindamycin, TMP/SMX, or placebo, in conjunction with I/D, in the treatment of limited abscesses in adults and children.
Patients for whom antibiotics are currently recommended include patients at the extremes of age and those with severe or extensive disease, rapidly progressing cellulitis, abscess in an anatomic location that precludes adequate drainage, systemic illness or hemodynamic instability, associated septic phlebitis, or failure to improve after incision and drainage alone [91]. As antimicrobial resistance has increased, choices of orally effective antimicrobials have diminished. Agents with in vitro activity against MRSA are provided in Table 2. Although antibiotic selection should be directed by one’s regional antibiogram, for many communities, clindamycin and TMP/SMX remain good first line therapeutic agents for suspected CA-MRSA SSTI. However, in a large retrospective study of Tennessee children, Williams et al. found that TMP/SMX was less effective than clindamycin in both treating an initial abscess and preventing recurrences of disease [99]. Among 6407 children who underwent drainage, 9% experienced treatment failures within 14 days, and 23% had recurrence within one year; both of these outcomes were more likely in children receiving TMP/SMX, compared with clindamycin. Among 41,000 children who did not undergo a drainage procedure, TMP/SMX remained significantly less effective than clindamycin for the initial treatment of SSTI. Despite these observed differences, however, the overall success of TMP/SMX was high and, given its availability, tolerability, and low cost, TMP/SMX remains a first-line SSTI agent targeting MSSA and MRSA alike.
Table 2.
Systemic antimicrobial agents for the treatment of Staphylococcus aureus SSTI
| Antimicrobial Agent | Recommended Pediatric Dose Range (Oral) | Comments |
|---|---|---|
| Clindamycin | 30–40 mg/kg/day divided q6–8h |
|
| Trimethoprim-Sulfamethoxazole | 10–20 mg/kg/day divided q12h |
|
| Doxycycline | 2.2 mg/kg/day divided q12h |
|
| Linezolid | 30 mg/kg/day divided q8h |
|
| Fluoroquinolones | (varies by individual quinolone) |
|
| Rifampin | 10 mg/kg/day |
|
EPIDEMIOLOGY OF RECURRENT SSTI
As many as 70% of patients with CA-MRSA SSTI will experience recurrent SSTI over one year (Table 3), even after successful initial treatment [59, 97, 99–105]. These recurrent infections may necessitate repeated courses of antibiotic therapy, further driving development of antibiotic resistance [106, 107]. The risk factors governing these recurrent infections are not yet clear, though there are certainly pathogen-level, host-level, and environmental-level variables that contribute to CA-MRSA transmission and risk of recurrence [108–110]. As described above, the prescription of systemic antibiotics for SSTI treatment [96, 97], as well as the choice of antibiotic prescribed [99], may influence the incidence of recurrent infection. This may be due to reduction of the staphylococcal colonization burden, thereby eliminating the endogenous source for infection. Indeed, several prospective studies have demonstrated eradication of MRSA carriage following treatment of SSTI with clindamycin [111, 112].
Table 3.
Incidence and Risk Factors Associated with Recurrent Skin and Soft Tissue Infection
| Study Reference | Population; Year(s) Study Performed | Study Design | Treatment or Intervention | Longitudinal Timeframe | Proportion of Patients with Recurrent SSTI | Factors Associated with Recurrent SSTI |
|---|---|---|---|---|---|---|
| Bocchini et al, 2013 [104] | 12,836 children presenting to Texas Children’s Hospital (TCH; Houston, TX) with community-associated S. aureus infection; 2001–2009 | Retrospective cohort study | N/A | 76 months | 5% presented to TCH with documented recurrent S. aureus infection (694 with recurrent S. aureus infection of any etiology; 637 with recurrent S. aureus SSTI) |
|
| Williams et al, 2011 [99] | 47,501 children with incident SSTI enrolled in Tennessee Medicaid; 2004–2007 | Retrospective cohort study | Treatment with clindamycin, trimethoprim-sulfamethoxazole (TMP/SMX), or a β-lactam antibiotic | 365 days | 14% overall had a documented recurrent SSTI (23% in patients undergoing drainage; 18% of those without drainage) |
|
| Chen et al, 2009 [100] | 95 children with purulent SSTI in Baltimore, MD; 2006–2007 | Subgroup analysis of a double-blind, randomized, controlled trial comparing cephalexin to clindamycin | Treatment with cephalexin or clindamycin (assignment not specified) | 3 months | 22% reported recurrent SSTI |
|
| Fritz et al, 2012 [103] [and Fritz unpublished data] | 183 children with acute CA-S. aureus SSTI and concurrent S. aureus colonization in St. Louis, MO; 2008–2009 | Randomized, controlled trial comparing individual vs. household decolonization | All patients were assigned a 5-day decolonization regimen of enhanced personal and household hygiene, intranasal 2% mupirocin ointment application twice daily, and daily 4% chlorhexidine body washes | 12 months | 63% reported recurrent SSTI (72% in index decolonization group; 52% in household decolonization group) |
|
| Miller et al, 2015 [105] | 330 adults and children treated for S. aureus SSTI in Los Angeles, CA and Chicago, IL; 2008–2010 | Prospective cohort study | N/A | 6 months | 51% reported recurrent SSTI |
|
| Kaplan et al, 2014 [59] | 987 children with suspected S. aureus SSTI or invasive infection in Houston, TX; 2009–2012 | Randomized, controlled trial comparing hygienic measures alone vs. hygienic measures plus bleach baths | Participants in the intervention arm bathed in dilute bleach water twice weekly for 3 months | 12 months | 19% reported medically-attended recurrent SSTI (21% hygiene group; 17% bleach bath group) |
|
Clustering of S. aureus infections occurs in households [113–124]. Additionally, a high proportion of household members of patients with MRSA infection are colonized with MRSA, frequently with strains identical to those recovered from index patients [119, 125]. Although these colonized contacts are often asymptomatic, they serve as important sources for ongoing transmission within households [5, 65, 106, 125–129], leading to reacquisition and recurrent infection. Households with young children may have greater risk of transmission through close personal contact [111, 119, 130].
PREVENTION STRATEGIES: DECOLONIZATION
S. aureus carriage is a risk factor for the development of subsequent infection [3, 6, 60–64], and recurrent S. aureus SSTI are frequently caused by the same strain type [104, 131, 132]. Thus, decolonization (i.e., “the use of antimicrobial or antiseptic agents to suppress or eliminate S. aureus carriage” [91]) is often prescribed in an attempt to prevent recurrent infections. Such therapies have traditionally been implemented in healthcare settings to prevent nosocomial S. aureus and MRSA infections [133–144]. During the ongoing CA-MRSA epidemic, these measures have been extrapolated to patients in community settings [91, 145, 146]. While application of these therapies for a discrete period is effective for MRSA eradication, their effectiveness in infection prevention varies by study, and maintenance of eradication often diminishes over time [101, 103, 133–136, 141–144, 147–149]. Thus, the optimal preventive strategy for recurrent S. aureus SSTI remains elusive, and a wide variety of treatment and decolonization practices exist [91, 145, 150].
Who should undergo decolonization?
Prior history of SSTI is a risk factor for recurrent SSTI [64, 151]. This association, coupled with the pursuit of judicious use of topical antimicrobials, suggests that decolonization is likely not necessary for patients experiencing a first SSTI. Indeed, the IDSA MRSA Clinical Practice Guidelines state that decolonization may be considered, upon optimizing wound care and hygiene (see below), for patients experiencing recurrent SSTI and for households in which there is ongoing transmission [91].
S. aureus transmission frequently leads to infections in multiple household members; thus, when decolonization is prescribed, it should be performed by all household members. A randomized trial of 183 households conducted by Fritz et al. compared the effectiveness of decolonization of the index patient alone (“index group”) to decolonization of all household members (“household group”). The 5-day decolonization regimen included hygiene education, twice daily application of 2% intranasal mupirocin, and daily body washes with 4% chlorhexidine. Three months following randomization, the incidence of SSTI was significantly lower in index patients assigned to the household decolonization group compared to those in the index group (28% vs. 47%, respectively, p=0.02). This benefit was also demonstrated for household contacts (at 3 months: 4% incidence in household group vs. 10% in index group, p=0.01) [103].
Hygiene strategies
Before staphylococcal eradication measures are prescribed, attention to basic wound care and personal hygiene should be addressed. Education should be provided to patients and their families regarding the transmissibility of S. aureus, particularly through contact with open wounds and contaminated surfaces. Patients should be encouraged to adopt enhanced hygiene practices, including regular bathing and frequent hand washing with soap and water or alcohol-based hand sanitizers. Patients and their contacts should avoid sharing personal hygiene items (e.g., towels, deodorant, cosmetics, brushes, razors, toothbrushes, or other items that come into contact with the skin). Additional measures that may reduce transmission and infection risk include using pump or pour lotions (rather than those in jars), keeping fingernails clean and trimmed short, avoiding loofas in the bath or shower, and changing underwear, sleepwear, towels, and washcloths daily [59, 91, 101, 103, 119, 146, 152].
Environmental surfaces serve as reservoirs for MRSA transmission and MRSA strains can persist in the environment for prolonged intervals, posing risk for the development of recurrent infections [34, 106, 109, 121–123, 128, 151, 153–156]. Thus, a barrier should be used between bare skin and surfaces touched by multiple people (e.g., exercise equipment). Additionally, patients with recurrent SSTI may consider performing environmental hygiene measures, focusing on frequently touched surfaces and using commercially available disinfectants [91, 152]. Routine laundry procedures, following the label directions on the detergent and the clothing or linens to be washed, are usually sufficient to disinfect items; use of hot water or bleach for all household laundry is not necessary [152].
Topical antimicrobial agents
While multiple agents and technologies have been proposed or evaluated for S. aureus decolonization [157], this review will focus on those most readily prescribed and available to patients. An important consideration of any decolonization regimen, regardless of efficacy, is the time and financial burden encumbered by patients, which heavily influences adherence and thus, the effectiveness of these measures.
Mupirocin (pseudomonic acid A) is produced naturally by Pseudomonas fluorescens [158–160]. Mupirocin targets the bacterial isoleucyl-tRNA synthetase, resulting in protein synthesis inhibition [161]. Mupirocin has antimicrobial activity against staphylococcal and streptococcal species and is prescribed for topical treatment of skin infections as well as eradication of S. aureus (both MSSA and MRSA) nasal carriage.
