Abstract
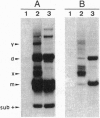
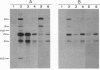
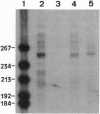
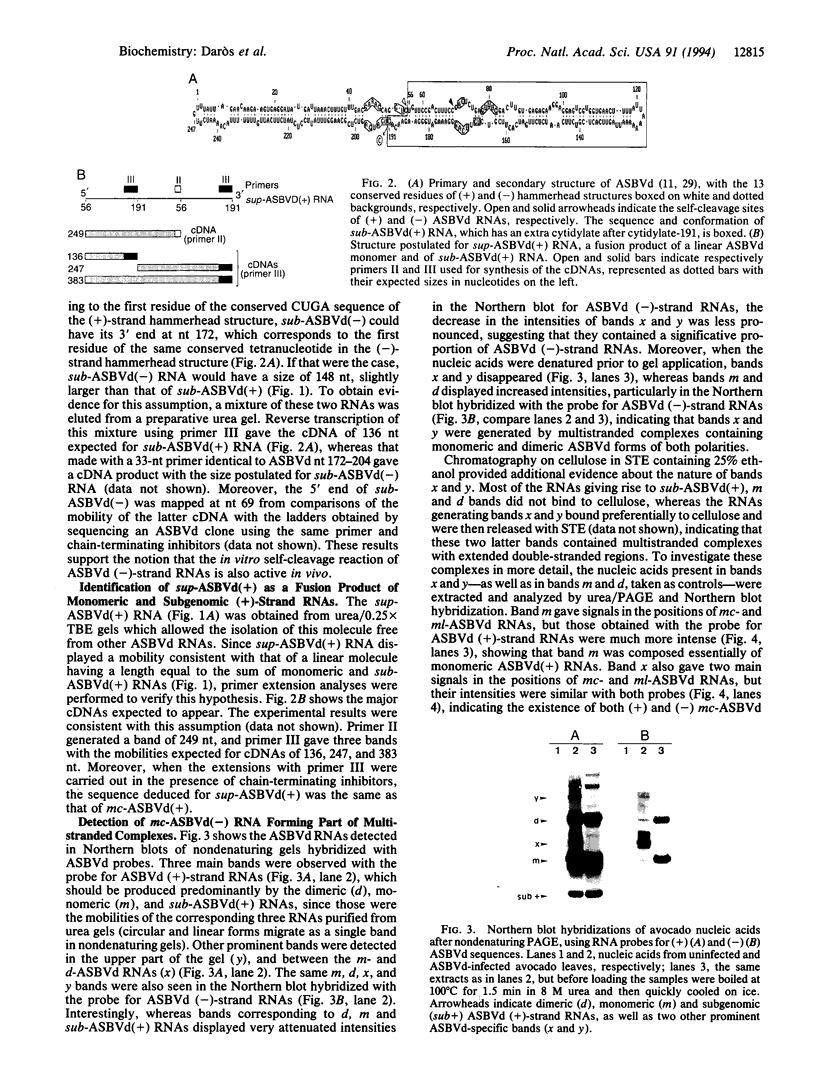
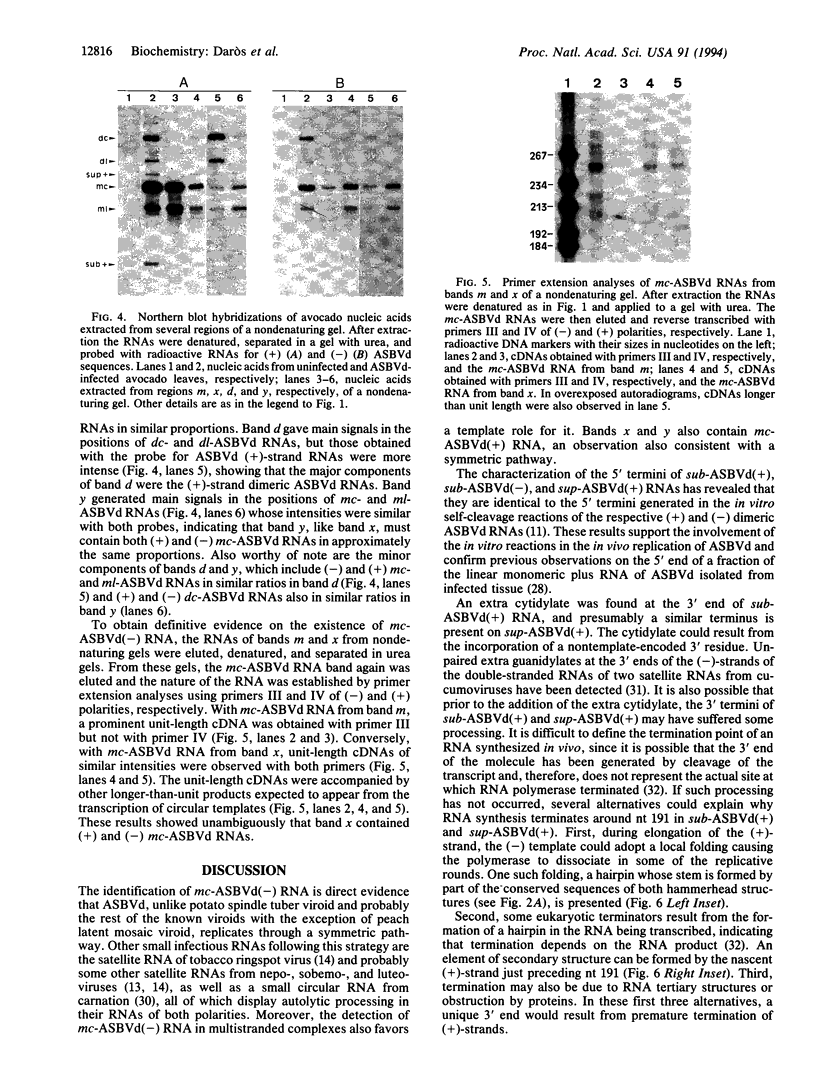
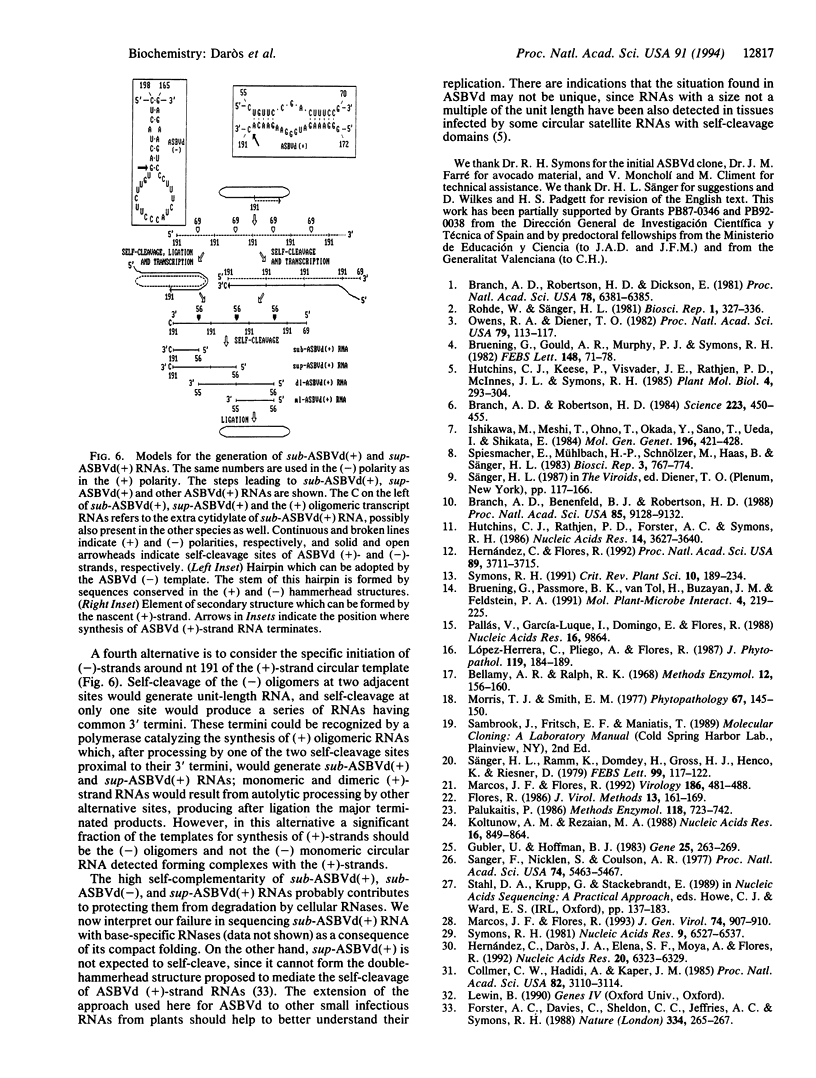
The structure of a series of RNAs extracted from avocado infected by the 247-nt avocado sunblotch viroid (ASBVd) was investigated. The identification of multistranded complexes containing circular ASBVd RNAs of (+) and (-) polarity suggests that replication of ASBVd proceeds through a symmetric pathway with two rolling circles where these two circular RNAs are the templates. This is in contrast to the replication of potato spindle tuber viroid and probably of most of its related viroids, which proceeds via an asymmetric pathway where circular (+)-strand and linear multimeric (-)-strand RNAs are the two templates. Linear (+) and (-) ASBVd RNAs of subgenomic length (137 nt and about 148 nt, respectively) and one linear (+)-strand ASBVd RNA of supragenomic length (383-384 nt) were also found in viroid-infected tissue. The two linear (+)-strand RNAs have the same 5'- and 3'-terminal sequences, with the supragenomic species being a fusion product of the monomeric and subgenomic (+)-strand ASBVd RNAs. The 3' termini of these two (+)-strand molecules, which at least in the subgenomic RNA has an extra nontemplate cytidylate residue, could represent sites of either premature termination of the (+)-strands or specific initiation of the (-)-strands. The 5' termini of sub- and supragenomic (+)-strand and the 5' terminus of the subgenomic (-)-strand ASBVd RNA are identical to those produced in the in vitro self-cleavage reactions of (+) and (-) dimeric ASBVd RNAs, respectively. These observations strongly suggest that the hammerhead structures which mediate the in vitro self-cleavage reactions are also operative in vivo.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Benenfeld B. J., Robertson H. D. Evidence for a single rolling circle in the replication of potato spindle tuber viroid. