Abstract
Objective:
To investigate the independent association of serum N-terminal fragment of the prohormone natriuretic peptide (NT-proBNP) with structural and functional features of abnormal brain aging in older individuals.
Methods:
In this cross-sectional study based on the Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study, we included 4,029 older community-dwelling individuals (born 1907 to 1935) with a measured serum level of NT-proBNP. Outcomes included parenchymal brain volumes estimated from brain MRI, cognitive function measured by tests of memory, processing speed, and executive functioning, and presence of depressive symptoms measured using the Geriatric Depression Scale. In a substudy, cardiac output of 857 participants was assessed using cardiac MRI.
Results:
In multivariate analyses, adjusted for sociodemographic and cardiovascular factors, higher levels of NT-proBNP were independently associated with lower total (p < 0.001), gray matter (p < 0.001), and white matter (p = 0.001) brain volumes. Likewise, in multivariate analyses, higher levels of NT-proBNP were associated with worse scores in memory (p = 0.005), processing speed (p = 0.001), executive functioning (p < 0.001), and more depressive symptoms (p = 0.002). In the substudy, the associations of higher NT-proBNP with lower brain parenchymal volumes, impaired executive function and processing speed, and higher depressive symptoms were independent of the level of cardiac output.
Conclusions:
Higher serum levels of NT-proBNP, independent of cardiovascular risk factors and a measure of cardiac function, are linked with alterations in brain structure and function. Roles of natriuretic peptides in the process of brain aging need to be further elucidated.
Brain natriuretic peptide (BNP) was first described in 1988 after isolation from porcine brain tissue.1 It was soon found that ventricular myocardium produces BNP, and thereafter, BNP has been regarded as a cardiac hormone.2 Upon release, BNP pro-hormone cleaves into the biologically active BNP hormone and the biologically inactive N-terminal fragment (NT-proBNP).3 The main stimulus for BNP and NT-proBNP synthesis is myocardial wall stress, and currently these biomarkers are widely used for diagnosis and prognosis of heart failure.4 Several reports showed that elevated levels of NT-proBNP not only predict higher risk of ischemic heart disease and cardiac arrhythmias but also cerebrovascular events.5–7 Recent evidence indicates that high NT-proBNP also associates with subclinical brain pathologies such as white matter hyperintensties.8
The structural and functional integrity of the brain is dependent on an adequate supply of cerebral blood flow.9 The heart provides a driving force for cerebral perfusion and acts in concert with the vessels in the heart-brain axis to ensure sufficient cerebral blood flow.10 Hence, impaired cardiac function might be a risk factor for accelerating development of pathologies in the brain.11 Recently, a limited number of studies investigated whether high NT-proBNP, as a marker for impaired cardiac function, is associated with impaired cognition.12,13 Most extant studies investigating this association are based on patient populations and no population-based cohort study of older individuals comprehensively evaluated the association of NT-proBNP with structural and functional features of abnormal brain aging. Furthermore, data are lacking on whether serum NT-proBNP is linked to the brain structural and functional characteristics, independent of cardiovascular risk factors and cardiac function. We aim to investigate the independent associations of serum NT-proBNP with structural and functional features of abnormal brain aging in older persons.
METHODS
Study population.
This analysis was performed in the framework of the first wave of the previously described population-based Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study (n = 5,764, 2002–2006). Details of the inclusion procedure for the AGES–Reykjavik Study have been reported previously.14 In the first wave of the AGES–Reykjavik Study, 12 participants had no data on NT-proBNP. From the remaining participants, 1,145 did not have complete data on brain MRI, 389 did not have complete data on cognitive function, and 189 did not have data on depressive symptoms. After exclusion of those with missing data on NT-proBNP or brain measures, there were 4,029 participants (figure e-1 on the Neurology® Web site at Neurology.org). Participants included in this analysis were younger and had lower burden of cardiovascular risk factors and diseases compared with the excluded participants. In this cross-sectional study, serum samples for the NT-proBNP assays, brain imaging, cognitive function, and depressive symptoms were obtained within a 3-week window.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the National Bioethics Committee in Iceland, which acts as the institutional review board for the Icelandic Heart Association, and by the Institutional Review Board National Institute on Aging. All participants gave informed consent before inclusion in the study.
Serum NT-proBNP.
The laboratory of the Icelandic Heart Association measured serum NT-proBNP using the Elecsys proBNPII sandwich immunoassay and 2 monoclonal antibodies on a Cobas e411 instruments (Roche Diagnostics, Basel, Switzerland). The analyses were performed using a single lot of reagents. The manufacturer's controls were used to monitor quality control with limits of acceptability defined by the manufacturer. The low control coefficient of variation was 2.7% and high control coefficient of variation was 3.2%. The limit of sensitivity was 5 pg/mL.
Brain imaging.