Retapamulin (Altabax, GlaxoSmithKline, Research Triangle Park, NC) is a semisynthetic antimicrobial derived from the natural compound pleuromutilin, produced by the edible mushroom Pleurotus mutilus [162, 163]. Retapamulin inhibits S. aureus protein synthesis by binding to the 50S ribosomal subunit [164, 165]. At present, retapamulin is approved for treatment of impetigo due to MSSA or Streptococcus pyogenes. Retapamulin has demonstrated activity against S. aureus strains exhibiting resistance to methicillin and mupirocin as well as several other systemic antibiotics [162, 163, 166]. A Phase I/IIa randomized, double-blind, placebo-controlled trial evaluated 3- and 5-day regimens of retapamulin (1%) ointment applied to the anterior nares twice daily in patients persistently colonized with S. aureus. Both retapamulin regimens demonstrated efficacy in S. aureus eradication 28 days following application, compared to placebo [167]. An ongoing randomized trial aims to determine the effectiveness of retapamulin in eradication of mupirocin-resistant MRSA from adult carriers [168].
Chlorhexidine gluconate is a broad-spectrum biguanide cationic bactericidal agent [169, 170]. At low concentrations, chlorhexidine disrupts cytoplasmic membrane integrity; at high concentrations, it causes microbial cytoplasmic contents to congeal [169]. Multiple preparations of chlorhexidine exist, including a liquid topical antiseptic available without a prescription (Hibiclens, Mölnlycke Health Care, Norcross, GA), an oral rinse, and impregnated cloths [171]. Attractive for the purposes of decolonization, chlorhexidine provides residual antibacterial activity on the skin [148, 172]. Of note, as chlorhexidine may result in ocular and ototoxicity, patients should be instructed to avoid the eyes and ears when using this agent.
Bleach, or sodium hypochlorite, has antimicrobial activity against S. aureus both in vivo and in vitro. Dilute bleach water baths have traditionally been recommended by dermatologists to treat eczema, presumably by suppressing S. aureus growth, which is correlated with disease severity [173–177]. The recommended dilution of bleach varies [91, 101, 146, 173, 176]. An in vitro assay determined that the hypochlorite concentration necessary for maximal S. aureus killing was 2.5 μL of 6% hypochlorite per mL of water (equal to approximately ½ cup bleach in ¼ bathtub full of water), with an exposure time of 15 minutes yielding a >4-log decrease in S. aureus [175]. In clinical practice, to minimize skin irritation, a dilution of ¼ cup household bleach in ¼ bathtub (~13 gal) of water is recommended; for non-standard bathtubs, 1 teaspoon of bleach should be added per gallon of water. Individuals should soak in the dilute bleach water for 15 minutes. As household bleach is readily available and inexpensive, bleach baths are attractive for the purpose of decolonization. Additionally, soaking body areas that are frequently colonized with S. aureus (e.g. the groin and axillae) likely provides optimal antimicrobial effect. However, in large families, this strategy may be cumbersome and impractical. A recent feasibility study evaluated a body wash gel preparation of sodium hypochlorite (CLn BodyWash, Top MD Skin Care, Inc., Dallas, TX) in atopic dermatitis patients whose eczematous lesions yielded S. aureus [177]. Over 12 weeks, patients experienced significant improvement in severity of their atopic dermatitis; parents reported that the body wash was easier to administer than dilute bleach water baths.
Oral antibiotics for decolonization
Trials evaluating the effectiveness of systemic antibiotics in eradicating S. aureus or MRSA carriage have produced disparate results. Many of these trials have demonstrated emergence of resistant organisms with the use of oral antibiotics. Additionally, systemic antimicrobials traditionally used for decolonization, in particular rifampin, have been associated with toxicities. Thus, oral antibiotics should be reserved for patients with acute infections and are generally not recommended for staphylococcal decolonization alone [91, 143, 178].
Effectiveness of decolonization in preventing SSTI
Several trials have evaluated decolonization measures among healthy individuals in community settings (Table 4). S. aureus colonizes the anterior nares and the skin at multiple anatomic sites [4–7, 56–58, 179], and a greater number of colonized sites confers increased risk of infection [59]. Additionally, the buttocks and lower extremities are frequent sites for S. aureus SSTI [49, 104, 145, 180]. Thus, a decolonization approach targeting intranasal and skin carriage has the greatest potential for success. Among recent trials, studies prescribing both intranasal mupirocin and antimicrobial body washes demonstrated significantly reduced incidence of SSTI [101, 103, 181], while studies employing only intranasal mupirocin [147] or only antimicrobial body washes [59, 110, 180] showed no significant effect on SSTI incidence.
Table 4.
Staphylococcus aureus Decolonization Trials Conducted in Healthy Populations
| Study Reference | Population; Year(s) Study Performed | Study Design | Intervention | Length of Follow-Up Period Following Randomization | Outcomes |
|---|---|---|---|---|---|
| Raz et al, 1996 [182] | 34 children and adults with a history of ≥3 staphylococcal SSTI in the prior year who were also colonized with S. aureus in Israel | Randomized double-blind placebo controlled trial | Baseline decolonization performed by all participants: mupirocin 2% ointment applied to the anterior nares twice daily for 5 days. Participants were then randomized to two groups:
|
12 months |
|
| Ellis et al, 2007 [147] | 134 U.S. Army personnel enrolled in the Health Care Specialist Course colonized with CA-MRSA, Fort Sam Houston, TX; 2005 | Cluster randomized, double-blind placebo controlled trial | Participants were randomized at the class level to perform:
|
8–10 weeks |
|
| Whitman et al, 2010 [110] | 1562 U.S. Marine recruits attending Officer Candidate School, Quantico, VA; 2007 | Cluster randomized, double-blind controlled trial | Participants were randomized at the platoon level to perform:
|
6 weeks |
|
| Fritz et al, 2011 [101] | 300 children and adults with acute CA-SSTI with S. aureus colonization in St. Louis, MO; 2007–2009 | Randomized, open-label four-arm controlled trial | Participants were randomized equally to perform 1 of 4 5-day decolonization protocols:
|
4–6 months |
|
| Fritz et al, 2012 [103] | 183 children with acute CA-S. aureus SSTI and concurrent S. aureus colonization and their household contacts (844 total participants) in St. Louis, MO; 2008–2009 | Randomized, open-label two-arm controlled trial | A 5-day decolonization regimen (including enhanced personal and household hygiene, intranasal 2% mupirocin ointment application twice daily, and daily 4% chlorhexidine body washes) was prescribed to two randomization groups:
|
12 months |
|
| Kaplan et al, 2014 [59] | 987 children with suspected S. aureus SSTI or invasive infection in Houston, TX; 2009–2012 | Randomized, single-blinded controlled trial | Participants were randomized equally to perform:
|
12 months |
|
| Ellis et al, 2014 [180] | 30,209 U.S. Army personnel enrolled in Infantry One Station Unit Training, Fort Benning, GA; 2010–2012 | Cluster randomized trial | Platoons were randomized to:
|
14 weeks |
|
Many trials to date have prescribed a brief decolonization regimen (e.g., 5 days) [101, 103]; although reduced SSTI incidence was demonstrated in the months immediately following decolonization, many participants experienced recurrent infection over longer intervals. These findings likely reflect ongoing exposure to colonized individuals and environmental reservoirs, and suggest that, especially for patients experiencing multiple infection recurrences, a periodic approach to decolonization may provide more effective, sustained protection [182]. A randomized trial of pediatric patients with SSTI by Kaplan et al. evaluated daily hygienic measures alone compared with hygienic measures accompanied by twice-weekly dilute bleach water baths performed for 3 months. Over the 12-month study period, the incidence of medically attended recurrent SSTI did not differ significantly between the group performing bleach baths compared to the hygiene-only group (17% vs. 21%, respectively, p=0.15) [59]. Of note, as not all patients with recurrent SSTI seek medical attention [7], the effectiveness of this intervention may have been underestimated.
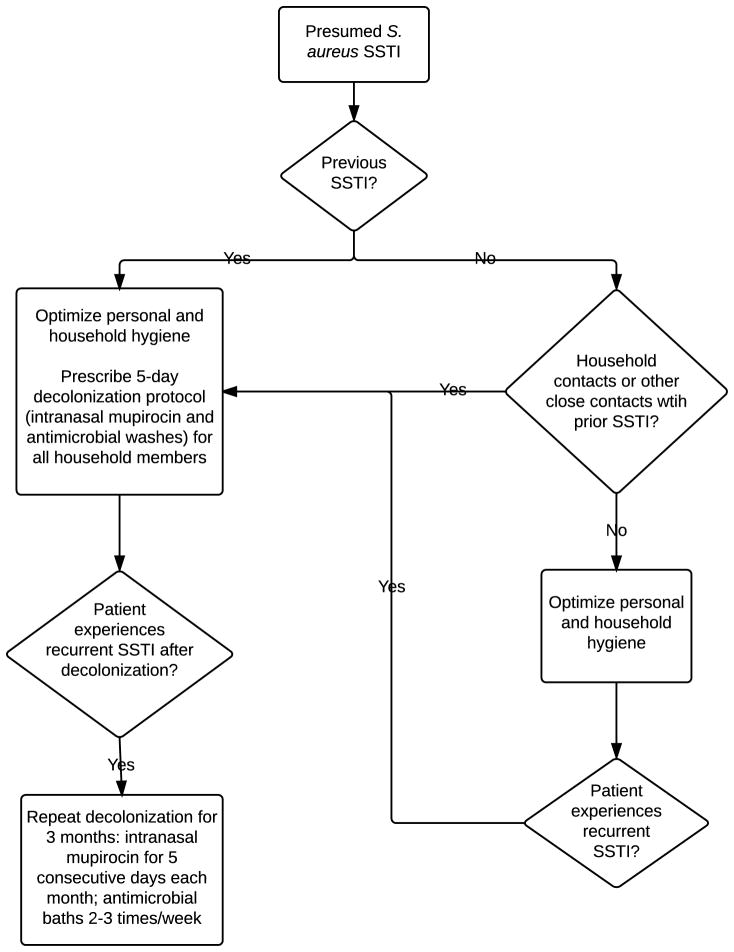
Based on existing evidence, guidance from the CDC and IDSA, and our clinical experience, we propose a preventive approach to recurrent staphylococcal SSTI which optimizes hygiene measures, targets nasal and skin colonization, and includes all household members (Figure 1).
