Abstract
Carney Complex (CNC) is a rare autosomal dominant syndrome, characterized by pigmented lesions of the skin and mucosa, cardiac, cutaneous and other myxomas, and multiple endocrine tumors. The disease is caused by inactivating mutations or large deletions of the PRKAR1A gene located at 17q22–24 coding for the regulatory subunit type I alpha of protein kinase A (PKA) gene. Most recently, components of the complex have been associated with defects of other PKA subunits, such as the catalytic subunits PRKACA (adrenal hyperplasia) and PRKACB (pigmented spots, myxomas, pituitary adenomas). In this report, we review CNC, its clinical features, diagnosis, treatment, and molecular etiology including PRKAR1A mutations and the newest on PRKACA and PRKACB defects especially as they pertain to adrenal tumors and Cushing’s syndrome.
Keywords: PPNAD, Carney complex, lentigines, cardiac myxomas, PRKAR1A
Introduction
Carney Complex (CNC) is a rare multiple neoplasia syndrome, inherited in an autosomal dominant manner or occurring sporadically as a result of a de novo genetic defect. It is characterized by pigmented lesions of the skin and mucosae, cardiac, cutaneous and other myxomatous tumors, and multiple other endocrine and non-endocrine neoplasms 1, 2. It was first described by Dr. J. Aidan Carney as “the complex of myxomas, spotting pigmentation and endocrine over-reactivity” 3, 4. It was designated as CNC by Bain 5 and in 1994 as Carney syndrome by MIM (Mendelian Inheritance in Man) 6
More than half of the cases are familial 2, 7. Most of the patients who in the past were diagnosed with LAMB (lentigines, atrial myxomas, myxoid neurofibromas and ephelide) or NAME (nevi, atrial myxoma, blue nevi) should be reclassified today as CNC 1, 3, 8, 9. CNC is in essence a multiple endocrine neoplasia syndrome but one that affects a number of other tissues 10. This unique condition has similarities to other syndromes/diseases such as the McCune-Albright, Peutz-Jeghers, Cowden, Bannayan-Zonana, and Birt-Hogg-Dube syndromes, neurofibromatosis, and other phacomatoses and hamartomatoses 10.
Epidemiology
CNC is a rare disease 4 with an unknown prevalence 11, 12. In the largest genotyped series of patients, 63% were females and 37% were males 12. The NIH-Mayo clinic, and other centers in the United States and the Cochin Hospital in France have collectively reported more than 750 cases including Caucasians, African-Americans, and Asians from all continents [North and South America, Europe, Asia (Japan, China, India)2, 11, 13. Approximately 70% of CNC cases had an affected parent (67 families), whereas the remaining had no known affected relatives and carried de novo germline mutations 2. In all inherited cases, CNC was passed on as an autosomal dominant trait with an almost 100% penetrance.
Clinical features
The clinical manifestations of CNC are quite variable and the full spectrum of the disease develops usually over a span of many years. Although the diagnosis is rarely made at birth, cases diagnosed as early as in the 2nd year of life and as late as in the 5th decade of life are known with a median age at detection of 20 years old 2, 13. Table 1 summarizes all the clinical manifestations found in CNC patients.
Table 1.
Summary of clinical manifestations of CNC
| Organ | Manifestation | % | |
|---|---|---|---|
| Skin | Lentigines | 70–80 | |
| Blue nevus |
|
40 | |
| Epitheliod blue nevus | |||
| Cutaneous myxomas | 30–50 | ||
| Café-au-lait spots | rare | ||
| Depigmented lesions | rare | ||
| Spitz nevus | rare | ||
|
| |||
| Pituitary | Somatomammotroph hyperplasia | 67 | |
| Asymptomatic elevation of GH, IGF-1 or prolactin | Up to 75 | ||
| GH-producing adenoma with acromegaly | 10–12 | ||
| Prolactinomas | rare | ||
|
| |||
| Eye | Facial and palpebral lentigines | 70 | |
| Pigmented lesions of the caruncle or conjuntival semilunar fold | 27 | ||
| Eyelid myxomas | 16 | ||
| Pigmented schwannomas of the uvea | rare | ||
|
| |||
| Thyroid | Cystic or Nodular disease | Up to 75 | |
| Benign thyroid adenomas | Up to 25 | ||
| Thyroid cancer (papillary or follicular type) | Up to 10 | ||
| Heart | Cardiac myxomas | 20–40 | |
|
| |||
| Pancreas | Acinar cell carcinoma |
|
2.5 |
| Adenocarcinoma | |||
| Intraductal pancreatic mucinous neoplasia (IPMN) | |||
|
| |||
| Liver | Hepatocellular adenoma | rare | |
|
| |||
| Adrenals | PPNAD | 25–60 | |
| Adrenocortical cancer | rare | ||
|
| |||
| Testes | LCCSCT | 41 | |
| Leydig-cell tumors | rare | ||
| Adrenocortical rest tumors | rare | ||
|
| |||
| Ovaries | Ovarian cyst |
|
14 |
| Seruscysadenomas | |||
| Cystic teratomas | |||
|
| |||
| Breast | Breast and nipple myxomas |
|
20 |
| Ductal adenomas | |||
| Myxoid fibroadenomas | |||
|
| |||
| Uterus | Uterine myxoid tumors | rare | |
|
| |||
| Bone | Osteochondromyxoma | rare | |
|
| |||
| Nerve sheath | PMS | Up to 10 | |
|
| |||
| Other organs | Paratiroid mixed tumor |
|
rare |
| Bronchogenic cyst | |||
| Colonic and gastric carcinoma | |||
| Peritoneal fibrous histiocytomas | |||
Cutaneous manifestations
Skin lesions are the most confident finding of CNC 1 and more than 80% of the patients report pigmented spots or skin “growths” that are easily recognizable typically early in life. They can vary from lentigines and blue nevi (in particular epithelioid blue nevi, small bluish domed papules with a smooth surface) 14 to cutaneous myxomas. Café-au-lait spots, irregular depigmented areas, many compound and, rarely, Spitz nevi have also been reported 12, 15–17.
Lentigines (flat small brown to black macules) usually appear before puberty, increase in number and pigment intensity during and after adolescence. They may be located everywhere on the body but a rather typical distribution exists on the face, the lips, genital area, and mucosa (Figure 1a–d). Although fading is common in old age, they can still be seen even in the very elderly 8, 18–20. African Americans may manifest with slightly raised dark papules 21, 22.
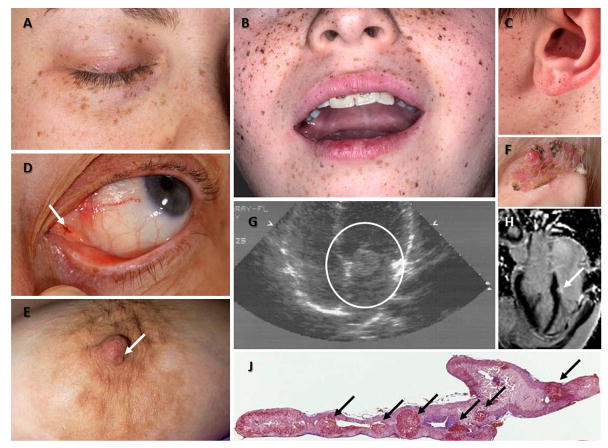
Figure 1.
Figure 1A, B, C: Manifestation of Carney Complex.