All participants underwent a high-resolution brain MRI scanning acquired on a study-dedicated 1.5T Signa TwinSpeed system (GE Healthcare, Waukesha, WI). The imaging protocol has been described previously.14,15 All images were acquired to give full brain coverage with slices angled parallel to the anterior commissure–posterior commissure line in order to give reproducible image views in the oblique-axial plane. Total brain, white, and gray matter volumes were computed automatically with a previously described algorithm. The pipeline allowed high throughput and minimal editing.16
Cognitive function and depressive symptoms.
A battery of 6 different cognitive tests was administered to all participants. From these tests, 3 cognitive domain composite scores were calculated: (1) the memory composite score included the immediate and delayed recall of a modified version of the California Verbal Learning Test17; (2) the speed of processing composite included the Figure Comparison Test,18 the Digit Symbol Substitution Test,19 and the Stroop Test,20 parts 1 and 2; and (3) the executive function composite included a short version of the Cambridge Neuropsychological Test Automated Battery Spatial Working Memory test, the Digits Backward test,19 and the Stroop Test, part 3. Composite measures were computed by converting raw scores on each test to standardized z scores and averaging the z scores across the tests in each composite. Depressive symptoms were assessed using the 15-item Geriatric Depression Scale (GDS-15).21 The GDS-15 is a well-established screening tool to detect depression in elderly people and consists of yes and no questions. Higher scores in the GDS-15 indicate greater number of depressive symptoms.
Other covariates.
Level of education and smoking status were assessed by questionnaire. Diabetes was defined as a history of diabetes, use of glucose-modifying medication, or fasting blood glucose of 7 mmol/L. Hypertension was defined as measured systolic blood pressure of 140 mm Hg or diastolic blood pressure of 90 mm Hg, self-reported doctor's diagnosis of hypertension, or use of antihypertensive medications. History of stroke was recorded using questionnaires and medical and neuroimaging reports. Prevalent coronary heart disease was defined as self-reported history of coronary artery disease, coronary artery bypass surgery or angioplasty, or angina pectoris on the Rose Angina Questionnaire, hospital records, or evidence on ECG of possible or probable myocardial infarction. The diagnosis of atrial fibrillation, from Minnesota codes, was made from a 12-lead ECG. In addition, hospital discharge diagnosis codes from all hospitals in Reykjavik were reviewed for the diagnosis of atrial fibrillation (ICD-9 code 427.9 or ICD-10 code I48).
Cardiac MRI and hemodynamic parameters.
We used MRI-based cardiac function measures acquired as a part of the previously described ICELAND-MI substudy. Details of the inclusion procedure and imaging protocol for this substudy have been reported previously.22 Briefly, the cardiac magnetic resonance scans were performed on a 1.5T scanner (GE Healthcare) using a 4-element cardiac phased array coil. End systolic phase was determined as the minimal cross-sectional area of a midventricular slice. Left ventricular end diastolic volume and left ventricular end systolic volume were computed by the summation of disks method. Left ventricular stroke volume (LVSV) in milliliters was calculated by subtracting left ventricular end systolic volume from left ventricular end diastolic volume. Cardiac output in liters/minute was calculated as LVSV/1,000 × heartbeats per minute. There were 970 participants who underwent cardiac MRI; of these, 34 had scans that could not be read. From the remaining participants (n = 936), 857 participants had complete data on serum NT-proBNP and brain structure and function. Participants included who underwent cardiac MRI, as compared with the total population, were slightly younger, were more frequently male, and had slightly higher body mass index and estimated glomerular filtration rate (all p < 0.05) (table e-1). The 2 groups did not differ in the prevalence of coronary heart disease, atrial fibrillation, and stroke by NT-proBNP level. Prevalence of diabetes was higher in patients with cardiac MRI as patients with diabetes mellitus were oversampled in the ICELAND-MI substudy.
Statistical analyses.
Differences in characteristics of study participants in tertiles of NT-proBNP were tested by analysis of variance and χ2 test. Linear regression methods were used to model the association between log-transformed NT-proBNP (due to skewed distribution) and brain measures. Analysis of covariance was used to calculate the adjusted means and standard errors for the brain measures in tertiles of NT-proBNP and evaluation of the differences between tertiles. We performed the analyses in 2 models. Model 1 was adjusted for age, sex, and intracranial volume (when MRI findings were used as the outcome). Model 2 was additionally adjusted for sociodemographic and cardiovascular factors including education, current smoking, hypertension, and/or use of antihypertensive medications, diabetes mellitus, systolic blood pressure, history of coronary heart disease, history of stroke, total cholesterol, body mass index, atrial fibrillation, and glomerular filtration rate. All of the continuous variables were added to the statistical models in their original format without categorization. In a subgroup analysis, based on the ICELAND-MI substudy, associations were further adjusted for cardiac output to test whether the associations between high NT-proBNP and brain structure and function measures are independent of cardiac function. All p values were calculated using the continuous value of log-transformed NT-proBNP levels. The interaction between NT-proBNP levels and cardiac output in relation to the brain outcomes was tested by adding an interaction term to the regression models. All analyses were performed using SPSS software (version 20.0.0; IBM Corp., Armonk, NY).