Figure 1. Recommended approach to prevention of recurrent staphylococcal SSTI.
For all patients with S. aureus SSTI, we recommend optimizing hygiene measures. For those experiencing recurrent SSTI, or for households in which multiple members have experienced S. aureus infection, we recommend decolonization with a regimen that includes the application of an intranasal antibiotic (twice daily for 5 days) and daily antimicrobial body washes (performed daily for 5 days; for individuals with sensitive skin, these washes may be performed every other day for 7–10 days). These measures should be performed by all household members and may be considered for other close contacts on a case-by-case basis. Patients and their household contacts should change their bedding at the onset and again at the completion of the decolonization regimen and towels should be changed daily during the 5-day protocol. For individuals experiencing recurrent SSTI after the optimization of personal and household hygiene measures and the performance of decolonization by all household members, clinicians may consider prescribing a three-month regimen of periodic decolonization, in which an intranasal antibiotic is applied to the anterior nares twice daily for five consecutive days each month and antimicrobial body washes are performed two to three times each week.
A POTENTIAL UNDESIRABLE REPERCUSSION OF DECOLONIZATION: ANTIMICROBIAL RESISTANCE
An important consideration for S. aureus decolonization is the emergence of staphylococcal strains resistant to topical antimicrobials, which has been demonstrated in vitro and in vivo (Table 5) [158–160, 170, 183–186]. This resistance in turn predicts failure of S. aureus decolonization efforts and has led to hospital outbreaks with resistant strains [159, 183, 187–195]. An additional concern is that the genes conferring resistance to mupirocin (most commonly mupA) and chlorhexidine (most commonly qac A/B or smr) are carried on plasmids that can also harbor genes conferring resistance to other systemic antibiotics [159, 169, 183, 188, 196–199]. A challenge to U.S. clinicians is the paucity of commercially available resistance testing for mupirocin and chlorhexidine; at present there are no interpretive breakpoints established by the Food and Drug Administration [159].
Table 5.
Staphylococcus aureus Mupirocin and Chlorhexidine Resistance Among Selected Populations
| Study Reference | Population; Year(s) Study Performed | Study Design/ Intervention | Mupirocin resistance | Chlorhexidine resistance | Other findings/Notes |
|---|---|---|---|---|---|
| McNeil et al. 2011 [197] | 68 children presenting with recurrent community-onset SSTI (136 S. aureus isolates) in Houston, TX; 2001–2009 | Retrospective study | 14.7% of S. aureus infecting isolates were positive for mupirocin resistance | N/A |
|
| Fritz et al, 2013 [190] | 1089 adults and children presenting with community-onset SSTI (2425 S. aureus isolates) in St. Louis, MO; 483 patients were enrolled in decolonization trials, of which 408 were assigned intranasal mupirocin and/or chlorhexidine body washes; 2007–2009 | Cross sectional study followed by a randomized controlled trial | 2.1% of patients were colonized or infected with a mupirocin-resistant S. aureus strain at baseline | 0.9% of patients were colonized or infected with a S. aureus strain harboring qacA/B at baseline |
|
| Al-Zubeidi et al, 2013 [131] | 105 children presenting to the emergency department with community-onset MRSA SSTI (248 isolates) in St. Louis, MO; 2005–2011 | Retrospective study | 6.7% of patients were infected with a mupirocin-resistant MRSA isolate | N/A | |
| Johnson et al, 2013 [184] | 281 MRSA isolates obtained from pediatric emergency department and hospitalized patients in Nashville, TN; 2004–2009 | Retrospective study | N/A | 18.5% of MRSA infecting isolates harbored qac A/B or smr (13.9% harbored smr only, 4.3% harbored qac A/B only and 1 isolate contained both smr and qacA/B) |
|
| McNeil et al, 2013 [185] | 179 patients with underlying malignancy with S. aureus infection (most commonly bacteremia, yielding 156 S. aureus isolates for analysis) in Houston, TX; 2001–2011 | Retrospective study | N/A | 7.6% of infecting S. aureus isolates harbored qacA/B |
|
| McNeil et al, 2013 [186] | 216 congenital heart disease patients with S. aureus infection (most commonly SSTI, surgical site infection, and bacteremia/infective endocarditis, yielding 183 S. aureus isolates for analysis) in Houston, TX; 2001–2011 | Retrospective study | N/A | 16.9% of infecting S. aureus isolates harbored qacA/B |
|
| McNeil et al, 2014 [183] | 400 children presenting with community-onset S. aureus SSTI in Houston, TX (200 isolates from patients with a first-time S. aureus SSTI episodes and 200 isolates from patients with ≥3 S. aureus SSTI episodes); 2010–2012 | Retrospective study | 9.8% of patients were infected with a mupirocin-resistant S. aureus isolate | 14% of patients were infected with a S. aureus isolate harboring smr |
|
FUTURE DIRECTIONS
Despite the effectiveness of topical antimicrobials in eradicating S. aureus carriage, patients continue to suffer a high burden of subsequent SSTI over time [101, 103, 110, 147]. Thus, novel strategies are needed for the prevention of recurrent staphylococcal infections.
Vaccine
Vaccine development for S. aureus has been stymied by multiple factors, including lack of understanding of human immunity to staphylococci, redundancy of virulence determinants within the staphylococcal genome, and failure of previous vaccine candidates. The first of these failures involved a capsular polysaccharide vaccine (StaphVAX, Nabi Pharmaceuticals) given to hemodialysis patients at high risk for S. aureus disease [200]. In this study, the vaccine adequately elicited anti-capsular antibodies but failed to protect recipients from clinical disease. This vaccine has been modified significantly to include other targets and is currently in clinical development (PentaStaph, GSK). The second clinical failure occurred with a monovalent IsdB-based vaccine (V710, Merck) [201] in which vaccine recipients, who were undergoing cardiothoracic surgery, experienced greater mortality than placebo recipients. Despite these failures, staphylococcal vaccine development, as well as strategies for passive immunization [202], continues. Once available, a suitable vaccine will be targeted to individuals at high risk for SSTI and those with recurrent infections.
Bacterial Interference and Probiotics
The endogenous microbiota (i.e., normal flora) exist in a delicate balance, vying for nutrients and adhesion sites. In this competition, commensal bacteria may interfere with the adherence and pathogenesis of potential pathogens, thereby protecting the host [203, 204]. Additionally, the endogenous microbiota may activate or augment host defenses against bacterial invaders. The concept of bacterial interference, or the use of a non-pathogenic organism to interfere with colonization and infection of a potentially pathogenic organism, is not novel. Indeed, the practice was implemented in the 1960s in an effort to abate S. aureus outbreaks among newborns due to epidemic strain type 80/81. The concept emerged from an observation that infants colonized with a non-epidemic strain type with apparent low pathogenicity, known as 502A, were at decreased risk for colonization and infection with strain type 80/81. Newborns intentionally inoculated with 502A in the nares and/or umbilicus were less likely to acquire strain type 80/81 and had a reduced incidence of infection with this epidemic strain [129, 205–210]. It is important to note, however, that following dissemination of this practice, reports emerged of infants developing pustulosis, conjunctivitis, and other infections, including one infant who died of meningitis and septicemia, all due to the 502A strain [211–213].
The balance of organisms within the host microbiota may play an important role in S. aureus colonization and development of symptomatic infection. In this contemporary CA-MRSA era, a U.S. military study comparing microbial communities within the anterior nares between soldiers with and without SSTI revealed a significantly higher abundance of Proteobacteria in the anterior nares of the non-SSTI group compared to soldiers with active SSTI [214]. Thus, perhaps manipulation of heterologous components of host microbiota may provide resilience against S. aureus colonization and infection. Uehara et al. conducted a trial in Japan in which persistent S. aureus nasal carriers were inoculated with a strain of Corynebacterium sp. (Co304) vs. sequential inoculation with saline and S. epidermidis. Of the 17 participants receiving Corynebacterium inoculation, S. aureus was completely eradicated in 71%. In contrast, eradication of S. aureus carriage was not demonstrated when NaCl and S. epidermidis were applied to the nares [215]. A trial conducted in Switzerland showed that consumption of a probiotic (a fermented milk drink containing Lactobacillus GG, L. acidophilus, Streptococcus thermophilus, and Bifidobacterium sp.) reduced nasal carriage of potentially pathogenic bacteria (S. aureus, Streptococcus pyogenes, β-hemolytic streptococci, and H. influenzae) compared with eating standard yogurt [216].
SUMMARY
Ultimately, the optimal regimen for long-term S. aureus eradication and prevention of recurrent infections remains unclear. Until a more definitive prevention strategy is available, disruption of colonization, targeting multiple anatomic sites with topical antimicrobials, and effective hygiene are the cornerstones of SSTI prevention. At present, the low rate of staphylococcal resistance to commonly prescribed topical agents makes these agents highly effective in temporarily decolonizing the anterior nares and skin. Given the transmission dynamics of S. aureus within households, decolonization of all household members optimizes this approach.
Key Points.
xThe majority of children with a Staphylococcus aureus skin and soft tissue infection will experience a recurrent infection within one year.
S. aureus infections cluster within households, likely due to colonization of family members and household environmental surfaces.
A combined approach of nasal and skin decolonization is often effective in temporarily eradicating staphylococcal colonization and reducing subsequent SSTI.
A household approach to decolonization is more effective in reducing SSTI occurrence than decolonization efforts aimed at the individual patient alone.