Characteristic distribution of the lentigines on the eyelids (A), the vermillion border of the lips and the cheeks (B), and the ears, including the ear canal (C) in patients with CNC; such typical pigmentation on the face is only present in less than one third of the patients but it is rather diagnostic when present. D: a pigmented macule (arrow) on the outer canthus of a patient with CNC who had minimal other pigmentation; inner or outer canthal pigmentation such as the one shown here is only seen in CNC and Peutz-Jeghers syndrome making it diagnostic for these two conditions. E: Nipple myxoma in a female patient with CNC. F: Ear myxoma complicated by chronic infection and tissue overgrowth in a toddler with CNC. G and H: Large myxoma (circled) between the left atrium and ventricle detected by echocardiography in an adolescent with CNC (G) who had surgery immediately thereafter, and a much smaller myxoma (arrow) of the left ventricle originating from the cardiac diaphragm detected by cardiac MRI in an older patient with CNC (H); this myxoma was followed by serial echocardiogram and has yet to be operated, as it is not growing and poses no immediate risks. J: 5x magnification hematoxylin and eosin staining of the adrenal gland of a patient with CNC: the characteristic nodules of PPNAD are shown by the arrows; the overall size of the gland is normal and the nodules may not be visible by imaging studies.
Epithelioid blue nevus (EBN) is an interesting subtype of blue nevus that is very rare in the general population, but is relatively commonly seen in patients with CNC. EBN presents with intensive pigmentation and poorly circumscribed proliferative regions with associated dermal fibrosis 16, 23. EBN is not pathognomonic for CNC, but is frequently associated with the disease and its presence should alert the clinician for the possible diagnosis of the complex 15.
Cutaneous myxomas (Figure 1D–F) are found in 30–55% of CNC patients and usually appear in the eyelid, external ear canal, breast nipples, and the genitalia 12, 15. These lesion can be localized in the dermis or the subcutaneous layer, and usually they are symptomless and less than 1 cm of diameter 24, 25. Rarely, a sharply circumscribed angiomyxoid nodule may be found 26.
Ophthalmologic manifestations
The most common ophthalmologic manifestations are facial and palpebral lentigines, pigmented lesions of the caruncle or conjuntival semilunar fold and eyelid myxomas 27, 28. There are some reports of pigmented schwannomas of the uvea 29. The differential diagnosis of pigmented lesions of the conjunctiva includes melanocytic nevus, melanosis (congenital or acquired), malignant melanoma or drug induce secondary pigmentation 28, 30.
Cardiac manifestations
The most common noncutaneous lesions found in CNC are cardiac myxomas (in 20–40% of the patients) 11 which can appear early in infancy 17 but the median age at detection is at 20 years 31. Cardiac myxomas in CNC occur anywhere in the heart and are equally present in males and females (Figure 1G, H).. Their epidemiology should be contrasted with that of sporadic cardiac myxomas that develop almost exclusively in the left atrium, and are far more frequent in older female patients. Cardiac myxomas present with symptoms related to intracardiac obstruction of blood flow or embolic phenomenona (into the systemic circulation) like strokes and/or heart failure. They are responsible for more than 50% of the mortality of the disease 1, 2, 12, 13, Myxomas can completely occlude a valvular orifice and may cause sudden death 14. This is why early detection and regular screening with echocardiography are essential 1, 32, 33; cardiac CT or MRI may also be used for the detection of these tumors, especially in post-operative hearts with altered anatomy 34.
Pituitary tumors
Up to 75% of CNC patients exhibit asymptomatic elevation of GH, IGF-1 or prolactin in the serum, abnormal response of GH to oral glucose tolerance test (OGTT) and “paradoxical” response to thyrotropin-releasing hormone without tumors detected on imaging studies 31, 35. The incidence of acromegaly due to pituitary tumors is around 10–12% in these patients 11–13, 36, 37. The adenomas usually appear during or after the third decade of life. Histological investigation of these tumors revealed somatomammotrophic hyperplasia (SH). SH is a putative precursor of GH-producing adenoma and may explain the protracted period of onset of clinical acromegaly in individuals with CNC 12, 14. Patients can be followed by measuring GH and IGF-1 levels and/or performing OGTT 11.
Rare prolactinomas have been also described 11 The tumors in operated CNC patients with acromegaly, frequently immunostained for both prolactin and alpha subunit in addition to GH. Interestingly, TSH, LH and FSH staining have also been seen in foci of normal pituitary cells entrapped within the tumor or hyperplasia 38.
Adrenocortical tumors
The most common endocrine tumor in CNC is primary pigmented nodular adrenocortical disease (PPNAD), a cause of (ACTH)-independent overproduction of cortisol 14. It affects between 25–60% of the individuals with CNC (70–71% female and 21% males) 12, 17, 31.
Few CNC patients present with PPNAD during the first 2–3 years of life 11; most present in the first 30 years of life (the observed “peak” of diagnosis is during the second and third decades of life) 2, 14. Histologic evidence of PPNAD has been found in almost every individual with CNC who underwent autopsy, showing that a number of patients have asymptomatic PPNAD.
In PPNAD (Figure 1J), the adrenal cortex is peppered with small pigmented nodules (<1cm) surrounded by usually atrophic cortex 4, 39. Cortisol production can be variable, often cyclical or periodic, is characterized by a paradoxical rise in response to dexamethasone administration 2, 17, 40.
PPNAD continues to be a diagnostic challenge to the radiologist due to the small overall size of the adrenals and the small size of the pigmented nodules. To observe numerous pigmented nodules, CT slice thickness have to be 3 mm or less, if the slice thickness is 5 mm or more the radiological report will be normal adrenals. The pigmented nodules are small, round, well delineated and hypodense compared with the rest of the adrenal parenchyma 4, 31.
Symptomatic individuals with PPNAD develop Cushing’s syndrome. The hypercortisolism of PPNAD is usually insidious in onset 14. Cyclical and other forms of atypical Cushing’s syndrome are also common among CNC patients 41
Adrenocortical cancer has also been recently described in CNC patients. In those cases, co-secretion of androgen and cortisol and rapid occurrence of metastasis were observed 42.
Thyroid neoplasms
CNC patients may have thyroid nodules (up to 60% of all patients and two-thirds among children and adolescents) 12, 31, 43; their histology varies from benign thyroid adenomas (mostly follicular) in 25% of the cases 1, 12 to otherwise nonspecific cystic disease in 75% of the cases 14, to the rare thyroid cancer (papillary or follicular type) in up to 10 % of the cases 2, 44. Thyroid nodules often appear during the first ten year of life in CNC patients 17. Despite thyroid nodularity, CNC patients are clinically and biochemically euthyroid 43, 45.
Psammomatous Melanotic Schwannomas (PMS)
PMS is a rare tumor of the nerve sheath that has been reported in patients with CNC (up to 10%) 12, 14. PMS has frequent calcification and multicentricity with heavy pigmentation and may be located anywhere in the central or peripheral nervous system 46, 47. The most frequent sites are in the gastrointestinal tract (esophagus, stomach, liver and rectum) and in the paraspinal sympathetic chain (28%) 11, 48. The chest wall, with involvement of adjacent ribs, is the third most common site 31. The spinal tumors present as pain and radiculopathy in adults (at a mean age of 32 years). Malignant degeneration occurs in approximately 10% of PMS associated with CNC 49, 50.
Testicular tumors
More than three quarters of males with CNC may have large cell calcifying Sertoli cell tumors (LCCSCT). Lesions may be bilateral, palpable and multifocal with some risk of malignancy and are associated with reduced fertility 22, 24, 51. LCCSCT are detected by ultrasonography as multiple and bilateral testicular microcalcifications that distinguishes them from germ cell and other testicular tumors 2, 26. Leydig-cell tumors or hypeprlasia and adrenocortical rest tumors (affected by PPNAD) have also been reported in CNC patients and should be considered especially in male patients with CNC and sexual precocity 24.
Breast tumors
Breast myxomas, often bilateral, occur in females with CNC after puberty. Both males and females may develop breast nipple myxomas at any age 11. In addition, ductal adenomas and myxoid fibroadenomas have been reported 12, 52, 53.