RESULTS
Characteristics of the participants in the whole population and in tertiles of NT-proBNP are presented in table 1. Average age of participants was 76.2 years and 44.1% were male.
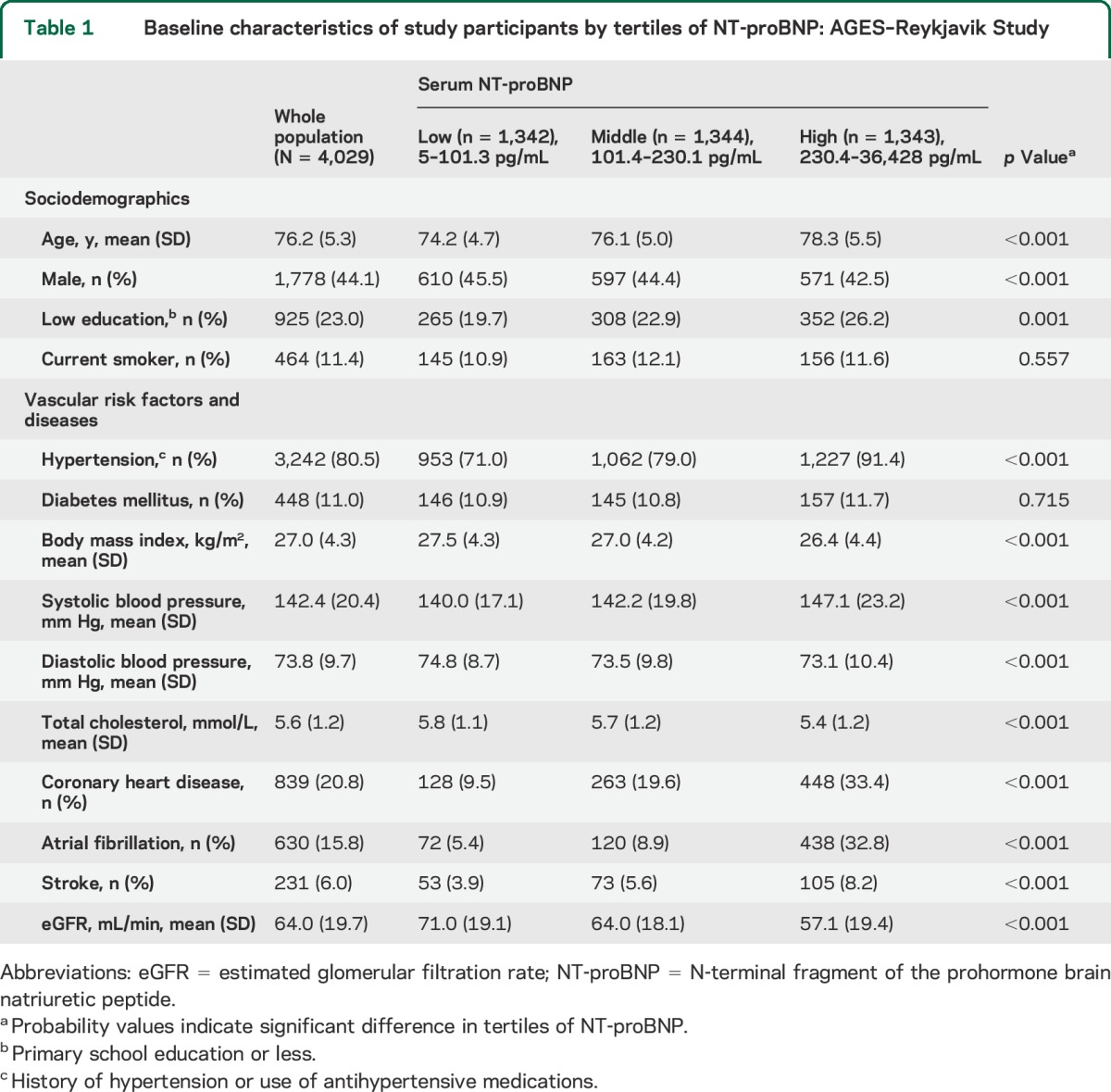
Table 1.
Baseline characteristics of study participants by tertiles of NT-proBNP: AGES–Reykjavik Study
In models adjusted for age, sex, and intracranial volume, higher NT-proBNP was associated with lower total brain parenchymal volume, gray matter volume, and white matter volume (all p < 0.001). Additional adjustments for sociodemographic and cardiovascular risk factors did not essentially change the associations. Fully adjusted mean values of total brain parenchymal volumes in the low, middle, and high tertiles of NT-proBNP were 1,061, 1,059, and 1,051 mL, respectively; 1% increase in NT-proBNP was associated with 0.128 mL lower total brain parenchymal volume (p < 0.001). Fully adjusted mean values of gray matter volumes in the low, middle, and high tertiles of NT-proBNP were 661, 559, and 652 mL, respectively; 1% increase in NT-proBNP was associated with 0.104 mL lower gray matter volume (p < 0.001). Fully adjusted mean values of white matter volumes in the low, middle, and high tertiles of NT-proBNP were 374, 373, and 370 mL, respectively; 1% increase in NT-proBNP was associated with 0.04 mL lower white matter volume (p = 0.001). Figure 1 presents the adjusted mean values for total brain parenchymal volume, gray matter volume, and white matter volume in 3 groups of participants with low, middle, and high NT-proBNP.