Footnotes
Disclosure Statement: This work was supported by grants from the National Institutes of Health (K23-AI091690, KL2-RR024994, and UL1-TR000448), the Agency for Healthcare Research and Quality (R01-HS021736), and the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (to S.A.F.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holt LE, Howland J, Holt LE, McIntosh R. Holt’s diseases of infancy and childhood; a textbook for the use of students and practitioners. 11. New York, London: D. Appleton-Century company; 1940. [Google Scholar]
- 2.Hersh AL, Chambers HF, Maselli JH, Gonzales R. National trends in ambulatory visits and antibiotic prescribing for skin and soft-tissue infections. Arch Intern Med. 2008;168(14):1585–91. doi: 10.1001/archinte.168.14.1585. [DOI] [PubMed] [Google Scholar]
- 3.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751–62. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 4.Faden H, Lesse AJ, Trask J, Hill JA, Hess DJ, Dryja D, et al. Importance of colonization site in the current epidemic of staphylococcal skin abscesses. Pediatr. 2010;125(3):e618–24. doi: 10.1542/peds.2009-1523. [DOI] [PubMed] [Google Scholar]
- 5.Fritz SA, Hogan PG, Hayek G, Eisenstein KA, Rodriguez M, Krauss M, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med. 2012;166(6):551–7. doi: 10.1001/archpediatrics.2011.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mertz D, Frei R, Jaussi B, Tietz A, Stebler C, Fluckiger U, et al. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin Infect Dis. 2007;45(4):475–7. doi: 10.1086/520016. [DOI] [PubMed] [Google Scholar]
- 8.Weems JJ., Jr The many faces of Staphylococcus aureus infection. Recognizing and managing its life-threatening manifestations. Postgrad Med. 2001;110(4):24–6. 29–31, 35–6. doi: 10.3810/pgm.2001.10.1042. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay JA, Holden MT. Staphylococcus aureus: Superbug, super genome? Trends Microbiol. 2004;12(8):378–85. doi: 10.1016/j.tim.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the united states. JAMA. 2007;298(15):1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 11.Barrett FF, McGehee RF, Jr, Finland M. Methicillin-resistant Staphylococcus aureus at Boston city hospital. Bacteriologic and epidemiologic observations. N Engl J Med. 1968;279(9):441–8. doi: 10.1056/NEJM196808292790901. [DOI] [PubMed] [Google Scholar]
- 12.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 13.Boyce JM, Causey WA. Increasing occurrence of methicillin-resistant Staphylococcus aureus in the United States. Infect Control. 1982;3(5):377–83. doi: 10.1017/s0195941700057337. [DOI] [PubMed] [Google Scholar]
- 14.Kline MW, Mason EO., Jr Methicillin-resistant Staphylococcus aureus: Pediatric perspective. Pediatr Clin North Am. 1988;35(3):613–24. doi: 10.1016/s0031-3955(16)36474-4. [DOI] [PubMed] [Google Scholar]
- 15.Mulligan ME, Murray-Leisure KA, Ribner BS, Standiford HC, John JF, Korvick JA, et al. Methicillin-resistant Staphylococcus aureus: A consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am J Med. 1993;94(3):313–28. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- 16.National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 17.Kuehnert MJ, Hill HA, Kupronis BA, Tokars JI, Solomon SL, Jernigan DB. Methicillin-resistant-Staphylococcus aureus hospitalizations, United States. Emerg Infect Dis. 2005;11(6):868–72. doi: 10.3201/eid1106.040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus -- Minnesota and North Dakota, 1997–1999. MMWR Morb Mortal Wkly Rep. 1999;48(32):707–10. [PubMed] [Google Scholar]
- 19.Gopal Rao G, Wong J. Interaction between methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) J Hosp Infect. 2003;55(2):116–8. doi: 10.1016/s0195-6701(03)00287-1. [DOI] [PubMed] [Google Scholar]
- 20.Hulten KG, Kaplan SL, Gonzalez BE, Hammerman WA, Lamberth LB, Versalovic J, et al. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2006;25(4):349–53. doi: 10.1097/01.inf.0000207404.50143.1e. [DOI] [PubMed] [Google Scholar]
- 21.Kluytmans-Vandenbergh MF, Kluytmans JA. Community-acquired methicillin-resistant Staphylococcus aureus: Current perspectives. Clin Microbiol Infect. 2006;12(Suppl 1):9–15. doi: 10.1111/j.1469-0691.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 22.Hanssen AM, Ericson Sollid JU. SCCmec in staphylococci: Genes on the move. FEMS Immunol Med Microbiol. 2006;46(1):8–20. doi: 10.1111/j.1574-695X.2005.00009.x. [DOI] [PubMed] [Google Scholar]
- 23.Tenover FC, McDougal LK, Goering RV, Killgore G, Projan SJ, Patel JB, et al. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44(1):108–18. doi: 10.1128/JCM.44.1.108-118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle-Vavra S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type VT or sccmec type IV. J Clin Microbiol. 2005;43(9):4719–30. doi: 10.1128/JCM.43.9.4719-4730.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katayama Y, Robinson DA, Enright MC, Chambers HF. Genetic background affects stability of mecA in Staphylococcus aureus. J Clin Microbiol. 2005;43(5):2380–3. doi: 10.1128/JCM.43.5.2380-2383.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48(7):2637–51. doi: 10.1128/AAC.48.7.2637-2651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katayama Y, Zhang HZ, Hong D, Chambers HF. Jumping the barrier to beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 2003;185(18):5465–72. doi: 10.1128/JB.185.18.5465-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daum RS, Ito T, Hiramatsu K, Hussain F, Mongkolrattanothai K, Jamklang M, et al. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002;186(9):1344–7. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- 29.Hiramatsu K, Katayama Y, Yuzawa H, Ito T. Molecular genetics of methicillin-resistant Staphylococcus aureus. Int J Med Microbiol. 2002;292(2):67–74. doi: 10.1078/1438-4221-00192. [DOI] [PubMed] [Google Scholar]
- 30.Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148(4):249–57. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 31.Han LL, McDougal LK, Gorwitz RJ, Mayer KH, Patel JB, Sennott JM, et al. High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a boston ambulatory health center. J Clin Microbiol. 2007;45(4):1350–2. doi: 10.1128/JCM.02274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otter JA, French GL. Nosocomial transmission of community-associated methicillin-resistant Staphylococcus aureus: An emerging threat. Lancet Infect Dis. 2006;6(12):753–5. doi: 10.1016/S1473-3099(06)70636-3. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez BE, Rueda AM, Shelburne SA, 3rd, Musher DM, Hamill RJ, Hulten KG. Community-associated strains of methicillin-resistant Staphylococccus aureus as the cause of healthcare-associated infection. Infect Control Hosp Epidemiol. 2006;27(10):1051–6. doi: 10.1086/507923. [DOI] [PubMed] [Google Scholar]
- 34.Hulten KG, Kaplan SL, Lamberth LB, Slimp K, Hammerman WA, Carrillo-Marquez M, et al. Hospital-acquired Staphylococcus aureus infections at Texas Children’s Hospital, 2001–2007. Infect Control Hosp Epidemiol. 2010;31(2):183–90. doi: 10.1086/649793. [DOI] [PubMed] [Google Scholar]
- 35.Diekema DJ, Richter SS, Heilmann KP, Dohrn CL, Riahi F, Tendolkar S, et al. Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: Results from a nationwide surveillance study. Infect Control Hosp Epidemiol. 2014;35(3):285–92. doi: 10.1086/675283. [DOI] [PubMed] [Google Scholar]
- 36.McDougal LK, Fosheim GE, Nicholson A, Bulens SN, Limbago BM, Shearer JE, et al. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob Agents Chemother. 2010;54(9):3804–11. doi: 10.1128/AAC.00351-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29(5):1128–32. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- 38.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367(9512):731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 39.Goering RV, McDougal LK, Fosheim GE, Bonnstetter KK, Wolter DJ, Tenover FC. Epidemiologic distribution of the arginine catabolic mobile element among selected methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates. J Clin Microbiol. 2007;45(6):1981–4. doi: 10.1128/JCM.00273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am. 2009;23(1):17–34. doi: 10.1016/j.idc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loughman JA, Fritz SA, Storch GA, Hunstad DA. Virulence gene expression in human community-acquired Staphylococcus aureus infection. J Infect Dis. 2009;199(3):294–301. doi: 10.1086/595982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290(22):2976–84. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan SL, Hulten KG, Gonzalez BE, Hammerman WA, Lamberth L, Versalovic J, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40(12):1785–91. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez BE, Martinez-Aguilar G, Hulten KG, Hammerman WA, Coss-Bu J, Avalos-Mishaan A, et al. Severe staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115(3):642–8. doi: 10.1542/peds.2004-2300. [DOI] [PubMed] [Google Scholar]
- 45.Fritz SA, Garbutt J, Elward A, Shannon W, Storch GA. Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive Staphylococcus aureus colonization in children seen in a practice-based research network. Pediatr. 2008;121(6):1090–8. doi: 10.1542/peds.2007-2104. [DOI] [PubMed] [Google Scholar]
- 46.Creech CB, 2nd, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J. 2005;24(7):617–21. doi: 10.1097/01.inf.0000168746.62226.a4. [DOI] [PubMed] [Google Scholar]
- 47.Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197(9):1226–34. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 48.McCaskill ML, Mason EO, Jr, Kaplan SL, Hammerman W, Lamberth LB, Hulten KG. Increase of the USA300 clone among community-acquired methicillin-susceptible Staphylococcus aureus causing invasive infections. Pediatr Infect Dis J. 2007;26(12):1122–7. doi: 10.1097/INF.0b013e31814536e0. [DOI] [PubMed] [Google Scholar]