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9128–9132. doi: 10.1073/pnas.85.23.9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D., Dickson E. Longer-than-unit-length viroid minus strands are present in RNA from infected plants. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6381–6385. doi: 10.1073/pnas.78.10.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening G., Passmore B. K., van Tol H., Buzayan J. M., Feldstein P. A. Replication of a plant virus satellite RNA: evidence favors transcription of circular templates of both polarities. Mol Plant Microbe Interact. 1991 May-Jun;4(3):219–225. doi: 10.1094/mpmi-4-219. [DOI] [PubMed] [Google Scholar]
- Collmer C. W., Hadidi A., Kaper J. M. Nucleotide sequence of the satellite of peanut stunt virus reveals structural homologies with viroids and certain nuclear and mitochondrial introns. Proc Natl Acad Sci U S A. 1985 May;82(10):3110–3114. doi: 10.1073/pnas.82.10.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores R. Detection of citrus exocortis viroid in crude extracts by dot-blot hybridization: conditions for reducing spurious hybridization results and for enhancing the sensitivity of the technique. J Virol Methods. 1986 May;13(2):161–169. doi: 10.1016/0166-0934(86)90084-4. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Davies C., Sheldon C. C., Jeffries A. C., Symons R. H. Self-cleaving viroid and newt RNAs may only be active as dimers. Nature. 1988 Jul 21;334(6179):265–267. doi: 10.1038/334265a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hernández C., Daròs J. A., Elena S. F., Moya A., Flores R. The strands of both polarities of a small circular RNA from carnation self-cleave in vitro through alternative double- and single-hammerhead structures. Nucleic Acids Res. 1992 Dec 11;20(23):6323–6329. doi: 10.1093/nar/20.23.6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández C., Flores R. Plus and minus RNAs of peach latent mosaic viroid self-cleave in vitro via hammerhead structures. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3711–3715. doi: 10.1073/pnas.89.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. A revised replication cycle for viroids: the role of longer than unit length RNA in viroid replication. Mol Gen Genet. 1984;196(3):421–428. doi: 10.1007/BF00436189. [DOI] [PubMed] [Google Scholar]