Figure 1. Brain parenchymal, gray matter, and white matter volumes in tertiles of serum NT-proBNP.
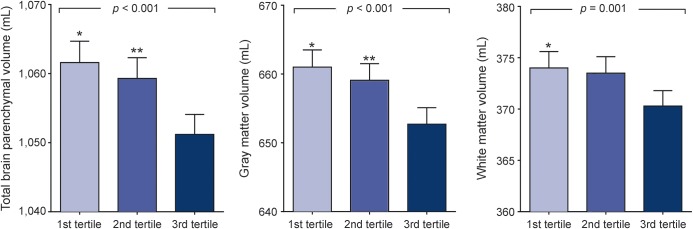
Bars represent mean values (standard error) of total brain parenchymal volume, gray matter volume, and white matter volume adjusted for age and sex, intracranial volume, education, current smoking, hypertension, diabetes mellitus, systolic blood pressure, history of coronary heart disease, history of stroke, total cholesterol, body mass index, atrial fibrillation, and glomerular filtration rate. The p values were calculated using the continuous value of log-transformed NT-proBNP. *Significant difference between low and high tertiles. **Significant difference between middle and high tertiles. NT-proBNP = N-terminal fragment of the prohormone brain natriuretic peptide.
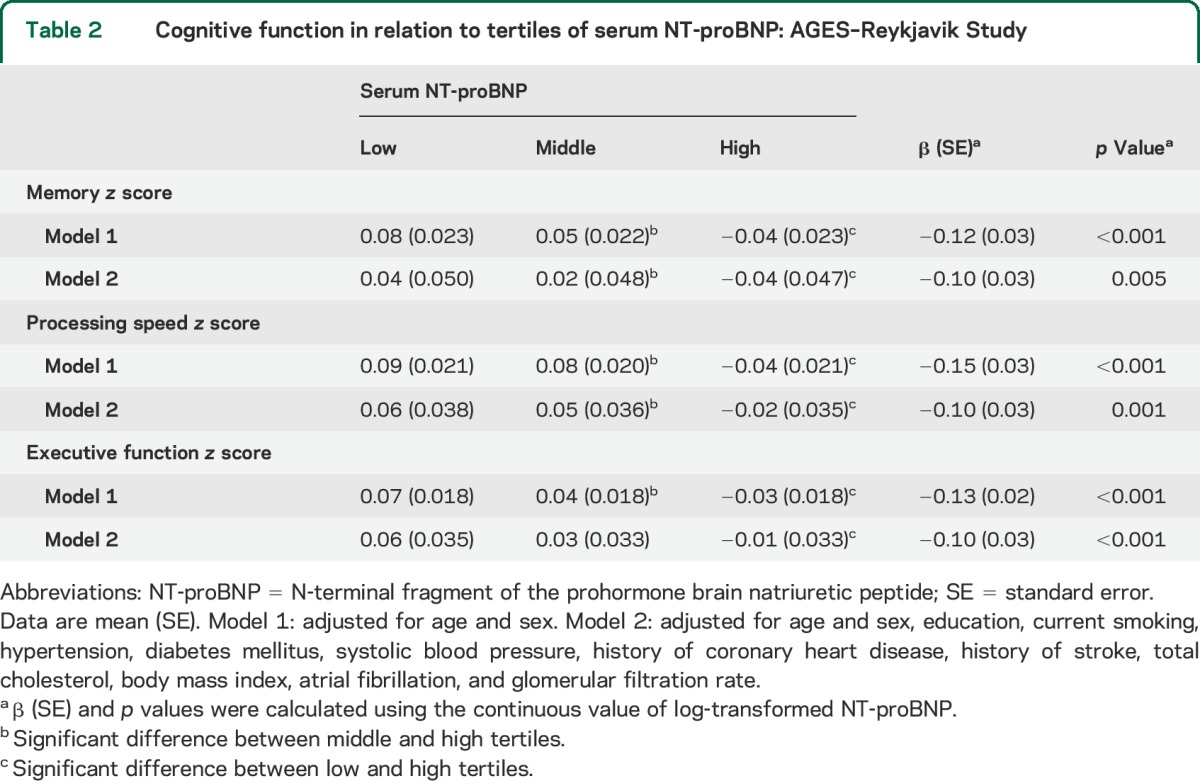
In age- and sex-adjusted models, higher NT-proBNP was associated with worse performance in memory, processing speed, and executive functioning (all p < 0.001). Additional adjustments for sociodemographic and cardiovascular risk factors did not essentially change the associations. Table 2 presents the adjusted mean values for memory, processing speed, and executive function composite scores in 3 groups of participants with low, middle, and high NT-proBNP.