- 49.Orscheln RC, Hunstad DA, Fritz SA, Loughman JA, Mitchell K, Storch EK, et al. Contribution of genetically restricted, methicillin-susceptible strains to the ongoing epidemic of community-acquired Staphylococcus aureus infections. Clin Infect Dis. 2009;49(4):536–42. doi: 10.1086/600881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witt WP, Weiss AJ, Elixhauser A. [Accessed January 29, 2015];Overview of hospital stays for children in the United States. 2012 Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. [PubMed]
- 51.Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: A 14-year study at Driscoll Children’s Hospital. Arch Pediatr Adolesc Med. 2005;159(10):980–5. doi: 10.1001/archpedi.159.10.980. [DOI] [PubMed] [Google Scholar]
- 52.Levison ME, Fung S. Community-associated methicillin-resistant Staphylococcus aureus: Reconsideration of therapeutic options. Curr Infect Dis Rep. 2006;8(1):23–30. doi: 10.1007/s11908-006-0031-7. [DOI] [PubMed] [Google Scholar]
- 53.Stankovic C, Mahajan PV. Healthy children with invasive community-acquired methicillin-resistant Staphylococcus aureus infections. Pediatr Emerg Care. 2006;22(5):361–3. doi: 10.1097/01.pec.0000215652.27137.c7. [DOI] [PubMed] [Google Scholar]
- 54.Creech CB, Johnson BG, Bartilson RE, Yang E, Barr FE. Increasing use of extracorporeal life support in methicillin-resistant Staphylococcus aureus sepsis in children. Pediatr Crit Care Med. 2007;8(3):231–5. doi: 10.1097/01.PCC.0000262801.81331.C7. quiz 47. [DOI] [PubMed] [Google Scholar]
- 55.Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis. 2009;49(1):65–71. doi: 10.1086/599348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eveillard M, de Lassence A, Lancien E, Barnaud G, Ricard JD, Joly-Guillou ML. Evaluation of a strategy of screening multiple anatomical sites for methicillin-resistant Staphylococcus aureus at admission to a teaching hospital. Infect Control Hosp Epidemiol. 2006;27(2):181–4. doi: 10.1086/500627. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson P, Ripa T. Staphylococcus aureus throat colonization is more frequent than colonization in the anterior nares. J Clin Microbiol. 2006;44(9):3334–9. doi: 10.1128/JCM.00880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters PJ, Brooks JT, Limbago B, Lowery HK, McAllister SK, Mindley R, et al. Methicillin-resistant Staphylococcus aureus colonization in HIV-infected outpatients is common and detection is enhanced by groin culture. Epidemiol Infect. 2011;139(7):998–1008. doi: 10.1017/S0950268810002013. [DOI] [PubMed] [Google Scholar]
- 59.Kaplan SL, Forbes A, Hammerman WA, Lamberth L, Hulten KG, Minard CG, et al. Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis. 2014;58(5):679–82. doi: 10.1093/cid/cit764. [DOI] [PubMed] [Google Scholar]
- 60.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344(1):11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 61.Toshkova K, Annemuller C, Akineden O, Lammler C. The significance of nasal carriage of Staphylococcus aureus as risk factor for human skin infections. FEMS Microbiol Lett. 2001;202(1):17–24. doi: 10.1111/j.1574-6968.2001.tb10774.x. [DOI] [PubMed] [Google Scholar]
- 62.Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39(6):776–82. doi: 10.1086/422997. [DOI] [PubMed] [Google Scholar]
- 63.Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis. 2004;39(7):971–9. doi: 10.1086/423965. [DOI] [PubMed] [Google Scholar]
- 64.Fritz SA, Epplin EK, Garbutt J, Storch GA. Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J Infect. 2009;59(6):394–401. doi: 10.1016/j.jinf.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macal CM, North MJ, Collier N, Dukic VM, Wegener DT, David MZ, et al. Modeling the transmission of community-associated methicillin-resistant Staphylococcus aureus: A dynamic agent-based simulation. J Transl Med. 2014;12(124) doi: 10.1186/1479-5876-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: A retrospective population-based study. BMC Infectious Diseases. 2013;13(252) doi: 10.1186/1471-2334-13-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ray GT, Suaya JA, Baxter R. Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2013;76(1):24–30. doi: 10.1016/j.diagmicrobio.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 68.David MZ, Mennella C, Mansour M, Boyle-Vavra S, Daum RS. Predominance of methicillin-resistant Staphylococcus aureus among pathogens causing skin and soft tissue infections in a large urban jail: Risk factors and recurrence rates. J Clin Microbiol. 2008;46(10):3222–7. doi: 10.1128/JCM.01423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright MO, Furuno JP, Venezia RA, Johnson JK, Standiford HC, Hebden JN, et al. Methicillin-resistant Staphylococcus aureus infection and colonization among hospitalized prisoners. Infect Control Hosp Epidemiol. 2007;28(7):877–9. doi: 10.1086/518461. [DOI] [PubMed] [Google Scholar]
- 70.Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections in correctional facilities -- Georgia, California, and Texas, 2001–2003. MMWR Morb Mortal Wkly Rep. 2003;52(41):992–6. [PubMed] [Google Scholar]
- 71.Creech CB, Saye E, McKenna BD, Johnson BG, Jimenez N, Talbot TR, et al. One-year surveillance of methicillin-resistant Staphylococcus aureus nasal colonization and skin and soft tissue infections in collegiate athletes. Arch Pediatr Adolesc Med. 2010;164(7):615–20. doi: 10.1001/archpediatrics.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus infections among competitive sports participants -- Colorado, Indiana, Pennsylvania, and Los Angeles county, 2000–2003. MMWR Morb Mortal Wkly Rep. 2003;52(33):793–5. [PubMed] [Google Scholar]
- 73.Kazakova SV, Hageman JC, Matava M, Srinivasan A, Phelan L, Garfinkel B, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352(5):468–75. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 74.Jimenez-Truque N, Saye EJ, Soper N, Saville BR, Thomsen I, Edwards KM, et al. Longitudinal assessment of colonization with Staphylococcus aureus in healthy collegiate athletes. Journal of the Pediatric Infectious Diseases Society. 2014 doi: 10.1093/jpids/piu108. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nouwen J, Schouten J, Schneebergen P, Snijders S, Maaskant J, Koolen M, et al. Staphylococcus aureus carriage patterns and the risk of infections associated with continuous peritoneal dialysis. J Clin Microbiol. 2006;44(6):2233–6. doi: 10.1128/JCM.02083-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nouwen JL, van Belkum A, Verbrugh HA. Determinants of Staphylococcus aureus nasal carriage. Neth J Med. 2001;59(3):126–33. doi: 10.1016/s0300-2977(01)00150-4. [DOI] [PubMed] [Google Scholar]
- 77.Wertheim HF, Walsh E, Choudhurry R, Melles DC, Boelens HA, Miajlovic H, et al. Key role for clumping factor b in Staphylococcus aureus nasal colonization of humans. PLoS Medicine. 2008;5(1):e17. doi: 10.1371/journal.pmed.0050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205(2):287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maresso AW, Schneewind O. Iron acquisition and transport in Staphylococcus aureus. BioMetals. 2006;19(2):193–203. doi: 10.1007/s10534-005-4863-7. [DOI] [PubMed] [Google Scholar]
- 80.de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199(5):687–95. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chavakis T, Hussain M, Kanse SM, Peters G, Bretzel RG, Flock JI, et al. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat Med. 2002;8(7):687–93. doi: 10.1038/nm728. [DOI] [PubMed] [Google Scholar]
- 82.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, et al. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol. 2011;79(3):814–25. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomita T, Kamio Y. Molecular biology of the pore-forming cytolysins from Staphylococcus aureus, alpha- and gamma-hemolysins and leukocidin. Biosci, Biotechnol, Biochem. 1997;61(4):565–72. doi: 10.1271/bbb.61.565. [DOI] [PubMed] [Google Scholar]
- 84.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13(12):1510–4. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 85.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3(12):948–58. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 86.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202(2):209–15. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clauditz A, Resch A, Wieland KP, Peschel A, Gotz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74(8):4950–3. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cosgrove K, Coutts G, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, et al. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol. 2007;189(3):1025–35. doi: 10.1128/JB.01524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mandell GL. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J Clin Invest. 1975;55(3):561–6. doi: 10.1172/JCI107963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology. 2003;149(Pt 10):2749–58. doi: 10.1099/mic.0.26353-0. [DOI] [PubMed] [Google Scholar]
- 91.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 92.Mistry RD. Skin and soft tissue infections. Pediatr Clin North Am. 2013;60(5):1063–82. doi: 10.1016/j.pcl.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 93.Llera JL, Levy RC. Treatment of cutaneous abscess: A double-blind clinical study. Ann Emerg Med. 1985;14(1):15–9. doi: 10.1016/s0196-0644(85)80727-7. [DOI] [PubMed] [Google Scholar]
- 94.Lee MC, Rios AM, Aten MF, Mejias A, Cavuoti D, McCracken GH, Jr, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23(2):123–7. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 95.Chen AE, Carroll KC, Diener-West M, Ross T, Ordun J, Goldstein MA, et al. Randomized controlled trial of cephalexin versus clindamycin for uncomplicated pediatric skin infections. Pediatr. 2011;127(3):e573–80. doi: 10.1542/peds.2010-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmitz GR, Bruner D, Pitotti R, Olderog C, Livengood T, Williams J, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56(3):283–7. doi: 10.1016/j.annemergmed.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55(5):401–7. doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 98.National Institute of Allergy and Infectious Diseases. Uncomplicated skin and soft tissue infections caused by community-associated methicillin-resistant Staphylococcus aureus ( NCT00730028) [Accessed 2/1/2015]; Available at: https://clinicaltrials.gov/ct2/show/NCT00730028.