Ovarian lesions
Ovarian cysts and tumors of the ovarian surface epithelium including serous cystadenomas and cystic teratomas have been reported in females with CNC. These are typically hypoechoic lesions by sonographic examination. They can grow or progress (rarely) to ovarian carcinoma 11, 12, 54
Bone lesions
Osteochondromyxoma is a rare component of CNC characterized by a myxomatous tumor of the bone that may affects any bone but has been seen most frequently in the nasal sinuses and the long bones of the upper and lower extremities 14. They usually appear early in life (before the age of 2) 2. These bone lesions are benign, but both local invasiveness and recurrence have been reported 11.
Other lesions
Hepatocellular adenoma was first reported in a 19-year-old female patient 55. Uterine myxoid tumors were also reported in a single patient 56. Pancreatic neoplasms including acinar cell carcinoma, adenocarcinoma, and intraductal pancreatic mucinous neoplasia, were seen in as many as 2.5% of CNC patients 12, 57. Other tumors like parathyroid mixed tumor, bronchogenic cysts, colonic, gastric carcinoma and peritoneal fibrous histiocytomas have also been described in rare cases 2, 11.
Diagnosis
CNC should be suspected in patients with a suggestive phenotype 31. Table 2 lists the criteria for the diagnosis of CNC1, 2, 5, 11, 15, 17. A number of clinical and biochemical manifestations may also suggestive but they are not clearly diagnostic of CNC (sometimes these are mentioned as minor criteria) (Table 2) 58. The median age of diagnosis is 20 years 13. Depending on the affected organ and the type of lesion there are other syndromes/disorders that will have a similar presentation (Table 3) 8, 14, 59–66.
Table 2.
Diagnostic criteria for CNC*
| Major Criteria |
|
|
|
|
|
| Supplemental criteria |
|
|
|
|
|
| Findings suggestive of or possibly associated with CNC, but not diagnostic for the disease (minor criteria) |
|
|
|
To make the diagnosis of CNC, a patient must either: (1) exhibit two of the major criteria confirmed by histology, imaging or biochemical testing or meet (2) one major criterion and one supplemental one
with histologic confirmation
Table 3.
Conditions to be considered in the differential diagnosis of CNC per tissue manifestation
| Organ | Related Disorders |
|---|---|
| Heart (cardiac myxomas) | Sporadic myxomas
Familial myxomas due to mutation of a protein of the myosin family |
| Skin (lentigines) | Familial lentiginosis, Peutz-Jeghers syndrome, LEOPARD syndrome, Noonan syndrome with lentiginosis, Bannayan-Riley-Ruvalcaba syndrome |
| Skin (café-au-lait spots) | McCune-Albright syndrome, Neurofibromatosis type 1, Neurofibromatosis type 2, Watson syndrome |
| Skin (blue nevi) | Solitary lesions |
| Thyroid (tumors) | Cowden syndrome, Sporadic thyroid tumors |
| Testes (Large-cell calcifying Sertoli cell tumor (LCCSCT)) | Peutz-Jeghers syndrome |
| Ovarian (tumors) | Peutz-Jeghers syndrome |
| Adrenals | Sporadic isolated primary pigmented nodular adrenocortical disease (PPNAD) Isolated micronodular adrenocortical hyperplasia |
| Adrenocortical tumors | Beckwith-Wiedemann syndrome, Li-Fraumeni syndrome, Multiple Endocrine Neoplasia type 1 (MEN1), Congenital adrenal hyperplasia resulting from 21-hydroxylase deficiency, McCune-Albright syndrome |
| Pituitary (GH-secreting adenoma) | MEN1, Isolated Familial Somatotropinomas (IFS), Sporadic somatotropinomas |
| Schwannomas | Neurofibromatosis type 1, Neurofibromatosis type 2, Isolated familial schwannomatosis |
Treatment
In CNC, each specific complication/tumor should be addressed separately. Cardiac myxomas should be removed surgically; most patients, however, have two or more open heart surgeries for recurrent tumors 11, 31, 67, 68. SH and/or GH-producing pituitary adenomas may be treated medically with somastotatin analogues or removed surgically 49. Similarly, for PPNAD, the best treatment is surgical, by bilateral adrenalectomy; however, medical adrenalectomy with inhibitors of steroidogenesis such as ketoconazole or mitotane may also be considered in selected cases 8, 17, 59, 69. Surgical excision of cutaneous and mammary myxomas may be needed but it is not always necessary as these tumors are completely benign. Fine-needle aspiration for thyroid nodules is recommended in suspicious cases 2, 11 and thyroid cancer may be treated as it is appropriate for the histologic subtype. Boys with LCCSCT may develop gynecomastia, premature epiphyseal fusion, and induction of central precocious puberty and for them surgery and/or treatment with aromatase inhibitors may be needed 14, 19, 70. PMS is more difficult to treat because many of these tumors are located around nerve roots, a location that typically renders them inoperable 49. There is no effective treatment for metastatic PMS; CNC patients that develop metastatic PMS die of complications of the spread of the tumor usually in the lungs, liver or brain 13, 19.
Surveillance and follow up of patients with CNC
Any patient with the diagnosis of CNC should be followed closely for clinical manifestations of the disease at least once a year. A study has shown that this type of follow up improves prognosis 13, 58.
The suggested studies 71–73 inlcude:
Annual echocardiogram, beginning in infancy; if a patient was diagnosed with a cardiac myxoma at least once, cardiac imaging may be done biannually.
Regular skin evaluations
Blood tests to check serum levels of GH, prolactin, and IGF-1 beginning in adolescence, as appropriate for the detection of GH and PRL excess; urinary free cortisol (UFC) and other testing for screening of Cushing’s syndrome, as appropriate.
Thyroid gland (neck) clinical examinations and with ultrasound, if needed.
Imaging may include adrenal computed tomography for the detection of PPNAD; pituitary magnetic reasonance imaging (MRI), and MRI of brain, spine, chest, abdomen, retroperitoneum, pelvis for the detection of PMS 14
In males, testicular examinations with ultrasound may be done annually for the detection and follow up of LCCSCT
In females, transabdominal ultrasound of the ovaries (baseline examination; it may be repeated, as needed) 10
In pre-pubertal children: close monitoring of linear growth rate and annual pubertal staging 14
Prognosis
The historic adjusted average life span for patients with CNC is 50–55 years but with careful surveillance life expectancy may be normal. The most common causes of death are related to complications of heart myxomas, such as emboli (strokes), post-operative cardiomyopathy and cardiac arrhythmias, as well as metastatic PMS, pancreatic and other cancers 14, 62, 74.
Genetics
CNC is caused by mutations in the PRKAR1A gene (OMIM 188830) coding for the regulatory subunit type I alpha of the protein kinase A (PKA, cAMP-dependent protein kinase) enzyme. PRKAR1A is situated at the 24.2–24.3 locus of the long arm of chromosome 17 and it has 11 exons, exons 2–11 are protein-coding 75. More than 70% of the patients diagnosed with CNC carry mutations on the PRKAR1A gene (CNC1 locus) and this percentage increases to 80% for those with Cushing’s syndrome due to primary pigmented nodular adrenocortical disease (PPNAD) 12, 76. Some families mapped to the CNC2 locus; the majority of these cases presented later in life10, 77. PRKACA copy number gain (CNG) is seen in patients with adrenal hypeprlasias but not in CNC. PRKACB CNG has only been seen in one patient with CNC. For the majority of the PRKAR1A-negative CNC cases the genetic cause remains unknown.