Table 2.
Cognitive function in relation to tertiles of serum NT-proBNP: AGES–Reykjavik Study
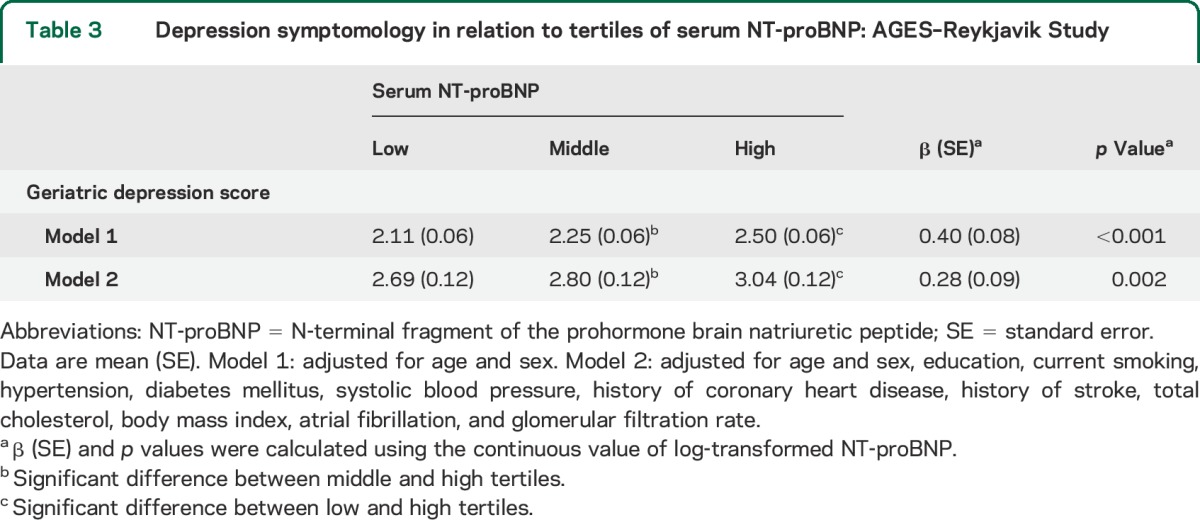
In age- and sex-adjusted models, higher NT-proBNP was associated with higher depressive scores (p < 0.001), and additional adjustments for sociodemographic and cardiovascular risk factors did not essentially change the associations. Table 3 presents the adjusted mean values for geriatric depression score in 3 groups of participants with low, middle, and high NT-proBNP.
Table 3.
Depression symptomology in relation to tertiles of serum NT-proBNP: AGES–Reykjavik Study
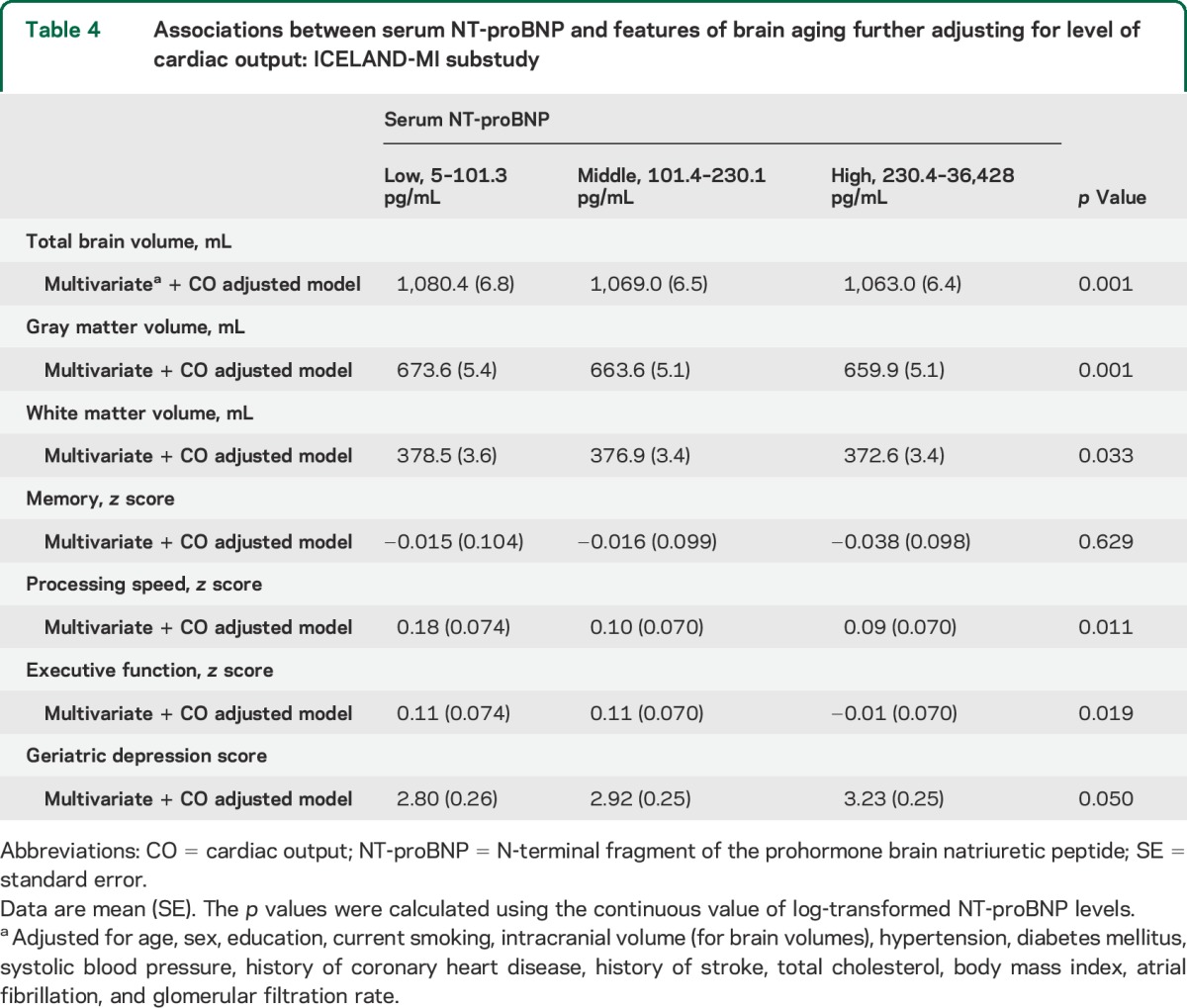
In the ICELAND-MI substudy, we found similar associations between NT-proBNP and the brain measures. Further adjustments of the analyses for levels of cardiac output did not essentially change the associations of NT-proBNP with brain structural volumes, depressive symptoms, processing speed, and executive function (table 4). However, the association between NT-proBNP and memory function was no longer statistically significant after correction for cardiac output. Furthermore, we tested whether an interaction exists between NT-proBNP levels and cardiac output in relation to the brain outcomes and found no significant interaction (all p > 0.05), although the sample size is likely not large enough to detect differences.
Table 4.
Associations between serum NT-proBNP and features of brain aging further adjusting for level of cardiac output: ICELAND-MI substudy
DISCUSSION
In this population-based study, we showed that higher serum NT-proBNP independent of cardiovascular risk factors, comorbidities, and cardiac output is associated with lower structural brain volumes, worse cognitive function, and higher depressive symptoms.
A growing body of evidence indicates that cardiovascular risk factors are linked with accelerated changes in the brain structure and function.23 Recently, it was suggested that impaired cardiac function can serve as a reversible risk factor for abnormal brain aging.24 In line with this hypothesis, previous studies showed that patients with heart failure are at an increased risk of developing dementia and structural brain changes.25,26 We recently showed that a graded decrease in cardiac functioning, as reflected in cardiac output and stroke volume, is linked with lower brain parenchymal volumes and cognitive impairment.27 All of this evidence suggests that lower cardiac output results in lower cerebral blood flow, which ultimately impairs brain structure and function. In this setting, a limited number of studies have investigated whether increased serum BNP and NT-proBNP, as the markers for left ventricular dysfunction, are associated with cognitive dysfunction. In a study of 950 community-dwelling individuals older than 60 years, it was shown that higher NT-proBNP levels, independent of cardiovascular factors, were associated with poor cognitive function.13 In contrast, in the multidisciplinary Kuopio 75+ Health Study, the association between BNP and cognitive impairment was only present in older participants with mild stages of cognitive impairment and even this association disappeared after adjustments for cardiovascular factors.28 In a population-based study of 464 older participants, it was shown that higher levels of BNP are associated with higher risk of incident dementia.29 However, there was no report as to whether correction for cardiovascular factors other