- 99.Williams DJ, Cooper WO, Kaltenbach LA, Dudley JA, Kirschke DL, Jones TF, et al. Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatr. 2011;128(3):e479–87. doi: 10.1542/peds.2010-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen AE, Cantey JB, Carroll KC, Ross T, Speser S, Siberry GK. Discordance between Staphylococcus aureus nasal colonization and skin infections in children. Pediatr Infect Dis J. 2009;28(3):244–6. doi: 10.1097/INF.0b013e31818cb0c4. [DOI] [PubMed] [Google Scholar]
- 101.Fritz SA, Camins BC, Eisenstein KA, Fritz JM, Epplin EK, Burnham CA, et al. Effectiveness of measures to eradicate Staphylococcus aureus carriage in patients with community-associated skin and soft-tissue infections: A randomized trial. Infect Control Hosp Epidemiol. 2011;32(9):872–80. doi: 10.1086/661285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller LG, Quan C, Shay A, Mostafaie K, Bharadwa K, Tan N, et al. A prospective investigation of outcomes after hospital discharge for endemic, community-acquired methicillin-resistant and -susceptible Staphylococcus aureus skin infection. Clin Infect Dis. 2007;44(4):483–92. doi: 10.1086/511041. [DOI] [PubMed] [Google Scholar]
- 103.Fritz SA, Hogan PG, Hayek G, Eisenstein KA, Rodriguez M, Epplin EK, et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: A randomized trial. Clin Infect Dis. 2012;54(6):743–51. doi: 10.1093/cid/cir919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bocchini CE, Mason EO, Hulten KG, Hammerman WA, Kaplan SL. Recurrent community-associated Staphylococcus aureus infections in children presenting to Texas Children’s Hospital in Houston, Texas. Pediatr Infect Dis J. 2013;32(11):1189–93. doi: 10.1097/INF.0b013e3182a5c30d. [DOI] [PubMed] [Google Scholar]
- 105.Miller LG, Eells SJ, David MZ, Ortiz N, Taylor AR, Kumar N, et al. Staphylococcus aureus skin infection recurrences among household members: An examination of host, behavioral, and pathogen-level predictors. Clin Infect Dis. 2015;60(5):753–63. doi: 10.1093/cid/ciu943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Uhlemann AC, Dordel J, Knox JR, Raven KE, Parkhill J, Holden MT, et al. Molecular tracing of the emergence, diversification, and transmission of S. aureus sequence type 8 in a New York community. Proc Natl Acad Sci USA. 2014;111(18):6738–43. doi: 10.1073/pnas.1401006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tacconelli E. Antimicrobial use: Risk driver of multidrug resistant microorganisms in healthcare settings. Curr Opin Infect Dis. 2009;22(4):352–8. doi: 10.1097/QCO.0b013e32832d52e0. [DOI] [PubMed] [Google Scholar]
- 108.Frazee BW, Lynn J, Charlebois ED, Lambert L, Lowery D, Perdreau-Remington F. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med. 2005;45(3):311–20. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 109.Miller LG, Diep BA. Clinical practice: Colonization, fomites, and virulence: Rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(5):752–60. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 110.Whitman TJ, Herlihy RK, Schlett CD, Murray PR, Grandits GA, Ganesan A, et al. Chlorhexidine-impregnated cloths to prevent skin and soft-tissue infection in Marine recruits: A cluster-randomized, double-blind, controlled effectiveness trial. Infect Control Hosp Epidemiol. 2010;31(12):1207–15. doi: 10.1086/657136. [DOI] [PubMed] [Google Scholar]
- 111.Cluzet VC, Gerber JS, Nachamkin I, Metlay JP, Zaoutis TE, Davis MF, et al. Duration of colonization and determinants of earlier clearance of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2015 doi: 10.1093/cid/civ075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hogan PG, Rodriguez M, Hunstad DA, Camins BC, Fritz SA. Effect of antibiotics on community-associated methicillin-resistant staphylococcus aureus (MRSA) colonization in patients with uncomplicated MRSA skin abscesses. Annual Meeting of the Infectious Diseases Society of America; Boston, MA. 2011. [Google Scholar]
- 113.Osterlund A, Kahlmeter G, Bieber L, Runehagen A, Breider JM. Intrafamilial spread of highly virulent Staphylococcus aureus strains carrying the gene for Panton-Valentine leukocidin. Scandinavian Journal of Infectious Diseases. 2002;34(10):763–4. doi: 10.1080/00365540260348554. [DOI] [PubMed] [Google Scholar]
- 114.Jones TF, Creech CB, Erwin P, Baird SG, Woron AM, Schaffner W. Family outbreaks of invasive community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;42(9):e76–8. doi: 10.1086/503265. [DOI] [PubMed] [Google Scholar]
- 115.L’Heriteau F, Lucet JC, Scanvic A, Bouvet E. Community-acquired methicillin-resistant Staphylococcus aureus and familial transmission. JAMA. 1999;282(11):1038–9. doi: 10.1001/jama.282.11.1038. [DOI] [PubMed] [Google Scholar]
- 116.Dietrich DW, Auld DB, Mermel LA. Community-acquired methicillin-resistant Staphylococcus aureus in Southern New England children. Pediatr. 2004;113(4):e347–52. doi: 10.1542/peds.113.4.e347. [DOI] [PubMed] [Google Scholar]
- 117.Hollis RJ, Barr JL, Doebbeling BN, Pfaller MA, Wenzel RP. Familial carriage of methicillin-resistant Staphylococcus aureus and subsequent infection in a premature neonate. Clin Infect Dis. 1995;21(2):328–32. doi: 10.1093/clinids/21.2.328. [DOI] [PubMed] [Google Scholar]
- 118.Huijsdens XW, van Santen-Verheuvel MG, Spalburg E, Heck ME, Pluister GN, Eijkelkamp BA, et al. Multiple cases of familial transmission of community-acquired methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2006;44(8):2994–6. doi: 10.1128/JCM.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nerby JM, Gorwitz R, Lesher L, Juni B, Jawahir S, Lynfield R, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2011;30(11):927–32. doi: 10.1097/INF.0b013e31822256c3. [DOI] [PubMed] [Google Scholar]
- 120.Mollema FP, Richardus JH, Behrendt M, Vaessen N, Lodder W, Hendriks W, et al. Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol. 2010;48(1):202–7. doi: 10.1128/JCM.01499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fritz SA, Hogan PG, Singh LN, Thompson RM, Wallace MA, Whitney K, et al. Contamination of environmental surfaces with Staphylococcus aureus in households with children infected with methicillin-resistant S aureus. JAMA Pediatr. 2014;168(11):1030–8. doi: 10.1001/jamapediatrics.2014.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Uhlemann AC, Knox J, Miller M, Hafer C, Vasquez G, Ryan M, et al. The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureus USA300: A case-control study. PLoS ONE. 2011;6(7):e22407. doi: 10.1371/journal.pone.0022407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knox J, Uhlemann AC, Miller M, Hafer C, Vasquez G, Vavagiakis P, et al. Environmental contamination as a risk factor for intra-household Staphylococcus aureus transmission. PLoS ONE. 2012;7(11):e49900. doi: 10.1371/journal.pone.0049900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davis MF, Iverson SA, Baron P, Vasse A, Silbergeld EK, Lautenbach E, et al. Household transmission of meticillin-resistant Staphylococcus aureus and other staphylococci. Lancet Infect Dis. 2012;12(9):703–16. doi: 10.1016/S1473-3099(12)70156-1. [DOI] [PubMed] [Google Scholar]
- 125.Rodriguez M, Hogan PG, Burnham CA, Fritz SA. Molecular epidemiology of Staphylococcus aureus in households of children with community-associated S aureus skin and soft tissue infections. J Pediatr. 2014;164(1):105–11. doi: 10.1016/j.jpeds.2013.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johansson PJ, Gustafsson EB, Ringberg H. High prevalence of MRSA in household contacts. Scand J Infect Dis. 2007;39(9):764–8. doi: 10.1080/00365540701302501. [DOI] [PubMed] [Google Scholar]
- 127.Huang YC, Ho CF, Chen CJ, Su LH, Lin TY. Nasal carriage of methicillin-resistant Staphylococcus aureus in household contacts of children with community-acquired diseases in Taiwan. Pediatr Infect Dis J. 2007;26(11):1066–8. doi: 10.1097/INF.0b013e31813429e8. [DOI] [PubMed] [Google Scholar]
- 128.Eells SJ, David MZ, Taylor A, Ortiz N, Kumar N, Sieth J, et al. Persistent environmental contamination with USA300 methicillin-resistant Staphylococcus aureus and other pathogenic strain types in households with S. aureus skin infections. Infect Control Hosp Epidemiol. 2014;35(11):1373–82. doi: 10.1086/678414. [DOI] [PubMed] [Google Scholar]
- 129.Shinefield HR, Ruff NL. Staphylococcal infections: A historical perspective. Infect Dis Clin North Am. 2009;23(1):1–15. doi: 10.1016/j.idc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 130.Fridkin SK. Methicillin-resistant Staphylococcus aureus in the community setting: The new reality. 2004 Annual Conference on Antimicrobial Resistance; Bethesda, MD. 2004. [Google Scholar]
- 131.Al-Zubeidi D, Burnham CD, Hogan PG, Collins R, Hunstad DA, Fritz SA. Molecular epidemiology of recurrent cutaneous methicillin-resistant Staphylococcus aureus infections in children. Journal of the Pediatric Infectious Diseases Society. 2014;3(3):261–64. doi: 10.1093/jpids/pit046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen CJ, Su LH, Lin TY, Huang YC. Molecular analysis of repeated methicillin-resistant Staphylococcus aureus infections in children. PLoS ONE. 2010;5(12):e14431. doi: 10.1371/journal.pone.0014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laupland KB, Conly JM. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: An evidence-based review. Clin Infect Dis. 2003;37(7):933–8. doi: 10.1086/377735. [DOI] [PubMed] [Google Scholar]
- 134.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346(24):1871–7. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- 135.Sandri AM, Dalarosa MG, Ruschel de Alcantara L, da Silva Elias L, Zavascki AP. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: Role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol. 2006;27(2):185–7. doi: 10.1086/500625. [DOI] [PubMed] [Google Scholar]
- 136.Tacconelli E, Carmeli Y, Aizer A, Ferreira G, Foreman MG, D’Agata EM. Mupirocin prophylaxis to prevent Staphylococcus aureus infection in patients undergoing dialysis: A meta-analysis. Clin Infect Dis. 2003;37(12):1629–38. doi: 10.1086/379715. [DOI] [PubMed] [Google Scholar]
- 137.Climo MW, Sepkowitz KA, Zuccotti G, Fraser VJ, Warren DK, Perl TM, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococcus, and healthcare-associated bloodstream infections: Results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37(6):1858–65. doi: 10.1097/CCM.0b013e31819ffe6d. [DOI] [PubMed] [Google Scholar]
- 138.Milstone AM, Elward A, Song X, Zerr DM, Orscheln R, Speck K, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: A multicentre, cluster-randomised, crossover trial. Lancet. 2013;381(9872):1099–106. doi: 10.1016/S0140-6736(12)61687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Calfee DP, Salgado CD, Milstone AM, Harris AD, Kuhar DT, Moody J, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(Suppl 2):S108–32. doi: 10.1017/s0899823x00193882. [DOI] [PubMed] [Google Scholar]
- 140.Huang SS, Septimus E, Kleinman K, Moody J, Hickok J, Avery TR, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–65. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Noto MJ, Domenico HJ, Byrne DW, Talbot T, Rice TW, Bernard GR, et al. Chlorhexidine bathing and health care-associated infections: A randomized clinical trial. JAMA. 2015;313(4):369–78. doi: 10.1001/jama.2014.18400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Rijen M, Bonten M, Wenzel R, Kluytmans J. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev. 2008;(4):CD006216. doi: 10.1002/14651858.CD006216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Loeb M, Main C, Walker-Dilks C, Eady A. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340. doi: 10.1002/14651858.CD003340. [DOI] [PubMed]
- 144.Wendt C, Schinke S, Wurttemberger M, Oberdorfer K, Bock-Hensley O, von Baum H. Value of whole-body washing with chlorhexidine for the eradication of methicillin-resistant Staphylococcus aureus: A randomized, placebo-controlled, double-blind clinical trial. Infect Control Hosp Epidemiol. 2007;28(9):1036–43. doi: 10.1086/519929. [DOI] [PubMed] [Google Scholar]
- 145.Creech CB, Beekmann SE, Chen Y, Polgreen PM. Variability among pediatric infectious diseases specialists in the treatment and prevention of methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27(3):270–2. doi: 10.1097/INF.0b013e31815c9068. [DOI] [PubMed] [Google Scholar]
- 146.Kaplan SL. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Semin Pediatr Infect Dis. 2006;17(3):113–9. doi: 10.1053/j.spid.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 147.Ellis MW, Griffith ME, Dooley DP, McLean JC, Jorgensen JH, Patterson JE, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: A cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51(10):3591–8. doi: 10.1128/AAC.01086-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Climo MW, Yokoe DS, Warren DK, Perl TM, Bolon M, Herwaldt LA, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–42. doi: 10.1056/NEJMoa1113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Doebbeling BN, Reagan DR, Pfaller MA, Houston AK, Hollis RJ, Wenzel RP. Long-term efficacy of intranasal mupirocin ointment. A prospective cohort study of Staphylococcus aureus carriage. Arch Intern Med. 1994;154(13):1505–8. [PubMed] [Google Scholar]
- 150.Mascitti KB, Gerber JS, Zaoutis TE, Barton TD, Lautenbach E. Preferred treatment and prevention strategies for recurrent community-associated methicillin-resistant Staphylococcus aureus skin and soft-tissue infections: A survey of adult and pediatric providers. Am J Infect Control. 2010;38(4):324–8. doi: 10.1016/j.ajic.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miller LG, Eells SJ, David MZ, Ortiz N, Taylor AR, Kumar N, et al. Staphylococcus aureus skin infection recurrences among household members: An examination of host, behavioral, and pathogen-level predictors. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Centers for Disease Control and Prevention. [Accessed 2/1/2015];Methicillin-resistant staphylcococcus aureus (MRSA) infections. Available at: http://www.cdc.gov/mrsa/community/index.html.