To date, more than 125 PRKAR1A pathogenic mutations have been reported (http://prkar1a.nichd.nih.gov/hmdb/intro.html) in 401 unrelated families of diverse ethnic origin (table 4) 12, 61, 78, 79. The PRKAR1A pathogenic mutations include single base substitutions, small (≤15 bp) deletions/insertions, combined rearrangements that are spread along the whole open reading frame (ORF) of the gene and large deletions that cover most of the exons and in some cases the whole gene locus 70, 78, 80. Most of these mutations are unique (presented in a single kindred) and only three pathogenic variants (c.82C>T, c.491_492delTG, and c.709-2_709-7 delATTTTT) have been identified in more than three unrelated pedigrees 10, 12, 81.
Table 4.
Genomic locus and genes/mutations associated with CNC
| Locus/Gene | Chromosomal locus | Mutation | Mutation type | Expression/protein |
|---|---|---|---|---|
| PRKAR1A(CNC1) | 17q24.12, 70, 78 | c.26G>A, c.206A>G, c.439A>G c.440G>A, c.502G>A, c.547G>T c.550G>A, c.638C>A, c.865G>T c.220C>T, c.438A>T, c.545 C>G |
Missense | Altered Protein |
| c.769G>A, c.786_787delGGinsCT, c.1A>G |
Missense | NMD | ||
| c.52_53delTG, c.63_64delCGinsGA, c.75_78dupTAAC, c.82C>T c.96_97insT, c.109C>T c.124C>T, c.187A>T, c.190C>T c.205C>T, c.286C>T, c.289C>T c.319G>T, c.496C>T, c.499C>T c.569G>A, c.570G>A, c.664A>T* c.671G>A, c.672G>A, c.682C>T c.693dupT, c.172 G>T, c.738T>G c.804dupT, c.910C>T, c.920C>G c.525 T>A, c.178_348del171 c.46C>T, c.491_492delTG c.951delA, c.1076_1077delTTinsAAATGGGCCAAGA |
Non-sense | NMD | ||
| c.-97G>A, c.18delC, c.43_58del16 c.54_55dupTG, c.69_70dupGA c.85_95del11, c.101_105del5 c.140delT, c.220_221delCG c.242delA, c.267delA c.279_282delTAGG c.316_317delAC, c.340delG c.350_353delTTAT, c.353_365del13, c.407_408delTG c.408_412delGCTGT c.418_419delCA, c.463delT c.466delG, c.528_531delGATTins11 c.531_(549+32)del51, c.545dupC c.558_559dupTA c.566_567delAAinsCAC c.587_588insGG, c.597delC c.619delT, c.623delG, c.624dupA c.652_653delAA, c.658_659delAA c.669_670delGT, c.679delG c.687delT*, c.479delC, c.711_712dupAA, c.718dupC c.725dupA, c.728_740del13 c.758_759delTC, c.763_764delAT c.764_768delTTTTA, c.812dupT c.845_846insA, c.963_985del23* |
Frameshift | NMD | ||
| c.1055_1058delGACC c.1067_1070delAACGinsGCCCA c.1083delA, c.1131_1132delTG c.1142_1145delTCTG, |
Frameshift | Elongated Protein | ||
| c.178-2A>G, c.348+1G>C c.440+1G>A, c.440+1_440+2insG c.440+2T>C, c.440+4delG c.502+1G>T,, c.550(−9–2)del8, c.708+1G>T c.709(−107–5)del103 c.709(−7–2)del6, c.709-7G>A c.769+5G>C, c.891+3A>G c.892-2A>G, c.973+1G>A c.974-1G>A*, c.177G>A c.177+1G>A, c.177+3 A>G |
Splice site | NMD/shorter protein/skip exon | ||
| 328bp-3Mb deletions | Large deletions (one exon-whole gene) | NMD/allele depletion | ||
| CNC2 | 2p1682 | NA | NA | NA |
| PRKACA | 19p13.183 | 294Kb-2.7Mb | Large gene amplification | overexpression |
| PRKACB | 1p31.184 | 1.6Mb | Large gene amplification | overexpression |
NMD: nonsense-mediated mRNA decay (NMD) mechanism http://prkar1a.nichd.nih.gov/hmdb/intro.html
Note: When a mutation is underlined, pathogenesis analyzed with in silico analysis; when the mutation is not underlined, its pathogenesis was confirmed in vitro.
A second genetic locus is associated with CNC and It is referred to as the “CNC2” locus (CNC1 being the PRKAR1A gene 17q locus) (Table 4). CNC2 is a 10Mb region in the 2p16 locus that has been detected through linkage analysis in PRKAR1A-negative patients with CNC and further delineated by copy number changes in CNC tumors 12, 78, 82. To date, the gene(s) residing in the 2p16 region that may be responsible for a CNC phenotype or progression of its tumors remain(s) unknown.
Two recent studies associated elements of the CNC phenotype with PRKACA and PRKACB gene defects (Table 4). Comparative genomic hybridization (CGH) in 35 patients with cortisol-producing bilateral adrenal hyperplasia and overt Cushing’s syndrome identified 5 patients with copy number gains of the genomic region that included the PRKACA gene on chromosome 19 83. A single individual with CNC, developmental delay and skeletal defects was identified; in her, the disease was caused by somatic PRKACB gene locus copy number gains on chromosome 1 84. The precise roles of PRKACA and PRKACB in the CNC phenotype remain to be elucidated.
Molecular pathogenesis of CNC: PKA and its subunits
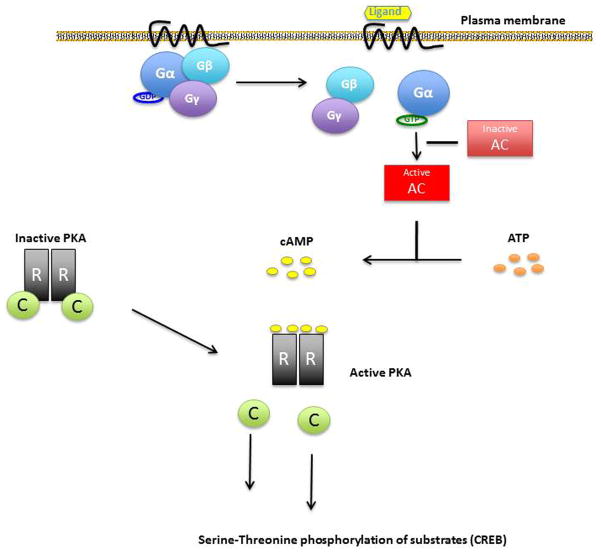
PRKAR1A gene encodes for the most widely expressed regulatory subunit of the PKA enzyme. The PKA heterotetramer consists of two regulatory (R) and two catalytic (C) subunits. Stimulation of adenyl cyclases through G protein subunit (Gs) activation leads to cAMP synthesis (Figure 2). cAMP, binds to the regulatory subunits and leads to their dissociation from the catalytic subunits. The catalytic subunits after their dissociation from the PKA complex phosphorylate many downstream factors such as cAMP response-binding protein (CREB) (Figure 2). PRKAR1A defects associated with CNC lead to PRKAR1A haploinsufficiency and, thus, loss of this regulatory subunit’s function: “unrestrained” catalytic subunit activity leads to increased cell proliferation in cAMP-responsive tissues and tumor formation in tissues affected by CNC 10,85.
Figure 2. cAMP pathway activation.
Receptor activation (ligand binding) makes Ga to exchange GDP to GTP, Ga is then freed from the Gβ-Gγ dimer and activates adenyl cyclase (AC). Activated AC produces cAMP from ATP, cAMP causes dissociation of the inactive protein kinase A (PKA) tetramer and then the catalytic subunits are freed to mediate serine-threonine phosphorylation of target molecules, including CREB. PRKAR1A inactivating mutations in Carney complex patients will result in less binding of the catalytic to the regulatory subunits and excessive cAMP signaling.