than hypertension influenced the associations. A limited number of studies investigated the association of BNP with subclinical changes identified in brain MRI. In a study on 3,127 stroke-free Framingham offsprings with mean age of 59 years, the investigators did not find an association between BNP level and brain parenchymal volumes.30 However, in the Atherosclerosis Risk in Communities Study, the investigators showed that higher NT-proBNP is associated with higher loads of white matter hyperintensities and silent brain infarcts.8
In the current study, using a large population of older individuals and various brain measures, we showed that higher serum NT-proBNP is linked with features of abnormal brain aging. Several explanations can be put forward for our findings. First, the associations could be attributable to shared vascular risk factors for both elevated NT-proBNP and features of brain aging. In this study, adjustments of the associations for conventional cardiovascular risk factors and cerebrovascular and cardiovascular diseases did not materially change the associations. However, we cannot rule out possible roles of unmeasured cardiovascular factors on this association. It is also possible that higher NT-proBNP reflects lower cardiac function, which might lead to long-lasting hypoperfusion of the brain.31 Availability of cardiac MRI data in a substudy of the population gave us the opportunity to test whether further correction of the analyses for level of cardiac output attenuates the associations. Despite a considerably smaller sample size of this substudy, the associations of NT-proBNP and brain measures, except memory function, only slightly attenuated after adjustment for level of cardiac output. The attenuation of results after adjustment for cardiac output could possibly result from cerebral perfusion in maintaining integrity of the brain structures such as hippocampus that are involved in memory function. Furthermore, it needs to be noted that participants who were included in this study had lower loads of cardiovascular risk factors and pathologies compared with those who were not included. This may lead to underestimation of the magnitude of the associations between serum NT-proBNP and features of abnormal brain aging. Overall, our findings suggest that NT-proBNP independent of impaired cardiac function may be related to age-related structural and functional brain changes including decline in brain tissue volume, cognitive impairment, and increased depressive symptoms. In line with our observation, a recent study showed that higher NT-proBNP levels are associated with cognitive decline in older persons at risk of cardiovascular disease but without heart failure.32
Although BNP was first discovered in the brain tissue, until recently, less attention has been given to its function in the brain.33 Growing evidence indicates that natriuretic peptides are involved in neural development, neurotransmitter release, synaptic transmission, and neuroprotection.34 It has been shown that receptors of the natriuretic peptides including BNP are abundantly present on the neurons of hypothalamus and cortical areas as well as glial and endothelial cells of the brain.35 Future experimental studies are required to test how elevated serum natriuretic peptides interact with their receptors in the brain and influence the process of brain aging.