- 153.Hardy KJ, Oppenheim BA, Gossain S, Gao F, Hawkey PM. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol. 2006;27(2):127–32. doi: 10.1086/500622. [DOI] [PubMed] [Google Scholar]
- 154.Milstone AM. Uncovering reservoirs of methicillin-resistant Staphylococcus aureus: Children contaminating households or households contaminating children? JAMA Pediatr. 2014;168(11):994–5. doi: 10.1001/jamapediatrics.2014.1260. [DOI] [PubMed] [Google Scholar]
- 155.Weber DJ, Rutala WA. Understanding and preventing transmission of healthcare-associated pathogens due to the contaminated hospital environment. Infect Control Hosp Epidemiol. 2013;34(5):449–52. doi: 10.1086/670223. [DOI] [PubMed] [Google Scholar]
- 156.Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65(Suppl 2):50–4. doi: 10.1016/S0195-6701(07)60015-2. [DOI] [PubMed] [Google Scholar]
- 157.McConeghy KW, Mikolich DJ, LaPlante KL. Agents for the decolonization of methicillin-resistant Staphylococcus aureus. Pharmacotherapy. 2009;29(3):263–80. doi: 10.1592/phco.29.3.263. [DOI] [PubMed] [Google Scholar]
- 158.Thomas CM, Hothersall J, Willis CL, Simpson TJ. Resistance to and synthesis of the antibiotic mupirocin. Nat Rev Microbiol. 2010;8(4):281–9. doi: 10.1038/nrmicro2278. [DOI] [PubMed] [Google Scholar]
- 159.Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clin Infect Dis. 2009;49(6):935–41. doi: 10.1086/605495. [DOI] [PubMed] [Google Scholar]
- 160.Hetem DJ, Bonten MJ. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect. 2013;85(4):249–56. doi: 10.1016/j.jhin.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 161.Morton TM, Johnston JL, Patterson J, Archer GL. Characterization of a conjugative staphylococcal mupirocin resistance plasmid. Antimicrob Agents Chemother. 1995;39(6):1272–80. doi: 10.1128/aac.39.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jones RN, Fritsche TR, Sader HS, Ross JE. Activity of retapamulin (sb-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrob Agents Chemother. 2006;50(7):2583–6. doi: 10.1128/AAC.01432-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rittenhouse S, Biswas S, Broskey J, McCloskey L, Moore T, Vasey S, et al. Selection of retapamulin, a novel pleuromutilin for topical use. Antimicrob Agents Chemother. 2006;50(11):3882–5. doi: 10.1128/AAC.00178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Champney WS, Rodgers WK. Retapamulin inhibition of translation and 50S ribosomal subunit formation in Staphylococcus aureus cells. Antimicrob Agents Chemother. 2007;51(9):3385–7. doi: 10.1128/AAC.00475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yan K, Madden L, Choudhry AE, Voigt CS, Copeland RA, Gontarek RR. Biochemical characterization of the interactions of the novel pleuromutilin derivative retapamulin with bacterial ribosomes. Antimicrob Agents Chemother. 2006;50(11):3875–81. doi: 10.1128/AAC.00184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Saravolatz LD, Pawlak J, Saravolatz SN, Johnson LB. In vitro activity of retapamulin against Staphylococcus aureus resistant to various antimicrobial agents. Antimicrob Agents Chemother. 2013 doi: 10.1128/AAC.00282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Naderer OJ, Anderson M, Roberts K, Lou Y, Zhu J, Min S, et al. Nasal decolonization of persistent Staphylococcus aureus (SA) carriers with twice daily application of retapamulin ointment, 1%, (Ret) for 3 or 5 days. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy and the Infectious Disease Society of Amercia 46th Annual Meeting; Washington, DC. 2008. [Google Scholar]
- 168.University of California, Irvine. [Accessed 2/2/2015];Retapamulin for reducing MRSA nasal carriage ( NCT01461668) Available at: https://clinicaltrials.gov/ct2/show/NCT01461668.
- 169.McDonnell G, Russell AD. Antiseptics and disinfectants: Activity, action, and resistance. Clin Microbiol Rev. 1999;12(1):147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Longtin J, Seah C, Siebert K, McGeer A, Simor A, Longtin Y, et al. Distribution of antiseptic resistance genes qacA, qacB, and smr in methicillin-resistant Staphylococcus aureus isolated in Toronto, Canada, from 2005 to 2009. Antimicrob Agents Chemother. 2011;55(6):2999–3001. doi: 10.1128/AAC.01707-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Edmiston CE, Jr, Seabrook GR, Johnson CP, Paulson DS, Beausoleil CM. Comparative of a new and innovative 2% chlorhexidine gluconate-impregnated cloth with 4% chlorhexidine gluconate as topical antiseptic for preparation of the skin prior to surgery. Am J Infect Control. 2007;35(2):89–96. doi: 10.1016/j.ajic.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 172.Milstone AM, Passaretti CL, Perl TM. Chlorhexidine: Expanding the armamentarium for infection control and prevention. Clin Infect Dis. 2008;46(2):274–81. doi: 10.1086/524736. [DOI] [PubMed] [Google Scholar]
- 173.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123(5):e808–14. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 174.Heggers JP, Sazy JA, Stenberg BD, Strock LL, McCauley RL, Herndon DN, et al. Bactericidal and wound-healing properties of sodium hypochlorite solutions: The 1991 lindberg award. J Burn Care Rehabil. 1991;12(5):420–4. doi: 10.1097/00004630-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 175.Fisher RG, Chain RL, Hair PS, Cunnion KM. Hypochlorite killing of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2008;27(10):934–5. doi: 10.1097/INF.0b013e318175d871. [DOI] [PubMed] [Google Scholar]
- 176.Paller AS, Mancini AJ. Hurwitz clinical pediatric dermatology. 3. Philadelphia, PA: Elsevier; 2006. [Google Scholar]
- 177.Ryan C, Shaw RE, Cockerell CJ, Hand S, Ghali FE. Novel sodium hypochlorite cleanser shows clinical response and excellent acceptability in the treatment of atopic dermatitis. Pediatr Dermatol. 2013;30(3):308–15. doi: 10.1111/pde.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Falagas ME, Bliziotis IA, Fragoulis KN. Oral rifampin for eradication of Staphylococcus aureus carriage from healthy and sick populations: A systematic review of the evidence from comparative trials. Am J Infect Control. 2007;35(2):106–14. doi: 10.1016/j.ajic.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 179.Buehlmann M, Frei R, Fenner L, Dangel M, Fluckiger U, Widmer AF. Highly effective regimen for decolonization of methicillin-resistant Staphylococcus aureus carriers. Infect Control Hosp Epidemiol. 2008;29(6):510–6. doi: 10.1086/588201. [DOI] [PubMed] [Google Scholar]
- 180.Ellis MW, Schlett CD, Millar EV, Wilkins KJ, Crawford KB, Morrison-Rodriguez SM, et al. Hygiene strategies to prevent methicillin-resistant Staphylococcus aureus skin and soft tissue infections: A cluster-randomized controlled trial among high-risk military trainees. Clin Infect Dis. 2014;58(11):1540–8. doi: 10.1093/cid/ciu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Miller LG, Tan J, Eells SJ, Benitez E, Radner AB. Prospective investigation of nasal mupirocin, hexachlorophene body wash, and systemic antibiotics for prevention of recurrent community-associated methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 2012;56(2):1084–6. doi: 10.1128/AAC.01608-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Raz R, Miron D, Colodner R, Staler Z, Samara Z, Keness Y. A 1-year trial of nasal mupirocin in the prevention of recurrent staphylococcal nasal colonization and skin infection. Arch Intern Med. 1996;156(10):1109–12. [PubMed] [Google Scholar]
- 183.McNeil JC, Hulten KG, Kaplan SL, Mason EO. Decreased susceptibilities to retapamulin, mupirocin, and chlorhexidine among Staphylococcus aureus isolates causing skin and soft tissue infections in otherwise healthy children. Antimicrob Agents Chemother. 2014;58(5):2878–83. doi: 10.1128/AAC.02707-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Johnson JG, Saye EJ, Jimenez-Truque N, Soper N, Thomsen I, Talbot TR, et al. Frequency of disinfectant resistance genes in pediatric strains of methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 2013;34(12):1326–7. doi: 10.1086/673983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McNeil JC, Hulten KG, Kaplan SL, Mahoney DH, Mason EO. Staphylococcus aureus infections in pediatric oncology patients: High rates of antimicrobial resistance, antiseptic tolerance and complications. Pediatr Infect Dis J. 2013;32(2):124–8. doi: 10.1097/INF.0b013e318271c4e0. [DOI] [PubMed] [Google Scholar]