Almost all PRKAR1A non-sense substitutions, small insertions/deletions, variations of splicing sites lead to frame shifts and/or premature stop codons (PSC) that result in shorter or otherwise defective mRNAs, which are not encoded to protein because they are degraded by the nonsense-mediated mRNA decay (NMD) surveillance mechanism 10, 79, 86. Large deletions that include the whole gene also lead to PRKAR1A haploinsufficiency and almost half the protein levels compared to normal cells 70, 80.
Rarely, missense mutations of the PRKAR1A gene, short in-frame insertions/deletions and splice variants that are expressed at the protein level (because NMD is not activated) lead to the disease not due to haploinsufficiency but to a defective protein that fails to respond appropriately to cAMP or does not bind effectively to the PKA catalytic subunits87;88. Mutations that give rise to alternate splice sites may also be pathogenic by either leading to mRNA that is subject to NMD or a protein that does not bind appropriately cAMP or the catalytic subunits 81. Some PRKAR1A mutations lead to a longer protein due to loss of the stop codon normally situated in the last coding exon that is not subject to NMD. These longer PRKAR1A proteins undergo proteosomal degradation resulting again in PRKAR1A haploinsufficiency 89.
There are groups of CNC patients that show specific genotype-phenotype correlation and this also explains the CNC heterogeneity 12, 79. For example mutation c.709-7del6 is present in most patients with isolated PPNAD 81 and most of the remaining were c.1A>G carriers 79. It is difficult to conceive what molecular mechanisms underlie these phenotypic differences, since all these mutations lead to NMD. One suggestion is that small amounts of mutant RIα protein are in fact produced at least in certain tissues. In that case these phenotype – genotype correlations are due to their high frequency, and that additional phenotypic differences between the various PRKAR1A mutations leading to NMD will emerge as the number of observations increases. On the other hand, mutations that lead to an alternate PRKAR1A protein and not NMD (See Table 1) are associated with an overall higher total number of CNC manifestations and this could be due to a dominant-negative effect of the expressed mutant protein 5, 12, 78.
Mouse models have been created in order to study the role of PRKAR1A in CNC and the ability to cause CNC phenotypes. Prkar1a+/- mice developed non-pigmented schwannomas and fibro-osseous bone lesions beginning around 6 months of age. Later in life 10% of the mice also developed thyroid tumors 90. Another mouse model that had significantly higher Prkar1a down-regulation and significantly higher cAMP signaling, produced a more severe CNC phenotype91.
In the cases where PRKACA and PRKACB amplification was found, the mechanism of disease is presumed to be increased free PKA catalytic subunit activity 83. Although the molecular stoichiometry of how this occurs remains to be studied in vitro and in animal studies, tissues from patients with PRKACA and PRKACB copy number gain both had increased PKA activity 83 This is less clearly understood in the single patient with PRKACB gene amplification, since based on what is known about PKA, two additional copies of the gene (and modestly higher Cβ protein levels) should have had minimal effects on total PKA activity; nevertheless, the defect led to an unquestionable phenotype of CNC with pigmented spots, myxomas, and a growth-hormone (GH)-producing pituitary tumor 84.
Genetic Counseling
The identification of a pathogenic variant in the PRKAR1A gene can be used for diagnosing CNC. Molecular testing may be suggested then for relatives, or for new patients with two or more diagnostic criteria. If sequencing of the PRKAR1A gene does not show a defect, copy number variant (CNV) analysis by comparative genomic hybridization (CGH) and/or deletion PRKAR1A gene deletion testing may be needed to rule out a PRKAR1A defect. If all testing is negative for PRKAR1A defects, other candidate genes or loci may be screened including the PRKACA, PRKACB and the phosphodiesterase genes. However, the latter are mostly limited to research at this point.
Acknowledgments
This study was supported by the intramural research program of NICHD, NIH. We thank Diane Cooper, MSLS, NIH Library, for providing assistance in writing this manuscript.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Sandrini F, Stratakis C. Clinical and molecular genetics of Carney complex. Mol Genet Metab. 2003;78:83–92. doi: 10.1016/s1096-7192(03)00006-4. [DOI] [PubMed] [Google Scholar]
- 2.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–4046. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 3.Atherton DJ, Pitcher DW, Wells RS, MacDonald DM. A syndrome of various cutaneous pigmented lesions, myxoid neurofibromata and atrial myxoma: the NAME syndrome. Br J Dermatol. 1980;103:421–429. doi: 10.1111/j.1365-2133.1980.tb07266.x. [DOI] [PubMed] [Google Scholar]
- 4.Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Boikos SA, Stratakis CA. Carney complex: pathology and molecular genetics. Neuroendocrinology. 2006;83:189–199. doi: 10.1159/000095527. [DOI] [PubMed] [Google Scholar]
- 6.Bain J. Carney’s complex. Mayo Clin Proc. 1986;61:508. doi: 10.1016/s0025-6196(12)61989-2. [DOI] [PubMed] [Google Scholar]
- 7.Akbas H, Kirali K, Daglar B, Kutay V, Isik O, Yakut C. Surgical treatment of left-atrial myxoma in Carney’s complex. Thorac Cardiovasc Surg. 1997;45:148–150. doi: 10.1055/s-2007-1013711. [DOI] [PubMed] [Google Scholar]
- 8.Stratakis CA. Genetics of Carney complex and related familial lentiginoses, and other multiple tumor syndromes. Front Biosci. 2000;5:D353–366. doi: 10.2741/stratakis. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes AR, Silverman RA, Harrist TJ, Perez-Atayde AR. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72–82. doi: 10.1016/s0190-9622(84)80047-x. [DOI] [PubMed] [Google Scholar]
- 10.Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 11.Espiard S, Bertherat J. Carney complex. Front Horm Res. 2013;41:50–62. doi: 10.1159/000345669. [DOI] [PubMed] [Google Scholar]
- 12.Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, Rene-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–2091. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boikos SA, Stratakis CA. Carney complex: the first 20 years. Curr Opin Oncol. 2007;19:24–29. doi: 10.1097/CCO.0b013e32801195eb. [DOI] [PubMed] [Google Scholar]
- 14.Stratakis CA, Salpea P, Raygada M. Carney Complex. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews(R) Seattle (WA): 2013. [Google Scholar]