Certain strengths and limitations of this study should be acknowledged. A study population of more than 4,000 community-dwelling older participants with extended data available on neurocognitive tests, brain MRI, depressive symptoms, and cardiovascular factors is a major strength of this study. In addition, measurement of cardiac function in a substudy using cardiac MRI, which is a reliable method and a less operator-dependent modality compared with conventional echocardiography, gave us a unique opportunity to show that the association of NT-proBNP with brain aging is independent of cardiac function. As a limitation, because of the cross-sectional design of this study, it is unclear whether increase in NT-proBNP preceded alterations in the brain structure and function. This calls for future longitudinal studies evaluating the relationship between higher NT-proBNP and accelerated brain aging in older adults. Furthermore, it needs to be pointed out that, despite all the adjustments, there is a possibility that we did not account for certain unmeasured confounders of the association between high NT-proBNP and features of abnormal brain aging. Given that higher NT-proBNP levels are observed in individuals with higher loads of vascular damage, it needs to be investigated whether treatment of cardiovascular diseases and risk factors would influence the link between NT-proBNP and brain structural and functional impairments.
Our findings indicate that higher serum NT-proBNP is associated with various brain structural and functional characteristics and this cross-sectional association is independent of cardiovascular risk factors and cardiac output. These findings should stimulate further research to elucidate mechanisms underlying this association and to clarify whether measurement of NT-proBNP can be a tool to identify older individuals at high risk of developing abnormal brain aging. There is increasing evidence that cardiac abnormalities are related to impairment in the brain structure and function. Although this link could be explained by hemodynamic and neurohormonal impairments due to cardiac dysfunction, it is possible that common systemic vascular processes drive both cardiac and brain pathologies.
Supplementary Material
GLOSSARY
- AGES
Age, Gene/Environment Susceptibility
- BNP
brain natriuretic peptide
- GDS-15
15-item Geriatric Depression Scale
- ICD
International Classification of Diseases
- NT-proBNP
N-terminal fragment of the prohormone brain natriuretic peptide
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Behnam Sabayan and Lenore J. Launer contributed in formulating the research question, analysis and interpretation of data, and drafting the manuscript. Mark A. van Buchem and Sigurdur Sigurdsson contributed in study design, acquisition of data, interpretation of data, and revising the manuscript. Qian Zhang contributed in analysis of data and revising the manuscript. Tamara B. Harris, Vilmundur Gudnason, Andrew E. Arai, and Lenore J. Launer contributed in formulating the AGES-RS study design, interpretation of data, and revising the manuscript.
STUDY FUNDING
The study was funded by the National Institute on Aging (NIA) (N01-AG-12100), Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament), with contributions from the Intramural Research Programs at the NIA and at the National Heart, Lung, and Blood Institute (Z01 HL004607-08 CE). The study was approved by the Icelandic National Bioethics Committee (VSN: 00-063) and the MedStar Research Institute (project 2003-145).