- 186.McNeil JC, Ligon JA, Hulten KG, Dreyer WJ, Heinle JS, Mason EO, et al. Staphylococcus aureus infections in children with congenital heart disease. Journal of the Pediatric Infectious Diseases Society. 2013;2(4):337–44. doi: 10.1093/jpids/pit037. [DOI] [PubMed] [Google Scholar]
- 187.Robicsek A, Beaumont JL, Thomson RB, Jr, Govindarajan G, Peterson LR. Topical therapy for methicillin-resistant Staphylococcus aureus colonization: Impact on infection risk. Infect Control Hosp Epidemiol. 2009;30(7):623–32. doi: 10.1086/597550. [DOI] [PubMed] [Google Scholar]
- 188.Lee AS, Macedo-Vinas M, Francois P, Renzi G, Schrenzel J, Vernaz N, et al. Impact of combined low-level mupirocin and genotypic chlorhexidine resistance on persistent methicillin-resistant Staphylococcus aureus carriage after decolonization therapy: A case-control study. Clin Infect Dis. 2011;52(12):1422–30. doi: 10.1093/cid/cir233. [DOI] [PubMed] [Google Scholar]
- 189.Simor AE, Phillips E, McGeer A, Konvalinka A, Loeb M, Devlin HR, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44(2):178–85. doi: 10.1086/510392. [DOI] [PubMed] [Google Scholar]
- 190.Fritz SA, Hogan PG, Camins BC, Ainsworth AJ, Patrick C, Martin MS, et al. Mupirocin and chlorhexidine resistance in Staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother. 2013;57(1):559–68. doi: 10.1128/AAC.01633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Upton A, Lang S, Heffernan H. Mupirocin and Staphylococcus aureus: A recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother. 2003;51(3):613–7. doi: 10.1093/jac/dkg127. [DOI] [PubMed] [Google Scholar]
- 192.Conly JM, Johnston BL. Mupirocin - are we in danger of losing it? Can J Infect Dis. 2002;13(3):157–9. doi: 10.1155/2002/692581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Miller MA, Dascal A, Portnoy J, Mendelson J. Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect Control Hosp Epidemiol. 1996;17(12):811–3. doi: 10.1086/647242. [DOI] [PubMed] [Google Scholar]
- 194.Vasquez JE, Walker ES, Franzus BW, Overbay BK, Reagan DR, Sarubbi FA. The epidemiology of mupirocin resistance among methicillin-resistant Staphylococcus aureus at a veterans’ affairs hospital. Infect Control Hosp Epidemiol. 2000;21(7):459–64. doi: 10.1086/501788. [DOI] [PubMed] [Google Scholar]
- 195.Batra R, Cooper BS, Whiteley C, Patel AK, Wyncoll D, Edgeworth JD. Efficacy and limitation of a chlorhexidine-based decolonization strategy in preventing transmission of methicillin-resistant Staphylococcus aureus in an intensive care unit. Clin Infect Dis. 2010;50(2):210–7. doi: 10.1086/648717. [DOI] [PubMed] [Google Scholar]
- 196.Mayer S, Boos M, Beyer A, Fluit AC, Schmitz FJ. Distribution of the antiseptic resistance genes qaca, qacb and qacc in 497 methicillin-resistant and -susceptible european isolates of Staphylococcus aureus. J Antimicrob Chemother. 2001;47(6):896–7. doi: 10.1093/jac/47.6.896. [DOI] [PubMed] [Google Scholar]
- 197.McNeil JC, Hulten KG, Kaplan SL, Mason EO. Mupirocin resistance in Staphylococcus aureus causing recurrent skin and soft tissue infections in children. Antimicrob Agents Chemother. 2011;55(5):2431–3. doi: 10.1128/AAC.01587-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cadilla A, David MZ, Daum RS, Boyle-Vavra S. Association of high-level mupirocin resistance and multidrug-resistant methicillin-resistant Staphylococcus aureus at an academic center in the midwestern United States. J Clin Microbiol. 2011;49(1):95–100. doi: 10.1128/JCM.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sheng WH, Wang JT, Lauderdale TL, Weng CM, Chen D, Chang SC. Epidemiology and susceptibilities of methicillin-resistant Staphylococcus aureus in Taiwan: Emphasis on chlorhexidine susceptibility. Diagn Microbiol Infect Dis. 2009;63(3):309–13. doi: 10.1016/j.diagmicrobio.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 200. [Accessed 2/20/2015];Nabi biopharmaceuticals announces results of staphvax(r) confirmatory phase iii clinical trial. Available at: http://www.prnewswire.com/news-releases/nabi-biopharmaceuticals-announces-results-of-staphvaxr-confirmatory-phase-iii-clinical-trial-55039197.html.
- 201.Fowler VG, Allen KB, Moreira ED, Moustafa M, Isgro F, Boucher HW, et al. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: A randomized trial. JAMA. 2013;309(13):1368–78. doi: 10.1001/jama.2013.3010. [DOI] [PubMed] [Google Scholar]
- 202.Sampedro GR, DeDent AC, Becker RE, Berube BJ, Gebhardt MJ, Cao H, et al. Targeting Staphylococcus aureus alpha-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J Infect Dis. 2014;210(7):1012–8. doi: 10.1093/infdis/jiu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cogen AL, Nizet V, Gallo RL. Skin microbiota: A source of disease or defence? Br J Dermatol. 2008;158(3):442–55. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Reid G, Howard J, Gan BS. Can bacterial interference prevent infection? Trends Microbiol. 2001;9(9):424–8. doi: 10.1016/s0966-842x(01)02132-1. [DOI] [PubMed] [Google Scholar]
- 205.Shinefield HR, Ribble JC, Boris M, Eichenwald HF. Bacterial interference: Its effect on nursery-acquired infection with Staphylococcus aureus. I. Preliminary observations on artificial colonzation of newborns. Amer J Dis Child. 1963;105:646–54. [PubMed] [Google Scholar]
- 206.Shinefield HR, Boris M, Ribble JC, Cale EF, Eichenwald HF. Bacterial interference: Its effect on nursery-acquired infection with Staphylococcus aureus. III. The Georgia epidemic. Amer J Dis Child. 1963;105:663–73. [PubMed] [Google Scholar]
- 207.Shinefield HR, Sutherland JM, Ribble JC, Eichenwald HF. Bacterial interference: Its effect on nursery-acquired infection with Staphylococcus aureus. II.The Ohio epidemic. Amer J Dis Child. 1963;105:655–62. [PubMed] [Google Scholar]
- 208.Shinefield HR, Ribble JC, Eichenwald HF, Boris M, Sutherland JM. Bacterial interference: Its effect on nursery-acquired infection with Staphylococcus aureus. V. An analysis and interpretation. Amer J Dis Child. 1963;105:683–8. [PubMed] [Google Scholar]
- 209.Boris M, Shinefield HR, Ribble JC, Eichenwald HF, Hauser GH, Caraway CT. Bacterial interference: It’s effect on nursery-acquired infection with Staphylococcus aureus: IV. The Louisiana epidemic. Amer J Dis Child. 1963;105:174–82. [PubMed] [Google Scholar]
- 210.Light IJ, Sutherland JM, Schott JE. Control of a staphylococcal outbreak in a nursery, use of bacterial interference. JAMA. 1965;193:699–704. doi: 10.1001/jama.1965.03090090005001. [DOI] [PubMed] [Google Scholar]
- 211.Drutz DJ, Van Way MH, Schaffner W, Koenig MG. Bacterial interference in the therapy of recurrent staphylococcal infections. Multiple abscesses due to the implantation of the 502A strain of Staphylococcus. N Engl J Med. 1966;275(21):1161–5. doi: 10.1056/NEJM196611242752104. [DOI] [PubMed] [Google Scholar]
- 212.Blair EB, Tull AH. Multiple infections among newborns resulting from colonization with Staphylococcus aureus 502A. Am J Clin Pathol. 1969;52(1):42–9. doi: 10.1093/ajcp/52.1.42. [DOI] [PubMed] [Google Scholar]
- 213.Houck PW, Nelson JD, Kay JL. Fatal septicemia due to Staphylococcus aureus 502A. Report of a case and review of the infectious complications of bacterial interference programs. Amer J Dis Child. 1972;123(1):45–8. [PubMed] [Google Scholar]
- 214.Johnson RC, Ellis MW, Lanier JB, Schlett CD, Cui T, Merrell DS. Correlation between nasal microbiome composition and remote purulent skin and soft tissue infections. Infect Immun. 2015;83(2):802–11. doi: 10.1128/IAI.02664-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Uehara Y, Nakama H, Agematsu K, Uchida M, Kawakami Y, Abdul Fattah AS, et al. Bacterial interference among nasal inhabitants: Eradication of Staphylococcus aureus from nasal cavities by artificial implantation of Corynebacterium sp. J Hosp Infect. 2000;44(2):127–33. doi: 10.1053/jhin.1999.0680. [DOI] [PubMed] [Google Scholar]
- 216.Gluck U, Gebbers JO. Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci) Am J Clin Nutr. 2003;77(2):517–20. doi: 10.1093/ajcn/77.2.517. [DOI] [PubMed] [Google Scholar]