- 15.Mateus C, Palangie A, Franck N, Groussin L, Bertagna X, Avril MF, Bertherat J, Dupin N. Heterogeneity of skin manifestations in patients with Carney complex. J Am Acad Dermatol. 2008;59:801–810. doi: 10.1016/j.jaad.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Horvath A, Stratakis CA. Carney complex and lentiginosis. Pigment Cell Melanoma Res. 2009;22:580–587. doi: 10.1111/j.1755-148X.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothenbuhler A, Stratakis CA. Clinical and molecular genetics of Carney complex. Best Pract Res Clin Endocrinol Metab. 2010;24:389–399. doi: 10.1016/j.beem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Carney JA, Stratakis CA. Epithelioid blue nevus and psammomatous melanotic schwannoma: the unusual pigmented skin tumors of the Carney complex. Semin Diagn Pathol. 1998;15:216–224. [PubMed] [Google Scholar]
- 19.Stratakis CA, Kirschner LS, Carney JA. Carney complex: diagnosis and management of the complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas. Am J Med Genet. 1998;80:183–185. doi: 10.1002/(sici)1096-8628(19981102)80:2<183::aid-ajmg19>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Stratakis CA, Kirschner LS. Isolated familial somatotropinomas: does the disease map to 11q13 or to 2p16? J Clin Endocrinol Metab. 2000;85:4920–4921. doi: 10.1210/jcem.85.12.7076-1. [DOI] [PubMed] [Google Scholar]
- 21.Stratakis CA. Genetics of Peutz-Jeghers syndrome, Carney complex and other familial lentiginoses. Horm Res. 2000;54:334–343. doi: 10.1159/000053283. [DOI] [PubMed] [Google Scholar]
- 22.Vandersteen A, Turnbull J, Jan W, Simpson J, Lucas S, Anderson D, Lin JP, Stratakis C, Pichert G, Lim M. Cutaneous signs are important in the diagnosis of the rare neoplasia syndrome Carney complex. Eur J Pediatr. 2009;168:1401–1404. doi: 10.1007/s00431-009-0935-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carney JA, Ferreiro JA. The epithelioid blue nevus. A multicentric familial tumor with important associations, including cardiac myxoma and psammomatous melanotic schwannoma. Am J Surg Pathol. 1996;20:259–272. doi: 10.1097/00000478-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Wilkes D, McDermott DA, Basson CT. Clinical phenotypes and molecular genetic mechanisms of Carney complex. Lancet Oncol. 2005;6:501–508. doi: 10.1016/S1470-2045(05)70244-8. [DOI] [PubMed] [Google Scholar]
- 25.Carney JA, Headington JT, Su WP. Cutaneous myxomas. A major component of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Arch Dermatol. 1986;122:790–798. doi: 10.1001/archderm.122.7.790. [DOI] [PubMed] [Google Scholar]
- 26.Hachisuka J, Ichikawa M, Moroi Y, Urabe K, Furue M. A case of Carney complex. Int J Dermatol. 2006;45:1406–1407. doi: 10.1111/j.1365-4632.2006.02889.x. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy RH, Waller RR, Carney JA. Ocular pigmented spots and eyelid myxomas. Am J Ophthalmol. 1987;104:533–538. doi: 10.1016/s0002-9394(14)74112-1. [DOI] [PubMed] [Google Scholar]
- 28.Chinchurreta-Capote A, Trueba A, Hernandez FJ, Pinas P, Lopez S, Tena ME, Aznarez N, Portillo E, Castillon L. Ocular findings in Carney complex. Arch Soc Esp Oftalmol. 2006;81:709–711. doi: 10.4321/s0365-66912006001200007. [DOI] [PubMed] [Google Scholar]
- 29.Saavedra E, Singh AD, Sears JE, Ratliff NB. Plexiform pigmented schwannoma of the uvea. Surv Ophthalmol. 2006;51:162–168. doi: 10.1016/j.survophthal.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Tsilou ET, Chan CC, Sandrini F, Rubin BI, Shen DF, Carney JA, Kaiser-Kupfer M, Stratakis CA. Eyelid myxoma in Carney complex without PRKAR1A allelic loss. Am J Med Genet A. 2004;130A:395–397. doi: 10.1002/ajmg.a.30279. [DOI] [PubMed] [Google Scholar]
- 31.Courcoutsakis NA, Tatsi C, Patronas NJ, Lee CC, Prassopoulos PK, Stratakis CA. The complex of myxomas, spotty skin pigmentation and endocrine overactivity (Carney complex): imaging findings with clinical and pathological correlation. Insights Imaging. 2013;4:119–133. doi: 10.1007/s13244-012-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mabuchi T, Shimizu M, Ino H, Yamguchi M, Terai H, Fujino N, Nagata M, Sakata K, Inoue M, Yoneda T, Mabuchi H. PRKAR1A gene mutation in patients with cardiac myxoma. Int J Cardiol. 2005;102:273–277. doi: 10.1016/j.ijcard.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 33.Lee B, Sir JJ, Park SW, Kim SB, Nah JC, Kang YK, Lee HK, Kim YI, Cho WH, Choi SK. Right-sided myxomas with extramedullary hematopoiesis and ossification in Carney complex. Int J Cardiol. 2008;130:e63–65. doi: 10.1016/j.ijcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 34.Bireta C, Popov AF, Schotola H, Trethowan B, Friedrich M, El-Mehsen M, Schoendube FA, Tirilomis T. Carney-Complex: multiple resections of recurrent cardiac myxoma. J Cardiothorac Surg. 2011;6:12. doi: 10.1186/1749-8090-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boikos SA, Stratakis CA. Pituitary pathology in patients with Carney Complex: growth-hormone producing hyperplasia or tumors and their association with other abnormalities. Pituitary. 2006;9:203–209. doi: 10.1007/s11102-006-0265-2. [DOI] [PubMed] [Google Scholar]
- 36.Pack SD, Kirschner LS, Pak E, Zhuang Z, Carney JA, Stratakis CA. Genetic and histologic studies of somatomammotropic pituitary tumors in patients with the “complex of spotty skin pigmentation, myxomas, endocrine overactivity and schwannomas” (Carney complex) J Clin Endocrinol Metab. 2000;85:3860–3865. doi: 10.1210/jcem.85.10.6875. [DOI] [PubMed] [Google Scholar]
- 37.Iwata T, Tamanaha T, Koezuka R, Tochiya M, Makino H, Kishimoto I, Mizusawa N, Ono S, Inoshita N, Yamada S, Shimatsu A, Yoshimoto K. Germline deletion and a somatic mutation of the PRKAR1A gene in a Carney complex-related pituitary adenoma. Eur J Endocrinol. 2015;172:K5–10. doi: 10.1530/EJE-14-0685. [DOI] [PubMed] [Google Scholar]
- 38.Jones GN, Manchanda PK, Pringle DR, Zhang M, Kirschner LS. Mouse models of endocrine tumours. Best Pract Res Clin Endocrinol Metab. 2010;24:451–460. doi: 10.1016/j.beem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shenoy BV, Carpenter PC, Carney JA. Bilateral primary pigmented nodular adrenocortical disease. Rare cause of the Cushing syndrome. Am J Surg Pathol. 1984;8:335–344. doi: 10.1097/00000478-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Louiset E, Stratakis CA, Perraudin V, Griffin KJ, Libe R, Cabrol S, Feve B, Young J, Groussin L, Bertherat J, Lefebvre H. The paradoxical increase in cortisol secretion induced by dexamethasone in primary pigmented nodular adrenocortical disease involves a glucocorticoid receptor-mediated effect of dexamethasone on protein kinase A catalytic subunits. J Clin Endocrinol Metab. 2009;94:2406–2413. doi: 10.1210/jc.2009-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stratakis CA, Kirschner LS. Clinical and genetic analysis of primary bilateral adrenal diseases (micro- and macronodular disease) leading to Cushing syndrome. Horm Metab Res. 1998;30:456–463. doi: 10.1055/s-2007-978914. [DOI] [PubMed] [Google Scholar]
- 42.Bertherat J. Adrenocortical cancer in Carney complex: a paradigm of endocrine tumor progression or an association of genetic predisposing factors? J Clin Endocrinol Metab. 2012;97:387–390. doi: 10.1210/jc.2011-3327. [DOI] [PubMed] [Google Scholar]