DISCLOSURE
B. Sabayan is partly supported by a grant from Internationale Stichting Alzheimer Onderzoek (ISAO). M.A. van Buchem, A.J.M. de Craen, S. Sigurdsson, Q. Zhang, T.B. Harris, V. Gudnason, A.E. Arai, and L.J. Launer report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature 1988;332:78–81. [DOI] [PubMed] [Google Scholar]
- 2.Mukoyama M, Nakao K, Hosoda K, et al. Brain natriuretic peptide as a novel cardiac hormone in humans: evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest 1991;87:1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HN, Januzzi JL., Jr Natriuretic peptide testing in heart failure. Circulation 2011;123:2015–2019. [DOI] [PubMed] [Google Scholar]
- 4.Bettencourt P. NT-proBNP and BNP: biomarkers for heart failure management. Eur J Heart Fail 2004;6:359–363. [DOI] [PubMed] [Google Scholar]
- 5.Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 2005;293:1609–1616. [DOI] [PubMed] [Google Scholar]
- 6.Weber M, Hamm C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006;92:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patton KK, Ellinor PT, Heckbert SR, et al. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation 2009;120:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadu RT, Fornage M, Virani SS, et al. Cardiovascular biomarkers and subclinical brain disease in the Atherosclerosis Risk in Communities Study. Stroke 2013;44:1803–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 2010;468:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz K, van Buchem MA, Daemen MJ. The heart-brain connection: mechanistic insights and models. Neth Heart J 2013;21:55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jefferson AL. Cardiac output as a potential risk factor for abnormal brain aging. J Alzheimers Dis 2010;20:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinkohl I, Sattar N, Welsh P, et al. Association of N-terminal pro-brain natriuretic peptide with cognitive function and depression in elderly people with type 2 diabetes. PLoS One 2012;7:e44569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels LB, Laughlin GA, Kritz-Silverstein D, et al. Elevated natriuretic peptide levels and cognitive function in community-dwelling older adults. Am J Med 2011;124:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility–Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA 2009;301:2563–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik Study. Neuroimage 2012;59:3862–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual: Adult Version (Research Edition). New York: The Psychological Corporation; 1987. [Google Scholar]
- 18.Salthouse T, Babcock R. Decomposing adult age differences in executive function. Dev Psychol 1991;27:763–776. [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale. Manual. New York: The Psychological Corporation; 1955. [Google Scholar]
- 20.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol 1935;18:643–662. [Google Scholar]
- 21.Incalzi RA, Cesari M, Pedone C, Carbonin PU. Construct validity of the 15-item Geriatric Depression Scale in older medical inpatients. J Geriatr Psychiatry Neurol 2003;16:23–28. [DOI] [PubMed] [Google Scholar]
- 22.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012;308:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flicker L. Cardiovascular risk factors, cerebrovascular disease burden, and healthy brain aging. Clin Geriatr Med 2010;26:17–27. [DOI] [PubMed] [Google Scholar]
- 24.Jefferson AL, Himali JJ, Beiser AS, et al. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation 2010;122:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med 2006;166:1003–1008. [DOI] [PubMed] [Google Scholar]
- 26.Vogels RL, van der Flier WM, van Harten B, et al. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail 2007;9:1003–1009. [DOI] [PubMed] [Google Scholar]
- 27.Sabayan B, van Buchem MA, Sigurdsson S, et al. Cardiac hemodynamics are linked with structural and functional features of brain aging: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. J Am Heart Assoc 2015;4:e001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiltunen M, Nieminen T, Kettunen R, et al. B-type natriuretic peptide and severity of cognitive disorder. Eur J Clin Invest 2013;43:1171–1177. [DOI] [PubMed] [Google Scholar]
- 29.Kerola T, Nieminen T, Hartikainen S, Sulkava R, Vuolteenaho O, Kettunen R. B-type natriuretic peptide as a predictor of declining cognitive function and dementia: a cohort study of an elderly general population with a 5-year follow-up. Ann Med 2010;42:207–215. [DOI] [PubMed] [Google Scholar]
- 30.Pikula A, Beiser AS, DeCarli C, et al. Multiple biomarkers and risk of clinical and subclinical vascular brain injury: the Framingham Offspring Study. Circulation 2012;125:2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruhn N, Larsen FS, Boesgaard S, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke 2001;32:2530–2533. [DOI] [PubMed] [Google Scholar]
- 32.Wijsman LW, Sabayan B, van Vliet P, et al. N-terminal pro-brain natriuretic peptide and cognitive decline in older adults at high cardiovascular risk. Ann Neurol 2014;76:213–222. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Z, Ma L. Regulation of axonal development by natriuretic peptide hormones. Proc Natl Acad Sci USA 2009;106:18016–18021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao LH, Yang XL. Natriuretic peptides and their receptors in the central nervous system. Prog Neurobiol 2008;84:234–248. [DOI] [PubMed] [Google Scholar]
- 35.Prado J, Baltrons MA, Pifarre P, Garcia A. Glial cells as sources and targets of natriuretic peptides. Neurochem Int 2010;57:367–374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