- Koltunow A. M., Rezaian M. A. Grapevine yellow speckle viroid: structural features of a new viroid group. Nucleic Acids Res. 1988 Feb 11;16(3):849–864. doi: 10.1093/nar/16.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos J. F., Flores R. Characterization of RNAs specific to avocado sunblotch viroid synthesized in vitro by a cell-free system from infected avocado leaves. Virology. 1992 Feb;186(2):481–488. doi: 10.1016/0042-6822(92)90013-f. [DOI] [PubMed] [Google Scholar]
- Marcos J. F., Flores R. The 5' end generated in the in vitro self-cleavage reaction of avocado sunblotch viroid RNAs is present in naturally occurring linear viroid molecules. J Gen Virol. 1993 May;74(Pt 5):907–910. doi: 10.1099/0022-1317-74-5-907. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. RNA intermediates in potato spindle tuber viroid replication. Proc Natl Acad Sci U S A. 1982 Jan;79(1):113–117. doi: 10.1073/pnas.79.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallás V., García-Luque I., Domingo E., Flores R. Sequence variability in avocado sunblotch viroid (ASBV). Nucleic Acids Res. 1988 Oct 25;16(20):9864–9864. doi: 10.1093/nar/16.20.9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde W., Sänger H. L. Detection of complementary RNA intermediates of viroid replication by Northern blot hybridization. Biosci Rep. 1981 Apr;1(4):327–336. doi: 10.1007/BF01114872. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiesmacher E., Mühlbach H. P., Schnölzer M., Haas B., Sänger H. L. Oligomeric forms of potato spindle tuber viroid (PSTV) and of its complementary RNA are present in nuclei isolated from viroid-infected potato cells. Biosci Rep. 1983 Aug;3(8):767–774. doi: 10.1007/BF01120988. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]