- 43.Stratakis CA, Courcoutsakis NA, Abati A, Filie A, Doppman JL, Carney JA, Shawker T. Thyroid gland abnormalities in patients with the syndrome of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas (Carney complex) J Clin Endocrinol Metab. 1997;82:2037–2043. doi: 10.1210/jcem.82.7.4079. [DOI] [PubMed] [Google Scholar]
- 44.Bossis I, Voutetakis A, Bei T, Sandrini F, Griffin KJ, Stratakis CA. Protein kinase A and its role in human neoplasia: the Carney complex paradigm. Endocr Relat Cancer. 2004;11:265–280. doi: 10.1677/erc.0.0110265. [DOI] [PubMed] [Google Scholar]
- 45.Pringle DR, Yin Z, Lee AA, Manchanda PK, Yu L, Parlow AF, Jarjoura D, La Perle KM, Kirschner LS. Thyroid-specific ablation of the Carney complex gene, PRKAR1A, results in hyperthyroidism and follicular thyroid cancer. Endocr Relat Cancer. 2012;19:435–446. doi: 10.1530/ERC-11-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carney JA. Psammomatous melanotic schwannoma. A distinctive, heritable tumor with special associations, including cardiac myxoma and the Cushing syndrome. Am J Surg Pathol. 1990;14:206–222. [PubMed] [Google Scholar]
- 47.Rodriguez FJ, Stratakis CA, Evans DG. Genetic predisposition to peripheral nerve neoplasia: diagnostic criteria and pathogenesis of neurofibromatoses, Carney complex, and related syndromes. Acta Neuropathol. 2012;123:349–367. doi: 10.1007/s00401-011-0935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Utiger CA, Headington JT. Psammomatous melanotic schwannoma. A new cutaneous marker for Carney’s complex. Arch Dermatol. 1993;129:202–204. doi: 10.1001/archderm.129.2.202. [DOI] [PubMed] [Google Scholar]
- 49.Watson JC, Stratakis CA, Bryant-Greenwood PK, Koch CA, Kirschner LS, Nguyen T, Carney JA, Oldfield EH. Neurosurgical implications of Carney complex. J Neurosurg. 2000;92:413–418. doi: 10.3171/jns.2000.92.3.0413. [DOI] [PubMed] [Google Scholar]
- 50.Shields LB, Glassman SD, Raque GH, Shields CB. Malignant psammomatous melanotic schwannoma of the spine: A component of Carney complex. Surg Neurol Int. 2011;2:136. doi: 10.4103/2152-7806.85609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown B, Ram A, Clayton P, Humphrey G. Conservative management of bilateral Sertoli cell tumors of the testicle in association with the Carney complex: a case report. J Pediatr Surg. 2007;42:E13–15. doi: 10.1016/j.jpedsurg.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Azzopardi JG, Salm R. Ductal adenoma of the breast: a lesion which can mimic carcinoma. J Pathol. 1984;144:15–23. doi: 10.1002/path.1711440103. [DOI] [PubMed] [Google Scholar]
- 53.Carney JA, Toorkey BC. Ductal adenoma of the breast with tubular features. A probable component of the complex of myxomas, spotty pigmentation, endocrine overactivity, and schwannomas. Am J Surg Pathol. 1991;15:722–731. doi: 10.1097/00000478-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Stratakis CA, Papageorgiou T, Premkumar A, Pack S, Kirschner LS, Taymans SE, Zhuang Z, Oelkers WH, Carney JA. Ovarian lesions in Carney complex: clinical genetics and possible predisposition to malignancy. J Clin Endocrinol Metab. 2000;85:4359–4366. doi: 10.1210/jcem.85.11.6921. [DOI] [PubMed] [Google Scholar]
- 55.Gennari M, Stratakis CA, Hovarth A, Pirazzoli P, Cicognani A. A novel PRKAR1A mutation associated with hepatocellular carcinoma in a young patient and a variable Carney complex phenotype in affected subjects in older generations. Clin Endocrinol (Oxf) 2008;69:751–755. doi: 10.1111/j.1365-2265.2008.03286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barlow JF, Abu-Gazeleh S, Tam GE, Wirtz PS, Ofstein LC, O’Brien CP, Woods GL, Drymalski WG. Myxoid tumor of the uterus and right atrial myxomas. S D J Med. 1983;36:9–13. [PubMed] [Google Scholar]
- 57.Gaujoux S, Tissier F, Ragazzon B, Rebours V, Saloustros E, Perlemoine K, Vincent-Dejean C, Meurette G, Cassagnau E, Dousset B, Bertagna X, Horvath A, Terris B, Carney JA, Stratakis CA, Bertherat J. Pancreatic ductal and acinar cell neoplasms in Carney complex: a possible new association. J Clin Endocrinol Metab. 2011;96:E1888–1895. doi: 10.1210/jc.2011-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stergiopoulos SG, Stratakis CA. Human tumors associated with Carney complex and germline PRKAR1A mutations: a protein kinase A disease! FEBS Lett. 2003;546:59–64. doi: 10.1016/s0014-5793(03)00452-6. [DOI] [PubMed] [Google Scholar]
- 59.Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libe R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet. 2006;38:794–800. doi: 10.1038/ng1809. [DOI] [PubMed] [Google Scholar]
- 60.Groussin L, Jullian E, Perlemoine K, Louvel A, Leheup B, Luton JP, Bertagna X, Bertherat J. Mutations of the PRKAR1A gene in Cushing’s syndrome due to sporadic primary pigmented nodular adrenocortical disease. J Clin Endocrinol Metab. 2002;87:4324–4329. doi: 10.1210/jc.2002-020592. [DOI] [PubMed] [Google Scholar]
- 61.Veugelers M, Bressan M, McDermott DA, Weremowicz S, Morton CC, Mabry CC, Lefaivre JF, Zunamon A, Destree A, Chaudron JM, Basson CT. Mutation of perinatal myosin heavy chain associated with a Carney complex variant. N Engl J Med. 2004;351:460–469. doi: 10.1056/NEJMoa040584. [DOI] [PubMed] [Google Scholar]
- 62.Fogt F, Zimmerman RL, Hartmann CJ, Brown CA, Narula N. Genetic alterations of Carney complex are not present in sporadic cardiac myxomas. Int J Mol Med. 2002;9:59–60. [PubMed] [Google Scholar]
- 63.Kjellman M, Larsson C, Backdahl M. Genetic background of adrenocortical tumor development. World J Surg. 2001;25:948–956. doi: 10.1007/s00268-001-0034-3. [DOI] [PubMed] [Google Scholar]
- 64.Frohman LA. Isolated familial somatotropinomas: clinical and genetic considerations. Trans Am Clin Climatol Assoc. 2003;114:165–177. [PMC free article] [PubMed] [Google Scholar]
- 65.Yamasaki H, Mizusawa N, Nagahiro S, Yamada S, Sano T, Itakura M, Yoshimoto K. GH-secreting pituitary adenomas infrequently contain inactivating mutations of PRKAR1A and LOH of 17q23–24. Clin Endocrinol (Oxf) 2003;58:464–470. doi: 10.1046/j.1365-2265.2003.01740.x. [DOI] [PubMed] [Google Scholar]
- 66.Sandrini F, Kirschner LS, Bei T, Farmakidis C, Yasufuku-Takano J, Takano K, Prezant TR, Marx SJ, Farrell WE, Clayton RN, Groussin L, Bertherat J, Stratakis CA. PRKAR1A, one of the Carney complex genes, and its locus (17q22–24) are rarely altered in pituitary tumours outside the Carney complex. J Med Genet. 2002;39:e78. doi: 10.1136/jmg.39.12.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCarthy PM, Piehler JM, Schaff HV, Pluth JR, Orszulak TA, Vidaillet HJ, Jr, Carney JA. The significance of multiple, recurrent, and “complex” cardiac myxomas. J Thorac Cardiovasc Surg. 1986;91:389–396. [PubMed] [Google Scholar]
- 68.Carney JA. Carney complex: the complex of myxomas, spotty pigmentation, endocrine overactivity, and schwannomas. Semin Dermatol. 1995;14:90–98. doi: 10.1016/s1085-5629(05)80003-3. [DOI] [PubMed] [Google Scholar]
- 69.Cignarelli M, Picca G, Campo M, Margaglione M, Marino A, Logoluso F, Giorgino F. A six month mitotane course induced sustained correction of hypercortisolism in a young woman with PPNAD and Carney complex. J Endocrinol Invest. 2005;28:54–60. doi: 10.1007/BF03345530. [DOI] [PubMed] [Google Scholar]
- 70.Salpea P, Horvath A, London E, Faucz FR, Vetro A, Levy I, Gourgari E, Dauber A, Holm IA, Morrison PJ, Keil MF, Lyssikatos C, Smith ED, Sanidad MA, Kelly JC, Dai Z, Mowrey P, Forlino A, Zuffardi O, Stratakis CA. Deletions of the PRKAR1A locus at 17q24.2-q24. 3 in Carney complex: genotype-phenotype correlations and implications for genetic testing. J Clin Endocrinol Metab. 2014;99:E183–188. doi: 10.1210/jc.2013-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Board CNE. ASoCOA- Cancer.Net, editor. Cancer Net. 2012. Carney Complex. [Google Scholar]
- 72.Developement NIoCHaH. Recommended clinical surveillance of patients with CNC (PDF) 2014 [Google Scholar]
- 73.Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K American Society of Clinical O. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 74.Imai Y, Taketani T, Maemura K, Takeda N, Harada T, Nojiri T, Kawanami D, Monzen K, Hayashi D, Murakawa Y, Ohno M, Hirata Y, Yamazaki T, Takamoto S, Nagai R. Genetic analysis in a patient with recurrent cardiac myxoma and endocrinopathy. Circ J. 2005;69:994–995. doi: 10.1253/circj.69.994. [DOI] [PubMed] [Google Scholar]
- 75.Zawadzki KM, Taylor SS. cAMP-dependent protein kinase regulatory subunit type IIbeta: active site mutations define an isoform-specific network for allosteric signaling by cAMP. J Biol Chem. 2004;279:7029–7036. doi: 10.1074/jbc.M310804200. [DOI] [PubMed] [Google Scholar]
- 76.Cazabat L, Ragazzon B, Groussin L, Bertherat J. PRKAR1A mutations in primary pigmented nodular adrenocortical disease. Pituitary. 2006;9:211–219. doi: 10.1007/s11102-006-0266-1. [DOI] [PubMed] [Google Scholar]
- 77.Stratakis CA, Carney JA, Lin JP, Papanicolaou DA, Karl M, Kastner DL, Pras E, Chrousos GP. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest. 1996;97:699–705. doi: 10.1172/JCI118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, Libe R, Remmers E, Rene-Corail F, Faucz FR, Clauser E, Calender A, Bertagna X, Carney JA, Stratakis CA. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): an update. Hum Mutat. 2010;31:369–379. doi: 10.1002/humu.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the carney complex. Hum Mol Genet. 2000;9:3037–3046. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 80.Horvath A, Bossis I, Giatzakis C, Levine E, Weinberg F, Meoli E, Robinson-White A, Siegel J, Soni P, Groussin L, Matyakhina L, Verma S, Remmers E, Nesterova M, Carney JA, Bertherat J, Stratakis CA. Large deletions of the PRKAR1A gene in Carney complex. Clin Cancer Res. 2008;14:388–395. doi: 10.1158/1078-0432.CCR-07-1155. [DOI] [PubMed] [Google Scholar]
- 81.Groussin L, Horvath A, Jullian E, Boikos S, Rene-Corail F, Lefebvre H, Cephise-Velayoudom FL, Vantyghem MC, Chanson P, Conte-Devolx B, Lucas M, Gentil A, Malchoff CD, Tissier F, Carney JA, Bertagna X, Stratakis CA, Bertherat J. A PRKAR1A mutation associated with primary pigmented nodular adrenocortical disease in 12 kindreds. J Clin Endocrinol Metab. 2006;91:1943–1949. doi: 10.1210/jc.2005-2708. [DOI] [PubMed] [Google Scholar]
- 82.Matyakhina L, Pack S, Kirschner LS, Pak E, Mannan P, Jaikumar J, Taymans SE, Sandrini F, Carney JA, Stratakis CA. Chromosome 2 (2p16) abnormalities in Carney complex tumours. J Med Genet. 2003;40:268–277. doi: 10.1136/jmg.40.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beuschlein F, Fassnacht M, Assie G, Calebiro D, Stratakis CA, Osswald A, Ronchi CL, Wieland T, Sbiera S, Faucz FR, Schaak K, Schmittfull A, Schwarzmayr T, Barreau O, Vezzosi D, Rizk-Rabin M, Zabel U, Szarek E, Salpea P, Forlino A, Vetro A, Zuffardi O, Kisker C, Diener S, Meitinger T, Lohse MJ, Reincke M, Bertherat J, Strom TM, Allolio B. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med. 2014;370:1019–1028. doi: 10.1056/NEJMoa1310359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Forlino A, Vetro A, Garavelli L, Ciccone R, London E, Stratakis CA, Zuffardi O. PRKACB and Carney complex. N Engl J Med. 2014;370:1065–1067. doi: 10.1056/NEJMc1309730. [DOI] [PubMed] [Google Scholar]
- 85.Yu S, Maillard RA, Gribenko AV, Lee JC. The N-terminal capping propensities of the D-helix modulate the allosteric activation of the Escherichia coli cAMP receptor protein. J Biol Chem. 2012;287:39402–39411. doi: 10.1074/jbc.M112.404806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Groussin L, Kirschner LS, Vincent-Dejean C, Perlemoine K, Jullian E, Delemer B, Zacharieva S, Pignatelli D, Carney JA, Luton JP, Bertagna X, Stratakis CA, Bertherat J. Molecular analysis of the cyclic AMP-dependent protein kinase A (PKA) regulatory subunit 1A (PRKAR1A) gene in patients with Carney complex and primary pigmented nodular adrenocortical disease (PPNAD) reveals novel mutations and clues for pathophysiology: augmented PKA signaling is associated with adrenal tumorigenesis in PPNAD. Am J Hum Genet. 2002;71:1433–1442. doi: 10.1086/344579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meoli E, Bossis I, Cazabat L, Mavrakis M, Horvath A, Stergiopoulos S, Shiferaw ML, Fumey G, Perlemoine K, Muchow M, Robinson-White A, Weinberg F, Nesterova M, Patronas Y, Groussin L, Bertherat J, Stratakis CA. Protein kinase A effects of an expressed PRKAR1A mutation associated with aggressive tumors. Cancer Res. 2008;68:3133–3141. doi: 10.1158/0008-5472.CAN-08-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greene EL, Horvath AD, Nesterova M, Giatzakis C, Bossis I, Stratakis CA. In vitro functional studies of naturally occurring pathogenic PRKAR1A mutations that are not subject to nonsense mRNA decay. Hum Mutat. 2008;29:633–639. doi: 10.1002/humu.20688. [DOI] [PubMed] [Google Scholar]
- 89.Patronas Y, Horvath A, Greene E, Tsang K, Bimpaki E, Haran M, Nesterova M, Stratakis CA. In vitro studies of novel PRKAR1A mutants that extend the predicted RIalpha protein sequence into the 3′-untranslated open reading frame: proteasomal degradation leads to RIalpha haploinsufficiency and Carney complex. J Clin Endocrinol Metab. 2012;97:E496–502. doi: 10.1210/jc.2011-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH, 2nd, Carney JA, Westphal H, Stratakis CA. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–4514. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 91.Griffin KJ, Kirschner LS, Matyakhina L, Stergiopoulos SG, Robinson-White A, Lenherr SM, Weinberg FD, Claflin ES, Batista D, Bourdeau I, Voutetakis A, Sandrini F, Meoli EM, Bauer AJ, Cho-Chung YS, Bornstein SR, Carney JA, Stratakis CA. A transgenic mouse bearing an antisense construct of regulatory subunit type 1A of protein kinase A develops endocrine and other tumours: comparison with Carney complex and other PRKAR1A induced lesions. J Med Genet. 2004;41:923–931. doi: 10.1136/jmg.2004.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]